Abstract
Introduction and Objectives
Numerous studies have assessed cost-effectiveness of different treatment modalities for stable angina. Direct comparisons, however, are uncommon. We therefore set out to compare the efficacy and mean cost per patient after 1 and 3 years of follow-up, of the following treatments as assessed in randomized controlled trials (RCT): medical therapy (MT), percutaneous coronary intervention (PCI) without stent (PTCA), with bare-metal stent (BMS), with drug-eluting stent (DES), and elective coronary artery bypass graft (CABG).
Methods
RCT comparing at least two of the five treatments and reporting clinical and cost data were identified by a systematic search. Clinical end-points were mortality and myocardial infarction (MI). The costs described in the different trials were standardized and expressed in US $ 2008, based on purchasing power parity. A network meta-analysis was used to compare costs.
Results
Fifteen RCT were selected. Mortality and MI rates were similar in the five treatment groups both for 1-year and 3-year follow-up. Weighted cost per patient however differed markedly for the five treatment modalities, at both one year and three years (P<0.0001). MT was the least expensive treatment modality: US $3069 and 13 864 after one and three years of follow-up, while CABG was the most costly: US $27 003 and 28 670 after one and three years. PCI, whether with plain balloon, BMS or DES came in between, but was closer to the costs of CABG.
Conclusions
Appreciable savings in health expenditures can be achieved by using MT in the management of patients with stable angina.
Introduction
Expenses related to the management of coronary artery disease represent a considerable burden for healthcare systems. The estimated direct and indirect cost of heart disease in 2010 in the USA was $177.13 billion [1]. The recent increase in expenditure can be explained by the rising number of invasive procedures, and by higher costs for percutaneous coronary intervention (PCI) due to the widespread use of drug-eluting stents (DES). In the USA, coronary revascularization is one of the most common major medical interventions provided by the healthcare system; between 2001 and 2008, the number of coronary revascularization procedures rose by 6% with over 1 million performed in 2006 [2]. In the same year, in the USA, over 70% of PCI were performed with DES [3]. Although DES do reduce the risk of repeat procedures as compared to bare-metal stents (BMS), widespread use of this technique has not led to the anticipated reduction in the total number of procedures performed [4].
Clinical data have failed to demonstrate clear superiority of any of the treatment modalities available (medical therapy alone, PCI or coronary artery bypass graft [CABG]) for stable coronary artery disease in terms of hard clinical events [5]–[7] for non-specific populations [8] (i.e., patients with diabetes, peripheral arterial disease, etc). Comparing the costs of these different management strategies therefore appears warranted and numerous studies have previously assessed the cost-effectiveness of the different pairwise therapeutic options [9]–[14].
In order to clarify this important public healthcare issue, we set out to compare, through a network meta-analysis, the efficacy and mean cost per patient (after one and three years of follow-up) of the following treatments as assessed in randomized controlled trials (RCT): MT, percutaneous coronary intervention without stent (PTCA), with BMS, with -DES, and elective CABG.
Methods
Search strategy
Our strategy involved searching Medline via PubMed, Embase and the Cochrane library and relevant websites (www.theheart.org, www.pcronline.com, www.tctmd.com, www.clinicaltrialresults.org, www.crtonline.org and stent manufacturer web pages). The search was also extended to proceedings of the American Heart Association, the American College of Cardiology, the British Cardiac Society and the European Society of Cardiology.
Keywords (used as free text words) for the PubMed search were "coronary artery disease", "myocardial revascularization" and "costs". We selected the following filters: "humans", "clinical trial" (for the type of study) and "English" for the language. The same keywords were used to search in the Cochrane Library. For the Embase search, two keywords were combined: "ischemic heart disease" and "cost". The search was filtered on the term "humans", and limited to RCT. Lastly, to avoid duplication, we excluded the PubMed database that is accessible via Embase (figure S1).
The search was restricted to the period between January 1, 1980 and June 1, 2012.
Two authors (TC and BS) independently reviewed titles, abstract, and the full text as required to determine whether the studies met our inclusion criteria. Any conflict between reviewers was resolved by re-review and discussion.
Inclusion and exclusion criteria
We conducted our analysis on adult patients with stable or stabilized unstable angina (stabilization was defined by symptoms older than 48 hours) or documented silent myocardial ischemia. All patients were assessed, regardless of whether they had single or multivessel coronary artery disease. Indeed, for these clinical situations, all three treatment modalities (MT only, PCI, CABG) are considered possible alternatives by learned societies, based on the results of RCT and the related meta-analyses [5]–[7], [15], [16]. We did not include RCT where patients had acute coronary syndromes (ACS), non-stabilized unstable angina (symptoms within 48 hours of randomization) or myocardial infarction in the previous 48 hours (MI), because revascularisation is usually considered the preferred therapeutic option for such patients [17], [18]. We also excluded studies performed in patients with in-stent restenosis because revascularization is the standard approach in these situations [19]–[21].
We selected all published randomized controlled trials that documented at least two of the five treatment modalities: MT, PTCA without stent, PCI with BMS or DES and elective CABG with cardiopulmonary bypass. Time periods with at least one event in any group were included in the analyses.
Studies were included in the clinical review if they reported 1) rates of death and MI, and 2) direct costs due to medical expenses for the management of the disease over a follow-up period of one year and/or three years. Indeed, costs relating to treatment of stable coronary artery disease are related to hospitalization due to complications such as subsequent revascularisation, MI and death.
We excluded all studies focusing on specific patient profiles, such as those with as diabetes mellitus, all studies with data based on economic models, and studies on non-conventional treatment modalities such as off-pump coronary artery bypass grafting, complete vessel treatment with PCI, etc. Lastly, all studies comprising clinical data alone were also excluded.
Data extraction and cost conversions
We recorded information from each trial about the publication (first author, journal, and year of publication); patient demographics (mean age, proportion of men, prior revascularization, prior MI, diabetic participants, and patients with multivessel disease); the type of treatments that were compared and the number of patients assigned to each group; years of patient enrolment; whether the trial was blinded; and follow-up duration.
We recorded death and MI rates in each arm of the studies. To study the economical outcome we sought the direct costs related to treatment in each study. We extracted the direct medical-care costs for the management of the disease. Costs were recorded with the currency and year of calculation.
A cascading adjustment was made to generate costs for the patient that would be comparable across the different countries. We used a comparison adjustment by purchasing power parity (PPP). The costs recorded ineach study were converted into US $ 2008 and then: 1) costs were collected in the original currency used in the study; 2) if necessary, costs were converted into the currency of the country where the economic study was conducted; 3) between the year the costs were calculated and 2008, we applied the consumer price index of the country where the economic study was conducted; 4) costs were converted to US $ 2008 using the PPP in 2008 (available on the Organisation for Economic Co-operation and Development website [15]). The currency conversion rate expresses the purchasing power of different currencies in one common unit (i.e. US $); it incorporates not only the exchange rates between currencies, but also the amount of currency needed to buy the same basket of goods and services in different countries. This method has been used previously in several studies [16]–[18].
Additionally, for each RCT, we recorded the source of the costs studied. Direct costs of treatment for stable coronary artery disease were related to hospitalisation (for an initial revascularization procedure or for management of complications), to outpatient care (medical visit, radiological and biological examinations, etc), and to outpatient drug prescriptions (antiplatelet drugs, antianginal drugs, etc).
