Abstract
Toll-like receptor 2 (TLR2) is critical in the immune response to mycobacteria. Herein, we report that the frequency of a human TLR2 Arg677Trp polymorphism (C2029T nucleotide substitution) in tuberculosis patients in Tunisia is significantly higher than in healthy controls (P < 0.0001). This finding suggests that this polymorphism could be a risk factor for tuberculosis.
Bacterial infection typically results in activation of the innate immune system as a first-line host defense mechanism. In humans, toll-like receptors (TLRs) contribute to this innate immune recognition of pathogens and shape the development of the adaptive immune response (8). TLRs are transmembrane proteins characterized by an extracellular leucine-rich domain that participates in ligand recognition (8, 6) and an intracellular tail that contains a conserved region called the Toll interleukin 1 receptor (IL-1R) homology domain. Stimulation of TLR initiates a signaling cascade that involves a number of proteins, such as MyD88 and IL-1 receptor-associated kinase (9). This signaling cascade leads to NF-κB activation, which induces the secretion of proinflammatory cytokines. TLR2 has been reported to be the principal mediator of macrophage activation in response to mycobacteria (12). Furthermore, a mutation in the mouse TLR2 intracellular domain (Pro681His) acts as a dominant-negative inhibitor of TLR2 signaling (11). Interestingly, a recent study showed that another mutation in the intracellular domain of human TLR2 (Arg677Trp) is associated with lepromatous leprosy (3). More recently, Bochud et al. have shown, using cell transfection studies, that this polymorphism inhibits Mycobacterium leprae- as well as Mycobacterium tuberculosis-mediated responses (2). We have investigated, in a case-control study, the occurrence of this polymorphism in patients infected with tuberculosis.
The study population consisted of 33 unrelated patients, aged from 25 to 70 years, with active pulmonary tuberculosis as confirmed by clinical, radiological, and bacteriological investigations. The control group included 33 healthy blood donors, aged from 22 to 50 years, with no history of tuberculosis or immune diseases. Consent to participate in the investigation was obtained from all subjects. All patients and control participants originated from Tunisia, North Africa. The Tunisian population is in the Hardy-Weinberg equilibrium; this has been demonstrated through the analysis of 10 short tandem repeat markers validated for use in national forensic biology (D. Fathallah, personal communication). Genomic DNA was extracted from frozen whole blood by using a phenol extraction procedure. To determine the TLR2 genotype, the genomic DNA was amplified using forward (5′-TACTGGGTGGAGAACCTTAT-3′) and reverse (5′-AGTTCATACTTGCACCACTC-3′) primers which span the region containing the Arg677Trp polymorphism. PCR was performed in a total volume of 50 μl consisting of 5 μl of 10× reaction buffer (20 mM Tris HCl [pH 8.8], 50 mM KCl, 15 mM MgCl2), 0.5 μl of deoxynucleoside triphosphate mix (25 mM solution), 5 U of AmpliTaq DNA polymerase (Amersham Biosciences, Little Chalfont, Buckinghamshire, United Kingdom), 25 pmol of each primer, and 5 μl of genomic DNA (100 ng). PCR was performed in the model 9700 GeneAmp PCR system (Applied Biosystems, Foster City, Calif.) with the following conditions: 5 min of initial denaturation at 95°C and then 30 cycles of 95°C for 30 s, 55°C for 30 s, and 72°C for 1 min, followed by one elongation step at 72°C for 1 min. PCR products were purified with a PCR purification kit (QIAGEN, Hilden, Germany) and were sequenced by using an ABI Prism 377 DNA sequencer (Applied Biosystems). Statistical analysis of genotype distribution between groups was determined by the Fisher exact test.
Direct sequencing of the 200-bp region containing the polymorphic site of interest detected an C→T replacement (Fig. 1) at nucleotide 2029 from the start codon of TLR2 cDNA. This C→T substitution results in replacement of a conserved arginine residue with tryptophan at amino acid 677. Table 1 shows the TLR2 genotype distribution in tuberculosis patients and healthy controls. Thirty-one (94%) out of 33 tuberculosis patients were heterozygous for the mutation (C/T), versus 10 (31%) out of 33 in the control population (P < 0.0001). No one in either group was homozygous for the mutation (T/T).
FIG. 1.
