Abstract
Staphylococcus aureus and Escherichia coli are among the most prevalent species of gram-positive and gram-negative bacteria, respectively, that induce clinical mastitis. The innate immune system comprises the immediate host defense mechanisms to protect against infection and contributes to the initial detection of and proinflammatory response to infectious pathogens. The objective of the present study was to characterize the different innate immune responses to experimental intramammary infection with E. coli and S. aureus during clinical mastitis. The cytokine response and changes in the levels of soluble CD14 (sCD14) and lipopolysaccharide-binding protein (LBP), two proteins that contribute to host recognition of bacterial cell wall products, were studied. Intramammary infection with either E. coli or S. aureus elicited systemic changes, including decreased milk output, a febrile response, and induction of the acute-phase synthesis of LBP. Infection with either bacterium resulted in increased levels of interleukin 1β (IL-1β), gamma interferon, IL-12, sCD14, and LBP in milk. High levels of the complement cleavage product C5a and the anti-inflammatory cytokine IL-10 were detected at several time points following E. coli infection, whereas S. aureus infection elicited a slight but detectable increase in these mediators at a single time point. Increases in IL-8 and tumor necrosis factor alpha were observed only in quarters infected with E. coli. Together, these data demonstrate the variability of the host innate immune response to E. coli and S. aureus and suggest that the limited cytokine response to S. aureus may contribute to the well-known ability of the bacterium to establish chronic intramammary infection.
Mastitis is an inflammation of the mammary gland that often develops in response to intramammary bacterial infection (51). Mastitis is an important public health problem in humans in developing countries, as well as in domesticated animals used in agriculture worldwide. In humans, mastitis is associated with increased mother-to-child transmission of human immunodeficiency virus (27) and bacterial pathogens (8, 46). Further, the inflammatory mediators produced during the host response to intramammary infection have been implicated in infant gut damage (54, 70). In addition to its implications in the pathogenesis of disease in humans, mastitis remains one of the most costly diseases in animal agriculture, with economic losses to the dairy industry approaching $2 billion annually in the United States alone (37).
Staphylococcus aureus and Escherichia coli account for the majority of clinical mastitis cases in cattle (7), whereas, S. aureus is the most prevalent pathogen associated with human mastitis (4). Striking differences exist between the courses of bovine intramammary infection caused by S. aureus and E. coli. Intramammary infection by E. coli is acute in nature and generally clears within a few days (57). In contrast, infection by S. aureus is often less severe but results in a chronic infection that can persist for the life of the animal (60).
The innate immune system represents the first line of defense in the host response to infection and is poised to immediately recognize and respond to the earliest stages of infection (24). Further, the innate immune system is able to respond to pathogens that have not been previously encountered. The inherent capability of the innate immune system to respond to a vast number of pathogens is mediated by its ability to recognize highly conserved motifs shared by diverse pathogens. These motifs, commonly referred to as pathogen-associated molecular patterns (PAMPs), include the bacterial cell wall components, lipopolysaccharide (LPS), peptidoglycan (PGN), and lipoteichoic acid (LTA) (1). The ability to recognize common PAMPs on distinct pathogens (e.g., the presence of LPS on all gram-negative bacteria) enables the innate immune system to respond to vast numbers of infectious agents with only a limited repertoire of host recognition elements.
Innate recognition of PAMPs is mediated by evolutionarily conserved pattern recognition receptors (PRRs) (1). Toll-like receptors (TLRs) comprise a family of PRRs that are capable of recognizing distinct PAMPs. At least 10 members of the TLR family have been identified in mammals, and each member is capable of recognizing a distinct PAMP (66). For example, TLR-2 recognizes PGN (72) and LTA (35, 52) from S. aureus and other gram-positive bacteria, whereas, TLR-4 recognizes LPS from gram-negative bacteria, including E. coli (22). PGN and LPS activations of distinct TLRs elicit different in vitro cellular responses (2, 38, 44). Further, differential activation of TLRs by whole gram-positive or gram-negative bacteria evokes distinct gene expression profiles in vitro (38).
A key component of the host innate immune response to infection is the upregulation of cytokine production (15, 28, 59). Two well-described proinflammatory cytokines, tumor necrosis factor alpha (TNF-α) and interleukin 1β (IL-1β), mediate the inflammatory response at both the local and systemic levels (15, 62). Locally, these cytokines induce vascular endothelial adhesion molecule expression, thereby promoting neutrophil transendothelial migration to the site of infection. Systemically, TNF-α and IL-1β are potent inducers of fever and the acute-phase response. Induction of the acute-phase response results in increased hepatic synthesis of proteins, such as LPS-binding protein (LBP) and C-reactive protein, which facilitate host detection of bacterial-wall products and complement activation, respectively (67). Neutrophil recruitment to the site of infection is further mediated by the upregulation of the chemoattractant IL-8 (20). IL-12 contributes to the innate immune response by stimulating the production of gamma interferon (IFN-γ), an activator of neutrophils and macrophages (64). Finally, resolution of the inflammatory process is mediated by the upregulation of IL-10, which downregulates proinflammatory cytokine production (45, 58).
Since establishment of infection is governed, in part, by the nature of the host response to the invading organism and E. coli and S. aureus are known to follow distinct clinical courses during intramammary infection, the present study examined the differential in vivo cytokine response elicited in the mammary gland during infection with either S. aureus or E. coli. In addition, we studied changes in the intramammary concentrations of LBP and soluble CD14 (sCD14), two accessory molecules that contribute to host innate recognition of components of the bacterial cell wall.
MATERIALS AND METHODS
Cows.
Sixteen healthy midlactating Holstein cows (214 ± 8.67 days in milk) were selected on the basis of milk somatic cell counts (SCC) of <500,000/ml and the absence of detectable bacterial growth from three daily consecutive aseptically collected milk samples plated on blood agar plates. The use and care of all animals in this study were approved by the Beltsville Agricultural Research Center's Animal Care and Use Committee.
Intramammary challenge with E. coli or S. aureus.
Prior to intramammary challenge, 10 ml of brain heart infusion broth (Becton Dickinson Diagnostic Systems, Inc., Sparks, Md.) was inoculated with either E. coli strain P4 (a gift of A. J. Bramley, Institute for Animal Health, Compton Laboratory, Newbury, England) or S. aureus strain 305 (American Type Culture Collection, Manassas, Va.) and incubated for 6 h at 37°C. These strains were originally isolated from clinical cases of mastitis and have been used as model organisms in other studies of mastitis (9, 40). One milliliter of the incubated cultures was transferred to aerating flasks containing 99 ml of tryptic soy broth and incubated overnight at 37°C. After incubation, the flasks were placed in an ice water bath and mixed by swirling. A 1-ml aliquot from each flask was serially diluted in PBS, and 1 ml of the resulting dilutions was mixed with 9 ml of premelted trypticase soy agar in petri dishes. The plates were allowed to solidify at room temperature and then were transferred to a 37°C incubator overnight. The aerating flasks containing the stock cultures were maintained at 4°C overnight. After the concentration of the stock culture was determined based on the prepared pour plates, the stock culture was diluted in phosphate-buffered saline (PBS) to yield a final concentration of ∼40 CFU/ml.
Immediately following the morning milking, one front or rear quarter of each of 16 cows was infused with either E. coli (n = 8) or S. aureus (n = 8). The contralateral quarter of each infected quarter was infused with 2 ml of PBS. Pour plating of the final prepared inoculum confirmed that the cows received 72 or 74 CFU of E. coli or S. aureus/quarter, respectively. Following challenge, aseptic milk samples were collected from all quarters at various times, serially diluted, and plated on blood agar plates. Following a 16-h incubation at 37°C, the numbers of CFU per milliliter were determined. Colonies composed of gram-positive cocci that were both catalase and coagulase positive were initially counted as S. aureus. Those colonies that displayed characteristics similar to those of E. coli and that were confirmed to be gram-negative, oxidase-negative rods were initially counted as E. coli. Further biochemical tests and gas chromatography were performed by the Animal Health Section, Maryland Department of Agriculture (College Park, Md.), to confirm the initial identification.
Determination of milk SCC and circulating-neutrophil counts.
To quantitate somatic cells, milk samples were heated to 60°C and subsequently maintained at 40°C until the cells were counted on an automated cell counter (Fossomatic model 90; Foss Food Technology, Hillerod, Denmark) as previously described (34). For the determination of circulating-neutrophil counts, tail vein blood samples were collected in Vacutainer glass tubes containing K3 EDTA (Becton Dickinson Corp., Franklin Lakes, N.J.), inverted 10 times, placed on a rocker for 15 min, and analyzed using a HEMAVET 3700 automated multispecies hematology system (CDC Technologies, Inc., Oxford, Conn.).