Assessment of methodological quality
Quality was evaluated using two checklists. Relevance of clinical data was assessed using methods put forward by the Heart Collaborative Review Group [19]. The 4 criteria considered are: the randomization process, the allocation concealment process, the potential for selection bias after allocation and the adequacy of masking. For each criterion, three or four answers are possible, "A" being the best. The “Drummond checklist” was used to measure the methodological quality of full economic evaluations conducted alongside single effectiveness studies [20]. This checklist evaluates 35 criteria grouped into three themes: study design, data collection, and analysis and interpretation of results. These checklists are presented in the figures S2 and S3.
Statistical analysis
We performed a network meta-analysis to compare MT versus PTCA versus PCI with BMS versus PCI and with DES versus CABG, separately all with regard to rates for death and MI.
Initially, Bayesian random effects models were used for multiple treatment comparisons; this approach preserves the within-trial randomised treatment comparison of each analysis. We compared the five treatments after one year of follow-up and then after three years of follow-up. Then, we used an extension of this model to compare the five treatment approaches throughout the whole follow-up period [21]–[24]. We used a random walk model based on piece-wise constant hazards to account for varying follow-up times [25]. In a random walk model, log hazard at time t depends on the log hazard at previous times. The model included random effects of the trials, adjacent time periods, interaction between trials and periods and treatment comparisons, and was fitted to the three pre-specified time periods (years 1 to 3).
Lastly, a sensitivity analysis was conducted to verify the robustness of the results. This focused only on studies that included outpatient costs (outpatient care and/or outpatient drugs) in addition to hospital costs.
Hazard ratios (HR) and cumulative incidences were estimated from the median of the posterior distribution. A HR lower than 1 indicates a benefit from the treatment. All results are given with 95% credibility intervals (CI) from the 2.5th and 97.5th percentiles of the posterior distribution. A result was considered significant when the CI of the HR did not contain 1. We also calculated the probability that each treatment was the best.
All results are based on 130 000 simulations with 30 000 burn-in. In all analyses, MT was considered as the reference treatment.
Mean costs, weighted by the number of patients in each study for each treatment, were calculated and compared by an ANOVA after 1 and 3 years of follow-up.
All analyses were carried out with WinBUGS version 1.4 and R version 2.12.
Results
We screened the titles and abstracts of 246 potentially eligible reports and examined the full text of 70 articles. We identified 15 RCT with 18 articles and two oral communications presented at major medical congresses that met our inclusion criteria (Figure 1): ACME [26] (The Veterans Affairs Cooperative Study: Angioplasty Compared to Medicine), ARTS [27], [28] (Arterial Revascularization Therapy Study), BENESTENT II [29] (Randomised comparison of implantation of heparin-coated stents with balloon angioplasty in selected patients with coronary artery disease), COURAGE [14] (Optimal Medical Therapy with or without PCI for Stable Coronary Disease), EAST [30] (Emory Angioplasty Versus Surgery Trial), ENDEAVOR II [12], [31]–[33] (Randomized Controlled Trial to Evaluate the Safety and Efficacy of the Medtronic AVE ABT-578 Eluting Driver Coronary Stent in De Novo Native Coronary Artery Lesions), ERACI [34], [35] (Argentine Randomized Trial of Percutaneous Transluminal Coronary Angioplasty Versus Coronary Artery Bypass Surgery in Multivessel Disease), MASS II [36] (The Medicine, Angioplasty, or Surgery Study II), RAVEL [37] (randomised study with the sirolimus eluting Bx Velocity balloon expandable stent in the treatment of patients with de novo native coronary artery lesions), RITA 2 [38] (The second Randomised Intervention Treatment of Angina), SIRIUS [11] (Sirolimus-Eluting Stent in De-Novo Native Coronary Lesions), SoS [10] (the Stent or Surgery trial), STRESS [39] (Stent Restenosis Study), SYNTAX [40] (Synergy between PCI with Taxus and Cardiac Surgery) and TAXUS IV [41] (A polymer-based, paclitaxel-eluting stent in patients with coronary artery disease). The table S4 entitled “List of excluded and selected studies” presents the main reasons for exclusion.
Figure 1. Flow diagram of the screening process.
The characteristics of included RCT are presented in table 1. Six of these trials involved patients with multivessel disease: ARTS [27], [28], COURAGE [14], EAST [30], ERACI [34], [35], MASS II [36] and SoS [10]. The other clinical trials included patients with single vessel disease. For eight trials, the duration of follow-up was one year: BENESTENT II [29], MASS II [36], RAVEL [37], SIRIUS [11], SoS [10], STRESS [39], SYNTAX [40] and TAXUS IV [41]. For four trials, 3-year follow-up was available: ACME [26], COURAGE [14], EAST [30] and RITA 2 [38]. Two trials included both 1 and 3-year follow-up data: ARTS [27], [28] and ERACI [34], [35]. Lastly, in ENDEAVOR II [12], [31]–[33], duration of follow-up was 1, 2, 3 and 4 years.
Table 1. Baseline characteristics of patients in the studies selected.
