Abstract
Gastric infection with Helicobacter pylori is one of the most common chronic infections in humans, causing substantial morbidity and mortality. The diagnosis of H. pylori infection usually involves upper endoscopy with biopsy since the only noninvasive method of comparable accuracy, the [13C]urea breath test, requires technical equipment that is not available in most gastroenterological units. Serological methods for detection of H. pylori infection have reached sufficient accuracy to be used as screening tests before endoscopy or for seroepidemiological surveys. In the present study we evaluated different interpretation criteria for use with immunoglobulin G immunoblotting for the diagnosis of H. pylori infection. We applied five different sets of interpretation criteria, four of which had been published previously, to the Western blot results of 294 patients with different gastrointestinal symptoms. Since it is known that less than 2% of patients who are infected with H. pylori fail to seroconvert, an optimally sensitive Western blotting system should be able to detect approximately 98% of active infections. When the different criteria were applied to our patient population, it became apparent that the abilities of the systems to detect active H. pylori infection were quite varied. The results for the sensitivity and specificity, according to the different applied criteria, ranged from 62.8 to 95.9% and from 85.7 to 100.0%, respectively. Positive predictive values and negative predictive values, according to the published criteria, ranged from 97.2 to 100.0% and from 37.7 to 82.4%, respectively. Recommendations for the optimal use of the different interpretation criteria are discussed.
Gastric infection with Helicobacter pylori is one of the most common chronic infections in humans, causing substantial morbidity and mortality (2). The diagnosis of H. pylori infection usually involves upper endoscopy with biopsy since the only noninvasive method of comparable accuracy, the [13C]urea breath test, requires technical equipment that is not available in most gastroenterological departments (15). However, in recent years, serological methods for detection of H. pylori infection have reached sufficient accuracy to be used as screening tests before endoscopy or for seroepidemiological surveys (17, 26, 31-33, 36, 37).
Most of the commercially available enzyme-linked immunosorbent assay (ELISA) systems are based on the detection of immunoglobulin G (IgG) antibodies against whole-cell preparations of H. pylori (14, 16, 17, 23, 24). Such preparations include antigens cross-reacting with other bacterial species, resulting in a specificity of these tests that rarely exceeds 90% (36, 37, 38). Even with a second-generation ELISA system employing highly purified, presumably non-cross-reactive components of the outer membrane of H. pylori as antigens, we found a sensitivity of 97.2% but a specificity of no more than 87.5% in a population of 71 H. pylori-positive and 56 H. pylori-negative patients (37). However, if the decision to treat a patient for H. pylori infection is based solely on the result of serological tests as has been suggested recently (31, 35), there is a definite need for tests offering a higher specificity. Western blotting may be a useful tool in this respect because it allows the direct visualization of antibody binding to antigens highly specific for H. pylori (40). Furthermore, it is well known that the H. pylori phenotype possessing the virulence island of genes called the cytotoxin-associated gene complex (CagA) is associated with severe gastric pathology, such as ulcer disease and cancer (4). Western blotting may help to recognize infections caused by CagA-expressing H. pylori strains because the cytotoxin-associated protein is highly immunogenic and usually stimulates an IgG immune response against the corresponding antibody of 118 to 136 kDa (40).
In spite of these potential advantages and the widespread availability of blotting equipment in clinical laboratories, Western blotting for H. pylori antibodies has not become general practice. This is due mainly to discrepant recommendations for blot interpretation which have been published by various authors and that have resulted in considerable confusion regarding interpretation criteria. Comparative studies in which these different interpretation criteria have been compared using identical patient populations are not available. In the present study, we therefore used a collection of sera from patients known to harbor or not to harbor H. pylori in order to compare the levels of accuracy of the immunoblot diagnoses depending on different previously published and our own criteria.
MATERIALS AND METHODS
Patients.
Sera were collected from all patients attending a gastroenterological department (Virchow-Klinikum, Humboldt University, Berlin, Germany) for performance of upper endoscopy over a period of 6 months. In total, 334 sera were collected. Both outpatients and in-hospital patients were examined. Reasons for referral were either gastric symptoms and signs (for the majority of patients) with the presence of esophageal diseases or the wish to exclude gastric diseases before abdominal surgery of other organs. Informed consent was obtained from each patient, and 10 ml of blood was collected from the intravenous cannula inserted for preendoscopic sedation. After clotting, sera were obtained by centrifugation and stored frozen at −80°C in several aliquots. During endoscopy, three biopsies were taken from the antral region and examined for the presence of H. pylori by culture, histology, and urease testing as described below. A patient was judged to be H. pylori positive when at least one of the three tests yielded a positive result. In addition to the sera of patients studied prospectively, nine sera from patients with gastric carcinoma were obtained from the Helicobacter serum collection of the Department of Internal Medicine I, University of Ulm, Ulm, Germany. Clinical and endoscopic diagnoses were documented for all patients.
Western blotting.
Sera were thawed on one occasion and examined by means of a commercially available test kit (Marblot; Viramed Co., Planegg-Steinkirchen, Germany). The antigen used in this test is a partially purified extract of H. pylori strain ATCC 43504. This strain is known to express the VacA and CagA proteins (30; L. Furtmayer, personal communication). Sera were tested at a dilution of 1:100 (20 μl of serum diluted in 2 ml of test buffer), and bound IgG was traced with alkaline phosphatase-conjugated anti-human IgG antibody and phosphatase substrate according to the protocol of the manufacturer. The developing time was 25 min. Each blot has a negative control (human) and a positive control (human IgG), as well as a readily developed positive blot strip. After drying, all blot strips were densitometrically scanned with a Hewlett-Packard Scan Jet II CX scanner and analyzed with a computer program for analysis of one-dimensional separations (Image Master 1D, version 1.10; Pharmacia Biotech AB).