Whey and plasma preparation.
For the preparation of whey, milk samples were centrifuged at 44,000 × g and 4°C for 30 min, and the fat layer was removed with a spatula. The skim milk was decanted into a clean tube and centrifuged again for 30 min as described above, and the translucent supernatant was collected and stored at −70°C. For the preparation of plasma, tail vein blood samples were collected as described above, inverted 10 times, and centrifuged at 1,500 × g for 15 min, and the clear plasma supernatant was collected, aliquoted, and stored at −70°C.
ELISAs for BSA, IL-8, IFN-γ, sCD14, and LBP.
Enzyme-linked immunosorbent assays (ELISAs) for bovine serum albumin (BSA), IL-8, sCD14, and LBP were performed as previously described (6). A commercially available kit (Biosource International, Inc., Camarillo, Calif.) was used to measure bovine IFN-γ in undiluted whey samples according to the manufacturer's instructions. Recombinant bovine IFN-γ (Serotec, Inc., Raleigh, N.C.) was used to generate a standard curve for the ELISA.
ELISAs for TNF-α and IL-1β.
Flat-bottom 96-well plates (Nalge Nunc International, Rochester, N.Y.) were coated overnight at 4°C with 100 μl of 0.05 M sodium carbonate, pH 9.6, containing either mouse anti-recombinant bovine TNF-α (diluted 1:1,000) (41) or mouse anti-ovine IL-1β (5 μg/ml) antibody (Serotec, Inc.). The plates were washed four times with 0.05% Tween 20 diluted in 50 mM Tris-buffered saline (TBS), pH 8.0, and subsequently blocked with 2% fish skin gelatin (Sigma Chemical Co., St. Louis, Mo.) for 1 h at room temperature. The plates were washed, and 100 μl of diluted whey samples (1:5 and 1:1, respectively, for the anti-TNF-α- and anti-IL-1β-coated plates) was added to each well. Standard curves of known amounts of either recombinant bovine TNF-α (Genentech Corp., South San Francisco, Calif.) or recombinant ovine IL-1β (a gift from CSIRO Livestock Industries, Victoria, Australia) were assayed in parallel. The plates were incubated for 1.5 h at room temperature and subsequently washed as described above. Rabbit anti-recombinant bovine TNF-α polyclonal serum (41) or rabbit anti-recombinant ovine IL-1β polyclonal serum (Serotec) was diluted 1:5,000 or 1:500, respectively, in TBS wash buffer containing 2% gelatin, and 100 μl was added to each well. The plates were incubated for 1 h at room temperature and washed. Goat anti-rabbit immunoglobulin G (IgG) conjugated to horseradish peroxidase (HRP) (Becton Dickinson Corp.) was diluted 1:5,000 (TNF-α plates) or 1:10,000 (IL-1β plates) in TBS wash buffer containing 2% gelatin, and 100 μl was added to each well of the respective plates. Following a 1-h incubation, the plates were washed, and 100 μl of 3,3′,5,5′-tetramethylbenzidine (TMB) substrate solution (Kirkegaard and Perry Laboratories Inc., Gaithersburg, Md.) was added to each well. The reaction was stopped by the addition of 100 μl of 2 M H2SO4, and the absorbance was read at 450 nm on a microplate reader (Bio-Tec Instruments, Inc., Winooski, Vt.). A background correction reading at 565 nm was subtracted from the 450-nm absorbance readings.
ELISA for IL-10 and IL-12.
Concentrations of IL-10 and IL-12 were determined as previously described with slight modification (25, 29). Briefly, flat-bottom 96-well plates were coated overnight at 4°C with 4 μg of mouse anti-bovine IL-10 (CC-318) or IL-12 (CC-301) antibody/ml diluted in 0.05 M sodium carbonate, pH 9.6. The plates were washed four times with 0.05% Tween 20 diluted in 50 mM TBS, pH 8.0, and subsequently blocked with 2% fish skin gelatin for 1 h at room temperature. The plates were washed, and 100 μl of diluted (1:5) or undiluted whey samples was added to the anti-IL-10- or anti-IL-12-coated plates, respectively. Following a 1-h incubation at room temperature, the plates were washed, and 100 μl of either biotin-conjugated mouse anti-bovine IL-10 (CC-320) or IL-12 (CC-326) antibody diluted to 1 or 8 μg/ml, respectively, was added to the wells of the respective plates. The plates were incubated for 1 h at room temperature and washed. HRP-conjugated streptavidin (Sigma Chemical Co.) was diluted 1:500 in TBS wash buffer containing 2% gelatin, and 100 μl of this solution was added to each well. The plates were incubated for 1 h at room temperature and washed, and TMB substrate solution as added to each well. The reaction was stopped by the addition of 100 μl of 2 M H2SO4, and the absorbance was read at 450 nm on a microplate reader. A background correction reading at 565 nm was subtracted from the 450-nm absorbance readings. Supernatants derived from COS-7 cells transfected with either IL-10- or IL-12-encoding plasmids and previously assayed for biological activity were used to generate a standard curve for each ELISA (25, 29). The concentration of IL-10 or IL-12 in milk was calculated by extrapolating from the respective standard curves, and the values were expressed as biological units of activity per milliliter.
ELISA for C5a.
Concentrations of complement cleavage factor 5a (C5a) in milk were measured by sandwich ELISA using anti-bovine C5a monoclonal antibody for antigen capture and rabbit anti-bovine C5a antiserum for detection (43). Flat-bottom 96-well plates were coated overnight at 4°C with goat anti-mouse IgG (Jackson Immunoresearch Laboratories, Inc., West Grove, Pa.) diluted to 5 μg/ml in 0.05 M sodium carbonate, pH 9.6. The plates were washed five times with 0.05% Tween 20 diluted in 50 mM TBS, pH 8.0, and subsequently blocked with 0.5% fish skin gelatin for 30 min at 37°C. The plates were washed, and 100 μl of anti-C5a monoclonal antibody/well diluted 1:5,000 in 0.05% Tween 20-TBS containing 0.1% gelatin was added to each well. Following a 1-h incubation at 37°C, the plates were washed, and 100 μl of whey diluted 1:10 in 0.05% Tween 20-TBS containing 0.1% gelatin and 1 mM EDTA was added to each well. The plates were incubated for 1.5 h at room temperature and washed, and 100 μl of rabbit anti-bovine C5a/C5 diluted 1:2,500 in 0.05% Tween 20-TBS containing 0.1% gelatin was added to each well. The plates were incubated for 30 min at 37°C and washed, and 100 μl of goat anti-rabbit IgG conjugated to HRP (1:5,000 dilution in 0.05% Tween 20-TBS containing 0.1% gelatin) was added to each well. Following a 30-min incubation at 37°C, the plates were washed, and 100 μl of TMB substrate solution was added to each well. The reaction was stopped by the addition of 100 μl of 2 M H2SO4, and the absorbance was read at 450 nm on a microplate reader. A background correction reading at 565 nm was subtracted from the 450-nm absorbance readings. Values expressed in nanograms per milliliter were calculated from a standard curve of known amounts of purified C5a.
Statistical methods.
Repeated-measures analysis of variance with the Dunnett post hoc comparison test was performed using the PROC MIXED model (SAS version 8.2; SAS Institute, Cary, N.C.) to compare the mean responses of the experimental groups and the preinfused (time zero) groups. For statistical analysis of milk SCC, data were transformed to log10 values. An unpaired t test (Prism version 4.0 for Windows; GraphPad Software Inc., San Diego, Calif.) was used to compare the maximal responses elicited by S. aureus and E. coli in a given experimental assay. A P value of <0.05 was considered significant.
RESULTS
Bacterial growth in infected quarters.
