Abstract
Reduced Heart Rate Variability is a strong predictor of cardiovascular risk factors, cardiovascular events and mortality; and thus may be associated with cognitive neurodegeneration. Yet this has been relatively unexplored, particularly in minority populations with high cardiovascular burden. We used data from the Sacramento Area Latino Study on Aging to examine the cross-sectional association of reduced heart rate variability with cognitive function among elderly Mexican Americans. A total of 869 participants (mean age of 75 years; 59% females) had their 6-minute heart rate variability measured using an ECG monitor and respiration pacer in response to deep breathing. We used the Mean Circular Resultant, known as R bar, as a measure of heart rate variability and categorized it into quartiles (Q1 to Q4 of R bar: reduced to high heart rate variability). Cognitive function was assessed using the Modified Mini Mental State Exam, a 100-point test of global cognitive function and the Spanish and English Verbal Learning Test, a 15-point test of verbal memory recall. In fully-adjusted linear regression models, participants in quartile 1 had a 4-point lower Modified Mini Mental State Exam score (p<0.01), those in quartile 2 had 2-point lower score (p=0.04), and those in quartile 3 had 1-point lower score (p=0.35), as compared to those in the highest quartile of R bar. Reduced R bar was not associated with verbal memory. Our results suggest that reduced heart rate variability is associated with worse performance on the test of global cognitive function, above and beyond traditional cardiovascular risk factors.
Keywords: Aging, autonomic function, cognition, epidemiology, heart rate variability
INTRODUCTION
Heart rate variability (HRV) has recently emerged as a non-invasive measure to quantitatively assess cardiovascular autonomic function.1,2 HRV is the beat-to-beat alterations to the sinus rhythm which result from the interactions between sympathetic and parasympathetic activity. Reduced HRV has been shown to be a strong predictor of cardiovascular events and mortality3–6 and has been proposed as a prognostic factor for cardiovascular disease risk stratification and management.1 Furthermore, HRV has been increasingly suggested to be associated with several vascular risk factors for cognitive impairment such as hypertension,7–9 diabetes,10,11 depression,12 and subclinical inflammation.13 In particular, reduced HRV has been recognized as a hallmark of early cardiac autonomic neuropathy14 which is in turn associated with impaired fasting plasma glucose15 and insulin sensitivity.16
The link between heart rate variability, cardiac autonomic neuropathy in particular, and cognitive impairment is of particular importance when we consider minority ethnic populations such as Mexican Americans who are at high risk for cardiovascular disease, type-2 diabetes, obesity, and insulin resistance compared to their non-Hispanic white counterparts.17–21 For example, subjects with type-2 diabetes have a two-fold increased risk for cognitive impairment and later dementia compared to those without diabetes.22 Identifying subclinical mechanisms and predictors of cognitive impairment in a population with poor cardiovascular prognosis will constitute a non-invasive clue for risk of cognitive impairment, and may lead to more targeted screening and early preventive strategies to delay the progression of cognitive impairment.
In spite of the potential association of HRV with cognitive function and with many of its risk factors, the direct association of HRV with cognitive function has been less explored and less understood, particularly in high-risk minority populations. In this study, we sought to determine the cross-sectional association of heart rate variability with cognitive function in a cohort of elderly Mexican Americans, and whether this association persists above and beyond traditional cardiovascular risk factors.
METHODS
Study population
Participants in this study were from the Sacramento Area Latino Study on Aging (SALSA). SALSA is a prospective cohort study of 1,789 community-dwelling Mexican Americans residing in California’s Sacramento Valley and aged 60–101 years at baseline in 1998–1999. The original goal of the SALSA study was to identify vascular, metabolic, and social correlates of dementia, cognitive function, functional limitation, and depressive symptoms among Mexican Americans, an understudied ethnic group. Biological and clinical data were collected on participants in home visits every 12 to 15 months for a maximum of 7 visits. SALSA has been approved by the Institutional Review Board (IRB) at the University of Michigan and the University of California, San Francisco and Davis. Details on the study design have been published elsewhere.23 Heart rate variability (HRV), our predictor of interest, was measured for a subsample of the SALSA participants (N=869) at study visit 5 or 6.
Measures
Assessment of heart rate variability
Heart rate variability (HRV) was measured using the ANS2000 (Autonomic Nervous System, D. E. Hokanson, Inc. Bellevue, Wash.), a validated device which has an ECG monitor and respiration pacer measuring variability in the heart rate in response to deep breathing.24 The examination took place in the morning with the participant being overnight fasting. Briefly, HRV is the change in the time of consecutive heart beats; with a heart beat measured as the time between the peak of one R wave to the peak of the next, also referred as the R-R interval. Changes in the length of the normal R-R interval are what define HRV. Most commonly, HRV is determined by either time or frequency domain measures. In this analysis, we use the Mean Circular Resultant (MCR), also known as the R bar, which is a time domain measure of HRV.
Calculation of Mean Circular Resultant (MCR)
RR variation was measured during a 6-minute test with participants in a supine position and breathing at a standard rate of 5 breaths per minute. The latter was achieved with the aid of a respiration pacer. RR variations were recorded for 6 minutes but only the middle 5 minutes were used for analysis, allowing room for beginning and ending. Thus, a total of 25 breaths cycles were included in the 5-minute analysis.
