Abstract
Melanopsin-based phototransduction is involved in non-image forming light responses including circadian entrainment, pupil constriction, suppression of pineal melatonin synthesis, and direct photic regulation of sleep in vertebrates. Given that the functions of melanopsin involve the measurement and summation of total environmental luminance, there would appear to be no need for the rapid deactivation typical of other G-protein coupled receptors. In this study, however, we demonstrate that heterologously expressed mouse melanopsin is phosphorylated in a light-dependent manner, and that this phosphorylation is involved in regulating the rate of G-protein activation and the lifetime of melanopsin’s active state. Furthermore, we provide evidence for light-dependent phosphorylation of melanopsin in the mouse retina using an in situ proximity ligation assay. Finally, we demonstrate that melanopsin preferentially interacts with the GRK2/3 family of G-protein coupled receptor kinases through co-immunoprecipitation assays. Based on the complement of G-protein receptor kinases present in the melanopsin-expressing retinal ganglion cells, GRK2 emerges as the best candidate for melanopsin’s cognate GRK.
Electronic supplementary material
The online version of this article (doi:10.1007/s00018-011-0891-3) contains supplementary material, which is available to authorized users.
Keywords: Melanopsin, Phosphorylation, In situ proximity assay
Introduction
As the largest superfamily of membrane proteins, G-protein coupled receptors (GPCRs) are involved in diverse signaling processes. Upon activation, GPCRs bind and activate heterotrimeric G-proteins, typically leading to the activation of a second messenger signaling cascade. In most systems, GPCR activity is rapidly attenuated following activation in a process termed desensitization or deactivation. This attenuation is typically accomplished by phosphorylation of the carboxy-terminal tail of the receptor by a member of the GPCR kinase (GRK) family of serine/threonine kinases. Phosphorylation serves to reduce the activation rate of G-proteins by as much as 70–80% [1], and the phosphorylated region serves as a recognition and activation site for the cytoplasmic protein, arrestin. Binding of arrestin to the GPCR’s cytoplasmic loops leads to complete quenching of receptor activity, and can also lead to internalization of the receptor. Furthermore, GPCR-bound arrestin can serve as a scaffold for the binding of other proteins, leading to the activation of other G-protein independent signaling cascades (reviewed in [2–6]).
Opsins, which mediate light detection in metazoans, make up one of the most extensively studied groups within the GPCR superfamily. Melanopsin is a member of the opsin family found in intrinsically photosensitive retinal ganglion cells (ipRGCs) in vertebrate retinas. Melanopsin-based phototransduction is responsible in part for non-image forming light responses including circadian photoentrainment, pupil constriction, suppression of pineal melatonin synthesis, and direct photic regulation of sleep [7, 8]. Melanopsin differs from other vertebrate opsins in that its sequence exhibits greater homology to rhabdomeric photopigments typically involved in invertebrate phototransduction than to other vertebrate ciliary opsins, and mounting evidence suggests that melanopsin may also use a more rhabdomeric type of signal transduction cascade, which typically employ a Gq/G11-based pathway leading to the activation of PLC and a transient receptor potential channel [9–11]. The onset of the melanopsin-based light response is sluggish compared to that seen in rods or cones, and the kinetics of deactivation are much slower [12]. This raises questions of whether the general mechanism of vertebrate GPCR deactivation is applicable to melanopsin-based signaling, and how melanopsin activity is quenched. These questions achieve greater relevance as melanopsin-based gene therapy is being investigated as a treatment for blindness caused by degeneration of the outer retina, and the extended deactivation kinetics of melanopsin remain a major obstacle to achieving adequate temporal resolution [13]. Here we provide evidence that melanopsin is phosphorylated in a light-dependent manner both in vivo and in vitro, and that this phosphorylation is involved in temporal control of the melanopsin-based light response in a heterologous expression system.
Materials and methods
cDNA constructs
In all experiments except those in Fig. 6, the long isoform of wild-type mouse melanopsin (Accession # AF147789) was used. This gene has not been modified with any added epitope. The gene for mouse melanopsin (OPN4), which had been tagged with the last eight amino acids of bovine rhodopsin (1D4 tag), was used only in the experiments in Fig. 6.
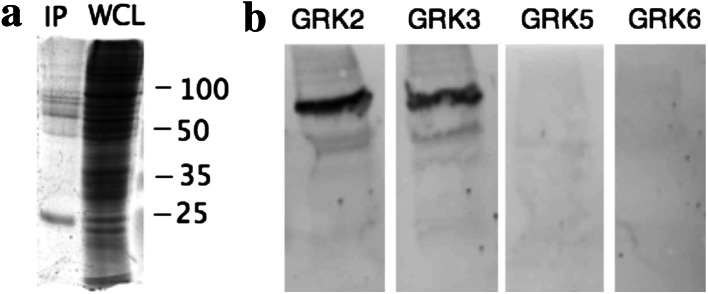
Fig. 6.
Crosslinking and co-immunoprecipitation of melanopsin. The reversible crosslinker DSP was used to crosslink melanopsin to interacting proteins. Melanopsin complexes were then immunoprecipitated using the 1D4 antibody bound to Sepharose beads. Beads were washed extensively and crosslinking was reversed by incubation with 50 mM DTT. a The SDS-PAGE analysis of the pulldown stained with Sypro Ruby: IP immunopulldown after reversal of crosslinking, WCL whole cell lysate. b Western-blot analysis of pulldown probed specifically with anti-GRK antibodies indicated
Immunoblotting
Two retinas from a single C57BL/6 mouse were dissected from eye cups and extracted by triturating in 200 μl of PBS, containing 1% dodecyl maltoside (RPI, Mount Prospect, IL), 1 mM phenylmethylsulfonylfluoride (PMSF; Sigma-Aldrich, St. Louis, MO), and 1 mM DTT (Fisher Scientific, Pittsburg, PA). An aliquot (10 μl) of the total retinal lysate was dissolved in Laemmli sample buffer (Bio-Rad, Hercules CA) and electrophoresed on a 10% SDS-polyacrylamide gel (120 V, 1 h). Gel-separated proteins were transferred to a PVDF membrane (Millipore, Billerica, MA) via tank transfer in Tris/glycine buffer (100 V, 1 h on ice). PVDF membranes were incubated in 0.5% I-block (Applied Biosystems, Foster City, CA) in 10 mM Tris–Cl (pH 7.4), containing 0.1% Tween-80. Blots were probed with antibodies specific for each member of the GRK family (GRK 1, 2, 3, 4, 5, and 6; Santa Cruz Biotech, Santa Cruz, CA) (1:500) and visualized by chemiluminescence using alkaline phosphatase-linked secondary antibody (AP-linked goat anti-rabbit IgG; Promega, Madison, WI) and Attophos (Promega, Madison, WI).
RT-PCR
RT-PCR was performed on total RNA from a single C57BL/6 mouse retina. RNA was purified using a MACHEREY-NAGEL kit (Bethlehem, PA). RT-PCR was performed with One Step RT-PCR kit (Qiagen, Valencia, Ca) as previously described [14] with gene-specific primers for all five GRKs present in the mouse genome (sequences are listed in Table 1 in Supplement).
RT-PCR was also performed on single melanopsin-expressing retinal ganglion cells. Briefly, individual melanopsin-expressing RGCs were obtained following dissociation of two mouse retinas with collagenase IV (Roche, Indianapolis, IN). Melanopsin-expressing cells were labeled using a custom-made anti-melanopsin N-terminal antibody (Covance, Denver, PA; Walker et al. [40]) and visualized with a secondary antibody conjugated to Alexa Fluor 488 (Invitrogen, Carlsbad, CA). Cells were diluted and allowed to settle onto nitrocellulose filters. Using a fluorescent microscope, single labeled cells were excised and placed immediately in 100 μl lysis buffer containing guanine thiocyanate from the Absolute RNA nanopure kit (Stratagene, La Jolla, CA). This suspension was subsequently used to isolate the RNA. RT-PCR was performed as described above.
Development of a stable mammalian cell line expressing melanopsin
The gene for mouse melanopsin (OPN4), which had been appended with the last eight amino acids of bovine rhodopsin (1D4 tag) and inserted into the expression vector pMT3 [14], was modified by PCR to contain flanking Kpn I and Not I sites. These restriction enzymes were used to clone the melanopsin-1D4 gene into a similarly digested pMCV-tetO vector (gift from P. Reeves) containing a tetracycline-regulated CMV promoter. This vector was transfected by calcium phosphate precipitation into a modified HEK-293 cell line, which constitutively expresses the tetracycline repressor protein. Geneticin selection was used to create a stable cell line [15]. Expression of melanopsin-1D4 was induced in ten plates with 2 μg/ml tetracycline and 5 mM sodium butyrate, as previously described for rhodopsin [15]. Cells were harvested 48 h after induction.
