Abstract
A 25-amino-acid synthetic peptide (C6 peptide) derived from an immunodominant conserved region (designated IR6) of the VlsE protein of Borrelia burgdorferi has been identified and used to construct immunoenzyme-based diagnostic procedures. These procedures have excellent sensitivity and specificity. Previous reports have demonstrated the usefulness of the C6 peptide as an antigen for the serodiagnosis of human and canine Lyme disease. Results indicated that assays based on the C6 peptide were nonreactive to sera from vaccinated nonexposed animals. The purpose of the present study was to confirm these results in a controlled trial by testing sera from experimentally vaccinated dogs known to be uninfected. Nine specific-pathogen-free beagles were assigned to one of three vaccine groups, each containing three dogs. Each group received one of three commercial Lyme vaccines: RECOMBITEK Lyme (Merial), LymeVax (Fort Dodge Animal Health), and Galaxy Lyme (Schering-Plough Animal Health). Each animal was administered a total of five doses of vaccine over a period of 39 weeks. Serum samples were collected prior to vaccination and then on a weekly basis from weeks 3 to 18 and from weeks 33 to 43. Selected samples were tested by the immunofluorescence assay (IFA) and the Western blot (WB) assay using whole-cell B. burgdorferi antigen extracts, and the results were compared to those obtained with two immunoenzyme-based procedures formatted by using the C6 peptide. Serum specimens from all animals were reactive to the IFA and WB assay at week 5 and became highly reactive following the administration of multiple doses of vaccine. All serum specimens were uniformly nonreactive in the C6 peptide immunoenzyme procedures at all time points throughout the study.
The results of conventional serologic assays that are used to confirm the clinical diagnosis of Lyme disease in dogs are complicated by the widespread use of commercial vaccines. These vaccines produce an antibody response that cross-reacts in whole-cell immunofluorescence assays (IFAs) and enzyme-linked immunosorbent assays (ELISAs) commonly used to detect antibody to Borrelia burgdorferi (2, 6, 18). Lyme disease vaccines elicit strong antibody responses to outer surface protein A (OspA), OspB, and other antigenic proteins of B. burgdorferi (6), and some of these proteins are abundant in the whole-cell antigen-based preparations used as antigens in conventional assays. The Western blot (WB) assay is commonly used to distinguish between naturally induced and vaccine-driven antibody responses by identifying sera that contain antibodies reacting with OspA and/or OspB (3, 4, 6). A strong antibody response to one or both of these proteins is a commonly accepted characteristic of serum from a previously vaccinated animal. However, the infection status of vaccinated dogs is at times difficult to ascertain because of the presence of bands of similar molecular weights in WBs of serum specimens from nonexposed, vaccinated animals and specimens from exposed, vaccinated dogs (3, 4).
Recently, a short segment of a B. burgdorferi surface protein named VlsE (Vmp-like sequence, expressed) has been used (9-16), in addition to the full-length protein (7), to construct antibody detection assays that have excellent sensitivity and virtually eliminate false-positive results. This peptide, named C6, is 25 amino acids in length and reproduces the sequence of an immunodominant and conserved region (IR6) of VlsE (12). A distinguishing feature of assays constructed with this peptide is the ability to obtain meaningful results with sera from vaccinated animals. The C6 peptide-based ELISA has been reported to be nonreactive with serum samples from animals vaccinated with either the OspA or the whole-cell (bacterin) Lyme disease vaccines (14, 15).
The purpose of this study was to conduct a controlled vaccination and testing trial, using experimental animals that were known to be uninfected, to unequivocally determine if serological assays using the C6 peptide developed by IDEXX Laboratories, Inc. (Westbrook, Maine), for B. burgdorferi antibody reacted with antibody resulting from vaccination. It was necessary to conduct such a vaccination study because of the ambiguity associated with reading WB results obtained from field populations of vaccinated dogs. We were not able to reliably distinguish populations of exposed and nonexposed vaccinated animals using the results of the WB assay as the sole criterion. Sera from vaccinated dogs known to be uninfected were needed to conclusively demonstrate the absence or presence of a reaction of C6 with vaccine-induced antibody.