Since it was found that every band recognized by the scanner was also clearly visible by the naked eye, visible bands of any optical density (OD) were included in the analysis.
The criteria used for interpretation were those published by von Wulffen et al. (39), Faulde et al. (13), Nilsson et al. (30), and Trautmann et al. (37) and our presently proposed criteria. In detail, these criteria were as follows.
(i) Criteria of von Wulffen et al.
In the work of von Wulffen et al. (39), only the 110-, 94-, 63-, and 28-kDa bands are considered. Based on the figures shown in that paper, we believe that these bands correspond to the 136-, 100-, 66-, and 24-kDa bands of our system. von Wulffen et al. proposed a semiquantitative grading system in which one point was assigned to each reactive band and half a point was assigned to a weakly reactive band. This resulted in a maximum score of 4 for each tested serum. IgG blots with a score of ≥2 were judged positive.
(ii) Criteria of Faulde et al.
According to the criteria of Faulde et al. (13), sera of patients with any combination of the 120-, 90-, 75-, 67-, 29.5-, and 19-kDa bands are judged positive. Based on the interpretation of figures from that paper, we assume that these bands correspond to the 136-, 87-, 74-, 66-, 30-, and 19.5-kDa bands of our system, respectively.
(iii) Criteria of Nilsson et al.
According to the work of Nilsson et al. (30), sera that stain with at least one high-molecular-mass protein (110 to 120, 94, or 87 kDa) or at least two of five of the low-molecular-mass proteins (33, 31, 30, 29, or 26 kDa), or combinations of both, are judged positive. Based on the figures shown in that paper, we assume that the high-molecular-mass bands indicated correspond to the 136-, 100-, and 87-kDa bands of our system. The precise identification of the low-molecular-mass bands from that paper was difficult since the blot photographs presented show some variation in the molecular sizes of the fast-migrating bands. Therefore, we applied these criteria to our system by assuming that any combination of two or more bands of ≤33 kDa can be judged positive.
(iv) Criteria of Trautmann et al.
By the method of Trautmann et al. (37), staining of at least two bands, with at least one band being ≥66 kDa, indicates a positive sample.
(v) Our own current criteria.
By the method discussed herein, sera in which one band of 136, 87, or 66 kDa by itself or in combination with other bands, or at least two bands of ≤33 kDa, stain with any intensity are judged positive.
Four bands (59, 55, 50, and 45 kDa) are thought to be specific and four bands (33, 30, 26, and 24 kDa) are highly specific, apart from CagA, VacA, and UreB, for H. pylori. Recently, a quantitative cutoff for the evaluation of these eight specific or highly specific bands was introduced by the manufacturer. This cutoff was established by adding to each test kit a cutoff control serum yielding a 59-kDa band of a certain intensity. Only bands giving a higher staining intensity (as determined by the naked eye) than that of this cutoff band should be judged positive. Since we performed the study before this cutoff system was introduced, we decided to include any visible band as described above. For the sake of clarity, we also added the number of potentially positive sera to the number of definitely positive sera.
RESULTS
Patients.
Forty patients had to be excluded from further analysis because of insufficient or unreliable information concerning their gastric H. pylori status (most often because the result of the histopathological examination was insufficient for a diagnosis of H. pylori infection).
A summary of the remaining 294 patients is given in Table 1. H. pylori infection was detected in 47 asymptomatic patients without gastric pathology, and 83 persons had no gastric abnormality and were H. pylori negative.
TABLE 1.
Summary of patient data
| Group | No. of patients | H. pylori status | Endoscopic and histopathologic diagnosis | Mean age (yr) ± 1 SD | No. of males (% of group) |
|---|---|---|---|---|---|
| 1 | 57 | + | Duodenal ulcer | 56.5 ± 16.9 | 37 (64.9) |
| 2 | 42 | + | Gastric ulcer | 57.8 ± 15.0 | 15 (35.7) |
| 3 | 38 | + | Gastritis | 59.6 ± 17.1 | 20 (52.6) |
| 4 | 47 | + | Normal | 49.0 ± 17.9 | 23 (48.9) |
| 5 | 83 | − | Normal | 50.7 ± 16.4 | 40 (48.2) |
| 6 | 9 | Eradicated | Normal | 57.3 ± 13.2 | 3 (33.3) |
| 7 | 6 | 5+, 1− | Gastric MALToma | 53.3 ± 14.3 | 4 (66.7) |
| 8 | 12 | 10+, 2− | Gastric carcinoma | NAa | NA |
| Σ | 294 | 54.5 ± 17.5b | 142 (50.3)b |
NA, not available.
Calculation does not include data from group 8.
Western blot reaction patterns of patients.
