Abstract
Searching for a mechanism underlying autoimmunity in autism, we postulated that gliadin peptides, heat shock protein 60 (HSP-60), and streptokinase (SK) bind to different peptidases resulting in autoantibody production against these components. We assessed this hypothesis in patients with autism and in those with mixed connective tissue diseases. Associated with antigliadin and anti-HSP antibodies, children with autism and patients with autoimmune disease developed anti-dipeptidylpeptidase I (DPP I), anti-dipeptidylpeptidase IV (DPP IV [or CD26]) and anti-aminopeptidase N (CD13) autoantibodies. A significant percentage of autoimmune and autistic sera were associated with elevated immunoglobulin G (IgG), IgM, or IgA antibodies against three peptidases, gliadin, and HSP-60. These antibodies are specific, since immune absorption demonstrated that only specific antigens (e.g., DPP IV absorption of anti-DPP IV), significantly reduced IgG, IgM, and IgA antibody levels. For direct demonstration of SK, HSP-60, and gliadin peptide binding to DPP IV, microtiter wells coated with DPP IV were reacted with SK, HSP-60, and gliadin. They were then reacted with anti-DPP IV or anti-SK, anti-HSP, and antigliadin antibodies. Adding SK, HSP-60, and gliadin peptides to DPP IV resulted in 27 to 43% inhibition of the DPP IV-anti-DPP IV reaction, but DPP IV-positive peptides caused 18 to 20% enhancement of antigen-antibody reactions. We propose that (i) superantigens (e.g., SK and HSP-60) and dietary proteins (e.g., gliadin peptides) in individuals with predisposing HLA molecules bind to aminopeptidases and (ii) they induce autoantibodies to peptides and tissue antigens. Dysfunctional membrane peptidases and autoantibody production may result in neuroimmune dysregulation and autoimmunity.
Autism is a developmental disorder of unknown etiology. Several factors have been implicated in its pathogenesis, including genetic, environmental, immunological, and neurological elements (14, 20, 62). Immune abnormalities in autism include changes in the numbers and activities of macrophages, T cells, B cells, and natural killer cell activity (53, 54). In addition, a shift occurs from T helper 1 to T helper 2 T cells in autism as evidenced by a decrease in the production of inteleukin-2 (IL-2) and gamma interferon, but there is an increase in the production of IL-4 (20). Another study reported that the innate and adaptive immune responses in children with autism were associated with tumor necrosis factor alpha, IL-1β, and/or IL-6 values >2 standard deviations (SD) above the control mean (26).
To better understand possible origins, autoimmune mechanisms and a theory have been proposed and investigated as underlying causes of autism (50). Immunogenetic analyses reveal that genes long implicated in lupus (15) and arthritis (46) are significantly increased in autistic populations (49, 55-57). The frequency of the complement C4B-null allele gene increases in autistic individuals, and there is an unusually high rate of autoimmune disorders in a family with an autistic child (34). Further larger-scale analyses revealed in families with autistic children the occurrence of autoimmune disease in first- and second-degree relatives (10). In general, increased prevalence of autoimmune disease in family members is common among probands with autoimmune disorders (4, 38, 48). If increased familial autoimmune disease in autistic probands were verified, then additional research into immunologic contributions to the pathophysiology of autism would seem to be warranted.
Membrane peptidases and the immune system.
Cell surface peptidases such as aminopeptidase N (CD13), aminopeptidase I (or dipeptidylpeptidase I [DPP I]), and dipeptidylpeptidase IV (DPP IV) play key roles in growth and differentiation of lymphocytes and leukemia or lymphoma cells (2, 29, 30, 31, 63). They cleave peptide mediators, resulting in activation or inactivation, and function as receptors as well as signal transduction and adhesion molecules (40). CD13 is expressed on stem cells and most developmental stages of myeloid cells (12, 39). During earliest stages of differentiation, T or B cells are CD13+ but become negative after maturation. In the intestinal brush border, 6 to 8% of protein is CD13, involved in terminal degradation of small peptides, amino acid scavenging, and inactivation of endorphins and enkephalins in synaptic membranes of the nervous system (32). CD13 is also the major receptor for the transmissible virus that causes severe gastroenteritis in piglets (11) and for coronavirus 229E that causes upper respiratory infections in humans (61).
DPP I is a cysteine protease found in lung, myelomonocytic cells, cytotoxic T cells, and mast cells (59). DPP I is apparently specialized in producing peptides that are presented in class I major histocompatibility complex molecules, which participate in antigen presentation (31, 63). DPP IV or CD26 is a closely related family of glycoproteins expressed on cell surfaces and through the plasma membrane. It is localized on epithelial brush border membranes, in the intestinal mucosa, and it cleaves X-Pro dipeptides from the NH2 terminus of several proteins (3, 27, 33).
Due to the key role that the membrane-bound DPP IV plays in T-cell-mediated immune responses and cytokine production, this enzyme has been analyzed in several autoimmune diseases, such as rheumatoid arthritis and systemic lupus erythematosus (36, 37). DPP IV is a receptor for streptokinase (SK) on rheumatoid synovial fibroblasts via the LTSRPA amino acid sequence (18). Binding of SK, the bacterial protein, to DPP IV resulted in the appearance of anti-SK and anti-DPP IV autoantibodies in patients with myocardial infarction treated with SK (8, 19). Based on these observations and the potent immunogenicity of SK in patients with autoimmune disease, SK could bind to DPP IV and induce significant levels of anti-SK and anti-DPP IV antibody production. We have recently detected antibodies to nine different, neuron-specific antigens in sera of autistic children. These antibodies bound to different encephalitogenic molecules (i.e., milk butyrophilin, Chlamydia pneumoniae and streptococcus M protein, as well as neurological antigens) (51). Chlamydia, streptococci, and many other infectious agents produce molecules called superantigens or heat shock proteins (HSPs), which can modulate host immune functions. Bacterial superantigens can transactivate the endogenous superantigens by stressful stimuli, including exposure to oxidative radicals, heavy metals, anoxia, and infection (28, 35, 60, 64). Induction of endogenous superantigens by infections might exert a profound impact on the immune response and nudge it into an autoimmune condition (16, 47).
Given these observations, and since HSPs are a potent immunogenic protein, we hypothesized that HSPs or dietary peptides may bind to DPP IV and other peptidases and induce autoantibody production. We assessed this hypothesis in a group of healthy control subjects compared to patients with (i) autism or (ii) mixed connective tissue disease. Our data suggest a potential role for HSP-60 and dietary peptides in this process. A definitive relationship between infections, dietary peptides, and autism has not been established. The present study was designed to identify epitopes from HSPs and gliadin that could bind to aminopeptidases in tissues and on lymphocyte receptors, resulting in autoantibody production and possible autoimmunity in autism.
MATERIALS AND METHODS
Patients.
Blood samples from 50 subjects (33 males and 17 females), 3 to 14 years of age (mean of 7.2 years), with a diagnosis of autism were sent by different clinicians to our laboratory for immunological examination. The clinical diagnosis of autism was made according to the DSM-III-R criteria, established by the American Psychiatric Association (Washington, D.C.), as well as by a developmental pediatrician, a pediatric neurologist, and/or a licensed psychologist. Samples were excluded if their medical histories included head injury, evidence of gliomas, failure to thrive, and other known factors that may contribute to abnormal development. Blood samples from 50 patients with confirmed diagnosis of mixed connective tissue disease (31 females and 19 males), 36 to 75 years of age with an antinuclear antibody (ANA) titer of 640 or greater and Sm/RnP speckled pattern chromosome negative were selected from our collection sera, preserved at −70°C. For comparison, serum samples from 50 healthy controls (25 children age 3 to 14 years and 25 adults age 36 to 75 years) with negative ANA titers and no known autoimmune diseases were included. The test requests were properly documented and kept in a confidential file. All persons gave their informed consent and allowed inclusion of their data in this report without disclosure of their identity.
Peptides. (i) Gliadin peptides.
Gliadin peptide QQLPQPQQPQQSFPQQQPF and Chlamydia trachomatis HSP-60 peptide LKQIAAHAGKEGAIIFQQVM (high-performance liquid chromatography grade) were synthesized by Bio-Synthesis, Inc. (Lewisville, Tex.).
(ii) Proteins.