Nine specific-pathogen-free dogs were vaccinated five times over a period of 39 weeks with three commonly used commercial Lyme disease vaccines. This frequency of vaccine administration was greater than that recommended by the vaccine manufacturers. The purpose of this experimental design was to induce high titers of antibody to each vaccine. Serum samples were obtained several times and tested by the IFA and WB assay to verify that the animals had received vaccine and that a vaccine-mediated response had been induced. The same samples were tested with a microtiter plate format C6-peptide ELISA and by a commercial lateral-flow B. burgdorferi antibody immunoassay (SNAP 3Dx test; IDEXX Laboratories, Inc.) constructed using the C6 peptide (8). Here we report the results of these studies.
MATERIALS AND METHODS
Serum samples from experimentally vaccinated dogs.
The experimental vaccinations were done in collaboration with Covance Research Products (Denver, Pa.) with a standard kennel facility located in Kalamazoo, Mich. A total of 10 purebred specific-pathogen-free beagle dogs (number 2; 5 to 7 months old, three males and seven females) were obtained from the Covance Research Products stock colony. All animals received the usual vaccines and medication per Covance Research Products standard operating procedure. Noncertified canine diet number 5L03 (PMI Feeds, Inc., St. Louis, Mo.) and tap water from a well supply were provided ad libitum. One dog served as a control, and each of the remaining nine dogs was assigned to one of three vaccine groups. Each vaccine group comprised three dogs, and all dogs in each group received one of the following vaccines: RECOMBITEK Lyme (Merial, Athens, Ga.), LymeVax (Fort Dodge Animal Health, Fort Dodge, Iowa), and Galaxy Lyme (Schering-Plough Animal Health, Omaha, Nebr.). Local veterinarians obtained the vaccines from veterinary distributors. Each animal received a total of 0.5 to 1.0 ml of vaccine at each administration. Each dose was divided into two intramuscular or subcutaneous injections (based on the vaccine manufacturers' instructions) and administered in two different injection sites. Vaccine was administered at weeks 0, 2, 33, 36, and 39. Test bleeds were obtained weekly between weeks 3 and 18 and weeks 33 and 43. Bleeds also were obtained immediately before the administration of the vaccine at weeks 0, 33, 36, and 39. Whole blood was collected via a jugular vein into glass Vacutainer tubes containing no anticoagulant. Serum was obtained from these samples and stored frozen at −20 to −80°C.
IFA and WB assay.
An indirect IFA was performed using whole-cell B. burgdorferi antigen-coated IFA slides (Bion Enterprises, Des Plaines, Ill.) and fluorescein isothiocyanate-labeled goat anti-canine immunoglobulin G (IgG) (Jackson Immunodiagnostics, West Grove, Pa.). The IFA slides were made with a low-passage-number B-31 strain of B. burgdorferi. The conjugate stock was diluted 1:50 in 50 mM carbonate buffer (pH 9.5) containing 150 mM NaCl. Dilutions of test samples were made in phosphate-buffered saline (pH 7.2) and incubated with the antigen-coated IFA slides. The slides were washed, incubated with fluorescein isothiocyanate-labeled conjugate, and viewed by UV light microscopy. Samples with IFA titers greater than or equal to 100 were positive. WBs for B. burgdorferi antibody were run with the QualiCode canine Lyme disease kit (Immunetics, Inc., Cambridge, Mass.) by using directions supplied with the kit. The WB strips were prepared with antigen obtained from a low-passage-number B-31 strain of B. burgdorferi. Specific bands were identified by comparison of the results obtained with the kit positive control to a template supplied with the kit.
Synthesis of the C6 peptide.