| Study | Inclu sion | Inclusion criteria | Blinded | Single- or multi- vessel disease | Number of centres | Number of patients | Mean age (years) | Diabetes mellitus (%) | Sex (% men) | Previous MI (%) | Previous revascularisation (%) | Follow-up (years) |
| PTCA versus MT | ||||||||||||
| ACME [26] | 1987–1990 | stable angina pectoris, a strikingly positive exercise tolerance test or an MI within the past 3 months | no | single | 8 | 200 | 62 | 18 | 100 | 30 | 0 | 3 |
| RITA 2 [38] | 1992–1996 | angiographically-documented coronary artery disease | no | single | 20 | 1 018 | 58 | 9 | 82 | 47 | 0 | 3 |
| PTCA versus CABG | ||||||||||||
| ERACI [34], [35] | 1988–1990 | severe stenosis >70% in ≥1 major epicardial coronary artery, severely limiting stable angina or refractory resting unstable angina despite optimal medical therapy, no or minimal symptoms but with a large area of myocardium at risk identified by exercise testing | no | multi | 1 | 127 | 57 | 11 | 85 | 50 | NA | 1 and 3 |
| EAST [30] | 1987–1990 | stable or unstable angina or objective signs of ischemia | no | multi | 1 | 392 | 61 | 23 | 74 | 41 | 0 | 3 |
| CABG versus MT | ||||||||||||
| MASS II [36] | 1995–2000 | symptomatic multivessel coronary disease (2 or more epicardial coronary arteries with ≥70% narrowing), | no | multi | 1 | 611 | 60 | 14 | 85 | 22 | 0 | 1 |
| CABG versus PCI with BMS | ||||||||||||
| MASS II [36] | 1995–2000 | symptomatic multivessel coronary disease (≥2 epicardial coronary arteries with ≥70% narrowing), | no | multi | 1 | 611 | 60 | 14 | 85 | 22 | 0 | 1 |
| SoS [10] | 1996–1999 | symptomatic patients with multivessel coronary artery disease | no | multi | 53 | 988 | 61 | 14 | 79 | 45 | 0 | 1 |
| ARTS [27], [28] | 1997–1998 | stable angina pectoris or unstable angina pectoris or silent myocardial ischemia | no | multi | 67 | 1 205 | 61 | 17 | 76 | 43 | 0 | 1 and 3 |
| CABG versus PCI with DES | ||||||||||||
| SYNTAX [40] | 2005–2007 | stable or unstable angina pectoris with ischemia; or patients with atypical chest pain or asymptomatic with demonstrated myocardial ischemia | no | multi | 85 | 1 740 | 65 | 28 | 78 | 32 | 0 | 1 |
| PCI with BMS versus PCI with DES | ||||||||||||
| SIRIUS [11] | 2001 | history of stable or unstable angina and signs of myocardial ischemia. | double | single | 53 | 1 058 | 62 | 26 | 71 | 31 | NA | 1 |
| TAXUS IV [41] | 2002 | stable or unstable angina or inducible ischemia | double | single | 73 | 1 314 | 63 | 24 | 72 | 30 | NA | 1 |
| RAVEL [37] | 2000–2001 | stable or unstable angina or silent ischemia | double | single | 19 | 238 | 61 | 19 | 76 | 36 | NA | 1 |
| ENDEA VOR II [12], [31]–[33] | 2003–2004 | patients with clinical evidence of ischemia or a positive functional test | double | single | 72 | 1 197 | 62 | 20 | 76 | 41 | 20 | 1, 2 and 3 |
| PCI with BMS versus PTCA | ||||||||||||
| STRESS [39] | 1991–1993 | symptomatic ischemic heart disease | no | single | 8 | 207 | 61 | 14 | 72 | 35 | 6 | 1 |
| MT versus PCI with BMS | ||||||||||||
| MASS II [36] | 1995–2000 | symptomatic multivessel coronary disease | no | multi | 1 | 611 | 60 | 14 | 85 | 22 | 0 | 1 |
| COURAGE [14] | 1999–2004 | chronic angina pectoris CCS I-III, stable post-MI patients, and asymptomatic patients with objective evidence of myocardial ischemia. | no | multi | 50 | 2 287 | 62 | 33 | 85 | 38 | 25 | 3 |
| CABG versus PCI with DES | ||||||||||||
| BENE STENT II [29] | 1995–1996 | stable angina or unstable angina | no | single | 50 | 823 | 54 | 12 | 78 | 26 | 9 | 1 |
MT: medical therapy, PTCA: percutaneous coronary angioplasty, CABG: coronary artery bypass graft, DES: drug-eluting stent, BMS: bare-metal stent, NA: not available.
Figure 2 shows the comparators and the duration of patient follow-up for each RCT. The BENESTENT II [29] trial was a comparison of a heparin-coated stent versus PTCA. We considered this particular stent as a bare-metal stent because drug-eluting stent referred to stents with antiproliferative coating. Only one trial compared three treatment modalities: MT, BMS and CABG: MASS II [36].
Figure 2. Comparators and duration of patient follow-up for the trials selected.
According to the recommendations put forward by the Heart Collaborative Review Group, eleven trials described appropriate methods of randomization: ARTS [27], [28], BENESTENT II [29], COURAGE [14], ENDEAVOR II, MASS II [36], RAVEL [37], SIRIUS [11], SoS [10], STRESS [39], SYNTAX [40] and TAXUS IV [41]. The methods used to conceal treatment allocation were considered adequate in ten trials: ARTS [27], [28], BENESTENT II [29], COURAGE [14], MASS II [36], RAVEL [37], SIRIUS [11], SoS [10], STRESS [39], SYNTAX [40] and TAXUS IV [41]. Four of the sixteen trials were double blind: ENDEAVOR II, RAVEL [37], SIRIUS [11] and TAXUS IV [41]. Quality assessment of the clinical methodology is reported in the table S1.
According to the Drummond checklist, one trial did not specify the method used for estimating quantities and unit cost (checklist item 17): ERACI [34], [35]. Three of the eight trials with a 3-year follow-up did not apply the discount rate as recommended (checklist item 23): ARTS [27], [28],, EAST [30] and ERACI [34], [35]. For eight trials, there was no approach to sensitivity analysis (checklist item 27): ARTS [27], [28], BENESTENT II [29], EAST [30], ENDEAVOR II [12], [31]–[33], ERACI [34], [35], MASS II [36], SoS [10] and STRESS [39]. Results of quality assessment of economical methodology are displayed in the table S2.
Clinical analysis
In all, the 15 trials included had enrolled 9 565 patients followed for one year and 6 443 patients for three years. The percentages of men or diabetic patients were similarly distributed among the treatment arms, regardless of duration of follow-up (P = 0.22 for the percentage of men and 0.23 for the percentage of diabetes mellitus).
After one year of follow-up, 202 patients died: three of the 203 patients on MT (1.5%), 60 of the 2 221 patients with CABG (2.7%), nine of the 578 patients with PTCA (1.6%), 60 of the 3 693 patients with BMS (1.6%) and 70 of the 2 796 patients with DES (2.5%). After three years of follow-up, 345 patients had died: 111 of the 1 759 patients on MT (6.3%), 43 of the 863 patients with CABG (5.0%), 39 of the 870 patients with PTCA (4.5%), 133 of the 2 336 patients with BMS (5.7%) and 19 of the 584 patients with DES (3.2%).
After one year of follow-up, 394 patients had a MI: eight of the 203 patients on MT (3.9%), 96 of the 2 221 patients with CABG (4.3%), 33 of the 578 patients with PTCA (5.7%), 171 of the 3 693 patients with BMS (4.6%) and 86 of the 2 769 patients with DES (3.1%). After three years of follow-up, 530 patients had a MI: 147 of the 1 759 patients on MT (8.3%), 80 of the 841 patients with CABG (9.5%), 67 of the 845 patients with PTCA (7.9%), 212 of the 2 336 patients with BMS (9.1%) and 19 of the 584 patients with DES (3.3%).
After one and three years of follow-up there was no statistically significant difference between the death and MI rates of the five treatments. Because of non-significant results, the rating of treatment efficacy is not informative (Table 2 and figure 3).
Table 2. Comparison of the rates of death and myocardial infarction between the 5 treatments (MT versus CABG versus PTCA versus BMS versus DES).
| Events | ||||||
| MT* | CABG | PTCA | BMS | DES | ||
| death within the first year of follow-up | HR (95% CI) | 1 | 2.61 (0.63; 12.55) | 3.78 (0.66; 25.28) | 3.10 (0.76; 15.18) | 4.01 (0.95; 21.12) |
| probability treatment is the best | 0.87 | 0.06 | 0.05 | 0.02 | 0.00 | |
| death within the first three years of follow-up | HR (95% CI) | 1 | 1.01 (0.41; 2.25) | 1.24 (0.57; 2.61) | 0.83 (0.41; 1.46) | 0.79 (0.23; 2.56) |
| probability treatment is the best | 0.11 | 0.15 | 0.05 | 0.24 | 0.49 | |
| MI within the first year of follow-up | HR (95% CI) | 1 | 1.07 (0.37; 2.89) | 1.67 (0.47; 5.47) | 1.70 (0.59; 4.57) | 1.14 (0.33; 3.25) |
| probability treatment is the best | 0.51 | 0.27 | 0.04 | 0.00 | 0.18 | |
| MI within the first three years of follow-up | HR (95% CI) | 1 | 1.48 (0.52; 5.20) | 1.36 (0.57; 3.97) | 1.76 (0.72; 3.45) | 1.03 (0.23; 6.11) |
| probability treatment is the best | 0.37 | 0.07 | 0.09 | 0.02 | 0.45 | |
HR: hazard ratio, MI: myocardial infarction, MT: medical therapy, PTCA: percutaneous coronary angioplasty, CABG: coronary artery bypass graft, DES: drug-eluting stent, BMS: bare-metal stent, CI: confidence interval.