DPP IV (CD26), aminopeptidase I, aminopeptidase N (CD13), SK, lipopolysaccharide (LPS), and human serum albumin (HSA) were purchased from Sigma (St. Louis, Mo.).
(iii) Antibodies.
Antibodies to DPP IV, DPP I, aminopeptidase N, SK, HSP-60, and gliadin peptides were prepared in rabbits according to standard protocols (23) by Cocalico Biologicals, Inc. (Reamstown, Pa.). These polyclonal antibodies were purified by affinity chromatography on protein A-Sepharose (17).
ELISA.
Enzyme-linked immunosorbent assay (ELISA) was used for testing antibodies against different antigens in the sera of patients with autism and autoimmune disease and with control subjects. Antigens and peptides were dissolved in methanol at a concentration of 1.0 mg/ml and then diluted 1:100 in 0.1 M carbonate-bicarbonate buffer (pH 9.5), and 50 μl was added to each well of a polystyrene flat-bottom ELISA plate. Plates were incubated overnight at 4°C and then washed three times with 200 μl of Tris-buffered saline (TBS) containing 0.05% Tween 20, pH 7.4. The nonspecific binding of immunoglobulins (Igs) was prevented by adding a mixture of 1.5% bovine serum albumin (BSA) and 1.5% gelatin in TBS and then incubating this mixture for 2 h at room temperature and then overnight at 4°C. Plates were washed as described above, and then serum samples diluted 1:200 in 1% HSA in TBS containing 1-mg/ml IgG Fc fragments (to avoid reactivity of specific antibodies with rheumatoid factors) were added to duplicate wells and incubated for 2 h at room temperature. Sera from patients with autoimmune disorders with known high titers of IgG, IgM, and IgA against DPP IV, gliadin, or HSP peptides were used in dilutions of 1:200 to 1:1,600 to construct a standard curve to rule out nonspecific antibody activities. Plates were washed, and then alkaline phosphatase-conjugated goat anti-human IgG, IgM, or IgA F(ab′)2 fragments (KPI, Gaithersburg, Md.) at an optimal dilution of 1:400 to 1:2,000 in 1% HSA-TBS was added to each well; the plates were then incubated for an additional 2 h at room temperature. After washing five times with TBS-Tween buffer, the enzyme reaction was started by adding 100 μl of paranitrophenylphosphate in 0.1 ml of diethanolamine buffer (1 mg/ml) containing 1 mM MgCl2 and sodium azide, pH 9.8. The reaction was stopped 45 min later with 50 μl of 1 N NaOH. The optical density was read at 405 nm (OD405) with a microtiter reader. To detect nonspecific binding, several control wells contained all reagents except human serum or wells were coated with different tissue antigens and other reagents were used. Coefficients of intra-assay and interassay variations for IgG, IgM, and IgA against DPP IV, DPP I, and CD13 were less than 10%.
Calculation of optimal serum dilution.
Two sera from healthy controls with low levels of antibodies to DPP IV, gliadin, and HSP peptides, two sera from patients with autoimmune disease; and two sera from autistic children with known high titers of antibodies were used to construct standard control curves. These sera were diluted 1:25 to 1:800. At dilutions of 1:50 to 1:400, the standard curves for samples with autoimmune disease or autism were linear (OD of 0.4 to 2.2) and antibodies to these antigens were not detected in healthy control sera (OD of <0.2). Hence, antibody detection in autistic and autoimmune sera was performed at 1:200 dilutions in the appropriate buffer.
Absorption of sera with specific and nonspecific antigens and peptides.
Three autistic patients' sera containing high levels of IgG and IgA antibodies to DPP IV (OD in ELISA of >0.5) and three control sera with insignificant levels of IgG and IgA antibodies were used in inhibition studies. In different test tubes, 1 ml of each serum sample was preincubated with 100 μl containing 100 μg of either HSA, LPS, DPP IV, DPP I, CD13, HSP-60, or gliadin peptides. After mixing, the tubes were kept for 1 h in a 37°C water bath followed by a 4-h incubation at 4°C and then centrifuged at 3,000 × g for 10 min. The supernatant was used for measuring antibody levels and comparing the ELISA OD of unabsorbed and absorbed sera with nonspecific and specific antigens.
Possible binding of SK, gliadin, and HSP-60 peptides to DPP IV.
Since interaction of DPP IV with SK has been shown to be associated with SK and anti-DPP IV autoantibodies (13), we sought out the possible binding of other peptides to DPP IV, DPP I, and CD13. A series of ELISA experiments were carried out to establish the binding specificity of peptides to DPP IV and other peptidases. The plates were coated with DPP IV first and then with BSA or HSA for inhibition of nonspecific binding. SK, gliadin, and HSP-60 were then added. To demonstrate binding of SK, gliadin, and HSP-60 to DPP IV, purified rabbit, anti-DPP IV, anti-SK, antigliadin, anti-HSP-60, and combined anti-DPP IV-anti-SK (anti-DPP IV + anti-SK), anti-DPP IV + antigliadin, and anti-DPP IV + anti-HSP antibodies were added to each of the different wells coated with either BSA, HSA, DPP IV + HSA, DPP IV + SK, DPP IV + gliadin, and DPP IV + HSP-60 (see Table 3).
TABLE 3.
Percent elevation of antibodies to DPP IV, DPP I, CD13, gliadin peptidase, and HSP-60 in controls and patients with autism and autoimmune disease
| Antigen | % Ig elevation
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IgG
|
IgM
|
IgA
|
||||||||||
| Control children | Autism | Control adults | Autoimmune | Control children | Autism | Control adults | Autoimmune | Control children | Autism | Control adults | Autoimmune | |
| DPP IV | 10 | 54 | 14 | 64 | 8 | 50 | 8 | 46 | 6 | 44 | 4 | 58 |
| DPP I | 8 | 56 | 14 | 60 | 10 | 46 | 16 | 54 | 10 | 46 | 14 | 56 |
| CD13 | 8 | 40 | 6 | 28 | 6 | 18 | 6 | 18 | 12 | 48 | 8 | 50 |
| Gliadin peptide | 12 | 42 | 18 | 62 | 8 | 50 | 20 | 48 | 6 | 44 | 18 | 52 |
| HSP-60 | 16 | 36 | 22 | 52 | 8 | 44 | 18 | 48 | 14 | 50 | 14 | 42 |
Plates were incubated for 1 h at 37°C and washed five times for removal of unbound competing antigens. Then purified rabbit anti-DPP IV, SK, HSP-60, or gliadin peptides were added to different wells. The concentration of bound rabbit antibodies was determined as described above but with alkaline phosphatase-labeled anti-rabbit IgG and not anti-human Igs.
Complex DPP IV and DPP IV enzyme activity in serum samples.
Levels of complex DPP IV were measured in 10 control sera with no DPP IV antibodies and 10 sera from autistic children with high levels of IgG anti-DPP IV antibodies. Levels were determined by adding 20 μl of control or patients' serum in 0.2 ml of serum diluent buffer to 96-well plates coated with monoclonal anti-DPP IV antibody and incubated for 1 h at 37°C. Plates were washed and incubated with antihuman IgG for 1 h at 37°C, followed by incubation with substrate and measurement of color development at 405 nm. DPP IV enzyme activity was measured with 0.2 mM Gly-Pro-P-nitroanilide in a reaction mixture (200 μl) containing 40 μl of serum samples and 50 mM Tris-HCl, pH 8.0. The hydrolysis of the substrate was monitored at 405 nm (8).
Statistical analysis. (i) General considerations.
The main objective of our data analysis was to examine the differences between the levels of IgG, IgM, and IgA antibodies among four groups (children with no conditions, adults with no conditions, children with autism, and adult patients with autoimmune disease) against anti-DPP IV autoantibody levels, anti-DPP I antibody levels, anti-aminopeptidase N (CD13) autoantibody levels, and antigliadin and HSP-60 peptide autoantibody levels. IgG, IgM, and IgA are considered three dependent variables, with one factor variable that divides our sample into the four groups mentioned above.
The general linear model (GLM) for Windows 11.5 with advanced option (SPSS, Inc., Chicago, Ill.) was used in this study. Using Hotelling's trace, the exact F statistics are provided. In addition, for the post hoc tests the Scheffé significant difference tests were performed. The GLM multivariate procedure provides analysis of variance for multiple dependent variables by one or more factor variables. The factor variables divide the population into various groupings of a joint distribution of dependent variables.