The calculation of MCR, also known as R bar, is based on vector analysis. The latter uses a technique that plots the time of the R wave spike on a circular graph that rotates in synchrony with the participant’s breath cycle. As such there is a record for the time at which the R wave spikes and the period of the respiratory cycle to which it corresponds. Both components are included in the statistical computation of MCR. For a detailed description of the MCR calculation see Weinberg and Pfeifer.25 In brief, the timing (Ti) and periodicity (λ) of an R wave spike is plotted as a point on the unit circle, and the mean vector of all R waves is then calculated as a function of these two parameters (Ti and λ). The length of the calculated mean vector is the MCR. MCR bar tends to be shorter when the R wave spikes are uniformly distributed, and MCR bar tends to be longer when the R wave spikes cluster towards one region of the circle i.e. depicting periodicity in the process. Therefore, lower MCR, which denotes reduced HRV, is treated as a risk factor for cognitive function in this analysis. MCR measurement is proved to be resistant to ectopic beats and less affected by intrinsic heart rate, and thus is a preferred method for the assessment of parasympathetic function. The HRV data was reviewed and edited into final form by Deng Y (coauthor). In the remaining text, we will refer to MCR as R bar.
Assessment of cognitive function
Cognitive function was assessed at the time of HRV measurement using the Modified Mini Mental State Exam (3MSE) and the Spanish and English Verbal Learning Test (SEVLT). The 3MSE is a 100-point test of global cognitive function and was validated and field-tested in both English and Spanish. Compared to the Mini-mental State Exam (MMSE), the 3MSE shows better reliability, test-re-test properties, better sensitivity and specificity and fewer ceiling effects.26,27 The SEVLT is a 15-point verbal memory recall test with four 15-word memory trials, an interference list, followed by a fifth trial which is usually used as the test score.28,29 SEVLT was developed for use in SALSA29 and has been validated in both English and Spanish and has been used in other studies. Higher scores on both tests indicate better cognitive performance.
Covariates
We used baseline socio-demographics such as age, country of birth (nativity) which we coded as US-born or Mexican-born, years of education completed, and marital status (married vs. other). For the remaining covariates, we used those collected at the same study visit of HRV measurement. We measured systolic (SBP) and diastolic (DBP) blood pressures using a digital blood pressure monitor. We measured standing height and weight and we calculated body mass index (BMI, Kg/m2). We defined type-2 diabetes as a self-report of a physician diagnosis, use of diabetes medication, or a fasting glucose level ≥ 126 mg/dl,30 and we defined hypertension as a report of a physician diagnosis, use of hypertension medication, a systolic blood pressure >140 mm Hg or a diastolic blood pressure >90 mm Hg.31 We defined stroke based on a physician diagnosis including hospitalization. We assessed cardiac failure as a self-report of congestive heart failure. We assessed depressive symptoms using the 20-item Center for Epidemiologic Studies- Depression Scale (CES-D) (range 0–60),32 and we defined elevated depressive symptoms as CESD ≥ 16. We assessed dementia using a 3-stage process that included: 1) cognitive screening tests, 2) the Spanish and English Neuropsychological Assessment Scales (SENAS)33 and the Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE), and 3) case adjudication by neurologists and neuropsychologists resulting in participants being classified as ‘normal’, ‘demented’, or ‘cognitively impaired but not demented (CIND)’. Consistent with prior publications, we combined the demented and CIND cases into one group which we refer to as dementia/CIND variable. Further details on the dementia screening process are published elsewhere.34
Statistical analysis
For ease of interpretation, we used quartile of R bar (quartile 1 to quartile 4= reduced to high R bar). Given that R bar measurement was only performed in a subsample of our SALSA cohort, we first presented a comparison of the baseline characteristics for participants with and without R bar data, using analysis of variance or chi-squared tests as appropriate (Table 1). Second, we presented the bivariate associations of quartile of R bar with important covariates, using analysis of variance or chi-squared tests as appropriate (Table 2 and Figure 1); this analysis uses covariates that were assessed at the same study visit at which R bar was measured. Finally, to examine the associations of quartile of R bar with performance on cognitive tests (3MSE and SEVLT), we fit unadjusted and multivariable adjusted linear regression models (Table 3). First, we fit an unadjusted model, then we added adjustment for socioeconomic and demographic risk factors including age, gender, education, and marital status in model 2, then we added adjustment for co-morbidities including type-2 diabetes, hypertension, stroke and depressive symptoms in model 3; the multivariable analysis also uses covariates assessed at the same study visit at which R bar was measured. The selection of these covariates was based on their associations with both R bar and cognitive outcomes. We provided the R-squared statistic to indicate the relative contribution of each set of covariates that we adjusted for in the linear regression models.
Table 1.