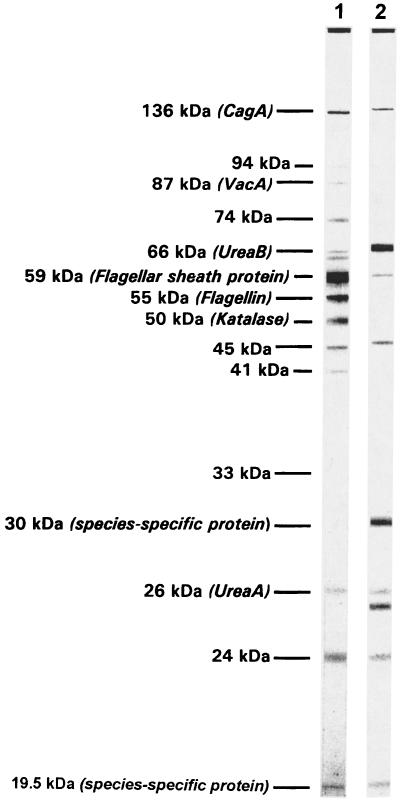
Figure 1 shows the band patterns of two strongly positive sera to illustrate the antigens present in the preparation and to demonstrate the variability of the IgG immune response. The CagA protein, which has been described by other authors as running with apparent molecular sizes between 110 and 138 kDa, was found to have a molecular size of 136 kDa in the present study. The 87-kDa band represents the VacA protein, the 66-kDa band represents the urease B subunit, and the 59- and 55-kDa proteins are thought to be components of the H. pylori flagella. The double-band staining of the 66-kDa (UreaB) band seen in lane 1 of Fig. 1 is typical for this band and has also been described when blots were reacted with a monoclonal antibody raised against this protein (18). The 59-kDa band may also be related to the 60-kDa, urease-associated heat shock protein identified in H. pylori (11), or the two bands may actually run so close to each other that they cannot be separated under the electrophoretic conditions used. It should, however, be noted that the heat shock protein has also been described as having a molecular size of 54 kDa (9), and therefore, it may also be located within the 54-kDa band present in our preparation. The 30- and 19.5-kDa proteins have been described as species-specific proteins of H. pylori, although their functional role is unclear at present (3, 7). The 26-kDa band represents the A subunit of the urease which has been described as running with apparent molecular sizes of 26 to 31 kDa (8, 19, 30).
FIG. 1.
Western blot reaction patterns of two strongly positive sera and identification of the major bands present in the antigen preparations.
Both of the two sera shown in Fig. 1 contained IgG antibodies binding to the CagA and VacA bands, although the VacA reaction was very faint in the serum shown in lane 2. The strongest reaction of the serum shown in lane 1 was directed against the 59-kDa probe. No antibody against the 30-kDa species-specific protein was present. By contrast, the serum represented in lane 2 showed the strongest reactions with the urease B subunit (66 kDa) and the 30-kDa protein. Both sera recognized the 26- and 24-kDa proteins, although some variations of the apparent molecular size, shown with double banding in lane 2, were obvious. This phenomenon, which has also been observed by others (30), may be explained by the different epitope specificities of the antibodies directed against individual surface proteins, which actually occupy a relatively broad space on the blot strip.
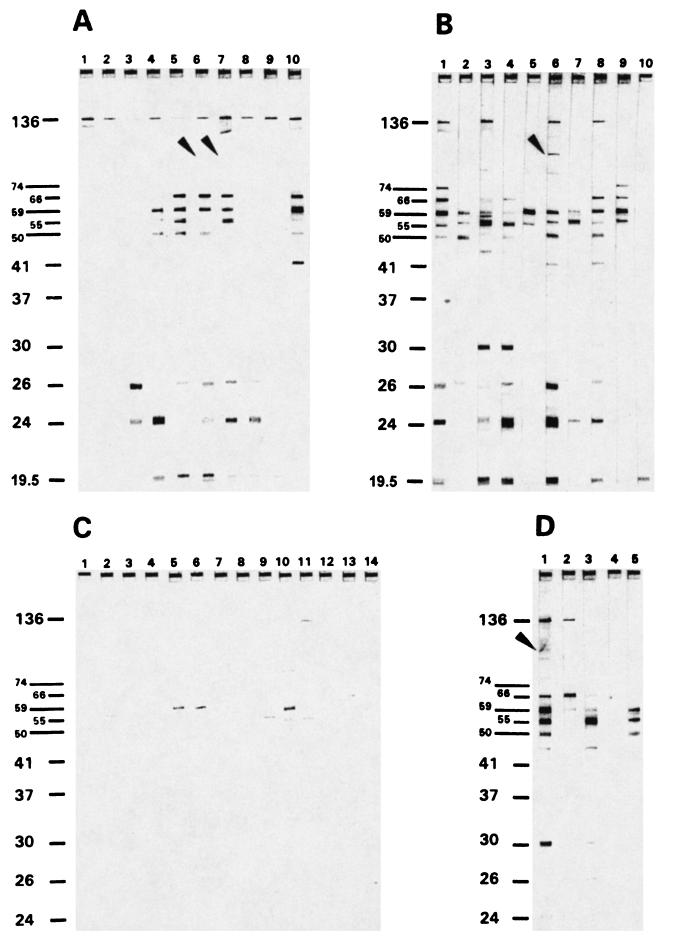
Typical band patterns produced by other sera from H. pylori-positive and -negative patients are shown in Fig. 2. Prominent bands seen in the majority of positive sera were the 136-, 74-, 66-, 59-, 55-, 50-, 26-, and 24-kDa bands. Again, the variability of the reaction with the 26- and 24-kDa bands was obvious, although these sera were run on blot strips produced from the same electrophoretic run. The 87-kDa (VacA) band stained weakly in blots of sera from some patients with active H. pylori infection (Fig. 2A and B) and one successfully treated patient (Fig. 2D) but was not found in blots for asymptomatic H. pylori-negative patients (Fig. 2C). The 136-kDa (CagA) band was seen in blots of sera from most of the patients with invasive H. pylori infection; however, it was clearly absent from some of them (Fig. 2B, lanes 2, 4, 5, and 7). It is faintly visible in lanes 9 and 10 of the original blots. Conversely, this band, although faintly stained, was also detectable on the original blots for a significant proportion of asymptomatic, H. pylori-negative patients (Fig. 2C, lanes 5, 7, 8, 9, 11, and 13).
FIG. 2.