The amino acid sequence of the B. burgdorferi sensu lato synthetic C6 peptide is Met-Lys-Lys-Asp-Asp-Gln-Ile-Ala-Ala-Ala-Met-Val-Leu-Arg-Gly-Met-Ala-Lys-Asp-Gly-Gln-Phe-Ala-Leu-Lys. These are the same 25 amino acids in the published sequence of IR6 found within the variable surface antigen (VlsE) of B. burgdorferi sensu lato (15). An amino-terminal Cys residue was added to the peptide to facilitate chemical coupling. The linear peptide raw material was synthesized at IDEXX on a Perkins-Elmer (Applied Biosystems Division, Foster City, Calif.) model 433A peptide synthesizer by using standard 9-fluorenylmethoxycarbonyl chemistry methods (1). The amino terminus of the peptide was esterified with acetic anhydride. The peptide was deprotected and cleaved from the resin in trifluoroacetic acid with scavengers by using standard methods for 9-fluorenylmethoxycarbonyl chemistry. The cleaved peptide product was precipitated, washed, lyophilized, and stored at 2 to 7°C or frozen for long-term storage in sealed bags with desiccant. The identity of the peptide was verified by mass spectral analysis. The C6 multiple antigenic peptide (C6-MAP) was synthesized with a four-branch lysine core (Applied Biosystems). The synthesis, cleavage, processing, and storage of C6-MAP were identical to those of the linear peptide.
Immunoassays for antibody to the C6 peptide.
The immunoassays for antibody to the C6 peptide were performed using the SNAP 3Dx test and a microtiter plate format ELISA (IDEXX Laboratories, Inc.). The SNAP 3Dx test is an immunoassay for simultaneous detection of canine heartworm antigen, antibodies to Ehrlichia canis, and antibodies to B. burgdorferi in canine serum, plasma, or whole blood. The SNAP assay format utilizes IDEXX's proprietary assay device, which provides reversible chromatographic flow of sample, and automatic, sequential flow of wash and enzyme substrate. The linear C6 synthetic peptide was conjugated to bovine serum albumin (BSA) and to horseradish peroxidase (HRP) by standard methods (5). The BSA-peptide conjugate was deposited onto a flow matrix. The HRP-peptide conjugate was added to a conjugate diluent containing nonspecific proteins and detergent. Two drops of test sample were mixed with 5 drops of conjugate and applied to the flow matrix as described in the kit package insert. The B. burgdorferi antibody, if present in the sample, would bind to the synthetic-peptide-HRP conjugate and to the synthetic-peptide-BSA conjugate. The deposited peptide-BSA conjugate is then exposed to wash and substrate reagents in the course of the assay. Color development in the area of deposition of the BSA-peptide conjugate would signify a positive result. The sensitivity and specificity of the SNAP 3Dx assay for B. burgdorferi antibody were determined by testing B. burgdorferi IFA-positive and -negative canine samples on SNAP 3Dx. The WB assay confirmed discrepant results. The SNAP 3Dx test was positive for 238 of 252 B. burgdorferi antibody-positive samples. The positive samples were from submissions to the IDEXX reference laboratory in Grafton, Mass. There were 18 IFA-reactive, SNAP 3Dx-negative samples that were tested by the WB assay and were shown to be from vaccinated animals with no evidence of natural infection (6.7% of IFA-positive samples). These samples were not considered to be part of the positive group. The sensitivity of the SNAP 3Dx test was 94.4% (238 of 252 samples).
Each of 203 B. burgdorferi antibody-negative samples tested on SNAP 3Dx was negative. The specificity of the SNAP 3Dx test in this population was 100% (203 of 203 samples).
The microtiter plate format ELISA was performed using C6-MAP-coated microtiter plates and a rabbit anti-canine IgG-HRP conjugate (Jackson Immunodiagnostics). The ELISA was optimized by testing dilutions of samples known to be positive and negative on microtiter plates (Immulon I; Dynatech Laboratories, Chantilly, Va.) coated with different levels of the C6-MAP in a checkerboard fashion with different concentrations of the anti-canine IgG-HRP conjugate. Optimized microtiter plates were coated with 1 μg of the C6-MAP per ml in 0.2 M phosphate buffer (pH 7.5) for 12 to 16 h at 3°C. Plates were washed three times with phosphate-buffered saline solution containing 0.05% Tween 20 (PBS-T), incubated with fish gelatin and sucrose solutions, and dried. Test samples were diluted 1:50 in PBS-T containing BSA, fish gelatin, and calf serum. A volume of 100 μl was added to individual microtiter wells and incubated for 30 min at room temperature. The contents of the microtiter wells were aspirated, washed three times with PBS-T, and incubated (at room temperature for 30 min) with rabbit anti-canine IgG-HRP conjugate diluted 1:3,000 in a conjugate diluent containing nonspecific proteins and detergent.