(ii) The Bonferroni, Scheffé, and Tukey's tests for significance.
The Bonferroni, Scheffé, and Tukey's significant difference tests are commonly used. The Bonferroni test, based on Student's t statistic, adjusts the observed significance level for the fact that multiple comparisons are made. In our post hoc analysis, the Bonferroni, Scheffé, and Tukey's honestly significant difference tests were performed, but the results were remarkably similar for all three methods. Therefore, only results for the Scheffé test are reported.
RESULTS
Statistical evaluation.
Table 1 shows the Hotelling's trace value, exact F statistic, and hypothesis degree of freedom for GLM multivariate tests. The results of the GLM indicate that overall, with no exception, significant differences exist between the groups (control children, control adults, and autism and autoimmune disease patients) for each dependent variable (DPP IV, DPP I, CD13, gliadin peptide, and HSP-60) (P < 0.0001).
TABLE 1.
GLM (multivariate tests), including Hotelling's trace value, exact F statistic, and hypothesis degree of freedoma
| Antibody | Hotelling's trace value | df | F |
|---|---|---|---|
| DPP IV | |||
| Intercept | 2.56 | 3 | 165.6 |
| Group | 0.54 | 9 | 11.5 |
| DPP I | |||
| Intercept | 2.53 | 3 | 163.6 |
| Group | 0.46 | 9 | 9.9 |
| CD13 | |||
| Intercept | 3.10 | 3 | 200.6 |
| Group | 0.36 | 9 | 7.7 |
| Gliadin peptide | |||
| Intercept | 1.95 | 3 | 126.1 |
| Group | 0.38 | 9 | 8.1 |
| HSP-60 peptide | |||
| Intercept | 1.64 | 3 | 105.7 |
| Group | 0.46 | 9 | 9.9 |
P < 0.0001 for all comparisons.
Anti-DPP IV autoantibody levels in control children with autism and patients with autoimmune disease.
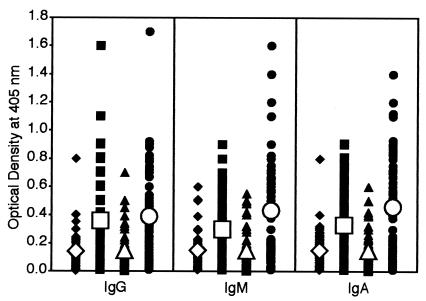
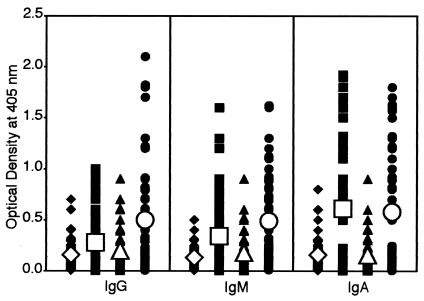
Using ELISAs, sera from 50 healthy subjects, 50 autistic children, and 50 patients with mixed connective tissue disease were analyzed for IgG, IgM, and IgA antibodies to DPP IV. Results expressed as mean OD ± SD are summarized in Fig. 1. The OD for IgG antibody values obtained with 1:200 dilution of healthy control sera were 0.05 to 0.8, and the mean ± SD were 0.15 ± 0.14. The corresponding IgG OD values from autistic children and patients with autoimmune disease sera were 0.01 to 1.1 and 0.01 to 1.5 with mean ± SD IgG values of 0.35 ± 0.28 and 0.43 ± 0.30. The result of post hoc multivariate comparison tests show that while the control groups are statistically alike for IgG, IgM, and IgA in anti-DPP IV, both the autism and autoimmune groups were significantly different compared to the control groups (P < 0.001) (Table 2, DPP IV). At a cutoff value of 0.29, OD levels of IgG antibody were calculated in controls and patients' sera; the results show that while 5 of 50 (10%) control children and 7 of 50 (14%) control adults had high IgG values, the patients' group showed IgG elevation in 54% (autistic children) and 64% (patients with autoimmune disease) (P < 0.0001) (Table 3). Levels of IgM anti-DPP IV in healthy controls and patients with autism and autoimmune disease are also shown in Fig. 1. These serum IgM antibodies were significantly higher in patients than in controls. The mean ± SD values were 0.14 ± 0.12 for controls and 0.33 ± 0.26 for patients with autism to 0.37 ± 0.36 for patients with autoimmune disease (P < 0.0001) (Fig. 1). When the 0.29 OD cutoff point was used, 8% of controls versus 50% of patients with autism and 46% of patients with autoimmune conditions showed elevated IgM antibody levels (P < 0.0001) (Table 3). Likewise, IgA antibody levels against DPP IV were examined in three groups. Individual and mean ± SD data depicted in Fig. 1 showed significant differences between the control and patient's group. The mean ± SD IgA antibody levels were 0.14 ± 0.11 in controls, 0.36 ± 0.33 in patients with autism, and 0.51 ± 0.40 under autoimmune conditions (P < 0.0001). The percent elevated serum IgA anti-DPP IV antibodies at an OD value of greater than 0.29 were significantly higher in patients with autism (44%) and autoimmune disease (58%) than in controls (4 to 6%) (Table 3).
FIG. 1.
Scattergram of serum titers of IgG, IgM, and IgA antibodies to DPP IV in healthy, young control subjects (♦), autistic patients (▪), healthy, older control subjects (▴), and patients with autoimmune disease (•), expressed as OD in the ELISA.
TABLE 2.
Multiple comparisons and means for groups in homogenous subsets with Scheffé's post hoc testsa
| Group | Mean for subset
|
|||||||
|---|---|---|---|---|---|---|---|---|
| IgG
|
IgM
|
IgA
|
||||||
| 1 | 2 | 3 | 1 | 2 | 3 | 1 | 2 | |
| DPP IV | ||||||||
| Control children | 0.1432 | 0.1694 | 0.1336 | |||||
| Control adults | 0.1994 | 0.1826 | 0.1654 | |||||
| Autism | 0.3488 | 0.3260 | 0.3620 | |||||
| Autoimmune | 0.4282 | 0.3700 | 0.5120 | |||||
| DPP I | ||||||||
| Control children | 0.1446 | 0.1492 | 0.1508 | |||||
| Control adults | 0.1492 | 0.1480 | 0.1546 | |||||
| Autism | 0.3580 | 0.2980 | 0.3320 | |||||
| Autoimmune | 0.3940 | 0.4300 | 0.4560 | |||||
| CD13 | ||||||||
| Control children | 0.1432 | 0.1310 | 0.1484 | |||||
| Control adults | 0.1994 | 0.1400 | 0.1218 | |||||
| Autism | 0.2900 | 0.1700 | 0.4040 | |||||
| Autoimmune | 0.2460 | 0.1680 | 0.3900 | |||||
| Gliadin peptide | ||||||||
| Control children | 0.1640 | 0.1460 | 0.1454 | |||||
| Control adults | 0.2140 | 0.2120 | 0.1920 | 0.1860 | ||||
| Autism | 0.3600 | 0.3600 | 0.3580 | 0.3680 | ||||
| Autoimmune | 0.5060 | 0.4640 | 0.4520 | |||||
| HSP-60 peptide | ||||||||
| Control children | 0.1620 | 0.1300 | 0.1618 | |||||
| Control adults | 0.1980 | 0.1770 | 0.1770 | 0.1636 | ||||
| Autism | 0.2800 | 0.3380 | 0.3380 | 0.6100 | ||||
| Autoimmune | 0.5020 | 0.4880 | 0.5820 | |||||
Means for groups in homogenous subsets are displayed based on the type III sum of squares (sample size of 200, with 50 subjects in each group). Means that are reported in the same subset are statistically similar. For example, means for IgG (DPP I) for the control children and control adult groups are 0.1446 and 0.1492, respectively, which are statistically alike. Similarly, the means for the autism (0.3580) and autoimmune (0.3940) groups are statistically the same. However, the means for control children are significantly different from the autism or autoimmune group. Similarly, the means for the control adults are statistically different from both the autism and autoimmune groups. Also note that the control groups (0.1492 and 0.1480) are similar for IgM (DPP I), but autism (0.2980) and autoimmune (0.4300) results are not statistically alike. Potentially, up to four subsets could be formed, simply because for each dependent variable we have four experimental groups. When this is the case, each mean's group should be reported in a separate subset. However, if all four means are statistically alike, all should be reported in one subset. For example, for CD13 IgM, all means are reported in the sample subset, indicating that no difference between the four groups was detected.
