Abstract
A 55-kDa protein named AILb-A, isolated from the seed extract of Aeginetia indica L., a parasitic plant, induces a Th1-type T-cell response and elicits a marked antitumor effect in tumor-bearing mice. In the present study, we examined the role of Toll-like receptors (TLRs), which have been implicated in pathogen-induced cell signaling, in AILb-A-induced immune responses. In the luciferase assay using a nuclear factor (NF)-κB-dependent reporter plasmid, AILb-A induced NF-κB activation in the cells transfected with TLR4, but not with those transfected with the TLR2 gene, in a dose-dependent manner. TLR4-mediated NF-κB activation induced by AILb-A but not by lipopolysaccharide (LPS) was also observed under serum-free conditions. In in vitro experiments using human peripheral blood mononuclear cells, AILb-A-induced cytokine production was markedly inhibited by anti-TLR4 but not by anti-CD14 antibody, while LPS-induced, TLR4-mediated cytokine production was inhibited by anti-CD14 as well as anti-TLR4 antibodies. Cytokine production, killer cell activities, maturation of dendritic cells, phosphorylation of mitogen-activated protein kinases, and nuclear translocation of interferon-regulatory factor 3 induced by AILb-A were severely impaired in TLR4-deficient but not TLR2-deficient mice. Transfection of TLR4-deficient mouse-derived macrophages with the TLR4 expression plasmid led AILb-A to induce cytokines. Finally, the antitumor effect of AILb-A was also impaired in TLR4-deficient and TLR4-mutated mice. These findings suggest that TLR4 mediates antitumor immunity induced by the plant-derived protein AILb-A.
Aeginetia indica L., a plant parasite found on roots of Japanese pampa grasses or sugar canes, has been used as a tonic and an antiinflammatory herbal agent in China and Japan. We previously reported that the butanol extract from seeds of A. indica L. (a compound termed AIL) mediates potent antitumor immunity in tumor-bearing mice. AIL-induced antitumor immunity seemed to be exerted through activating antitumor CD4+ T cells, while the role of CD8+ T cells was not usually essential for this system (8). We also found that spleen cells from AIL-treated mice secreted gamma interferon (IFN-γ), tumor necrosis factor (TNF), interleukin-2 (IL-2), and IL-6 when restimulated in vitro with AIL, and that CD4+ T cells were the main producers of these cytokines (9). Recently, our investigators generated a monoclonal antibody (MAb), F3, that recognizes a 55-kDa protein in AIL, and elimination of the protein from AIL by immunoprecipitation using the F3 MAb significantly reduced the antitumor ability of AIL in tumor-bearing mice (10). Since it can be considered that the 55-kDa protein is most responsible for AIL-mediated antitumor host responses, we isolated this protein (AILb-A) by affinity chromatography on N-hydroxysuccinimide (NHS)-activated Sepharose high-performance (HP) column-bound F3 MAb. AILb-A was the protein with a molecular mass of 55 kDa not containing any carbohydrate determinants, and it markedly induced helper T cell 1 (Th1)-type cytokines and apoptosis-inducing factors such as TNF-α, TNF-β, Fas ligand, TNF-related apoptosis-inducing ligand, and perforin on human peripheral blood mononuclear cells (PBMCs) in vitro (26). Furthermore, AILb-A induced a Th1-dominant state and elicited marked antitumor effects in syngeneic Meth-A tumor-bearing BALB/c mice, in which the Th2 response is genetically dominant. It has been strongly suggested that AILb-A may be a useful immunotherapeutic agent for patients with malignancies (25).
The toll gene controls dorsoventral pattern formation during the early embryonic development of Drosophila melanogaster (22). Interestingly, toll participates in antimicrobial immune responses upon infection in adult Drosophila (18). Recently, several mammalian homologues of the Drosophila Toll receptor protein (Toll-like receptors [TLRs]) have been identified. TLRs are transmembrane proteins and represent a newly recognized family of vertebrate pattern recognition receptors in the innate immune system (1, 31). Subsequent to ligand binding, TLRs initiate the signaling via sequential recruitment of myeloid differentiation protein 88 (MyD88), IL-1 receptor-associated kinase, and TNF receptor-associated factor 6, which in turn activate downstream mediators such as nuclear factor (NF)-κB and mitogen-activated protein kinases (MAPKs) (21, 23). In addition, the MyD88-independent and IFN-regulatory factor 3 (IRF3)-mediated pathways of TLR signaling have been identified (15). Among the 10 identified TLRs, TLR2 recognizes peptidoglycan (PGN), lipoprotein, lipoarabinomannan, and lipoteichoic acid derived from gram-positive bacteria, mycobacteria, or mycoplasma (19, 34, 37). TLR4 recognizes gram-positive and gram-negative bacterial cell wall components, including lipopolysaccharide (LPS) and lipoteichoic acid (29, 30, 34). TLR9 recognizes bacterial unmethylated CpG DNA and is the receptor that distinguishes bacterial DNA from self DNA (12). MD-2 (32) and CD14 (20) act as significant coreceptors in TLR signaling. Especially, MD-2 is physically associated with TLR4 on the cell surface and the TLR4/MD-2 complex confers responsiveness to bacterial components (32). Furthermore, it was also reported that the TLR4/MD-2 complex mediates LPS-mimetic signal transduction by the anticancer agent Taxol, a plant-derived diterpene, in the mouse but not in humans (16).
We hypothesized that the signaling induced by AILb-A, a plant-derived protein, may be mediated by TLRs. In the present study, we conducted in vitro and in vivo experiments to examine the role of TLRs in AILb-A-induced signaling and anticancer immunity.
MATERIALS AND METHODS
Isolation of AILb-A, a 55-kDa protein, by using an affinity column-bound F3 MAb.
The antigen recognized by the F3 MAb was purified from a butanol extract of the seed of A. indica L. (AILb) by an affinity chromatography on a HiTrap NHS-activated Sepharose HP column (bed volume, 5 ml; Amersham Pharmacia Biotech AB, Uppsala, Sweden) with bound F3 as described previously (26). Five milliliters of AILb in phosphate-buffered saline (PBS; 1 mg/ml) was layered on the F3-binding HiTrap NHS-activated Sepharose HP column and incubated for 2 h at room temperature. After the column was washed with a large volume of PBS, the antigen bound to F3 was eluted with 0.15 M NaCl-NH3 buffer, pH 11, at a flow rate of 30 ml/h at room temperature. The eluates were dialyzed immediately against PBS and then lyophilized. This preparation, reacting positively with F3 in an enzyme-linked immunosorbent assay (ELISA), was designated AILb-A and was used for the present study. AILb-A was the protein with a molecular mass of 55 kDa not containing any carbohydrate determinants (10, 26). Absence of LPS contamination in AILb-A was confirmed by Endospecy test (data not shown).
Established cell lines and culture media.
The derivation of the IL-3-dependent mouse proB Ba/F3 cell lines stably expressing human TLR2 (Ba/hTLR2), mouse TLR2 (Ba/mTLR2), human TLR4 (Ba/hTLR4), human TLR4 and human MD-2 (Ba/hTLR4/hMD-2), mouse TLR4 (Ba/mTLR4), and mouse TLR4 and mouse MD-2 (Ba/mTLR4/mMD-2) have been described elsewhere. All of the transfectants have been also transfected with p55IgκLuc, an NF-κB reporter construct (2, 16, 32). The construct p55IgκLuc contains the NF-κB p55 regulatory sequence from the immunoglobulin kappa light chain promoter upstream of a Photonius pyralis luciferase gene. The construct is used as a measure of NF-κB activity. A control cell line that stably expressed only p55IgκLuc (Ba/κB) was also established. Parental Ba/F3 cells do not express TLR2, TLR4, or MD-2 (32).
