Abstract
The development of effective prophylactic and therapeutic vaccines against genital herpes has proven problematic. Difficulties are associated with the complexity of the virus life cycle (latency) and our relatively poor understanding of the mechanism of immune control of primary and recurrent disease. The types of effector cells and the mechanisms responsible for their activation and regulation are particularly important. Studies from my and other laboratories have shown that recurrent disease is prevented by virus-specific T helper 1 (Th1) cytokines (viz., gamma interferon) and activated innate immunity. Th2 cytokines (viz., interleukin-10 [IL-10]) and regulatory (suppressor) T cells downregulate this immune profile, thereby allowing unimpeded replication of reactivated virus and recurrent disease. Accordingly, an effective therapeutic vaccine must induce Th1 immunity and be defective in Th2 cytokine production, at least IL-10. These concepts are consistent with the findings of the most recent clinical trials, which indicate that (i) a herpes simplex virus type 2 (HSV-2) glycoprotein D (gD-2) vaccine formulated with a Th1-inducing adjuvant has prophylactic activity in HSV-2- and HSV-1-seronegative females, an activity attributed to the adjuvant function, and (ii) a growth-defective HSV-2 mutant (ICP10ΔPK), which is deleted in the Th2-polarizing gene ICP10PK, induces Th1 immunity and has therapeutic activity in both genders. The ICP10ΔPK vaccine prevents recurrent disease in 44% of treated subjects and reduces the frequency and severity of recurrences in the subjects that are not fully protected. Additional studies to evaluate these vaccines are warranted.
Herpes simplex virus type 2 (HSV-2) is the primary cause of genital herpes, a common sexually transmitted disease with at least 40 to 60 million infected individuals in the U.S. alone. Significant morbidity and mortality are associated with HSV-2 transmission to the neonate; a minimum of 2,500 cases occur annually (3, 113). HSV-2 infection increases the risk of human immunodeficiency virus (HIV) acquisition (8, 59, 77). Following primary infection at skin or mucosal surfaces, viral DNA is rapidly transported to the innervating ganglia by retrograde axonal transport. Here the viral genome circularizes and establishes a life-long latency in sensory neurons. During latency the viral genome is largely transcriptionally inactive. Low-level transcription of viral sequences known as latency-associated transcripts occurs, but their function is a point of contention in the field. Infection induces both humoral and T-cell immunity. Stress stimuli trigger the resumption of virus gene transcription and replication, resulting in clinically overt skin and mucosal lesions and/or asymptomatic shedding. Approximately two-thirds of HSV-2-infected subjects develop clinically overt recurrent disease (12). Latency-associated transcripts as well as other viral and inducible cellular genes were implicated in latency reactivation (12, 13, 47, 49, 57, 75, 106, 111). Regardless of the mechanism of latency reactivation, at least 37 to 57% of infected subjects claim that HSV-2 interferes with sexual relationships, triggers anxiety and depression, and ruins their lives (103). The worldwide social and economic burden created by HSV-2 infection (35) has stimulated major interest in vaccines that prevent infection and/or recurrent disease. However, success has been elusive. This work is a brief review of the present status of HSV-2 vaccines, emphasizing the role of immunity in the control of infection and recurrent disease. Recent promising developments are stressed.
IMMUNE CONTROL OF HSV INFECTION
Studies of animal models have shown that neutralizing antibody is inefficient in preventing the axonal spread of the virus. Innate immunity (natural killer [NK] cells, interferon [IFN], and macrophages) is an important component of protection from HSV infection (1, 6, 12), but T cells, primarily CD8+-cytotoxic T cells (CTL), are a major determinant of protective immunity (68). This is a particular challenge for the immune system, because HSV has developed various immune evasion mechanisms. Notable among these is the direct targeting of antigen presentation by major histocompatibility complex (MHC) class I through binding (by viral protein ICP47) of the cellular protein that transports antigenic peptides into the endoplasmic reticulum (50, 104). Other immune evasion mechanisms include resistance to attack by complement, mediated by viral glycoprotein C (36); prevention of antibody binding, mediated by the viral glycoproteins gE and gI (72); and blocking of CD8+ CTL activity through US3-mediated inhibition of caspase activation (25). However, most of the HSV-specific T cells in human lesional skin secrete IFN-γ (55, 67), which partially restores MHC class I expression. IFN-γ also stimulates expression of MHC class II by infected keratinocytes (KC), thereby leading to their recognition and destruction by CD4+ T cells (28). Accordingly, the role of the immune evasion mechanisms in recurrent disease remains unclear.
Latency-reactivated virus presumably circumvents immune inhibition by replicating before immunity is alerted or by shifting the immunological balance towards tolerance via the induction of responses which downregulate Th1 antiviral immunity. For example, Th2 cytokines (viz., interleukin-10 [IL-10]) downregulate costimulatory molecules and the maturation and function of antigen-presenting dendritic cells (DC) (2, 31, 40). Virus infection of DC inhibits their maturation and migration, which are necessary for efficient CTL priming (76, 82). Regulatory (suppressor) T cells, which were previously discredited as a mechanism of immune regulation, are also gaining increasing support. They include CD4+ CD25+ cells that function via cell-to-cell interaction and the production of transforming growth factor β (TGF-β) and Tr1 cells that function via secretion of TGF-β and IL-10 (33, 56), and they are consistent with earlier reports that reactivated virus escapes immune inhibition by inducing suppressor T cells (7, 8, 48, 84-87). KC infected by the reactivated virus can act as potent inducers of immune suppression by shifting the profile of cytokine production towards Th2 (24, 44).
TH1 IMMUNITY PREVENTS RECURRENT DISEASE
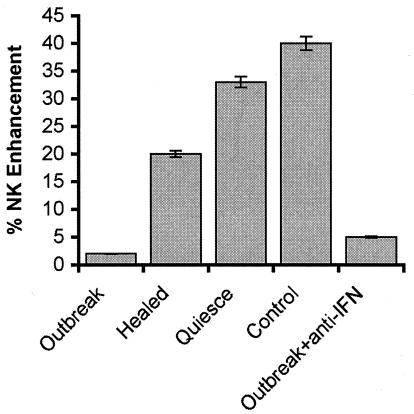
In the 1980s researchers had already attempted to determine the role of immunity in preventing recurrences by elucidating the T-cell responses of infected subjects who do not develop recurrent disease. To ensure functional relevance these responses were compared to those of patients with recurrent disease, studied during the acute episode (days 1 to 3 postonset), at convalescence (days 4 to 9 postonset) and in the interim between recurrences (quiescence) (4, 8, 84-87). It was found that HSV-stimulated T cells from patients who do not develop recurrent disease produce IFN-γ and stimulate NK lytic activity. IFN-γ was not produced by T cells collected during recurrences or at prodrome (24 to 48 h before a clinical outbreak), but its production was restored during quiescence (Fig. 1). Similar results were obtained with the guinea pig model of recurrent disease (32, 48). The IFN-γ-producing immune profile of patients who do not suffer from recurrent disease was called relevant immunity in order to distinguish it from the immunity exhibited by patients with recurrent disease, and it was shown that during recurrences this profile is replaced by an IL-6-IL-10-producing profile (7, 8, 69). Since that time, IFN-γ has been identified as a Th1 cytokine that has antiviral potential, activates NK cells, and induces CD8+ CTL. Numerous independent studies confirmed that Th1 cytokines are overproduced during recurrence-free episodes, while downregulatory Th2 cytokines (viz., IL-10) are overproduced during recurrent episodes (27, 30, 61, 92, 114). Accordingly, it was concluded that the onset of recurrent disease is regulated at two checkpoints. The first is the decision for resumed transcription of latent HSV DNA. This checkpoint is under the control of stress-induced cellular proteins, such as the AP-1 transcription factor (106). Virus genes that respond to AP-1 (viz., the large subunit of HSV-2 ribonucleotide reductase, also known as ICP10) initiate the resumption of virus replication (13, 112, 115). The second is the decision to allow unimpeded replication of the reactivated virus through downregulation of the antiviral Th1 immunity. This checkpoint is under the control of a newly synthesized viral protein(s) that is Th2 polarizing.