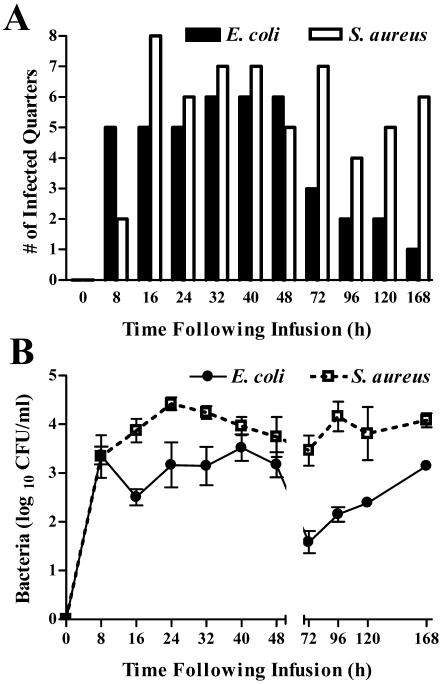
Within 16 h of challenge, viable bacteria were recovered from all eight quarters challenged with S. aureus (Fig. 1A). Transient decreases in the number of quarters from which viable S. aureus organisms were recovered occurred at 48 and 96 h postinfection and may reflect the cyclical shedding of S. aureus into milk that has been described (13). In those eight quarters infused with E. coli, viable bacteria were recovered at least once from each infected quarter within 32 h of challenge; however, at no single time point were bacteria recovered from all eight quarters. The viable bacteria recovered from the S. aureus- and E. coli-infused quarters were all confirmed to be the same species of bacteria infused at time zero. Saline-infused quarters remained free of detectable pathogens throughout the study. Within 8 h of challenge, comparable numbers of E. coli (3.36 ± 0.18 log10 CFU/ml) and S. aureus (3.34 ± 0.48 log10 CFU/ml) were recovered from infected glands (Fig. 1B). At later time points, however, consistently higher numbers of CFU were recovered from S. aureus-infected quarters than from those infected with E. coli. By the final day of the study, E. coli was recovered from only a single quarter, whereas S. aureus was recovered from six of the eight initially infected quarters (Fig. 1A).
FIG. 1.
Intramammary growth of E. coli and S. aureus following experimental challenge. Following intramammary infusion of eight quarters with 72 CFU of E. coli or eight quarters with 74 CFU of S. aureus, sterile milk samples were collected from all infused quarters at various times and plated. (A) Number of quarters in which viable bacteria were recovered. (B) Means (± standard error) of log10 CFU per milliliter in those quarters where bacteria were recovered.
Systemic response to intramammary challenge with either E. coli or S. aureus.
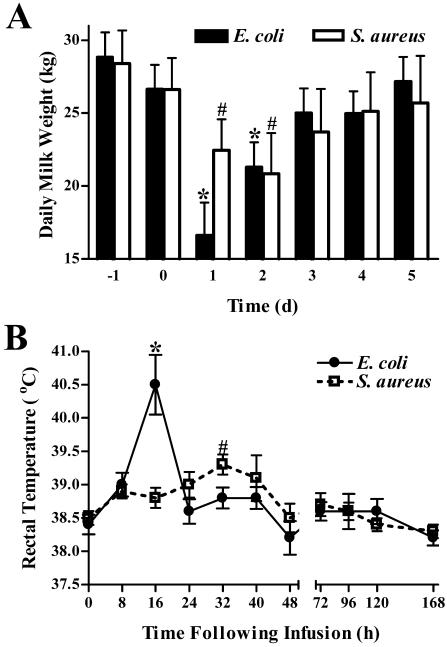
Daily milk weights for cows infected with either E. coli or S. aureus dropped by ∼42 and ∼21%, respectively, on the day following challenge (Fig. 2A). The mean milk output of E. coli-infected cows (16.62 ± 2.23 kg) was significantly lower than that of cows infected with S. aureus (22.44 ± 2.14 kg) on the first day after challenge (P = 0.041). Milk output remained significantly depressed for 2 days following E. coli or S. aureus challenge. Elevated rectal temperatures were detected at 16 and 32 h postinfection with E. coli or S. aureus, respectively (Fig. 2B). Peak elevations in body temperature were higher in cows receiving E. coli (40.5 ± 0.45°C) than in those infused with S. aureus (39.3 ± 0.15°C) (P = 0.024).
FIG. 2.
Effects of intramammary infection with E. coli or S. aureus on daily milk weights and temperatures. (A) Total milk weight (sum of morning and evening outputs) data were collected 1 day prior to infection (−1), the day of infection (0), and for 5 days following infection. The vertical bars represent the means (plus standard error) of milk weights. * and #, significantly decreased compared to prechallenge levels (day −1) in cows challenged with E. coli or S. aureus, respectively (P < 0.05). (B) Rectal temperatures were measured immediately prior to and at various times following intramammary infection as an indicator of the systemic response. Mean (± standard error) temperatures are shown. * and #, significantly increased compared to time zero in cows challenged with E. coli or S. aureus, respectively (P < 0.05).
Intramammary infection with either E. coli or S. aureus induces an increase in milk SCC with or without, respectively, a corresponding decrease in circulating neutrophils.
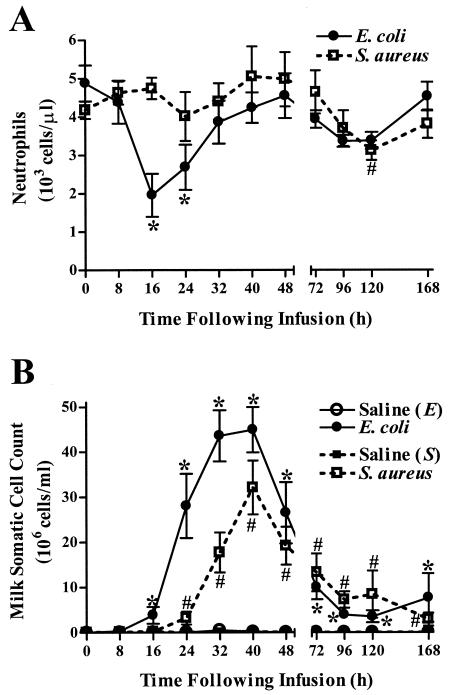
A decrease in the number of circulating neutrophils was evident within 16 h of E. coli challenge and reached a minimum of 1,963 ± 562 cells/μl (Fig. 3A). In contrast, significant decreases in circulating neutrophils in cows challenged with S. aureus were not observed until 120 h after infection. Increases in milk somatic cells, which are primarily composed of neutrophils during the acute phase of infection (50), were evident within 16 and 24 h of E. coli or S. aureus challenge, respectively (Fig. 3B). Milk SCC peaked within 40 h of infection in quarters challenged with either bacterium. There was no significant difference (P = 0.12) between the maximal milk SCC reached in quarters infected with E. coli (44.9 × 106 ± 5.04 × 106 cells/ml) and in those infected with S. aureus (32.1 × 106 ± 5.9 × 106 cells/ml). Milk SCC in all infected quarters remained elevated relative to prechallenge levels throughout the study.
FIG. 3.
Effects of intramammary infection with E. coli or S. aureus on circulating neutrophils and milk SCC. (A) Total differential neutrophil counts were determined in blood samples collected immediately prior to and at various times following intramammary infection. Mean (± standard error) cell counts are shown. * and #, significantly decreased compared to time zero in cows challenged with E. coli or S. aureus, respectively (P < 0.05). (B) Milk SCC were quantified in milk samples collected from both infected and PBS-infused quarters throughout the study. Mean (± standard error) milk SCC are shown. * and #, significantly increased in E. coli- or S. aureus-infected quarters, respectively, relative to time zero (P < 0.05).
Intramammary infection with E. coli or S. aureus elicits an increase in mammary vascular permeability.
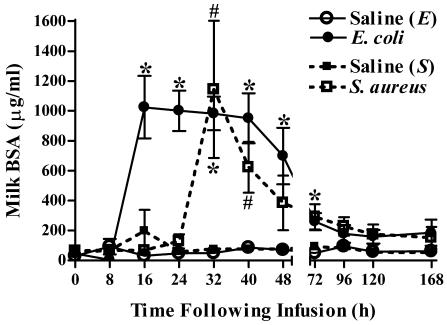
Quarters infused with either E. coli or S. aureus demonstrated increases in levels of BSA in milk, which reflected increases in mammary vascular permeability (Fig. 4). Initial increases in BSA levels in E. coli-infused quarters preceded by 16 h those increases observed in quarters inoculated with S. aureus. The maximal increases in BSA levels were comparable in quarters infected with either bacterium. Within 48 to 72 h of infection, milk BSA levels in all infected quarters returned to prechallenge levels.
FIG. 4.
Intramammary infection with E. coli or S. aureus increases concentrations of BSA in milk. As a marker of mammary vascular permeability, BSA levels were assayed by ELISA in milk samples obtained from quarters immediately prior to and at various times following intramammary infusion with saline, E. coli, or S. aureus. Mean (± standard error) BSA levels are shown. * and #, significantly increased in E. coli- or S. aureus-infected quarters, respectively, relative to time zero (P < 0.05).
