Abstract
An overly aggressive immune response to the intestinal microflora in a genetically susceptible host background has been implicated in the pathogenesis of inflammatory bowel diseases. We measured the impact of a probiotic preparation (SIM) containing inulin on the severity of colitis and on intestinal microflora profiles of HLA-B27-β2-microglobulin transgenic (TG) rats. SIM is a mixture of lactobacilli, bifidobacteria, and inulin. Two-month-old TG rats received either SIM or water. Control TG rats received metronidazole, alone or in combination with SIM, for 8 weeks. Nontransgenic rats received SIM or water. The cecal content was removed for analysis of the intestinal microflora by PCR combined with denaturing gradient gel electrophoresis. The colon was scored for histological evidence of inflammation, colonic myeloperoxidase activity and interleukin-1β RNA levels were measured photometrically or by real-time quantitative PCR. At 4 months, the colonic inflammation of TG rats treated with SIM was histologically diminished compared to that in untreated TG rats (2.2 ± 0.2 versus 2.9 ± 0.1; P ≤ 0.03). The administration of SIM altered the microflora profiles of TG rats by increasing the diversity and stimulating specifically the growth of Bifidobacterium animalis. The probiotic bacteria added to SIM were below the detection level in cecal stool samples at the end of the study period. The administration of SIM resulted in a measurable impact on the cecal microflora profiles of TG rats with attenuation of colitis. The lack of detection of any added probiotic bacteria in the cecal content suggests that prebiotic inulin is the major effective compound.
The precise etiology of inflammatory bowel diseases (IBD) is still unknown (7, 30, 31), but most investigators share the hypothesis that IBD are the result of an overly aggressive immune response to the intestinal microflora on a genetically susceptible host background (31). This hypothesis is supported by a rapidly increasing number of in vitro experiments, animal studies, and human investigations (7).
Most recently, differences in the composition of the intestinal microflora of patients with acute Crohn's disease in comparison to Crohn's disease in remission have been reported (35, 36). Several animal models of experimental colitis develop intestinal inflammation if raised conventionally but remain disease-free under germfree conditions (7). For example, rats transgenic (TG) for human HLA-B27-β2-microglobulin develop severe, immune-mediated colitis if raised in a specific-pathogen-free environment but fail to develop disease if raised germfree (25, 38). The role of the intestinal microflora in the pathogenesis of colitis in this animal model was further demonstrated by the prevention of disease through the administration of metronidazole and the treatment of established colitis with vancomycin and imipenem (24). The preventive effect of metronidazole has been confirmed in a human trial (29). Compelling evidence of a host-dependent immunologic response to residual bacterial flora was provided by Duchmann and colleagues (6), who demonstrated that both local and systemic tolerance toward autologous flora were broken in 2,4,6-trinitrobenzene sulfonic acid (TNBS)-induced colitis, and could be restored by treatment with interleukin-10 (IL-10) or antibodies to IL-12, suggesting a TH1 type of immunoresponse.
Not all bacteria have equal proinflammatory capabilities, as has been demonstrated in gnotobiotic HLA-B27 TG rats (25, 27). More recently, increasing evidence suggests even beneficial effects on chronic intestinal inflammation from commensal bacteria such as several lactobacilli, bifidobacteria, or apathogenic Escherichia coli (12, 33).
Some bacteria are under active investigation for their potentially anti-inflammatory capabilities. In animal models, Lactobacillus spp. were demonstrated to reduce mucosal permeability (19), prevent the onset of colitis, and reverse established intestinal inflammation (34) in IL-10−/− mice and Lactobacillus rhamnosus GG prevents recurrence of intestinal inflammation after antibiotic induction of remission in HLA-B27 TG rats (5).
Probiotic microorganisms are defined as viable nutritional agents conferring benefits to the health of the human host (17). Clinically, beneficial effects of probiotic administration have been demonstrated for the treatment of infectious diarrhea in infants (13), amelioration of side effects of antibiotic therapy (3), and prevention of allergies (2). Clinical trials with different probiotic preparations have also suggested a potential role in the treatment of IBD (33) and have included single-strain preparations (16, 28), as well as a combination of several bacterial species (10). However, the detailed mechanisms by which these bacteria mediate their effects are unknown. Alteration of the composition of the intestinal microflora is only temporary when a probiotic is administered (37), but other effects have been described. Experimental animal and in vitro studies have suggested that probiotic bacteria enhance the intestinal mucosal barrier (20), possibly by an increase in the expression of the genes encoding MUC2 and MUC3 (18). An influence on the intestinal immune system has also been suggested, leading to decreased levels of the proinflammatory cytokines gamma interferon, IL-6, and tumor necrosis factor alpha (32) but also to increased levels of secretory immunoglobulin A (22).
These observations suggest that alteration of the composition of the intestinal microflora with antibiotics or probiotics may influence the course of chronic intestinal inflammation (33). Another approach to modify the bacterial flora is to use prebiotic agents. The polysaccharide inulin is a prebiotic compound that is not absorbed or hydrolyzed in the small intestine (4). In vitro studies show that inulin is fermented mainly by bifidobacteria (9). Administration of inulin to human volunteers on a defined diet has been reported to result in increased numbers of bifidobacteria and reduced bacteroides, clostridial, and fusobacterial populations in feces (8, 14). Welters et al. studied the effect of inulin on patients with pouchitis. Compared to placebo-treated patients, 3 weeks of inulin supplementation led to a reduction of inflammation of the pouch mucosa. Furthermore, decreased numbers of Bacteroides fragilis were noted, but the concentration of bifidobacteria was not commented upon in that study (42).
