Abstract
Dendritic cells (DC) are highly-specialized, bone marrow-derived antigen-presenting cells that induce or regulate innate and adaptive immunity. Regulatory or “tolerogenic” DC play a crucial role in maintaining self tolerance in the healthy steady-state. These regulatory innate immune cells subvert naïve or memory T cell responses by various mechanisms. Regulatory DC (DCreg) also exhibit the ability to induce or restore T cell tolerance in many animal models of autoimmune disease or transplant rejection. There is also evidence that adoptive transfer of DCreg can regulate T cell responses in non-human primates and humans. Important insights gained from in vitro studies and animal models have led recently to the development of clinical grade human DCreg, with potential to treat autoimmune disease or enhance transplant survival while reducing patient dependency on immunosuppressive drugs. Phase I trials have been conducted in type-1 diabetes and rheumatoid arthritis, with results that emphasize the feasibility and safety of DCreg therapy. This mini-review will outline how observations made using animal models have been translated into human use, and discuss the challenges faced in further developing this form of regulatory immune cell therapy in the fields of autoimmunity and transplantation.
Keywords: dendritic cells, cell therapy, tolerance, autoimmunity, transplantation
Introduction
Current therapies for autoimmune diseases and allograft rejection generally involve the indefinite use of non-specific immunosuppressive drugs, which may result in infectious complications, predisposition to certain types of cancer, and the attendant toxicities and adverse side effects of these agents. Use of these drugs also fails to induce antigen (Ag)-specific tolerance. An emerging strategy for the treatment of both autoimmunity and graft rejection is the use of regulatory immune cells [1–3], that include regulatory T cells (Treg) [4,5] and regulatory myeloid cells [6], including regulatory dendritic cells (DCreg) [7–9]. These cellular therapies have potential to promote Ag-specific tolerance.
While DCreg exist naturally in blood and both lymphoid and non-lymphoid tissues, they are rare cells, but can be generated readily in vitro from precursors in blood or bone marrow (BM) using appropriate cytokine growth factors. DCreg can be propagated in culture using biologic or pharmacologic agents, among other techniques, and promising results have been obtained using such approaches. In addition, adoptive cell transfer in animal models and humans has raised expectation that DCreg can be used to control adverse T cell-mediated immune responses in the clinic.
DC as regulators of immunity and tolerance
DC are a heterogeneous group of professional, antigen-presenting immune cells, that are widely distributed through lymphoid and non-lymphoid tissues [10], and that regulate immune responses, maintaining the balance between tolerance and immunity. Different subtypes of DC are distributed throughout the body and act as sentinels in peripheral tissues or in lymphoid organs, where they encounter potential Ags. When pathogen invasion occurs, tissue-resident immature DCs capture microorganisms via endocytic surveillance receptors [11], that trigger their maturation. This maturation process consists of profound phenotypic and functional modifications, that include expression of MHC-peptide complexes on the cell surface, increased expression of T cell co-stimulatory molecules (such as CD40, CD80, CD86, OX40L, or inducible costimulatory ligand [ICOSL]), together with the secretion of cytokines (including IL-1β, IL-2, IL-6, IL-10 and IL-12) [10,11]. In vivo, this maturation process is accompanied by changes in the expression of cell traffick regulating molecules, such as up-regulation of cell surface CCR7, a chemokine receptor that enables migration of DC to lymph nodes [12]. Therein, mature DCs present Ag-derived peptides in association with MHC-II molecules to naive T helper (Th) lymphocytes, that recognize the MHC-II/peptide complex via the T cell receptor (TCR) [10,13]. After Ag recognition, and with the appropriate additional interactions mediated by co-stimulatory molecules, naive T lymphocytes become effector T cells, with different helper or cytotoxic activities. Thus, DC function as a crucial link between innate and adaptive immunity.
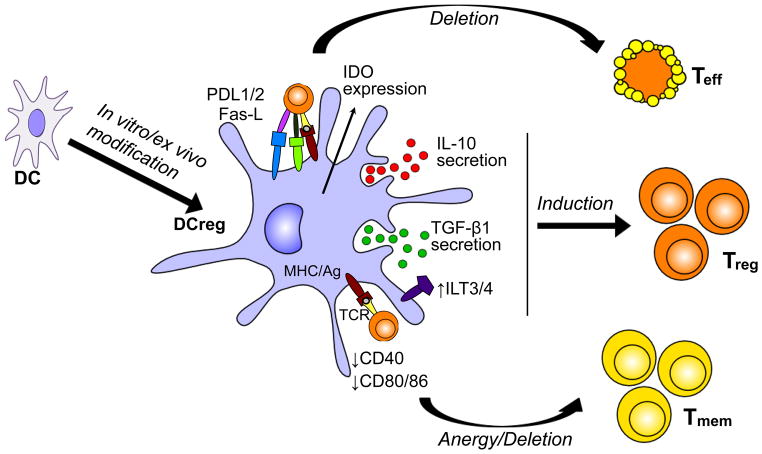
It is currently acknowledged that DC are important not only in the generation of T cell-mediated immune responses, but also in the induction and maintenance of central and peripheral tolerance. Under steady-state conditions, DC may present self-Ags (from captured apoptotic bodies) and silence autoreactive T cells [14]. These naturally-occurring DCreg also maintain tolerance in peripheral tissues to commensal microorganisms, Ags derived from food and the airways, etc, within the steady-state environment. DCreg are characterized by low expression of co-stimulatory molecules (mainly CD40, CD80, CD86), and usually by reduced production of pro-inflammatory IL-12 and increased secretion of anti-inflammatory IL-10 [15], together with reduced ability to induce T cell proliferation. While these properties can help explain their ability to induce regulatory T cells (Treg) rather than T effector cells, several other mechanisms may play a role in tolerance induction by DCreg [16]. The molecular mechanisms involved in the tolerogenic function of DCreg in the periphery (Figure 1) include induction of anergy, promotion of Treg differentiation and induction of T cell death (deletion).