Examples of the immunoblot reaction patterns of representative sera from H. pylori-positive and H. pylori-negative patients. Markers to the left of each panel are in kilodaltons. (A) Patients from group 1 (H. pylori positive with duodenal ulcer [n = 57]). (B) Patients from group 2 (H. pylori positive with gastric ulcer [n = 42]). The 136-kDa band is absent from lanes 2, 4, 5, and 7; it is, however, faintly visible (on the original blots) in lanes 9 and 10. (C) Patients from group 5 (H. pylori negative with no gastric disease [n = 83]). The 136-kDa band is absent from lanes 1, 2, 3, 4, 6, 10, 12, 13, and 14 and faintly visible in lanes 5, 7, 8, 9, and 11. (D) Patients from group 6 (currently H. pylori negative with no gastric disease, previously underwent H. pylori eradication therapy [n = 9]). (A, B, and D) Arrowheads indicate the 87-kDa (VacA) band.
The intensity of band staining was clearly correlated with disease activity (Table 2). The mean OD values of positive sera were strongest for the 59-kDa band, followed by the 55-, 136-, and 50-kDa bands. Sera from patients who did not at that time harbor H. pylori showed positive reactions most often with the 55- and 59-kDa (flagellin complex) probes (Table 2). Seventeen (20.5%) sera of H. pylori-negative persons without gastric disease clearly reacted with the 136-kDa (CagA) probe.
TABLE 2.
Antibody responses of patient groups to individual antigensa
| Molecular size (kDa) of antigen | Specificity | Groups 1 & 2 (n = 99)
|
Group 4 (n = 47)
|
Group 5 (n = 83)
|
|||
|---|---|---|---|---|---|---|---|
| No. of reactive sera (%) | Mean OD of positive sera ± 1 SD | No. of reactive sera (%) | Mean OD of positive sera ± 1 SD | No. of reactive sera (%) | Mean OD of positive sera ± 1 SD | ||
| 136 (CagA) | HS | 75 (75.8) | 0.48 ± 0.42 | 24 (51.1) | 0.49 ± 0.45 | 17 (20.5) | 0.18 ± 0.19 |
| 87 (VacA) | HS | 35 (35.4) | 0.10 ± 0.08 | 11 (23.4) | 0.06 ± 0.06 | 2 (2.4) | 0.07 ± 0.03 |
| 66 (UreB) | NS | 66 (66.7) | 0.34 ± 0.45 | 26 (55.3) | 0.53 ± 0.74 | 26 (31.3) | 0.14 ± 0.27 |
| 59 (HspB) | Specific | 81 (81.8) | 1.45 ± 1.26 | 32 (68.1) | 1.62 ± 1.33 | 30 (36.1) | 0.19 ± 0.39 |
| 55 (FlaA/FlaB) | Specific | 88 (88.9) | 0.54 ± 0.61 | 41 (87.2) | 0.65 ± 0.81 | 49 (59.0) | 0.15 ± 0.23 |
| 50 | Specific | 65 (65.7) | 0.40 ± 0.56 | 27 (57.4) | 0.45 ± 0.62 | 10 (12.0) | 0.26 ± 0.45 |
| 45 | Specific | 26 (26.3) | 0.20 ± 0.20 | 18 (38.3) | 0.27 ± 0.42 | 8 (9.6) | 0.11 ± 0.14 |
| 33 | HS | 10 (10.1) | 0.26 ± 0.40 | 4 (8.5) | 0.05 ± 0.03 | 10 (12.0) | 0.06 ± 0.06 |
| 30 | HS | 20 (20.2) | 0.29 ± 0.24 | 12 (25.5) | 0.35 ± 0.62 | 9 (10.8) | 0.06 ± 0.03 |
| 26 (UreA) | HS | 53 (53.5) | 0.33 ± 0.48 | 20 (42.6) | 0.26 ± 0.27 | 24 (28.9) | 0.10 ± 0.13 |
| 24 | HS | 57 (57.6) | 0.33 ± 0.44 | 27 (57.4) | 0.40 ± 0.69 | 26 (31.3) | 0.07 ± 0.06 |
| 19.5 | 53 (53.5) | NA | 23 (49.0) | NA | 14 (16.9) | NA | |
Group 1, H. pylori positive with duodenal ulcer; group 2, H. pylori positive with gastric ulcer; group 4, H. pylori positive with no gastric disease; group 5, H. pylori negative with no gastric disease. HS, highly specific for H. pylori; NS, nonspecific for H. pylori; NA, not available.
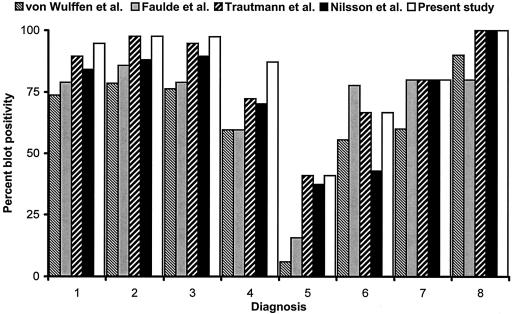
Table 3 summarizes the calculated seropositivity rates according to the different interpretation criteria. Figure 3 shows the graphic representation of these rates. The lowest seropositivity rate in the group of H. pylori-negative patients (group 5) was obtained with the criteria of von Wulffen et al., while the highest rate was reached with the criteria that we propose in this study.
TABLE 3.