Comparison of baseline characteristics for participants with and without HRV data in SALSA
| Characteristics | With HRV data (N=869) | Without HRV data (N=920) | p-value |
|---|---|---|---|
| Age (years), mean (SD) | 69.4 (6.1) | 71.8 (7.8) | <0.01 |
| % Females | 509 (58.6) | 529 (57.5) | 0.74 |
| %Mexican born | 421 (48.5) | 487 (52.9) | 0.04 |
| %Married | 521 (60.0) | 525 (57.1) | 0.27 |
| Education (years), mean (SD) | 8.2 (5.4) | 6.4 (5.1) | <0.01 |
| Body Mass Index (Kg/m2), mean (SD) | 29.8 (5.5) | 29.7 (6.3) | 0.66 |
| %Congestive Heart Failure (CHF) | 9 (1.0) | 43 (4.7) | <0.01 |
| % Stroke | 58 (6.7) | 110 (12.0) | 0.01 |
| %Diabetes | 233 (26.8) | 353 (38.4) | <0.01 |
| % Hypertension | 557 (64.1) | 651 (70.8) | <0.01 |
| % CESD≥16 | 184 (21.2) | 255 (27.7) | <0.01 |
| %Dementia/CIND | 35 (4.0) | 80 (8.7) | <0.01 |
Table 2.
Bivariate associations of heart rate variability, as R bar, with sample characteristics at the time of HRV assessment
| Characteristics | Quartiles of R bar (N=869)
|
|||||
|---|---|---|---|---|---|---|
| Overall | Q1 (0.10–3.3) | Q2 (3.4–9.4) | Q3 (9.5–18.3) | Q4 (18.4–82.4) | p-value | |
| Age, years, mean (SD) | 75.6 (6.1) | 76.9 (6.3) | 76.8 (6.2) | 75.2 (5.7) | 73.3 (5.4) | <0.01 |
| Gender, n (%)* | 0.01 | |||||
| Females | 509 (58.6) | 144 (66.7) | 116 (53.2) | 130 (59.9) | 119 (54.6) | |
| Males | 357 (41.1) | 71 (32.9) | 102 (46.8) | 85 (39.2) | 99 (45.4) | |
| Nativity, n (%)* | 0.51 | |||||
| Mexican born | 421 (48.5) | 109 (50.5) | 112 (51.4) | 102 (47.0) | 98 (45.0) | |
| U.S.-born | 445 (51.2) | 106 (49.1) | 106 (48.6) | 113 (52.1) | 120 (55.1) | |
| Marital Status, n (%)* | 0.02 | |||||
| Married | 521 (60.0) | 110 (50.9) | 133 (61.0) | 138 (63.6) | 140 (64.2) | |
| Other | 344 (40.0) | 104 (48.2) | 85 (39.0) | 77 (35.5) | 78 (35.8) | |
| Education, years, mean (SD)* | 8.2 (5.4) | 6.8 (5.0) | 7.6 (5.3) | 9.0 (5.5) | 9.4 (5.4) | <0.01 |
| BMI, Kg/m2, mean (SD) | 29.3 (5.3) | 29.8 (5.8) | 29.5 (5.1) | 29.3 (5.3) | 28.6 (5.0) | 0.09 |
| Systolic Blood Pressure, mmHg | 142.6 (21.9) | 145.3 (22.7) | 145.2 (24.0) | 139.6 (20.6) | 140.4 (19.6) | <0.01 |
| Diastolic Blood Pressure, mmHg | 77.0 (11.4) | 76.6 (11.6) | 76.8 (11.5) | 77.2 (11.5) | 77.6 (11.3) | 0.79 |
| Fasting plasma glucose, mg/dl | 110.3 (40.3) | 113.4 (46.4) | 112.5 (45.3) | 111.1 (39.4) | 104.6 (27.6) | 0.11 |
| Insulin, μU/ml | 26.2 (32.2) | 31.6 (48.9) | 26.9 (35.0) | 24.0 (16.9) | 22.7 (16.9) | 0.03 |
| Interleukin-6, mean (SD) | 3.8 (7.3) | 4.4 (5.9) | 3.9 (7.6) | 3.5 (5.5) | 3.4 (9.3) | 0.64 |
| C-reactive protein, mean (SD) | 4.8 (8.0) | 5.4 (9.1) | 4.7 (7.3) | 4.9 (9.3) | 4.1 (6.1) | 0.42 |
Measured at baseline
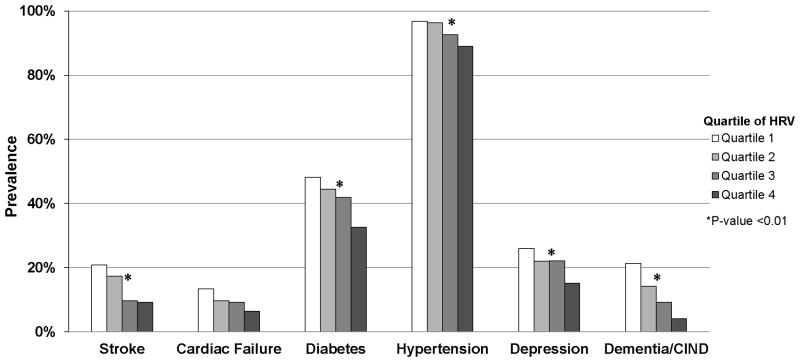
Figure 1.
Unadjusted associations of quartile of heart rate variability, as R bar, with sample co-morbidities at the time of heart rate variability assessment.
Table 3.