Figure 1.
Tolerogenic characteristics of DCreg. DCreg express and secrete regulatory molecules that mediate tolerogenic effects on T cells, leading to the induction of T cell deletion, T cell anergy or Treg expansion/induction.
Strategies for generating DCreg
As DC are involved in the regulation of both tolerance and immunity, they could have many clinical applications for treatment of immune-based diseases. Indeed, the potential of DCs for clinical application has been under extensive investigation for some time [17–19]. Many strategies have been described for the generation of potent regulatory/tolerogenic DC in mouse or human systems. These DC are most frequently generated in vitro from murine BM precursors [20] or human blood monocytes [21]. Although a wide variety of conditions have been reported to support DC generation, the growth factor most commonly used to generate conventional murine or human DC is granulocyte-macrophage colony-stimulating factor (GM-CSF), combined with IL-4 [22]. DCreg features can be induced in vitro by exposure of DC to pharmacological agents, anti-inflammatory biologicals, or following their genetic modification [2,23].
Diverse biomolecules that are encountered physiologically under tolerogenic conditions in vivo, can induce DCreg differentiation in vitro. For instance, incubation of DC with IL-10 confers an ability to induce Tregs [24] that have suppressive capacity in models of organ allograft rejection, allergy, and graft-versus-host disease (GVHD) [23]. Signaling through the IL-10 receptor maintains DC in their immature state, even in the presence of maturation signals [25]. Transforming growth factor-β (TGF-β), a cytokine produced by Treg and other cells, allows DC to attenuate the neuropathology associated with experimental allergic encephalomyelitis (EAE) [26], a model of multiple sclerosis (MS). When treated in vitro with pro-inflammatory stimuli and the active metabolite of vitamin D: 1α,25-dihydroxyvitamin D3 (vitD3), human DC express indoleamine dioxygenase (IDO), CCL2, IL-10, TGF-β, tumor necrosis factor (TNF) receptor apoptosis-inducing ligand (TRAIL), and the inhibitory receptors CD300LF and CYP24A1, that have been implicated in immune tolerance [27]. Also, hepatocyte growth factor induces a tolerogenic phenotype (low IL-12; high IL-10) in human monocyte-derived DC [28] that induce Treg. Several other factors, such as estrogen, vasoactive intestinal peptide (VIP), binding immunoglobulin protein (BiP), thymic stromal lymphopoietin (TSLP), GM-CSF, prostaglandin (PG) E2, and TNFα, may also promote Treg-inducing ability of DCreg [29,30].
Pharmacological agents have been employed very successfully to manipulate DC function, both in vitro and in many disease models [31,32]. These include: anti-inflammatory agents (such as acetylsalicylic acid), histamine, adenosine receptor agonists, and immunosuppressive drugs such as corticosteroids, cyclosporine A, rapamycin, deoxyspergualin, tacrolimus (FK506), mycophenolate mofetil (MMF), and BAY-117085 [33]. Treatment with prednisolone or dexamethasone (Dex) leads to DCreg differentiation with the ability to instruct Treg [34,35], and negatively modulate the nuclear factor (NF)κB pathway, inflammatory cytokines, chemokines, and Ag-presenting molecules [36]. Inhibition of the mechanistic target of rapamycin (mTOR) by rapamycin promotes DCreg that stimulate Treg expansion in vivo and in vitro [37–39]. BAY-117085 is an irreversible NF-κB inhibitor, and DC treated with this agent induce Treg and suppress established experimental autoimmune arthritis [33].
Several genetic manipulations have been used to modulate the maturation of DC to induce DCreg [2]. Towards this end, selected genes can be transferred to DCs through viral or non-viral delivery systems (including liposomes and electroporation) [40], or knocked-down by selective gene silencing using e.g. anti-sense oligodeoxynucleotides (ODNs) and small interfering RNAs (siRNA) [41]. Using these techniques, DCreg have been generated by either inducing the expression of different immunomodulatory molecules (such as IL-4, IL-10, TGF-β, cytotoxic T lymphocyte Ag (CTLA)-4, or programmed death ligand (PDL)-1, among others) or, in contrast, by inhibiting specific molecules involved in DC activation (i.e. IL-12p35, CD40, or CD86) (reviewed in [2,9]). These genetically-induced DCreg have been shown, in some instances, to induce T cell hyporesponsiveness and to prolong allograft survival in mice [42], to induce Treg differentiation [43], and to suppress autoimmune diabetes or delayed-type hypersensitivity in mice [44].