Anti-DPP I antibody levels in controls, children with autism, and patients with autoimmune disease.
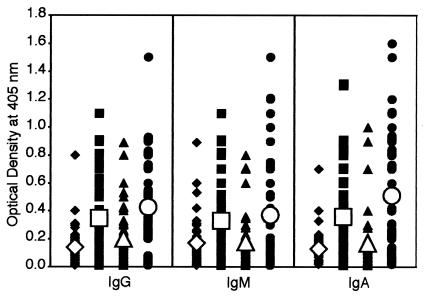
Analysis of anti-DPP I IgG, IgM, and IgA levels in controls and patients with autism or autoimmune disease showed significant differences between the antibody values and percent elevation of these antibodies against DPP I (Fig. 2). The mean ± SD OD values were 0.14 ± 0.11 to 0.16 ± 0.15 in controls, 0.30 ± 0.22 for patients with autism, and 0.46 ± 0.36 in patients with autoimmune disease (P < 0.0001) (Fig. 2).
FIG. 2.
Scattergram of serum titers of IgG, IgM, and IgA antibodies to DPP I in healthy, young control subjects (♦), autistic patients (▪), healthy, older control subjects (▴), and patients with autoimmune disease (•) expressed as OD in the ELISA.
The post hoc multivariate comparisons for DPP I in Table 2 reveal that the control groups for IgG, IgM, and IgA are statistically identical. For IgG and IgA, the autism and autoimmune groups are also alike. However, for IgM, a significant difference between the autoimmune and autism groups was detected. Overall, the autism and autoimmune groups are statistically different compared to control groups. Percent elevations of IgG, IgM, and IgA antibodies were 8 to 16% for controls, 46 to 56% for the autism group, and 54 to 60% for the autoimmune group (Table 3).
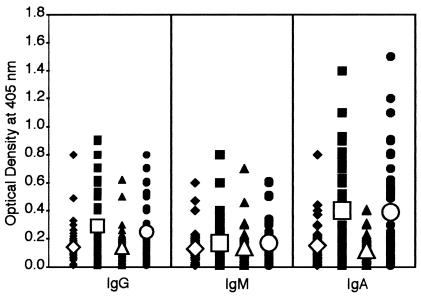
Anti-aminopeptidase N (CD13) autoantibody levels in controls, children with autism, and patients with autoimmune disease.
Similar to the analysis of DPP IV and DPP I data, levels of IgG, IgM, and IgA antibodies to CD13 were significantly higher in patients with autism or autoimmune disease than in controls (Fig. 3). In comparison to DPP IV and DPP I, the percent elevation of CD13 autoantibodies in both groups of patients were significantly lower for IgM (P < 0.31) but not for IgG and IgA (P < 0.0001) (Table 3). The results for CD13 in Table 2 show that for IgG and IgA, similar to control groups, autoimmune and autism are alike. Yet a significant difference was detected between the control groups and the autism and autoimmune groups. According to the data for anti-CD13 IgM, no differences between the four groups were detected.
FIG. 3.
Scattergram of serum titers of IgG, IgM, and IgA antibodies to aminopeptidase N (CD13) in healthy, young control subjects (♦), autistic patients (▪), healthy, older control subjects (▴), and patients with autoimmune disease (•), expressed as OD in the ELISA.
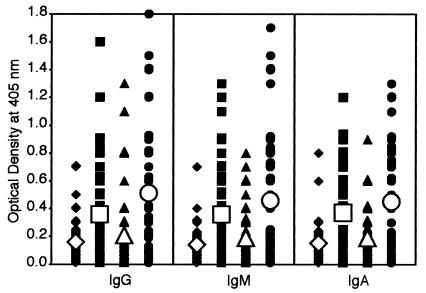
Antigliadin and HSP-60 peptide autoantibody levels in controls, children with autism, and patients with autoimmune disease.
Concomitant with an increase of IgG, IgM, and IgA against DPP IV, DPP I, and CD13, we observed a statistically significant increase of antigliadin and anti-HSP-60 antibodies in most patients' sera. Antibodies for controls were 0.14 ± 0.11 to 0.15 ± 0.17, and those for patients with autism and autoimmune disease 0.36 ± 0.32 to 0.51 ± 0.43 (P < 0.0001) (Fig. 4 and Table 3). The results for gliadin peptide in Table 2 show that IgM and IgA for control groups against gliadin peptide are statistically alike. Similarly, the autism and autoimmune groups are identical, yet the control groups are different compared with the autism and autoimmune groups. Finally, the results for HSP-60 in Table 2 show that for IgA against HSP-60, the control groups are statistically not different from the autoimmune and autism groups. However, for IgG, no similarity between the autoimmune groups and other groups (autism and controls) was detected. The autism and control groups are similar to but different from those of the autoimmune group. For IgM, no differences between the autism group and control adults were observed, but the autism group was statistically different compared with control children.
FIG. 4.
Scattergram of serum titers of IgG, IgM, and IgA antibodies to gliadin peptide in healthy, young control subjects (♦), autistic patients (▪), healthy, older control subjects (▴), and patients with autoimmune disease (•), expressed as OD in the ELISA.
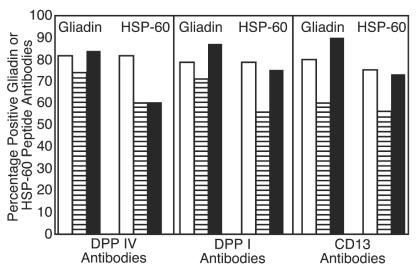
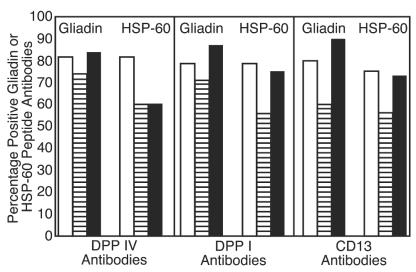
These values as well as percent elevation of IgG, IgM, and IgA antibodies against gliadin are presented in Tables 2 and 3. For examination of possible involvement of gliadin and HSP-60 peptides in the production of autoantibodies to different peptidases, calculations of the simultaneous elevation in these antibodies in patients' sera were made (see Fig. 6 and 7). Between 57 and 90% of sera from children with autism who had high IgG, IgM, or IgA against DPP IV, DPP I, or CD13 had simultaneous elevation in these antibodies to gliadin or HSP-60 peptides (Fig. 6). This correlation between IgG, IgM, and IgA antibodies to DPP IV, DPP I, CD13, and gliadin and HSP-60 peptides in sera of patients with autoimmune disease was 50 to 100% (Fig. 7). When concomitant detection of antibodies to all five tested antigens (DPP IV, DPP I, CD13, gliadin peptide, and HSP-60) was measured, 72, 30, and 24% of sera from children with autism versus 41, 25, and 26% of sera, respectively, from patients with autoimmune disease had simultaneous elevations in IgA, IgG, and IgM antibody levels to all tested antigens.
FIG. 6.
Percentage of positive sera from patients with autism for IgA (□), IgG (▤), and IgM (▪) antibodies to gliadin and HSP-60 peptides, which are positive for anti-DPP IV, anti-DPP I, or anti-CD13 antibodies.
FIG. 7.
Percentage of sera from patients with autoimmune disease positive for IgA (□), IgG (▤), and IgM (▪) antibodies to gliadin and HSP-60 peptides, which are positive for anti-DPP IV, anti-DPP I, or anti-CD13 antibodies.
Absorption of anti-DPP IV antibodies with DPP IV, DPP I, CD13, gliadin, HSP-60 peptide, HSA, and LPS.