The transfectants were grown in RPMI 1640 medium (Life Technologies, Inc., Gaithersburg, Md.) containing 10% heat-inactivated fetal bovine serum (FBS; Bio-Whittaker, Walkersville, Md.) and 100 U of mouse IL-3/ml. The human erythroleukemic cell line K-562 (4), mouse fibrosarcoma cell line Meth-A (3), mouse Moloney lymphoma cell line YAC-1 (17), and mouse squamous cell carcinoma cell line SCCVII (33) were cultured in RPMI 1640 supplemented with 10% heat-inactivated FBS. LL/2, a Lewis lung carcinoma cell line (6), was grown in Eagle's minimum essential medium (Life Technologies, Inc.) supplemented with 10% FBS. All cell lines were grown in a humidified atmosphere of 95% air and 5% CO2 at 37°C.
Animals and immunomodulators.
All mice were maintained according to the National Institutes of Health standards established in the Guidelines for the Care and Use of Laboratory Animals (http://oacu.od.nih.gov/regs/guide/guide2.htm), and all experimental protocols were approved by the Animal Investigation Committee of Tokushima University, Tokushima, Japan. The mutant mice deficient in TLR2 (TLR2−/−) or in TLR4 (TLR4−/−) with a C57BL/6 background were generated by gene targeting, as described previously (14, 28). Wild-type C57BL/6, C3H/HeN, and C3H/HeJ mice were purchased from Japan SLC Inc. (Shizuoka, Japan). It has been reported that C3H/HeJ mice have a point mutation in the TLR4 gene, that the mutation resulted in an amino acid change from proline to histidine at position 712, and that the resultant amino acid substitution in the cytoplasmic domain of the protein abolishes LPS signaling in these mice (14, 29, 30). C57BL/6 and C3H/HeN mice express wild-type TLR2 and TLR4.
LPS derived from Escherichia coli 055:B5, a specific ligand for TLR4, and PGN (Fluka 77140), a specific ligand for TLR2, were purchased from Sigma (St. Louis, Mo.).
Luciferase assay.
The stable transfectants derived from Ba/F3 (5 × 105/well) were inoculated into a 48-well dish (Corning Incorporated, Corning, N.Y.), and then AILb-A (0.1, 1, or 10 μg/ml), LPS (1 μg/ml), or PGN (2 μg/ml) was added. After stimulation at 37°C for 5 h, cells were harvested and lysed in 100 μl of luciferase cell culture lysis reagent (Promega, Madison, Wis.), and luciferase activity was measured using 20 μl of lysate and 100 μl of luciferase assay substrate (Promega). The luminescence was quantitated as relative light units by using a luminometer (Bio-Orbit, Turku, Finland). Relative luciferase activity was obtained by dividing the relative light unit value for each treated sample with that of each untreated sample. The experiment was repeated three times. A 5-h incubation period was chosen on the basis of our previous studies (M. Okamoto and M. Sato, unpublished observations) (2, 16, 32).
Treatment of human PBMCs with AILb-A.
The PBMCs isolated from heparinized venous blood by Ficoll-Hypaque gradient density centrifugation according to standard procedures were stimulated with AILb-A (1 μg/ml) or LPS (1 μg/ml) in the presence or absence of specific Ab against human TLR4 (HTA125 [32]; 10 μg/ml) or against human CD14, a coreceptor for LPS (10 μg/ml; PharMingen, San Diego, Calif.) for 24 h. Then, the supernatants were analyzed for cytokines and the cytotoxic activity of the PBMCs was assayed by a 51Cr release test.
Treatment of mouse peritoneal macrophages (PMs) and splenocytes with AILb-A.
Mice were injected intraperitoneally with 2 ml of 3% thioglycolate medium (Difco, Detroit, Mich.). Three days later, peritoneal exudate cells were harvested and cultured in RPMI 1640 medium containing 10% heat-inactivated FBS. After 2 h of cultivation, cells were washed with serum-free medium to remove nonadherent cells. Adherent monolayer cells were used as PMs. PMs (5 × 105/ml) were stimulated with AILb-A (0.1, 1, or 10 μg/ml), LPS (1 μg/ml), or PGN (2 μg/ml) for 48 h, and then cytokines in the supernatants were measured by ELISA. Mouse splenocytes were also prepared and stimulated with AILb-A (0.1, 1, or 10 μg/ml) or LPS (1 μg/ml), and then cytotoxic activity of the splenocytes was analyzed by 51Cr release test.
Transient transfection.
PMs (5 × 105/ml) derived from TLR4−/− mice were transiently transfected with 0.75 or 1.5 μg of pEFBOS expression plasmid containing the mouse TLR4 gene using SuperFect transfection reagent (Qiagen, Hilden, Germany). Twenty-four hours later, the PMs were treated with AILb-A (0.1, 1, or 10 μg/ml) or LPS (1 μg/ml) for 48 h at 37°C, and then cytokines in the supernatants were measured.
Preparation of bone marrow-derived DCs.
Bone-marrow dendritic cell (DC) culture was obtained using methods described previously (24). Briefly, mouse bone marrow cells were harvested from the femur and tibia of sacrificed mice. Contaminating erythrocytes were lysed with 0.83 M NH4Cl buffer, and lymphocytes were depleted with a mixture of antibodies (RL-172 [anti-CD4], TIB-105 [anti-CD8], and TIB-140 [anti-B220]; all from American Type Culture Collection, Manassas, Va.) and rabbit complement (Accurate Chemical and Scientific Corp., Westbury, N.Y.). These cells were cultured overnight in RPMI 1640 medium containing 10% heat-inactivated FBS to remove the adherent macrophages, and then nonadherent cells were placed in fresh culture medium supplemented with 1,000 U of recombinant murine granulocyte-macrophage colony-stimulating factor (PeproTech, London, England)/ml and 1,000 U of recombinant murine IL-4 (PeproTech)/ml to induce immature DCs (iDCs). After 6 days of cultivation, AILb-A (1 μg/ml) or TNF-α (10 ng/ml; Endogen, Boston, Mass.) was added to the iDC cultures to induce their maturation. After 2 days of adding these agents, cells were collected and analyzed for expression of cell surface molecules. The culture supernatants were assayed for IL-12.
Flow cytometric analysis of cell surface antigens.
Cell surface staining was performed using the following anti-murine MAbs. Fluorescein isothiocyanate-labeled anti-I Ab (major histocompatibility complex [MHC] class II), anti-CD80, and anti-CD86 were purchased from PharMingen. The cells were resuspended in PBS containing 0.1% sodium azide and 0.2% bovine serum albumin and then incubated for 30 min at 4°C with a saturating concentration of each MAb. After the cells were washed twice, their fluorescence intensity was determined using a flow cytometer (EPICS XL-MCL; Beckman Coulter, Fullerton, Calif.).
Animal model.
Syngeneic LL/2 cells (5 × 105/mouse) were injected subcutaneously into the footpads of wild-type C57BL/6 and TLR4−/− mice. SCCVII cells (105/mouse) were inoculated subcutaneously into the dorsal foot of C3H/HeN and C3H/HeJ mice. Approximately 10 days after the inoculation and when tumors were approximately 40 mm3 in volume, the mice were randomly divided into two groups consisting of 10 mice with almost equal mean tumor volumes. The mice were given 0.2 ml of physiological saline or 10 μg of AILb-A peritumorally on days 11, 15, 18, 22, and 25. The mice were monitored twice a week, and the tumor volume was estimated by measuring tumor size and using the following formula: tumor volume = 0.4 × L × W2, where L represents the largest diameter and W represents the smallest diameter. On day 26, the mice were killed, sera were collected to measure cytokines, and tumor infiltrating lymphocytes (TILs) and draining lymph node (LN) cells were isolated to examine killer cell activities.
Cytokine assay.
The assay for cytokines was performed using ELISA kits. The ELISA kits for human TNF-α, human IL-12, mouse IFN-γ, mouse TNF-α, and mouse IL-12 were purchased from BioSource International, Inc. (Camarillo, Calif.).
Assay for cytotoxic activities.