Construction of a mouse melanopsin mutant lacking all potential carboxy-terminal phosphorylation sites (phospho-null melanopsin)
Site-directed mutagenesis was used to create a unique Kpn I site by introducing a silent mutation at position 993(G-A) in the nucleic acid sequence of the wild-type mouse melanopsin cDNA. Flanking Kpn I and Not I sites were used to insert a synthetic DNA duplex (Integrated DNA Technology, Coralville, IA), which matched the coding sequence of mouse melanopsin (positions 990–1,566) except that all serine and threonine residues were converted to alanines.
In vitro phosphorylation assay
Membrane extracts from HEK-293 cells, which had been transiently transfected with the mouse melanopsin gene, were fractionated and isolated on a 30% sucrose gradient [16]. Melanopsin concentration was determined by quantitative dot blot. Phosphorylation was determined using an in vitro kinase assay protocol modified from Benovic et al. [17]. Membranes containing approximately 250 pmol of melanopsin or phospho-null melanopsin were reconstituted with 20 μM 11-cis-retinal (gift of R. Crouch) at 4°C for 1 h in 100 μl of buffer containing 20 mM Tris–Cl, 7.5 mM MgCl2, and 2 mM EDTA. HEK-293 cell membrane preparations were shown to contain endogenous GRK2, GRK5, and GRK6, so no exogenous kinase was added. Membrane samples were centrifuged at 14,000 rpm for 5 min, and the pellets were resuspended in 50 μl of kinase buffer containing 500 μM ATP, 1 mM GTP, 1 mM DTT, 1 mM PMSF, and 1–2 μCi of ATP-γ-32P (Perkin-Elmer, Waltham, MA). Melanopsin-containing membranes that were not reconstituted with 11-cis-retinal served as a negative control. Membranes were placed on ice and exposed to bright white light for 30 min. Duplicate samples were kept in the dark as a control, and membranes containing phospho-null melanopsin were assayed in parallel. Phosphorylation was measured by the incorporation of 32P as measured in triplicate using a filter-binding assay [14]. Filter-bound 32P was determined by scintillation counting in a LC6500 multi-scintillation counter (Beckman Coulter, Fullerton, CA) using Biodegradable Counting Scintillant (Amersham Biosciences, Piscataway, NJ).
In situ proximity ligation assay
For experiments with heterologously expressed melanopsin, HEK-293 cells were transiently transfected with DNA encoding wild-type melanopsin, seeded onto poly-lysine-coated coverslips 24 h post-transfection, and grown again overnight. Cells were incubated with 20 μM 11-cis-retinal for 1 h, and fixed in ice-cold 4% PFA in the dark, or after a 10-min incubation in the light. For experiments with endogenous melanopsin, mice were dark-adapted overnight and killed either under dim red light or after a 15-min incubation in room light. Retinas were fixed in ice-cold 4% PFA for 2 h, cryoprotected in 30% sucrose, embedded in OCT, and sectioned at 16 μm on a cryostat. Melanopsin-expressing cells or retinal sections were permeabilized in PBS containing 0.3% Triton-X, and blocked in PBS containing 0.3% Triton-X and 10% normal goat serum. Cells were probed with anti-melanopsin C-terminal antibody (1:100) (Thermo Scientific, Cat #PA1-781) and anti-phosphoserine (1:100) (Sigma-Aldrich, St. Louis, Mo, Cat# P5747) or anti-phosphotyrosine (1:100) (Sigma-Aldrich, St. Louis, Mo, Cat# P18669). Specificity of the anti-melanopsin C-terminal antibody has been previously shown [18], and was confirmed by Western blot on wild-type and melanopsin knockout mice (data not shown). The anti-phosphoserine and phosphotyrosine antibodies are purified mouse monoclonal antibodies that are specific for phosphoserine or phosphotyrosine as indicated by the manufacturer. Duolink (Olink Biosciences, Uppsala, Sweden) in situ proximity ligation assays (PLA) were performed according to the manufacturer’s protocol. The PLA assay is used to determine the proximity of two epitopes to one another using antibodies specific for each epitope. Antibodies against the carboxy tail of melanopsin and against phosphoserine or phosphotyrosine were used. Secondary antibodies conjugated with oligonucleotide linkers are then added. If the primary antibodies bind in close enough proximity (<40 nm), then the oligos are able to anneal together and form a piece of circular DNA. This circle can then be amplified by a polymerase using rolling circle amplification to produce a concatemer of single stranded copies of the DNA. Fluorescently labeled oligonucleotide probes are then added that are specific for the DNA that was amplified, resulting in a single fluorescent spot. This spot will appear only if the primary antibodies were bound in close enough proximity. This assay has been used to detect the in vivo phosphorylation of proteins [25, 26]. PLA probes were diluted in 0.1% Triton X-100/PBS/1% fetal calf serum and incubated in a pre-heated humidity chamber for 1 h at 37°C, followed by hybridization, ligation, amplification, and detection. Nuclei were visualized with a DAPI stain that was included in the Duolink kit. Fluorescence was visualized by confocal microscopy (TCS SP5 Leica Microsystems, Wetzlar, Germany).
G-protein activation assay
Membrane preparations as described above were also used to determine the effect of phosphorylation on the rate of G-protein activation using a procedure modified from Newman et al. [14]. In this experiment, the GTPγ35S was replaced with fluorescently tagged GTPγS (Bodipy FL (Ex 490) Invitrogen, Carlsbad, CA). When free in solution, the Bodipy fluorescence is quenched by the electron rich guanine ring and increases in fluorescence upon binding the G-protein [42]. To determine the effect of melanopsin phosphorylation on the rate of G-protein activation, melanopsin-containing membranes reconstituted with 11-cis-retinal were pre-incubated in the light for 5 min in the presence or absence of 1 mM ATP. Assays were performed in kinase buffer as described above. After pre-incubation in light, GTPγS-BodipyFL and purified bovine transducin (previously described in [19]) were added to a final concentrations of 2 μM and 1 μM respectively. Samples were rapidly mixed in a 96-well plate, and fluorescence was measured on a Tecan Infinite M200 microplate reader (Tecan Group Ltd., Männedorf, Switzerland) in triplicate. The rate of transducin activation was calculated from the slope of the average fluorescence increase per unit time using Microsoft Excel (Microsoft, Bellevue, WA). The authors are aware that transducin is not melanopsin’s cognate G-protein. The exact identity of melanopsin’s cognate G-protein has not been definitively identified, although it is most likely in the Gq family. We have used transducin in these assays as an indicator of melanopsin activation. This assay has been previously used for melanopsin, mGluR-6, and the α2 adrenergic receptor [14, 20, 21].
Kinetic measurements of melanopsin activity based on fluorescent Ca2+-imaging
HEK-293 cells were transfected with full-length, wild-type, or phospho-null C-tail mouse melanopsin cDNA in the pMT3 expression vector using Lipofectamine plus per the manufacturer’s instructions (Invitrogen, Carlsbad, CA). Cells were allowed to grow 24 h post transfection and then released from the plate with 0.25% trypsin (Invitrogen), counted, and re-plated for fluorescent Ca2+-imaging at a density of 8 × 104 cells per well in a 96-well plate with a clear bottom and black walls (BD, San Jose, CA). Twenty-four hours after re-plating, cells were washed two times with Hank’s balanced salt solution (HBSS) plus 20 mM HEPES, pH 7.4, and subsequently incubated in HBSS/HEPES, supplemented with 4 μM Fluo-4 (Molecular Probes, Eugene, OR), 0.02% pluronic acid (Invitrogen, Carlsbad, CA), and 20 μM 11-cis-retinal. The 11-cis-retinal was dissolved in ethanol at a concentration of 2 mM and is added at a dilution of 1/1,000 in the dark. After the addition of 11-cis-retinal, the HEK-293 cells were placed in a modified incubator that allows them to be kept in the dark. Since expressed melanopsin is localized to the plasma membrane of the HEK-293 cells the addition of the chromophore is able to reconstitute the visual pigment. Fluorescent measurements were performed on a Tecan Infinite M200 microplate reader (Tecan Group Ltd., Männedorf, Switzerland) (EX 485 nm, EM 520 nm), sampling at a rate of 1 Hz for 60 s. The intensity of the light flash is according to manufacturer specifications. The procedure was modified from Zhang et al. [22]. The background fluorescence was subtracted to account for variations in transfection efficiency and dye loading.