Studies of the composition of the intestinal microflora have been hampered, however, because the majority of the members of the ecosystem have yet to be cultured under laboratory conditions (39). We used a nucleic acid-based screening method to monitor the impact of treatments on the composition of the microflora to overcome this problem. PCR in combination with denaturing gradient gel electrophoresis (DGGE) detects the numerically predominant members of the microflora without the need for bacterial culture (44).
The aim of our study was to evaluate the anti-inflammatory and microflora-modulating effect of a pre- and probiotic preparation containing inulin and four bacterial species by using the TG HLA-B27 rat model of spontaneous colitis.
MATERIALS AND METHODS
Preparation and administration of the probiotic.
The Symbiotic Instant Mixture (SIM; Nutrichem Diät-Pharma GmbH, Roth, Germany) consists of the prebiotic compound inulin, a polysaccharide, and the probiotic microorganisms L. acidophilus La-5 and Bifidobacterium lactis Bb-12. The manufacturer's claim of a total bacterial concentration of ca. 8 × 107 CFU/ml could be confirmed, and the preparation of the product was standardized according to the company's instructions. Briefly, a covered bowl containing 150 g of a lyophilized powder, mixed with lukewarm nonchlorinated tap water (500 ml), was placed inside an isolated container, which was filled with boiling-hot water; this was followed by incubation for 16 h. Since the bacterial concentration of SIM and the specific activity of metronidazole remained stable for up to 4 days, the drinking-water, probiotic, and antibiotic solutions were replenished twice weekly and were fed ad libitum, leading to a daily concentration of ca. 2 × 109 CFU of probiotic microorganism/rats as calculated by measuring the water consumption of the animals. To reduce the photodegradation of SIM and metronidazole, darkened bottles were used.
Animals.
HLA-B27-β2-microglobulin TG rats and nontransgenic (NT) controls of the same breed (Fischer F344) were obtained from Taconic, Inc. (Germantown, Wis.) and housed individually in isolated, ventilated cages on standard bedding. All rats were fed standard rat chow ad libitum. The use of the animals was approved by the animal care committee of the local government.
Treatment protocols.
TG animals (n = 5/group) at the age of 8 weeks, which is prior to the onset of colitis, received either tap water or SIM for 2 months. To validate the experiments and to confirm the data with previous observations, several control groups were added: (i) NT rats without treatment to serve as negative controls (n = 5); (ii) NT rats with SIM to prove by body weight that the caloric uptake with SIM did not differ from tap water (n = 5); (iii) TG rats treated with metronidazole (n = 5; 50 mg/kg [body weight]; Bayer Vital, Leverkusen, Germany) to confirm the bacterial influence on this model as reported previously (24); and (iv) TG rats (n = 5) treated with SIM and metronidazole (Bayer Vital, Leverkusen, Germany) to test whether modification of the bacterial flora by antibiotics, prebiotics, and probiotics simultaneously would enhance the beneficial effect of the single compound treatment or counteract its effects. After 8 weeks of treatment, the animals were weighed and then killed by CO2 asphyxiation; intestinal tissue was removed for histological analysis of the inflammation and determination of the colonic myeloperoxidase (MPO) activity and the concentration of colonic IL-1β. Cecal contents were removed aseptically for analysis of the microflora.
Histological grading of colitis.
Colonic tissues were removed for histological analysis. The tissues were cut into 5-μm slices (colon and rectum) and stained with hematoxylin and eosin as previously described (25). The sections were scored blindly by two investigators (H. C. Rath and M. Schultz) for histological evidence of inflammation with a scoring system described in detail previously (25). In brief, the tissue samples were assessed for edema, the influx of inflammatory cells, damage to the mucosal architecture, crypt abscesses, and ulcerations on a scale from 0 to 4.
Determination of MPO activity.
Methods of tissue preparation and the assay of MPO activity (units/gram of tissue weight) were described previously (11).
Determination of IL-1β in colonic tissue by quantitative reverse transcription-PCR.
Approximately 1 cm of the colon was taken and IL-1β RNA concentration was measured as described previously (23). In brief, the tissue was placed in an ice-cold RNAlater solution (Ambion, Austin, Tex.). RNA was extracted by using the RNeasy kit (Qiagen, Hilden, Germany) in combination with the Qiagen Shredder kit according to the manufacturer's recommendations. Quantification of cytokine RNA was performed by using a light cycler (Roche Diagnostics GmbH, Mannheim, Germany) according to the manufacturer's recommendations. All primers (IL-1β, F100; IL-1β, R450) were purchased from MWG-Biotech (Ebersberg, Germany). IL-1β values were expressed as units/gram of tissue.
Analysis of the intestinal microflora.
To assess the effect of the treatments on the composition of the intestinal microflora, the cecal content was removed aseptically and stored frozen until analysis.
