Abstract
Cytokine levels were compared between schistosomiasis patients affected by intense fibrosis defined by ultrasound examination and graded from F-0 to F-3. The concentrations of interleukin-1β (IL-1β), IL-4, IL-5, IL-10, IL-13, gamma interferon, and tumor necrosis factor alpha (TNF-α) were determined by enzyme-linked immunosorbent assay of serum samples. Levels of IL-4, IL-5, and TNF-α in the sera of F-3 patients were significantly higher than those found in F-0 individuals, while levels of IL-13 were lower. Levels of IL-4, IL-5, and TNF-α in serum were significantly higher in F-3 males than in F-0 males or F-3 females. Conversely, levels of IL-13 were significantly lower in F-3 females than in F-0 females and males.
The pathology resulting from parasitic infection caused by the helminth Schistosoma mansoni includes an acute inflammatory granulomatous reaction, followed by a tissue repair process that has evolved to block the toxic effects of parasite eggs trapped in host tissues (especially in the liver) and prevent extensive cell damage (25). If this healing process is excessive, it may lead to the development of more severe forms of disease, accompanied by severe clinical manifestations, such as hepatosplenomegaly, portal-vein hypertension, gastrointestinal bleeding, and heart or kidney failure, ultimately caused by an extensive fibrosis in hepatic periportal spaces designated Symmers' fibrosis. Direct evaluation of the degree of fibrosis development was enabled by abdominal ultrasonography, an accurate, noninvasive, and reliable technique and a more precise procedure than clinical examination (14, 20). The reasons why severe fibrosis occurs only in a fraction of infected individuals, even when they are submitted to the same environmental conditions, are still unknown.
Epidemiologic studies of populations living in areas where S. mansoni is endemic have suggested that fibrosis development is related to infection intensity and duration, gender-related factors, and inherited factors (21, 24). A field study of a Sudanese population demonstrated the prevalence of high fibrosis grades (F-2 and F-3) in young adult and old males but only of F-2 in young adult females, suggesting that males have a tendency to earlier development of higher fibrosis grades (24).
A codominant major gene located in the 5q31-q33 region (Sm1) controls human resistance to the parasite, influencing the intensity of infection, probably by acting on cytokine production (23, 28). Another codominant major gene, named Sm2, that accounts for familial distribution of the fibrosis-affected phenotype and gender-dependent penetrance was mapped on chromosome 6, in the 6q21-q22 region, closely linked to the gamma interferon (IFN-γ) receptor alpha gene (10).
Several Th2 cytokines, such as interleukin-4 (IL-4), IL-5, IL-10, and IL-13, were associated with the murine immune response against S. mansoni (2, 3, 5, 12, 16, 22, 29, 32). Recent studies have demonstrated that tumor necrosis factor alpha (TNF-α) and some Th2 cytokines, such as IL-4 and IL-13, increase fibrosis development in experimental models of S. mansoni infection (2, 4). IFN-γ seems to act in the opposite direction, since it elicits antiproliferative and antifibrogenic activities (33).
However, there are still large gaps in our understanding of the role of lymphocytes and effector cells, and their cytokines, in initiating and extending the fibrotic process. Differences in severity between individuals could be related to diverse levels of cytokine production, and therefore it is essential to examine the cytokine profile associated with the physiopathology of severe fibrosis development in human schistosomiasis mansoni.
This study was performed with Brazilians who were born and live in rural areas where the helminth S. mansoni is highly endemic: the Caraí district, located in the central region of the Jequitinhonha Valley in the northeastern part of Minas Gerais State, and Caatinga do Moura, a village in the northeastern part of Bahia State (20, 28). Different degrees of hepatic fibrosis were diagnosed by ultrasound in accordance with World Health Organization guidelines (14, 18). All infections were treated with oxamniquine. Studies were conducted with the informed consent of all blood donors, following approval of the patient protocols used throughout this study by the Ethics Committee of Human Subjects. Grades of fibrosis were determined by echography with a Hitachi linear-array scanner with a 3.5-MHz transducer. Degrees of involvement were estimated on the basis of previously defined criteria, in accordance with World Health Organization guidelines, which range from absence of fibrosis (grade F-0) to severe fibrosis (grade F-3) (14, 20).
Blood samples were collected by intravenous puncture without anticoagulant. Sera were collected after centrifugation, aliquoted, and stored frozen at −80°C until tested.