The cytotoxic activity of human PBMCs was analyzed against K-562 cells, which are sensitive for human NK cell activity, and that of mouse TILs, LN cells, and splenocytes was assayed against YAC-1 cells, which are markedly sensitive target cells for mouse NK cells and Meth-A cells and resistant to mouse NK cells, LL/2, and SCCVII, which are inoculated cell lines and, thus, sensitive targets for the cytotoxic T lymphocytes recognizing the antigens expressed in LL/2 and SCCVII, respectively, in the present animal model by a 51Cr release test. The 51Cr release was carried out as described previously (35). For cell-mediated cytotoxicity assays, 1.0 × 105, 2.0 × 105, or 4.0 × 105 effector cells were mixed in the wells of 96-well microtiter plates (Falcon; Becton Dickinson Labware, Lincoln Park, N.J.) with 1.0 × 104 51Cr-labeled target cells (E:T = 10, 20, or 40) in a total volume of 200 μl of medium and incubated at 37°C for 4 h. The percent specific 51Cr release was calculated according to the following formula: [(E − S)/(M − S)] × 100, where E is experimental 51Cr release, S is spontaneous 51Cr release, and M is maximum 51Cr release.
Western blot analysis.
PMs (5 × 105/ml) were stimulated with AILb-A (1 μg/ml), LPS (1 μg/ml), or PGN (2 μg/ml) for 1 h and then harvested by centrifugation. Cells were suspended in lysis buffer (20 mM Tris-HCl buffer [pH 7.4] containing 1% sodium dodecyl sulfate [SDS], 1% Triton X-100, 1% sodium deoxycholate, and 1% protease inhibitor cocktail [Sigma]) and sonicated for 30 s. The samples were centrifuged at 4°C for 15 min, and supernatants were transferred into new tubes. The samples (30 μg of protein/lane) were electrophoresed on SDS-10% polyacrylamide gels, transferred onto polyvinylidene difluoride membranes (Bio-Rad, Hercules, Calif.), and immunoblotted with an Ab specific for p38 MAPK, phospho-p38 MAPK, c-Jun NH2-terminal kinase (JNK), or phospho-JNK. These specific proteins were then detected by using an ECL kit (Amersham, Arlington Heights, Ill.). All of these Abs were purchased from Cell Signaling Technology, Inc. (Beverly, Mass.).
Immunofluorescence staining of PMs.
PMs (2 × 104/ml) were seeded on glass plates and stimulated with AILb-A (1 μg/ml) or LPS (1 μg/ml) for 5 h. The cells were washed twice with PBS, fixed in PBS containing 3% paraformaldehyde and 0.3% Triton X-100 for 5 min, and additionally incubated in 3% paraformaldehyde for 20 min. After blocking with 3% bovine serum albumin, the PMs were incubated with anti-IRF-3 Ab (Santa Cruz Biotechnology, Inc., Santa Cruz, Calif.) for 60 min, washed with PBS three times, and then incubated with rhodamine-conjugated anti-rabbit immunoglobulin G Ab (PharMingen). The glass plates were washed and drained, and then microscopic analysis was conducted under the conditions of fluorescent light.
Statistical analysis.
The data were evaluated by using one-way analysis of variance. P values of <0.05 were considered significant.
RESULTS
TLR4-mediated NF-κB activation by AILb-A.
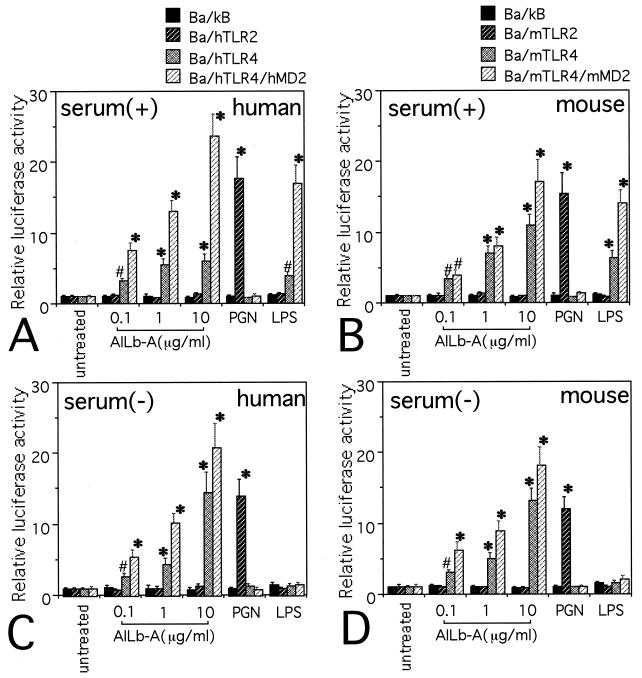
We first examined the responsiveness of Ba/F3 transfectants to AILb-A by a luciferase assay using an NF-κB-dependent reporter construct. Ba/F3 transfectants were cultured for 5 h in the medium containing 10% heat-inactivated FBS in the presence of AILb-A, LPS, or PGN. Results are shown in Fig. 1. AILb-A induced NF-κB activation in Ba/hTLR4 and Ba/mTLR4 cells. Further increased levels of NF-κB activities were observed in Ba/hTLR4/hMD-2 and Ba/mTLR4/mMD-2 cells stimulated with AILb-A. LPS also induced NF-κB activation in Ba/hTLR4, Ba/mTLR4, Ba/hTLR4/hMD-2, and Ba/mTLR4/mMD-2 cells. Ba/hTLR2 and Ba/mTLR2 but not TLR4-expressing cells responded to PGN to activate NF-κB (Fig. 1A and B). These data from the experiments using LPS and PGN were consistent with previous reports (2, 16, 32). We next tested the role of a serum factor(s) in AILb-A-induced, TLR4-mediated NF-κB activation. It has been reported that LPS-induced signaling is dependent on serum as a source of LPS-binding protein and/or soluble type CD14 (11). Moreover, it was suggested that LPS-induced signals are dependent on soluble CD14 in serum in Ba/F3 transfectants that do not express the CD14 molecule (16, 32). Interestingly, AILb-A induced NF-κB activation in TLR4-expressing cells even under serum-free conditions, and the levels of reporter activities increased by AILb-A under serum-free conditions were similar to those in the presence of serum, although serum was required for LPS-induced NF-κB activation in these cells (Fig. 1C and D).
FIG. 1.
TLR4-mediated NF-κB activation by AILb-A. The stable transfectants derived from Ba/F3 (5 × 105/well) were stimulated with AILb-A (0.1, 1, or 10 μg/ml), PGN (2 μg/ml), or LPS (1 μg/ml) at 37°C for 5 h in the presence (A and B) or absence (C and D) of 10% FBS. After the stimulation, cells were harvested and lysed, and then luciferase activity was measured. Bars denote standard deviations of triplicate samples, *, P < 0.01; #, P < 0.05 compared with respective untreated controls.
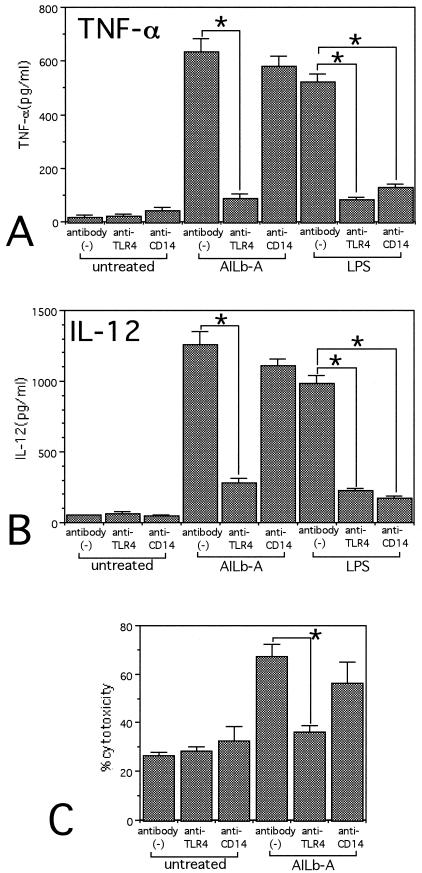
Inhibition of cytokine- and killer cell-inducing activities of AILb-A in human PBMCs by anti-human TLR4 MAb.