While different methods to generate DCreg have shown very promising results in murine models of transplantation and autoimmune disease, there are some discrepancies in the effectiveness of these approaches between mice and humans. For this reason, careful studies that compare different DCreg-generating strategies are essential. For instance, the study performed by Naranjo-Gómez et al [45] compared the use of different agents to generate human DCreg for prospective clinical use, and demonstrated significant differences in DCreg features, highlighting the importance of appropriate agent selection. On the other hand, a recent study by Boks et al [46] that also compared different agents for generating clinical grade DCreg, concluded that IL-10-treated DC possessed the most potent tolerogenic phenotype, with promise for clinical use.
Clinical application of DCreg
One of the major concerns associated with injection of DCreg into humans, is the functional stability of the DCreg product. There is the possibility that these cells could revert to immunogenic DC in response to inflammatory signals (such as pro-inflammatory cytokines, Toll-like receptor ligands or CD40 ligation) encountered in vivo, that could worsen allograft rejection or autoimmune disease in the patient. Thus, clinically-applicable DCreg must be tested rigorously for robust stability before infusion into humans. In experimental hematopoietic stem cell transplantation, a form of DCreg referred to as “alternatively-activated” DC has been used to prevent murine GVHD [47]. In this instance, DCreg of donor origin are propagated from BM in the presence of IL-10 and TGF-β, then stimulated with lipopolysaccharide (LPS). However, LPS is potentially toxic and must therefore be avoided for human use. Alternatives such as non-toxic monophosphoryl lipid A (MPLA), a TLR4-stimulator, have been used successfully as adjuvants in vaccine research [48], and may therefore be suitable alternatives to LPS for this purpose. Interestingly, DCreg stimulated with MPLA can promote tolerance in vitro [49]. When comparing MPLA with a cytokine cocktail containing IL-1β, TNFα and PGE for stimulating human DCreg, cells matured with this cocktail showed greater stability and suppressive function, as well as superior migratory activity, than those matured with MPLA.
A further challenge associated with the prospective use of DCreg for therapy and promotion/restoration of Ag-specific tolerance in human autoimmune disease or organ transplantation is the need to personalize a “made-to-order” cell product that needs to be pulsed with either auto- or alloAg. In the case of autoimmune disease, some examples are the use of myelin peptides such as myelin oligodendrocyte glycoprotein (MOG) or citrullinated peptides in the context of multiple sclerosis and rheumatoid arthritis (RA), respectively. In transplantation, donor Ag may take the form of allogeneic donor cell extract [50], purified exosomes or apoptotic bodies expressing donor MHC [51]. These approaches add to the labor intensity and cost of rendering a good manufacturing product (GMP) product for human use.
While the two major approaches to DCreg therapy used in experimental organ transplantation have been the infusion of either donor DCreg or alloAg-pulsed recipient DCreg as “negative vaccines” [52], Beriou et al [53] have investigated the efficacy of peri-transplant administration of unpulsed, recipient DCreg. These authors showed that unpulsed autologous DCreg (treated with an NF-Kβ inhibitor) were efficient at promoting cardiac, skin and islet allograft survival in rodents, and suggest that these DCreg acquire donor alloAg in vivo and induce T cell tolerance within host secondary lymphoid tissue [53]. Such an approach may minimize the risk of host allosensitization, but has not yet been tested in a large animal model.
Current Progress
Despite the challenges associated with translation of DCreg therapy from rodents to humans, advances such as the development of closed cell-culture systems and current GMP-compliant reagents, have allowed strategies for the production of clinical grade DCreg to move forward (Table 1). This has led to early clinical trials in type-1 diabetes and RA, with others in prospect.
Table 1.
Agents used to generate human regulatory dendritic cells (DCreg) for (prospective) clinical use
| Agent(s) Used | Reported Properties of DCreg | Antigen (if used) | Intended Application | Author/Reference |
|---|---|---|---|---|
| Dexamethasone VitaminD3 TLR-agonist (e.g. LPS or MPLA) |
|
Synovial fluid containing joint- associated auto- Ag | Rheumatoid Arthritis | [60] Hilkens, et al |
| Antisense oligonucleotides targeting CD40, CD80 and CD86 |
|
No Ag used | Type-1 Diabetes | [54] Giannoukakis, et al |
| BAY 11-7082 |
|
Four citrullinated peptides | Rheumatoid Arthritis | [57] Thomas, et al |
| VitaminD3 Cytokine cocktail (TNFα, PGE2, IL-1β) |
|
Seven myelin peptides | Multiple Sclerosis | [61]Raïch- Regué, et al |
| IL-10 Cytokine cocktail (TNFα, PGE2, IL-1β) |
|
No Ag used | N/A | [46] Boks, et al |
ILT3/4; immunoglobulin-like transcript 3/4.
The first human clinical trial of DCreg was undertaken at the University of Pittsburgh by Giannoukakis et al [54] in adult, type-1 diabetic patients. In this trial, autologous DCreg were treated ex vivo with anti-sense oligonucleotides targeting CD40, CD80 and CD86. DC generated in this way induce T cell unresponsiveness and/or apoptosis in non-obese diabetic (NOD) mice and can prevent or reverse autoimmune diabetes [55]. The principal outcome of the phase I clinical trial was that the autologous DCreg were shown to be safe and well-tolerated, as none of the patients experienced any adverse effects [54]. Although the DCreg treatment did not noticeably reduce symptoms of autoimmunity, it did increase the frequency a B220+CD11c− B-cell population, that might potentially be beneficial in relieving symptoms of type-1 diabetes.