Levels of IL-1β, IL-4, IL-5, IL-10, IL-13, IFN-γ, and TNF-α in serum were determined by enzyme-linked immunosorbent assay (ELISA) with specific monoclonal antibody (MAb) pairs. Microplates (Nunc, Roskilde, Denmark) were sensitized overnight with an anti-IL-1β MAb (Genzyme, Cambridge, Mass.), an anti-TNF-α MAb (Genzyme), an anti-IL-4 MAb (Mabtech, Nacka, Sweden), an anti-IL-5 MAb (Pharmingen, San Diego, Calif.), an anti-IL-10 MAb (Pharmingen), an anti-IL-13 MAb (Mabtech), or an anti-IFN-γ MAb (Mabtech). Nonspecific binding was prevented by incubating the plates with 3% bovine serum albumin (Sigma, St. Louis, Mo.) in phosphate-buffered saline (PBS). Plates were incubated overnight with 100 μl of a 1:2 dilution of serum samples in PBS, 2% bovine serum albumin, and standard cytokines (Pharmingen and R&D, Minneapolis, Minn.). Plates were then washed four times with 0.05% Tween in PBS and incubated with a rabbit anti-IL-1β antibody (Genzyme), a rabbit anti-TNF-α antibody (Genzyme), a biotinylated anti-IL-4 MAb (Mabtech), a biotinylated anti-IL-5 MAb (Pharmingen), a biotinylated anti-IL-10 MAb (Pharmingen), a biotinylated anti-IL-13 antibody (Mabtech), or a biotinylated anti-IFN-γ MAb (Mabtech) for 4 h. Plates were washed and incubated for 2 h with alkaline phosphatase-conjugated goat anti-rabbit immunoglobulin G (Pierce) or alkaline phosphatase conjugated to streptavidin. Finally, plates were washed four times and incubated with p-nitrophenyl phosphate (Sigma). The A405 was read in a microplate reader (Bio-Rad, Hercules, Calif.). The sensitivity of the tests was 10 to 20 pg/ml.
Data were analyzed statistically by the Mann-Whitney test or the Kruskal-Wallis test, with the level of significance set at P ≤ 0.05.
Although many studies have revealed the involvement of T cells and effector cells in the human immune response against S. mansoni (1, 7-9, 28, 30), the correlation between the outcomes of different clinical forms of this parasitic disease and the establishment of distinct cytokine secretion patterns has not been completely defined. Analysis of cytokines in sera from a great number of individuals with different degrees of hepatic fibrosis, related to distinct fibrosis levels determined by echography, is one way to address this issue. In the present paper, involvement of the immune response in fibrosis development and the cytokine patterns presented by patients with no hepatic fibrosis (F-0) were compared to those of patients with more severe fibrosis (F-3).
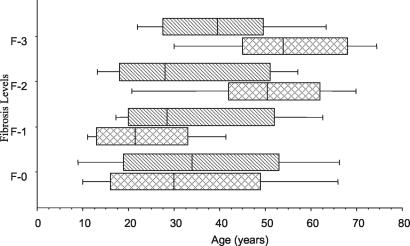
We focused our interest especially on polar groups, i.e., the F-3 group (n = 34) on the one hand and the F-0 group (n = 114) on the other hand. The other patients were characterized as F-1 (n = 36) and F-2 (n = 31). Figure 1 shows the distribution of the individuals analyzed in this study by age, by Symmers' fibrosis grade, and by gender. In the F-2 and F-3 grades, the mean age of fibrosis development is clearly divergent between the genders (P ≤ 0.05, Kruskal-Wallis test), as females had moderate (F-2) or intense (F-3) fibrosis, especially at more advanced ages, while males had the same frequency of fibrosis grades independent of age. This observation is similar to other studies of Brazilian and Sudanese populations (20, 24).
FIG. 1.
Distribution of schistosomiasis patients by fibrosis intensity, age, and gender. Patients were grouped by gender (males, striped; females, crosshatched), and fibrosis was graded by echography. Vertical lines represent the median values, boxes represent the 25th to the 75th percentiles, and horizontal lines represent the 10th to the 90th percentiles.