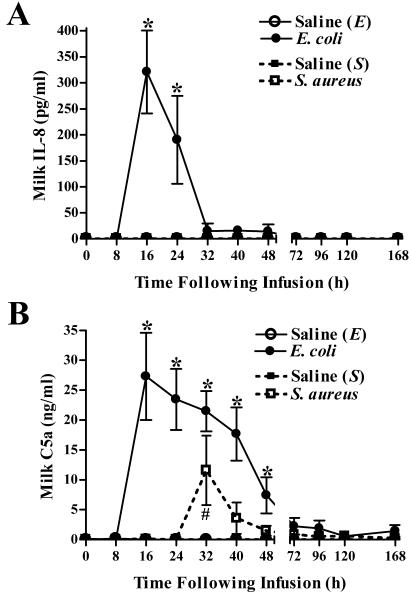
Differential changes in levels of IL-8 and C5a following intramammary challenge with either E. coli or S. aureus.
Intramammary infection with E. coli induced an increase in concentrations of the chemoattractants IL-8 and C5a in milk within 16 h of challenge (Fig. 5). The elevated levels of IL-8 and C5a in milk persisted through >24 and 48 h, respectively, following challenge. S. aureus elicited a transient increase in C5a concentrations in milk at 32 h postinfection (Fig. 5B) but failed to induce IL-8 production (Fig. 5A). The increase in C5a in S. aureus-infected quarters varied considerably among cows, and two of the eight infected quarters showed no measurable increases. Increases in C5a were detected in all quarters challenged with E. coli. The mean peak concentrations of C5a following E. coli infection (27.29 ± 7.29 ng/ml) approached, but did not reach, levels that were significantly different from those in S. aureus-infected quarters (11.59 ± 5.79 ng/ml) (P = 0.057).
FIG. 5.
Effects of intramammary infection with E. coli or S. aureus on concentrations of IL-8 and C5a in milk. Levels of IL-8 (A) and C5a (B) in milk following intramammary bacterial or saline challenge were determined by ELISA. Mean (± standard error) IL-8 and C5a concentrations are shown. * and #, significantly increased in E. coli- or S. aureus-infected quarters, respectively, relative to time zero (P < 0.05).
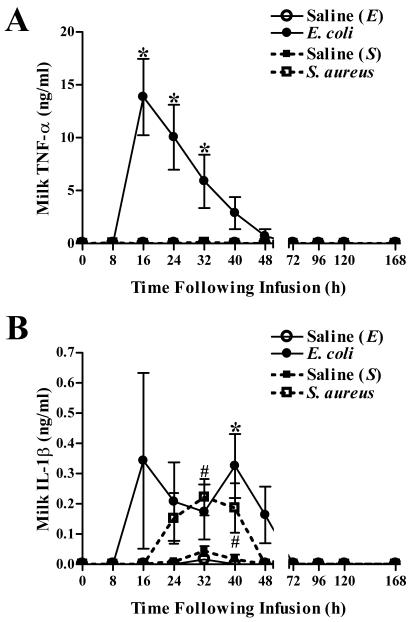
Differential induction of TNF-α and IL-1β following intramammary challenge with either E. coli or S. aureus.
E. coli and S. aureus infections both induced increases in mammary IL-1β levels, but only E. coli elicited the production of TNF-α (Fig. 6). Initial increases in TNF-α levels in the milk from E. coli-infected quarters were evident within 16 h and persisted through 32 h postchallenge (Fig. 6A). Increases in IL-1β production were highly variable following E. coli challenge and were significantly increased relative to prechallenge levels only at 40 h postinfection. Increases in IL-1β levels were evident in S. aureus-infected quarters 32 h after challenge and persisted for an additional 8 h (Fig. 6B). Maximal concentrations of IL-1β were comparable following infection with either bacterium.
FIG. 6.
Effects of E. coli and S. aureus infection on levels of TNF-α and IL-1β in milk. An ELISA was used to measure the concentrations of TNF-α (A) and IL-1β (B) in milk obtained from quarters infused with saline, E. coli, or S. aureus. Mean (± standard error) TNF-α and IL-1β concentrations are shown. * and #, significantly increased in E. coli- or S. aureus-infected quarters, respectively, relative to time zero (P < 0.05).
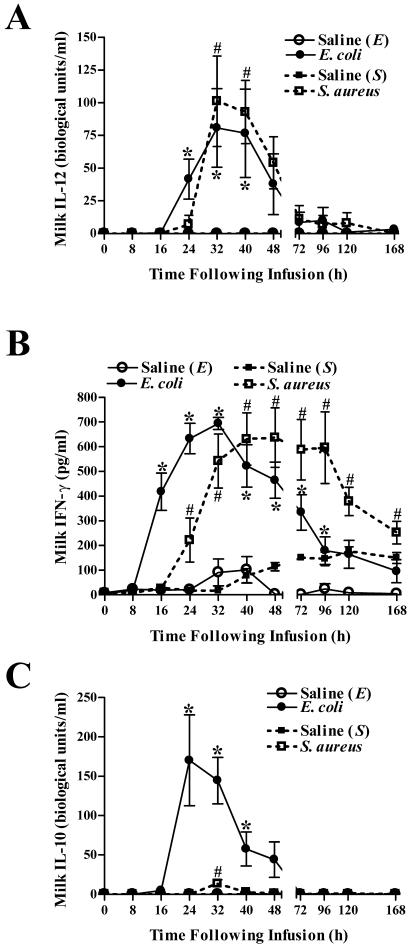
Intramammary infection with E. coli or S. aureus induces production of IL-12, IFN-γ, and IL-10.
Increased levels of IL-12 in milk were detectable within 24 h of E. coli infection and peaked 8 h later (Fig. 7A). Initial increases in milk IL-12 concentrations following S. aureus infection occurred 32 h after challenge. Peak levels of IL-12 were comparable in quarters infected with either E. coli or S. aureus. Similar to IL-12, infection with either bacterium elicited comparable production of IFN-γ; however, initial increases in E. coli-challenged quarters preceded those in S. aureus-infused quarters by 8 h (Fig. 7B). The increased milk IFN-γ levels remained elevated in S. aureus-infected quarters throughout the study, whereas those in quarters infected with E. coli returned to baseline by 120 h.
FIG. 7.
Intramammary infection with E. coli or S. aureus elicits an increase in levels of IL-12, IFN-γ, and IL-10 in milk. ELISAs were used to measure the levels of IL-12 (A), IFN-γ (B), and IL-10 (C) in milk obtained from quarters infused with saline, E. coli, or S. aureus. Mean (± standard error) IL-12, IL-10, and IFN-γ concentrations are shown. * and #, significantly increased in E. coli- or S. aureus-infected quarters, respectively, relative to time zero (P < 0.05).
Initial increases in IL-10, an anti-inflammatory cytokine, were observed in milk within 24 h of E. coli infection and returned to prechallenge levels >16 h later (Fig. 7C). A slight, but significant, transient increase in milk IL-10 levels was observed in quarters challenged with S. aureus 32 h after infection. Peak levels of IL-10 in E. coli-challenged quarters (170.1 ± 57.7 biological units/ml) were significantly higher than those detected in quarters challenged with S. aureus (13.7 ± 4.9 biological units/ml) (P = 0.009).
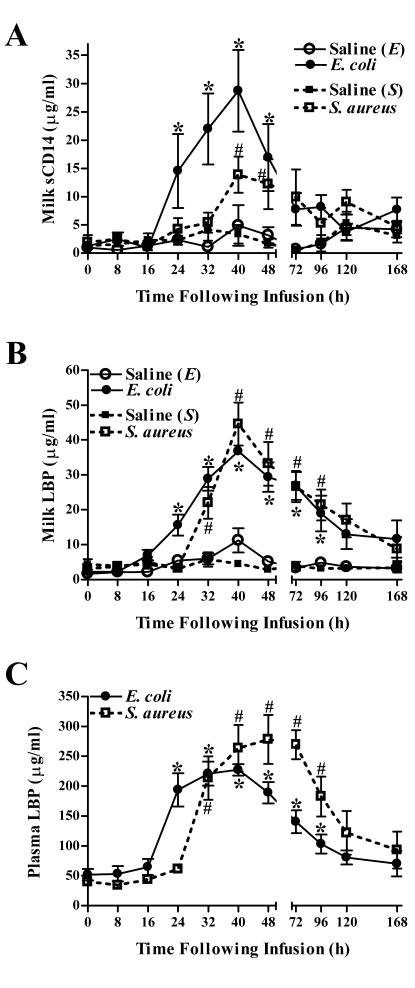
E. coli and S. aureus intramammary infection augments levels of both sCD14 and LBP in milk.
To determine whether intramammary infection with either bacterium could alter mammary gland sCD14 and LBP levels, two proteins involved in host cell recognition of bacterial-wall products, the levels of these molecules in milk were quantified by ELISA (Fig. 8A and B). Relative to prechallenged quarters, increased levels of both sCD14 and LBP were detected within 24 h of E. coli infection. Peak levels of both molecules were observed 40 h after infection. Increases in concentrations of LBP and sCD14 in milk were observed in S. aureus-infused quarters within 32 and 40 h, respectively, of challenge. Similar to E. coli-challenged glands, peak levels of both molecules were observed 40 h following infection. sCD14, but not LBP, reached a maximal concentration in E. coli-infected quarters (28.74 ± 7.24 μg/ml) that was significantly higher than that in quarters challenged with S. aureus (13.89 ± 3.17 μg/ml) (P = 0.041).