Also in the field of autoimmunity, a phase I study of DCreg in RA is being performed by Thomas et al at the University of Queensland, Australia. In this trial, DCreg have been generated by treatment with BAY 11-7082, an inhibitor of NFκB signaling, to prevent their maturation. BAY 11-7082-treated DC are distinct phenotypically from the DCreg used in the autoimmune diabetes trial. Thus, they are also deficient in CD40, but they express high levels of CD86 [33]. In the diabetes trial [54], DC were not loaded with Ag, while in the RA trial, the DC were pulsed with four citrullinated peptides. This combination of DCreg with citrullinated peptides was given the name Rheumavax, and patients received a single dose intradermally. Citrullinated self-Ags have emerged as a major group of post-translationally modified auto-Ags in RA and are found in inflamed RA joints [56]. As in the autoimmune diabetes trial at the University of Pittsburgh, the vaccine was well-tolerated and no adverse effects were observed. In some cases, severe disease symptoms appeared to improve, but no difference was noted in participants with low disease activity. This study demonstrates that autologous DCreg loaded with disease-appropriate Ag are safely tolerated, with no notable adverse effects [57]. While neither phase I trial demonstrated measurable efficacy, the demonstration of autologous DCreg safety in humans is an important step in further development of regulatory DC therapy.
A further clinical trial using DCreg for the treatment of RA is currently being developed by Hilkens and Isaacs at Newcastle University in the UK (C.M.U. Hilkens and J.D. Isaacs, University of Newcastle (U.K.), ClinicalTrials.gov identifier: NCT01352858). In their protocol, monocyte-derived DC are modified pharmacologically with Dex and vitD3, and treated additionally with a TLR4 agonist,- clinical grade MPLA [58]. The TLR4 ligand is required to stimulate the DC to process and present Ag efficiently on MHC class II, and may also confer on DC the ability to migrate in a CCR7-dependent manner to lymph nodes, where they can interact with T cells, and induce autoAg-specific Treg. DCreg generated using this protocol are characterized by high expression of MHC class II, intermediate expression of the co-stimulatory molecules CD80 and CD86 and low expression of CD40 [58]. Therefore, despite maintaining the ability to present Ag, they have lower T cell stimulatory capacity than mature DC. Furthermore, these DCreg are characterized by high levels of IL-10 and TGF-β, and low levels of IL-12, IL-23 and TNFα. Experiments performed by the same group have shown that DC generated with Dex, VitD3 and LPS significantly reduce the severity and progression of arthritis in a mouse model of collagen-induced arthritis [59]. It is hoped that a similar protocol can be used to generate human DC with the ability to inhibit autoreactive T cells in the rheumatoid joint. As the DCreg can present Ag in association with low co-stimulatory molecules, they have the ability to induce hyporesponsiveness in Ag-experienced memory T cells, whilst polarizing naïve T cells towards anti-inflammatory cytokine production [60].
A recent study by Raïch-Regué et al [61] has shown that myelin peptide-pulsed human DC can induce anergy in myelin-specific T cells obtained from relapsing-remitting MS patients. The authors were able to differentiate DCreg from monocytes of both healthy and MS patients, and found that, when treated with VitD3, the cells showed decreased expression of co-stimulatory molecules (CD40, CD80 and CD86) and reduced IL-12 production, as well as enhanced IL-10 secretion [61]. A recent study in EAE, a model of MS, has shown that treatment with VitD3 induces tolerogenic DC, that can reduce the severity of disease [62]. In the human in vitro study, DCreg induced stable, Ag-specific hyporesponsiveness in T cells from MS patients, and exhibited an anti-inflammatory cytokine profile, even when re-challenged with LPS [61]. This demonstrates that DCreg are capable of inducing stable hyporesponsiveness in myelin-autoreactive T cells, indicating the promise for DCreg therapy for treatment of MS.
While no clinical trials of DCreg have been reported thus far in transplantation, recent studies performed in a robust, clinically-relevant, non-human primate model provide important evidence of both the safety and efficacy of DCreg in organ transplantation [63,64]. Rhesus macaque monocyte-derived DC were generated in vitD3 and IL-10 [65] and expressed low MHC class II and co-stimulatory molecules, but comparatively high levels of programmed death ligand-1 (=B7-H1) [63]. These DC were administered together with CTLA4Ig, a clinically-approved co-stimulation blocking agent, 7 days before renal transplantation. Following transplantation, CTLA4Ig was given for up to 8 weeks, and rapamycin for 6 months. A significant increase was observed in median graft survival time in the DCreg-treated monkeys, accompanied by depression of donor-reactive memory T cells [63]. Importantly, there was no evidence of host sensitization to alloAgs. This demonstration supports testing of DCreg together with co-stimulation blockade and mTOR inhibition to reduce patients’ dependence on immunosuppressive drugs and promote rejection-free, long-term allograft survival.
Conclusion
DCreg are a promising novel immune cell therapy option for the inhibition of autoimmunity and allograft rejection. They offer the potential to promote or restore Ag-specific tolerance and to reduce patients’ dependence on non-specific immunosuppressive drugs. The success of DCreg generation in vitro and their use in animal models has led to the development of promising protocols for application in humans. While as with other forms of cell therapy, there are clearly challenges to be overcome and numerous questions to be answered (summarized in Table 2), the recent demonstration of the safety of DCreg in patients and their efficacy in a NHP organ transplant model, support the development of further clinical trials in both autoimmunity and transplantation.