The data from the luciferase assay strongly suggested that AILb-A-induced signaling is mediated by TLR4 but not by TLR2. We next examined the involvement of TLR4 in AILb-A-induced cytokine production and killer cell activity in an in vitro human system. We added the anti-human TLR4 MAb HTA125 into the human PBMC culture in the presence of AILb-A. After 24 h of cultivation, TNF-α and IL-12 in the supernatants were measured by ELISA. Addition of anti-human TLR4 MAb significantly inhibited the production of TNF-α and IL-12 by the PBMCs stimulated with AILb-A as well as those stimulated with LPS. In contrast, addition of the blocking Ab against CD14, a significant cofactor for TLR4-mediated LPS signaling, inhibited only LPS-induced cytokine production but not that induced by AILb-A. The different role(s) of CD14 was shown between AILb-A-induced and LPS-induced TLR4 signaling (Fig. 2A and B). The increase in cytotoxic activity of AILb-A-stimulated PBMCs against K-562 was also inhibited by addition of anti-TLR4 MAb but not by anti-CD14 (Fig. 2C).
FIG. 2.
Effect of anti-human TLR4 MAb in cytokine production and cytotoxic activity induced by AILb-A. PBMCs were stimulated with AILb-A (1 μg/ml) or LPS (1 μg/ml) in the presence or absence of specific MAbs against human TLR4 (HTA125; 10 μg/ml) or against human CD14, a coreceptor for LPS (10 μg/ml) for 24 h. Then, the supernatants were analyzed for TNF-α (A) and IL-12 (B) by ELISA. Cytotoxic activity against K-562 of the PBMCs was assayed by 51Cr release test (C). Bars denote standard deviations of triplicate samples, *, P < 0.01.
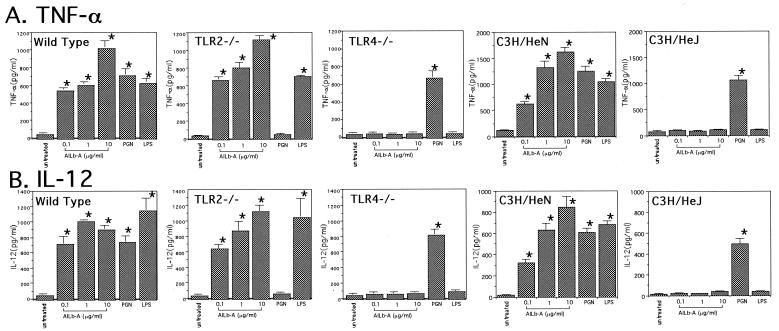
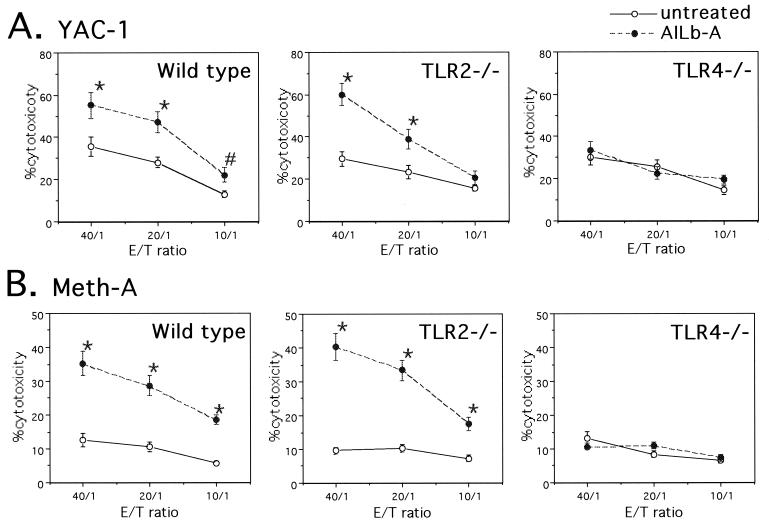
Impairment of cytokine- and killer cell-inducing activities of AILb-A in TLR4−/− mice.
Mouse-derived PMs were treated with AILb-A, LPS, or PGN for 24 h, and then the supernatants were examined for cytokines. Although AILb-A induced TNF-α and IL-12 in PMs derived from wild-type C57BL/6 and C3H/HeN mice as well as in those from TLR2−/− mice in a dose-dependent manner, TLR4−/− and C3H/HeJ mice, which have a point mutation in the TLR4 gene, did not respond to AILb-A to induce cytokines. Impairment of cytokine-inducing activity of PGN was observed only in TLR2−/− mouse-derived PMs, and PMs from TLR4−/− and from C3H/HeJ mice did not respond to LPS (Fig. 3). Cytotoxic activity of AILb-A-treated mouse splenocytes was also examined. AILb-A treatment significantly augmented killer cell activities both against YAC-1 and Meth-A of splenocytes derived from wild-type C57BL/6 and TLR2−/− mice but not from TLR4−/− mice (Fig. 4).
FIG. 3.
Impairment of cytokine-inducing activity of AILb-A in PMs derived from TLR4−/− and TLR4 mutant mice. Thioglycolate-elicited PMs (5 × 105/ml) derived from wild-type C57BL/6, TLR2−/−, TLR4−/−, C3H/HeN, and C3H/HeJ mice were treated with AILb-A (0.1, 1, or 10 μg/ml), PGN (2 μg/ml), or LPS (1 μg/ml) for 48 h, and then TNF-α (A) and IL-12 (B) in the supernatants were measured by ELISA. Bars denote standard deviations of triplicate samples, *, P < 0.01 compared with untreated controls.
FIG. 4.
Impairment of cytotoxic activity of splenocytes induced by AILb-A in TLR4−/− mice. Splenocytes (2 × 106/ml) derived from wild-type C57BL/6, TLR2−/−, or TLR4−/− mice were treated with 1 μg of AILb-A/ml for 24 h, and then the cytotoxic activities against YAC-1 (A) and Meth-A (B) of the splenocytes were analyzed by 51Cr release test. Bars denote standard deviations of triplicate samples, *, P < 0.01; #, P < 0.05 compared with untreated controls.
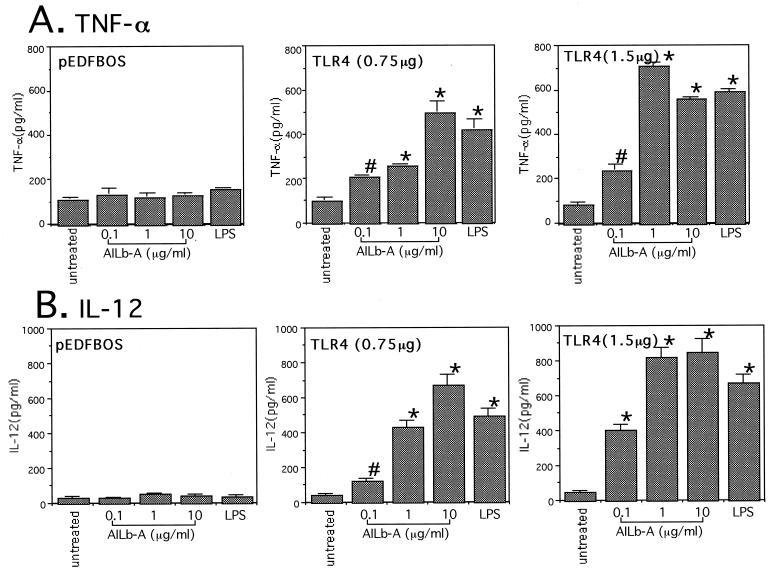
Acquisition of responsiveness to AILb-A to induce cytokines by transfection of TLR4−/− mouse-derived PMs with the expression plasmid containing the mouse TLR4 gene.
To confirm the involvement of TLR4 in AILb-A-induced cytokine production, we transiently transfected TLR4−/− mouse-derived PMs with a pEFBOS expression plasmid containing mouse TLR4 cDNA and then stimulated these PMs with AILb-A or LPS. After 48 h of stimulation, the supernatants were analyzed for cytokines. TLR4−/− mouse-derived PMs transfected with empty vector responded to neither AILb-A nor LPS to produce the cytokines tested, TNF-α and IL-12. The PMs acquired the responsiveness to AILb-A to produce cytokines by transfection of the expression vector for mouse TLR4 in a manner dependent on dose of DNA. LPS also induced cytokines in the PMs with the mouse TLR4 gene but not in empty vector-transfected PMs (Fig. 5).