FIG. 8.
Effects of intramammary infection with E. coli or S. aureus on levels of sCD14 and LBP. Concentrations of sCD14 (A) and LBP (B) in milk obtained from quarters infused with either saline, E. coli, or S. aureus, as well as levels of LBP in plasma (C) obtained from these infected cows, were assayed by ELISA. Mean (± standard error) concentrations of both proteins are shown. * and #, significantly increased in milk (A and B) or plasma (C) obtained from E. coli- or S. aureus-infected cows, respectively, relative to time zero (P < 0.05).
Systemic levels of LBP, a liver-derived protein whose expression is increased during the acute-phase response to infection, were also assayed. Circulating blood LBP levels increased within 24 and 32 h following intramammary infection with E. coli or S. aureus, respectively, and remained above prechallenge levels until >96 h after infection with either bacterium (Fig. 8C). Increases in levels of LBP in blood for cows challenged with either bacterium were temporally coincident with increases in levels of LBP in milk (Fig. 8B). There were no significant differences between the peak circulating levels of LBP in cows challenged with E. coli or S. aureus.
DISCUSSION
Intramammary infection with E. coli or S. aureus elicited both a localized and a systemic response. Systemic signs following infection with either bacterium included decreased milk output (Fig. 2A), a febrile response (Fig. 2B), and elevated levels of the acute-phase protein LBP (Fig. 8C). Localized signs included increased permeability of the mammary vasculature (Fig. 4) and elevated milk SCC (Fig. 3B) in the infected quarters. Cows infected with E. coli demonstrated an earlier and more dramatic increase in body temperature, as well as a more pronounced decrease in milk output.
TNF-α and IL-1β are potent inducers of fever and the acute-phase response (15). In E. coli-challenged quarters, maximal increases in intramammary TNF-α and IL-1β were observed at 16 h postinfection (Fig. 6), a time that was coincident with increased body temperature (Fig. 2B) and that immediately preceded increased levels of circulating LBP (Fig. 8C). These temporal changes in the expression of TNF-α and IL-1β in milk are consistent with a previous study using a different strain of E. coli (56). In contrast to E. coli-infected quarters, there was no detectable TNF-α production in quarters challenged with S. aureus, and peak levels of IL-1β were not detected until 32 h postinfection (Fig. 6). This increase in IL-1β correlated with the onset of the febrile response in S. aureus-infected animals and the induction of the acute-phase synthesis of LBP. The absence of TNF-α production in cows challenged with S. aureus is consistent with a previous report (48) and may account for both the delayed and diminished febrile response and the delayed induction of acute-phase hepatic synthesis relative to that observed in cows infected with E. coli.
Increases in milk SCC were observed within 16 and 24 h of infection by E. coli or S. aureus, respectively (Fig. 3B). Saad and Ostensson have reported that neutrophils constitute >90% of the milk somatic cells present in infected quarters during the acute stages of mastitis (50). Correspondingly, the initial increase in milk SCC in E. coli-infected cows paralleled decreases in circulating neutrophils (Fig. 3A). In contrast, the delayed initial increase in milk SCC in S. aureus-infected quarters occurred in the absence of detectable decreases in circulating neutrophils. This delay in recruitment of neutrophils to the mammary gland following S. aureus infection may enable recruitment from bone marrow stores of white blood cells over a longer period, thus precluding a decrease in circulating neutrophils.
Similar to the findings of Shuster et al. (56), initial neutrophil recruitment to quarters infected with E. coli was temporally coincident with increases in IL-8, C5a, and TNF-α in milk, all of which are chemotactic (Fig. 5 and 6A). Consistent with a previous report (48), there was no detectable production of either IL-8 or TNF-α in quarters infected with S. aureus; however, a transient increase in C5a was detected within 32 h of infection. The increase in C5a occurred at a time when large increases in milk SCC were observed; however, slight but significant initial increases in milk SCC were observed within 24 h of S. aureus infection. Earlier increases in C5a may have gone undetected due to sensitivity limitations of the assay. Alternatively, upregulation of other chemoattractants, such as leukotriene B4, may have contributed to neutrophil recruitment (23, 49).
Initial increases in milk C5a at 16 and 32 h postinfection with E. coli or S. aureus, respectively, occurred in parallel with increased mammary vascular permeability, as evidenced by elevated milk BSA levels (Fig. 4). Since physiological levels of milk complement proteins are relatively low (42), these findings suggest a sequence in which serum-derived complement components leak into the quarter and subsequent activation results in the generation of detectable levels of complement fragments.
In comparison to TNF-α and IL-8, for which peak levels were observed 16 h following infection with E. coli, maximal amounts of IL-12 and IFN-γ were observed at 32 h (Fig. 7). Both IL-12 and IFN-γ levels remained elevated for >40 and 96 h, respectively, postchallenge. Similar changes in the expression of these cytokines were observed following S. aureus infection; however, the initial increases were detected 8 h later than those in quarters infected with E. coli. The parallel increases in IL-12 and IFN-γ in response to infection with either bacterium are consistent with the ability of each cytokine to stimulate the production of the other (12, 33, 36). Sustained and highly elevated increases in IL-10 in milk were observed following E. coli infection, yet only a slight transient increase was detected following S. aureus challenge. This is consistent with a previous study demonstrating that gram-negative bacteria are more potent inducers of IL-10 than gram-positive bacteria (21).
Elevated levels of IL-10 in E. coli-infected quarters were observed at times in which heightened levels of TNF-α declined, consistent with reports that IL-10 inhibits TNF-α production (5, 11). In contrast, increased levels of IL-12 and IFN-γ were temporally coincident with elevated levels of IL-10 in E. coli-challenged quarters, despite reports from studies using other model organisms and/or in vitro systems that the latter downregulates the expression of IL-12 and IFN-γ (14, 61). Interestingly, IFN-γ levels in S. aureus-infected quarters, which were virtually devoid of IL-10, remained elevated for a longer time than those in quarters infected with E. coli, where IL-10 levels were elevated. Thus, IL-10 may have exerted its classical downregulatory effect on the expression of IFN-γ in E. coli-infected quarters by limiting the duration of expression of IFN-γ.
Upregulation of both of the innate immune PRRs, Tlr-2 and Tlr-4, has recently been reported in cows with mastitis induced by gram-positive and gram-negative infections (17). LPS activation of Tlr-4 is dependent on the presence of either membrane-associated CD14 or sCD14 (18, 68). Another protein, LBP, facilitates LPS interaction with CD14, thus, enhancing host cell activation by LPS (53, 63). A protective role for CD14 and LBP in mediating host responses to LPS and infection by gram-negative bacteria has been established using an array of experimental approaches, including the use of LBP−/− (16, 26) or CD14−/− (71) mice or CD14 neutralizing antibodies (32, 69) and administration of exogenous LBP (30). Recently, it was demonstrated that coadministration of sCD14 with an inoculum of E. coli enhanced intramammary bacterial clearance, suggesting a beneficial role for sCD14 in mediating mammary innate immune responses to infection by gram-negative bacteria (31). In addition to a requisite role in LPS activation of Tlr-4, CD14 and LBP have been reported to facilitate Tlr-2 activation by LTA and PGN derived from S. aureus and other gram-positive bacteria (52, 72). Thus, sCD14 and LBP contribute to the elicitation of host cell responses to bacterial cell wall products derived from both gram-positive and gram-negative bacteria via Tlr-2- and Tlr-4-mediated activation, respectively.