Overall percentages of blot positivity according to different interpretation criteria
| Group | No. of patients | Diagnosis (H. pylori status) | % Blot positivity according to criteria proposed by:
|
||||
|---|---|---|---|---|---|---|---|
| von Wulffen et al. (39) | Faulde et al. (13) | Trautmann et al. (37) | Nilsson et al. (30) | Authors of present study | |||
| 1 | 57 | Duodenal ulcer (+) | 73.7 | 78.9 | 89.5 | 84.2 | 94.7 |
| 2 | 42 | Gastric ulcer (+) | 78.6 | 85.8 | 97.6 | 88.1 | 97.6 |
| 3 | 38 | Gastritis (+) | 76.3 | 78.9 | 94.7 | 89.5 | 97.4 |
| 4 | 47 | Normal (+) | 59.6 | 59.6 | 72.3 | 70.2 | 87.2 |
| 5 | 83 | Normal (−) | 6.0 | 15.7 | 41.0 | 37.4 | 41.0 |
| 6 | 9 | Normal (eradicated) | 55.6 | 77.7 | 66.7 | 42.9 | 66.7 |
| 7 | 6 | Gastric MALToma (5 +)a | 60.0 | 80.0 | 80.0 | 80.0 | 80.0 |
| 8 | 12 | Gastric carcinoma (10 +)a | 90.0 | 80.0 | 100.0 | 100.0 | 100.0 |
Values for groups 7 and 8 were calculated using data from H. pylori-positive patients only.
FIG. 3.
Overall blot positivity of eight diagnosis groups of H. pylori-positive patients according to different interpretation criteria. Group 1, H. pylori positive with duodenal ulcer (n = 57); group 2, H. pylori positive with gastric ulcer (n = 42); group 3, H. pylori positive with gastritis (n = 38); group 4, H. pylori positive with no gastric disease (n = 47); group 5, H. pylori negative with no gastric disease (n = 83); group 6, currently H. pylori negative with no gastric disease, previously underwent H. pylori eradication therapy (n = 9); group 7, 5 H. pylori-positive patients with gastric mucosa-associated lymphoid tissue lymphoma (MALToma) (n = 6); group 8, 10 H. pylori-positive patients with gastric carcinoma (n = 12). Only H. pylori-positive patients were considered for this figure.
DISCUSSION
Although a number of authors have studied the humoral immune response against H. pylori by Western immunoblotting, most of the publications dealing with this subject are merely descriptive (1, 5, 12, 19, 23, 24, 38). We found only four studies (13, 30, 37, 39) in which interpretation criteria were proposed. In the present study, we evaluated the performance of blotting according to these criteria.
One of the interpretation systems used a quantitative cutoff value for band intensity (39), while the others considered bands of any intensity. Furthermore, bands representing the flagellar complex (59 and 55 kDa) were to be included in the analysis, although these proteins have been shown to be immunologically cross-reactive among flagellated bacteria of various species (22, 25, 29). The cutoff value that is included in the revised version of the criteria obviously aims to exclude weakly positive bands of these molecular sizes. The other interpretation systems do not consider these bands (30, 37, 39).
In applying the different criteria to our patient population, it became apparent that the abilities of the systems to detect active H. pylori infection were quite varied. Since it is known that less than 2% of patients who harbor H. pylori fail to seroconvert (17, 20, 21, 34, 35, 37), an optimally sensitive Western blotting system should be able to detect approximately 98% of active infections. The only interpretation system that approached this sensitivity was our currently used system (Tables 3 and 4). The main difference between our current interpretation criteria and those proposed by Nilsson et al. (30) is that those authors considered a 94-kDa band in their analysis instead of the 66-kDa (urease B) band included in our system. The 94-kDa band described by Nilsson et al. most probably corresponds to the 100-kDa band of our system because, in their figures, it is located approximately in the middle between the VacA and CagA bands (30). Weak antibody binding to this band was seen in a significant proportion of positive sera in the study of Nilsson et al., while we found this band in only a minority of positive sera (e.g., Fig. 2B, lanes 6 and 8). The fact that we saw only insignificant responses to this band may be related to differences in the preparations of the immunoblot antigen, although the strain used in our study was the same as that used by Nilsson et al., namely, the reference strain H. pylori ATCC 43504 (30). Instead of this band, we included the 66-kDa (urease B) antigen in our interpretation, based on the following observations from the literature: experiments in which sera reactive with the 66-kDa probe were absorbed with Campylobacter organisms confirmed the specificity of this band for H. pylori (23). Recently, genetic and phenotypic studies have been performed with the urease genes and proteins from various bacterial species and genera (21). It was reported that there was antigenic cross-reactivity between the urease B protein from Clostridium perfringens and that of H. pylori (10). However, since only 2% of the clostridial strains were found to be urease positive, and since infections due to C. perfringens are very rare in the general population, we believe that this cross-reaction is of no relevance for the interpretation of H. pylori immunoblots. The low detection rate of the 100-kDa band and the consideration of the urease B band are responsible for the higher overall seropositivity rates found with our system than with the system of Nilsson et al. (30). Further, however weaker, arguments for the inclusion of this band are that monoclonal antibodies that react with a protein of 64 to 66 kDa that most probably corresponded to the urease B subunit have been shown to be specific for H. pylori because they did not react with strains belonging to other urease-producing species and genera, such as Klebsiella, Enterobacter, Pseudomonas, and, most notably, Campylobacter (18). A similar monoclonal antibody has been used to construct a competitive ELISA system to detect H. pylori-specific IgG antibody in the sera of patients. This system was both highly sensitive and specific for the detection of H. pylori infection (28).
TABLE 4.