* medical therapy was the reference treatment.
Figure 3. Cumulative incidences of death and MI.

Economic analysis
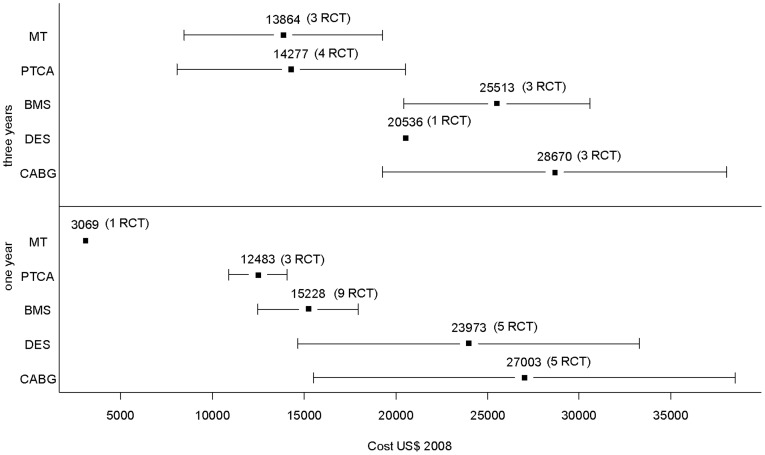
Table 3 and figure 4 present the evaluation of cost per patient for each RCT: cost published in the article (year of publication and currency used) and cost per patient adjusted in US $ 2008. Figure 5 presents the mean cost per patient for each treatment. After one year of follow-up, the mean weighted cost per patient in US $ 2008 was: $3069 with MT, $27 003 with CABG, $12 483 with PTCA, $15 228 with BMS, and $23 973 with DES. After three years of follow-up, the mean weighted cost was: $13 864 with MT, $28 670 with CABG, $14 277 with PTCA, $25 513 with BMS, and $20 536 with DES. There was a statistically significant difference of weighted cost per patient for the comparison of the five treatments: P value was <0.0001 after one year and after three years. Between one and three years of follow-up, the greatest increase in average weighted cost per patient was observed with MT (+ $10 795, +352% compared with the cost per patient after one year). During this period, weighted cost with treatment by CABG was stable (+ $1667, +6% compared with cost per patient after one year). We performed a comparison of the weighted cost of each treatment in at least two clinical studies. For all these comparisons, at one and three years of follow-up, the differences observed were significant (P<0.0001): CABG versus PTCA after one year, CABG versus BMS after one year, CABG versus DES after one year, etc.
Table 3. Cost per patient for each treatment.
| Trial | FU (year) | Cost per patient as published (currency, year, country) | Cost followed | multi or single vessel disease | Cost per patient adjusted (US $ 2008) | |
| MT | MASS II | 1 | 2 285 (US $, 1998, Netherlands) | H+D | MVD | 3 069 |
| ACME | 3 | 6 311 (Aus $, 1994, Australia) | H+C+D | SVD | 6 299 | |
| RITA 2 | 3 | 3 613 (£, 1999, UK) | H+C+D | SVD | 6 633 | |
| COURAGE | 3 | 15 653 (US $, 2004, USA) | H+C+D | MVD | 17 842 | |
| CABG | ARTS | 1 | 13 638 (US $, 1998, Netherlands) | H+C | MVD | 19 100 |
| ERACI | 1 | 12 938 (US $, 1991, Argentina) | H | MVD | 23 733 | |
| MASS II | 1 | 11 794 (US $, 1998, Netherlands) | H+D | MVD | 15 846 | |
| SoS | 1 | 8 905 (£2000, UK) | H+D | MVD | 16 222 | |
| SYNTAX | 1 | 39 581 (US $, 2007, USA) | H+C+D | MVD | 41 101 | |
| ARTS | 3 | 16 100 (€, 1998, Netherlands) | H+C | MVD | 23 596 | |
| EAST | 3 | 25 310 (US $, 1997, USA) | H | MVD | 46 083 | |
| ERACI | 3 | 13 000 (US $, 1991, Argentina) | H | MVD | 23 847 | |
| PTCA | BENESTENT II | 1 | 16 727 (Dfl, 1996, Netherlands) | H | SVD | 11 596 |
| STRESS | 1 | 10 865 (US $, 1994, USA) | H | SVD | 15 782 | |
| ERACI | 1 | 6 952 (US $, 1991, Argentina) | H | MVD | 12 753 | |
| ACME | 3 | 6 790 (Aus $, 1994, Australia) | H+C+D | SVD | 6 777 | |
| RITA 2 | 3 | 6 299 (£1999, UK) | H+C+D | SVD | 11 565 | |
| ERACI | 3 | 7 523 (US $, 1991, Argentina) | H | MVD | 13 800 | |
| EAST | 3 | 23 734 (US $, 1997, USA) | H | MVD | 25 310 | |
| BMS | BENESTENT II | 1 | 18 812 (Dfl, 1996, Netherlands) | H | SVD | 13 041 |
| ENDEAVOR II | 1 | 16 641 (US $, 2008, USA) | H | SVD | 16 641 | |
| RAVEL | 1 | 9 915 (€, 2001, Netherlands) | H+D | SVD | 13 339 | |
| SIRIUS | 1 | 16 504 (US $, 2002, USA) | H+C+D | SVD | 19 755 | |
| STRESS | 1 | 11 656 (US $, 1994, USA) | H | SVD | 16 931 | |
| TAXUS IV | 1 | 14 011 (US $, 2004, USA) | H+D | SVD | 15 971 | |
| ARTS | 1 | 10 665 (US $, 1998, Netherlands) | H+C | MVD | 14 936 | |
| MASS II | 1 | 8 676 (US $, 1998, Netherlands) | H+D | MVD | 11 656 | |
| SoS | 1 | 6 296 (£2000, UK) | H+D | MVD | 11 469 | |
| ENDEAVOR II | 3 | 20 348 (US $, 2008, USA) | H | SVD | 20 348 | |
| ARTS | 3 | 14 302 (€, 1998, Netherlands) | H+C | MVD | 20 961 | |
| COURAGE | 3 | 26 847 (US $, 2004, USA) | H+C+D | MVD | 30 602 | |
| DES | ENDEAVOR II | 1 | 17 422 (US $, 2008, USA) | H | SVD | 17 422 |
| RAVEL | 1 | 9 969 (€, 2001, Netherlands) | H+D | SVD | 13 412 | |
| SIRIUS | 1 | 16 813 (US $, 2002, USA) | H+C+D | SVD | 20 124 | |
| TAXUS IV | 1 | 15 447 (US $, 2004, USA) | H+D | SVD | 16 624 | |
| SYNTAX | 1 | 35 991 (US $, 2007, USA) | H+C+D | MVD | 37 373 | |
| ENDEAVOR II | 3 | 20 536 (US $, 2008, USA) | H | SVD | 20 536 |
FU: follow-up, MT: medical therapy, PTCA: percutaneous coronary angioplasty, CABG: coronary artery bypass graft, DES: drug-eluting stent, BMS: bare-metal stent, SVD: single vessel disease, MVD: multi vessel disease, H: hospital cost, C: costs related to outpatient care, D: costs related to outpatient drugs.