DNA extraction.
Nucleic acid extraction was performed essentially as described by Walter et al. (41). Briefly, 100 mg of cecal content was weighed into a sterile tube containing 300 mg of sterile zirconium beads (diameter, 0.1 mm) and suspended in 1 ml of TN150 buffer (10 mM Tris-HCl, 150 mM NaCl [pH 8.0]). The suspension was vortexed thoroughly and centrifuged at 14,600 × g for 5 min. The supernatant was discarded, and the pellet washed twice with 1 ml of TN150 buffer. After the final wash, the pellet was suspended in 1 ml of TN150 buffer. The cells were lysed by physical disruption in a mini-bead beater (Biospec Products, Bartlesville, Okla.) at 5,000 rpm for 3 min and placed on ice to cool. Subsequently, the samples were extracted three times with phenol and chloroform-isoamyl alcohol, and the DNA was precipitated with 2 volumes of cold ethanol (−20°C) and 0.1 volume of 3 M sodium acetate and then stored overnight at −20°C. The DNA was collected by centrifugation at 14,600 × g for 20 min at −4°C, and the pellet was dried at 37°C for 1 h. Finally, DNA was dissolved in 30 μl of TE buffer (10 mM Tris-HCl, 1 mM EDTA, [pH 7.5]).
PCR amplification with universal bacterial primers and group-specific primers.
Amplification of target DNA was performed by using TaqDNA polymerase (Roche) and a PCR-Express thermal cycler (Hybaid, Teddington, United Kingdom). PCR amplifications of total bacterial community DNA were carried out with the primer pair HDA1-GC-HDA2 and a PCR thermocycling program as described previously (41). In order to specifically detect lactic acid bacteria in cecal samples, amplifications with group-specific primers were conducted. Lactic acid bacteria-specific primers (Lac primers) Lac1 and Lac2-GC were used according to the procedure reported previously (40). In order to identify the PCR amplicons obtained with the Lac primers and to supplement identifications made by sequencing fragments eluted from gel slices, an identification ladder was prepared consisting of the following reference strains listed in order of migration distance in the DGGE gel: L. plantarum (ATCC 14917T), L. johnsonii (ATCC 33200T), L. gasseri (ATCC 33323T), L. acidophilus (ATCC 4356T), L. crispatus (ATCC 33820T), L. salivarius (ATCC 11741T), L. ruminis (ATCC 277780T), L. rhamnosus (ATCC 7469T), and L. reuteri (DSM 20016T).
DGGE analysis of PCR products.
The DGGE was performed as previously described (41) by using a 16- by 16-cm by 1-mm thick 6% polyacrylamide gel (acrylamide/bisacrylamide ratio = 37.5:1) containing a 30 to 55% (HDA1-GC-HDA2 amplicons) or a 30 to 45% (Lac-amplicons) gradient of urea and formamide. Representative (consensus) profiles of each rat group were prepared by pooling 1 μl of DNA extracted from each rat. The pooled DNA was used as a template in PCR. This enabled the group profiles to be compared intragel (38). Therefore, interpretations of similarity and/or diversity of profiles between different electrophoretic gels did not occur. Profiles were compared by using QuantityOne, version 4.2, software which is part of the Discovery Series (Bio-Rad Laboratories) and were performed by using Dice's similarity coefficient (Dsc) analysis.
Identification of bacteria by sequencing PCR-DGGE fragments.
DNA fragments of interest were excised aseptically from the polyacrylamide gel, placed in 100 μl of diffusion buffer (QIAEX II; 0.5 M ammonium acetate, 10 mM magnesium acetate, 1 mM EDTA, 0.1% sodium dodecyl sulfate [pH 8.0]), and incubated overnight at 4°C to allow elution of the DNA. Recovery of DNA was performed by using the QIAEX II kit (Qiagen) according to the manufacturer's instructions. Cloning and sequencing of the eluted fragments was achieved by using the protocols described by Knarreborg et al. (15). Sequencing was carried out by the Centre for Gene Research, University of Otago, Dunedin, New Zealand. The sequences (∼200 bp) retrieved were compared to GenBank database by using the basic local alignment search tool (BLAST) algorithm (40).
Culture of SIM bacteria.
SIM powder, labeled as containing L. acidophilus, L. delbrueckii subsp. bulgaricus, Streptococcus thermophilus, B. lactis Bb-12, and inulin, was suspended in sterile water, and 10-fold dilutions were prepared to 10−6. Portions (100 μl) of each dilution were spread plated onto plates of M17 agar (Difco Laboratories, Detroit, Mich.), Lactobacillus MRS agar (Difco), and Rogosa SL agar (Difco). Sets of plates were incubated aerobically, microaerophilically, or anaerobically at 30, 37, or 42°C. L. acidophilus and B. lactis Bb-12 were the only bacteria that were isolated. DNA was extracted from pure cultures of these bacteria as described previously (41) and used to generate PCR amplicons that served as markers in DGGE gels.
Statistical analysis.
Data are expressed as means ± the standard error of the means. Statistical analysis for significant differences was performed by using analysis of variance, the Student t test for parametric samples, and the Mann-Whitney rank sum test for nonparametric samples (Sigma Stat, version 2.03; SPSS, Inc.).