FIG. 1.
Recurrent disease is associated with inhibition of IFN-γ-mediated NK cell activation. T cells were collected from HSV-2-infected subjects who do not develop recurrent disease (control) and from those with recurrent disease during a clinical outbreak, convalescence, or the interval between recurrences (quiescence). They were cultured with HSV-2 antigen (20 μg/ml) in the presence or absence of rabbit IFN-γ antibody (104 neutralizing U/ml) for 1 and 7 days. At this time the cells were pelleted by centrifugation, the IFN-γ antibody complex in the supernatants was removed by adsorption with agarose beads coated with goat antibody to rabbit immunoglobulin G, and the supernatants were used in NK-enhancing assays. The NK assay used peripheral blood leukocytes from normal healthy volunteers as effectors and K562 cells as targets (40:1 ratio). NK enhancement was calculated from the following formula: % NK enhacement = 1 − (% specific lysis effector cells/% specific lysis effector cells + supernatant) × 100 (86). In this assay the percent specific lysis by effector cells was 24% ± 3%. The average percent NK enhancing activity by effector cells exposed to 50 U of standard IFN-γ was 40% ± 5%. IFN-γ levels in culture supernatants (determined by enzyme-linked immunosorbent assay) were 1,024 U/ml in the patients without recurrent disease and 16 U/ml during recurrent disease.
Based on these interpretations, it was predicted that a leak in the first, but not second, regulatory checkpoint would result in asymptomatic virus shedding, and the existence of such shedding was first demonstrated in 1985 (5, 9). Today, asymptomatic shedding is an established aspect of HSV pathogenesis. The second prediction implicit in the two-checkpoint interpretation is that clinical recurrences are associated with the suppression of virus-specific Th1 responses. Remaining questions relate to the role of downregulatory Tr cells in recurrent disease and the contribution of asymptomatic shedding to immune downregulation. Regardless, the available data suggest that for a therapeutic vaccine to be effective, it must (i) induce Th1 immunity, including CD8+ CTL; (ii) activate innate immunity (viz., NK activity); (iii) fail to induce appropriate levels of Th2 cytokines; and (iv) overcome the effects or inhibit the function of regulatory (suppressor) T cells.
VIRAL PROTEIN TARGETS FOR VACCINE CONSTRUCTION
Two approaches were taken to identify viral proteins that are targets for vaccine development. The first was a positive selection approach directed at identifying the most immunogenic HSV proteins to be used in vaccine construction. One experimental protocol to identify such proteins examined the specificity of T cells from infected animals or humans by stimulation with vaccinia virus recombinants that express various HSV proteins. This approach led to the conclusions that glycoproteins gD and gB are key targets for the activation of CD4+ T cells and that ICP27 is a key target for the activation of CD8+ T cells (14, 67). It also showed that glycosylation is essential for T-cell activation by viral glycoproteins and demonstrated that DC play a determining role in the generation of protective immunity (10, 107-109). Another experimental protocol to identify the most immunogenic HSV proteins examined the response of T cells from HSV lesions to HSV-1- and -2-infected B cells. It identified several tegument proteins as the major T-cell targets (54), confirming widely held beliefs that many viral proteins can induce T-cell responses.
The second approach to identify viral targets for vaccine construction was a negative selection effort. It was directed at identifying the Th2-polarizing proteins so that they can be deleted from a potential vaccine construct. This latter approach was used, and it showed that the functionally independent protein kinase (PK) domain of the HSV-2 large subunit of ribonucleotide reductase (known as ICP10) has Th2-polarizing activity. In infected cells, including KC, ICP10PK upregulates the Th2 cytokines IL-6, IL-10, and IL-13 and downregulates RANTES, which is a chemoattractant for Th1 but not Th2 cells (C. C. Smith, B. B. Goswami, M. Gober, E. F. Pereira, and L. Aurelian, submitted for publication). Significantly, ICP10PK is required for virus replication and latency reactivation (13, 47, 49, 93), suggesting that its deletion will interfere with virus replication and latency establishment while reducing or eliminating Th2 polarization and toleragenic potential.
HSV-2 VACCINE CANDIDATES
All the presently developed HSV-2 vaccine candidates are based on the targets described above. They include protein subunits, killed virus, live recombinant or attenuated viruses, and viral DNA preparations. Because the formulation (viz., adjuvant or conjugate vaccine vehicle), route of delivery (viz., subcutaneous or mucosal), and protocol (viz., prime and boost) affect the magnitude, composition, duration, and compartmentalization of the immune response, various modifications were examined. The vaccination goals and target populations also have defining roles in the selection of a specific composition. In the case of HSV-2, the original goals were to develop a prophylactic vaccine that induces local (mucosal) and systemic immunity which prevents infection and, thereby, virus transmission. This goal was predicated on the belief that neutralizing antibody and activated T cells at mucosal surfaces are the likeliest means to prevent infection, and its construction targets the viral glycoproteins gD and gB, which are involved in cell entry (21). However, it is becoming increasingly evident that the original vaccination goals may be unrealistic. Accordingly, the more recent goals of prophylactic vaccination are to prevent or reduce the clinical symptoms of primary infection. In this context, efforts focus on the selection of ideal adjuvants and the definition of optimal immunization route and protocol (17, 19, 89).
(i) Subunit vaccines.
Significant effort has been invested in the development of subunit HSV-2 vaccines. Their advantages are that they are safe (in HIV-infected subjects as well), chemically defined, selective, and stable at ambient temperature. Their production is simple, easily standardized, and cost-effective. Glycoproteins were selected as antigenic targets for these vaccines because they are localized on the surface of infected cells and virions and because they are immunogenic (see above). Immune-stimulating complexes (ISCOM) consisting of glycoproteins gB, gC, gD, and gE prepared from infected cells with the zwitterionic detergent Empigen BB and glycoside Quil A induced antibody and provided protection from lethal HSV-1 and -2 infection in mice. They also reduced the symptoms of vaginal HSV-2 infection in guinea pigs (63, 70, 88). Glycoproteins gD and/or gB prepared from infected cells or constructed by recombinant methods reduced HSV-2 disease in animal models (16, 23, 74, 97, 105), modestly decreased the frequency and severity of recurrences, and reduced the incidence of virus shedding, but only when given together with potent adjuvants (46, 79). The adjuvant effect was also strong in vaccination by the mucosal route (37).