Multivariable associations of heart rate variability, as R bar, with cognitive function from linear regression models
| 3MSE* | Unadjusted | Demographic-adjusted | Fully-adjusted | |||
|---|---|---|---|---|---|---|
|
| ||||||
| β (SE) | p-value | β (SE) | p-value | β (SE) | p-value | |
| Heart rate Variability | ||||||
| Quartile 1 | −11.4 (1.4) | <0.01 | −6.5 (1.2) | <0.01 | −4.0 (1.0) | <0.01 |
| Quartile 2 | −6.9 (1.3) | <0.01 | −3.0 (1.2) | 0.01 | −2.0 (1.0) | 0.04 |
| Quartile 3 | −3.0 (1.3) | 0.03 | −1.4 (1.2) | 0.25 | −0.9 (0.9) | 0.35 |
| Quartile 4 | ref | ref | ref | ref | ref | Ref |
| R-squared statistic | 8.7% | 30.4% | 34.4% | |||
| SEVLT† | Unadjusted | Demographic-adjusted | Fully-adjusted | |||
|---|---|---|---|---|---|---|
|
| ||||||
| β (SE) | p-value | β (SE) | p-value | β (SE) | p-value | |
| Heart rate Variability | ||||||
| Quartile 1 | −1.02 (0.33) | <0.01 | −0.07 (0.30) | 0.82 | 0.30 (0.30) | 0.31 |
| Quartile 2 | −0.94 (0.31) | <0.01 | −0.04 (0.29) | 0.90 | 0.19 (0.28) | 0.49 |
| Quartile 3 | −0.09 (0.31) | 0.77 | 0.26 (0.28) | 0.35 | 0.35 (0.27) | 0.20 |
| Quartile 4 | ref | ref | ref | ref | ref | Ref |
| R square | 2.1% | 23.0% | 24.7% | |||
Model 1 is unadjusted; Model 2 additionally adjusts for age, gender, education, marital status; Model 3 additionally adjusts for diabetes, stroke, and elevated depressive symptoms (CESD≥16).
SEVLT: Model 1 is unadjusted; Model 2 additionally adjusts for age, gender, education; Model 3 additionally adjusts for diabetes, stroke, hypertension, and elevated depressive symptoms (CESD≥16).
RESULTS
Our results from Table 1 suggest that participants for whom HRV data was collected (N=869) were significantly younger, had more years of education, were less likely to have had a co-morbidity at baseline such as cardiac failure, stroke, type-2 diabetes, hypertension, dementia/CIND, and elevated depressive symptoms compared to SALSA participants for whom HRV data was not collected.
Of the study sample included in this analysis (Table 2), a total of 58.6% were females, 48.5% were born in Mexico, 60% were married, and the mean years of education was 8 years (SD=5.4). At the time of HRV measurement, the mean age of our study sample was 75.6 years (SD=6.1) with a mean BMI of 29.3 Kg/m2, mean SBP of 142.6 mmHg and a mean DBP of 77.0 mmHg. Reduced R bar (i.e. lower quartile) was associated with older age, being a female, non-married, and with lower mean years of education. Reduced R bar was also associated with higher SBP and higher insulin levels.
In Figure 1, we present the bivariate association of quartile of R bar with vascular-related co-morbidities at the time of HRV measurement. In our sample, a total of 14.3% reported having had a stroke, 9.7% had cardiac failure, 41.8% had type-2 diabetes, 93.7% had hypertension, 12.2% had a diagnosis of dementia/CIND and 21.3% had elevated depressive symptoms, at the time of HRV measurement (data not shown). Reduced R bar (lower quartile) was significantly associated with higher prevalence of stroke, type-2 diabetes, hypertension, dementia/CIND diagnosis, and elevated depressive symptoms (p<0.01). R bar was not associated with the presence of cardiac failure (p>0.05).
Our multivariable analysis of the associations between R bar and cognitive scores on the 3MSE are presented in Table 3. Our results showed that reduced R bar (lower quartile) was associated with worse cognitive scores on the 3MSE. In particular, results from unadjusted models showed that compared to subjects with the highest quartile of R bar, those in quartile 1 had an 11-point lower 3MSE score (p<0.01), those in quartile 2 had a 7-point lower 3MSE score (p<0.01), and those in quartile 3 had a 3-point lower 3MSE score (p=0.03). Adjusting for socio-demographics attenuated the associations but remained significant. In fully-adjusted models, compared to subjects with the highest quartile of R bar, those with quartile 1 had a 4-point lower 3MSE score (p<0.01), those with quartile 2 had a 2-point lower 3MSE score (p=0.04), and those with quartile 3 had a 1-point lower 3MSE score (p=0.35). For these same models, the R-squared statistic was 8.7% in the unadjusted model, 30.4% in the demographic-adjusted model and 34.4% in the fully-adjusted model.
Our multivariable analysis of the associations between quartile of R bar and cognitive scores on the SEVLT are also presented in Table 3. In unadjusted models, having reduced R bar was associated with lower SEVLT scores though the effect estimates were modest. Adjusting for socio-demographics in model 2 and for co-morbidities in model 3 attenuated the associations and became non-significant.