Table 2.
Challenges facing regulatory dendritic cell (DCreg)-based therapy
| Autoimmunity |
| Choice and loading of auto-Ags |
|
|
| Transplantation |
| Source of DC (donor or recipient) |
| Form of donor alloAg used |
| Identification of optimal combination regimens (with other immunosuppressive or tolerogenic strategies) |
| Overcoming late graft rejection |
|
|
| Both |
| Optimal route, timing, dose, and frequency of administration |
| Guarantee of stability, tolerogenicity and specificity |
| Outcomes that can be monitored adequately i.e. by identification of biomarkers of tolerance |
| Quality control: GMP compliance, safety and standardization of protocols |
| Choice of growth factors, tolerance-inducing agent and maturation stimulus |
| Overcoming immunological memory |
Highlights.
Regulatory dendritic cells (DCreg) control naïve and memory T cell responses
DCreg suppress experimental autoimmune disease and transplant rejection
Phase I clinical trials demonstrate the safety of autologous DCreg in autoimmunity
Clinical trials to establish the efficacy of DCreg therapy are merited
Acknowledgments
The authors’ studies have been supported by grants from the National Institutes of Health (R01 AI67541, U01 AI51698 and U01 AI91197 to AWT) and by a European Society of Organ Transplantation/American Society of Transplantation research grant and a Thomas E. Starzl Postdoctoral Fellowship in Transplantation Biology (DR-R). We thank our colleagues in the laboratory and clinic for valuable discussion and Ms. Miriam Freeman for excellent administrative support.
Abbreviations
- Ag
antigen
- DCreg
regulatory dendritic cells
- Dex
dexamethasone
- MPLA
monophosphoryl lipid A
- MS
multiple sclerosis
- RA
rheumatoid arthritis
Footnotes
Disclosure
AWT is co-inventor of US patents for the use of dendritic cells to promote transplant tolerance.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Dalia Raïch-Regué, Email: raichregued@upmc.edu.
Megan Glancy, Email: 0912720G@student.gla.ac.uk.
References
- 1.Wood KJ, Bushell A, Hester J. Regulatory immune cells in transplantation. Nat Rev Immunol. 2012;12:417–430. doi: 10.1038/nri3227. [DOI] [PubMed] [Google Scholar]
- 2.Morelli AE, Thomson AW. Tolerogenic dendritic cells and the quest for transplant tolerance. Nat Rev Immunol. 2007;7:610–621. doi: 10.1038/nri2132. [DOI] [PubMed] [Google Scholar]
- 3.Lombardi G, Sagoo P, Scotta C, Fazekasova H, Smyth L, Tsang J, et al. Cell therapy to promote transplantation tolerance: a winning strategy? Immunotherapy. 2011;3:28–31. doi: 10.2217/imt.11.42. [DOI] [PubMed] [Google Scholar]
- 4.Brusko TM, Putnam AL, Bluestone JA. Human regulatory T cells: role in autoimmune disease and therapeutic opportunities. Immunol Rev. 2008;223:371–390. doi: 10.1111/j.1600-065X.2008.00637.x. [DOI] [PubMed] [Google Scholar]
- 5.McMurchy AN, Bushell A, Levings MK, Wood KJ. Moving to tolerance: clinical application of T regulatory cells. Semin Immunol. 2011;23:304–313. doi: 10.1016/j.smim.2011.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rosborough BR, Raich-Regue D, Turnquist HR, Thomson AW. Regulatory myeloid cells in transplantation. Transplantation. doi: 10.1097/TP.0b013e3182a860de. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ezzelarab M, Thomson AW. Tolerogenic dendritic cells and their role in transplantation. Semin Immunol. 2011;23:252–263. doi: 10.1016/j.smim.2011.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gross CC, Wiendl H. Dendritic cell vaccination in autoimmune disease. Curr Opin Rheumatol. 2013;25:268–274. doi: 10.1097/BOR.0b013e32835cb9f2. [DOI] [PubMed] [Google Scholar]
- 9.Hilkens CM, Isaacs JD, Thomson AW. Development of dendritic cell-based immunotherapy for autoimmunity. Int Rev Immunol. 2010;29:156–183. doi: 10.3109/08830180903281193. [DOI] [PubMed] [Google Scholar]
- 10.Banchereau J, Briere F, Caux C, Davoust J, Lebecque S, Liu YJ, et al. Immunobiology of dendritic cells. Annu Rev Immunol. 2000;18:767–811. doi: 10.1146/annurev.immunol.18.1.767. [DOI] [PubMed] [Google Scholar]
- 11.Mellman I, Steinman RM. Dendritic cells: specialized and regulated antigen processing machines. Cell. 2001;106:255–258. doi: 10.1016/s0092-8674(01)00449-4. [DOI] [PubMed] [Google Scholar]
- 12.Scandella E, Men Y, Gillessen S, Forster R, Groettrup M. Prostaglandin E2 is a key factor for CCR7 surface expression and migration of monocyte-derived dendritic cells. Blood. 2002;100:1354–1361. doi: 10.1182/blood-2001-11-0017. [DOI] [PubMed] [Google Scholar]