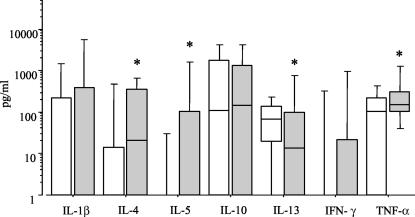
Results shown in Fig. 2 demonstrate different patterns of serum cytokine expression between individuals not affected (F-0) and those with extensive fibrosis (F-3). Levels of IL-4, IL-5, and TNF-α in sera from F-3 patients were statistically significantly higher (P ≤ 0.05, Mann-Whitney test) than those in sera from F-0 patients. Conversely, IL-13 levels were significantly lower in patients with advanced fibrosis.
FIG. 2.
Serum cytokine profiles related to fibrosis intensity. Levels of IL-1-β, IL-4, IL-5, IL-10, IL-13, IFN-γ, and TNF-α were determined by ELISA in serum samples from individuals with schistosomiasis and intense Symmers' fibrosis (F-3) (grey bars) or nonaffected individuals (F-0) (open bars). Horizontal lines represent the median values, boxes represent the 25th to the 75th percentiles, and vertical lines represent the 10th to the 90th percentiles. Asterisks indicate statistically significant differences (P ≤ 0.05).
Previous studies have revealed that Th2 cells, and their cytokines, are required for fibrosis development in an experimental model (19) and that TNF-α and IL-4 displayed profibrotic activities (2). Studies with human T-cell clones revealed that TNF-α is necessary to stimulate in vitro granuloma formation (30) and that low IFN-γ production and high TNF-α production are associated with severe fibrosis (17). The results presented here point out the association of high levels of IL-4, IL-5, and TNF-α with the development of Symmers' fibrosis. On the other hand, IL-13 levels were significantly lower in patients with extensive fibrosis. Our results also show that no significant differences appeared for IL-1β, IL-10, and IFN-γ.
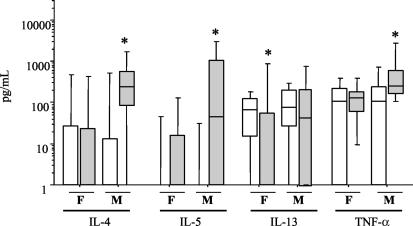
As we found significant differences among males and females with higher fibrosis levels (Fig. 1), we also analyzed serum cytokine patterns by gender (Fig. 3). These results revealed completely different patterns among males and females, based on fibrosis grades. Significantly high levels of IL-4, IL-5, and TNF-α were observed in F-3 male patients, while the levels of these cytokines in F-3 females were equivalent to those in nonaffected patients.
FIG. 3.
Male and female serum cytokine profiles related to fibrosis intensity. Levels of IL-4, IL-5, IL-13, and TNF-α were determined by ELISA in serum samples from males (M) and females (F) with schistosomiasis and intense Symmers' fibrosis (F-3) (grey bars) or nonaffected persons (F-0) (open bars). Horizontal lines represent the median values, boxes represent the 25th to the 75th percentiles, and vertical lines represent the 10th to the 90th percentiles Asterisks indicate statistically significant differences (P ≤ 0.05).
In fact, previous epidemiological studies described differences in fibrosis development between the genders (20, 24). Nonimmune physiological factors, such as steroid hormones, could have an important role in the orientation of immune response in females, leading to modulation of cytokine production and consequently to slow fibrosis development. It was demonstrated that progesterone favors the switch to Th2 to the detriment of Th1 responses (6). Estradiol seems to modulate TNF-α production and increase IL-10 production, in contrast to our results (11). A gender-dependent specific immune response during chronic human schistosomiasis haematobia was also described with high IL-10 and TGF-β production and low TNF-α and IFN-γ production by females (27). Our results demonstrated that S. mansoni-infected females with intense fibrosis have lower IL-4, IL-5, and TNF-α levels in serum; this finding suggested that other mediators may be involved in the development of Symmers' fibrosis in females. It is interesting that males are affected by severe fibrosis predominantly and early and that high levels of profibrotic cytokines were observed in F-3 males. It is well documented in the literature that TNF-α and IL-4 are able to stimulate collagen synthesis (15, 31), leading us to suggest that these cytokines are involved in fibrosis development in S. mansoni-infected male patients. It also seems that these cytokines do not contribute to fibrosis development in females. Other mediators may account for fibrosis in females, who are affected at a lower intensity and frequency than are males, perhaps owing to lower levels of profibrotic cytokines.