FIG. 5.
Acquisition of responsiveness to AILb-A by transfection of TLR4−/− mouse-derived PMs with the expression vector containing the mouse TLR4 gene. PMs (5 × 105/ml) derived from TLR4−/− mice were transiently transfected with 0.75 or 1.5 μg of pEFBOS expression plasmid containing the mouse TLR4 gene. Twenty-four hours later, the PMs were treated with AILb-A (0.1, 1, or 10 μg/ml) or LPS (1 μg/ml) for 48 h at 37°C, and then TNF-α (A) and IL-12 (B) in the supernatants were measured by ELISA. Bars denote standard deviations of triplicate samples. *, P < 0.01; #, P < 0.05 compared with untreated controls.
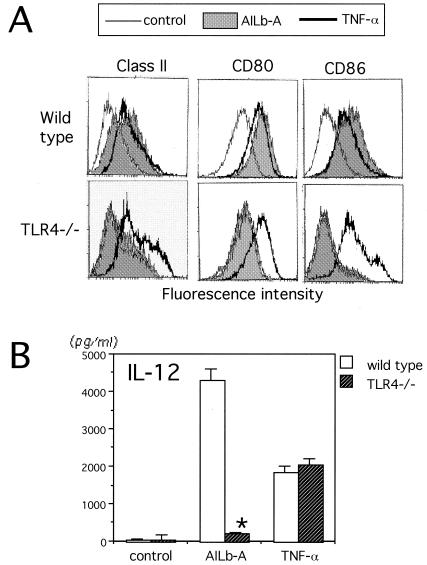
Impairment of AILb-A-induced DC maturation in TLR4−/− mice.
Mouse bone marrow-derived iDCs were treated with AILb-A or TNF-α, a known DC-maturating cytokine, for 48 h, and then expression of surface molecules was analyzed by flow cytometric analysis. DCs derived from wild-type C57BL/6 mice showed enhanced surface expression of MHC class II, CD80, and CD86 when stimulated with AILb-A, while TLR4−/− mouse-derived DCs did not show any enhanced expression of the surface molecules in response to AILb-A (Fig. 6A). IL-12 in the supernatants from the DC culture was also examined by ELISA. IL-12 was induced by AILb-A stimulation in wild-type mouse-derived DCs but not in DCs derived from TLR4−/− mice (Fig. 6B). Both wild-type and TLR4−/−-derived DCs exhibited similar responses to TNF-α (Fig. 6).
FIG. 6.
Impairment of AILb-A-induced DC maturation in TLR4−/− mice. (A) Mouse bone marrow-derived iDCs induced by granulocyte-macrophage colony-stimulating factor and IL-4 were treated with AILb-A or TNF-α for 48 h, and then expression of surface molecules, MHC class II, CD80, and CD86 was analyzed by flow cytometric analysis. (B) IL-12 in the supernatants from the DC culture was examined by ELISA. Bars indicate standard deviations of triplicate samples. *, P < 0.01 compared with respective controls using wild-type DCs.
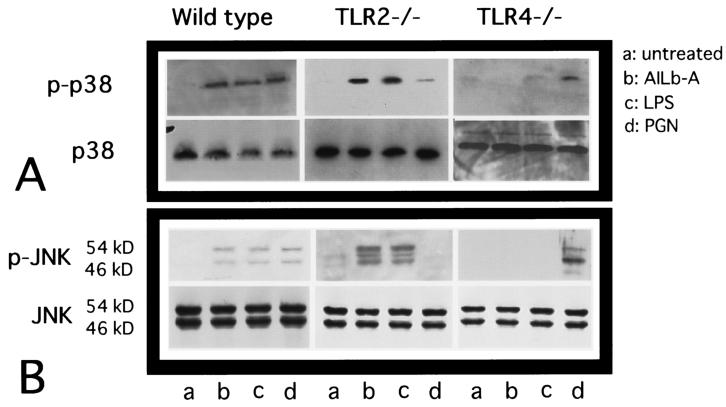
Involvement of TLR4 in the phosphorylation of MAPKs induced by AILb-A.
TLR4-mediated LPS signaling has been shown to initiate multiple intracellular signaling events, including the stimulation of pathways that lead to the activation of two distinct MAPKs, the p38 and JNK proteins (21, 23), as well as NK-κB. We next examined the involvement of TLR4 in the AILb-A-induced phosphorylation of these MAPKs by Western blot analysis of the cellular lysates from mouse-derived PMs using phospho-specific Abs. AILb-A stimulation activated all two MAPK proteins tested in wild-type and TLR2−/− mouse-derived PMs but not in the PMs from TLR4−/− mice (Fig. 7A and B, upper panels).
FIG. 7.
Involvement of TLR4 in AILb-A-induced phosphorylation of p38 MAPK and JNK. PMs (5 × 105/ml) were stimulated with AILb-A (1 μg/ml), LPS (1 μg/ml), or PGN (2 μg/ml) for 1 h and then lysed in 1× SDS sample buffer. The samples (30 μg of protein/lane) were electrophoresed on SDS-10% polyacrylamide gels, transferred onto polyvinylidene difluoride membranes, and immunoblotted with an Ab specific for phospho-p38 MAPK (A, upper panel) or phospho-JNK (B, upper panel). The same blots were stripped and reblotted with an Ab specific both for phosphorylated and for nonphosphorylated forms of each respective signaling protein (A and B, lower panels). These are representative blots from three independent experiments.
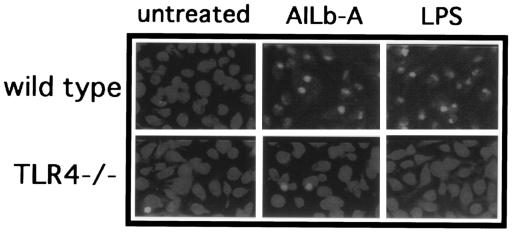
Involvement of TLR4 in AILb-A-induced IRF-3 activation.
PMs derived from wild-type or TLR4−/− mice were stimulated with AILb-A or LPS for 5 h. The cells were stained with anti-IRF-3 Ab, and the cellular localization of IRF-3 was determined. IRF-3 normally localized in the cytoplasm in the macrophages from both wild-type and TLR4−/− mice. Following AILb-A stimulation, IRF-3 protein accumulated in the nuclei in wild-type mouse-derived macrophages but not in those from TLR4−/− mice (Fig. 8).
FIG. 8.
Cellular localization of IRF-3 protein in AILb-A-stimulated PMs. PMs (2 × 104/ml) were stimulated with AILb-A (1 μg/ml) or LPS (1 μg/ml) for 5 h. The cells were stained with anti-IRF-3 Ab, and the cellular localization of IRF-3 protein was determined under the conditions of fluorescent light.
Involvement of TLR4 in AILb-A-induced antitumor immunity.