- 13.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 14.Steinman RM, Nussenzweig MC. Avoiding horror autotoxicus: the importance of dendritic cells in peripheral T cell tolerance. Proc Natl Acad Sci U S A. 2002;99:351–358. doi: 10.1073/pnas.231606698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Steinman RM, Hawiger D, Nussenzweig MC. Tolerogenic dendritic cells. Annu Rev Immunol. 2003;21:685–711. doi: 10.1146/annurev.immunol.21.120601.141040. [DOI] [PubMed] [Google Scholar]
- 16.Adler HS, Steinbrink K. Tolerogenic dendritic cells in health and disease: friend and foe! Eur J Dermatol. 2007;17:476–491. doi: 10.1684/ejd.2007.0262. [DOI] [PubMed] [Google Scholar]
- 17.Thomson AW, Robbins PD. Tolerogenic dendritic cells for autoimmune disease and transplantation. Ann Rheum Dis. 2008;67(Suppl 3):iii90–96. doi: 10.1136/ard.2008.099176. [DOI] [PubMed] [Google Scholar]
- 18.Gilboa E. DC-based cancer vaccines. J Clin Invest. 2007;117:1195–1203. doi: 10.1172/JCI31205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Steinman RM. Decisions about dendritic cells: past, present, and future. Annu Rev Immunol. 2012;30:1–22. doi: 10.1146/annurev-immunol-100311-102839. [DOI] [PubMed] [Google Scholar]
- 20.Inaba K, Inaba M, Romani N, Aya H, Deguchi M, Ikehara S, et al. Generation of large numbers of dendritic cells from mouse bone marrow cultures supplemented with granulocyte/macrophage colony-stimulating factor. J Exp Med. 1992;176:1693–1702. doi: 10.1084/jem.176.6.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goxe B, Latour N, Chokri M, Abastado JP, Salcedo M. Simplified method to generate large quantities of dendritic cells suitable for clinical applications. Immunol Invest. 2000;29:319–336. doi: 10.3109/08820130009060870. [DOI] [PubMed] [Google Scholar]
- 22.Romani N, Gruner S, Brang D, Kampgen E, Lenz A, Trockenbacher B, et al. Proliferating dendritic cell progenitors in human blood. J Exp Med. 1994;180:83–93. doi: 10.1084/jem.180.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maldonado RA, von Andrian UH. How tolerogenic dendritic cells induce regulatory T cells. Adv Immunol. 2010;108:111–165. doi: 10.1016/B978-0-12-380995-7.00004-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jonuleit H, Schmitt E, Steinbrink K, Enk AH. Dendritic cells as a tool to induce anergic and regulatory T cells. Trends Immunol. 2001;22:394–400. doi: 10.1016/s1471-4906(01)01952-4. [DOI] [PubMed] [Google Scholar]
- 25.Corinti S, Albanesi C, la Sala A, Pastore S, Girolomoni G. Regulatory activity of autocrine IL-10 on dendritic cell functions. J Immunol. 2001;166:4312–4318. doi: 10.4049/jimmunol.166.7.4312. [DOI] [PubMed] [Google Scholar]
- 26.Laouar Y, Town T, Jeng D, Tran E, Wan Y, Kuchroo VK, et al. TGF-beta signaling in dendritic cells is a prerequisite for the control of autoimmune encephalomyelitis. Proc Natl Acad Sci U S A. 2008;105:10865–10870. doi: 10.1073/pnas.0805058105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Szeles L, Keresztes G, Torocsik D, Balajthy Z, Krenacs L, Poliska S, et al. 1,25-dihydroxyvitamin D3 is an autonomous regulator of the transcriptional changes leading to a tolerogenic dendritic cell phenotype. J Immunol. 2009;182:2074–2083. doi: 10.4049/jimmunol.0803345. [DOI] [PubMed] [Google Scholar]
- 28.Rutella S, Bonanno G, Procoli A, Mariotti A, de Ritis DG, Curti A, et al. Hepatocyte growth factor favors monocyte differentiation into regulatory interleukin (IL)-10++IL-12low/neg accessory cells with dendritic-cell features. Blood. 2006;108:218–227. doi: 10.1182/blood-2005-08-3141. [DOI] [PubMed] [Google Scholar]
- 29.Soumelis V, Reche PA, Kanzler H, Yuan W, Edward G, Homey B, et al. Human epithelial cells trigger dendritic cell mediated allergic inflammation by producing TSLP. Nat Immunol. 2002;3:673–680. doi: 10.1038/ni805. [DOI] [PubMed] [Google Scholar]
- 30.Lutz MB, Suri RM, Niimi M, Ogilvie AL, Kukutsch NA, Rossner S, et al. Immature dendritic cells generated with low doses of GM-CSF in the absence of IL-4 are maturation resistant and prolong allograft survival in vivo. Eur J Immunol. 2000;30:1813–1822. doi: 10.1002/1521-4141(200007)30:7<1813::AID-IMMU1813>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 31.Hackstein H, Thomson AW. Dendritic cells: emerging pharmacological targets of immunosuppressive drugs. Nat Rev Immunol. 2004;4:24–35. doi: 10.1038/nri1256. [DOI] [PubMed] [Google Scholar]