Conversely, the level of IL-13 was significantly lower in F-3 females, while F-3 males had levels equivalent to those of nonaffected individuals.
The gender-related cytokine patterns associated with Symmers' fibrosis grades, shown in this work, may lead to a better understanding of the immunological mechanisms involved in fibrosis development in schistosomiasis and a variety of other chronic diseases. The localization of cytokine receptor genes such as those for IL-13R and IL-2Rγ in the Xq24 and Xq13 regions of chromosome X (13, 26) may help us to explain the observed gender-dependent differences in immune mediators associated with fibrosis revealed in this work.
Acknowledgments
We thank the Fundação Nacional de Saúde (especially Ludgero) for support in the areas of endemicity and the medical staff of the Faculdade de Medicina do TriÂngulo Mineiro for examination of patients from the area of endemicity of Caatinga do Moura. We are grateful to all volunteers from the areas of endemicity.
REFERENCES
- 1.Araujo, M. I., A. R. de Jesus, O. Bacellar, E. Sabin, E. Pearce, and E. M. Carvalho. 1996. Evidence of a T helper type 2 activation in human schistosomiasis. Eur. J. Immunol. 26:1399-1403. [DOI] [PubMed] [Google Scholar]
- 2.Cheever, A. W., M. E. Williams, T. A. Wynn, F. D. Finkelman, R. A. Seder, T. M. Cox, S. Hieny, P. Caspar, and A. Sher. 1994. Anti-IL-4 treatment of Schistosoma mansoni-infected mice inhibits development of T cells and non-B, non-T cells expressing Th2 cytokines while decreasing egg-induced hepatic fibrosis. J. Immunol. 153:753-759. [PubMed] [Google Scholar]
- 3.Chensue, S. W., P. D. Terebuh, K. S. Warmington, S. D. Hershey, H. L. Evanoff, S. L. Kunkel, and G. I. Higashi. 1992. Role of IL-4 and IFN-γ in Schistosoma mansoni egg-induced hypersensitivity granuloma formation: orchestration, relative contribution, and relationship to macrophage function. J. Immunol. 148:900-906. [PubMed] [Google Scholar]
- 4.Chiaramonte, M. G., D. D. Donaldson, A. W. Cheever, and T. A. Wynn. 1999. An IL-13 inhibitor blocks the development of hepatic fibrosis during a T-helper type 2-dominated inflammatory response. J. Clin. Investig. 104:777-785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chiaramonte, M. G., L. R. Schopf, T. Y. Neben, A. W. Cheever, D. D. Donaldson, and T. A. Wynn. 1999. IL-13 is a key regulatory cytokine for Th2 cell-mediated pulmonary granuloma formation and IgE responses induced by Schistosoma mansoni eggs. J. Immunol. 162:920-930. [PubMed] [Google Scholar]
- 6.Choi, B. C., K. Polgar, L. Xiao, and J. A. Hill. 2000. Progesterone inhibits in-vitro embryotoxic Th1 cytokine production to trophoblast in women with recurrent pregnancy loss. Hum. Reprod. 15(Suppl.)1:46-59. [DOI] [PubMed] [Google Scholar]
- 7.Colley, D. G. 1981. T lymphocytes that contribute to the immunoregulation of granuloma formation in chronic murine schistosomiasis. J. Immunol. 126:1465-1468. [PubMed] [Google Scholar]
- 8.Contigli, C., B. L. Doughty, J. C. Cone, and A. M. Goes. 1994. Recognition of different Schistosoma mansoni antigens by specific human T cell clones. Cell. Immunol. 154:77-87. [DOI] [PubMed] [Google Scholar]
- 9.Couissinier-Paris, P., and A. J. Dessein. 1995. Schistosoma-specific helper T cell clones from subjects resistant to infection by Schistosoma mansoni are Th0/2. Eur. J. Immunol. 25:2295-2302. [DOI] [PubMed] [Google Scholar]