To examine whether antibodies to DPP IV are specific or cross-reactive, we performed an absorption analysis using nonspecific antigens (LPS and HSA), specific antigen (DPP IV), and other antigens (DPP I, CD13, gliadin, and HSP-60) to which antibodies were detected simultaneously. Results summarized in Table 4 showed that DPP IV, DPP I, and CD13 significantly absorbed IgG and IgA antibodies when a specific antigen (DPP IV) was used, as expected. For example, anti-DPP IV-positive sera were absorbed by DPP IV up to 66%, with DDP I up to 25%, and with CD13 up to 33% but not with gliadin, HSP-60, HSA, or LPS (Table 4). This inhibition of antibodies by different antigens varied among samples and IgG versus IgA.
TABLE 4.
Level of anti-DPP IV antibodies in serum and inhibition after absorption with different specific and nonspecific antigensa
| Sample | IgG level (% inhibition) after absorption with 1-mg/ml:
|
IgA level (% inhibition) after absorption with 1-mg/ml:
|
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PBS | HSA | LPS | DPP IV | DPPI | CD13 | HSP-60 | Gliadin peptide | PBS | HSA | LPS | DPP IV | DPPI | CD13 | HSP-60 | Gliadin peptide | |
| Patient | ||||||||||||||||
| 1 | 1.19 | 1.23 (NS) | 1.18 (NS) | 0.45 (63) | 0.98 (18) | 0.81 (32) | 1.17 (NS) | 1.27 (NS) | 0.81 | 0.75 (NS) | 0.83 (NS) | 0.52 (36) | 0.76 (NS) | 0.71 (NS) | 0.89 (NS) | 0.92 (NS) |
| 2 | 0.92 | 0.95 (NS) | 1.0 (NS) | 0.32 (66) | 0.73 (21) | 0.68 (27) | 0.89 (NS) | 0.92 (NS) | 1.35 | 1.42 (NS) | 1.47 (NS) | 0.93 (32) | 1.0 (26) | 0.95 (30) | 1.28 (NS) | 1.47 (NS) |
| 3 | 0.61 | 0.57 (NS) | 0.59 (NS) | 0.24 (61) | 0.46 (25) | 0.41 (33) | 0.67 (NS) | 0.63 (NS) | 0.93 | 0.98 (NS) | 0.92 (NS) | 0.57 (39) | 0.73 (22) | 0.66 (29) | 0.95 (NS) | 0.88 (NS) |
| Control | ||||||||||||||||
| 1 | 0.22 | 0.23 (NS) | 0.21 (NS) | 0.16 (NS) | 0.20 (NS) | 0.23 (NS) | 0.27 (NS) | 0.25 (NS) | 0.24 | 0.22 (NS) | 0.26 (NS) | 0.14 (NS) | 0.18 (NS) | 0.21 (NS) | 0.23 (NS) | 0.19 (NS) |
| 2 | 0.14 | 0.12 (NS) | 0.11 (NS) | 0.10 (NS) | 0.15 (NS) | 0.16 (NS) | 0.13 (NS) | 0.18 (NS) | 0.21 | 0.18 (NS) | 0.23 (NS) | 0.20 (NS) | 0.19 (NS) | 0.26 (NS) | 0.22 (NS) | 0.23 (NS) |
| 3 | 0.16 | 0.14 (NS) | 0.12 (NS) | 0.18 (NS) | 0.13 (NS) | 0.18 (NS) | 0.11 (NS) | 0.14 (NS) | 0.16 | 0.19 (NS) | 0.21 (NS) | 0.17 (NS) | 0.15 (NS) | 0.18 (NS) | 0.22 (NS) | 0.18 (NS) |
Antibody levels in serum are expressed as OD (by ELISA). NS, not significant.
Possible binding of SK, gliadin, and HSP-60 peptides to DPP IV.
For demonstration of SK, gliadin, and HSP-60 binding to DPP IV, polyclonal antibodies were added to different wells. Only a rabbit anti-DPP IV reaction with wells coated with DPP IV resulted in an ELISA OD of 2.38. Addition of SK, gliadin, and HSP-60 caused 32, 43, and 27% inhibition of the DPP IV + anti-DPP IV ELISA values. Furthermore, on wells coated with DPP IV + SK, DPP IV + gliadin, or DPP IV + HSP-60, only rabbit antibody to SK, gliadin, or HSP-60 was capable of reacting with the specific antigen added to DPP IV and resulted in an OD above 2 SD of the background (Table 3). This ELISA OD is a further indication for binding of SK, gliadin, or HSP-60 to DPP IV. Similar results were obtained if, instead of DPP IV on the plate, DPP I or CD13 was used (data not shown).
Detection of complex DPP IV and DPP IV enzyme activity.
The mean ± SD for this complex of IgG with DPP IV in controls was 0.24 ± 0.19, and that in patients with autism was 1.3 ± 0.8 (P < 0.001). This binding of anti-DPP IV to DPP IV in serum may result in lower activity of DPP IV. In fact, when DPP IV activity was measured with a specific substrate, the OD of 10 specimens negative for DPP IV antibodies was 2.8 ± 1.2 and that for 10 specimens with high levels of DPP IV antibodies was 2.2 ± 0.9. Overall, in comparison to sera without DPP IV antibodies, the OD for samples with high levels of DPP IV antibodies was lower by 20% (P < 0.05).
DISCUSSION
Immunological research has suggested autoimmunity as a pathogenic factor in autism (42, 49, 55-58). This possible autoimmunity in autism was further strengthened by our earlier investigation and demonstration of antibodies to nine different neuron-specific antigens and their cross-reactive proteins and peptides from milk, C. pneumoniae, and Streptococcus group A (51). In that study, we suggested a role for milk proteins and bacterial antigens in the pathogenesis of autistic behavior. Based on these findings and earlier reports about the elevation of anti-DPP IV and anti-SK antibodies in rheumatoid arthritis, systemic lupus erythematosus (7), and patients with myocardial infarction (8), we first examined the existence of DPP IV autoantibodies in blood samples of children with autism and compared them to levels in patients with mixed connective tissue disease (Fig. 1 and Table 3). Second, we asked whether or not antibodies to other peptidases, such as DPP I and CD13, are detected in the sera of patients with autoimmune disease and in children with autism. To our surprise, we found almost similar levels of IgG, IgM, and IgA antibodies to DPP I and CD13 in patients with mixed connective tissue disease and autism, but not in controls (Fig. 2 and 3).
These antibodies to DPP IV appear to be specific, since in our absorption studies DPP IV was capable of reducing significantly (up to 63%) the levels of IgG and IgA anti-DPP IV antibodies from highly positive sera. DPP I and CD13 reduced levels of these antibodies to a less significant degree (up to 33%) (Table 4). This partial absorption of anti-DPP IV antibodies by DPP I and CD13 may indicate that production of anti-DPP I and anti-CD13 autoantibodies is due to different mechanisms of action and not due to cross-reactivity between DPP IV, DPP I, and CD13.
Based on these findings, we hypothesized that infectious agents, superantigens (including SK and HSP-60), and dietary proteins such as gliadin peptides in individuals with predisposing HLA molecules may bind to different aminopeptidases and induce autoantibodies to peptides and tissue antigens. To test this hypothesis, and based on gliadin peptide binding to transglutaminase in the brush border of celiac disease patients (43), we measured antigliadin and anti-HSP-60 peptide antibodies in blood samples of children with autism and patients with autoimmune disease. Data presented in Fig. 5 and Table 3 showed that similar to anti-DPP IV, anti-DPP I, and anti-CD13 antibody levels, a significant number of autistic and autoimmune sera exhibited antigliadin peptide and anti-HSP-60 antibodies.
FIG. 5.
Scattergram of serum titers of IgG, IgM, and IgA antibodies to HSP-60 in healthy, young control subjects (♦), autistic patients (▪), healthy, older control subjects (▴), and patients with autoimmune disease (•), expressed as OD in the ELISA.