The microtiter plate wells were aspirated, washed three times as described above, and incubated with substrate solution containing 3,3′,5,5′-tetramethylbenzidine at room temperature. Optical density (OD) values were determined spectrophotometrically at 650 nm. The microtiter plate assay was qualified by testing field samples that were known to be positive and presumed to be negative. One hundred twenty-one samples reactive in the whole-cell B. burgdorferi IFA were obtained from the IDEXX reference laboratory in Grafton, Mass., and tested with the C6 microtiter plate test. One hundred four of these samples were positive. The 17 negative samples were tested by WB assay and found to be from either vaccinated animals with no evidence of natural infection (n = 16) or a nonexposed animal (n = 1). The sensitivity of the microtiter plate test in this population was 100%. Microtiter plate test specificity was evaluated by testing 39 canine samples from a region of Europe (England) in which Lyme disease is not endemic. The specificity of the microtiter plate test in this population was 97.4% (38 of 39). The mean OD of the 39 canine samples was 0.051 (standard deviation, 0.013), and the assay cutoff was an OD of 0.096. Several very weak SNAP 3Dx-positive canine field samples were used as positive controls for the microtiter plate test.
This study was conducted in accordance with applicable Covance Research Products, Inc., standard operating procedures. All procedures are in compliance with U.S. Animal Welfare Act regulations (9 Code of Federal Regulations 3).
RESULTS
Serum samples.
Sera from animals were obtained on a weekly basis, and samples were tested by the IFA, the WB assay, and the C6 microtiter plate ELISA and with the C6 SNAP 3Dx kit. All samples were negative for canine heartworm antigen and antibody to E. canis throughout the study. All animals were negative for antibody to B. burgdorferi by all assays at the start of the study.
IFA.
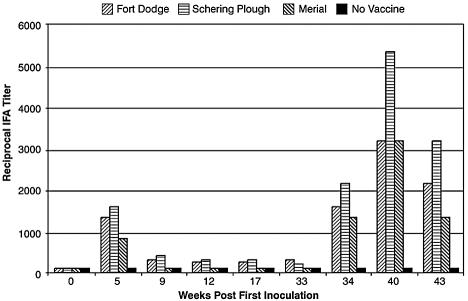
Sera from all of the vaccinated animals had elevated IFA titers ranging from 800 to 1,600 at week 5 following the initial vaccination. These values declined 4- to 16-fold (titer range, 100 to 400) by week 33 but increased markedly following administration of additional doses of vaccine at weeks 33, 36, and 39. The IFA titers reached their highest levels (titer range, 1,600 to 6,400) 1 week following the fifth vaccination (week 40). The mean IFA titer for each vaccine group throughout the course of the study is shown in Fig. 1. Assay results for key time points are given in Table 1.
FIG. 1.
Mean reciprocal IFA titer results for serum samples from experimentally vaccinated animals. IFA results are shown for serum specimens collected throughout the study. Reciprocal IFA titers are given for the mean obtained from three dogs in each vaccine group. The IFA titer was <100 for serum samples from the single unvaccinated control dog at all time points. Dogs were vaccinated at weeks 0, 2, 33, 36, and 39 as described in Materials and Methods. Serum samples were obtained prior to vaccination at weeks 0 and 33. Vaccine manufacturers were Fort Dodge, Schering-Plough, and Merial.
TABLE 1.