DISCUSSION
In this study, we examined the cross-sectional association between heart rate variability and cognitive performance among community-dwelling elderly Mexican Americans, an ethnic group at high-risk for cardiovascular disease risk factors. We provided evidence that reduced HRV was associated with worse performance on the global test of cognitive function above and beyond traditional cardiovascular risk factors, but was not associated with verbal memory.
The literature has discussed several potential mechanisms through which heart rate variability may influence brain structure and function. For example, the baroflex mechanism regulates blood flow and maintains proper perfusion to vital organs, including the brain, through modulation of the heart rate and contractibility.7,8,35 In other words, cardiac autonomic function and the sympathetic and parasympathetic activity interact to maintain blood pressure within a normal range. Indeed, HRV and blood pressure variability (BPV) have been shown to be inversely associated.7,36 Furthermore, fluctuations in blood pressure (i.e. BPV) are associated with cognitive impairment37,38 and with structural brain changes related to hypertension, such as cerebral white matter lesions,39 and to stroke such as lacunar infarctions.40 Furthermore, HRV may influence cognitive function through cardiac autonomic neuropathy and its associations with type-2 diabetes,10,11 impaired plasma glucose,15 and insulin sensitivity.16 Sensitivity analysis of our main regression models suggest that in our cohort of elderly Latinos, years of education explain the most, among other socio-demographics, of the observed association between heart rate variability and cognitive function. Co-morbidities including diabetes, stroke and elevated depressive symptoms further explain some of the HRV-cognitive function association. Given that education triggers a cascade of behavioral and risk factor change, which in turn may influence co-morbidities, targeting education may provide a potential opportunity for prevention and management. However, given the cross-sectional nature of our study, the interpretation of our findings should be done with caution until future prospective studies confirm the predictive role of HRV and the role of potential underlying mechanisms.
Our findings were consistent with some studies that examined the associations of heart rate variability and cognitive function. For example, cross-sectional results from a sub-sample (N=311) of elderly disabled women in the Women’s Health and Aging Study have shown that the lowest quartile of a HRV index of decreased parasympathetic activity was independently associated with 3 to 6 times greater odds of cognitive impairment based on the Mini Mental State Exam.41 Recent results from the Vietnam Era Twin Registry (N=416) have shown that reduced HRV was associated with worse verbal recall scores but not with visual, learning or memory tests.2 Further reports among patients with mild cognitive impairment (N=42) have shown that reduced HRV (time and frequency measures) was independently associated with increased white matter lesions.42 Finally, results from a case-control study of a small sample of patients admitted for cognitive disturbances have shown that measures of HRV were lower in subjects with Alzheimer’s disease than in those with mild cognitive impairment or cognitively normal.43 In the latter study, HRV was significantly associated with the degree of cognitive impairment. However, our findings were not consistent with those from the Whitehall II cohort study of middle-aged men and women. Results from the Whitehall study showed no cross-sectional or longitudinal associations between HRV and cognitive function or decline; cognition was assessed based on several domains including memory, vocabulary, phonemic and semantic fluency.44
Our study has some limitations that are worth noting. Given the cross-sectional nature of our study, we could not examine the temporality of the association i.e. whether HRV preceded and consequently resulted in cognitive impairment. As such, it may be possible that reduced HRV is a consequence of cardiac autonomic dysfunction that accompanies cognitive impairment or may be a cross-correlate of other factors influencing cognitive impairment. Thus, despite a statistically significant association between reduced HRV and worse cognitive function from our fully-adjusted models, the interpretation of our findings needs to be done with caution. Evaluation of the temporality of the HRV-cognitive function association and the role of other covariates as potential confounders or mediators cannot be determined from our cross-sectional findings. Disentangling the timing of these associations and the role of HRV as a predictor and a potential prognostic factor of cognitive impairment needs to be elucidated in future prospective studies. Furthermore, while we adjusted for potential confounders, we cannot rule out the possibility of residual confounding in the observed associations. In particular, given the role of education in shaping cognitive function, the lack of data on the quality of education may have resulted in residual confounding despite our adjustment for years of education completed. We also acknowledge that HRV measurement in the SALSA sample was not random. Those for whom HRV was measured were younger and healthier than those for whom HRV was not measured. As such we cannot rule out the possibility of selection bias, yet towards the null. Finally, while we had information on many of the co-morbidities that play a role in the link between HRV and cognitive function, such as hypertension and diabetes, we did not have imaging data on our HRV sample. As such, we could not examine whether HRV may also be associated with structural brain changes in our cohort.
However, our study has many strengths. Importantly, our results take advantage of a unique and under-studied ethnic group. Mexican Americans are disproportionately burdened with obesity and type-2 diabetes which are linked to cardiac autonomic neuropathy and are major risk factors for cognitive impairment as well. Exploring these associations will help us better define predictors of cognitive impairment and will help in elucidating underlying subclinical pathways. Furthermore, unlike the vast majority of the literature that included small sample sizes and patient or clinical samples, our data comes from a large population-based study of community-dwelling older adults and our analysis includes a relatively large sample size.