Figure 4. Cost per patient adjusted in US $ 2008 after 1 and 3 years of follow-up (each mark represents a clinical study).
Figure 5. Mean weighted cost per patient in US $ 2008 and standard deviation (number of RCT available).
For five trials, only hospital costs were assessed: BENESTENT II [29], ENDEAVOR II [12], [31]–[33], EAST [30], ERACI [34], [35] and STRESS [39]. For ARTS [27], [28], costs related to outpatient medical visits were studied in addition to hospital costs. In four studies, the costs assessed were related to hospitalization and outpatient drugs: MASS II [36], RAVEL [37] TAXUS IV [41] and SoS [10]. In five studies, costs assessed were related to hospitalization, outpatient care (medical visit and/or cardiovascular testing) and outpatient drugs: ACME [26], COURAGE [14], RITA 2 [38], SIRIUS [11] and SYNTAX [40].
Sensitivity analysis
In this analysis, we excluded trials reporting only hospital costs: BENESTENT II [29], ENDEAVOR II [12], [31]–[33], EAST [30], ERACI [34], [35] and STRESS [39]. Consequently, not all treatment modalities could be compared at one year and three years of patient follow-up: at one year, data on PTCA alone were not available for the sensitivity analysis; likewise, data on DES after three years of follow-up could not be used in the sensitivity analysis.
After one year of follow-up, results of the sensitivity analysis were consistent with the main results. MT remained the least expensive, followed by BMS, then DES and lastly CABG (P<0.0001). After 3 years however, the results were different. Treatment with PTCA appeared to be the least expensive and CABG was still the most costly strategy (P<0.0001). Table S3.
Discussion
The present network meta-analysis confirms the absence of a statistically significant difference between medical therapy, angioplasty without stent, angioplasty with BMS, angioplasty with DES and coronary artery bypass graft on mortality and myocardial infarction rates at one and three years of follow-up. These results concord with those reported in recent meta-analyses and therefore justify the cost-comparison of the different treatment strategies [5]–[7], [42].
Our economic analysis demonstrates a significant difference of weighted costs per patient between the five treatment options. Medical therapy is the least expensive with a weighted cost per patient of US $3069 after one year of follow-up and US $13 864 after three years of follow-up. Coronary artery bypass grafting is the most costly treatment modality: US $27 003 and US $28 670 at one and three years respectively. Between one and three years of follow-up, however, the largest increase in average weighted cost per patient was observed with MT (+ $10 795, +352% compared with the cost after one year), followed by BMS (+ $10 285, +67%), then PTCA (+ $1794, +14%) and CABG (+ $1667, +6%). This significant increase in expenditures, particularly for the MT group, can probably be explained by the need for (additional) revascularization during mid-term follow-up. The sensitivity analysis, performed on the 10 studies that followed, in addition to hospital costs, outpatient costs (outpatient care and/or outpatient drugs), yielded results that are consistent with those of the primary analysis after one year of follow-up. At three years, balloon PTCA and MT had comparable low costs, while there was little difference in the costs of BMS and CABG.
The apparent decrease in cost from one year to three years with DES is artefactual, and due to the fact that only one trial (ENDEAVOR II [12], [31]–[33]) reported 3-year results, whereas several trials were pooled to derive one-year costs. When considering change in costs from one to three years in ENDEAVOR II, an 18% increase was observed, which is consistent with the reduced need for additional revascularization with DES, compared with BMS [12]. The cost increase in ENDEAVOR II is in line with that found in ENDEAVOR III, a clinical trial comparing two different DES: +23% for the sirolimus-eluting stent and +24% for the zotarolimus-eluting stent [43]. In addition, after three years of follow-up we observed a lower cost per patient for the treatment with PTCA compared with treatment with BMS. This surprising observation can probably be explained by the different proportion of patients with SVD: 70% of patients treated by PTCA versus 25% in the BMS group after three years of follow-up.
Overall, the increased initial cost related to initial performance of myocardial revascularization was not counterbalanced by equivalent savings during the three subsequent years of follow-up, although the difference at one year was notably attenuated at three years. A cost advantage for MT compared with myocardial revascularisation was also observed in BARI 2D after four years of follow-up [44]. In this study, which only included patients with type 2 diabetes mellitus, medical costs per patient were higher for CABG or PCI than for MT. Costs (in US$ 2007) were 80 900, 73 400 and 65 600 respectively after four years of follow-up.
Because of the relatively small number of studies in the meta-analysis, and as we did not use individual data, we were unable to perform separate analyses for patients with single-vessel disease versus multivessel disease. It is possible that the benefit of MT in terms of costs might be more limited in patients with multi-vessel coronary artery disease who are more likely to need subsequent myocardial revascularization.
Nowadays, plain balloon angioplasty (PTCA) is only used in very rare instances. We did keep these studies in our analysis, however, in order to provide additional data on the treatment modalities studied in the non-PTCA arms of the trials (CABG or medical treatment); indeed, excluding the six studies using balloon angioplasty would have resulted in also excluding two studies with medical treatment (ACME and RITA 2), two studies with CABG (ERACI and EAST) and two studies with BMS (BENESTENT 2 and STRESS), thereby much decreasing the statistical power of our analyses.
We also adopted the approach to consider BMS and DES studies separately. In fact, although DES are increasingly used, a substantial proportion of procedures still use BMS, with wide between-country variations; the proportion of DES in published studies varied from 23% (Sweden, 2007, Gudnason et al.), 45% (France, 2004-2008, Puymirat et al.), 61% (Spain, 2009, Diaz J et al.) and 70% (USA, 2011, Dehmer GJ et al.) [45]-[48].
Critical appraisal of costing methods
We must emphasize that the definition of costs in each study varies. In the management of coronary artery disease, direct costs correspond to three items of expenditure: hospitalization (for invasive treatment and/or care of complications of the disease), outpatient care (medical visits, radiological and biological tests, home visits by nurses, etc.) and outpatient medications (anti-platelet drugs, anti-anginal drugs, etc). All 15 studies included in our economical analysis assessed hospitalization-related costs. Among the 15 RCT, 10 measured both hospital and ambulatory costs (assessment of ambulatory care and /or medication costs). For seven of these 10 RCT, separate hospital and ambulatory cost analyses were available. After one year, ambulatory costs represented an average of 8% of the total cost (from 2.9% in TAXUS-IV to 15% in SoS); after three years, only one study (RITA-2) provided a detailed analysis of the respective proportion of hospital and ambulatory costs; as expected, the percentage of the total cost related to ambulatory care was higher than that observed at one year. Furthermore, a number of the studies analysed the cost of all cardiovascular drugs (ARTS, SYNTAX, RITA 2 etc), whereas the SIRIUS trial analysed the cost of thienopyridines only.