RESULTS
Impact on severity of colitis.
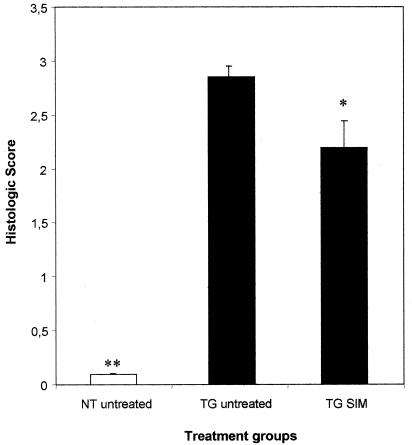
TG rats developed a spontaneous chronic colitis as revealed by loose stool and histological evidence of inflammation (2.9 ± 0.1 versus 0.1 ± 0.1; P ≤ 0.001 versus NT; Fig. 1) at the age of 4 months, confirmed by MPO activity (0.741 ± 0.149 versus 0.214 ± 0.034; P ≤ 0.001 versus NT) and IL-1β level (122 × 10−6 ± 9 × 10−6 versus 9 × 10−6 ± 4 × 10−6; P ≤ 0.008 versus NT). Prophylactic treatment with SIM reduced the severity of spontaneous chronic colitis in TG rats compared to untreated TG controls, as demonstrated by histology (2.2 ± 0.2 versus 2.9 ± 0.1; P ≤ 0.03; Fig. 1 and 2). The influence of the commensal flora on the chronic colitis was confirmed by the beneficial effect of preventive administration of metronidazole on the degree of histological intestinal inflammation (2.0 ± 0.1 versus 2.9 ± 0.1; P < 0.001 versus TG rats) confirmed by colonic IL-1β (5 × 10−6 ± 1 × 10−6 versus 122 × 10−6 ± 9 × 10−6; P < 0.008 versus TG rats). However, the simultaneous treatment with metronidazole and SIM did not result in additional benefit compared to metronidazole or SIM alone (data not shown). There was no difference in body weight between NT animals treated with SIM and those receiving plain water, suggesting that SIM did not influence caloric uptake (data not shown).
FIG. 1.
Histologic score of the colon from TG rats and normal controls after 8 weeks of treatment. The rats were either treated with the pre- and probiotic combination (SIM), or with plain drinking water. *, P ≤ 0.03; **, P ≤ 0.001 versus TG water.
FIG. 2.
(A) Colonic inflammation in untreated TG rats, characterized by massive infiltration of inflammatory cells, mucosal edema, and disturbed crypt architecture (magnification, ×100) (B) hematoxylin-and-eosin-stained section from the colon of TG rats treated with SIM (magnification, ×100).
Impact on the intestinal microflora.
Comparison of cecal microflora profiles of individual rats. The Dsc values (Table 1) show that the cecal microflora profiles varied between animals within each group. Profiles of control animals were, on average, about 70% similar. The cecal microflora profiles were very similar (80%) in NT rats fed SIM, but there was considerable variation between the profiles of TG rats fed SIM (56% similar). Profiles of TG rats administered metronidazole or metronidazole plus SIM showed animal-to-animal variation similar to that of the control animals.
TABLE 1.
Dsc values from a comparison of the cecal microflora profiles of rats
| Group (treatment) | Mean intragroup, rat-to-rat variation (Dsc [SEM])a |
|---|---|
| NT (untreated) | 68.3 (2.0) |
| TG (untreated) | 69.4 (4.1) |
| NT (SIM) | 81.5 (3.7) |
| TG (SIM) | 56.0 (8.1) |
| TG (metronidazole) | 74.1 (5.8) |
| TG (SIM + metronidazole) | 75.2 (1.9) |
Values are expressed as percentages (Dice's similarity coefficient).
Comparison of cecal microflora profiles of treatment groups.
Group consensus profiles generated from pooled DNAs revealed alterations in the composition of the cecal microflora according to treatment (Table 2). Sample replicates intragel were >80% similar. In summary, feeding SIM had a greater effect on the consensus profiles than did metronidazole.
TABLE 2.
Dsc values from the comparison of group consensus microflora profiles
| Comparison (treatment) | Dsc (%) between groups |
|---|---|
| TG vs NT | 79.1 |
| TG vs TG (SIM) | 65.3 |
| TG vs TG (metronidazole) | 80.7 |
| TG vs TG (SIM + metronidazole) | 57.9 |
| NT vs NT (SIM) | 76.2 |
| NT (SIM) vs TG (SIM) | 68.5 |
TG and NT consensus profiles differed mainly in the presence of a fragment originating from B. animalis in the TG group (Fig. 3, H2/4). Furthermore, rats fed SIM had B. animalis fragments that were of greater staining intensity than those of non-SIM-fed animals (Fig. 3, H5/14 relative to H2/4). The interpretation of increased staining intensity was appropriate because a fragment of an unidentified bacterium (Fig. 3, arrows) had the same intensity of staining in all of the profiles and thus acted as an internal control. The increased intensity of staining was not due to the B. lactis Bb-12 content of SIM because the DNA fragment representative of this culture migrated to a different location in the gel than that of B. animalis (Fig. 4). Interestingly, the TG consensus profile of rats administered metronidazole lacked a fragment originating from a Clostridium species (Fig. 3, H2/3). Other sequence analyses of DNA fragments eluted from the DGGE gels showed that many of the bacteria were of yet-to-be-cultivated species of gut origin (Fig. 3 and Table 3).