Intramammary challenge with E. coli induced a significant increase in both sCD14 and LBP concentrations in infected quarters (Fig. 8). Initial increases in both proteins were observed within 24 h of infection and reached maximal levels 16 h later. Like E. coli infection, S. aureus infection resulted in similar increases in sCD14 and LBP, with the levels of both proteins peaking 40 h following infection. Because LBP and sCD14 act in concert to facilitate host recognition of bacterial-wall products, the simultaneous increase in the concentrations of both of these proteins following infection with either bacterium would be expected to be optimally beneficial to the host. The fact that initial increases in IL-8 and TNF-α in E. coli-infected quarters preceded changes in LBP and sCD14 concentrations suggests that the induction of initial proinflammatory responses may occur in the presence of physiological levels of sCD14 and LBP. Interestingly, peak levels of milk SCC and of IL-12 and IFN-γ production were all observed at times of maximal elevation of both sCD14 and LBP, thereby raising the possibility that maximal neutrophil recruitment and elevation of these cytokines are dependent upon augmented levels of sCD14 and/or LBP. Elevated levels of sCD14 and LBP at a time of maximal neutrophil recruitment would be expected to be advantageous to the host, as both of the molecules enhance neutrophil function (19, 55, 65).
Increased concentrations of LBP in quarters infected with either bacterium were temporally coincident with elevated plasma LBP levels (Fig. 8C) and occurred at a time of increased mammary vascular permeability (Fig. 4). This finding is compatible with leakage of LBP from the vascular compartment into the infected quarters. The assay used to detect sCD14 in milk was unable to determine sCD14 concentrations in plasma, presumably due to interference from plasma components. This plasma interference effect has been reported in other ELISAs as well (39). Therefore, whether the increases in sCD14 were a result of vascular leakage remains unknown. Lee et al. have suggested that increases in intramammary sCD14 during the course of mastitis may result from CD14 shedding from the neutrophil surface (31). Consistent with this hypothesis, increases in sCD14 levels (Fig. 8A) paralleled elevations in milk SCC (Fig. 3B) in quarters infected with either E. coli or S. aureus. Further, higher levels of sCD14 were found in E. coli-infected quarters, where there were higher milk SCC than in quarters challenged with S. aureus.
To our knowledge, the present study is the first to examine differences in the inflammatory responses during clinical mastitis elicited by E. coli and S. aureus. The interpretation of results from the only other study to examine the intramammary innate immune responses to these two bacteria was limited by the different clinical outcomes following infection (48). In that study, cows infected with E. coli developed clinical mastitis, whereas those infected with S. aureus developed subclinical mastitis. Thus, the differences identified in that report could have been attributed to the differential severity of inflammation that ensued. In the present study, all of the cows developed clinical mastitis. Another difference between the previous and present studies concerns the relative titers of E. coli and S. aureus recovered from infected glands. The study by Riollet et al. reported ∼2-log-unit-higher recovery of bacteria from the quarters infused with E. coli than from those infected with S. aureus (48). Thus, the lack of a proinflammatory cytokine response elicited in S. aureus-infected quarters in that study could have been attributed to the lower titers of S. aureus relative to those of E. coli. In the present report, comparable or higher numbers of CFU of S. aureus per milliliter than of E. coli were recovered. Thus, the present study establishes that the differences between the innate immune responses to these two bacteria cannot be ascribed to differences in titers. Finally, the previous report by Riollet et al. examined only a limited number of cytokines, including IL-1β, IL-8, and TNF-α (48). We have now extended those findings by studying several additional aspects of the innate immune response, including (i) differential induction of the cytokines IL-10, IL-12, and IFN-γ; (ii) changes in the levels of the innate immune accessory molecules sCD14 and LBP; (iii) systemic changes reflecting milk output, febrile response, and induction of the acute-phase response; and (iv) changes in milk SCC relative to decreases in circulating neutrophils.
Consistent with the findings of Riollet et al. (48), we found that E. coli, but not S. aureus, could elicit production of IL-8 (Fig. 5A) and TNF-α (Fig. 6A). In contrast, we observed an increase in IL-1β in response to infection with either bacterium (Fig. 6B), whereas the previous study reported increases in IL-1β only in response to E. coli. Since the strain of S. aureus used in the present study led to the development of clinical mastitis whereas that in the previous study elicited subclinical mastitis, the differential severity of the clinical course may have influenced the production of IL-1β. Alternatively, the discrepancy in findings may be attributed to the high variability associated with the assay. A previous study showed an increase in IL-1β mRNA in milk somatic cells following S. aureus challenge (47), consistent with our finding of increased levels of soluble IL-1β as assayed by ELISA.
Interestingly, increases in milk somatic cell TNF-α mRNA have been reported in two studies following S. aureus challenge (3, 47), yet at the protein level, we and others (48) have been unable to detect corresponding increases in TNF-α by ELISA. Thus, detectable increases in mRNA levels are not necessarily indicative of increased secreted levels of protein. In the present study, we have directly assayed for all cytokines and innate immune accessory molecules by ELISA. To our knowledge, this is the first comprehensive report to differentiate E. coli- and S. aureus-elicited intramammary changes in levels of soluble IL-10, IL-12, IFN-γ, sCD14, and LBP.
Although the innate immune system has evolved to respond to the multitude of bacterial species that exist in nature, the present report clearly establishes that different in vivo responses are elicited by distinct bacterial pathogens. Because the ability of bacteria to establish infection is mediated in part by the ability of the host to respond to the invading organism (10), the differences reported here between the host responses to E. coli and S. aureus may contribute to the limited acute intramammary infection associated with the former and the more chronic infectious state characteristic of the latter.
Acknowledgments
We acknowledge J. Bilheimer, M. Bowman, and E. Cates for their technical assistance.
REFERENCES
- 1.Aderem, A., and R. J. Ulevitch. 2000. Toll-like receptors in the induction of the innate immune response. Nature 406:782-787. [DOI] [PubMed] [Google Scholar]
- 2.Agrawal, S., A. Agrawal, B. Doughty, A. Gerwitz, J. Blenis, T. Van Dyke, and B. Pulendran. 2003. Cutting edge: different Toll-like receptor agonists instruct dendritic cells to induce distinct Th responses via differential modulation of extracellular signal-regulated kinase-mitogen-activated protein kinase and c-Fos. J. Immunol. 171:4984-4989. [DOI] [PubMed] [Google Scholar]
- 3.Alluwaimi, A. M., C. M. Leutenegger, T. B. Farver, P. V. Rossitto, W. L. Smith, and J. S. Cullor. 2003. The cytokine markers in Staphylococcus aureus mastitis of bovine mammary gland. J. Vet. Med. B 50:105-111. [DOI] [PubMed] [Google Scholar]
- 4.Amir, L. H., H. Harris, and L. Andriske. 1999. An audit of mastitis in the emergency department. J. Hum. Lact. 15:221-224. [DOI] [PubMed] [Google Scholar]