Statistical performance of H. pylori IgG immunoblotting according to different interpretation criteriaa
| Authors of interpretation criteria | Performance values [n/n (%)]
|
|||
|---|---|---|---|---|
| Sensitivity | Specificity | Positive predictive value | Negative predictive value | |
| von Wulffen et al. (39) | 137/218 (62.8) | 49/49 (100) | 152/152 (100) | 49/130 (37.7) |
| Faulde et al. (13) | 152/218 (69.7) | 49/49 (100) | 165/165 (100) | 49/115 (42.6) |
| Trautmann et al. (37) | 196/218 (89.9) | 49/49 (100) | 230/230 (100) | 49/71 (69.0) |
| Nilsson et al. (30) | 183/218 (83.9) | 49/49 (100) | 214/214 (100) | 49/84 (59.3) |
| Lepper et al. (this study) | 209/218 (95.9) | 42/49 (85.7) | 243/250 (97.2) | 42/51 (82.4) |
Only patients from groups 1 to 5 (n = 267) were included in these calculations. The number of truly seropositive patients in group 5 (n = 83) was assumed to be 34 (41%; see Discussion), and the number of truly seronegative patients in group 5 was assumed to be 49.
In order to evaluate the specificity of blotting, it would be necessary to know the number of truly H. pylori-seronegative persons. From the data shown in Table 3 it is clearly evident that this value cannot be identical with the number of patients without current H. pylori infection (group 5), because all interpretation systems recognized some patients of this group as seropositive. Since we took at least three biopsies per patient and examined them by three different methods, each having a sensitivity of >90% to detect H. pylori (6), we do not believe that sampling errors of the biopsy technique can explain the mismatch between the results of reference methods and serology. Rather, it must be assumed that an unknown percentage of patients in this group had residual IgG antibodies after former H. pylori infection. While spontaneous elimination of H. pylori may occur more frequently than is usually assumed (16), persons included in this group may also have been incidentally cured during antibiotic treatment of respiratory or other infections (34). In this respect, it is of interest that Meyer et al. saw the same mismatch between the prevalence of H. pylori antibodies in serum and [13C]urea breath test results (27); among 100 healthy volunteers, 49 had H. pylori antibodies, whereas only 24 had a positive breath test as an indicator of active infection (27).
In order to estimate the true number of H. pylori-seropositive persons in our population (group 5), the following assumptions were made: it has been shown that approximately 50% of healthy, H. pylori-seropositive persons from industrialized countries have antibody against CagA in their sera (32, 33). This figure was again confirmed in our study with the patients of group 4, in which a 51% prevalence of CagA antibodies was found (Table 2). Since 17 patients (20.5%) in group 5 had antibodies against the CagA protein (e.g., Fig. 2C, lanes 5, 7 to 9, 11, and 13), and since we believe that this finding was unequivocal due to the clear delineation of the corresponding 136-kDa band, the overall number of H. pylori-seropositive persons in this group can be assumed to be twice as high, namely, ∼34. Using 49 as a denominator and assuming that all positive Western blot results belonged to this seropositive group, the performance figures for the specificity given in Table 4 were calculated. In order to calculate the sensitivity, we added the 34 patients assumed to be seropositive to the number of truly positive patients with either a positive or a negative Western blot result. Since the tests used to verify the H. pylori status can detect only active infections, in contrast to Western blotting, and the possibility of a sampling error cannot be excluded for every patient, we believe that the addition of the seropositive patients of this group avoids the overestimation of the sensitivity. The resulting 218 patients were used as a denominator to calculate the sensitivity values shown in Table 4.
These data show that the interpretation systems of Wulffen et al. and Faulde et al. had low sensitivities and low negative predictive values. However, these systems and the system of Nilsson et al. yielded results that were 100% specific, i.e., the absence of (former and present) H. pylori infection can be detected with 100% probability on the basis of these criteria. The interpretation system that combined a relatively high sensitivity (89.9%) with 100% specificity was that of Trautmann et al. (37). The positive predictive values of all of the systems were acceptable, but the negative predictive values were relatively low except with our newly developed criteria (Table 4). This means that a negative test result obtained with these systems does not reliably exclude the possibility of the presence of past or current H. pylori infection.
Which of these criteria should be applied when performing H. pylori Western blots? The answer to this question may depend on the purpose of the serological examination. If serology is to be used for seroepidemiological surveys, then Western blotting is a very useful tool because it detects both former and current H. pylori infections. A system that combines the highest possible positive and negative predictive values may be most suitable for this purpose, and therefore, our newly developed criteria can be recommended for this application. If serology is to be used as a preendoscopic screening method, then the sensitivity must be very high (in order not to miss a patient who might harbor H. pylori), but the specificity is of lesser concern because false-positive serological findings will be corrected by the results of the invasive tests that are going to follow. Our presently proposed criteria are recommendable for this purpose. If the results of serological tests are used to decide about H. pylori-targeted therapy in patients with dyspepsia without performing endoscopy as has been recently suggested (31, 35), then the specificity and the positive predictive value must be as high as possible in order to avoid the unnecessary and potentially harmful treatment of patients who are not actually infected. In this case, the criteria of Nilsson et al. and Trautmann et al. may be most suitable. An additional advantage of Western blotting in this scenario is its ability to detect infection with CagA-positive H. pylori strains, because treatment can be limited to the subgroup of CagA-seropositive patients (27).
Finally, it has been suggested that Western blotting may be used as a second-line method in a two-step serological workup (30). The first step is an ELISA which identifies all truly and potentially H. pylori-positive patients with a high sensitivity. If serology is to be used for deciding about treatment, the ELISA method can be adjusted in such a way that it detects only currently H. pylori-infected patients. Western blotting is then used as a second step in order to identify false-positive ELISA results. In this case, the sensitivity of blotting is of no primary importance, but a high specificity (i.e., ability to detect truly negative patients) is desired. The criteria of Nilsson et al. and Trautmann et al. would be most suitable for this purpose.