Several human trials were done with the subunit vaccines. A vaccine prepared from purified envelope glycoproteins (Merck, Sharp & Dohme) was studied in sexual partners. In a phase IIa trial the numbers of individuals developing infection were similar between the vaccine and placebo groups (65). A vaccine consisting of recombinant gB-2 and gD-2 formulated with the adjuvant MF-59 (Chiron) induced specific neutralizing antibody and T-cell lymphoproliferative responses in phase I studies. However, in two phase III studies that assessed prevention of HSV-2 infection, the overall efficacy was 9% and vaccination had no significant effect on the duration of the first clinical episode of genital HSV-2 or the frequency of subsequent recurrences (22, 100). The most recently studied subunit vaccine is from GlaxoSmithKline (GSK), and it is gD-2 formulated with a mixture of alum and 3-deacylated monophosphoryl lipid A (3-dMPL) as adjuvant. In two phase III trials, this vaccine reduced clinical symptoms of primary HSV-2 infection (approximately 70% efficacy), but only in women and only if they were initially HSV-1 and HSV-2 seronegative (98). The effect of gender is unlikely to be due to gender-specific immunological differences, because similar immunity was induced in males, although they were not protected (98). The absence of efficacy in HSV-1-infected women suggests that previously established immunity may interfere with vaccination or that the latter does not enhance the levels of protection provided by the previous HSV-1 infection. In either case, a significant effect on the proportion of the disease is unlikely, because approximately 80 to 95% of adults are infected with HSV-1 (113). The GSK vaccine did not seem to provide protection from HSV infection, and activity was attributed to the adjuvant used in its composition (98). Consistent with this interpretation, symptom reduction was seen for the 3-dMPL, but not MF-59, formulation, and infection was not prevented by either formulation in guinea pig studies (19). Moreover, disease symptoms are also reduced by immune modulators (viz., imiquimod and resiquimod) (42, 96). The subunit vaccines do not have therapeutic activity in humans (30, 101).
(ii) Killed and live attenuated virus vaccines.
Most human studies of killed virus vaccines suffer from the absence of appropriate controls. The few studies that were placebo controlled yielded conflicting results, with evidence of therapeutic activity in some studies but not others (34, 60, 73). Live attenuated viruses have the advantage of imparting long-lasting and broad immunity. Their disadvantages are safety concerns, including the risk of homologous recombination during production in cell lines engineered to supply the absent viral gene product(s) in trans. Two strategies were used to develop live HSV-2 vaccines. The first strategy used viral vectors that express HSV glycoproteins. Recombinant HSV-1 strains expressing the HSV-2 glycoproteins gD-2, gE-2, and gG-2 and part of gI-2 were studied in guinea pigs, owl monkeys, and human clinical trials with limited success (64). A human adenovirus recombinant expressing HSV-2 glycoprotein gB-2 was studied in the mouse genital model (60), and the severity of HSV-2 infection in the guinea pig genital model was decreased by immunization with the Oka vaccine strain of varicella-zoster virus expressing gD-2 (43). Vaccinia virus recombinants expressing gD-2 were studied in the mouse and guinea pig models. These studies showed that glycosylation epitopes and DC-mediated antigen presentation play determinant roles in protective immunity (10, 107-109).
The second strategy used to develop live vaccines was to generate mutants rendered nonvirulent by the deletion of one or more genes while retaining the viral glycoproteins previously identified as immunogenic targets. An HSV-2 mutant with deletions of both copies of the γ134.5 gene and the UL55 and UL56 open reading frames reduced disease severity and frequency in guinea pigs (95). Another HSV-2 mutant with a deletion of ICP8, a gene required for viral DNA synthesis, reduced HSV-2 replication, disease severity, and the frequency of recurrent disease in the guinea pig (29). An HSV-1 mutant deficient in virion host shutoff protein (vhs) reduced recurrent stromal keratitis but not virus shedding after intraperitoneal inoculation in mice (51) (presumably related to vhs-mediated DC inactivation [83]; however, the mutant retained virulence [51]). An HSV-2 mutant deficient in glycoprotein gH, known as DISC (disabled infectious single cycle), reduced HSV-2 replication and provided protection from HSV-2-induced disease (62). DISC also caused a 36% reduction in recurrent lesions in the guinea pig when given systemically but not when administered by the mucosal route (20, 62). In phase I clinical trials done by Cantab (now Xenova/GSK), DISC was well tolerated and induced neutralizing antibody and lymphoproliferative T-cell responses. Eighty-three percent of the vaccine recipients also developed HSV-specific CTL. However, clinical endpoints were not met in phase II trials to assess DISC's efficacy as a therapeutic vaccine, and further development was halted (Xenova Press Release, 19 February 2002, http:/www.xenova.co.uk/PressReleases/pr_20020219_01.html).
(iii) Viral DNA vaccines.
Plasmid DNA encoding glycoproteins gD and/or gB or the immediate-early protein ICP27 induced protective immunity in some animal models. Immunity was incomplete in other models (18, 38, 81). Expression of cell-associated forms of gD-2 induced primarily Th1 responses, whereas expression of secreted gD-2 resulted in a Th2 response (45). In guinea pigs, immunization with DNA encoding cytosolic gD-2 did not protect from acute or recurrent disease, whereas protection was seen in mice. The vaccines did not improve virus clearance from the inoculation site or significantly reduce recurrent disease in guinea pigs when used for therapeutic vaccination (99). Protection, when present, was mediated by CD4+ T cells (58), and coadministration of DNA encoding Th1 cytokines (90), IFN-α1 (41), or chemokines for Th1 cells (91), increased the potency of these agents. DNA vaccine potency was also increased by coadministration of DNA encoding antiapoptotic proteins (53) or by prime-boost immunization with DNA and modified vaccinia virus vectors expressing gD-2 (66). Bacterial artificial chromosomes containing a replication-competent packaging-defective HSV-1 genome were also shown to induce protective immunity in the mouse model of fatal disease, representing a new generation of DNA-based vaccination strategies (102).
ICP10ΔPK HAS THERAPEUTIC ACTIVITY IN ANIMALS
The negative selection approach that was chosen in order to identify targets for vaccine construction was designed to identify viral proteins with Th2-polarizing capability to be deleted in vaccine construction. The choice of this approach was based on previous findings, which are discussed above, that Th2 polarization plays a pivotal role in the onset of recurrent disease. Because it was found that ICP10PK is Th2 polarizing (Smith et al., submitted), it was deleted from the vaccine candidate ICP10ΔPK. The data also indicated that ICP10PK is required for virus replication and latency establishment and reactivation (13, 93), leading to the conclusion that ICP10ΔPK will meet safety criteria for vaccine development.
(i) Safety considerations.
As predicted, ICP10ΔPK was growth and latency compromised. In cultured cells, growth onset was significantly delayed and virus titers were significantly reduced (93). ICP10ΔPK did not replicate or cause disease in infected animals (mice and guinea pigs), even when given at very high titers (1 × 107 to 5 × 107 PFU). It was latency defective, with only 12% of the ganglia containing viral DNA, and had 100-fold lower DNA levels than those in ganglia from HSV-2-infected animals (11, 110). Because latency reactivation is related to HSV DNA copy number, the latency defect of ICP10ΔPK is, at least, partially related to its impaired replication. However, ICP10-specific antisense oligonucleotides inhibited the reactivation of the wild-type virus (13), suggesting that ICP10ΔPK also has a specific reactivation defect. Additional safety considerations that make ICP10ΔPK particularly promising as a vaccine candidate include (i) growth complementation by a cellular (rather than viral in trans) function, thereby avoiding the risk of virulence-restoring recombination during production; (ii) failure to reactivate upon HSV-2 infection; and (iii) loss of transforming activity (11, 12). The latter is clinically relevant, because HSV-2 can cause extensive hyperproliferative lesions in infected humans (15) and because it increases the risk of cervical cancer (94). Because ICP10ΔPK is latency compromised, the risk of potential recombination with latent HSV-2 DNA carried by the patient is also relatively low.