FIG. 3.
DGGE gel showing microflora profiles generated from pooled DNA samples (n = 5) with the universal bacterial primers HDA1-GC and HDA2. Lane 1, NT control; lane 2, TG control; lane 3, NT control; lane 4, NT SIM; lane 5, TG SIM; lane 6, TG control; lane 7, TG metronidazole; lane 8, TG SIM-metronidazole; lane 9, TG SIM; lane 10, TG control. DNA fragments whose bacterial origin was identified are labeled. Arrows indicate a DNA fragment of even staining intensity present in all profiles.
FIG. 4.
DGGE gel showing profiles generated from DNA samples with the universal bacterial primers HDA1-GC and HDA2. Lane 1, TG SIM; lane 2, B. lactis Bb-12 culture from SIM; lane 3, L. acidophilus cultured from SIM. Arrow indicates B. animalis DNA fragment.
TABLE 3.
Identification of bacterial origins of DNA fragments in DGGE gels by BLAST search
| Clone | Identification (origin) | Identity (%) |
|---|---|---|
| H1/1 | Uncultured bacterium clone p-57-a5 (pig gut); uncultured bacterium adhufec 25 (human gut); unidentified rumen bacterium RCR10 (rumen); unidentified rumen bacterium RC20 (rumen) | 98 |
| H1/2 | Unidentified rumen bacterium (rumen) | 94 |
| H2/3 | Clostridium species strain ASF 356 (mouse gut) | 99 |
| H2/4 | B. animalis | 100 |
| H3/5 | Unidentified bacterium clone p-195-05 (pig gut) | 99 |
| H3/6 | Uncultured bacterium clone p-195-05 (pig gut) | 100 |
| H4/7 | Uncultured bacterium clone 616-a5 (pig gut); uncultured bacterium clone p-2743-24E5 (pig gut); uncultured bacterium clone p-1590-c5 (pig gut); uncultured bacterium p-2418-55G5 (pig gut); uncultured bacterium adhufec 225 (human gut); uncultured bacterium adhufec 150 (human gut); uncultured bacterium clone HuCA13 (human gut) | 97 |
| H4/7A | Flexistipes group UNSWp12 (mouse gut) | 98 |
| H4/8 | Unidentified rumenbacterium 12-111 (rumen) | 95 |
| H5/10 | Uncultured bacterium clone HuCB6 (human gut) | 98 |
| H5/11 | Uncultured Bacteroides species (human gut) | 100 |
| H5/13 | Uncultured bacterium clone p-1763-b3 (pig gut) | 89 |
| H5/14 | B. animalis | 100 |
Detection of lactic acid bacteria and SIM isolates in cecal samples of rat groups.
Group consensus profiles generated from pooled DNAs by using the Lac primers showed a high degree of similarity between the profiles of the various groups (Fig. 5). Resident L. johnsonii and L. reuteri were present in all groups and were identified by reference to the identification ladder. Other unidentified lactic acid bacteria were present in all of the animals. L. acidophilus (Fig. 4) fed with SIM was not detectable in the profiles.
FIG. 5.
DGGE gel showing profiles generated from pooled DNA samples (n = 5) by using the lactic acid bacteria group-specific primers Lac1 and Lac2-GC. Lane 1, Lactobacillus identification ladder; lane 2, NT control; lane 3, TG control; lane 4, TG metronidazole; lane 5, NT SIM; lane 6, TG SIM; lane 7, TG SIM-metronidazole; lane 8, L. acidophilus cultured from SIM.
DISCUSSION
Histological observations and to some extent of colonic IL-1β levels showed that SIM administration reduced the severity of colitis in the TG rats relative to control animals. The reduced severity was associated with alterations to the microflora profiles generated from large bowel samples collected from the animals.