We then asked the following question: what percentage of autism and autoimmune sera that is positive for antibodies to DPP IV, DPP I, and CD13 also have concomitant elevation in gliadin and HSP-60 antibodies? The answer, depicted in Fig. 6 and 7, is that between 50 and 100% of patients with autism and autoimmune disease who had elevated DPP IV, DPP I, and CD13 antibodies also had antigliadin and anti-HSP-60 antibodies. Since in inhibition studies (Table 4) gliadin and HSP were not capable of reducing the levels of DPP IV antibodies (meaning there is no antigenic similarity between gliadin, HSP, and DPP IV) similar to SK binding to DPP IV (44, 45), we postulated that gliadin and HSP-60 peptides binding to aminopeptidase is responsible for antigliadin, anti-HSP, and anti-DPP IV, anti-DPP I, and anti-CD13 autoantibody production. Further analysis of results showed that while 26, 25, and 41% of autoimmune sera had simultaneous elevation of IgG, IgM, and IgA antibodies to all tested antigens, the autistic sera had 24, 30, and 74% elevations in antibodies to DPP IV, DPP I, CD13, gliadin, and HSP-60. This high level of IgA production in 36 of 50 (72%) of children with autism against three peptidases, gliadin, and HSP peptide is further indication of reaction between these molecules and tissue peptidases. Indeed, when the Scheffé significant difference test was employed for the four groups, overall the autism and autoimmune groups were very similar for the levels of IgG, IgM, and IgA antibodies, but they were significantly different from the control groups (Table 2). These results are only supportive but not strong evidence for the binding of gliadin and HSP peptides to aminopeptidases. This similarity in antibody response to membrane peptidases, SK, HSPs, and gliadin peptides in children with autism and patients with autoimmune disease is further evidence of suggested autoimmunity in autism.
Using rabbit anti-DPP IV, anti-SK, antigliadin, and anti-HSP-60 antibodies, a series of ELISA experiments were carried out to demonstrate binding specificity of SK, gliadin, and HSP peptides to DPP IV-coated plates. Binding of SK, gliadin, and HSP-60 to DPP IV resulted in 27 to 43% inhibition of rabbit antibody binding to DPP IV. In addition, introduction of rabbit anti-SK, antigliadin, or anti-HSP antibody to the plates coated with DPP IV first and then SK, gliadin, or HSP resulted in enhancement of ODs above background by 18 to 27%. These results further support the binding of SK, gliadin, or HSP to DPP IV in vitro and possibly in vivo (Table 5). From our results we conclude that the binding of bacterial superantigens and dietary peptides to DPP IV, DPP I, or CD13 is responsible for autoantibody production in children with autism and in patients with autoimmune diseases.
TABLE 5.
Inhibition of antibody and peptide binding to DPP IV with specific and nonspecific rabbit antibodiesa
| Microwell coating | First antibody | ELISA OD492 | % Induction of antibody bindingb | % Inhibition of antibody binding |
|---|---|---|---|---|
| BSA | Rabbit anti-DPP IV | 0.25 | BG | |
| Rabbit anti-SK | 0.21 | BG | ||
| Rabbit anti-Gliadin | 0.17 | BG | ||
| Rabbit anti-HSP-60 | 0.22 | BG | ||
| HSA | Rabbit anti-DPP IV | 0.15 | BG | |
| Rabbit anti-SK | 0.19 | BG | ||
| Rabbit anti-gliadin | 0.20 | BG | ||
| Rabbit anti-HSP-60 | 0.24 | BG | ||
| DPP IV + HSA | Rabbit anti-DPP IV | 2.38 | 100 | |
| Rabbit anti-SK | 0.35 | NS | ||
| Rabbit antigliadin | 0.26 | NS | ||
| Rabbit anti-HSP-60 | 0.31 | NS | ||
| DPP IV + SK | Rabbit anti-DPP IV | 1.62 | 32 | |
| Rabbit anti-SK | 0.83 | 20 | ||
| Rabbit antigliadin | 0.18 | NS | ||
| Rabbit anti-HSP-60 | 0.35 | NS | ||
| Rabbit anti-DPP IV + Anti-SK | 2.21 | 79 | ||
| DPP IV + gliadin | Rabbit anti-DPP IV | 1.36 | 43 | |
| Rabbit anti-SK | 0.27 | NS | ||
| Rabbit antigliadin | 0.69 | 18 | ||
| Rabbit anti-HSP-60 | 0.15 | NS | ||
| Rabbit anti-DPP IV + antigliadin | 2.12 | 81 | ||
| DPP IV + HSP-60 | Rabbit anti-DPP IV | 1.74 | 27 | |
| Rabbit anti-SK | 0.36 | NS | ||
| Rabbit antigliadin | 0.25 | NS | ||
| Rabbit anti-HSP-60 | 0.96 | 18 | ||
| Rabbit anti-DPP IV + Anti-HSP | 2.59 | 96 |
Shown are inhibition of anti-DPP IV antibody binding to DPP IV by BSA, HSA, SK, gliadin, and HSP-60 and binding of SK, gliadin, and HSP-60 to DPP IV by specific and nonspecific rabbit antibodies. Note that all alkaline phosphatase-conjugated goat anti-rabbit IgG results were positive.
BG, background; NS, not significant.
Taking into consideration the short half-lives of IgG, IgM, and IgA in circulation, these findings suggest that SK, HSP, and gliadin peptides persist for long periods in the host tissue (1, 6-8). This persistence of SK, HSP, and gliadin peptide in circulation results in autoantibodies as well as in reduction of serum peptidase activity and increased complex formation between peptidases and their respective antibodies (45).
Production of DPP IV autoantibodies and reduced levels of circulating DPP IV in children with autism are perhaps part of the repair mechanism for nonspecific colitis and ileal-lymphoid-nodular hyperplasia reported in children with pervasive developmental disorder (52). This contradictory effect on the immune system was explained by a bimodal action of DPP IV in immune function (25). Finally, by virtue of this exopeptidase activity, we should not forget that DPP IV plays a key regulatory role in the metabolism of peptides. Thus inactivation of peptidases by autoantibodies may result in accumulation of dietary peptides such as gluteomorphins and casomorphins, in the gut and in circulation, resulting in possible neuroimmune dysregulation. The molecular mimicry between these peptides and human tissue antigens such as myosin (9) and cerebellar antigens (5, 13, 21) may result in sensitization of cross-reactive T cells that could mediate the demyelinating process. The activated T cells may then stimulate B cells to produce specific antibodies directed against nerve cell components or recruit macrophages as effector cells (24). A combination of humoral and cellular factors including cytokines may therefore participate in the cause of the disease.
Furthermore, the binding of antibodies to CD13- and CD26-bearing lymphocytes may interfere with major histocompatibility complex class I antigen presentation, cytokine production, inhibition of autoreactive lymphocyte apoptosis, and autoimmunity. Whether or not these autoantibodies that bind to tissue enzymes and lymphocyte receptors can cause some of the symptoms in patients with mixed connective tissue disease and in autism remains to be determined. In other words, it is not possible to make strong distinctions between induction of autoimmunity and the etiology of autoimmune disease (16). Only the injection of labeled gliadin and HSP peptides into animal models and demonstration of increased levels of antibodies in the blood and induction of abnormal metabolism of neuropeptides in the brain can support this hypothesis. Further identification of pathogenic peptides and their oral administration, as suggested for patients with celiac disease (41) and patients with idiopathic dilated cardiomyopathy (22), may help to design oral tolerance or other immune suppression strategies in patients with autism and autoimmune disease. Indeed, oral tolerance induced with HSP-65 of Mycobacterium tuberculosis proved to be a novel means of suppressing autoimmune atherogenesis.
REFERENCES
- 1.Anderson, R. P., P. Degano, A. J. Godkin, D. P. Jewell, and A. V. Hill. 2000. In vivo antigen challenge in celiac disease identifies a single transglutaminase-modified peptide as the dominant antigen T-cell epitope. Nat. Med. 6:337-342. [DOI] [PubMed] [Google Scholar]
- 2.Ansorge, S., F. Buhling, T. Hoffmann, T. Kahne, K. Neubert, and D. Reinhold. 1995. DPP IV/CD26 on human lymphocytes: functional roles in cell growth and cytokine regulation, p. 163-184. In B. Fleischer (ed.), Dipeptidyl peptidase IV (CD26) in metabolism and the immune response. Springer Verlag, Berlin, Germany.
- 3.Barret, A. J., N. D. Rawlings, and J. F. Woessner (ed.). 1998. Handbook of proteolytic enzymes, p. 379-382. Academic Press, New York, N.Y.