Assay results for experimentally vaccinated dogsa
| Vaccine | Sample time | SNAP 3Dx result | IFA titer, dog 1, 2, 3 | WB result, (molecular mass[es] of reactive band[s] [kDa]) | C6 peptide microtiter plate ELISA result (mean ODb) |
|---|---|---|---|---|---|
| LymeVax (Fort Dodge-Animal Health) | Prebleed | Neg | All < 100 | Neg | 0.053 |
| Wk 5 | Neg | 1,600, 1,600, 800 | 41, 34, 31, 18 | 0.060 | |
| Wk 33 | Neg | 400, 400, 100 | 41, 39, 34, 31, 21, 18 | 0.061 | |
| Wk 34 | Neg | 1,600, 1,600, 1,600 | 93, 66, 60, 58, 41, 39, 34, 31, 21, 18 | 0.062 | |
| Wk 40 | Neg | 6,400, 1,600, 1,600 | 93, 66, 60, 58, 41, 39, 34, 31, 30, 28, 23, 21, 18 | 0.073 | |
| Wk 43 | Neg | 3,200, 1,600, 1,600 | Not tested | 0.069 | |
| Galaxy (Schering-Plough Animal Health) | Prebleed | Neg | All < 100 | Neg | 0.043 |
| Wk 5 | Neg | 1,600, 1,600, 1,600 | 66, 58, 41, 39, 34, 31, 21, 18 | 0.079 | |
| Wk 33 | Neg | 400, 100, 100 | 66, 58, 39, 34, 31, 21 | 0.072 | |
| Wk 34 | Neg | 3,200, 1,600, 1,600 | 93, 66, 60, 58, 41, 39, 34, 31, 30, 23, 21, 18 | 0.069 | |
| Wk 40 | Neg | 6,400, 6,400, 3,200 | 93, 66, 60, 58, 41, 39, 34, 31, 30, 23, 21, 18 | 0.078 | |
| Wk 43 | Neg | 3,200, 3,200, 3,200 | Not tested | 0.072 | |
| RECOMBITEK Lyme (Merial) | Prebleed | Neg | All < 100 | 41 | 0.043 |
| Wk 5 | Neg | 800, 800, 800 | 41, 31 | 0.087 | |
| Wk 33 | Neg | 100, 100, 100 | 41, 31 | 0.058 | |
| Wk 34 | Neg | 1,600, 1,600, 800 | 93, 66, 41, 31, 18 | 0.060 | |
| Wk 40 | Neg | 3,200, 3,200, 3,200 | 93, 66, 41, 31, 18 | 0.064 | |
| Wk 43 | Neg | 1,600, 1,600, 800 | Not tested | 0.065 | |
| None (control) | Prebleed | Neg | All < 100 | Neg | 0.055 |
| Wk 5 | Neg | All < 100 | Neg | 0.054 | |
| Wk 33 | Neg | All < 100 | Neg | 0.066 | |
| Wk 34 | Neg | All < 100 | Neg | 0.055 | |
| Wk 40 | Neg | All < 100 | Neg | 0.053 | |
| Wk 43 | Neg | All < 100 | Not tested | 0.067 |
Neg, negative.
OD, optical density at 650 nm. Assay cutoff, 0.097.
WB assay.