Moreover, the methods used to calculate the cost per patient vary in the studies analyzed. In practice, as published by Reed et al. [49], the calculation of the average cost depends on two parameters. The first is the approach used in the clinical trial to estimate the resource consumed. Indeed, resource-use can be based on data from patients in all countries participating in the clinical trial or from patients belonging to one center or one country. The second is the costing approach; again, the unit cost applied for the whole trial population can be derived from individual countries, from a single country, or from one center. According to these two parameters, studies can be classified into six groups: fully pooled with multi-country costing, fully pooled with one-country costing, partially split with multi-country costing, partially split with one-country costing, fully split with multi-country costing and fully split with one-country costing. The 15 trials we analyzed belong to two of these six groups. Eight trials are classified as “fully pooled with one-country costing”: ARTS [27], [28], BENESTENT II [29], COURAGE [14],, ENDEAVOR II [12], [31]–[33], RAVEL [37], RITA 2 [38], SoS [10] and SYNTAX [40]. The seven other studies are classified as “fully split with one-country costing”: ACME [26], EAST [30], ERACI [34], [35], MASS II [36], SIRIUS [11], STRESS [39] and TAXUS IV [41]. Our conclusions may therefore be limited by the methodological variability of the 16 trials we analyzed, although the results were fairly consistent regardless of the costing methods used.
Limitations
Our meta-analysis included trials that were conducted at a time when the technique of PCI used would be considered completely obsolete in today's terms. In such earlier studies, the rates of subsequent revascularisation following PCI were definitely higher than those that would currently be observed, leading to higher follow-up costs than would be found nowadays.
Also, as some trials planned angiographic follow-up for all patients, including those who were asymptomatic, the rates of repeat revascularization may have been higher that those that would have been observed in a real-life situation, because of the “oculostenotic reflex” that mandatory coronary angiography during follow-up may have induced. In fact, only six studies did not include routine angiographic follow-up: ARTS [27], [28], COURAGE [14], ERACI [34], [35], RITA 2 [38], SoS [10] and SYNTAX [40]. Studies in which angiographic follow-up was planned, tended to have higher treatment costs.
Another limitation of our analysis is that the period over which we selected the studies expands over two decades, with the oldest trial (EAST) recruiting patients from 1987 to 1990, and the most recent (SYNTAX) between 2005 and 2007. During this period, the cost of BMS and DES has decreased substantially. Among the trials selected, five reported the unit cost of stents (RAVEL, SIRIUS, TAXUS IV, SYNTAX et ENDEAVOR II): the cost of BMS remained relatively stable (US $900 to 700) while the absolute price decrease for DES was greater (from US $2900 to 2100). The differential cost between BMS and DES remained stable (≈ US $2000), except for the two most recent trials (SYNTAX, 2007 and ENDEAVOR II, 2008), where the difference was smaller. Although we lacked the power to fully take these changes into consideration, they should be kept in mind when analysing our results.
In addition, it must be emphasized that the nature of costs varied across the 15 RCT. All measured hospital costs, while 10 also assessed ambulatory costs. A specific sensitivity analysis, however, was performed to take this variability into account.
Lastly, we used data from one single randomized clinical trial in two situations: medical therapy with one year follow-up (MASS II [36]) and treatment with DES with three years of follow-up (ENDEAVOR II [12], [31]–[33]). As mentioned above, this may have led to inconsistencies, such as the seemingly lower costs from one to three years in patients with DES.
Conclusions
This network meta-analysis documents considerable differences in treatment costs at 3-year follow-up, when comparing five treatment modalities that provided similar clinical results, in terms of death and risk of myocardial infarction. Medical therapy in patients without acute coronary syndromes therefore appears to be the most cost-effective option, which may achieve appreciable savings in healthcare expenditures. Our findings, however, may be limited by methodological considerations pertaining to the way costs are evaluated in long-term randomized trials, and by the fact that we did not take into account potential differences between treatment modalities in terms of symptoms.
Supporting Information
Search strategy in Medline, Embase and Cochrane library.
(DOC)
Checklist of clinical quality assessment according to the Heart Collaborative Review Group.
(DOC)
Drummond checklist.
(DOC)
Clinical quality assessment.
(DOC)
Economical quality assessment.
(DOC)
Sensitivity analyse for the comparison MT versus CABG versus PTCA versus BMS versus DES.
(DOC)
List of selected or rejected articles and reason in case of exclusion.
(DOC)
PRISMA checklist.
(DOC)
Funding Statement
The authors have no support or funding to report.
References
- 1. Fihn SD, Gardin JM, Abrams J, Berra K, Blankenship JC, et al. (2012) 2012 ACCF/AHA/ACP/AATS/PCNA/SCAI/STS Guideline for the diagnosis and management of patients with stable ischemic heart disease: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, and the American College of Physicians, American Association for Thoracic Surgery, Preventive Cardiovascular Nurses Association, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol 60: e44–e164 10.1016/j.jacc.2012.07.013 [DOI] [PubMed] [Google Scholar]
- 2. Epstein AJ, Polsky D, Yang F, Yang L, Groeneveld PW (2011) Coronary revascularization trends in the United States, 2001-2008. JAMA J Am Med Assoc 305: 1769–1776 10.1001/jama.2011.551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Eisenberg MJ (2006) Drug-eluting stents: the price is not right. Circulation 114: 1745–1754 discussion 1754 10.1161/CIRCULATIONAHA.106.646190 [DOI] [PubMed] [Google Scholar]
- 4. Kirtane AJ, Gupta A, Iyengar S, Moses JW, Leon MB, et al. (2009) Safety and efficacy of drug-eluting and bare metal stents: comprehensive meta-analysis of randomized trials and observational studies. Circulation 119: 3198–3206 10.1161/CIRCULATIONAHA.108.826479 [DOI] [PubMed] [Google Scholar]
- 5. Pursnani S, Korley F, Gopaul R, Kanade P, Chandra N, et al. (2012) Percutaneous coronary intervention versus optimal medical therapy in stable coronary artery disease: a systematic review and meta-analysis of randomized clinical trials. Circ Cardiovasc Interv 5: 476–490 10.1161/CIRCINTERVENTIONS.112.970954 [DOI] [PubMed] [Google Scholar]