The cecal microflora of TG animals differed from that of NT rats principally in the presence of a DNA fragment originating from B. animalis. The intensity of staining of this fragment was increased in TG and NT animals administered SIM, probably reflecting the prebiotic effect of inulin, known to stimulate bifidobacterial numbers when administered as a dietary supplement (9). B. animalis was at undetectable levels in the bowel of NT rats but apparently increased to detectable numbers when SIM was administered. The major effect of SIM administration, however, was in increasing the diversity among microflora profiles of TG rats. This may reflect poor homeostatic regulation in the bowel ecosystem of TG rats, relative to that of NT rats, which was therefore more susceptible to change when inulin in the SIM was administered. It is unlikely that the bacterial components of SIM were important in reducing the severity of colitis because only two of the four bacterial species used in the preparation of the product could be cultivated, and neither of these species (L. acidophilus and B. lactis Bb-12) were detected in the microflora profiles when universal bacterial PCR primers were used. This indicated that they were <109 CFU/g of cecal content (44). Nor was L. acidophilus detected in the cecal profiles when group-specific PCR primers were used that have a lower limit of detection of 106 CFU/g (40). These findings suggest inulin as the primarily effective compound in this preparation. An anti-inflammatory effect of feeding prebiotics was also shown for this animal model in a recent study by Yacyshyn et al. with carboxymethyl cellulose (43). The beneficial effect of a modulation of the colonic bacterial composition on the chronic intestinal inflammation in HLA-B27 TG rats is consistent with previous findings. It has been shown that adding B. vulgatus to a cocktail with five defined anaerobic bacterial strains introduced in germfree HLA-B27 TG rats resulted in severe colitis and gastritis, whereas the same bacterial cocktail without B. vulgatus had no proinflammatory effect (25). Moreover, variation of the cecal bacterial composition by creating a self-filling blind loop revealed a relative overgrowth of obligate anaerobic bacteria, especially Bacteroides spp., with a consecutive exacerbation of colitis (26). Madsen et al. demonstrated in IL-10−/− mice a beneficial effect of broad-spectrum antibiotic therapy, including metronidazole, on spontaneous chronic colitis by reducing Clostridium spp. below detectable levels and increasing Lactobacillus spp. (21).
Metronidazole administration reduces the severity of colitis (24), probably due to its antibacterial activity leading to the removal of a DNA fragment originating from a rodent Clostridium species from the bacterial profiles in rats. Metronidazole has also been reported to have, itself, anti-inflammatory activity, which could explain the more stable composition of the microflora of rats administered both SIM and metronidazole relative to SIM alone (1).
Three avenues of research emerge from the present study. First, the role of B. animalis in the etiology of colitis should be investigated in gnotobiotic rat studies. This species was undetectable in NT rats fed standard laboratory chow but was detectable in TG animals fed the same diet and even increased in animals fed SIM, leading to ameliorated intestinal inflammation. Perhaps the inflamed conditions pertaining to the TG bowel provide conditions in which the bifidobacteria proliferate. Second, the role of clostridia in the pathogenesis of colitis should be investigated since a Clostridium species was detected in untreated animals but not in metronidazole-treated rats. Third, a dose-response investigation of dietary supplementation with inulin could be helpful as to its ability to modulate the composition of the bowel microflora in relation to the severity of colitis.
REFERENCES
- 1.Arndt, H., K. D. Palitzsch, M. B. Grisham, and D. N. Granger. 1994. Metronidazole inhibits leukocyte-endothelial cell adhesion in rat mesenteric venules. Gastroenterology 106:1271-1276. [DOI] [PubMed] [Google Scholar]
- 2.Bienenstock, J., R. E. Riley, G. S. Neigh, S. Waserman, and P. Keith. 2002. Probiotics in the management and prevention of atopy. Clin. Rev. Allergy Immunol. 22:275-285. [DOI] [PubMed] [Google Scholar]
- 3.Cremonini, F., S. Di Caro, E. C. Nista, F. Bartolozzi, G. Capelli, G. Gasbarrini, and A. Gasbarrini. 2002. Meta-analysis: the effect of probiotic administration on antibiotic-associated diarrhea. Aliment. Pharmacol. Ther. 16:1461-1467. [DOI] [PubMed] [Google Scholar]
- 4.Crittenden, R. G. 1999. Prebiotics, p. 141-156. In G. W. Tannock (ed.), Probiotics: a critical review. Horizon Scientific Press, Wymondham, United Kingdom.
- 5.Dieleman, L. A., M. S. Goerres, A. Arends, D. Sprengers, C. Torrice, F. Hoentjen, W. Grenther, and R. B. Sartor. 2003. Lactobacillus GG prevents recurrence of colitis in HLA-B27 transgenic rats after antibiotic treatment. Gut 52:370-376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Duchmann, R., E. Schmitt, P. Knolle, K. Meyer zum Büschenfelde, and M. Neurath. 1996. Tolerance toward resident intestinal flora in mice is abrogated in experimental colitis and restored by treatment with interleukin-10 or antibodies to interleukin-12. Eur. J. Immunol. 26:934-938. [DOI] [PubMed] [Google Scholar]
- 7.Fiocchi, C. 1998. Inflammatory bowel disease: etiology and pathogenesis. Gastroenterology 115:182-205. [DOI] [PubMed] [Google Scholar]