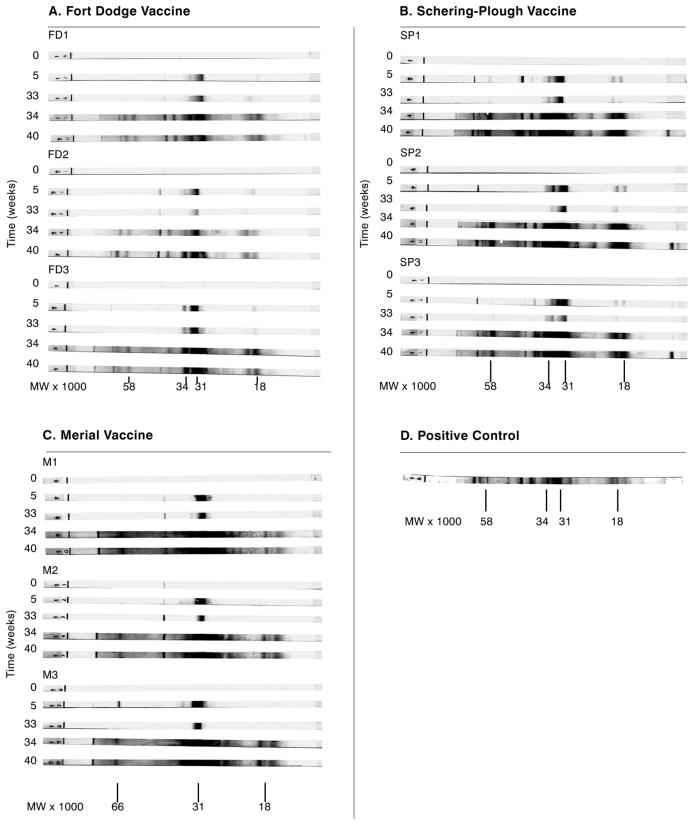
At week 5 after the first vaccine dose, sera from dogs immunized with bacterin vaccines (Schering-Plough and Fort Dodge) yielded prominent WB bands at positions corresponding to 31 and 34 kDa (OspA and OspB, respectively) and less intense bands at positions corresponding to molecular masses of 18, 21, 39, 41, 58, 60, 66, and 93 kDa (Fig. 2A and B). Sera from animals immunized with the recombinant OspA vaccine (Merial) had a single prominent WB band corresponding to 31 kDa and less intense bands corresponding to molecular masses of approximately 18 and 41 kDa (Fig. 2C). WB bands obtained with sera from all of the vaccinated dogs became slightly less intense during the period between week 5 and week 33, when vaccine was not administered (Fig. 2). Bands became more intense and numerous at later time points in the study (weeks 34 and 40) (Table 1; Fig. 2) following the administration of the additional doses of vaccine on weeks 33, 36, and 39.
FIG. 2.
Western blot results for serum samples obtained from experimentally vaccinated dogs prior to and at various times (weeks) following initial vaccination. Vaccine was administered to dogs at weeks 0, 2, 33, 36, and 39; WB results are shown for sera collected at weeks 0, 5, 33, 34, and 40. Serum samples were obtained prior to vaccination at weeks 0 and 33. (A) Fort Dodge LymeVax vaccine, administered to dogs FD1, FD2, and FD3; (B) Schering-Plough Galaxy Lyme vaccine, administered to dogs SP1, SP2, and SP3; (C) Merial RECOMBITEK Lyme vaccine, administered to dogs M1, M2, and M3; (D) positive control. Sera from the unvaccinated control dog failed to produce clearly visible bands in the WB assay at any time. WBs were run with the QualiCode canine Lyme disease kit (Immunetics, Inc.) as described in Materials and Methods.
C6 immunoenzyme assays.
The C6 microtiter plate and the SNAP 3Dx assay results were negative for all samples from all vaccinated and control animals at all time points (Table 1).
DISCUSSION
A sensitive and specific serologic assay that is not cross-reactive with Lyme disease vaccine antibodies would be immensely useful in monitoring exposure to B. burgdorferi in vaccinated dogs. The design of the experiments whose results are reported herein was aimed at offering conclusive evidence that C6-based immunoenzyme assays, already shown to be sensitive and specific (11, 14, 15), did not react with serum antibodies from vaccinated dogs that were known to be free of a B. burgdorferi infection. The results obtained amply demonstrate the absence of such cross-reactivity in the face of vaccine antibody titers, as measured by IFA, that are probably higher than titers obtained with a regular two-dose vaccine administration regimen. In fact, serum specimens from dogs that had received the recommended two-dose regimen of whole-cell (bacterin) or recombinant OspA vaccines had IFA titers ranging from 800 to 1,600 at 3 weeks following the administration of vaccine (week 5), whereas IFA titers reached values of up to 6,400 after the administration of additional vaccine doses. Nonetheless, these serum specimens exhibited no reactivity in the C6-based tests.