(ii) Prophylactic and therapeutic activity.
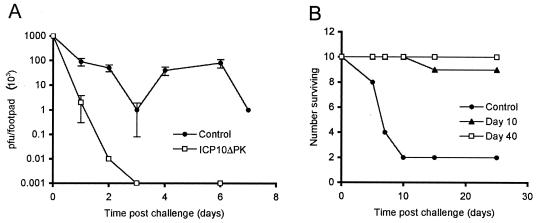
In the mouse, ICP10ΔPK vaccination inhibited HSV-2 replication (Fig. 2A) and provided virtually absolute protection from fatal and cutaneous HSV-2 disease (Fig. 2B) (11). In the guinea pig, it reduced HSV-2 cutaneous lesions (6% with disease versus 100% in the placebo group) and vaginal disease (10% with disease versus 100% in the placebo group). The proportion of ganglia positive for HSV-2 DNA (8 out of 25 [32%]) and the levels of HSV-2 DNA/ganglion (3 × 103 molecules) were also significantly lower in immunized than control animals (70% latently infected ganglia; 2 × 105 molecules/ganglion). Similar results were obtained with animals challenged with HSV-1, indicating that protection is not restricted to HSV-2 (11). The ability of the vaccine to decrease latency establishment is epidemiologically relevant, because over time it should lead to a general reduction in virus transmission in the population at large and decrease disease prevalence, even in unvaccinated subjects. ICP10ΔPK also had therapeutic activity. Vaccination of guinea pigs previously infected with HSV-2 caused a 75 to 90% reduction in the number of animals with recurrent disease and reduced the frequency and duration of the recurrent episodes experienced by the rare animals that were not absolutely protected (110).
FIG. 2.
ICP10ΔPK immunization prevents HSV-2 replication and disease. (A) Mice (Swiss Webster, 5 weeks old, groups of 10) were given two doses of ICP10ΔPK (3 × 106 PFU) or phosphate-buffered saline (control) by subcutaneous inoculation in the rear right footpad at 10-day intervals and were challenged with HSV-2 (107 PFU) in the contralateral footpad. HSV-2 titers (PFU) in the footpad were determined by plaque assay at various times after challenge. (B) Mice were immunized with ICP10ΔPK or phosphate-buffered saline (control) and were challenged with HSV-2 (5 × 107 PFU) at 10 or 40 days after the last immunization. They were monitored for skin lesions, paralysis, and/or death. Similar protection was observed for animals challenged with HSV-1 instead of HSV-2.
(iii) Mechanism of protection.
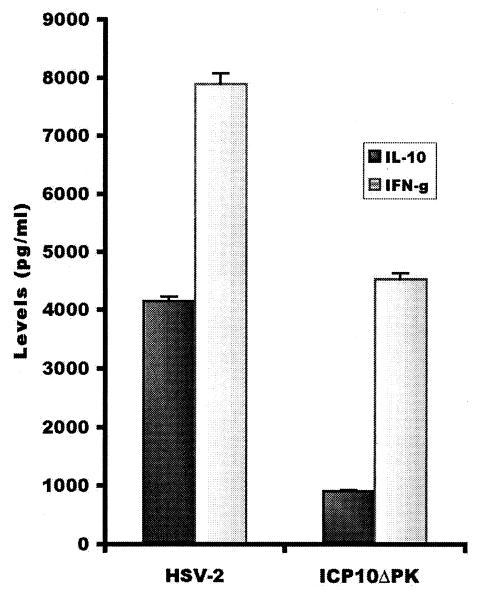
ICP10ΔPK was defective in Th2 cytokine production in infected keratinocytes, and it elicited a Th1 response in infected animals (Fig. 3). This response included CD8+ CTL (39). Its generation involved increased DC activation (IL-12 production) (39), which is Th1 polarizing (71, 78, 80, 116). This immune profile is responsible for vaccine activity, because HSV-2 replication and disease were inhibited by adoptive transfer of the CD8+ CTL and/or CD4+ Th1 cells (39). CD4+ T cells that infiltrate the HSV lesions likely function by producing IFN-γ, which is also found in human lesional skin (55). However, because HSV blocks recognition by CD8+ T cells (50, 104), it is likely that the infiltrating CD8+ T cells provide additional signals that contribute to their protective function in adoptive transfer, such as chemokines (viz., IL-8, stroma-derived factor 1α, macrophage inflammatory protein 1α, and macrophage inflammatory protein 1β) that function collectively to drive and expand immunity (52). By contrast to ICP10ΔPK, HSV-2 upregulates IL-6, IL-13, and IL-10 and downregulates the Th1-specific chemokine RANTES (Smith et al., submitted), inhibiting IL-12 production by DC (39) and polarizing the response in favor of Th2 (78).
FIG. 3.
ICP10ΔPK induces Th1 immunity and is defective in IL-10 production. T cells from lymph nodes of mice (Swiss Webster, 5 weeks old) given ICP10ΔPK or HSV-2 (106 PFU) were cultured with HSV-2 antigen (10 μg/ml) for 2 days, and supernatants were assayed for IFN-γ (Th1 cytokine) and IL-10 (Th2 cytokine) by enzyme-linked immunosorbent assay. Results are expressed as cytokine levels.
ICP10ΔPK HAS THERAPEUTIC ACTIVITY IN PHASE I AND PHASE II CLINICAL TRIALS
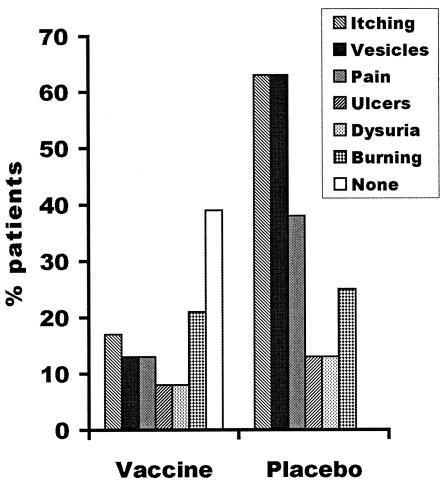
The results of a phase I and II clinical trial of ICP10ΔPK were recently reported (26). In this study the vaccine (2 × 105 PFU) or its vehicle formulation (placebo) was given subcutaneously at 7 days after the initiation of the presenting lesion, followed by additional injections at days 14 and 28. Recurrences were considered ended when all the symptoms disappeared. The vaccine was tolerated well. At 6 months after treatment, HSV-2 recurrences were completely prevented in 37.5% of the vaccinated patients but were prevented in none of the placebo-treated patients. Vaccinated patients who experienced disease had significantly lower frequency of episodes and mean total illness days relative to the placebo group (P = 0.028). The severity of the observed episodes was also reduced in the vaccinated patients compared to that in the placebo-treated patients. This was expressed in a reduction of vesicles, pain, and itching (Fig. 4). The mean number of recurrent episodes/month for the vaccinated patients was significantly reduced compared to that documented for the previous year (P < 0.001), and similar protection was still seen at 12 months after treatment (G. Casanova, unpublished results).