- 8.Gibson, G. R., E. R. Beatty, X. Wang, and J. H. Cummings. 1995. Selective stimulation of bifidobacteria in the human colon by oligofructose and inulin. Gastroenteology 108:975-982. [DOI] [PubMed] [Google Scholar]
- 9.Gibson, G. R., and X. Wang. 1994. Bifidogenic properties of different types of fructo-oligosaccharides. Food. Microbiol. 11:491-498. [Google Scholar]
- 10.Gionchetti, P., F. Rizzello, A. Venturi, P. Brigidi, D. Matteuzzi, G. Bazzocchi, G. Poggioli, M. Miglioli, and M. Campieri. 2000. Oral bacteriotherapy as maintenance treatment in patients with chronic pouchitis: a double-blind, placebo-controlled trial. Gastroenterology 119:305-309. [DOI] [PubMed] [Google Scholar]
- 11.Grisham, M. B., J. N. Benoit, and D. N. Granger. 1990. Assessment of leukocyte involvement during ischemia and reperfusion of intestine. Methods Enzymol. 186:729-742. [DOI] [PubMed] [Google Scholar]
- 12.Guarner, F., and J. R. Malagelada. 2003. Gut flora in health and disease. Lancet 361:512-519. [DOI] [PubMed] [Google Scholar]
- 13.Isolauri, E. 2003. Probiotics for infectious diarrhea. Gut 52:436-437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kleesen, B., B. Sykura, H.-J. Zunft, and M. Blaut. 1997. Effects of inulin and lactose on fecal microflora, microbial activity, and bowel habit in elderly constipated persons. Am. J. Clin. Nutr. 65:1397-1402. [DOI] [PubMed] [Google Scholar]
- 15.Knarreborg, A., M. A. Simon, R. M. Engberg, B. B. Jensen, and G. W. Tannock. 2002. Effects of dietary fat source and subtherapeutic levels of antibiotic on the bacterial community in the ileum of broiler chickens at various ages. Appl. Environ. Microbiol. 68:5981-5984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kruis, W., P. Fric, M. Stolte, and The Mutaflor Study Group. 2001. Maintenance of remission in ulcerative colitis is equally effective with Escherichia coli Nissle 1917 and with standard mesalamine. Gastroenterology 120:680. [Google Scholar]
- 17.Lilly, D. M., and R. H. Stilwell. 1965. Probiotics: growth promoting factors produced by microorganisms. Science 47:747-748. [DOI] [PubMed] [Google Scholar]
- 18.Mack, D. R., S. Michail, S. Wei, L. McDougall, and M. A. Hollingsworth. 1999. Probiotics inhibit enteropathogenic Escherichia coli adherence in vitro by inducing intestinal mucin gene expression. Am. J. Physiol. 276:G941-G950. [DOI] [PubMed] [Google Scholar]
- 19.Madsen, K. L., J. S. Doyle, L. D. Jewell, M. M. Taverini, and R. N. Fedorak. 1999. Lactobacillus species prevents colitis in interleukin-10 gene-deficient mice. Gastroenterology 116:1107-1114. [DOI] [PubMed] [Google Scholar]
- 20.Madsen, K. L., A. Cornish, P. Soper, C. McKaigney, H. Jijon, C. Yachimec, J. Doyle, L. Jewell, and C. De Simone. 2001. Probiotic bacteria enhance murine and human intestinal epithelial barrier function. Gastroenterology 121:580-591. [DOI] [PubMed] [Google Scholar]
- 21.Madsen, K. L., J. S. Doyle, M. M. Tavernini, L. D. Jewell, R. P. Rennie, and R. N. Fedorak. 2000. Antibiotic therapy attenuates colitis in interleukin 10 gene-deficient mice. Gastroenterology 118:1094-1105. [DOI] [PubMed] [Google Scholar]
- 22.Malin, M., H. Suomalainen, M. Saxelin, and E. Isolauri. 1996. Promotion of IgA immune response in patients with Crohn's disease by oral bacteriotherapy with Lactobacillus GG. Ann. Nutr. Metabol. 40:137-145. [DOI] [PubMed] [Google Scholar]
- 23.Obermeier, F., N. Dunger, L. Deml, H. Herfarth, and J. Schölmerich. 2002. CpG motifs of bacterial DNA exacerbate colitis of dextran sodium-treated mice. Eur. J. Immunol. 32:2084-2092. [DOI] [PubMed] [Google Scholar]
- 24.Rath, H. C., M. Schultz, R. Freitag, L. A. Dieleman, F. Li, H. J. Linde, J. Schölmerich, and R. B. Sartor. 2001. Different subsets of enteric bacteria induce and perpetuate experimental colitis in rats and mice. Infect. Immun. 69:2277-2285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rath, H. C., H. H. Herfarth, J. S. Ikeda, W. B. Grenther, T. E. Hamm, Jr., E. Balish, J. D. Taurog, R. E. Hammer, K. H. Wilson, and R. B. Sartor. 1996. Normal luminal bacteria, especially Bacteroides species, mediate chronic colitis, gastritis, and arthritis in HLA-B27/human β2-microglobulin transgenic rats. J. Clin. Investig. 98:945-953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rath, H. C., J. S. Ikeda, H. J. Linde, J. Schölmerich, K. H. Wilson, and R. B. Sartor. 1999. Varying cecal bacterial loads influences colitis and gastritis in HLA-B27 transgenic rats. Gastroenterology 116:310-319. [DOI] [PubMed] [Google Scholar]
- 27.Rath, H. C., K. H. Wilson, and R. B. Sartor. 1999. Differential induction of colitis and gastritis in HLA-B27 transgenic rats selectively colonized with Bacteroides vulgatus of Escherichia coli. Infect. Immun. 67:2969-2974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rembacken, B. J., A. M. Snelling, P. M. Hawkey, D. M. Chalmers, and A. T. Axon. 1999. Non-pathogenic Escherichia coli versus mesalazine for the treatment of ulcerative colitis: a randomised trial. Lancet 354:635-639. [DOI] [PubMed] [Google Scholar]
- 29.Rutgeerts, P., M. Hiele, K. Geboes, M. Peeters, F. Penninckx, R. Aerts, and R. Kerremans. 1995. Controlled trial of metronidazole treatment for prevention of Crohn's recurrence after ileal resection. Gastroenterology 108:1617-1621. [DOI] [PubMed] [Google Scholar]
- 30.Sartor, R. B. 1999. Microbial factors in the pathogenesis of Crohn's disease, ulcerative colitis, and experimental intestinal inflammation, p. 153-178. In J. B. Kirsner (ed.), Inflammatory bowel disease. The Williams & Wilkins Co., Baltimore, Md.