RNAi experiments
HEK-293 cells were transfected with siRNA directed against GRK2 and GRK3 using the N-TER nanoparticle siRNA transfection system (Sigma-Aldrich, St. Louis, MO). For each gene two Mission siRNAs (Sigma-Aldrich, St. Louis, MO) targeting different parts of the gene were used (GRK2: SasI Hs01 00039322 and SasI Hs02 00332179. GRK3: SasI Hs02 0039102 and SasI Hs01 0025367). The cell penetrating peptide N-TER was mixed with the two oligos for each gene per the manufacturer’s instructions. N-TER-siRNA nanoparticles were added to cells seeded into a 96-well plate already transfected with melanopsin as described above. Final concentration of each siRNA oligo in the well was 25 nM.
Crosslinking co-immunoprecipitation
The procedure was adapted from Hasabi et al. [23]. One plate of an induced stable cell line expressing melanopsin-1D4 was washed twice with 5 ml DMEM without serum, and then incubated in the dark at 37°C for 1 h in 5 ml of DMEM supplemented with 50 μM 11-cis-retinal. After reconstitution with 11-cis-retinal, 500 μl of 25 mM dithiobis (succinimidyl propionate) (DSP; Pierce, Rockford, IL) was added to each plate, which was immediately placed under bright white light and incubated with gentle rocking for 30 min at room temperature. After two washes with PBS, cells were harvested, flash frozen on dry ice, and stored at −80°C. After thawing, cells were lysed in 1% DM in PBS with 1 mM PMSF on a rotating wheel at 4°C for 1 h. The lysate was centrifuged at 14,000 rpm for 5 min, and the supernatant was removed and added to an anti-1D4 antibody column (Amersham Bioscience, Piscataway, NJ) and incubated overnight at 4°C. The following morning, beads were washed three times with lysis buffer and twice with 0.1% DM in PBS. Following the final wash, the beads were resuspended in PBS containing 1 mM PMSF and 50 mM DTT and incubated at 37°C for 1 h to reverse cross-linking. After centrifugation, the supernatant was removed for immunoblot analysis with antibodies specific for GRK2, GRK3, GRK5, and GRK6 (Santa Cruz Biotech, CA).
Immunohistochemistry
Whole eyes were removed from C57BL/6 mice and fixed in 4% PFA. The anterior segment and lens were removed, and the eye cups were cryoprotected with 30% sucrose. Tissue was then embedded in OCT and sectioned at 16 μm on a CTD Harris cryostat (International Equipment Company, Needham Heights, MA). Sections were stained with anti-GRK2 and anti-GRK3 (1:500, Santa Cruz Biotech), and probed with a FITC-conjugated anti-rabbit secondary antibody (1:500, Invitrogen). Sections were stained with an anti-melanopsin N-terminal antibody (1:1,000) and a FITC-conjugated anti-rabbit secondary antibody (1:500, Invitrogen). Retinas were double-labeled with antibodies targeting GRK2 and 3 (1:100, Santa Cruz Biotech); because these antibodies were also raised in rabbit, they were directly labeled with a Zenon 588 labeling kit (Invitrogen). Fluorescence was visualized by confocal microscopy (TCS SP5, Leica Microsystems, Wetzlar, Germany). Negative control experiments were performed to ensure the specificity of the reactions. Sections were incubated with just the FITC-conjugated anti-rabbit secondary antibody, and just the primary melanopsin antibody. In both cases, no signal was observed.
Results
Melanopsin is phosphorylated in a light-dependent manner in vivo
The first step in the stereotypical deactivation of a GPCR is the phosphorylation of the carboxy-terminal tail of the receptor by a member of the GRK family [4]. In order to determine if melanopsin is phosphorylated in a light-dependent manner in vivo, we examined phosphorylation in mouse retina using an in situ proximity ligation assay (Olink Biosciences, Uppsala, Sweden). In this assay the proximity of two epitopes is determined by the ability of complimentary oligonucleotides attached to secondary antibodies to anneal and form a circular piece of DNA. The circular template is then amplified and detected with fluorescently labeled nucleotide probes specific for the amplified DNA. Here we used an anti-melanopsin C-terminal antibody [18] and a general anti-phosphoserine antibody, such that anywhere in the cell where the anti-melanopsin and anti-phosphoserine antibodies bind in close proximity (<40 nm), a fluorescent spot would be generated [24]. This approach has previously been used to detect protein phosphorylation in situ [25, 26]. Using this approach, we detected significant interaction between the antibodies in light-exposed retinas, as indicated by the fluorescent puncta seen in the inner plexiform (Fig. 1a) and ganglion cell layers, but virtually no interaction in retinas maintained in the dark (Fig. 1b). This labeling is consistent with the known location of the cell bodies and dendritic processes of melanopsin-containing RGCs, and implies that melanopsin is phosphorylated in a light-dependent manner in the murine retina.
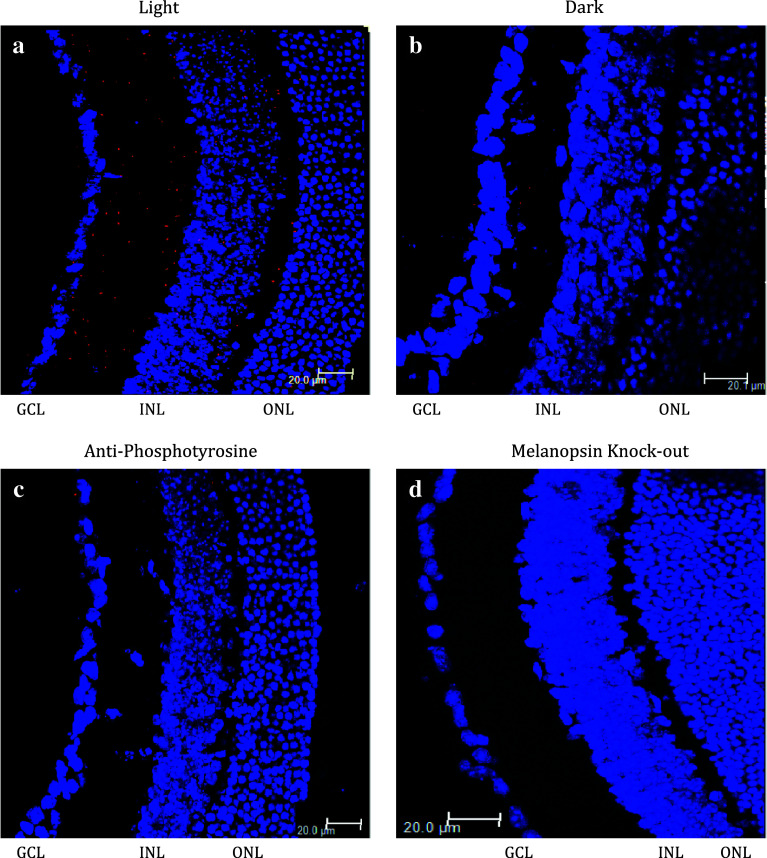
Fig. 1.
Light-dependent phosphorylation of melanopsin in vivo. Animals were dark-adapted overnight and killed either in the light (a, c, and d) or in the dark (b). Eyes were removed, fixed, and sectioned. Phosphorylation was probed using a PLA assay (Olink Biosciences) that is predicated on detecting the interaction between antibodies in close proximity (see “Methods” for details). The red dots indicate an interaction between the two antibodies and indicate melanopsin phosphorylation. Sections were probed with anti-melanopsin C-terminal antibody and anti-phosphoserine (a, b, and d) or anti-melanopsin C-terminal and anti-phosphotyrosine (c). The retinas in panels a, b, and c were from WT mice while the retina in d was from an opn4 knockout mouse. DAPI staining of cell nuclei is shown in blue
The specificity of the anti-mouse melanopsin carboxy-terminal antibody is demonstrated in supplemental Fig. 1. In this experiment, the genes for wild-type melanopsin, the phospho-null melanopsin mutant, and the c-tail deletion mutant Δ385 were transfected into HEK cells and probed with both the N and C terminal mouse melanopsin antibodies. Cells expressing all melanopsin constructs interacted with the N-terminal antibody, while the C-terminus antibody only binds to the wild-type and phospho-null proteins.
The specificity of this assay is indicated by the lack of signal observed when the above experiment was performed on light exposed retina from opn4 −/− mice, in which both copies of the melanopsin gene have been replaced with DNA encoding LacZ (Fig. 1d). This indicates that we are only observing the interaction of the anti melanopsin antibody with the phosphoserine antibody when melanopsin is expressed and light activated. In addition, no signal was observed in the light when an anti-phosphotyrosine was used in place of the phosphoserine antibody, thereby confirming the specificity of the phosphorylation reaction (Fig. 1c).