FIG. 4.
ICP10ΔPK immunization decreases the severity of the HSV-2 recurrences in humans. Results are shown for patients in the 2 × 105-PFU trial.
A second trial used the vaccine at a dose of 2 × 106 PFU, with injections at 10 and 28 days after the initiation of the presenting lesions and a 1-year follow-up. Again the vaccine was tolerated well. Recurrences were completely eliminated in 43.5% of the vaccinated patients, while the placebo group had 13% without recurrences (P = 0.024). There was also a significant reduction in the number of episodes per patient (P = 0.04), the number of episodes relative to the previous year (P < 0.001), and the number of recurrent episodes in the vaccinated group relative to the placebo group (P = 0.04) (G. Casanova, R. Cancela, L. Alonzo, R. Benuto, C. Mdel Magana, D. R. Hurley, E. Fishbein, C. Lara, T. Gonzalez, R. Ponce, J. W. Burnett, and G. J. Calton, unpublished data). The safety and efficacy of the vaccine in HIV-infected subjects have not been determined, but recent studies suggest that ICP10ΔPK does not cause disease in nude mice even at the high dose (G. Calton, personal communication). Additional studies are needed in order to perfect the dosage necessary to further reduce the frequency of recurrent episodes, establish the maximal duration of protection in larger numbers of patients, and define the effect of the vaccine on asymptomatic virus shedding.
CONCLUSIONS
Difficulties associated with the development of effective HSV vaccines include the virus life cycle and our relatively poor understanding of the role of immunity in preventing infection, clinical symptoms, and/or unimpeded replication of reactivated endogenous virus. The role of distinct effector T cells in preventing infection as opposed to recurrent disease, the effect of previously acquired immunity on vaccine efficacy, and the function of the adjuvants are important considerations. A wealth of information indicates that recurrent disease is prevented by virus-specific Th1 immunity, most notably by IFN-γ, and its ability to enhance the innate immunity, particularly NK cell lytic activity. Tr cells and Th2 cytokines (viz., IL-10) downregulate this immune profile, thereby allowing for unimpeded replication of reactivated virus and recurrent disease. Accordingly, an effective therapeutic vaccine must induce Th1 immunity and lack toleragenic epitopes, including those that elicit IL-10 production. Guarded optimism is generated by recent findings for a prophylactic (gD-2; GSK) and a therapeutic (ICP10ΔPK; AuRx) vaccine, supporting their further evaluation.
REFERENCES
- 1.Ahmad, A., E. Sharif-Askari, L. Fawaz, and J. Menezes. 2000. Innate immune response of the human host to exposure with herpes simplex virus type 1: in vitro control of the virus infection by enhanced natural killer activity via interleukin-15 induction. J. Virol. 74:7196-7203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akdis, C. A., and K. Blaser. 2001. Mechanisms of interleukin-10-mediated immune suppression. Immunology 103:131-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Armstrong, G. L., J. Schillinger, L. Markowitz, A. J. Nahmias, R. E. Johnson, G. M. McQuillan, and M. E. St. Louis. 2001. Incidence of herpes simplex virus type 2 infection in the United States. Am. J. Epidemiol. 153:912-920. [DOI] [PubMed] [Google Scholar]
- 4.Aurelian, L., and J. F. Sheridan. 1982. Herpesvirus infection: inhibition of leukocyte migration inhibition factor production in the diagnosis of recurrent disease. Clin. Immunol. Newsl. 2:169-172 [Google Scholar]
- 5.Aurelian, L., and I. I. Kessler. 1985. Subclinical herpes virus infections of the genital tract are commonly associated with viral shedding. Cervix 3:235-248. [Google Scholar]
- 6.Aurelian, L., C. L. Rinehart, M. Wachsman, M. Kulka, and P. O. P. Ts'o. 1987. Augmentation of natural immune defense mechanisms and therapeutic potential of a mismatched double-stranded polynucleotide in cutaneous herpes simplex virus type 2 infection. J. Gen. Virol. 68:2831-2838. [DOI] [PubMed] [Google Scholar]
- 7.Aurelian, L. 1989. Herpes simplex, p. 73-100. In S. Spector, M. Bendinelli, and H. Friedman (ed.), Virus induced immunosuppression. Plenum Publishing Corp., New York, N.Y.
- 8.Aurelian, L. (ed.) 1990. Herpesviruses, the immune system and AIDS. Kluwer Academic Publishers, Boston, Mass.
- 9.Aurelian, L., M. Wachsman, and J. W. Burnett. 1990. Clinical and subclinical HSV infection resulting from exposure to asymptomatic patients. Br. J. Dermatol. 122:117-119. [DOI] [PubMed] [Google Scholar]
- 10.Aurelian, L., C. C. Smith, M. Wachsman, and E. Paoletti. 1991. Immune responses to herpes simplex virus in guinea pigs (footpad model) and mice immunized with vaccinia virus recombinants containing herpes simplex virus glycoprotein D. Rev. Infect. Dis. 13:S924-S934. [DOI] [PubMed] [Google Scholar]
- 11.Aurelian, L., H. Kokuba, and C. C. Smith. 1999. Vaccine potential of a herpes simplex virus type 2 mutant deleted in the PK domain of the large subunit of ribonucleotide reductase (ICP10). Vaccine 17:1951-1963. [DOI] [PubMed] [Google Scholar]
- 12.Aurelian, L. 2000. Herpes simplex viruses, p. 384-409. In S. Specter, R. L. Hodinka, and S. A. Young (ed.), Clinical virology manual, 3rd ed. ASM Press, Washington, D.C.