- 6. Hlatky MA, Boothroyd DB, Bravata DM, Boersma E, Booth J, et al. (2009) Coronary artery bypass surgery compared with percutaneous coronary interventions for multivessel disease: a collaborative analysis of individual patient data from ten randomised trials. Lancet 373: 1190–1197 10.1016/S0140-6736(09)60552-3 [DOI] [PubMed] [Google Scholar]
- 7. Stergiopoulos K, Brown DL (2012) Initial coronary stent implantation with medical therapy vs medical therapy alone for stable coronary artery disease: meta-analysis of randomized controlled trials. Arch Intern Med 172: 312–319 10.1001/archinternmed.2011.1484 [DOI] [PubMed] [Google Scholar]
- 8. Hlatky MA, Boothroyd DB, Baker L, Kazi DS, Solomon MD, et al. (2013) Comparative effectiveness of multivessel coronary bypass surgery and multivessel percutaneous coronary intervention: a cohort study. Ann Intern Med 158: 727–734 10.7326/0003-4819-158-10-201305210-00639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hill RA, Boland A, Dickson R, Dündar Y, Haycox A, et al.. (2007) Drug-eluting stents: a systematic review and economic evaluation. Health Technol Assess Winch Engl 11: iii, xi–221. [DOI] [PubMed]
- 10. Weintraub WS, Mahoney EM, Zhang Z, Chu H, Hutton J, et al. (2004) One year comparison of costs of coronary surgery versus percutaneous coronary intervention in the stent or surgery trial. Heart Br Card Soc 90: 782–788 10.1136/hrt.2003.015057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cohen DJ, Bakhai A, Shi C, Githiora L, Lavelle T, et al. (2004) Cost-effectiveness of sirolimus-eluting stents for treatment of complex coronary stenoses: results from the Sirolimus-Eluting Balloon Expandable Stent in the Treatment of Patients With De Novo Native Coronary Artery Lesions (SIRIUS) trial. Circulation 110: 508–514 10.1161/01.CIR.0000136821.99814.43 [DOI] [PubMed] [Google Scholar]
- 12. Eisenstein EL, Wijns W, Fajadet J, Mauri L, Edwards R, et al. (2009) Long-term clinical and economic analysis of the Endeavor drug-eluting stent versus the Driver bare-metal stent: 4-year results from the ENDEAVOR II trial (Randomized Controlled Trial to Evaluate the Safety and Efficacy of the Medtronic AVE ABT-578 Eluting Driver Coronary Stent in De Novo Native Coronary Artery Lesions). JACC Cardiovasc Interv 2: 1178–1187 10.1016/j.jcin.2009.10.011 [DOI] [PubMed] [Google Scholar]
- 13. Hlatky MA, Rogers WJ, Johnstone I, Boothroyd D, Brooks MM, et al. (1997) Medical care costs and quality of life after randomization to coronary angioplasty or coronary bypass surgery. Bypass Angioplasty Revascularization Investigation (BARI) Investigators. N Engl J Med 336: 92–99 10.1056/NEJM199701093360203 [DOI] [PubMed] [Google Scholar]
- 14. Weintraub WS, Boden WE, Zhang Z, Kolm P, Zhang Z, et al. (2008) Cost-effectiveness of percutaneous coronary intervention in optimally treated stable coronary patients. Circ Cardiovasc Qual Outcomes 1: 12–20 10.1161/CIRCOUTCOMES.108.798462 [DOI] [PubMed] [Google Scholar]
- 15.OED website. Available: http://www.oecd.org/std/prices-ppp/ (n.d.).
- 16.Bertoldi EG, Rohde LE, Zimerman LI, Pimentel M, Polanczyk CA (2011) Cost-effectiveness of cardiac resynchronization therapy in patients with heart failure: The perspective of a middle-income country's public health system. Int J Cardiol. doi:10.1016/j.ijcard.2011.06.046. [DOI] [PubMed]
- 17. Kühr EM, Ribeiro RA, Rohde LEP, Polanczyk CA (2011) Cost-effectiveness of supervised exercise therapy in heart failure patients. Value Health J Int Soc Pharmacoeconomics Outcomes Res 14: S100–107 10.1016/j.jval.2011.05.006 [DOI] [PubMed] [Google Scholar]
- 18. Antioch KM, Jennings G, Botti M, Chapman R, Wulfsohn V (2002) Integrating cost-effectiveness evidence into clinical practice guidelines in Australia for acute myocardial infarction. Eur J Health Econ HEPAC Health Econ Prev Care 3: 26–39 10.1007/s10198-001-0088-z [DOI] [PubMed] [Google Scholar]
- 19.Villanueva EV, Wasiak J, Petherick ES (2003) Percutaneous transluminal rotational atherectomy for coronary artery disease. Cochrane Database Syst Rev Online: CD003334. doi:10.1002/14651858.CD003334. [DOI] [PubMed]
- 20. Evers S, Goossens M, de Vet H, van Tulder M, Ament A (2005) Criteria list for assessment of methodological quality of economic evaluations: Consensus on Health Economic Criteria. Int J Technol Assess Health Care 21: 240–245. [PubMed] [Google Scholar]
- 21. Woods BS, Hawkins N, Scott DA (2010) Network meta-analysis on the log-hazard scale, combining count and hazard ratio statistics accounting for multi-arm trials: a tutorial. BMC Med Res Methodol 10: 54 10.1186/1471-2288-10-54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lu G, Ades AE (2004) Combination of direct and indirect evidence in mixed treatment comparisons. Stat Med 23: 3105–3124 10.1002/sim.1875 [DOI] [PubMed] [Google Scholar]
- 23. Higgins JP, Whitehead A (1996) Borrowing strength from external trials in a meta-analysis. Stat Med 15: 2733–2749 [DOI] [PubMed] [Google Scholar]
- 24. Smith TC, Spiegelhalter DJ, Thomas A (1995) Bayesian approaches to random-effects meta-analysis: a comparative study. Stat Med 14: 2685–2699. [DOI] [PubMed] [Google Scholar]
- 25. Lu G, Ades AE, Sutton AJ, Cooper NJ, Briggs AH, et al. (2007) Meta-analysis of mixed treatment comparisons at multiple follow-up times. Stat Med 26: 3681–3699 10.1002/sim.2831 [DOI] [PubMed] [Google Scholar]
- 26. Kinlay S (1996) Cost-effectiveness of coronary angioplasty versus medical treatment: the impact of cost-shifting. Aust N Z J Med 26: 20–26. [DOI] [PubMed] [Google Scholar]
- 27. Serruys PW, Unger F, Sousa JE, Jatene A, Bonnier HJ, et al. (2001) Comparison of coronary-artery bypass surgery and stenting for the treatment of multivessel disease. N Engl J Med 344: 1117–1124 10.1056/NEJM200104123441502 [DOI] [PubMed] [Google Scholar]
- 28. Legrand VMG, Serruys PW, Unger F, van Hout BA, Vrolix MCM, et al. (2004) Three-year outcome after coronary stenting versus bypass surgery for the treatment of multivessel disease. Circulation 109: 1114–1120 10.1161/01.CIR.0000118504.61212.4B [DOI] [PubMed] [Google Scholar]
- 29. Serruys PW, van Hout B, Bonnier H, Legrand V, Garcia E, et al. (1998) Randomised comparison of implantation of heparin-coated stents with balloon angioplasty in selected patients with coronary artery disease (Benestent II). Lancet 352: 673–681. [DOI] [PubMed] [Google Scholar]
- 30. Weintraub WS, Mauldin PD, Becker E, Kosinski AS, King SB 3rd (1995) A comparison of the costs of and quality of life after coronary angioplasty or coronary surgery for multivessel coronary artery disease. Results from the Emory Angioplasty Versus Surgery Trial (EAST). Circulation 92: 2831–2840. [DOI] [PubMed] [Google Scholar]
- 31.Meredith I (n.d.) Trial updates & long term follow-up - ENDEAVOR I: 3-year, ENDEAVOR II: 2-year clinical results. May 16-19 2006 Eur Paris 2006.