- 10.Dessein, A. J., D. Hillaire, N. E. Elwali, S. Marquet, Q. Mohamed-Ali, A. Mirghani, S. Henri, A. A. Abdelhameed, O. K. Saeed, M. M. Magzoub, and L. Abel. 1999. Severe hepatic fibrosis in Schistosoma mansoni infection is controlled by a major locus that is closely linked to the interferon-gamma receptor gene. Am. J. Hum. Genet. 65:709-721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gilmore, W., L. P. Weiner, and J. Correale. 1997. Effect of estradiol on cytokine secretion by proteolipid protein-specific T cell clones isolated from multiple sclerosis patients and normal control subjects. J. Immunol. 158:446-451. [PubMed] [Google Scholar]
- 12.Grzych, J. M., E. Pearce, A. Cheever, Z. A. Caulada, P. Caspar, S. Heiny, F. Lewis, and A. Sher. 1991. Egg deposition is the major stimulus for the production of Th2 cytokines in murine schistosomiasis mansoni. J. Immunol. 146:1322-1327. [PubMed] [Google Scholar]
- 13.Guo, J., F. Apiou, M. P. Mellerin, B. Lebeau, Y. Jacques, and S. Minvielle. 1997. Chromosome mapping and expression of the human interleukin-13 receptor. Genomics 42:141-145. [DOI] [PubMed] [Google Scholar]
- 14.Hatz, C., J. M. Jenkins, Q. M. Ali, M. F. Abdel-Wahab, G. G. Cerri, and M. Tanner. 1992. A review of the literature on the use of ultrasonography in schistosomiasis with special reference to its use in field studies. 2. Schistosoma mansoni. Acta Trop. 51:15-28. [DOI] [PubMed] [Google Scholar]
- 15.He, Y., and W. Liu. 1996. The preliminary research on the relationship between TNF- and egg-induced granuloma and hepatic fibrosis of schistosomiasis japonica. J. Tongji Med. Univ. 16:205-208. [DOI] [PubMed] [Google Scholar]
- 16.Henderson, G. S., X. Lu, T. L. McCurley, and D. G. Colley. 1992. In vivo molecular analysis of lymphokines involved in the murine immune response during Schistosoma mansoni infection. II. Quantification of IL-4 mRNA, IFN-γ mRNA, and IL-2 mRNA levels in the granulomatous livers, mesenteric lymph nodes, and spleens during the course of modulation. J. Immunol. 148:2261-2269. [PubMed] [Google Scholar]
- 17.Henri, S., C. Chevillard, A. Mergani, P. Paris, J. Gaudart, C. Camilla, H. Dessein, F. Montero, N. E. Elwali, O. K. Saeed, M. Magzoub, and A. J. Dessein. 2002. Cytokine regulation of periportal fibrosis in humans infected with Schistosoma mansoni: IFN-γ is associated with protection against fibrosis and TNF-α with aggravation of disease. J. Immunol. 169:929-936. [DOI] [PubMed] [Google Scholar]
- 18.Homeida, M., S. Ahmed, A. Dafalla, S. Suliman, I. Eltom, T. Nash, and J. L. Bennett. 1988. Morbidity associated with Schistosoma mansoni infection as determined by ultrasound: a study in Gezira, Sudan. Am. J. Trop. Med. Hyg. 39:196-201. [DOI] [PubMed] [Google Scholar]
- 19.Kaplan, M. H., J. R. Whitfield, D. L. Boros, and M. J. Grusby. 1998. Th2 cells are required for the Schistosoma mansoni egg-induced granulomatous response. J. Immunol. 160:1850-1856. [PubMed] [Google Scholar]
- 20.Lambertucci, J. R., G. F. Cota, R. A. Pinto-Silva, J. C. Serufo, R. Gerspacher-Lara, S. C. Drummond, C. M. Antunes, V. Nobre, and A. Rayes. 2001. Hepatosplenic schistosomiasis in field-based studies: a combined clinical and sonographic definition. Mem. Inst. Oswaldo Cruz 96(Suppl.):147-150. [DOI] [PubMed] [Google Scholar]
- 21.Lambertucci, J. R., R. Gerspacher-Lara, R. A. Pinto-Silva, M. M. Barbosa, R. Teixeira, H. F. Barbosa, J. C. Serufo, D. F. Resende, S. C. Drummond, and A. A. Rayes. 1996. The Queixadinha Project: morbidity and control of schistosomiasis in an endemic area in the northeast of Minas Gerais, Brazil. Rev. Soc. Bras. Med. Trop. 29:127-135. [DOI] [PubMed] [Google Scholar]