Melanopsin is phosphorylated in a light-dependent manner in vitro
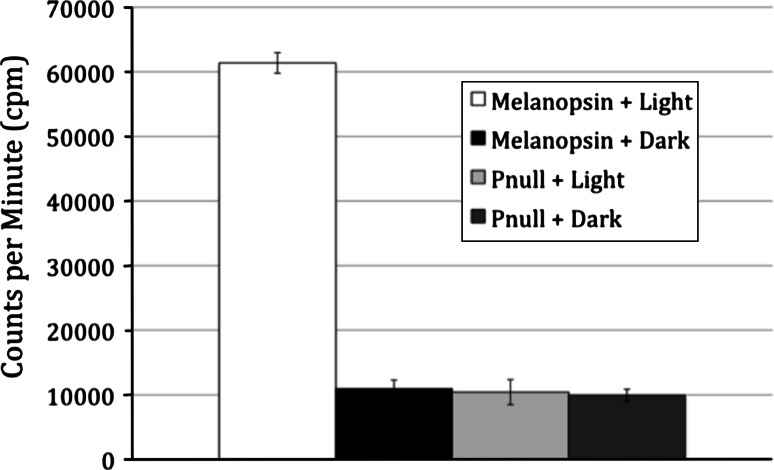
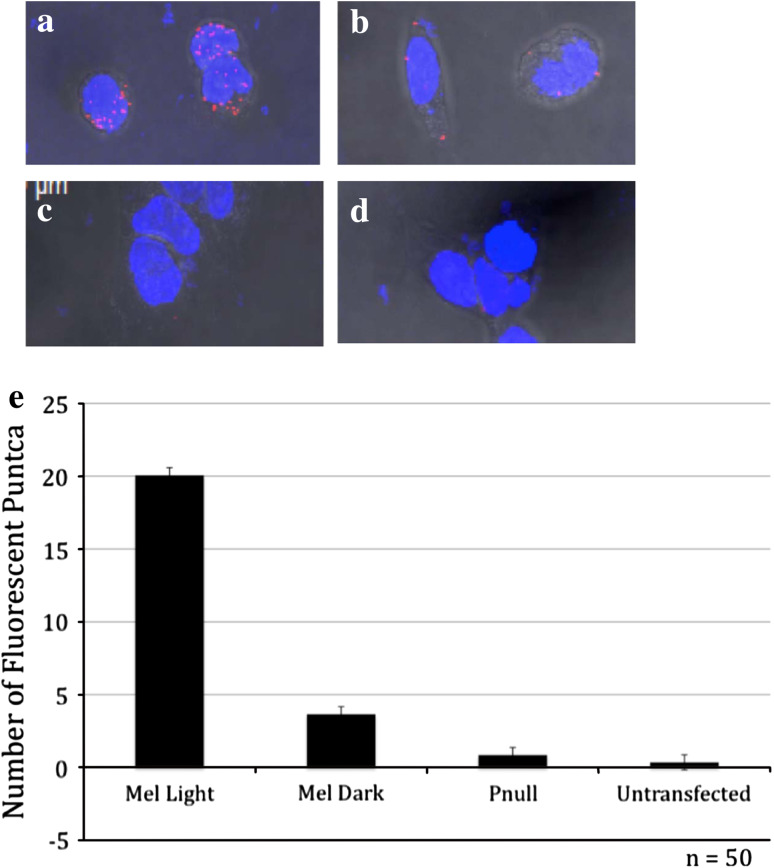
In order to more easily examine the effect of phosphorylation on melanopsin-based signaling, we developed an in vitro assay system. Except where noted, we used the long isoform of wild-type melanopsin (Accession # AF147789) in all studies. First, we demonstrated light-dependent phosphorylation of melanopsin in our heterologous expression system using an in vitro kinase assay. Phosphorylation of melanopsin was measured by quantitating the incorporation of radioactive phosphate from ATP-γ-32P in a filter-binding assay. We found that the degree of phosphorylation was dependent on both the presence of the chromophore (11-cis-retinal) and exposure to light. We observed minimal 32P incorporation when melanopsin-containing membranes were reconstituted with 11-cis-retinal and incubated in the dark. In contrast, after illumination, membranes containing reconstituted melanopsin had an approximately fivefold increase in radioactivity (Fig. 2a). In order to identify the carboxy tail as the hypothesized site of phosphorylation, we constructed a melanopsin mutant in which all 37 serines and threonines in the C-terminal domain were changed to alanines. When this phospho-null construct was tested in the kinase assay, the light-dependent increase in 32P incorporation was virtually eliminated. It should be noted that the increase in background level of 32P incorporation in the phospho-null samples is due to the higher amount of membrane added to the reaction to achieve comparable concentrations of the less abundant phospho-null melanopsin. When similar reactions were separated by SDS-PAGE and exposed to X-ray film, we observed that the majority of 32P was incorporated into a protein band with an apparent molecular weight of ~60 kDa, the appropriate M r for melanopsin; this radioactive band was absent in untransfected cells (Supplemental Fig. 1). Some phosphorylation of melanopsin in the un-reconstituted membranes may be due to incorporation of retinoids produced by the HEK-293 cells in which the protein was expressed [27]. We also demonstrated light-dependent phosphorylation of melanopsin in vitro using transfected cells and the in situ proximity ligation assay (PLA). There was an approximately seven-fold increase in the number of fluorescent spots in cells exposed to light compared to those maintained in the dark (Fig. 3), and cells transfected with the phospho-null construct or untransfected cells were virtually devoid of the PLA signal. These results demonstrate that, like other vertebrate opsins and GPCRs, melanopsin’s carboxy-terminal tail is phosphorylated in an activity-dependent manner.
Fig. 2.
Light-dependent phosphorylation of melanopsin in vitro. Membrane preparations containing ~250 pmol of mouse melanopsin were resuspended in kinase buffer after reconstitution with 50 μM 11-ci-retinal (opsin + chromophore). Samples were either exposed to white light for 30 min, or left in the dark. Incorporation of 32P as measured by a filter-binding assay and scintillation counting of WT melanopsin or phospho-null mutant melanopsin. Data represents the average of three measurements; error bars represent standard deviation
Fig. 3.