- 32.Meredith I, Wijns W (n.d.) Clinical Trial Update. ENDEAVOR I & II clinical program: long term follow-up. 4 Sept 2005 ESC Stockh 2005.
- 33.Zeiher A (n.d.) ENDEAVOR clinical Program Update. ENDEAVOR I: 4-year clinical follow-up. ENDEAVOR II: 3-year clinical follow-up. May 22 2007 Eur Barc 2007.
- 34. Rodriguez A, Boullon F, Perez-Baliño N, Paviotti C, Liprandi MI, et al. (1993) Argentine randomized trial of percutaneous transluminal coronary angioplasty versus coronary artery bypass surgery in multivessel disease (ERACI): in-hospital results and 1-year follow-up. ERACI Group. J Am Coll Cardiol 22: 1060–1067. [DOI] [PubMed] [Google Scholar]
- 35. Rodriguez A, Mele E, Peyregne E, Bullon F, Perez-Baliño N, et al. (1996) Three-year follow-up of the Argentine Randomized Trial of Percutaneous Transluminal Coronary Angioplasty Versus Coronary Artery Bypass Surgery in Multivessel Disease (ERACI). J Am Coll Cardiol 27: 1178–1184. [DOI] [PubMed] [Google Scholar]
- 36. Favarato D, Hueb W, Gersh BJ, Soares PR, Cesar LAM, et al. (2003) Relative cost comparison of treatments for coronary artery disease: the First Year Follow-Up of MASS II Study. Circulation 108 Suppl 1 II21–23 10.1161/01.cir.0000087381.98299.7b [DOI] [PubMed] [Google Scholar]
- 37. Van Hout BA, Serruys PW, Lemos PA, van den Brand MJBM, van Es G-A, et al. (2005) One year cost effectiveness of sirolimus eluting stents compared with bare metal stents in the treatment of single native de novo coronary lesions: an analysis from the RAVEL trial. Heart Br Card Soc 91: 507–512 10.1136/hrt.2004.034454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sculpher M, Smith D, Clayton T, Henderson R, Buxton M, et al. (2002) Coronary angioplasty versus medical therapy for angina. Health service costs based on the second Randomized Intervention Treatment of Angina (RITA-2) trial. Eur Heart J 23: 1291–1300. [DOI] [PubMed] [Google Scholar]
- 39. Cohen DJ, Krumholz HM, Sukin CA, Ho KK, Siegrist RB, et al. (1995) In-hospital and one-year economic outcomes after coronary stenting or balloon angioplasty. Results from a randomized clinical trial. Stent Restenosis Study Investigators. Circulation 92: 2480–2487. [DOI] [PubMed] [Google Scholar]
- 40. Cohen DJ, Lavelle TA, Van Hout B, Li H, Lei Y, et al. (2012) Economic outcomes of percutaneous coronary intervention with drug-eluting stents versus bypass surgery for patients with left main or three-vessel coronary artery disease: one-year results from the SYNTAX trial. Catheter Cardiovasc Interv Off J Soc Card Angiogr Interv 79: 198–209 10.1002/ccd.23147 [DOI] [PubMed] [Google Scholar]
- 41. Bakhai A, Stone GW, Mahoney E, Lavelle TA, Shi C, et al. (2006) Cost effectiveness of paclitaxel-eluting stents for patients undergoing percutaneous coronary revascularization: results from the TAXUS-IV Trial. J Am Coll Cardiol 48: 253–261 10.1016/j.jacc.2006.02.063 [DOI] [PubMed] [Google Scholar]
- 42. Schömig A, Mehilli J, de Waha A, Seyfarth M, Pache J, et al. (2008) A meta-analysis of 17 randomized trials of a percutaneous coronary intervention-based strategy in patients with stable coronary artery disease. J Am Coll Cardiol 52: 894–904 10.1016/j.jacc.2008.05.051 [DOI] [PubMed] [Google Scholar]
- 43. Eisenstein EL, Leon MB, Kandzari DE, Mauri L, Edwards R, et al. (2009) Long-term clinical and economic analysis of the Endeavor zotarolimus-eluting stent versus the cypher sirolimus-eluting stent: 3-year results from the ENDEAVOR III trial (Randomized Controlled Trial of the Medtronic Endeavor Drug [ABT-578] Eluting Coronary Stent System Versus the Cypher Sirolimus-Eluting Coronary Stent System in De Novo Native Coronary Artery Lesions). JACC Cardiovasc Interv 2: 1199–1207 10.1016/j.jcin.2009.10.009 [DOI] [PubMed] [Google Scholar]
- 44. Hlatky MA, Boothroyd DB, Melsop KA, Kennedy L, Rihal C, et al. (2009) Economic outcomes of treatment strategies for type 2 diabetes mellitus and coronary artery disease in the Bypass Angioplasty Revascularization Investigation 2 Diabetes trial. Circulation 120: 2550–2558 10.1161/CIRCULATIONAHA.109.912709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Gudnason T, Gudnadottir GS, Lagerqvist B, Eyjolfsson K, Nilsson T, et al. (2013) Comparison of interventional cardiology in two European countries: a nationwide internet based registry study. Int J Cardiol 168: 1237–1242 10.1016/j.ijcard.2012.11.054 [DOI] [PubMed] [Google Scholar]
- 46.Puymirat E, Blanchard D, Perier M-C, Piadonataccio M, Gilard M, et al.. (2013) Study Design and Baseline Characteristics of the National Observational Study of Diagnostic and Interventional Cardiac Catheterization by the French Society of Cardiology. Am J Cardiol. doi:10.1016/j.amjcard.2013.03.030. [DOI] [PubMed]
- 47. Díaz JF, de La Torre JM, Sabaté M, Goicolea J (2012) Spanish Cardiac Catheterization and Coronary Intervention Registry. 21st official report of the Spanish Society of Cardiology Working Group on Cardiac Catheterization and Interventional Cardiology (1990-2011). Rev Esp Cardiol Engl Ed 65: 1106–1116 10.1016/j.recesp.2012.07.021 [DOI] [PubMed] [Google Scholar]
- 48. Dehmer GJ, Weaver D, Roe MT, Milford-Beland S, Fitzgerald S, et al. (2012) A contemporary view of diagnostic cardiac catheterization and percutaneous coronary intervention in the United States: a report from the CathPCI Registry of the National Cardiovascular Data Registry, 2010 through June 2011. J Am Coll Cardiol 60: 2017–2031 10.1016/j.jacc.2012.08.966 [DOI] [PubMed] [Google Scholar]
- 49. Reed SD, Anstrom KJ, Bakhai A, Briggs AH, Califf RM, et al. (2005) Conducting economic evaluations alongside multinational clinical trials: toward a research consensus. Am Heart J 149: 434–443 10.1016/j.ahj.2004.11.001 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Search strategy in Medline, Embase and Cochrane library.
(DOC)
Checklist of clinical quality assessment according to the Heart Collaborative Review Group.
(DOC)
Drummond checklist.
(DOC)
Clinical quality assessment.
(DOC)
Economical quality assessment.
(DOC)
Sensitivity analyse for the comparison MT versus CABG versus PTCA versus BMS versus DES.
(DOC)
List of selected or rejected articles and reason in case of exclusion.
(DOC)
PRISMA checklist.
(DOC)