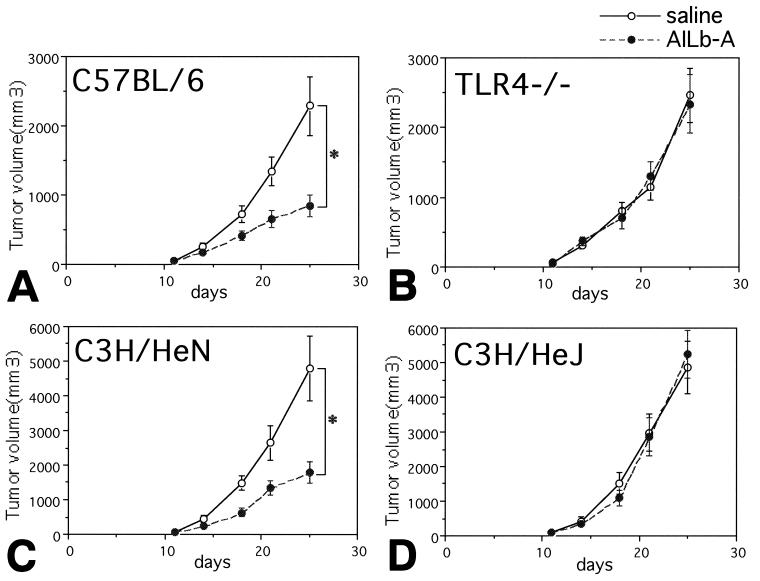
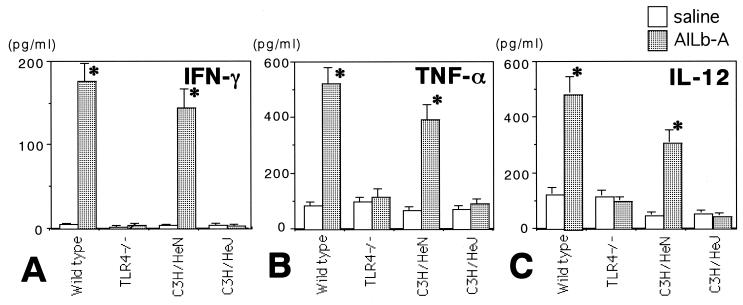
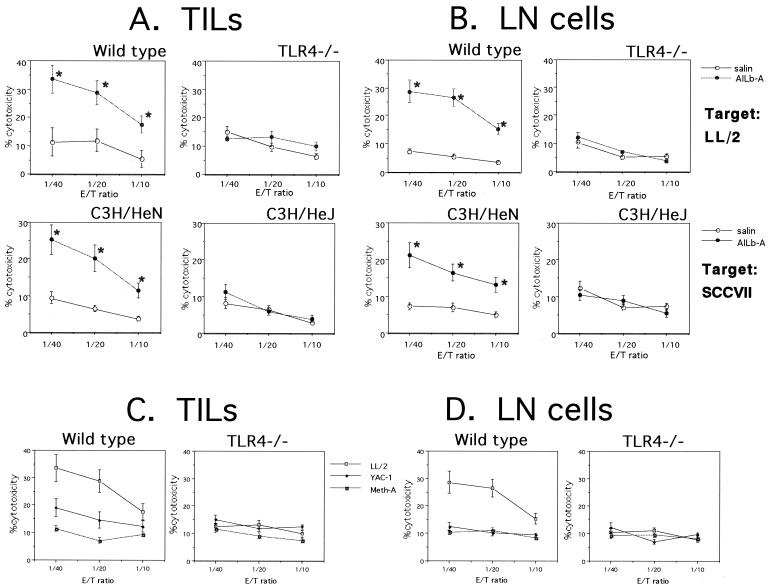
Finally, involvement of TLR4 signaling in AILb-A-induced antitumor immunity was examined. Syngeneic LL/2-bearing C57BL/6 and TLR4−/− mice as well as syngeneic SCCVII-bearing C3H/HeN and C3H/HeJ mice were treated with AILb-A. The peritumoral injection of AILb-A resulted in significant inhibition of tumor growth in wild-type C57BL/6 and C3H/HeN mice bearing syngeneic tumors; however, no antitumor effect of AILb-A was observed in TLR4−/− and C3H/HeJ mice (Fig. 9). The sera derived from these tumor-bearing mice were examined for cytokines that might be important for antitumor immunity. The serum levels of all cytokines tested, including IFN-γ, TNF-α, and IL-12, were significantly increased by peritumoral administration of AILb-A in wild-type C57BL/6 and C3H/HeN mice but not in TLR4−/− and C3H/HeJ mice (Fig. 10). The cytotoxic activities of TILs and LN cells derived from the mice administered AILb-A were also analyzed. Although cytotoxic activity against LL/2 of TILs derived from wild-type C57BL/6 mice and that against SCCVII of C3H/HeN-derived TILs were markedly increased by AILb-A administration, the killing activity was not enhanced in TLR4−/− and C3H/HeJ mice (Fig. 11A). In the cytotoxic activities of LN cells, similar results were obtained (Fig. 11B). In nonspecific killing of the cells against YAC-1 and Meth-A, cytolysis of wild-type mouse-derived TILs but not of LN cells against YAC-1 was slightly enhanced by AILb-A treatment. Nonspecific killing activity of TLR4−/−-derived lymphocytes was not increased by AILb-A administration (Fig. 11C and D).
FIG. 9.
Involvement of TLR4 in AILb-A-induced tumor regression. LL/2-bearing wild-type C57BL/6 mice (A) and TLR4−/− mice (B) and SCCVII-bearing C3H/HeN (C) and C3H/HeJ (D) mice received physiological saline or 10 μg of AILb-A peritumorally on days 11, 15, 18, 22, and 25 after tumor cell inoculation. The tumor volumes were measured twice a week. Bars denote standard deviations of 10 animals. *, P < 0.01 compared with controls given saline.
FIG. 10.
Cytokines in the sera derived from tumor-bearing mice given AILb-A. LL/2-bearing wild-type C57BL/6 and TLR4−/− mice and SCCVII-bearing C3H/HeN and C3H/HeJ mice received physiological saline or 10 μg of AILb-A peritumorally on days 11, 15, 18, 22, and 25 after tumor cell inoculation. On day 26, mice were sacrificed and sera were collected. Then, IFN-γ (A), TNF-α (B), and IL-12 (C) were measured by ELISA. Bars denote standard deviations of four serum samples. *, P < 0.01 compared with controls given saline.
FIG. 11.
Cytotoxic activities of TILs and LN cells derived from tumor-bearing mice given AILb-A. LL/2-bearing wild-type C57BL/6 and TLR4−/− mice and SCCVII-bearing C3H/HeN and C3H/HeJ mice were killed on day 26, and TILs (A and C) and LN cells (B and D) were prepared for analysis of cytotoxic activity by 51Cr release assay. Inoculated tumor cells, LL/2 and SCCVII, were used as target cells (A and B). In some experiments, nonspecific target cells, YAC-1 and Meth-A, were used (C and D). Bars denote standard deviations of four samples. *, P < 0.01 compared with controls given saline.
DISCUSSION
We have reported that a 55-kDa protein (AILb-A) isolated from a parasitic plant, A. indica L., induces Th1-type cytokines and mediates potent anticancer immunity in vitro and in vivo (25, 26), and the use of this protein for the treatment of human cancer should be evaluated. However, the molecular mechanism by which AILb-A enhances anticancer host responses remains unclear. The present study was conducted in an attempt to elucidate the molecular mechanism of action of AILb-A. TLR2, recognizing bacterial protein components, and TLR4, recognizing a plant-derived component in addition to bacterial components, were chosen as candidate signaling receptors for AILb-A. TLRs that are expressed mainly on macrophages and DCs were originally identified as receptor molecules that recognize many types of pathogens and activate an innate immune system (1, 31). Recently, several studies proposed that the signaling via TLRs induces anticancer immunity through maturation of antigen-presenting cells as well as through leading a Th1-type T-cell response. Seya et al. demonstrated that maturation of DCs and cytokine induction by the cell wall skeleton of Mycobacterium bovis bacillus Calmette-Guérin, an anticancer immuno-adjuvant, are induced via both TLR2 and TLR4 (36). Hemmi et al. discovered a new molecule of the TLR family, TLR9, recognizing unmethylated bacterial CpG-DNA, whose clinical use is expected for cancer therapy as a potent Th1 inducer (12). We have also reported that TLR4 signaling is intimately involved in anticancer immunity induced by OK-432, a streptococcal preparation that is commonly used for the treatment of cancer patients (27, 28). TLR-mediated activation of innate as well as adaptive immunity may be greatly important, and TLR ligands may be useful application for cancer therapy.