PERSPECTIVES
In conclusion, in our community-dwelling cohort of elderly Mexican Americans, we provided evidence that reduced heart rate variability, indicative of cardiac autonomic dysfunction and reduced parasympathetic control of the heart rate, was cross-sectionally associated with worse performance on the test of global cognitive function. Our findings shed the light on the role of autonomic dysfunction as a predictor of cognitive impairment. Future studies are needed to explore these associations prospectively as well as to explore underlying pathways including brain imaging studies.
Novelty and Significance.
1) What Is New?
The first study to examine the association of heart rate variability and cognitive performance among community-dwelling elderly Mexican Americans.
Our study includes a relatively large sample size that provides accurate estimates of the associations of interest and a well-established measure of heart rate variability.
2) What Is Relevant?
Heart rate variability is a hallmark of cardiac autonomic dysfunction and is associated with major cardiovascular risk factors of cognitive impairment.
Mexican Americans are an understudied ethnic group with a high-risk cardiovascular risk factor profile.
3) Summary
Reduced heart rate variability was associated with worse cognitive performance among community-dwelling older adults.
The association of heart rate variability and cognition persists above and beyond traditional cardiovascular risk factors.
Our findings provide insight on the role of heart rate variability as a subclinical predictor of cognitive impairment.
Acknowledgments
Sources of Funding
This work is supported by grants from the National Institute on Aging (AG12975, AG033751) and National Institute of Diabetes and Digestive and Kidney Diseases (DK60753). Dr. Zeki Al Hazzouri was supported by the American Heart Association/American Stroke Association/American Academy of Neurology Foundation Lawrence M. Brass, M.D. Stroke Research Postdoctoral Fellowship.
Footnotes
Conflict(s) of Interest/Disclosure(s) Statement: None.
References
- 1.Heart rate variability: standards of measurement, physiological interpretation and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Circulation. 1996;93:1043–1065. [PubMed] [Google Scholar]
- 2.Shah AJ, Su S, Veledar E, Bremner JD, Goldstein FC, Lampert R, Goldberg J, Vaccarino V. Is heart rate variability related to memory performance in middle-aged men? Psychosom Med. 2011;73:475–482. doi: 10.1097/PSY.0b013e3182227d6a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.La Rovere MT, Bigger JT, Jr, Marcus FI, Mortara A, Schwartz PJ. Baroreflex sensitivity and heart-rate variability in prediction of total cardiac mortality after myocardial infarction. ATRAMI (Autonomic Tone and Reflexes After Myocardial Infarction) Investigators. Lancet. 1998;351:478–484. doi: 10.1016/s0140-6736(97)11144-8. [DOI] [PubMed] [Google Scholar]
- 4.Tsuji H, Larson MG, Venditti FJ, Jr, Manders ES, Evans JC, Feldman CL, Levy D. Impact of reduced heart rate variability on risk for cardiac events. The Framingham Heart Study. Circulation. 1996;94:2850–2855. doi: 10.1161/01.cir.94.11.2850. [DOI] [PubMed] [Google Scholar]
- 5.Bigger JT, Jr, Fleiss JL, Steinman RC, Rolnitzky LM, Kleiger RE, Rottman JN. Frequency domain measures of heart period variability and mortality after myocardial infarction. Circulation. 1992;85:164–171. doi: 10.1161/01.cir.85.1.164. [DOI] [PubMed] [Google Scholar]
- 6.Liao D, Cai J, Rosamond WD, Barnes RW, Hutchinson RG, Whitsel EA, Rautaharju P, Heiss G. Cardiac autonomic function and incident coronary heart disease: a population-based case-cohort study. The ARIC Study. Atherosclerosis Risk in Communities Study. Am J Epidemiol. 1997;145:696–706. doi: 10.1093/aje/145.8.696. [DOI] [PubMed] [Google Scholar]
- 7.Sloan RP, DeMeersman RE, Shapiro PA, Bagiella E, Chernikhova D, Kuhl JP, Zion AS, Paik M, Myers MM. Blood pressure variability responses to tilt are buffered by cardiac autonomic control. The American journal of physiology. 1997;273:H1427–1431. doi: 10.1152/ajpheart.1997.273.3.H1427. [DOI] [PubMed] [Google Scholar]
- 8.Sloan RP, Shapiro PA, Bagiella E, Myers MM, Gorman JM. Cardiac autonomic control buffers blood pressure variability responses to challenge: a psychophysiologic model of coronary artery disease. Psychosom Med. 1999;61:58–68. doi: 10.1097/00006842-199901000-00010. [DOI] [PubMed] [Google Scholar]
- 9.Singh JP, Larson MG, Tsuji H, Evans JC, O’Donnell CJ, Levy D. Reduced heart rate variability and new-onset hypertension: insights into pathogenesis of hypertension: the Framingham Heart Study. Hypertension. 1998;32:293–297. doi: 10.1161/01.hyp.32.2.293. [DOI] [PubMed] [Google Scholar]
- 10.Carnethon MR, Golden SH, Folsom AR, Haskell W, Liao D. Prospective investigation of autonomic nervous system function and the development of type 2 diabetes: the Atherosclerosis Risk In Communities study, 1987–1998. Circulation. 2003;107:2190–2195. doi: 10.1161/01.CIR.0000066324.74807.95. [DOI] [PubMed] [Google Scholar]