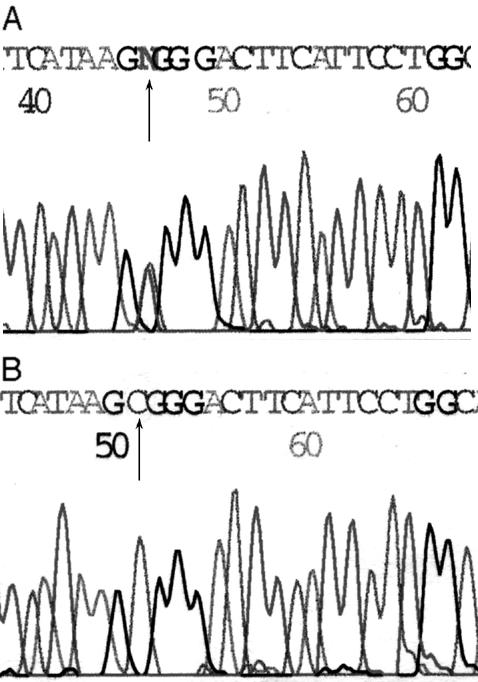
Sequencing results of TLR2 genomic DNA. (A) Heterozygous (wild type/mutation) genotype; (B) homozygous (wild type/wild type) genotype.
TABLE 1.
Distribution of TLR2 genotypes in tuberculosis patients and healthy controls
| TLR2 genotype | No. of tuberculosis patients (%) (n = 33) | No. of healthy controls (%) (n = 33) |
|---|---|---|
| C/C | 2 (6) | 23 (69) |
| C/T | 31 (94)a | 10 (31) |
| T/T | 0 (0) | 0 (0) |
P < 0.0001 when this value was compared with that for normal controls as calculated by a Fisher exact test.
The importance of TLRs in human diseases has been stressed in recent studies of polymorphisms in TLR4. Indeed, two missense mutations (Asp299Gly and Thr399Ile) affecting the extracellular domain of human TLR4 have been reported to be associated with hyporesponsiveness to inhaled endotoxin (1). Another polymorphism located in the Toll IL-1R domain of human TLR2 (Arg753Gln) was associated with staphylococcal infection (7). Several studies have investigated the genetic component of human susceptibility to mycobacterial infections. Recently, a missense mutation affecting the intracellular domain of human TLR2 (Arg677Trp) in a Korean population was described and was found to be associated with lepromatous leprosy (3). In two other studies, this TLR2 polymorphism was shown to be associated with lower serum IL-12 levels in lepromatous leprosy patients (4) and to abolish the response to M. leprae and M. tuberculosis as indicated by cell transfection studies (2). These data provide evidence that polymorphisms of the TLR2 gene may lead to increased susceptibility to infections by bacteria containing TLR2 agonists. In the present case-control study we showed that the frequency of the C/T genotype in tuberculosis patients is significantly higher than that in normal control subjects (94 versus 31%; P <0.0001). To date, such an association between this heterozygous genotype and the disease trait has not been reported for tuberculosis; therefore, detection of this polymorphism among tuberculosis patients may provide important information for the assessment of risk profiles regarding susceptibility to tuberculosis. Since unknown risk factors may interfere with this interpretation, a study of healthy household contacts in the control group is warranted. On the other hand, a recent study has shown that the Arg677Trp polymorphism previously reported among Koreans and Tunisians does not occur among Germans (10). Thus, the association between this polymorphism and tuberculosis might not be observed in some populations.
Interestingly, the Arg677Trp mutation affects a conserved arginine residue situated in close proximity to the locus corresponding to the dominant-negative mutation of the TLR2 gene (Pro681His), that abolishes the interaction with MyD88, which is required for signaling (9). Moreover, this mutation may affect the association of TLR2 homodimers and/or of TLR2/TLR1 heterodimers that have been involved in responses to the putative M. leprae 19-kDa lipoprotein (5). This lipoprotein shares 47% amino acid sequence identity with the 19-kDa lipoprotein of M. tuberculosis.
TLR2 is critical in the immune response to mycobacterial infection and is required for IL-12 induction (13). The IL-12-dependent gamma interferon pathway is known to play a major role in cell-mediated immunity to intramacrophagic pathogens by promoting Th1 responses. Furthermore, mutations in IL-12R and IL-12p40 subunits have been shown to be associated with susceptibility to weakly pathogenic mycobacteria and to tuberculosis. TLR2-mediated M. tuberculosis signaling and the consequences of the Arg677Trp polymorphism on IL-12 production by monocytes from tuberculosis patients are under investigation.