Light-dependent phosphorylation of transfected HEK Cells. Proximity ligation assay (PLA) showing phosphorylation of heterologously expressed melanopsin in the light, but not in the dark. PLA assays were performed on HEK-293 transiently transfected with wild-type melanopsin or phospho-null mutant melanopsin. Cells were split onto poly-lysine coated cover slips 1 day after transfection and moved to an incubator in the dark room. Forty-eight hours post-transfection cell were reconstituted with 11-cis-retinal for 1 h; some cells were then exposed to bright light illumination for 10 min. Cells were fixed on ice in ice cold 4% PFA for 30 min in the dark and probed with the PLA kit. Representative images were taken for each condition: wild-type melanopsin in the light (a), wild-type melanopsin in the dark (b), phospho-null melanopsin mutant in the light (c), and untransfected cells in the light (d). Histograms showing average numbers of fluorescent spots per cell (e). Error bars represent standard deviation. n = 100 cells/condition
Phosphorylation of melanopsin inhibits G-protein activation
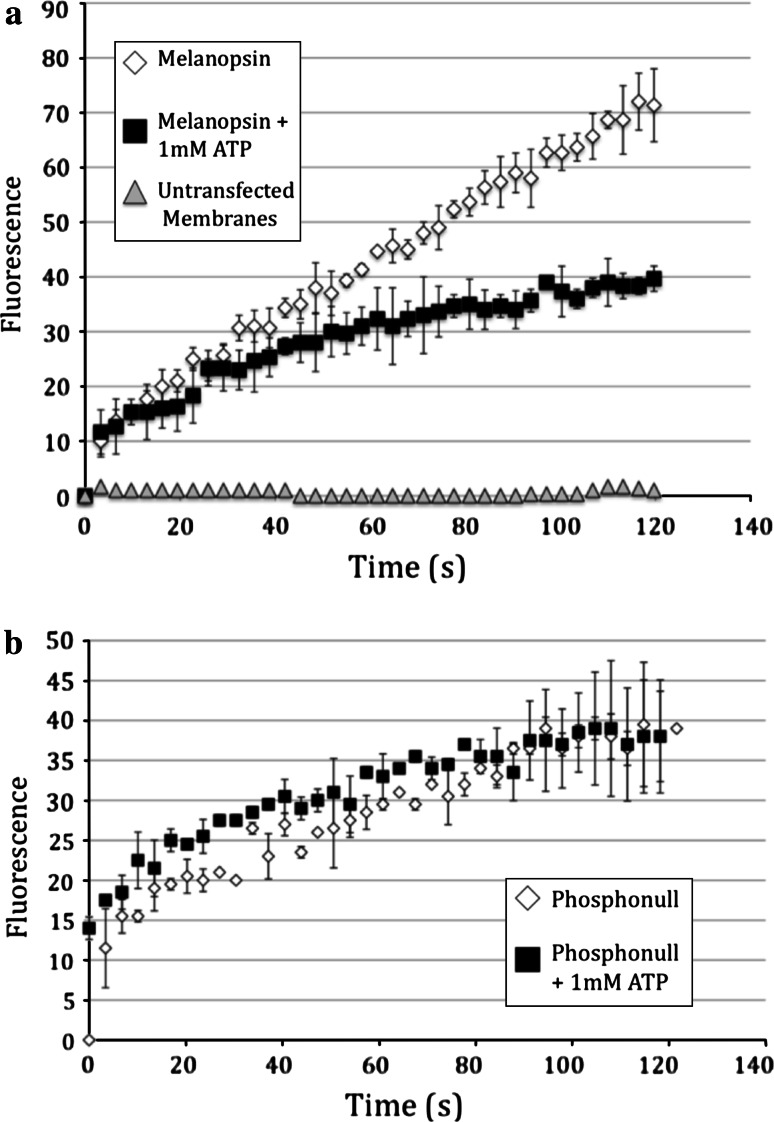
One of the measurable effects of phosphorylation on GPCRs is a reduction in the rate of G-protein activation [1]. We tested this prediction for melanopsin using purified bovine transducin as the G-protein with a fluorescent G-protein activation assay. While transducin is almost certainly not the endogenous G-protein in melanopsin-expressing RGCs [9, 28], melanopsin has been previously shown to activate bovine transducin in a light-dependent manner [14]. We first examined the suitability of a fluorescently labeled GTPγS to function in this assay as a replacement for the radiolabeled GTP. BODIPY FL GTPγS, with an excitation wavelength of 490 nm, was selected for its similarity to the λmax of melanopsin. In this assay the fluorescent dye is quenched in solution by the electron transfer from the electron rich guanine, and there is a fluorescence increase upon binding to the G-protein [27–29]. To investigate the effect of phosphorylation on G-protein activation, melanopsin-containing membranes were incubated under bright white light illumination in the presence or absence of ATP for 5 min. Incubation in the light in the presence of ATP cause a ~50% reduction in the rate of transducin activation (Fig. 4a). Importantly, there was no significant ATP-dependent reduction in the rate of G-protein activation by the phospho-null mutant (Fig. 4b).
Fig. 4.
Effect of phosphorylation on G-protein activation. Membrane preparations containing wild-type melanopsin (a) or the melanopsin phospho-null mutant (b) were mixed with bovine retinal transducin in the presence of BODIPY-GTPγS to monitor the rate of G-protein activation. Reactions were split in half, with one half receiving 1 mM ATP. All reactions were incubated in the light for 5 min prior to the addition of transducin and BODIPY-GTPγS
Melanopsin phosphorylation affects signaling kinetics
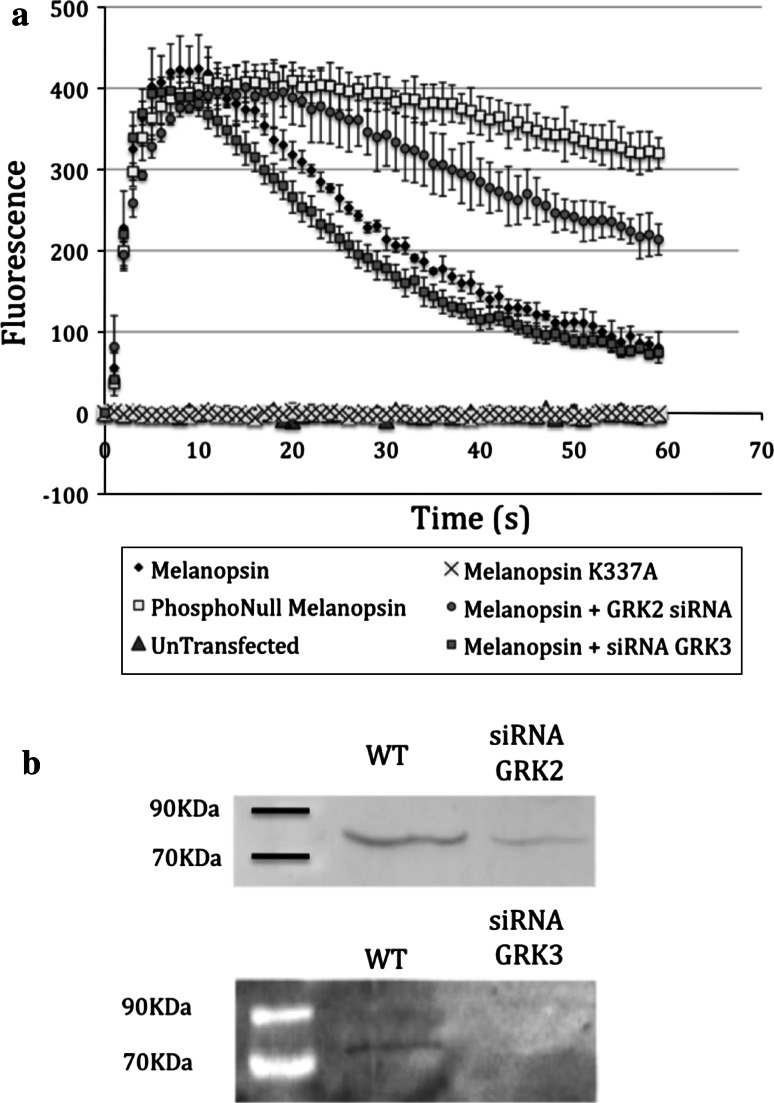
In order to test the physiological significance of melanopsin phosphorylation on signaling amplitude and kinetics, we used fluorescent Ca2+-imaging in a heterologous-expression system. As shown previously [30, 31], expression of melanopsin renders HEK-293 cells light sensitive, and light-dependent activation of melanopsin leads to an increase in intracellular calcium. Thus, intracellular calcium can be used as a surrogate to monitor the kinetics and amplitude of melanopsin-based signal transduction. The excitation maximum for both the Ca2+-sensitive dye Fluo-4 and melanopsin are within 10 nm of each other (490 and 480 nm, respectively) which allowed us to use a single wavelength for both activation of melanopsin and excitation of the Ca2+-indicator dye. We began by demonstrating efficacy of this system with HEK-293 cells transiently transfected with melanopsin. When cells were transfected with a plasmid containing wild-type melanopsin, the calcium level rose quickly and peaked within 12 s after the onset of light exposure. In the continued presence of light, the fluorescence peak was followed by a declining phase in which fluorescence returned to near baseline levels. Neither untransfected HEK cells nor cells expressing a non-functional melanopsin mutant (K337A), a mutant that is unable to bind chromophore [14], showed any increase in calcium. In contrast, when cells were transfected with the phospho-null C-tail melanopsin mutant, the decay phase was virtually abolished, and the fluorescence levels remained near the original peaks during the 60-s duration of the assay. Deactivation kinetics were also slowed by the addition of siRNA directed against GRK2, while siRNA against GRK3 had no effect on the rate of deactivation (Fig. 5a). GRK2 and 3 knockdown were confirmed by Western blot (Fig. 5b). Additionally, siRNA knockdown of GRKs 5 or 6 had no measurable effect on signaling kinetics (data not shown). The use of multiple related gene targets also serves as a scrambled gene control for the RNAi method.
Fig. 5.