Acknowledgments
We thank Viramed Ltd., Planegg-Steinkirchen, Germany, for kindly supplying the blotting kit.
REFERENCES
- 1.Andersen, L. P., and F. Espersen. 1992. Immunoglobulin G antibodies to Helicobacter pylori in patients with dyspeptic symptoms investigated by the Western immunoblot technique. J. Clin. Microbiol. 30:1743-1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blaser, M. J. 1990. Helicobacter pylori and the pathogenesis of gastroduodenal inflammation. J. Infect. Dis. 161:626-633. [DOI] [PubMed] [Google Scholar]
- 3.Bölin, I., H. Lönroth, and A.-M. Svennerholm. 1995. Identification of Helicobacter pylori by immunological dot blot method based on reaction of a species-specific monoclonal antibody with a surface-exposed protein. J. Clin. Microbiol. 33:381-384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Covacci, A., S. Falkow, D. E. Berg, and R. Rappuoli. 1997. Did the inheritance of a pathogenicity island modify the virulence of Helicobacter pylori? Trends Microbiol. 5:205-208. [DOI] [PubMed] [Google Scholar]
- 5.Czinn, S., H. Carr, L. Sheffler, and S. Aronoff. 1989. Serum IgG antibody to the outer membrane proteins of Campylobacter pylori in children with gastroduodenal disease. J. Infect. Dis. 159:586-589. [DOI] [PubMed] [Google Scholar]
- 6.Deltenre, M., Y. Glupczynski, C. DePrez, J. F. Nyst, A. Burette, M. Labbé, C. Jonas, and E. DeKoster. 1989. The reliability of urease tests, histology and culture in the diagnosis of Campylobacter pylori infection. Scand. J. Gastroenterol. 24(Suppl. 160):19-24. [DOI] [PubMed] [Google Scholar]
- 7.Drouet, E. B., G. A. Denoyel, M. Boude, E. Wallano, M. Andujar, and H. P. de Montclos. 1991. Characterization of an immunoreactive species-specific 19-kilodalton outer membrane protein from Helicobacter pylori by using a monoclonal antibody. J. Clin. Microbiol. 29:1620-1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dunn, B. E., G. P. Campbell, G. I. Perez-Perez, and M. J. Blaser. 1990. Purification and characterization of urease from Helicobacter pylori. J. Biol. Chem. 265:9464-9469. [PubMed] [Google Scholar]
- 9.Dunn, B. E., R. M. Roop II, C.-C. Sung, S. A. Sharma, G. I. Perez-Perez, and M. J. Blaser. 1992. Identification and purification of a cpn60 heat shock protein homolog from Helicobacter pylori. Infect. Immun. 60:1946-1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dupuy, B., G. Daube, M. R. Popoff, and S. T. Cole. 1997. Clostridium perfringens urease genes are plasmid borne. Infect. Immun. 65:2313-2320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Evans, D. J., Jr., D. G. Evans, L. Engstrand, and D. Y. Graham. 1992. Urease-associated heat shock protein of Helicobacter pylori. Infect. Immun. 60:2125-2127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Faulde, M., J. P. Schröder, and D. Sobe. 1992. Serodiagnosis of Helicobacter pylori infections by detection of immunoglobulin G antibodies using an immunoblot and enzyme immunoassay technique. Eur. J. Clin. Microbiol. Infect. Dis. 11:589-594. [DOI] [PubMed] [Google Scholar]
- 13.Faulde, M., J. Cremer, and L. Zöller. 1993. Humoral immune response against Helicobacter pylori as determined by immunoblot. Electrophoresis 14:945-951. [DOI] [PubMed] [Google Scholar]
- 14.Glupczynski, Y., A. Burette, H. Goossens, C. DePrez, and J. P. Butzler. 1992. Effect of antimicrobial therapy on the specific serological response to Helicobacter pylori infection. Eur. J. Clin. Microbiol. Infect. Dis. 11:582-588. [DOI] [PubMed] [Google Scholar]
- 15.Graham, D. Y., P. D. Klein, D. J. Evans, Jr., D. G. Evans, L. C. Alpert, A. R. Opekun, and T. W. Boutton. 1987. Campylobacter pylori detected noninvasively by the 13C-urea breath test. Lancet i:1174-1177. [DOI] [PubMed] [Google Scholar]
- 16.Granström, M., Y. Tindberg, and M. Blennow. 1997. Seroepidemiology of Helicobacter pylori infection in a cohort of children monitored from 6 months to 11 years of age. J. Clin. Microbiol. 35:468-470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hirschl, A. M., G. Brandstätter, B. Dragosics, E. Hentschel, M. Kundi, M. L. Rotter, K. Schütze, and M. Taufer. 1993. Kinetics of specific IgG antibodies for monitoring the effect of anti-Helicobacter pylori chemotherapy. J. Infect. Dis. 168:763-766. [DOI] [PubMed] [Google Scholar]
- 18.Husson, M. O., and H. Leclerc. 1991. Detection of Helicobacter pylori in stomach tissue by use of a monoclonal antibody. J. Clin. Microbiol. 29:2831-2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Klaamas, K., M. Held, T. Wadström, A. Lipping, and O. Kurtenkov. 1996. IgG immune response to Helicobacter pylori antigens in patients with gastric cancer as defined by ELISA and immunoblotting. Int. J. Cancer 67:1-5. [DOI] [PubMed] [Google Scholar]
- 20.Kosunen, T. U., K. Seppälä, S. Sarna, and P. Sipponen. 1992. Diagnostic value of decreasing IgG, IgA, and IgM antibody titres after eradication of Helicobacter pylori infection. Lancet 339:893-895. [DOI] [PubMed] [Google Scholar]