- 5.Armstrong, L., N. Jordan, and A. Millar. 1996. Interleukin 10 (IL-10) regulation of tumour necrosis factor alpha (TNF-alpha) from human alveolar macrophages and peripheral blood monocytes. Thorax 51:143-149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bannerman, D. D., M. J. Paape, W. R. Hare, and E. J. Sohn. 2003. Increased levels of LPS-binding protein in bovine blood and milk following bacterial lipopolysaccharide challenge. J. Dairy Sci. 86:3128-3137. [DOI] [PubMed] [Google Scholar]
- 7.Barkema, H. W., Y. H. Schukken, T. J. Lam, M. L. Beiboer, H. Wilmink, G. Benedictus, and A. Brand. 1998. Incidence of clinical mastitis in dairy herds grouped in three categories by bulk milk somatic cell counts. J. Dairy Sci. 81:411-419. [DOI] [PubMed] [Google Scholar]
- 8.Bingen, E., E. Denamur, N. Lambert-Zechovsky, Y. Aujard, N. Brahimi, P. Geslin, and J. Elion. 1992. Analysis of DNA restriction fragment length polymorphism extends the evidence for breast milk transmission in Streptococcus agalactiae late-onset neonatal infection. J. Infect. Dis. 165:569-573. [DOI] [PubMed] [Google Scholar]
- 9.Bramley, A. J. 1976. Variations in the susceptibility of lactating and non-lactating bovine udders to infection when infused with Escherichia coli. J. Dairy Res. 43:205-211. [DOI] [PubMed] [Google Scholar]
- 10.Burvenich, C., V. Van Merris, J. Mehrzad, A. Diez-Fraile, and L. Duchateau. 2003. Severity of E. coli mastitis is mainly determined by cow factors. Vet. Res. 34:521-564. [DOI] [PubMed] [Google Scholar]
- 11.Cassatella, M. A., L. Meda, S. Bonora, M. Ceska, and G. Constantin. 1993. Interleukin 10 (IL-10) inhibits the release of proinflammatory cytokines from human polymorphonuclear leukocytes. Evidence for an autocrine role of tumor necrosis factor and IL-1 beta in mediating the production of IL-8 triggered by lipopolysaccharide. J. Exp. Med. 178:2207-2211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Collins, R. A., E. B. Camon, P. J. Chaplin, and C. J. Howard. 1998. Influence of IL-12 on interferon-gamma production by bovine leucocyte subsets in response to bovine respiratory syncytial virus. Vet. Immunol. Immunopathol. 63:69-72. [DOI] [PubMed] [Google Scholar]
- 13.Daley, M. J., E. R. Oldham, T. J. Williams, and P. A. Coyle. 1991. Quantitative and qualitative properties of host polymorphonuclear cells during experimentally induced Staphylococcus aureus mastitis in cows. Am. J. Vet. Res. 52:474-479. [PubMed] [Google Scholar]
- 14.D'Andrea, A., M. Aste-Amezaga, N. M. Valiante, X. Ma, M. Kubin, and G. Trinchieri. 1993. Interleukin 10 (IL-10) inhibits human lymphocyte interferon gamma-production by suppressing natural killer cell stimulatory factor/IL-12 synthesis in accessory cells. J. Exp. Med. 178:1041-1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dinarello, C. A. 1996. Cytokines as mediators in the pathogenesis of septic shock. Curr. Top. Microbiol. Immunol. 216:133-165. [DOI] [PubMed] [Google Scholar]
- 16.Fierer, J., M. A. Swancutt, D. Heumann, and D. Golenbock. 2002. The role of lipopolysaccharide binding protein in resistance to Salmonella infections in mice. J. Immunol. 168:6396-6403. [DOI] [PubMed] [Google Scholar]
- 17.Goldammer, T., H. Zerbe, A. Molenaar, H. J. Schuberth, R. M. Brunner, S. R. Kata, and H. M. Seyfert. 2004. Mastitis increases mammary mRNA abundance of beta-defensin 5, toll-like-receptor 2 (TLR2), and TLR4 but not TLR9 in cattle. Clin. Diagn. Lab. Immunol. 11:174-185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guha, M., and N. Mackman. 2001. LPS induction of gene expression in human monocytes. Cell. Signal. 13:85-94. [DOI] [PubMed] [Google Scholar]
- 19.Hailman, E., T. Vasselon, M. Kelley, L. A. Busse, M. C. Hu, H. S. Lichenstein, P. A. Detmers, and S. D. Wright. 1996. Stimulation of macrophages and neutrophils by complexes of lipopolysaccharide and soluble CD14. J. Immunol. 156:4384-4390. [PubMed] [Google Scholar]
- 20.Harada, A., N. Sekido, T. Akahoshi, T. Wada, N. Mukaida, and K. Matsushima. 1994. Essential involvement of interleukin-8 (IL-8) in acute inflammation. J. Leukoc. Biol. 56:559-564. [PubMed] [Google Scholar]
- 21.Hessle, C., B. Andersson, and A. E. Wold. 2000. Gram-positive bacteria are potent inducers of monocytic interleukin-12 (IL-12) while gram-negative bacteria preferentially stimulate IL-10 production. Infect. Immun. 68:3581-3586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hirschfeld, M., Y. Ma, J. H. Weis, S. N. Vogel, and J. J. Weis. 2000. Cutting edge: repurification of lipopolysaccharide eliminates signaling through both human and murine toll-like receptor 2. J. Immunol. 165:618-622. [DOI] [PubMed] [Google Scholar]
- 23.Hoedemaker, M., L. A. Lund, and W. C. Wagner. 1992. Influence of arachidonic acid metabolites and steroids on function of bovine polymorphonuclear neutrophils. Am. J. Vet. Res. 53:1534-1539. [PubMed] [Google Scholar]
- 24.Hoffmann, J. A., F. C. Kafatos, C. A. Janeway, and R. A. Ezekowitz. 1999. Phylogenetic perspectives in innate immunity. Science 284:1313-1318. [DOI] [PubMed] [Google Scholar]
- 25.Hope, J. C., L. S. Kwong, G. Entrican, S. Wattegedera, H. M. Vordermeier, P. Sopp, and C. J. Howard. 2002. Development of detection methods for ruminant interleukin (IL)-12. J. Immunol. Methods 266:117-126. [DOI] [PubMed] [Google Scholar]
- 26.Jack, R. S., X. Fan, M. Bernheiden, G. Rune, M. Ehlers, A. Weber, G. Kirsch, R. Mentel, B. Furll, M. Freudenberg, G. Schmitz, F. Stelter, and C. Schutt. 1997. Lipopolysaccharide-binding protein is required to combat a murine gram-negative bacterial infection. Nature 389:742-745. [DOI] [PubMed] [Google Scholar]
- 27.John, G. C., R. W. Nduati, D. A. Mbori-Ngacha, B. A. Richardson, D. Panteleeff, A. Mwatha, J. Overbaugh, J. Bwayo, J. O. Ndinya-Achola, and J. K. Kreiss. 2001. Correlates of mother-to-child human immunodeficiency virus type 1 (HIV-1) transmission: association with maternal plasma HIV-1 RNA load, genital HIV-1 DNA shedding, and breast infections. J. Infect. Dis. 183:206-212. [DOI] [PubMed] [Google Scholar]
- 28.Koj, A. 1996. Initiation of acute phase response and synthesis of cytokines. Biochim. Biophys. Acta 1317:84-94. [DOI] [PubMed] [Google Scholar]
- 29.Kwong, L. S., J. C. Hope, M. L. Thom, P. Sopp, S. Duggan, G. P. Bembridge, and C. J. Howard. 2002. Development of an ELISA for bovine IL-10. Vet. Immunol. Immunopathol. 85:213-223. [DOI] [PubMed] [Google Scholar]
- 30.Lamping, N., R. Dettmer, N. W. Schroder, D. Pfeil, W. Hallatschek, R. Burger, and R. R. Schumann. 1998. LPS-binding protein protects mice from septic shock caused by LPS or gram-negative bacteria. J. Clin. Investig. 101:2065-2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee, J. W., M. J. Paape, T. H. Elsasser, and X. Zhao. 2003. Recombinant soluble CD14 reduces severity of intramammary infection by Escherichia coli. Infect. Immun. 71:4034-4039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Le Roy, D., F. Di Padova, Y. Adachi, M. P. Glauser, T. Calandra, and D. Heumann. 2001. Critical role of lipopolysaccharide-binding protein and CD14 in immune responses against gram-negative bacteria. J. Immunol. 167:2759-2765. [DOI] [PubMed] [Google Scholar]
- 33.Ma, X. 2001. TNF-alpha and IL-12: a balancing act in macrophage functioning. Microbes Infect. 3:121-129. [DOI] [PubMed] [Google Scholar]
- 34.Miller, R. H., M. J. Paape, and J. C. Acton. 1986. Comparison of milk somatic cell counts by Coulter and Fossomatic Counters. J. Dairy Sci. 69:1942-1946. [DOI] [PubMed] [Google Scholar]
- 35.Morath, S., A. Stadelmaier, A. Geyer, R. R. Schmidt, and T. Hartung. 2002. Synthetic lipoteichoic acid from Staphylococcus aureus is a potent stimulus of cytokine release. J. Exp. Med. 195:1635-1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Munder, M., M. Mallo, K. Eichmann, and M. Modolell. 1998. Murine macrophages secrete interferon gamma upon combined stimulation with interleukin (IL)-12 and IL-18: A novel pathway of autocrine macrophage activation. J. Exp. Med. 187:2103-2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.National Mastitis Council. 1999. Current concepts of bovine mastitis, 4th ed. The National Mastitis Council, Inc., Madison, Wis.