The findings obtained from the present study clearly demonstrated that AILb-A is a novel ligand for TLR4 and augments anticancer immunity via TLR4 signaling. AILb-A-induced TLR4 signaling mediated the enhancement of transcriptional activity of NF-κB, phosphorylation of MAPKs, nuclear translocation of IRF-3, cytokine induction, cytotoxic activity of lymphocytes, and DC maturation in vitro. Furthermore, AILb-A induced IL-12 both on macrophages and on DCs. It was thus suggested that in in vivo situations, AILb-A may induce a Th1-dominant state and killer cells and exhibit an antitumor effect. Actually, in vivo AILb-A induced IFN-γ production and increased cytotoxic activities of TILs and LN cells against each inoculated tumor. None of these activities described above was observed in TLR4−/− mice; thus, TLR4 signaling was suggested to be intimately involved in the Th1 response, killer cell activities, and tumor regression induced by AILb-A. Identification of TLR4 as a receptor molecule for AILb-A represents great progress in elucidation of the molecular mechanism of the AILb-A-induced anticancer host response as well as in its clinical use. When AILb-A is used as a therapeutic application, expression of TLR4 in the patients may be a useful marker to discriminate between responders and nonresponders to therapy with the agent. Furthermore, TLR4 gene transfer may be effective in augmenting the responsiveness to AILb-A in patients in which TLR4 expression is low or the TLR4 gene is mutated. Mutation of the TLR4 gene in humans has already been reported (5). We have also reported that TLR4 mRNA was not detected in 2 of 28 oral cancer patients examined (28). This hypothesis is strongly supported by the results from the present experiments that the transient transfection of the TLR4−/− mouse-derived macrophages with expression plasmid containing TLR4 gene recovered the responsiveness of the cells to AILb-A to produce cytokines.
A risk of preparation of a biologically active component in the laboratory is contamination with gram-negative bacterial endotoxin LPS. Especially this is a significant risk factor in the evaluation of the activity of AILb-A, because AILb-A exhibits its activity via TLR4 signaling, similarly to LPS. A luciferase assay using TLR4-expressing cells showed that TLR4-mediated NF-κB activation by LPS was dependent on a serum factor(s), while that by AILb-A was not. Furthermore, anti-CD14 Ab neutralized only LPS-induced cytokine production and killer cell activity but not that induced by AILb-A. A different role of CD14 was shown between AILb-A-induced and LPS-induced TLR4 signaling. It is to be clarified that AILb-A-induced TLR4 signaling is not due to LPS contamination in this preparation, and also that AILb-A-induced TLR4 signaling is involved in a different mechanism(s) from that induced by LPS. Unlike AILb-A, LPS exhibits lethal side effects and is not suitable as a therapeutic agent. There may be some other differences between AILb-A and LPS in the signal transduction mechanism downstream of TLR4. Recently, Toll-IL-1 receptor domain-containing adapter protein, an adapter molecule that mediates TLR signaling (13, 38), Toll-interacting protein, a negative regulator of TLR signaling (7, 39), and the MyD88-independent signaling pathway (15) were identified, while the mechanism of managing the different actions between AILb-A and LPS is still uncertain. The downstream signal transduction mechanism of AILb-A-induced TLR4 activation to eliminate cancer cells should be elucidated. It is now under investigation in our laboratories.
We are now cloning the gene encoding the AILb-A protein. We believe that immuno-gene therapy using the gene encoding this protein, which induces DC maturation, a Th1-type T-cell response, and killer cells and, further, whose receptor molecule has been identified, may be a useful application for cancer therapy.
Acknowledgments
This investigation was supported in part by a Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
REFERENCES
- 1.Aderem, A., and R. J. Ulevitch. 2000. Toll-like receptors in the induction of the innate immune response. Nature 406:782-787. [DOI] [PubMed] [Google Scholar]
- 2.Akashi, S., R. Shimazu, H. Ogata, Y. Nagai, K. Takeda, M. Kimoto, and K. Miyake. 2000. Cell surface expression and lipopolysaccharide signaling via the Toll-like receptor 4-MD-2 complex on mouse peritoneal macrophages. J. Immunol. 164:3471-3475. [DOI] [PubMed] [Google Scholar]
- 3.Alfieri, A. A., and E. W. Hahn. 1978. An in situ method for estimating cell survival in a solid tumor. Cancer Res. 38:3006-3011. [PubMed] [Google Scholar]
- 4.Anderson, L. C., K. Nilsson, and C. G. Gahmberg. 1979. K-562, a human erythroleukemic cell line. Int. J. Cancer 23:143-147. [DOI] [PubMed] [Google Scholar]
- 5.Arbour, N. C., E. Lorenz, B. C. Schutte, J. Zabner, J. N. Kline, M. Jones, K. Frees, J. L. Watt, and D. A. Schwartz. 2000. TLR4 mutations are associated with endotoxin hyporesponsiveness in humans. Nat. Genet. 25:187-191. [DOI] [PubMed] [Google Scholar]
- 6.Bertram, J. S., and P. Janik. 1980. Establishment of a cloned line of Lewis lung carcinoma cells adapted to cell culture. Cancer Lett. 11:63-73. [DOI] [PubMed] [Google Scholar]
- 7.Burns, K., J. Clatworthy, L. Martin, F. Martinon, C. Plumpton, B. Maschera, A. Lewis, K. Ray, J. Tschopp, and F. Volpe. 2000. Tollip, a new component of the IL-1RI pathway, links IRAK to the IL-1 receptor. Nat. Cell Biol. 2:346-351. [DOI] [PubMed] [Google Scholar]
- 8.Chai, J. G., T. Bando, S. Kobashi, M. Oka, H. Nagasawa, S. Nakai, K. Maeda, K. Himeno, M. Sato, and S. Ohkubo. 1992. An extract of seeds from Aeginetia indica L., a parasitic plant, induces potent antigen-specific antitumor immunity in Meth A-bearing BALB/c mice. Cancer Immunol. Immunother. 35:181-185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chai, J. G., T. Bando, H. Nagasawa, K. Himeno, M. Sato, and S. Ohkubo. 1994. Seed extract of Aeginetia indica L. induces cytokine production and lymphocyte proliferation in vitro. Immunopharmacology 27:13-21. [DOI] [PubMed] [Google Scholar]
- 10.Chai, J. G., M. Okamoto, T. Bando, H. Nagasawa, H. Hisaeda, T. Sakai, K. Himeno, M. Sato, and S. Ohkubo. 1995. Dissociation between the mitogenic effect and antitumor activity of seed extract from Aeginetia indica L. Immunopharmacology 30:209-215. [DOI] [PubMed] [Google Scholar]
- 11.Fenton, M. J., and D. T. Golenbock. 1998. LPS-binding proteins and receptors. J. Leukoc. Biol. 64:25-32. [DOI] [PubMed] [Google Scholar]
- 12.Hemmi, H., O. Takeuchi, T. Kawai, T. Kaisho, S. Sato, H. Sanjo, M. Matsumoto, K. Hoshino, H. Wagner, K. Takeda, and S. Akira S. 2000. A Toll-like receptor recognizes bacterial DNA. Nature 408:740-745. [DOI] [PubMed] [Google Scholar]
- 13.Horng, T., G. M. Barton, and R. Medzhitov. 2001. TIRAP: an adapter molecule in the Toll signaling pathway. Nat. Immunol. 2:835-841. [DOI] [PubMed] [Google Scholar]