- 11.Carnethon MR, Yan L, Greenland P, Garside DB, Dyer AR, Metzger B, Daviglus ML. Resting heart rate in middle age and diabetes development in older age. Diabetes Care. 2008;31:335–339. doi: 10.2337/dc07-0874. [DOI] [PubMed] [Google Scholar]
- 12.Nahshoni E, Aravot D, Aizenberg D, Sigler M, Zalsman G, Strasberg B, Imbar S, Adler E, Weizman A. Heart rate variability in patients with major depression. Psychosomatics. 2004;45:129–134. doi: 10.1176/appi.psy.45.2.129. [DOI] [PubMed] [Google Scholar]
- 13.Sajadieh A, Nielsen OW, Rasmussen V, Hein HO, Abedini S, Hansen JF. Increased heart rate and reduced heart-rate variability are associated with subclinical inflammation in middle-aged and elderly subjects with no apparent heart disease. Eur Heart J. 2004;25:363–370. doi: 10.1016/j.ehj.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 14.Ziegler D, Zentai CP, Perz S, Rathmann W, Haastert B, Doring A, Meisinger C. Prediction of mortality using measures of cardiac autonomic dysfunction in the diabetic and nondiabetic population: the MONICA/KORA Augsburg Cohort Study. Diabetes Care. 2008;31:556–561. doi: 10.2337/dc07-1615. [DOI] [PubMed] [Google Scholar]
- 15.Panzer C, Lauer MS, Brieke A, Blackstone E, Hoogwerf B. Association of fasting plasma glucose with heart rate recovery in healthy adults: a population-based study. Diabetes. 2002;51:803–807. doi: 10.2337/diabetes.51.3.803. [DOI] [PubMed] [Google Scholar]
- 16.Festa A, D’Agostino R, Jr, Hales CN, Mykkanen L, Haffner SM. Heart rate in relation to insulin sensitivity and insulin secretion in nondiabetic subjects. Diabetes Care. 2000;23:624–628. doi: 10.2337/diacare.23.5.624. [DOI] [PubMed] [Google Scholar]
- 17.Umpierrez GE, Gonzalez A, Umpierrez D, Pimentel D. Diabetes mellitus in the Hispanic/Latino population: an increasing health care challenge in the United States. The American journal of the medical sciences. 2007;334:274–282. doi: 10.1097/MAJ.0b013e3180a6efe3. [DOI] [PubMed] [Google Scholar]
- 18.Haffner SM, Miettinen H, Gaskill SP, Stern MP. Metabolic precursors of hypertension. The San Antonio Heart Study. Arch Intern Med. 1996;156:1994–2001. [PubMed] [Google Scholar]
- 19.Sundquist J, Winkleby MA. Cardiovascular risk factors in Mexican American adults: a transcultural analysis of NHANES III, 1988–1994. Am J Public Health. 1999;89:723–730. doi: 10.2105/ajph.89.5.723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sundquist J, Winkleby MA, Pudaric S. Cardiovascular disease risk factors among older black, Mexican-American, and white women and men: an analysis of NHANES III, 1988–1994. Third National Health and Nutrition Examination Survey. J Am Geriatr Soc. 2001;49:109–116. doi: 10.1046/j.1532-5415.2001.49030.x. [DOI] [PubMed] [Google Scholar]
- 21.Chatterji P, Joo H, Lahiri K. Racial/ethnic- and education-related disparities in the control of risk factors for cardiovascular disease among individuals with diabetes. Diabetes Care. 2012;35:305–312. doi: 10.2337/dc11-1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Whitmer RA, Sidney S, Selby J, Johnston SC, Yaffe K. Midlife cardiovascular risk factors and risk of dementia in late life. Neurology. 2005;64:277–281. doi: 10.1212/01.WNL.0000149519.47454.F2. [DOI] [PubMed] [Google Scholar]
- 23.Haan MN, Mungas DM, Gonzalez HM, Ortiz TA, Acharya A, Jagust WJ. Prevalence of dementia in older latinos: the influence of type 2 diabetes mellitus, stroke and genetic factors. J Am Geriatr Soc. 2003;51:169–177. doi: 10.1046/j.1532-5415.2003.51054.x. [DOI] [PubMed] [Google Scholar]
- 24.Hokanson DE. Autonomic Nervous System (ANS) 2000- Manual. http://www.deh-inc.com/index.cfm?fuseaction=productdetail&productid=32.
- 25.Weinberg CR, Pfeifer MA. An improved method for measuring heart-rate variability: assessment of cardiac autonomic function. Biometrics. 1984;40:855–861. [PubMed] [Google Scholar]
- 26.Teng EL, Chui HC. The Modified Mini-Mental State (3MS) examination. J Clin Psychiatry. 1987;48:314–318. [PubMed] [Google Scholar]
- 27.Tombaugh TN. Test-retest reliable coefficients and 5-year change scores for the MMSE and 3MS. Arch Clin Neuropsychol. 2005;20:485–503. doi: 10.1016/j.acn.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 28.Gonzalez HM, Mungas D, Haan MN. A verbal learning and memory test for English- and Spanish-speaking older Mexican-American adults. Clin Neuropsychol. 2002;16:439–451. doi: 10.1076/clin.16.4.439.13908. [DOI] [PubMed] [Google Scholar]
- 29.Gonzalez HM, Mungas D, Haan MN. A semantic verbal fluency test for English- and Spanish-speaking older Mexican-Americans. Arch Clin Neuropsychol. 2005;20:199–208. doi: 10.1016/j.acn.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 30.The American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2006;29 (Suppl 1):S43–48. [PubMed] [Google Scholar]
- 31.American Heart Association. [Accessed March 19, 2010.];High blood pressure AHA recommendations. http://www.americanheart.org/presenter.jhtml?identifier=4623.