- 4.Broadley, S. A., J. Dean, S. J. Sawcer, D. Clayton, and D. A. S. Compston. 2000. Autoimmune disease in first-degree relatives of patients with multiple sclerosis. Brain 123:1102-1111. [DOI] [PubMed] [Google Scholar]
- 5.Bürk, K., S. Bösch, C. A. Müller, et al. 2001. Sporadic cerebellar ataxia associated with gluten sensitivity. Brain 124:1013-1019. [DOI] [PubMed] [Google Scholar]
- 6.Chatchatee, P., K. M. Järviven, L. Bardina, L Vila, K. Beyere, and H. A. Sampson. 2001. Identification of IgE and IgG binding epitope on β- and κ-casein in cow's milk-allergic patients. Clin. Exp. Allergy 31:1256-1262. [DOI] [PubMed] [Google Scholar]
- 7.Chuchacovich, M., H. Gatica, H. S. V. Pizzo, and M. Gonzalez-Gronow. 2001. Characterization of human serum dipeptidylpeptidase IV (CD26) and analysis of its autoantibodies in patients with rheumatoid arthritis and other autoimmune diseases. Clin. Exp. Rheumatol. 19:673-680. [PubMed] [Google Scholar]
- 8.Chuchacovich, M., H. Gatica, P. Vial, J. Yovanovich, S. V. Pizzo, and M. Gonzalez-Gronow. 2002. Streptokinase promotes development of dipeptidyl peptidase IV (CD26) autoantibodies after fibrinolytic therapy in myocardial infarction patients. Clin. Diagn. Lab. Immunol. 9:1253-1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ciervo, A., P. Visca, A. Petrucca, L. M. Biasucci, A. Maseri, and A. Cassone. 2002. Antibodies to 60-kilodalton heat shock protein and outer membrane protein 2 of Chlamydia pneumoniae in patients with coronary heart disease. Clin. Diagn. Lab. Immunol. 9:66-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Comi, A. M., A. W. Zimmerman, V. H. Frye, P. A. Law, and J. N. Peeden. 1999. Familial clustering of autoimmune disorders and evaluation of medical risk factors in autism. J. Child. Neurol. 14:388-394. [DOI] [PubMed] [Google Scholar]
- 11.Delmas, B., J. Gelfi, R. L'Haridon, K. Vogell, H. Sjostrom, O. Noren, and H. Laude. 1992. Aminopeptidase is a major receptor for the enteropathogenic coronavirus TGEV. Nature 357:417-420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Drexler, H. G. 1987. Classification of acute myeloid leukemias—a comparison of FAB immunophenotyping. Leukemia 1:697-705. [PubMed] [Google Scholar]
- 13.Dropcho, E. J., Y. Chen, J. B. Posner, and L. J. Old. 1987. Cloning of a brain protein identified by autoantibodies from a patient with paraneoplastic cerebellar degeneration. J. Immunol. 84:4552-4556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Edelson, S. B., and D. S. Cantor. 2000. The neurotoxic etiology of the autistic spectrum disorder: a replicative study. Toxicol. Ind. Health 16:239-247. [Google Scholar]
- 15.Fielder, A. H., M. J. Walport, J. R. Batchelor, et al. 1983. Family study of the major histocompatibility complex in patients with systemic lupus erythematosus: importance of null alleles of C4A and C4B in determining disease susceptibility. Br. Med. J. 286:425-428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Frustaci, A., L. Cuoco, C. Chimenti, M. Pieroni, G. Fioravanti, N. Gentilon, A. Maseri, and G. Gasbarrini. 2002. Celiac disease associated with autoimmune myocarditis. Circulation 105:2611-2618. [DOI] [PubMed] [Google Scholar]
- 17.Goding, J. W. 1978. Use of staphylococcal protein A as an immunological reagent. J. Immunol. Methods 20:241-253. [DOI] [PubMed] [Google Scholar]
- 18.Gonzalez-Gronow, M., M. R. Weber, G. Gawdi, and S. V. Pizzo. 1998. Dipeptidylpeptidase IV (CD26) is a receptor for streptokinase on rheumatoid synovial fibroblasts. Fibrinolysis Proteolysis 12:129-135. [Google Scholar]
- 19.Gonzalez-Gronow, M., M. Cuchacovich, D. M. Grigg, and S. V. Pizzo. 1996. Analysis of autoantibodies to plasminogen in the serum of patients with rheumatoid arthritis. J. Mol. Med. 74:463-469. [DOI] [PubMed] [Google Scholar]
- 20.Gupta, S., T. Lee, and S. Aggarval. 1998. Alterations in Th1 and Th2 subsets of CD4+ and CD8+ T-cells in autism. J. Neuroimmunol. 14:499-504. [DOI] [PubMed] [Google Scholar]
- 21.Hadjivassiliou, M., S. Boscolos, G. A. B. Davies-Jones, R. A. Grünwald, T. Not, D. S. Sanders, J. E. Simpson, E. Tongiorgi, C. A. Williamson, and N. M. Woodroofe. 2002. The humoral response in the pathogenesis of gluten ataxia. Neurology 58:1221-1226. [DOI] [PubMed] [Google Scholar]
- 22.Harat, S. D., N. Yacov, B. Gilburd, Y. Shoenfeld, and J. George. 2002. Oral tolerance with heat shock protein-65 attenuates mycobacterium tuberculosis-induced and high-fat-diet-driven atherosclerotic lesions. J. Am. Coll. Cardiol. 40:1333-1338. [DOI] [PubMed] [Google Scholar]
- 23.Harlow, E., and D. Lane (ed.). 1988. Antibodies: a laboratory manual, p. 98-108. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N. Y.
- 24.Hartun, H. P., G. Stoll, and K. V. Toyka. 1993. Immune reactions in the peripheral nervous system, p. 418-444. In P. J. Dyck, P. K. Thomas, J. W. Griffin, et al. (ed.) Peripheral neuropathy. W. B. Saunders, Philadelphia, Pa.
- 25.Hildebrandt, M., W. Reutter, P. Arck, M. Rose, and B. Klapp. 2000. A guardian angel: the involvement of dipeptidylpeptidase IV in psychoneuroendocrine function, nutrition and immune defense. Clin. Sci. 99:93-104. [PubMed] [Google Scholar]
- 26.Jyonouchi, H., S. N. Sun, and H. Le. 2001. Proinflammatory and regulatory cytokine production associated with innate and adaptive immune responses in children with autism spectrum disorders and developmental regression. J. Neuroimmunol. 120:170-179. [DOI] [PubMed] [Google Scholar]
- 27.Kameoka, J., T. Tanaka, Y. Nojima, S. F. Schlossman, and C. Morimoto. 1993. Direct association of adenosine deaminase with a T-cell activation antigen, CD26. Science 261:466-469. [DOI] [PubMed] [Google Scholar]
- 28.Kol, A., T. Bourcier, A. H. Lichtman, and P. Libby. 1991. Chlamydial and human heat shock protein 60s activate human vascular endothelium, smooth muscle cells and macrophages. J. Clin. Investig. 103:571-577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Letarte, M., S. Vera, R. Tran, J. B. L. Addis, R. J. Onizuka, E. J. Quackenbush, C. V. Jongeneel, and R. R. McInnes. 1988. Common acute lymphocyte leukemia antigen is identical to endopeptidase. J. Exp. Med. 168:1247-1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Look, A. T., R. A. Ashmun, L. H. Shapiro, and S. H. Peiper. 1989. Human myeloid plasma membrane glucoprotein CD13 (gP150) is identical to aminopeptidase N. J. Clin. Investig. 83:1299-1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mabee, C. L., M. J. McGuire, and D. L. Thiele. 1998. Dipeptidylpeptidase I and granzyme A are coordinately expressed during CD8+ T-cell development and differentiation. J. Immunol. 150:5880-5885. [PubMed] [Google Scholar]
- 32.Matsas, R., S. L. Stephenson, J. Hryszko, A. J. Turner, and A. J. Kenny. 1985. The metabolism of neuropeptides; phase separation of synaptic membrane preparations with Triton X-114 reveals presence of aminopeptidase N. Biochem. J. 231:445-449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Misumi, Y., Y. Hayashi, F. Arakawa, and Y. Ikehara. 1992. Molecular cloning and sequence analysis of human dipeptidyl peptidase IV, a serine proteinase on the cell surface. Biochim. Biophys. Acta 1131:333-336. [DOI] [PubMed] [Google Scholar]