- 38.Nau, G. J., A. Schlesinger, J. F. Richmond, and R. A. Young. 2003. Cumulative Toll-like receptor activation in human macrophages treated with whole bacteria. J. Immunol. 170:5203-5209. [DOI] [PubMed] [Google Scholar]
- 39.Nemzek, J. A., J. Siddiqui, and D. G. Remick. 2001. Development and optimization of cytokine ELISAs using commercial antibody pairs. J. Immunol. Methods 255:149-157. [DOI] [PubMed] [Google Scholar]
- 40.Newbould, F. H. 1977. Evaluation of induced infections as a research method. J. Am. Vet. Med. Assoc. 170:1208-1209. [PubMed] [Google Scholar]
- 41.Paape, M. J., P. M. Rautiainen, E. M. Lilius, C. E. Malstrom, and T. H. Elsasser. 2002. Development of anti-bovine TNF-alpha mAb and ELISA for quantitating TNF-alpha in milk after intramammary injection of endotoxin. J. Dairy Sci. 85:765-773. [DOI] [PubMed] [Google Scholar]
- 42.Rainard, P., and B. Poutrel. 1995. Deposition of complement components on Streptococcus agalactiae in bovine milk in the absence of inflammation. Infect. Immun. 63:3422-3427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rainard, P., P. Sarradin, M. J. Paape, and B. Poutrel. 1998. Quantification of C5a/C5a(desArg) in bovine plasma, serum and milk. Vet. Res. 29:73-88. [PubMed] [Google Scholar]
- 44.Re, F., and J. L. Strominger. 2001. Toll-like receptor 2 (TLR2) and TLR4 differentially activate human dendritic cells. J. Biol. Chem. 276:37692-37699. [DOI] [PubMed] [Google Scholar]
- 45.Redpath, S., P. Ghazal, and N. R. Gascoigne. 2001. Hijacking and exploitation of IL-10 by intracellular pathogens. Trends Microbiol. 9:86-92. [DOI] [PubMed] [Google Scholar]
- 46.Rench, M. A., and C. J. Baker. 1989. Group B streptococcal breast abscess in a mother and mastitis in her infant. Obstet. Gynecol. 73:875-877. [PubMed] [Google Scholar]
- 47.Riollet, C., P. Rainard, and B. Poutrel. 2001. Cell subpopulations and cytokine expression in cow milk in response to chronic Staphylococcus aureus infection. J. Dairy Sci. 84:1077-1084. [DOI] [PubMed] [Google Scholar]
- 48.Riollet, C., P. Rainard, and B. Poutrel. 2000. Differential induction of complement fragment C5a and inflammatory cytokines during intramammary infections with Escherichia coli and Staphylococcus aureus. Clin. Diagn. Lab. Immunol. 7:161-167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rose, D. M., S. N. Giri, S. J. Wood, and J. S. Cullor. 1989. Role of leukotriene B4 in the pathogenesis of Klebsiella pneumoniae-induced bovine mastitis. Am. J. Vet. Res. 50:915-918. [PubMed] [Google Scholar]
- 50.Saad, A. M., and K. Ostensson. 1990. Flow cytofluorometric studies on the alteration of leukocyte populations in blood and milk during endotoxin-induced mastitis in cows. Am. J. Vet. Res. 51:1603-1607. [PubMed] [Google Scholar]
- 51.Schalm, O. W., E. J. Carroll, and N. C. Jain. 1971. Bovine mastitis. Lea & Febiger, Philadelphia, Pa.
- 52.Schroder, N. W., S. Morath, C. Alexander, L. Hamann, T. Hartung, U. Zahringer, U. B. Gobel, J. R. Weber, and R. R. Schumann. 2003. Lipoteichoic acid (LTA) of Streptococcus pneumoniae and Staphylococcus aureus activates immune cells via Toll-like receptor (TLR)-2, lipopolysaccharide-binding protein (LBP), and CD14, whereas TLR-4 and MD-2 are not involved. J. Biol. Chem. 278:15587-15594. [DOI] [PubMed] [Google Scholar]
- 53.Schumann, R. R., and E. Latz. 2000. Lipopolysaccharide-binding protein. Chem. Immunol. 74:42-60. [DOI] [PubMed] [Google Scholar]
- 54.Semba, R. D. 2000. Mastitis and transmission of human immunodeficiency virus through breast milk. Ann. N. Y. Acad. Sci. 918:156-162. [DOI] [PubMed] [Google Scholar]
- 55.Shapira, L., C. Champagne, B. Gordon, S. Amar, and T. E. Van Dyke. 1995. Lipopolysaccharide priming of superoxide release by human neutrophils: role of membrane CD14 and serum LPS binding protein. Inflammation 19:289-295. [DOI] [PubMed] [Google Scholar]
- 56.Shuster, D. E., M. E. Kehrli, Jr., P. Rainard, and M. Paape. 1997. Complement fragment C5a and inflammatory cytokines in neutrophil recruitment during intramammary infection with Escherichia coli. Infect. Immun. 65:3286-3292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Smith, K. L., and J. S. Hogan. 1993. Environmental mastitis. Vet. Clin. N. Am. Food Anim. Pract. 9:489-498. [DOI] [PubMed] [Google Scholar]
- 58.Spits, H., and R. de Waal Malefyt. 1992. Functional characterization of human IL-10. Int. Arch. Allergy Immunol. 99:8-15. [DOI] [PubMed] [Google Scholar]
- 59.Suffredini, A. F., G. Fantuzzi, R. Badolato, J. J. Oppenheim, and N. P. O'Grady. 1999. New insights into the biology of the acute phase response. J. Clin. Immunol. 19:203-214. [DOI] [PubMed] [Google Scholar]
- 60.Sutra, L., and B. Poutrel. 1994. Virulence factors involved in the pathogenesis of bovine intramammary infections due to Staphylococcus aureus. J. Med. Microbiol. 40:79-89. [DOI] [PubMed] [Google Scholar]
- 61.Takenaka, H., S. Maruo, N. Yamamoto, M. Wysocka, S. Ono, M. Kobayashi, H. Yagita, K. Okumura, T. Hamaoka, G. Trinchieri, and H. Fujiwara. 1997. Regulation of T cell-dependent and -independent IL-12 production by the three Th2-type cytokines IL-10, IL-6, and IL-4. J. Leukoc. Biol. 61:80-87. [DOI] [PubMed] [Google Scholar]
- 62.Thijs, L. G., A. B. Groeneveld, and C. E. Hack. 1996. Multiple organ failure in septic shock. Curr. Top. Microbiol. Immunol. 216:209-237. [DOI] [PubMed] [Google Scholar]
- 63.Tobias, P. S., R. I. Tapping, and J. A. Gegner. 1999. Endotoxin interactions with lipopolysaccharide-responsive cells. Clin. Infect. Dis. 28:476-481. [DOI] [PubMed] [Google Scholar]
- 64.Trinchieri, G. 1997. Cytokines acting on or secreted by macrophages during intracellular infection (IL-10, IL-12, IFN-gamma). Curr. Opin. Immunol. 9:17-23. [DOI] [PubMed] [Google Scholar]
- 65.Troelstra, A., B. N. Giepmans, K. P. Van Kessel, H. S. Lichenstein, J. Verhoef, and J. A. Van Strijp. 1997. Dual effects of soluble CD14 on LPS priming of neutrophils. J. Leukoc. Biol. 61:173-178. [DOI] [PubMed] [Google Scholar]
- 66.Underhill, D. M. 2003. Toll-like receptors: networking for success. Eur. J. Immunol. 33:1767-1775. [DOI] [PubMed] [Google Scholar]
- 67.Uthaisangsook, S., N. K. Day, S. L. Bahna, R. A. Good, and S. Haraguchi. 2002. Innate immunity and its role against infections. Ann. Allergy Asthma Immunol. 88:253-264. [DOI] [PubMed] [Google Scholar]
- 68.Viriyakosol, S., and T. Kirkland. 1995. Knowledge of cellular receptors for bacterial endotoxin—1995. Clin. Infect. Dis. 21(Suppl. 2):S190-S195. [DOI] [PubMed] [Google Scholar]
- 69.Wenneras, C., P. Ave, M. Huerre, J. Arondel, R. Ulevitch, J. Mathison, and P. Sansonetti. 2001. Blockade of CD14 aggravates experimental shigellosis. J. Endotoxin Res. 7:442-446. [PubMed] [Google Scholar]
- 70.Willumsen, J. F., S. M. Filteau, A. Coutsoudis, K. E. Uebel, M. L. Newell, and A. M. Tomkins. 2000. Subclinical mastitis as a risk factor for mother-infant HIV transmission. Adv. Exp. Med. Biol. 478:211-223. [DOI] [PubMed] [Google Scholar]
- 71.Yang, K. K., B. G. Dorner, U. Merkel, B. Ryffel, C. Schutt, D. Golenbock, M. W. Freeman, and R. S. Jack. 2002. Neutrophil influx in response to a peritoneal infection with Salmonella is delayed in lipopolysaccharide-binding protein or CD14-deficient mice. J. Immunol. 169:4475-4480. [DOI] [PubMed] [Google Scholar]
- 72.Yoshimura, A., E. Lien, R. R. Ingalls, E. Tuomanen, R. Dziarski, and D. Golenbock. 1999. Cutting edge: recognition of Gram-positive bacterial cell wall components by the innate immune system occurs via Toll-like receptor 2. J. Immunol. 163:1-5. [PubMed] [Google Scholar]