The results obtained with the WB assay further illustrate the extent to which the absence of cross-reactivity of the C6 tests is reliable. Serum samples from dogs that had been vaccinated with the whole-bacterin vaccines reacted initially with OspA (31 kDa) and OspB (34 kDa), while samples from animals that had received the recombinant OspA vaccine reacted primarily, as expected, with the OspA protein. However, after additional vaccine doses were administered, serum from animals that had received bacterin-type vaccines displayed multiple WB bands, and even the OspA-vaccinated animals showed serum antibody specificities other than just OspA. As antibody affinity matures, following repeated administration of antigen, high-affinity antibodies tend to react more broadly, as shown in these WBs. Despite this broadening of antigenic specificity of the vaccine-induced antibodies, reactivity with the C6 peptide remained undetectable. The WB results also illustrate the inherent difficulty of using WB assays to detect B. burgdorferi infection or exposure in a vaccinated dog, as bands reflecting infection or exposure would have to be discerned from the potentially numerous vaccine-derived bands.
In closing, we put forward what we believe is a likely explanation for the absence of anti-C6 antibody in vaccinated dog serum. Serodiagnosis with the C6 peptide has been found to be highly specific (11, 14, 15). Except for sequences present in the vlsE gene and vls loci of B. burgdorferi, no other sequences homologous to C6 could be identified by the BLAST search algorithm in the National Center for Biotechnology Information protein sequence database (15). The uniqueness of the C6 sequence readily explains the absence of cross-reactivity between antibodies elicited by the OspA vaccine and C6. The failure of the bacterin-type vaccines to elicit anti-C6 antibody is probably due to the loss of the lp 28-1 plasmid, which contains the vlsE gene, by the spirochete stocks used to generate such vaccines. As few as five culture passages are sufficient to generate B. burgdorferi clones that lack the lp 28-1 plasmid (17), and it is likely that the spirochetal isolates used to produce bacterin vaccines have undergone many more such passages and have therefore lost the ability to express VlsE.
The C6 peptide technology offers a more reliable single-test alternative to the standard two-tier method (18) of Lyme disease diagnosis in dogs. Diagnostic assays utilizing the C6 peptide provide a unique capability that is particularly needed in areas of the country where Lyme disease vaccines are frequently administered and where the disease is endemic. At issue is the problem of distinguishing between animals that are naturally infected and those that are vaccinated. In these populations, C6 peptide-based assays for Lyme disease antibody circumvent problems related to vaccine-induced antibody and provide an effective procedure for routine testing.
REFERENCES
- 1.Barony, G., and R. B. Merrifield. 1980. The peptides: analysis, synthesis, & biology. Academic Press, Inc., New York, N.Y.
- 2.Barthold, S. W., S. A. Levy, E. Fikrig, L. K. Bockenstedt, and A. L. Smith. 1995. Serologic responses of dogs naturally exposed to or vaccinated against Borrelia burgdorferi infection. J. Am. Vet. Med. Assoc. 207:1435-1440. [PubMed] [Google Scholar]
- 3.Gauthier, D. T., and L. S. Mansfield. 1999. Western immunoblot analysis for distinguishing vaccination and infection status with Borrelia burgdorferi (Lyme disease) in dogs. J. Vet. Diagn. Investig. 11:259-265. [DOI] [PubMed] [Google Scholar]
- 4.Guerra, M. A., E. D. Walker, and U. Kitron. 2000. Quantitative approach for the serodiagnosis of canine Lyme disease by the immunoblot procedure. J. Clin. Microbiol. 38:2628-2632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hashida, S., M. Imagawa, S. Inoue, K. H. Ruan, and E. Ishikawa. 1984. More useful maleimide compounds for the conjugation of Fab′ to horseradish peroxidase through the thiol groups in the hinge. J. Appl. Biochem. 6:56-63. [PubMed] [Google Scholar]