- 32.Gonzalez HM, Haan MN, Hinton L. Acculturation and the prevalence of depression in older Mexican Americans: baseline results of the Sacramento Area Latino Study on Aging. J Am Geriatr Soc. 2001;49:948–953. doi: 10.1046/j.1532-5415.2001.49186.x. [DOI] [PubMed] [Google Scholar]
- 33.Mungas D, Reed BR, Crane PK, Haan MN, Gonzalez H. Spanish and English Neuropsychological Assessment Scales (SENAS): further development and psychometric characteristics. Psychol Assess. 2004;16:347–359. doi: 10.1037/1040-3590.16.4.347. [DOI] [PubMed] [Google Scholar]
- 34.Zeki Al Hazzouri A, Haan MN, Kalbfleisch JD, Galea S, Lisabeth LD, Aiello AE. Life-course socioeconomic position and incidence of dementia and cognitive impairment without dementia in older Mexican Americans: results from the Sacramento area Latino study on aging. Am J Epidemiol. 2011;173:1148–1158. doi: 10.1093/aje/kwq483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Saul JP, Berger RD, Albrecht P, Stein SP, Chen MH, Cohen RJ. Transfer function analysis of the circulation: unique insights into cardiovascular regulation. The American journal of physiology. 1991;261:H1231–1245. doi: 10.1152/ajpheart.1991.261.4.H1231. [DOI] [PubMed] [Google Scholar]
- 36.Sloan RP, Demeersman RE, Shapiro PA, Bagiella E, Kuhl JP, Zion AS, Paik M, Myers MM. Cardiac autonomic control is inversely related to blood pressure variability responses to psychological challenge. The American journal of physiology. 1997;272:H2227–2232. doi: 10.1152/ajpheart.1997.272.5.H2227. [DOI] [PubMed] [Google Scholar]
- 37.Kanemaru A, Kanemaru K, Kuwajima I. The effects of short-term blood pressure variability and nighttime blood pressure levels on cognitive function. Hypertension research: official journal of the Japanese Society of Hypertension. 2001;24:19–24. doi: 10.1291/hypres.24.19. [DOI] [PubMed] [Google Scholar]
- 38.Bellelli G, Pezzini A, Bianchetti A, Trabucchi M. Increased blood pressure variability may be associated with cognitive decline in hypertensive elderly subjects with no dementia. Arch Intern Med. 2002;162:483–484. doi: 10.1001/archinte.162.4.483. [DOI] [PubMed] [Google Scholar]
- 39.Gomez-Angelats E, de La Sierra A, Sierra C, Parati G, Mancia G, Coca A. Blood pressure variability and silent cerebral damage in essential hypertension. Am J Hypertens. 2004;17:696–700. doi: 10.1016/j.amjhyper.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 40.Kukla C, Sander D, Schwarze J, Wittich I, Klingelhofer J. Changes of circadian blood pressure patterns are associated with the occurence of lucunar infarction. Arch Neurol. 1998;55:683–688. doi: 10.1001/archneur.55.5.683. [DOI] [PubMed] [Google Scholar]
- 41.Kim DH, Lipsitz LA, Ferrucci L, Varadhan R, Guralnik JM, Carlson MC, Fleisher LA, Fried LP, Chaves PH. Association between reduced heart rate variability and cognitive impairment in older disabled women in the community: Women’s Health and Aging Study I. J Am Geriatr Soc. 2006;54:1751–1757. doi: 10.1111/j.1532-5415.2006.00940.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Galluzzi S, Nicosia F, Geroldi C, Alicandri A, Bonetti M, Romanelli G, Zulli R, Frisoni GB. Cardiac autonomic dysfunction is associated with white matter lesions in patients with mild cognitive impairment. J Gerontol A Biol Sci Med Sci. 2009;64:1312–1315. doi: 10.1093/gerona/glp105. [DOI] [PubMed] [Google Scholar]
- 43.Zulli R, Nicosia F, Borroni B, Agosti C, Prometti P, Donati P, De Vecchi M, Romanelli G, Grassi V, Padovani A. QT dispersion and heart rate variability abnormalities in Alzheimer’s disease and in mild cognitive impairment. J Am Geriatr Soc. 2005;53:2135–2139. doi: 10.1111/j.1532-5415.2005.00508.x. [DOI] [PubMed] [Google Scholar]
- 44.Britton A, Singh-Manoux A, Hnatkova K, Malik M, Marmot MG, Shipley M. The association between heart rate variability and cognitive impairment in middle-aged men and women. The Whitehall II cohort study. Neuroepidemiology. 2008;31:115–121. doi: 10.1159/000148257. [DOI] [PMC free article] [PubMed] [Google Scholar]