- 13.Aurelian, L., and C. C. Smith. 2000. Herpes simplex virus type 2 growth and latency reactivation by cocultivation are inhibited with antisense oligonucleotides complementary to the translation initiation site of the large subunit of ribonucleotide reductase (RR1). Antisense Nucleic Acid Drug Dev. 10:77-85. [DOI] [PubMed] [Google Scholar]
- 14.Banks, T. A., S. Nair, and B. T. Rouse. 1993. Recognition by and in vitro induction of cytotoxic T lymphocytes against predicted epitopes of the immediate-early protein ICP27 of herpes simplex virus. J. Virol. 67:613-616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Beasley, K. L., G. E. Cooley, G. F. Kao, M. H. Lowitt, J. W. Burnett, and L. Aurelian. 1997. Herpes simplex vegetans: atypical genital herpes infection in a patient with common variable immunodeficiency. J. Am. Acad. Dermatol. 37:860-863. [DOI] [PubMed] [Google Scholar]
- 16.Bernstein, D. I., and L. R. Stanberry. 1999. Herpes simplex virus vaccines. Vaccine 17:1681-1689. [DOI] [PubMed] [Google Scholar]
- 17.Bernstein, D. I. 2000. Effect of route of vaccination with vaccinia virus expressing HSV-2 glycoprotein D on protection from genital HSV-2 infection. Vaccine 18:1351-1358. [DOI] [PubMed] [Google Scholar]
- 18.Bourne, N., G. N. Milligan, M. R. Schleiss, D. I. Bernstein, and L. R. Stanberry. 1996. DNA immunization confers protective immunity on mice challenged intravaginally with herpes simplex virus type 2. Vaccine 14:1230-1234. [DOI] [PubMed] [Google Scholar]
- 19.Bourne, N., F. J. Bravo, M. Francotte, D. I. Bernstein, M. G. Myers, M. Slaoui, and L. R. Stanberry. 2003. Herpes simplex virus (HSV) type 2 glycoprotein D subunit vaccines and protection against genital HSV-1 or HSV-2 disease in guinea pigs. J. Infect. Dis. 187:542-549. [DOI] [PubMed] [Google Scholar]
- 20.Boursnell, M. E. G., C. Entwistle, D. Blakeley, C. Roberts, I. A. Duncan, S. E. Chisholm, G. M. Martin, et al. 1997. A genetically inactivated herpes simplex virus type 2 (HSV-2) vaccine provides effective protection against primary and recurrent HSV-2 disease. J. Infect. Dis. 175:16-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Browne, H., B. Bruun, and T. Minson. 2001. Plasma membrane requirements for cell fusion induced by herpes simplex virus type 1 glycoproteins gB, gD, gH, and gL. J. Gen. Virol. 82:1419-1422. [DOI] [PubMed] [Google Scholar]
- 22.Burke, R. L. 1993. Current developments in herpes simplex virus vaccines. Semin. Virol. 4:187-197. [Google Scholar]
- 23.Byars, N. E., E. B. Fraser-Smith, R. A. Pecyk, M. Welch, G. Nakano, R. L. Burke, A. Hayward, and A. C. Allison. 1994. Vaccinating guinea pigs with recombinant glycoprotein D of herpes simplex virus in an efficacious adjuvant formulation elicits protection against vaginal infection. Vaccine 12:200-209. [DOI] [PubMed] [Google Scholar]
- 24.Cao, Y., H. Zhou, J. Tao, Z. Zheng, N. Li, B. Shen, T. S. Shih, J. Hong, J. Zhang, and K. Y. Chou. 2003. Keratinocytes induce local tolerance to skin graft by activating interleukin-10-secreting T cells in the context of costimulation molecule B7-H1. Transplantation 75:1390-1396. [DOI] [PubMed] [Google Scholar]
- 25.Cartier, A., E. Broberg, T. Komai, M. Henriksson, and M. G. Masucci. 2003. The herpes simplex virus-1 Us3 protein kinase blocks CD8T cell lysis by preventing the cleavage of Bid by granzyme B. Cell Death Differ. 10:1320-1328. [DOI] [PubMed] [Google Scholar]
- 26.Casanova, G., R. Cancela, L. Alonzo, R. Benuto, C. Mdel Magana, D. R. Hurley, E. Fishbein, C. Lara, T. Gonzalez, R. Ponce, J. W. Burnett, and G. J. Calton. 2002. A double-blind study of the efficacy and safety of the ICP10ΔPK vaccine against recurrent genital HSV-2 infections. Cutis 70:235-239. [PubMed] [Google Scholar]
- 27.Croft, M., L. Carter, S. L. Swain, and R. W. Dutton. 1994. Generation of polarized antigen-specific CD8 effector populations: reciprocal action of interleukin (IL)-4 and IL-12 in promoting type 2 versus type 1 cytokine profiles. J. Exp. Med. 180:1715-1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cunningham, A. L., and J. R. Noble. 1989. Role of keratinocytes in human recurrent herpetic lesions. Ability to present herpes simplex virus antigen and act as targets for T lymphocyte cytotoxicity in vitro. J. Clin. Investig. 83:490-496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Da Costa, X. J., N. Bourne, L. R. Stanberry, and D. M. Knipe. 1997. Construction and characterization of a replication-defective herpes simplex virus 2 ICP8 mutant strain and its use in immunization studies in a guinea pig model of genital disease. Virology 232:1-12. [DOI] [PubMed] [Google Scholar]
- 30.Deshpande, S. P., U. Kumaraguru, and B. T. Rouse. 2000. Why do we lack an effective vaccine against herpes simplex virus infections? Microbes Infect. 2:973-978. [DOI] [PubMed] [Google Scholar]
- 31.De Smedt, T., M. Van Mechelen, G. De Becker, J. Urbain, O. Leo, and M. Moser. 1997. Effect of interleukin-10 on dendritic cell maturation and function. Eur. J. Immunol. 27:1229-1235. [DOI] [PubMed] [Google Scholar]
- 32.Donnenberg, A. D., E. Chaikof, and L. Aurelian. Immunity to herpes simplex virus type 2. II. Cell-mediated immunity in latently infected guinea pigs. Infect. Immun. 30:99-109. [DOI] [PMC free article] [PubMed]
- 33.Dubois, B., L. Chapat, A. Goubier, and D. Kaiserlian. 2003. CD4+ CD25+ T cells as key regulators of immune responses. Eur. J. Dermatol. 13:111-116. [PubMed] [Google Scholar]
- 34.Dundarov, S., P. Andonov, B. Bakalov, K. Nechev, and C. Tomov. 1982. Immunotherapy with inactivated polyvalent herpes vaccines. Dev. Biol. Stand. 52:351-358. [PubMed] [Google Scholar]
- 35.Fisman, D. N., M. Lipsitch, E. W. Hook III, and S. J. Goldie. 2002. Projection of the future dimensions and costs of the genital herpes simplex type 2 epidemic in the United States. Sex. Transm. Dis. 29:608-622. [DOI] [PubMed] [Google Scholar]
- 36.Friedman, H. M., L. Wang, M. K. Pangburn, J. D. Lambris, and J. Lubinski. 2000. Novel mechanism of antibody-independent complement neutralization of herpes simplex virus type 1. J. Immunol. 165:4528-4536. [DOI] [PubMed] [Google Scholar]
- 37.Gallichan, W. S., R. N. Woolstencroft, T. Guarasci, M. J. McCluskie, H. L. Davis, and K. L. Rosenthal. 2001. Intranasal immunization with CpG oligodeoxynucleotides as an adjuvant dramatically increases IgA and protection against herpes simplex virus-2 in the genital tract. J. Immunol. 166:3451-3457. [DOI] [PubMed] [Google Scholar]
- 38.Ghiasi, H., S. Cia, S. Slanina, A. B. Nesburn, and S. L. Wechsler. 1995. Vaccination of mice with herpes simplex virus type 1 glycoprotein D DNA produces low levels of protection against lethal HSV-1 challenge. Antivir. Res. 28:147-157. [DOI] [PubMed] [Google Scholar]