- 6.Jacobson, R. H., Y. F. Chang, and S. J. Shin. 1996. Lyme disease: laboratory diagnosis of infected and vaccinated symptomatic dogs. Semin. Vet. Med. Surg. (Small Anim.) 11:172-182. [DOI] [PubMed] [Google Scholar]
- 7.Lawrenz, M. B., J. M. Hardham, R. T. Owens, J. Nowakowski, A. C. Steere, G. P. Wormser, and S. J. Norris. 1999. Human antibody responses to VlsE antigenic variation protein of Borrelia burgdorferi. J. Clin. Microbiol. 37:3997-4004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Levy, S., T. P. O'Connor, J. L. Hanscom, and P. Shields. 2002. Utility of an in-office C6 ELISA test kit for determination of infection status of dogs naturally exposed to Borrelia burgdorferi. Vet. Ther. 3:308-315. [PubMed] [Google Scholar]
- 9.Liang, F. T., and M. T. Philipp. 1999. Analysis of antibody response to invariable regions of VlsE, the variable surface antigen of Borrelia burgdorferi. Infect. Immun. 67:6702-6706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liang, F. T., and M. T. Philipp. 2000. Epitope mapping of the immunodominant invariable region of Borrelia burgdorferi VlsE in three host species. Infect. Immun. 68:2349-2352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liang, F. T., E. Aberer, M. Cinco, L. Gern, C. M. Hu, Y. N. Lobet, M. Ruscio, P. E. Voet, Jr., V. E. Weynants, and M. T. Philipp. 2000. Antigenic conservation of an immunodominant invariable region of the VlsE lipoprotein among European pathogenic genospecies of Borrelia burgdorferi SL. J. Infect. Dis. 182:1455-1462. [DOI] [PubMed] [Google Scholar]
- 12.Liang, F. T., A. L. Alvarez, Y. Gu, J. M. Nowling, R. Ramamoorthy, and M. T. Philipp. 1999. An immunodominant conserved region within the variable domain of VlsE, the variable surface antigen of Borrelia burgdorferi. J. Immunol. 163:5566-5573. [PubMed] [Google Scholar]
- 13.Liang, F. T., L. C. Bowers, and M. T. Philipp. 2001. C-terminal invariable domain of VlsE is immunodominant but its antigenicity is scarcely conserved among strains of Lyme disease spirochetes. Infect. Immun. 69:3224-3231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liang, F. T., R. H. Jacobson, R. K. Straubinger, A. Grooters, and M. T. Philipp. 2000. Characterization of a Borrelia burgdorferi VlsE invariable region useful in canine Lyme disease serodiagnosis by enzyme-linked immunosorbent assay. J. Clin. Microbiol. 38:4160-4166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liang, F. T., A. C. Steere, A. R. Marques, B. J. B. Johnson, J. N. Miller, and M. T. Philipp. 1999. Sensitive and specific serodiagnosis of Lyme disease by enzyme-linked immunosorbent assay with a peptide based on an immunodominant conserved region of Borrelia burgdorferi VlsE. J. Clin. Microbiol. 37:3990-3996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Philipp, M. T., L. C. Bowers, P. T. Fawcett, M. B. Jacobs, F. T. Liang, A. R. Marques, P. D. Mitchell, J. E. Purcell, M. S. Ratterree, and R. M. Straubinger. 2001. Antibody response to IR6, a conserved immunodominant region of the VlsE lipoprotein, wanes rapidly after antibiotic treatment of Borrelia burgdorferi infection in experimental animals and in humans. J. Infect. Dis. 184:870-878. [DOI] [PubMed] [Google Scholar]
- 17.Purser, J. E., and S. J. Norris. 2000. Correlation between plasmid content and infectivity in Borrelia burgdorferi. Proc. Natl. Acad. Sci. USA 97:13865-13870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sheets, J. T., C. A. Rossi, B. J. Kearney, and G. E. Moore. 2000. Evaluation of a commercial enzyme-linked immunosorbent assay for detection of Borrelia burgdorferi exposure in dogs. J. Am. Vet. Med. Assoc. 216:1418-1422. [DOI] [PubMed] [Google Scholar]