Time course of the calcium response of HEK-293 cells. a HEK-293 cells were transiently transfected with DNA for wild-type melanopsin, phospho-null melanopsin mutant, or the K337A chromophore-binding melanopsin mutant as indicated. Some cells transfected with wild-type melanopsin were also treated with siRNA targeting GRK2 or GRK3. Cells were re-seeded into a 96-well plate at a density of 80,000 per well, and loaded with the calcium sensitive dye Flou-4. Melanopsin signaling was monitored by measuring intracellular calcium levels. b Western-blot analysis of the experiment described above. Whole cell lysates were probed with anti-GRK antibodies as indicated. The lane labeled WT refers to cells transfected with wild-type melanopsin, lanes labeled with siRNA indicate cells transfected with wild-type melanopsin and targeted with the indicated siRNA
Melanopsin interacts with GRK2 and 3 but not GRK 5 and 6
Given that there are multiple GRK family members present in HEK cells, it is important to determine which GRKs are actually interacting with melanopsin. In order to investigate the specificity of interaction of the available GRKs with melanopsin, we used a reversible crosslinker, dithiobis(succinimidyl) propionate (DSP; Pierce Chemical Co., Rockford, IL) in conjunction with co-immunoprecipitation. The anti-melanopsin antibody was not suitable for this experiment because both it and the anti-GRK antibodies were raised in rabbit. To avoid this complication, an inducible stable cell line was created expressing a mouse melanopsin with a 1D4 tag appended to the carboxy tail. The 1D4 tag consists of the last eight amino acids of bovine rhodopsin. Following immunoprecipitation with anti-1D4 antibody conjugated beads and reversal of the cross-linking under reducing conditions, the immunoprecipitated proteins were analyzed by SDS-PAGE. Staining for total protein with Sypro Ruby (Bio-Rad, Hercules, CA) revealed 12 distinct bands (Fig. 6), with a >eightfold increase of total protein cross-linked to melanopsin in the light compared to the dark (data not shown). When eluted proteins were analyzed on an immunoblot probing for the GRKs expressed in HEK cells, GRK2 and GRK3 were detected while GRK5 and GRK6 were not. These results clearly demonstrate that melanopsin has a higher affinity for the GRK2/3 subgroup of the GRK family than GRK5/6 subgroup.
GRKs in melanopsin-containing RGCs
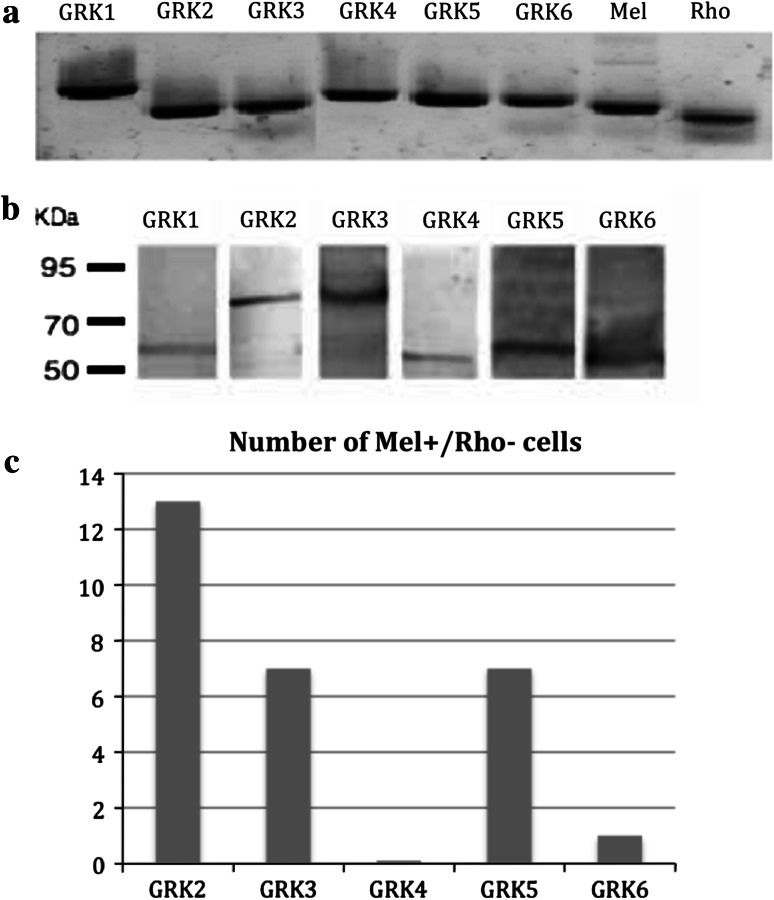
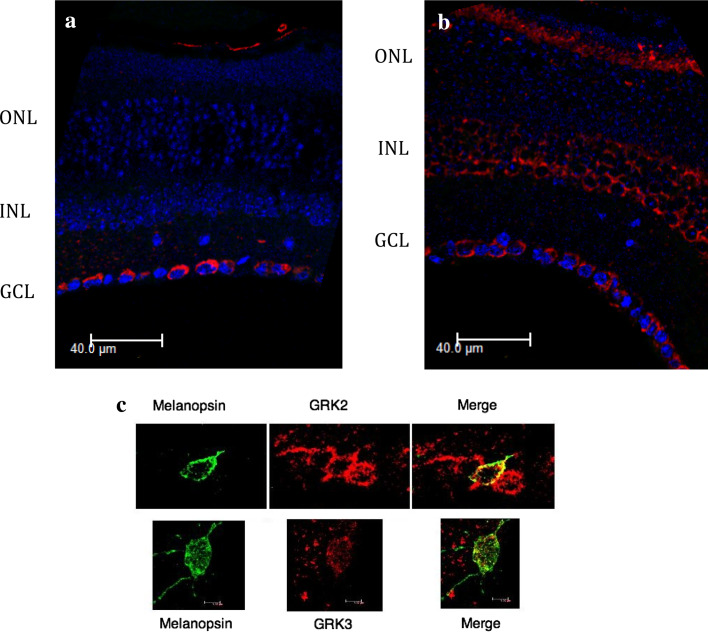
In order to investigate the in vivo physiological role of melanopsin phosphorylation by GRKs, we first identified candidate GRKs that are present in melanopsin-containing RGCs. As determined by both RT-PCR and immunoblotting, all GRKs are expressed in the retina (Fig. 7a). This is a somewhat surprising result given that the expression of all 6 GRKs in the murine retina has not been previously reported. To determine which GRKs are expressed in melanopsin-containing RGCs, single-cell RT-PCR was performed following retinal dissociation and labeling of single melanopsin-expressing RGCs using an anti-melanopsin N-terminal antibody. In order to be deemed valid, cells had to be both melanopsin positive and rhodopsin negative in the RT-PCR. GRK2 was present in all cells assayed, and GRK3 and GRK5 were present in approximately half of the cells (Fig. 7b). No other GRKs were detected consistently in the single cell analysis. Retinal sections were probed with antibodies against GRK2 and GRK3 to determine distribution of these widely distributed kinases in the retina. GRK2 was found to be expressed primarily in the entire ganglion cell layer, while GRK3 was also expressed throughout the ganglion cell layer and in the inner nuclear layer and the outer segments of the photoreceptive layer (Fig. 8a, b). Double immunolabeling of retinal sections also demonstrated co-localization of GRK2 and GRK3 with melanopsin (Fig. 8). These data provide three strong candidates for GRKs that may interact with melanopsin in vivo.
Fig. 7.
G-protein coupled receptor kinase (GRK) expression in the retina. All 6 GRKs expressed in mice are expressed in the retina, as determined by both RT-PCR (a) and immunoblot analysis (b). Single cell RT-PCR of immuno-labeled ipRGCs screening for GRK expression. n = 13. In order to be counted, cells were required to be both positive for melanopsin expression and negative for rhodopsin
Fig. 8.
Co-localization of melanopsin with GRK2 and 3. Retinal sections were stained with anti-GRK2 (1:500, Santa Cruz Biotech) (a) or anti-GRK3 (1:500, Santa Cruz Biotech) (b). Mouse retinal sections were stained with anti-melanopsin (1:1,000) and FITC-conjugated anti rabbit antibody (Invitrogen). GRK2 and 3 were detected using specific antibodies (1:100, Santa Cruz Biotech), directly labeled with a Zenon 588 labeling kit (Invitrogen) (c)
Discussion
Here we provide the first evidence that the non-visual photopigment melanopsin is phosphorylated in a light-dependent manner in vivo, and that phosphorylation plays an important role in receptor deactivation in vitro. Phosphorylation of the activated receptor provides a molecular mechanism for deactivation that is consistent with other vertebrate opsins, and GPCRs in general. The general model of vertebrate GPCR deactivation is a two-step model. The first step is activity-dependent phosphorylation, which both decreases signaling efficiency and creates a binding signal for arrestin. The subsequent binding of arrestin serves to completely quench the response and in many cases leads to internalization of the receptor [2–6]. Deactivation of invertebrate opsins, as established in Drosophila melanogaster, differs in that phosphorylation is not required for binding of arrestin [32], though it plays a similar role in turning down the gain of the response [33]. Thus, the ERG from mutant flies expressing a C-terminally truncated form of rhodopsin show no defect in signaling or changes in deactivation kinetics [32]. Despite the similarities between melanopsin and invertebrate opsins in sequence and signal transduction [9, 34], melanopsin appears to be deactivated in a manner similar to that of other vertebrate opsins. As shown here, mutation of the carboxy-terminal tail phosphorylation sites of melanopsin led to an absence of the deactivation in a heterologous system implying that phosphorylation of the carboxy tail plays an important role. This finding should not be surprising in that the signal termination machinery in vertebrates would require melanopsin to conform to a vertebrate-type deactivation mechanism. The ability of phosphorylation to adjust the gain of a light-dependent response could be particularly important in melanopsin-expressing RGCs, as the melanopsin is thought to be under constant illumination. Reducing G-protein activation through phosphorylation may provide a mechanism to protect the cell from excitotoxicity while still maintaining the possibility of long-term signaling throughout the brightly lit day. It remains to be determined if light-dependent phosphorylation plays a role in the deactivation of the melanopsin-based signal in vivo, or the previously-reported light adaptation of the melanopsin-based light response [35].