- 32.Adorini L, Giarratana N, Penna G. Pharmacological induction of tolerogenic dendritic cells and regulatory T cells. Semin Immunol. 2004;16:127–134. doi: 10.1016/j.smim.2003.12.008. [DOI] [PubMed] [Google Scholar]
- 33.Martin E, Capini C, Duggan E, Lutzky VP, Stumbles P, Pettit AR, et al. Antigen-specific suppression of established arthritis in mice by dendritic cells deficient in NF-kappaB. Arthritis Rheum. 2007;56:2255–2266. doi: 10.1002/art.22655. [DOI] [PubMed] [Google Scholar]
- 34.Unger WW, Laban S, Kleijwegt FS, van der Slik AR, Roep BO. Induction of Treg by monocyte-derived DC modulated by vitamin D3 or dexamethasone: differential role for PD-L1. Eur J Immunol. 2009;39:3147–3159. doi: 10.1002/eji.200839103. [DOI] [PubMed] [Google Scholar]
- 35.Anderson AE, Sayers BL, Haniffa MA, Swan DJ, Diboll J, Wang XN, et al. Differential regulation of naive and memory CD4+ T cells by alternatively activated dendritic cells. J Leukoc Biol. 2008;84:124–133. doi: 10.1189/jlb.1107744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Leung DY, Bloom JW. Update on glucocorticoid action and resistance. J Allergy Clin Immunol. 2003;111:3–22. doi: 10.1067/mai.2003.97. quiz 23. [DOI] [PubMed] [Google Scholar]
- 37.Battaglia M, Stabilini A, Migliavacca B, Horejs-Hoeck J, Kaupper T, Roncarolo MG. Rapamycin promotes expansion of functional CD4+CD25+FOXP3+ regulatory T cells of both healthy subjects and type 1 diabetic patients. J Immunol. 2006;177:8338–8347. doi: 10.4049/jimmunol.177.12.8338. [DOI] [PubMed] [Google Scholar]
- 38.Horibe EK, Sacks J, Unadkat J, Raimondi G, Wang Z, Ikeguchi R, et al. Rapamycin-conditioned, alloantigen-pulsed dendritic cells promote indefinite survival of vascularized skin allografts in association with T regulatory cell expansion. Transpl Immunol. 2008;18:307–318. doi: 10.1016/j.trim.2007.10.007. [DOI] [PubMed] [Google Scholar]
- 39.Fischer R, Turnquist HR, Taner T, Thomson AW. Use of rapamycin in the induction of tolerogenic dendritic cells. Handbook of experimental pharmacology. 2009;188:215–232. doi: 10.1007/978-3-540-71029-5_10. [DOI] [PubMed] [Google Scholar]
- 40.Humbert JM, Halary F. Viral and non-viral methods to genetically modify dendritic cells. Current gene therapy. 2012;12:127–136. doi: 10.2174/156652312800099580. [DOI] [PubMed] [Google Scholar]
- 41.Fjose A, Ellingsen S, Wargelius A, Seo HC. RNA interference: mechanisms and applications. Biotechnol Annu Rev. 2001;7:31–57. doi: 10.1016/s1387-2656(01)07032-6. [DOI] [PubMed] [Google Scholar]
- 42.Yang DF, Qiu WH, Zhu HF, Lei P, Wen X, Dai H, et al. CTLA4-Ig-modified dendritic cells inhibit lymphocyte-mediated alloimmune responses and prolong the islet graft survival in mice. Transpl Immunol. 2008;19:197–201. doi: 10.1016/j.trim.2008.05.005. [DOI] [PubMed] [Google Scholar]
- 43.Tomasoni S, Aiello S, Cassis L, Noris M, Longaretti L, Cavinato RA, et al. Dendritic cells genetically engineered with adenoviral vector encoding dnIKK2 induce the formation of potent CD4+ T-regulatory cells. Transplantation. 2005;79:1056–1061. doi: 10.1097/01.tp.0000161252.17163.31. [DOI] [PubMed] [Google Scholar]
- 44.Ruffner MA, Robbins PD. Dendritic cells transduced to express interleukin 4 reduce diabetes onset in both normoglycemic and prediabetic nonobese diabetic mice. PloS one. 2010;5:e11848. doi: 10.1371/journal.pone.0011848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Naranjo-Gomez M, Raich-Regue D, Onate C, Grau-Lopez L, Ramo-Tello C, Pujol-Borrell R, et al. Comparative study of clinical grade human tolerogenic dendritic cells. J Transl Med. 2011;9:89. doi: 10.1186/1479-5876-9-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Boks MA, Kager-Groenland JR, Haasjes MS, Zwaginga JJ, van Ham SM, ten Brinke A. IL-10-generated tolerogenic dendritic cells are optimal for functional regulatory T cell induction--a comparative study of human clinical-applicable DC. Clin Immunol. 2012;142:332–342. doi: 10.1016/j.clim.2011.11.011. [DOI] [PubMed] [Google Scholar]
- 47.Sato K, Yamashita N, Yamashita N, Baba M, Matsuyama T. Regulatory dendritic cells protect mice from murine acute graft-versus-host disease and leukemia relapse. Immunity. 2003;18:367–379. doi: 10.1016/s1074-7613(03)00055-4. [DOI] [PubMed] [Google Scholar]
- 48.Cluff CW. Monophosphoryl lipid A (MPL) as an adjuvant for anti-cancer vaccines: clinical results. Adv Exp Med Biol. 2010;667:111–123. doi: 10.1007/978-1-4419-1603-7_10. [DOI] [PubMed] [Google Scholar]