- 21.Kuipers, E. J., A. S. Peña, G. van Kamp, A. M. Uyterlinde, G. Pals, N. F. M. Pels, E. Kurz-Pohlmann, and S. G. M. Meuwissen. 1993. Seroconversion for Helicobacter pylori. Lancet 342:328-331. [DOI] [PubMed] [Google Scholar]
- 22.Lee, A., S. M. Logan, and T. J. Trust. 1987. Demonstration of a flagellar antigen shared by a diverse group of spiral-shaped bacteria that colonize intestinal mucus. Infect. Immun. 55:828-831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lelwala-Guruge, J., C. Schalén, I. Nilsson, A. Ljungh, T. Tyszkiewicz, M. Wikander, and T. Wadström. 1990. Detection of antibodies to Helicobacter pylori cell surface antigens. Scand. J. Infect. Dis. 22:457-465. [DOI] [PubMed] [Google Scholar]
- 24.Lelwala-Guruge, J., I. Nilsson, A. Ljungh, and T. Wadström. 1992. Cell surface proteins of Helicobacter pylori as antigens in an ELISA and a comparison with three commercial ELISA. Scand J. Infect. Dis. 24:457-465. [DOI] [PubMed] [Google Scholar]
- 25.Logan, S. M., and T. J. Trust. 1983. Molecular identification of surface protein antigens of Campylobacter jejuni. Infect. Immun. 42:675-682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mégraud, F., M.-P. Brassens-Rabbé, F. Denis, A. Belbouri, and D. Quynh Hoa. 1989. Seroepidemiology of Campylobacter pylori in various populations. J. Clin. Microbiol. 27:1870-1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Meyer, B., B. Werth, C. Beglinger, S. Dill, J. Drewe, W. A. Vischer, R. H. Eggers, F. E. Bauer, and G. A. Stalder. 1991. Helicobacter pylori infection in healthy people: a dynamic process? Gut 32:347-350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Negrini, R., I. Zanella, A. Savio, C. Poiesi, R. Verardi, S. Ghielmi, A. Albertini, O. Sangaletti, M. Lazzaroni, and G. Bianchi Porro. 1992. Serodiagnosis of Helicobacter pylori-associated gastritis with a monoclonal antibody competitive enzyme-linked immunosorbent assay. Scand. J. Gastroenterol. 27:599-605. [DOI] [PubMed] [Google Scholar]
- 29.Newell, D. G. 1987. Identification of the outer membrane proteins of Campylobacter pyloridis and antigenic cross-reactivity between C. pyloridis and C. jejuni. J. Gen. Microbiol. 133:163-170. [DOI] [PubMed] [Google Scholar]
- 30.Nilsson, I., Å. Ljungh, P. Aleljung, and T. Wadström. 1997. Immunoblot assay for serodiagnosis of Helicobacter pylori infections. J. Clin. Microbiol. 35:427-432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ofman, J. J., J. Etchason, S. Fullerton, K. L. Kahn, and A. H. Soll. 1997. Management strategies for Helicobacter pylori-seropositive patients with dyspepsia: clinical and economic consequences. Ann. Intern. Med. 126:280-291. [DOI] [PubMed] [Google Scholar]
- 32.Parsonnet, J., M. Replogle, S. Yang, and R. Hiatt. 1997. Seroprevalence of CagA-positive strains among Helicobacter pylori-infected, healthy young adults. J. Infect. Dis. 175:1240-1242. [DOI] [PubMed] [Google Scholar]
- 33.Parsonnet, J., G. D. Friedman, N. Orentreich, and H. Vogelman. 1997. Risk for gastric cancer in people with CagA positive or CagA negative Helicobacter pylori infection. Gut 40:297-301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pavièiæ, M. J. A. M. P., F. Namavar, T. Verboom, A. J. van Winkelhoff, and J. de Graaff. 1993. In vitro susceptibility of Helicobacter pylori to several antimicrobial combinations. Antimicrob. Agents Chemother. 37:1184-1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rabeneck, L., and D. Y. Graham. 1997. Helicobacter pylori: when to test, when to treat. Ann. Intern. Med. 126:315-316. [DOI] [PubMed] [Google Scholar]
- 36.Thomas, J. E., A. M. Whatmore, M. R. Barer, E. J. Eastham, and M. A. Kehoe. 1990. Serodiagnosis of Helicobacter pylori infection in childhood. J. Clin. Microbiol. 28:2641-2646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Trautmann, M., M. Moldrzyk, K. Vogt, J. Körber, T. Held, and R. Marre. 1994. Use of a receiver operating characteristic in the evaluation of two commercial enzyme immunoassays for detection of Helicobacter pylori infection. Eur. J. Clin. Microbiol. Infect. Dis. 13:812-819. [DOI] [PubMed] [Google Scholar]
- 38.von Wulffen, H., J. Heesemann, G. H. Bützow, T. Löning, and R. Laufs. 1986. Detection of Campylobacter pyloridis in patients with antrum gastritis and peptic ulcers by culture, complement fixation test, and immunoblot. J. Clin. Microbiol. 24:716-720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.von Wulffen, H., H. J. Grote, S. Gatermann, T. Löning, B. Berger, and C. Buhl. 1988. Immunoblot analysis of immune response to Campylobacter pylori and its clinical associations. J. Clin. Pathol. 41:653-659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.von Wulffen, H. 1992. An assessment of serological tests for detection of Helicobacter pylori. Eur. J. Clin. Microbiol. Infect. Dis. 11:577-582. [DOI] [PubMed] [Google Scholar]