In order to identify a candidate GRK responsible for melanopsin phosphorylation in vivo, RT-PCR was performed on single isolated ipRGCs. This analysis indicated that GRK2, 3, and 5 are expressed in a high percentage of melanopsin-containing RGCs and are viable candidates for the endogenous kinase. The co-IP data demonstrates a strong interaction between melanopsin and GRK2 and 3, and suggests that they are better candidates for the endogenous melanopsin kinase than GRK5, which demonstrated no detectable interaction with melanopsin. Given that robust phosphorylation is seen in the in vitro kinase assay in the absence of GRK3, GRK2 emerges as the strongest candidate for the endogenous kinase. Furthermore, immunolabeling demonstrated that GRK2 is expressed in all RGCs, including melanopsin-expressing RGCs. Recent reports have indicated, however, that there are cell-type specific differences in phosphorylation of GPCRs [36], and one must, therefore, be careful extrapolating the data obtained in a heterologous expression system to the endogenous cell. Unfortunately, it is very difficult to do these types of analyses on endogenous melanopsin-expressing RGCs due to their scarcity. Complicating matters further, there is increasing evidence of heterogeneity within the ipRGC population, with as many as five types of melanopsin-expressing cells identified [18, 37–39]. Some of the physiological differences among these cell types includes the rate of response deactivation, which may be caused by differences in the expression patterns and levels of GRKs. It is likely that our method of selecting single cells is biased toward the M1 melanopsin cell type, which is the most abundant and stains most effectively with the melanopsin N-terminal antibody used in single cell selection [18, 37]. While methods have been developed to concentrate and enrich cellular populations for melanopsin-expressing cells [40, 41], it will remain challenging to tease these biochemical answers from this highly non-uniform system.
Further understanding of how melanopsin deactivation is regulated will be helpful in developing melanopsin gene therapy as a treatment for blindness caused by retinal degeneration. When expressed ectopically in large numbers of ganglion cells, melanopsin has been shown to cause light-dependent depolarization and restore some visual function in mice [13]. One of the major drawbacks of using melanopsin for gene therapy is the extended deactivation kinetics, which limit the temporal resolution of melanopsin-based vision. In the future, it may be possible to accelerate melanopsin deactivation by co-expressing the appropriate GRKs in the retinal ganglion cells.
In conclusion, we have shown here that melanopsin is phosphorylated in a light-dependent manner in vivo and in vitro and that this phosphorylation inhibits G-protein activation. Furthermore, light-dependent phosphorylation is important for rapid termination of the melanopsin-based calcium response in transfected HEK-293 cells. We have also demonstrated a physical interaction between melanopsin and both GRK2 and GRK3, but not GRK5 or GRK6, and show that GRK2, GRK3, and GRK5 are expressed in the melanopsin-containing RGCs of the mouse retina.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
This study was supported by Grants from the National Science Foundation to P.R.R (IOS0721608), and from the National Eye Institute to P. R. R. (R01EY019053). J. B. was supported by an NIH training grant (T32 GM066706). We would like to thank R. Crouch for the gift of 11-cis-retinal and D. Ujla for constructing the phospho-null melanopsin mutant.
Abbreviations
- GPCR
G-protein coupled receptor
- GRK
G-protein coupled receptor kinase
- PLA
Proximity-dependent ligation assay
- ipRGC
Intrinsically photosensitive retinal ganglion cell
References
- 1.Miller JL, Fox DA, Litman BJ. Amplification of phosphodiesterase activation is greatly reduced by rhodopsin phosphorylation. Biochemistry. 1986;25:4983–4988. doi: 10.1021/bi00366a002. [DOI] [PubMed] [Google Scholar]
- 2.Dromey JR, Pfleger KD. G-protein coupled receptors as drug targets: the role of beta-arrestins. Endocr Metab Immune Disord Drug Targets. 2008;8:51–61. doi: 10.2174/187153008783928352. [DOI] [PubMed] [Google Scholar]
- 3.Kelly E, Bailey CP, Henderson G. Agonist-selective mechanisms of GPCR desensitization. Br J Pharmacol. 2008;153(Suppl 1):S379–S388. doi: 10.1038/sj.bjp.0707604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ribas C, Penela P, Murga C, Salcedo A, Garcia-Hoz C, Jurado-Pueyo M, Aymerich I, Mayor F., Jr The G-protein-coupled receptor kinase (GRK) interactome: role of GRKs in GPCR regulation and signaling. Biochim Biophys Acta. 2007;1768:913–922. doi: 10.1016/j.bbamem.2006.09.019. [DOI] [PubMed] [Google Scholar]
- 5.Penela P, Murga C, Ribas C, Tutor AS, Peregrin S, Mayor F., Jr Mechanisms of regulation of G-protein-coupled receptor kinases (GRKs) and cardiovascular disease. Cardiovasc Res. 2006;69:46–56. doi: 10.1016/j.cardiores.2005.09.011. [DOI] [PubMed] [Google Scholar]
- 6.Pitcher JA, Freedman NJ, Lefkowitz RJ. G-protein-coupled receptor kinases. Annu Rev Biochem. 1998;67:653–692. doi: 10.1146/annurev.biochem.67.1.653. [DOI] [PubMed] [Google Scholar]
- 7.Lupi D, Oster H, Thompson S, Foster RG. The acute light-induction of sleep is mediated by OPN4-based photoreception. Nat Neurosci. 2008;11:1068–1073. doi: 10.1038/nn.2179. [DOI] [PubMed] [Google Scholar]
- 8.Altimus CM, Guler AD, Villa KL, McNeill DS, Legates TA, Hattar S. Rods-cones and melanopsin detect light and dark to modulate sleep independent of image formation. Proc Natl Acad Sci USA. 2008;105:19998–20003. doi: 10.1073/pnas.0808312105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Graham DM, Wong KY, Shapiro P, Frederick C, Pattabiraman K, Berson DM. Melanopsin ganglion cells use a membrane-associated rhabdomeric phototransduction cascade. J Neurophysiol. 2008;99:2522–2532. doi: 10.1152/jn.01066.2007. [DOI] [PubMed] [Google Scholar]
- 10.Warren EJ, Allen CN, Brown RL, Robinson DW. The light-activated signaling pathway in SCN-projecting rat retinal ganglion cells. Eur J Neurosci. 2006;23:2477–2487. doi: 10.1111/j.1460-9568.2006.04777.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schmidt TM, Taniguchi K, Kofuji P. Intrinsic and extrinsic light responses in melanopsin-expressing ganglion cells during mouse development. J Neurophysiol. 2008;100:371–384. doi: 10.1152/jn.00062.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Berson DM, Dunn FA, Takao M. Phototransduction by retinal ganglion cells that set the circadian clock. Science. 2002;295:1070–1073. doi: 10.1126/science.1067262. [DOI] [PubMed] [Google Scholar]
- 13.Lin B, Koizumi A, Tanaka N, Panda S, Masland RH. Restoration of visual function in retinal degeneration mice by ectopic expression of melanopsin. Proc Natl Acad Sci USA. 2008;105:16009–16014. doi: 10.1073/pnas.0806114105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Newman LA, Walker MT, Brown RL, Cronin TW, Robinson PR. Melanopsin forms a functional short-wavelength photopigment. Biochemistry. 2003;42:12734–12738. doi: 10.1021/bi035418z. [DOI] [PubMed] [Google Scholar]