- 14.Hoshino, K., O. Takeuchi, T. Kawai, H. Sanjo, T. Ogawa, Y. Takeda, K. Takeda, and S. Akira. 1999. Toll-like receptor 4 (TLR4)-deficient mice are hyporesponsive to lipopolysaccharide: evidence for TLR4 as the Lps gene product. J. Immunol. 162:3749-3752. [PubMed] [Google Scholar]
- 15.Kawai, T., O. Takeuchi, T. Fujita, J. Inoue, P. F. Muhlradt, S. Sato, K. Hoshino, and S. Akira. 2001. Lipopolysaccharide stimulates the MyD88-independent pathway and results in activation of IFN-regulatory factor 3 and the expression of a subset of lipopolysaccharide-inducible genes. J. Immunol. 167:5887-5894. [DOI] [PubMed] [Google Scholar]
- 16.Kawasaki, K., S. Akashi, R. Shimazu, T. Yoshida, K. Miyake, and M. Nishijima. 2000. Mouse Toll-like receptor 4′MD-2 complex mediates lipopolysaccharide-mimetic signal transduction by Taxol. J. Biol. Chem. 275:2251-2254. [DOI] [PubMed] [Google Scholar]
- 17.Lamon, E. W., R. A. Gatti, R. Kiessling, and E. M. Fenyo. 1975. Comparison of the allospecific and viral-specific immune responses to irradiated versus formaldehyde-fixed allogeneic Moloney lymphoma cells in CBA mice. Cancer Res. 35:962-969. [PubMed] [Google Scholar]
- 18.Lemaitre, B., E. Nicolas, L. Michaut, J. M. Reichhart, and J. A. Hoffmann. 1996. The dorsoventral regulatory gene cassette spatzle/Toll/Cactus controls the potent antifungal response in Drosophila adults. Cell 86:973-983. [DOI] [PubMed] [Google Scholar]
- 19.Lien, E., T. J. Sellati, A. Yoshimura, T. H. Flo, G. Rawadi, R. W. Finberg, J. D. Carroll, T. Espevik, R. R. Ingalls, J. D. Radolf, and D. T. Golenbock. 1999. Toll-like receptor 2 functions as a pattern recognition receptor for diverse bacterial products. J. Biol. Chem. 274:33419-33425. [DOI] [PubMed] [Google Scholar]
- 20.Means, T. K., A. Yoshimura, S. Wang, D. T. Golenbock, and M. J. Fenton. 1999. The CD14 ligands lipoarabinomannan and lipopolysaccharide differ in their requirement for Toll-like receptors. J. Immunol. 163:6748-6755. [PubMed] [Google Scholar]
- 21.Medzhitov, R., P. Preston-Hurlburt, E. Kopp, A. Stadlen, C. Chen, S. Ghosh, and C. A. Janeway, Jr. 1998. MyD88 is an adaptor protein in the hToll/IL-1 receptor family signaling pathways. Mol. Cell 2:253-258. [DOI] [PubMed] [Google Scholar]
- 22.Morisato, D., and K. V. Anderson. 1994. The spaetzle gene encodes a component of the extracellular signaling pathway establishing the dorsal-ventral pattern of the Drosophila embryo. Cell 76:677-688. [DOI] [PubMed] [Google Scholar]
- 23.Muzio, M., N. Polentarutti, D. Bosisio, P. P. Manoj Kumar, and A. Mantovani. 2000. Toll-like receptor family and signalling pathway. Biochem. Soc. Trans. 28:563-566. [DOI] [PubMed] [Google Scholar]
- 24.Nishioka, Y., M. Hirao, P. D. Robbins, M. T. Lotze, and H. Tahara. 1999. Induction of systemic and therapeutic antitumor immunity using intratumoral injection of dendritic cells genetically modified to express interleukin 12. Cancer Res. 59:4035-4041. [PubMed] [Google Scholar]
- 25.Ohe, G., M. Okamoto, T. Oshikawa, S. Furuichi, H. Nishikawa, T. Tano, K. Uyama, T. Bando, H. Yoshida, T. Sakai, K. Himeno, M. Sato, and S. Ohkubo. 2001. Th1-cytokine induction and anti-tumor effect of 55 kDa protein isolated from Aeginetia indica L., a parasitic plant. Cancer Immunol. Immunother. 50:251-259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Okamoto, M., G. Ohe, T. Oshikawa, H. Nishikawa, S. Furuichi, T. Bando, H. Yoshida, T. Sakai, K. Himeno, M. Sato, and S. Ohkubo. 2000. Purification and characterization of cytokine-inducing protein of seed extract from Aeginetia indica L., a parasitic plant. Immunopharmacology 49:377-389. [DOI] [PubMed] [Google Scholar]
- 27.Okamoto, M., T. Oshikawa, G. Ohe, H. Nishikawa, S. Furuichi, T. Tano, Y. Moriya, M. Saito, and M. Sato. 2001. Severe impairment of anti-cancer effect of lipoteichoic acid-related molecule isolated from a penicillin-killed Streptococcus pyogenes in toll-like receptor 4-deficient mice. Int. Immunopharmacol. 1:1789-1795. [DOI] [PubMed] [Google Scholar]
- 28.Okamoto, M., T. Oshikawa, T. Tano, G. Ohe, S. Furuichi, H. Nishikawa, S. U. Ahmed, S. Akashi, K. Miyake, O. Takeuchi, S. Akira, Y. Moriya, S. Matsubara, Y. Ryoma, M. Saito, and M. Sato. 2003. Involvement of toll-like receptor 4 signaling in interferon-γ production and antitumor effect by streptococcal agent OK-432. J. Natl. Cancer Inst. 95:316-326. [DOI] [PubMed] [Google Scholar]
- 29.Poltorak, A., X. He, I. Smirnova, M. Y. Liu, C. V. Huffel, X. Du, D. Birdwell, E. Alejos, M. Silva, C. Galanos, M. Freudenberg, P. Ricciardi-Castagnoli, B. Layton, and B. Beutler. 1998. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science 282:2085-2088. [DOI] [PubMed] [Google Scholar]
- 30.Qureshi, S. T., L. Lariviere, G. Leveque, S. Clermont, K. J. Moore, P. Gros, and D. Malo. 1999. Endotoxin-tolerant mice have mutations in Toll-like receptor 4 (Tlr4). J. Exp. Med. 189:615-625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rock, F. L., G. Hardiman, J. C. Timans, R. A. Kasteleins, and J. F. Bazann. 1998. A family of human receptors structurally related to Drosophila Toll. Proc. Natl. Acad. Sci. USA 95:588-593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shimazu, R., S. Akashi, H. Ogawa, Y. Nagai, K. Fukudome, K. Miyake, and M. Kimoto. 1999. MD-2, a molecule that confers lipopolysaccharide responsiveness on Toll-like receptor 4. J. Exp. Med. 189:1777-1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Suit, H. D., R. S. Sedlacek, and A. Zietman. 1988. Quantitative transplantation assays of spontaneous tumors of the C3H mouse as allografts in athymic NCr/Sed-nu/nu nude mice and isografts in C3Hf/Sed mice. Cancer Res. 48:4525-4528. [PubMed] [Google Scholar]
- 34.Takeuchi, O., K. Hoshino, T. Kawai, H. Sanjo, H. Takada, T. Ogawa, K. Takeda, and S. Akira. 2001. Differential roles of TLR2 and TLR4 in recognition of gram-negative and gram-positive bacterial cell wall components. Immunity 11:443-451. [DOI] [PubMed] [Google Scholar]
- 35.Timonen, T., and E. Saksela. 1977. A simplified isotope release assay for cell-mediated cytotoxicity against anchorage-dependent target cells. J. Immunol. Methods 18:123-132. [DOI] [PubMed] [Google Scholar]
- 36.Tsuji, S., M. Matsumoto, O. Takeuchi, S. Akira, I. Azuma, A. Hayashi, K. Toyoshima, and T. Seya. 2000. Maturation of human dendritic cells by cell wall skeleton of Mycobacterium bovis bacillus Calmette-Guérin: involvement of Toll-like receptors. Infect. Immun. 68:6883-6890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Underhill, D. M., A. Ozinsky, K. D. Smith, and A. Aderem. 1999. Toll-like receptor-2 mediates mycobacteria-induced proinflammatory signaling in macrophages. Proc. Natl. Acad. Sci. USA 96:14459-14463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yamanoto, M., S. Sato, H. Hemmi, H. Sanjo, S. Uematsu, T. Kaisho, K. Hoshino, O. Takeuchi, M. Kobayashi, T. Fujita, K. Takeda, and S. Akira. 2001. Essential role for TIRAP in activation of the signaling cascade shared by TLR2 and TLR4. Nature 420:324-329. [DOI] [PubMed] [Google Scholar]
- 39.Zhang, G., and S. Ghosh. 2002. Negative regulation of Toll-like receptor-mediated signaling by Tollip. J. Biol. Chem. 277:7059-7065. [DOI] [PubMed] [Google Scholar]