- 34.Money, J., N. A. Bobrow, and F. C. Clarke. 1971. Autism and autoimmune disease: a family study. J. Autism Child. Schizophr. 1:146-160. [DOI] [PubMed] [Google Scholar]
- 35.Murray, D. L., D. H. Ohlendorf, and P. M. Schlievert. 1995. Staphylococcal and streptococcal superantigens: their role in human diseases. ASM News 61:229-235. [Google Scholar]
- 36.Muscat, C., A. Bertotto, E. Agea, O. Bistoni, R. Ercolani, R. Tognelli, F. Spinozzi, M. Cesarotti, and R. Gerli. 1994. Expression and functional role of 1F7 (CD26) antigen on peripheral blood and synovial fluid cells in rheumatoid arthritis patients. Clin. Exp. Immunol. 98:252-256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nakao, H., K. Eguchi, A. Kawakami, K. Migita, Y. Otsubo, C. Ueki, H. Shimomura, M. Tezuka, K. Maeda Matsunaga, and S. Nagataki. 1989. Increment of Tal positive cells in peripheral blood from patients with rheumatoid arthritis. J. Rheumatol. 16:904-914. [PubMed] [Google Scholar]
- 38.Prahalad, S., E. S. Shear, S. D. Thompson, E. H. Giannini, and D. N. Glass. 2002. Increased prevalence of familial autoimmunity in simplex and multiplex families with juvenile rheumatoid arthritis. Arthritis Rheum. 46:1851-1856. [DOI] [PubMed] [Google Scholar]
- 39.Riemann, D., A. Kehlen, and J. Langner. 1999. CD13—not just a marker in leukemia typing. Immunol. Today 20:83-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sentandrau, M. A., and F. Toldra. 2000. Purification and biochemical properties of dipeptidylpeptidase I from porcine skeletal muscle. J. Agric. Food Chem. 48:5014-5022. [DOI] [PubMed] [Google Scholar]
- 41.Shan, L., Ø. Molberg, I. Parrot, F. Hausch, F. Filiz, G. M. Gray, L. M. Sollid, and C. Khosla. 2002. Structural basis for gluten intolerance in celiac sprue. Science 297:2275-2279. [DOI] [PubMed] [Google Scholar]
- 42.Singh, V. K., R. P. Warren, R. Averett, and M. Ghaziuddin. 1997. Circulating autoantibodies to neuronal and glial filament protein in autism. Pediatr. Neurol. 17:88-90. [DOI] [PubMed] [Google Scholar]
- 43.Sollid, L. M. 2002. Coeliac disease: dissecting a complex inflammatory disorder. Nat. Rev. Immunol. 2:647-655. [DOI] [PubMed] [Google Scholar]
- 44.Squire, I. B., W. Lawley, S. Fletcher, E. Holme, W. S. Hillis, C. Hewitt, and K. L. Woods. 1999. Humoral and cellular immune responses up to 7.5 years after administration of streptokinase for acute myocardial infarction. Eur. Heart J. 20:1245-1252. [DOI] [PubMed] [Google Scholar]
- 45.Stancikova, M., Z. Lojda, J. Lukac, and M. Ruzickova. 1992. Dipeptidylpeptidase IV in patients with systemic lupus erythematosus. Clin. Exp. Rheumatol. 10:381-385. [PubMed] [Google Scholar]
- 46.Stastny, P. 1978. Association of the B-cell alloantigen DRw4 with rheumatoid arthritis. N. Engl. J. Med. 298:869-871. [DOI] [PubMed] [Google Scholar]
- 47.Stöllberger, C., and J. Finsterer. 2002. Role of infections and immune factors in coronary and cerebrovascular arteriosclerosis. Clin. Diagn. Lab. Immunol. 9:207-215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sweeten, T. L., S. L. Bowyer, D. J. Posey, et al. 2003. Increased prevalence of familial autoimmunity in probands with pervasive development disorders. Pediatrics 112:420-424. [DOI] [PubMed] [Google Scholar]
- 49.Torres, A. R., A. Maciulis, E. G. Stubbs, A. Cutler, and D. Odell. 2002. The transmission disequilibrium test suggests that HLA-DR4 and DR13 are linked to autism spectrum disorder. Hum. Immunol. 63:311-316. [DOI] [PubMed] [Google Scholar]
- 50.van Gent, T., C. J. Heijnen, and P. D. A. Treffers. 1997. Autism and the immune system. J. Child. Psychol. Psychiatry 38:337-349. [DOI] [PubMed] [Google Scholar]
- 51.Vojdani, A., A. W. Campbell, E. Anyanwu, A. Kashanian, K. Bock, and E. Vojdani. 2002. Antibodies to neuron-specific antigens in children with autism: possible cross-reaction with encephalitogenic proteins from milk, Chlamydia pneumoniae and streptococcus group A. J. Neuroimmunol. 129:168-177. [DOI] [PubMed] [Google Scholar]
- 52.Wakefield, A. J., S. H. Murch, A. Anthony, J. Linnell, D. M. Casson, M. Malik, M. Berelowitz, M. A. Thomson, P. Harvey, A. Valetine, S. E. Davies, and J. A. Walker-Smith. 1998. Ileal-lymphoid-nodular hyperplasia, non-specific colitis, and pervasive developmental disorder in children. Lancet 351:637-641. [DOI] [PubMed] [Google Scholar]
- 53.Warren, R. P., A. Foster, N. C. Margaretten, N. C. Pace, and A. Foster. 1986. Immune abnormalities in patients with autism. J. Autism Dev. Disord. 16:189-197. [DOI] [PubMed] [Google Scholar]
- 54.Warren, R. P., A. Foster, and N. C. Margarette. 1987. Reduced natural killer activity in autism. J. Am. Acad. Child Adolesc. Psychiatry 26:333-335. [DOI] [PubMed] [Google Scholar]
- 55.Warren, R. P., V. K. Singh, P. Cole, et al. 1991. Increased frequency of the null allele at the complement C4B locus in autism. Clin. Exp. Immunol. 83:438-440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Warren, R. P., V. K. Singh, P. Cole, et al. 1992. Possible association of the extended MHC haplotype B44-SC30-DR4 with autism. Immunogenetics 36:203-207. [DOI] [PubMed] [Google Scholar]
- 57.Warren, R. P., V. K. Singh, R. E. Avernett, et al. 1996. Immunogenetic studies in autism and related disorders. Mod. Chem. Neuropathol. 28:77-81. [DOI] [PubMed] [Google Scholar]
- 58.Weizman, A., R. Weizman, G. A. Szekely, H. Wijsenbeek, and E. Livni. 1982. Abnormal immune response to brain tissue antigen in the syndrome of autism. Am. J. Psychiatry 139:1462-1465. [DOI] [PubMed] [Google Scholar]
- 59.Wolters, P. J., M. Laig-Webster, and G. H. Caughey. 2000. Dipeptidyl peptidase I cleaves matrix-associated proteins and is expressed mainly by mast cells in normal dog airways. Am. J. Respir. Cell. Mol. Biol. 22:183-190. [DOI] [PubMed] [Google Scholar]
- 60.Yamazaki, K., Y. Ohsawa, K. Tabeta, H. Ito, K. Ueki, T. Oda, H. Yoshie, and G. J. Seymour. 2002. Accumulation of heat shock protein 60-reactive T cells in the gingival tissues of periodontitis patients. Infect. Immun. 70:2492-2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yeager, C. L., R. A. Ashmun, R. K. Williams, C. B. Cardellichio, L. H. Shapiro, A. T. Look, and K. V. Holmes. 1992. Human aminopeptidase N is a receptor for human coronavirus 229E. Nature 357:420-422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yonk, L. J., R. P. Warren, R. A. Burger, P. Cole, J. D. Odell, W. R. Warren, E. White, and V. K. Singh. 1990. CD4+ helper T-cell depletion in autism. Immunol. Lett. 25:344-346. [DOI] [PubMed] [Google Scholar]
- 63.York, I. A., S. C. Chang, T. Saric, et al. 2002. The ER aminopeptidase ERAP1 enhances or limits antigen presentation by trimming epitopes to 8-9 residues. Nat. Immunol. 3:1177-1184. [DOI] [PubMed] [Google Scholar]
- 64.Young, R. A., and T. J. Elliot. 1989. Stress proteins, infection, and immune surveillance. Cell 59:5-8. [DOI] [PubMed] [Google Scholar]