- 49.Harry RA, Anderson AE, Isaacs JD, Hilkens CM. Generation and characterisation of therapeutic tolerogenic dendritic cells for rheumatoid arthritis. Ann Rheum Dis. 2010;69:2042–2050. doi: 10.1136/ard.2009.126383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Taner T, Hackstein H, Wang Z, Morelli AE, Thomson AW. Rapamycin-treated, alloantigen-pulsed host dendritic cells induce Ag-specific T cell regulation and prolong graft survival. Am J Transplant. 2005;5:228–236. doi: 10.1046/j.1600-6143.2004.00673.x. [DOI] [PubMed] [Google Scholar]
- 51.Morelli AE. The immune regulatory effect of apoptotic cells and exosomes on dendritic cells: its impact on transplantation. Am J Transplant. 2006;6:254–261. doi: 10.1111/j.1600-6143.2005.01197.x. [DOI] [PubMed] [Google Scholar]
- 52.Moreau A, Varey E, Beriou G, Hill M, Bouchet-Delbos L, Segovia M, et al. Tolerogenic dendritic cells and negative vaccination in transplantation: from rodents to clinical trials. Frontiers in immunology. 2012;3:218. doi: 10.3389/fimmu.2012.00218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Beriou G, Moreau A, Cuturi MC. Tolerogenic dendritic cells: applications for solid organ transplantation. Curr Opin Organ Transplant. 2012;17:42–47. doi: 10.1097/MOT.0b013e32834ee662. [DOI] [PubMed] [Google Scholar]
- 54.Giannoukakis N, Phillips B, Finegold D, Harnaha J, Trucco M. Phase I (safety) study of autologous tolerogenic dendritic cells in type 1 diabetic patients. Diabetes Care. 2011;34:2026–2032. doi: 10.2337/dc11-0472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Machen J, Harnaha J, Lakomy R, Styche A, Trucco M, Giannoukakis N. Antisense oligonucleotides down-regulating costimulation confer diabetes-preventive properties to nonobese diabetic mouse dendritic cells. J Immunol. 2004;173:4331–4341. doi: 10.4049/jimmunol.173.7.4331. [DOI] [PubMed] [Google Scholar]
- 56.Thomas R. Dendritic cells and the promise of antigen-specific therapy in rheumatoid arthritis. Arthritis research & therapy. 2013;15:204. doi: 10.1186/ar4130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Thomas R, Street S, Ramnoruth N. Safety and preliminary evidence of efficacy in a phase I clinical trial of autologous tolerizing dendritic cells exposed to citrullinated peptides (Rheumavax) in patients with rheumatoid arthritis. Ann Rheum Dis. 2011;70:169. [Google Scholar]
- 58.Stoop JN, Robinson JH, Hilkens CM. Developing tolerogenic dendritic cell therapy for rheumatoid arthritis: what can we learn from mouse models? Ann Rheum Dis. 2011;70:1526–1533. doi: 10.1136/ard.2011.151654. [DOI] [PubMed] [Google Scholar]
- 59.Stoop JN, Harry RA, von Delwig A, Isaacs JD, Robinson JH, Hilkens CM. Therapeutic effect of tolerogenic dendritic cells in established collagen-induced arthritis is associated with a reduction in Th17 responses. Arthritis Rheum. 2010;62:3656–3665. doi: 10.1002/art.27756. [DOI] [PubMed] [Google Scholar]
- 60.Hilkens CM, Isaacs JD. Tolerogenic dendritic cell therapy for rheumatoid arthritis: where are we now? Clin Exp Immunol. 2013;172:148–157. doi: 10.1111/cei.12038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Raich-Regue D, Grau-Lopez L, Naranjo-Gomez M, Ramo-Tello C, Pujol-Borrell R, Martinez-Caceres E, et al. Stable antigen-specific T-cell hyporesponsiveness induced by tolerogenic dendritic cells from multiple sclerosis patients. Eur J Immunol. 2012;42:771–782. doi: 10.1002/eji.201141835. [DOI] [PubMed] [Google Scholar]
- 62.Farias AS, Spagnol GS, Bordeaux-Rego P, Oliveira CO, Fontana AG, de Paula RF, et al. Vitamin D3 induces IDO(+) tolerogenic DCs and enhances Treg, reducing the severity of EAE. CNS neuroscience & therapeutics. 2013;19:269–277. doi: 10.1111/cns.12071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ezzelarab MB, Zahorchak AF, Lu L, Morelli AE, Chalasani G, Demetris AJ, et al. Regulatory dendritic cell infusion prolongs kidney allograft survival in nonhuman primates. Am J Transplant. 2013;13:1989–2005. doi: 10.1111/ajt.12310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Azimzadeh AM, Bromberg JS. Transplantation: Negative vaccination to modulate transplant immunity. Nature reviews Nephrology. 2013;9:557–559. doi: 10.1038/nrneph.2013.172. [DOI] [PubMed] [Google Scholar]
- 65.Zahorchak AF, Kean LS, Tokita D, Turnquist HR, Abe M, Finke J, et al. Infusion of stably immature monocyte-derived dendritic cells plus CTLA4Ig modulates alloimmune reactivity in rhesus macaques. Transplantation. 2007;84:196–206. doi: 10.1097/01.tp.0000268582.21168.f6. [DOI] [PubMed] [Google Scholar]