- 22.Lukacs, N. W., C. L. Addison, J. Gauldie, F. Graham, K. Simpson, R. M. Strieter, K. Warmington, S. W. Chensue, and S. L. Kunkel. 1997. Transgene-induced production of IL-4 alters the development and collagen expression of T helper cell 1-type pulmonary granulomas. J. Immunol. 158:4478-4484. [PubMed] [Google Scholar]
- 23.Marquet, S., L. Abel, D. Hillaire, H. Dessein, J. Kalil, J. Feingold, J. Weissenbach, and A. J. Dessein. 1996. Genetic localization of a locus controlling the intensity of infection by Schistosoma mansoni on chromosome 5q31-q33. Nat. Genet. 14:181-184. [DOI] [PubMed] [Google Scholar]
- 24.Mohamed-Ali, Q., N. E. Elwali, A. A. Abdelhameed, A. Mergani, S. Rahoud, K. E. Elagib, O. K. Saeed, L. Abel, M. M. Magzoub, and A. J. Dessein. 1999. Susceptibility to periportal (Symmers) fibrosis in human Schistosoma mansoni infections: evidence that intensity and duration of infection, gender, and inherited factors are critical in disease progression. J. Infect. Dis. 180:1298-1306. [DOI] [PubMed] [Google Scholar]
- 25.Phillips, S. M., W. A. Reid, B. L. Doughty, and A. G. Bentley. 1980. The immunologic modulation of morbidity in schistosomiasis. Studies in athymic mice and in vitro granuloma formation. Am. J. Trop. Med. Hyg. 29:820-831. [DOI] [PubMed] [Google Scholar]
- 26.Puck, J. M., S. M. Deschenes, J. C. Porter, A. S. Dutra, C. J. Brown, H. F. Willard, and P. S. Henthom. 1993. The interleukin-2 receptor gamma chain maps to Xq13.1 and is mutated in X-linked severe combined immunodeficiency, SCIDX1. Hum. Mol. Genet. 2:1099-1104. [DOI] [PubMed] [Google Scholar]
- 27.Remoue, F., D. To Van, A. M. Schacht, M. Picquet, O. Garraud, J. Vercruysse, A. Ly, A. Capron, and G. Riveau. 2001. Gender-dependent specific immune response during chronic human schistosomiasis haematobia. Clin. Exp. Immunol. 124:62-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rodrigues, V., Jr., K. Piper, P. Couissinier-Paris, O. Bacelar, H. Dessein, and A. J. Dessein. 1999. Genetic control of schistosome infections by the SM1 locus of the 5q31-q33 region is linked to differentiation of type 2 helper T lymphocytes. Infect. Immun. 67:4689-4692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Secor, W. E., S. J. Stewart, and D. G. Colley. 1990. Eosinophils and immune mechanisms. VI. The synergistic combination of granulocyte-macrophage colony-stimulating factor and IL-5 accounts for eosinophil-stimulation promoter activity in Schistosoma mansoni-infected mice. J. Immunol. 144:1484-1489. [PubMed] [Google Scholar]
- 30.Silva-Teixeira, D. N., C. Contigli, and A. M. Goes. 1998. Cytokine profile associated to effector functions of human T cell clones specific for Schistosoma mansoni antigens. Hum. Immunol. 59:219-224. [DOI] [PubMed] [Google Scholar]
- 31.Tiggelman, A. M., W. Boers, C. Linthorst, M. Sala, and R. A. Chamuleau. 1995. Collagen synthesis by human liver (myo)fibroblasts in culture: evidence for a regulatory role of IL-1β, IL-4, TGF-β and IFN-γ. J. Hepatol. 23:307-317. [PubMed] [Google Scholar]
- 32.Wynn, T. A., A. W. Cheever, D. Jankovic, R. W. Poindexter, P. Caspar, F. A. Lewis, and A. Sher. 1995. An IL-12-based vaccination method for preventing fibrosis induced by schistosome infection. Nature 376:594-596. [DOI] [PubMed] [Google Scholar]
- 33.Zhang, L., J. Mi, Y. Yu, H. Yao, H. Chen, M. Li, and X. Cao. 2001. IFN-γ gene therapy by intrasplenic hepatocyte transplantation: a novel strategy for reversing hepatic fibrosis in Schistosoma japonicum-infected mice. Parasite Immunol. 23:11-17. [DOI] [PubMed] [Google Scholar]