- 39.Gyotoku, T., F. Ono, and L. Aurelian. 2002. Development of HSV-specific CD4+ Th1 responses and CD8+ cytotoxic T lymphocytes with antiviral activity by vaccination with the HSV-2 mutant ICP10ΔPK. Vaccine 20:2796-2807. [DOI] [PubMed] [Google Scholar]
- 40.Hara, M., C. O. Kingsley, M. Niimi, S. Read, S. E. Turvey, A. R. Bushell, P. J. Morris, F. Powrie, and K. J. Wood. 2001. IL-10 is required for regulatory T cells to mediate tolerance to alloantigens in vivo. J. Immunol. 166:3789-3796. [DOI] [PubMed] [Google Scholar]
- 41.Härle, P., S. Noisakran, and D. J. J. Carr. 2001. The application of a plasmid DNA encoding IFN-α1 postinfection enhances cumulative survival of herpes simplex virus type 2 vaginally infected mice. J. Immunol. 166:1803-1812. [DOI] [PubMed] [Google Scholar]
- 42.Harrison, C. J., R. L. Miller, and D. I. Bernstein. 2001. Reduction of recurrent HSV disease using imiquimod alone or combined with a glycoprotein vaccine. Vaccine 19:1820-1826. [DOI] [PubMed] [Google Scholar]
- 43.Heineman, T. C., B. L. Connelly, N. Bourne, L. R. Stanberry, and J. Cohen. 1995. Immunization with recombinant varicella-zoster virus expressing herpes simplex virus type 2 glycoprotein D reduces the severity of genital herpes in guinea pigs. J. Virol. 69:8109-8113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Herrick, C. A., L. Xu, A. N. McKenzie, R. E. Tigelaar, and K. Bottomly. 2003. IL-13 is necessary, not simply sufficient, for epicutaneously induced Th2 responses to soluble protein antigen. J. Immunol. 170:2488-2495. [DOI] [PubMed] [Google Scholar]
- 45.Higgins, T. J., K. M. Herold, R. L. Arnold, S. P. McElhiney, K. E. Shroff, and C. J. Pachuk. 2000. Plasmid DNA-expressed secreted and nonsecreted forms of herpes simplex virus glycoprotein D2 induce different types of immune responses. J. Infect. Dis. 182:1311-1320. [DOI] [PubMed] [Google Scholar]
- 46.Ho, R. Y. J., R. L. Burke, and T. C. Merigan. 1989. Antigen-presenting liposomes are effective in treatment of recurrent herpes simplex virus genitalis in guinea pigs. J. Virol. 63:2951-2958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hunsperger, E. A., and C. L. Wilcox 2003. Caspase-3-dependent reactivation of latent herpes simplex virus type 1 in sensory neuronal cultures. J. Neurovirol. 9:390-398. [DOI] [PubMed] [Google Scholar]
- 48.Iwasaka, T., J. F. Sheridan, and L. Aurelian. 1983. Immunity to herpes simplex virus type 2: recurrent lesions are associated with the induction of suppressor cells and soluble suppressor factors. Infect. Immun. 42:955-964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jacobson, J. G., D. A. Leib, D. J. Goldstein, C. L. Bogard, P. A. Schaffer, S. K. Weller, and D. M. Coen. 1989. A herpes simplex virus ribonucleotide reductase deletion mutant is defective for productive acute and reactivatable latent infections of mice and for replication in mouse cells. Virology 173:276-283. [DOI] [PubMed] [Google Scholar]
- 50.Jugovic, P., A. M. Hill, R. Tomazin, H. Ploegh, and D. C. Johnson. 1998. Inhibition of major histocompatibility complex class I antigen presentation in pig and primate cells by herpes simplex virus type 1 and 2 ICP47. J. Virol. 72:5076-5084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Keadle, T. L., A. K. A. Laycock, J. L. Morris, D. A. Leib, L. A. Morrison, J. S. Pepose, and P. M. Stuart. 2002. Therapeutic vaccination with vhs-herpes simplex virus reduces the severity of recurrent herpetic stromal keratitis in mice. J. Gen. Virol. 83:2361-2365. [DOI] [PubMed] [Google Scholar]
- 52.Kim, J. J., L. K. Nottingham, J. I. Sin, A. Tsai, L. Morrison, J. Oh, K. Dang, Y. Hu, K. Kazahaya, M. Bennett, T. Dentchev, D. M. Wilson, A. A. Chalian, J. D. Boyer, M. G. Agadjanyan, and D. B. Weiner. 1998. CD8 positive T cells influence antigen-specific immune responses through the expression of chemokines. J. Clin. Investig. 102:1112-1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kim, T. W., C. F. Hung, M. Ling, J. Juang, L. He, J. M. Hardwick, S. Kumar, and T.-C. Wu. 2003. Enhancing DNA vaccine potency by coadministration of DNA encoding antiapoptotic proteins. J. Clin. Investig. 112:109-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Koelle, D. M., L. Corey, R. L. Burke, R. J. Eisenberg, G. H. Cohen, R. Pichyangkura, and S. J. Triezenberg. 1994. Antigenic specificities of human CD4+ T-cell clones recovered from recurrent genital herpes simplex virus type 2 lesions. J. Virol. 68:2803-2810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kokuba, H., L. Aurelian, and J. W. Burnett. 1999. Herpes simplex virus associated erythema multiforme (HAEM) is mechanistically distinct from drug-induced EM: IFN-γ is expressed in HAEM lesions and TNF-α in drug-induced EM lesions. J. Investig. Dermatol. 113:808-815. [DOI] [PubMed] [Google Scholar]
- 56.Levings, M. K., R. Sangregorio, C. Sartirana, A. L. Moschin, M. Battaglia, P. C. Orban, and M. G. Roncarolo. 2002. Human CD25+ CD4+ T suppressor cell clones produce transforming growth factor beta, but not interleukin 10, and are distinct from type 1 T regulatory cells. J. Exp. Med. 196:1335-1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Loiacono, C. M., R. Myers, and W. J. Mitchell. 2002. Neurons differentially activate the herpes simplex virus type 1 immediate-early gene ICP0 and ICP27 promoters in transgenic mice. J. Virol. 76:2449-2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Manickan, E., R. J. Rouse, Z. Yu, W. S. Wire, and B. T. Rouse. 1995. Genetic immunization against herpes simplex virus. Protection is mediated by CD4+ T lymphocytes. J. Immunol. 155:259-265. [PubMed] [Google Scholar]
- 59.McClelland, R. S., C. C. Wang, J. Overbaugh, B. A. Richardson, L. Corey, R. L. Ashley, K. Mandaliya, J. Ndinya-Achola, J. J. Bwayo, and J. K. Kreiss. 2002. Association between cervical shedding of herpes simplex virus and HIV-1. AIDS 16:2425-2430. [DOI] [PubMed] [Google Scholar]
- 60.McDermott, M. R., J. R. Smiley, P. Leslie, J. Brais, H. E. Rudzroga, and J. Bienenstock. 1984. Immunity in the female genital tract after intravaginal vaccination of mice with an attenuated strain of herpes simplex virus type 2. J. Virol. 51:747-773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.McKenna, D. B., W. A. Neill, and M. Norval. 2001. Herpes simplex virus-specific immune responses in subjects with frequent and infrequent orofacial recrudescences. Br. J. Dermatol. 144:459-464. [DOI] [PubMed] [Google Scholar]