Acknowledgments
We thank Sonia Abdelhak for providing some of the DNA samples from tuberculosis patients. We also thank Houda Elloumi-Zghal and Belhassen Kaabi for fruitful discussion and Meherzia Ben Fadhel for technical assistance.
REFERENCES
- 1.Arbour, N. C., E. Lorenz, B. Schutte, J. Zabner, J. Kline, M. Jones, K. Frees, J. L. Watt, and D. A. Schwartz. 2000. TLR4 mutations are associated with endotoxin hyporesponsiveness in humans. Nat. Genet. 25:187-191. [DOI] [PubMed] [Google Scholar]
- 2.Bochud, P. Y., T. R. Hawn, and A. Aderem. 2003. Cutting edge: a toll-like receptor 2 polymorphism that is associated with lepromatous leprosy is unable to mediate mycobacterial signaling. J. Immunol. 170:3451-3454. [DOI] [PubMed] [Google Scholar]
- 3.Kang, T. J., and G. T. Chae. 2001. Detection of Toll-like receptor 2 (TLR2) mutation in the lepromatous leprosy patients. FEMS Immunol. Med. Microbiol. 31:53-58. [DOI] [PubMed] [Google Scholar]
- 4.Kang, T. J., S. B. Lee, and G. T. Chae. 2002. A polymorphism in the toll-like receptor 2 is associated with IL-12 production from monocyte in lepromatous leprosy. Cytokine 20:56-62. [DOI] [PubMed] [Google Scholar]
- 5.Krutzik, S. R., M. T. Ochoa, P. A. Sieling, S. Uematsu, Y. W. Ng, A. Legaspi, P. T. Liu, S. T. Cole, P. J. Godowski, Y. Maeda, E. N. Sarno, M. V. Norgard, P. J. Brennan, S. Akira, T. H. Rea, and R. L. Modlin. 2003. Activation and regulation of Toll-like receptors 2 and 1 in human leprosy. Nat. Med. 9:525-532. [DOI] [PubMed] [Google Scholar]
- 6.Lien, E., T. K. Means, H. Heine, A. Yoshimura, S. Kusumoto, K. Fukase, M. J. Fenton, M. Oikawa, N. Qureshi, B. Monks, R. W. Finberg, R. R. Ingalls, and D. T. Golenbock. 2000. Toll-like receptor 4 imparts ligand-specific recognition of bacterial lipopolysaccharide. J. Clin. Investig. 105:497-504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lorenz, E., J. P. Mira, K. L. Cornish, N. C. Arbour, and D. A. Schwartz. 2000. A novel polymorphism in the toll-like receptor 2 gene and its potential association with staphylococcal infection. Infect. Immun. 68:6398-6401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Medzhitov, R., P. Preston-Hurlburt, and C. A. Janeway, Jr. 1997. A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature 388:394-397. [DOI] [PubMed] [Google Scholar]
- 9.Medzhitov, R., P. Preston-Hurlburt, E. Kopp, A. Stadlen, C. Chen, S. Ghosh, and C. A. Janeway, Jr. 1998. MyD88 is an adaptor protein in the hToll/IL-1 receptor family signaling pathways. Mol. Cell 2:253-258. [DOI] [PubMed] [Google Scholar]
- 10.Schroder, N. W., C. Hermann, L. Hamann, U. B. Gobel, T. Hartung, and R. R. Schumann. 2003. High frequency of polymorphism Arg753Gln of the Toll-like receptor-2 gene detected by a novel allele-specific PCR. J. Mol. Med. 81:368-372. [DOI] [PubMed] [Google Scholar]
- 11.Underhill, D. M., A. Ozinsky, A. M. Hajjar, A. Stevens, C. B. Wilson, M. Bassetti, and A. Aderem. 1999. The Toll-like receptor 2 is recruited to macrophage phagosomes and discriminates between pathogens. Nature 401:811-815. [DOI] [PubMed] [Google Scholar]
- 12.Underhill, D. M., A. Ozinsky, K. D. Smith, and A. Aderem. 1999. Toll-like receptor-2 mediates mycobacteria-induced proinflammatory signaling in macrophages. Proc. Natl. Acad. Sci. USA 96:14459-14463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang, T., W. P. Lafuse, and B. S. Zwilling. 2000. Regulation of toll-like receptor 2 expression by macrophages following Mycobacterium avium infection. J. Immunol. 165:6308-6313. [DOI] [PubMed] [Google Scholar]