- 31.Sartor, R. B. 2000. New therapeutic approaches to Crohn's disease. N. Engl. J. Med. 342:1664-1666. [DOI] [PubMed] [Google Scholar]
- 32.Schultz, M., H. J. Linde, N. Lehn, K. Zimmermann, J. Grossmann, W. Falk, and J. Schölmerich. 2003. Immunomodulatory consequences of oral administration of Lactobacillus rhamnosus strain GG (L. GG) in healthy volunteers. J. Dairy. Res. 70:165-173. [DOI] [PubMed] [Google Scholar]
- 33.Schultz, M., and H. C. Rath. 2002. The role of probiotics in inflammatory bowel disease, p. 175-239. In G. W. Tannock (ed.), Probiotics and prebiotics: where are we going? Horizon Scientific Press, Wymondham, United Kingdom.
- 34.Schultz, M., C. Veltkamp, L. A. Dieleman, R. B. Wyrick, S. L. Tonkonogy, and R. B. Sartor. 2002. Lactobacillus plantarum 299v in treatment and prevention of spontaneous colitis in IL-10-deficient mice. Inflamm. Bowel Dis. 8:71-80. [DOI] [PubMed] [Google Scholar]
- 35.Seksik, P., L. Rigottier-Gois, G. Gramet, M. Sutren, P. Pochart, P. Marteau, R. Jian, and J. Doré. 2003. Alterations of the dominant faecal bacterial groups in patients with Crohn's disease of the colon. Gut 52:237-242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Swidsinski, A., A. Ladhoff, A. Pernthaler, S. Swidsinski, V. Loening-Baucke, M. Ortner, J. Weber, U. Hoffmann, S. Schreiber, M. Dietel, and H. Lochs. 2002. Mucosal flora in inflammatory bowel disease. Gastroenterology 122:44-54. [DOI] [PubMed] [Google Scholar]
- 37.Tannock, G. W., K. Munro, H. J. Harmsen, G. W. Welling, J. Smart, and P. K. Gopal. 2000. Analysis of the fecal microflora of human subjects consuming a probiotic product containing Lactobacillus rhamnosus DR20. Appl. Environ. Microbiol. 66:2578-2588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Taurog, J. D., J. A. Richardson, J. T. Croft, W. A. Simmons, M. Zhou, J. L. Fernandes-Sueiro, E. Balish, and R. E. Hammer. 1994. The germfree state prevents development of gut and joint inflammatory disease in HLA-B27 transgenic rats. J. Exp. Med. 180:2359-2364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vaughan, E. E., F. Schut, H. G. Heilig, E. G. Zoetendal, W. M. De Vos, and A. D. Akkermans. 2000. A molecular view of the intestinal ecosystem. Curr. Issues Intestinal Microbiol. 1:1-12. [PubMed] [Google Scholar]
- 40.Walter, J., C. Hertel, G. W. Tannock, C. M. Lis, K. Munro, and W. P. Hammes. 2001. Detection of Lactobacillus, Pediococcus, Leuconostoc, and Weissella species in human feces by using group-specific PCR primers and denaturing gradient gel electrophoresis. Appl. Environ. Microbiol. 67:2578-2585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Walter, J., G. W. Tannock, A. Tilsala-Timisjarvi, S. Rodtong, D. M. Loach, K. Munro, and T. Alatossava. 2000. Detection and identification of gastrointestinal Lactobacillus species by using dentauring gradient gel electrophoresis and species-specific PCR primers. Appl. Environ. Microbiol. 66:297-303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Welters, C. F., E. Heinemann, F. B. Thunnissen, A. E. van den Bogaard, P. B. Soeters, and C. G. Baeten. 2002. Effect of dietary inulin supplementation on inflammation of pouch mucosa in patients with an ileal pouch-anal anastomosis. Dis. Colon Rectum 45:621-627. [DOI] [PubMed] [Google Scholar]
- 43.Yacyshyn, B., G. Armstrong, G. W. Tannock, and M. B. Bowen-Yacyshyn. 2003. Carboxy-Methycellulose decreasescolonic inflammation and changes bacterial flora in the HLA-B27 transgenic rat. Gastroenterology 118:T1152. [Google Scholar]
- 44.Zoetendal, E. G., A. D. Akkermans, and W. M. De Vos. 1998. Temperature gradient gel electrophoresis analysis of 16S rRNA from human fecal samples reveals stable and host-specific communities of active bacteria. Appl. Environ. Microbiol. 64:3854-3859. [DOI] [PMC free article] [PubMed] [Google Scholar]