- 15.Reeves PJ, Kim JM, Khorana HG. Structure and function in rhodopsin: a tetracycline-inducible system in stable mammalian cell lines for high-level expression of opsin mutants. Proc Natl Acad Sci USA. 2002;99:13413–13418. doi: 10.1073/pnas.212519199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weiss ER, Osawa S, Shi W, Dickerson CD. Effects of carboxyl-terminal truncation on the stability and G-protein-coupling activity of bovine rhodopsin. Biochemistry. 1994;33:7587–7593. doi: 10.1021/bi00190a011. [DOI] [PubMed] [Google Scholar]
- 17.Benovic JL, Regan JW, Matsui H, Mayor F, Jr, Cotecchia S, Leeb-Lundberg LM, Caron MG, Lefkowitz RJ. Agonist-dependent phosphorylation of the alpha 2-adrenergic receptor by the beta-adrenergic receptor kinase. J Biol Chem. 1987;262:17251–17253. [PubMed] [Google Scholar]
- 18.Baver SB, Pickard GE, Sollars PJ. Two types of melanopsin retinal ganglion cell differentially innervate the hypothalamic suprachiasmatic nucleus and the olivary pretectal nucleus. Eur J Neurosci. 2008;27:1763–1770. doi: 10.1111/j.1460-9568.2008.06149.x. [DOI] [PubMed] [Google Scholar]
- 19.Robinson PR, Cohen GB, Zhukovsky EA, Oprian DD. Constitutively active mutants of rhodopsin. Neuron. 1992;9:719–725. doi: 10.1016/0896-6273(92)90034-B. [DOI] [PubMed] [Google Scholar]
- 20.Weng K, Lu C, Daggett LP, Kuhn R, Flor PJ, Johnson EC, Robinson PR. Functional coupling of a human retinal metabotropic glutamate receptor (hmGluR6) to bovine rod transducin and rat Go in an in vitro reconstitution system. J Biol Chem. 1997;272:33100–33104. doi: 10.1074/jbc.272.52.33100. [DOI] [PubMed] [Google Scholar]
- 21.Cerione RA, Regan JW, Nakata H, Codina J, Benovic JL, Gierschik P, Somers RL, Spiegel AM, Birnbaumer L, Lefkowitz RJ, et al. Functional reconstitution of the alpha 2-adrenergic receptor with guanine nucleotide regulatory proteins in phospholipid vesicles. J Biol Chem. 1986;261:3901–3909. [PubMed] [Google Scholar]
- 22.Zhang JY, Nawoschik S, Kowal D, Smith D, Spangler T, Ochalski R, Schechter L, Dunlop J. Characterization of the 5-HT6 receptor coupled to Ca2+ signaling using an enabling chimeric G-protein. Eur J Pharmacol. 2003;472:33–38. doi: 10.1016/S0014-2999(03)01855-7. [DOI] [PubMed] [Google Scholar]
- 23.Hasbi A, Devost D, Laporte SA, Zingg HH. Real-time detection of interactions between the human oxytocin receptor and G-protein-coupled receptor kinase-2. Mol Endocrinol. 2004;18:1277–1286. doi: 10.1210/me.2003-0440. [DOI] [PubMed] [Google Scholar]
- 24.Soderberg O, Gullberg M, Jarvius M, Ridderstrale K, Leuchowius KJ, Jarvius J, Wester K, Hydbring P, Bahram F, Larsson LG, Landegren U. Direct observation of individual endogenous protein complexes in situ by proximity ligation. Nat Methods. 2006;3:995–1000. doi: 10.1038/nmeth947. [DOI] [PubMed] [Google Scholar]
- 25.Jarvius M, Paulsson J, Weibrecht I, Leuchowius KJ, Andersson AC, Wahlby C, Gullberg M, Botling J, Sjoblom T, Markova B, Ostman A, Landegren U, Soderberg O. In situ detection of phosphorylated platelet-derived growth factor receptor beta using a generalized proximity ligation method. Mol Cell Proteomics. 2007;6:1500–1509. doi: 10.1074/mcp.M700166-MCP200. [DOI] [PubMed] [Google Scholar]
- 26.Leuchowius KJ, Jarvius M, Wickstrom M, Rickardson L, Landegren U, Larsson R, Soderberg O, Fryknas M, Jarvius J. High content screening for inhibitors of protein interactions and post-translational modifications in primary cells by proximity ligation. Mol Cell Proteomics. 2010;9:178–183. doi: 10.1074/mcp.M900331-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen Y, Moiseyev G, Wu BX, Ma JX, Crouch RK. Visual cycle retinoid processing proteins are present in HEK293S cells. Vis Res. 2003;43:L3037–L3044. doi: 10.1016/j.visres.2003.08.001. [DOI] [PubMed] [Google Scholar]
- 28.Hattar S, Lucas RJ, Mrosovsky N, Thompson S, Douglas RH, Hankins MW, Lem J, Biel M, Hofmann F, Foster RG, Yau KW. Melanopsin and rod-cone photoreceptive systems account for all major accessory visual functions in mice. Nature. 2003;424:76–81. doi: 10.1038/nature01761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kimple RJ, De Vries L, Tronchere H, Behe CI, Morris RA, Gist Farquhar M, Siderovski DP. RGS12 and RGS14 GoLoco motifs are G alpha(i) interaction sites with guanine nucleotide dissociation inhibitor activity. J Biol Chem. 2001;276:29275–29281. doi: 10.1074/jbc.M103208200. [DOI] [PubMed] [Google Scholar]
- 30.Qiu X, Kumbalasiri T, Carlson SM, Wong KY, Krishna V, Provencio I, Berson DM. Induction of photosensitivity by heterologous expression of melanopsin. Nature. 2005;433:745–749. doi: 10.1038/nature03345. [DOI] [PubMed] [Google Scholar]
- 31.Giesbers ME, Shirzad-Wasei N, Bosman GJ, de Grip WJ. Functional expression, targeting and Ca2+ signaling of a mouse melanopsin-eYFP fusion protein in a retinal pigment epithelium cell line. Photochem Photobiol. 2008;84:990–995. doi: 10.1111/j.1751-1097.2008.00347.x. [DOI] [PubMed] [Google Scholar]
- 32.Vinos J, Jalink K, Hardy RW, Britt SG, Zuker CS. A G-protein-coupled receptor phosphatase required for rhodopsin function. Science. 1997;277:687–690. doi: 10.1126/science.277.5326.687. [DOI] [PubMed] [Google Scholar]
- 33.Lee SJ, Xu H, Montell C. Rhodopsin kinase activity modulates the amplitude of the visual response in Drosophila . Proc Natl Acad Sci USA. 2004;101:11874–11879. doi: 10.1073/pnas.0402205101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Panda S, Nayak SK, Campo B, Walker JR, Hogenesch JB, Jegla T. Illumination of the melanopsin signaling pathway. Science. 2005;307:600–604. doi: 10.1126/science.1105121. [DOI] [PubMed] [Google Scholar]
- 35.Wong KY, Dunn FA, Berson DM. Photoreceptor adaptation in intrinsically photosensitive retinal ganglion cells. Neuron. 2005;48:1001–1010. doi: 10.1016/j.neuron.2005.11.016. [DOI] [PubMed] [Google Scholar]
- 36.Tobin AB, Butcher AJ, Kong KC. Location, location, location…site-specific GPCR phosphorylation offers a mechanism for cell-type-specific signalling. Trends Pharmacol Sci. 2008;29:413–420. doi: 10.1016/j.tips.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schmidt TM, Kofuji P. Functional and morphological differences among intrinsically photosensitive retinal ganglion cells. J Neurosci. 2009;29:476–482. doi: 10.1523/JNEUROSCI.4117-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tu DC, Zhang D, Demas J, Slutsky EB, Provencio I, Holy TE, Van Gelder RN. Physiologic diversity and development of intrinsically photosensitive retinal ganglion cells. Neuron. 2005;48:987–999. doi: 10.1016/j.neuron.2005.09.031. [DOI] [PubMed] [Google Scholar]
- 39.Ecker JL, Dumitrescu ON, Wong KY, Alam NM, Chen SK, LeGates T, Renna JM, Prusky GT, Berson DM, Hattar S. Melanopsin-expressing retinal ganglion-cell photoreceptors: cellular diversity and role in pattern vision. Neuron. 2010;67:49–60. doi: 10.1016/j.neuron.2010.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Walker MT, Brown RL, Cronin TW, Robinson PR. Photochemistry of retinal chromophore in mouse melanopsin. Proc Natl Acad Sci USA. 2008;105:8861–8865. doi: 10.1073/pnas.0711397105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hartwick AT, Bramley JR, Yu J, Stevens KT, Allen CN, Baldridge WH, Sollars PJ, Pickard GE. Light-evoked calcium responses of isolated melanopsin-expressing retinal ganglion cells. J Neurosci. 2007;27:13468–13480. doi: 10.1523/JNEUROSCI.3626-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McEwen DP, Gee KR, Kang HC, Neubig RR. Fluorescent BODIPY-GTP analogs: real-time measurement of nucleotide binding to G-proteins. Anal Biochem. 2001;291:109–117. doi: 10.1006/abio.2001.5011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.