- 62.McLean, C. S., M. Erturk, R. Jennings, D. Ni Challanain, A. C. Minson, I. Duncan, M. E. G. Boursnell, and S. C. Inglis. 1994. Protective vaccination against primary and recurrent disease caused by herpes simplex virus (HSV) type 2 using a genetically disabled HSV-1. J. Infect. Dis. 170:1100-1109. [DOI] [PubMed] [Google Scholar]
- 63.Meignier, B., T. M. Jourdier, B. Norrild, L. Pereira, and B. Roizman. 1987. Immunization of experimental animals with reconstituted glycoprotein mixtures of herpes simplex virus 1 and 2: protection against challenge with virulent virus. J. Infect. Dis. 155:921-930. [DOI] [PubMed] [Google Scholar]
- 64.Meignier, B., R. Longnecker, P. Mavroma-Nazos, A. E. Sears, and B. Roizman. 1988. Virulence and establishment of latency by genetically engineered deletion mutants of herpes simplex virus 1. Virology 162:251-254. [DOI] [PubMed] [Google Scholar]
- 65.Mertz, G. J., R. Ashley, R. L. Burke, J. Benedetti, C. Critchlow, C. C. Jones, and L. Corey. 1990. Double-blind placebo-controlled trial of a herpes simplex virus type 2 glycoprotein vaccine in persons at high risk for a genital herpes infection. J. Infect. Dis. 161:653-660. [DOI] [PubMed] [Google Scholar]
- 66.Meseda, C. A., K. L. Elkins, M. J. Merchlinsky, and J. P. Weir. 2002. Prime-boost immunization with DNA and modified vaccinia virus Ankara vectors expressing herpes simplex virus-2 glycoprotein D elicits greater specific antibody and cytokine responses than DNA vaccine alone. J. Infect. Dis. 186:1065-1073. [DOI] [PubMed] [Google Scholar]
- 67.Mikloska, Z., and A. L. Cunningham. 1998. Herpes simplex virus type 1 glycoproteins gB, gC, and gD are major targets for CD4 T-lymphocyte cytotoxicity in HLA-DR expressing human epidermal keratinocytes. J. Gen. Virol. 79:353-361. [DOI] [PubMed] [Google Scholar]
- 68.Milligan, G. N., D. I. Bernstein, and N. Bourne. 1998. T lymphocytes are required for protection of the vaginal mucosae and sensory ganglia of immune mice against reinfection with herpes simplex virus type 2. J. Immunol. 160:6093-6100. [PubMed] [Google Scholar]
- 69.Miura, S., M. Kulka, C. C. Smith, S. Imafuku, J. W. Burnett, and L. Aurelian. 1994. Cutaneous UV radiation (UVR) inhibits herpes simplex virus induced lymphoproliferation in latently infected subjects. Clin. Immunol. Immunopathol. 72:62-69. [DOI] [PubMed] [Google Scholar]
- 70.Mohamedi, S. A., A. W. Heath, and R. Jennings. 2001. A comparison of oral and parenteral routes for therapeutic vaccination with HSV-2 ISCOMs in mice; cytokine profiles, antibody responses and protection. Antivir. Res. 49:83-99. [DOI] [PubMed] [Google Scholar]
- 71.Moulin, V., F. Andris, K. Thielemans, C. Maliszewski, J. Urbain, and M. B. Moser. 2000. Lymphocytes regulate dendritic cell (DC) function in vivo: increased interleukin 12 production by DCs from B cell-deficient mice results in T helper cell type 1 deviation. J. Exp. Med. 192:475-482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Nagashunmugam, T., J. Lubinski, L. Wang, L. T. Goldstein, B. S. Weeks, P. Sundaresan, E. H. Kang, G. Dubin, and H. M. Friedman. 1998. In vivo immune evasion mediated by the herpes simplex virus type 1 immunoglobulin G Fc receptor. J. Virol. 72:5351-5359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Nasemann, T., and G. Schaeg. 1973. Herpes simplex virus type II: microbiology and clinical experiences with attenuated vaccine. Hautarzt 24:133-139. [PubMed] [Google Scholar]
- 74.O'Hagan, D., C. Goldbeck, M. Ugozzoli, G. Ott, and R. L. Burke. 1999. Intranasal immunization with recombinant gD2 reduces disease severity and mortality following genital challenge with herpes simplex virus type 2 in guinea pigs. Vaccine 17:2229-2236. [DOI] [PubMed] [Google Scholar]
- 75.Perng, G.-C., B. Maguen, J. Ling, K. R. Mott, N. Osorio, S. M. Slanina, A. Yukht, H. Ghiasi, A. B. Nesburn, M. Inman, G. Henderson, C. Jones, and S. L. Wechsler. 2002. A gene capable of blocking apoptosis can substitute for the herpes simplex virus type 1 latency-associated transcript gene and restore wild-type reactivation levels. J. Virol. 76:1224-1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Pollara, G., K. Speidel, L. Samady, M. Rajpopat, Y. McGrath, J. Ledermann, R. S. Coffin, D. R. Katz, and B. Chain. 2003. Herpes simplex virus infection of dendritic cells: balance among activation, inhibition, and immunity. J. Infect. Dis. 187:165-178. [DOI] [PubMed] [Google Scholar]
- 77.Reynolds, S. J., A. R. Risbud, M. E. Shepherd, J. M. Zenilman, R. S. Brookmeyer, R. S. Paranjape, A. D. Divekar, R. R. Gangakhedkar, M. V. Ghate, R. C. Bollinger, and S. M. Mehendale. 2003. Recent herpes simplex virus type 2 infection and the risk of human immunodeficiency virus type 1 acquisition in India. J. Infect. Dis. 187:1513-1521. [DOI] [PubMed] [Google Scholar]
- 78.Ria, F., G. Penna, and L. Adorini. 1998. Th1 cells induce and Th2 inhibit antigen-dependent IL-12 secretion by dendritic cells. Eur. J. Immunol. 28:2003-2016. [DOI] [PubMed] [Google Scholar]
- 79.Richards, C. M., A. T. Aman, T. R. Hirst, T. J. Hill, and N. A. Williams. 2001. Protective mucosal immunity to ocular herpes simplex virus type 1 infection in mice by using Escherichia coli heat-labile enterotoxin B subunit as an adjuvant. J. Virol. 75:1664-1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ridge, J. P., F. Di Rosa, and P. Matzinger. 1998. A conditioned dendritic cell can be a temporal bridge between a CD4+ T-helper and a T-killer cell. Nature 393:474-478. [DOI] [PubMed] [Google Scholar]
- 81.Rouse, B. T., S. Nair, R. J. Rouse, Z. Yu, N. Kuklin, K. Karem, and E. Manickan. 1998. DNA vaccines and immunity to herpes simplex virus. Curr. Top. Microbiol. Immunol. 226:69-78. [DOI] [PubMed] [Google Scholar]
- 82.Salio, M., M. Cella, M. Suter, and A. Lanzavecchia. 1999. Inhibition of dendritic cell maturation by herpes simplex virus. Eur. J. Immunol. 29:3245-3253. [DOI] [PubMed] [Google Scholar]
- 83.Samady, L., E. Costigliola, L. MacCormac, Y. McGrath, S. Cleverley, C. E. Lilley, J. Smith, D. S. Latchman, B. Chain, and R. S. Coffin. 2003. Deletion of the virion host shutoff protein (vhs) from herpes simplex virus (HSV) relieves the viral block to dendritic cell activation: potential of vhs-hsv vectors for dendritic cell-mediated immunotherapy. J. Virol. 77:3768-3776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sheridan, J. F., A. D. Donnenberg, L. Aurelian, and D. J. Elpern. 1982. Immunity to herpes simplex virus type 2. IV. Impaired lymphokine production during recrudescence correlates with an imbalance in T lymphocyte subsets. J. Immunol. 129:326-331. [PubMed] [Google Scholar]
- 85.Sheridan, J. F., and L. Aurelian. 1983. Immunity to herpes simplex virus type 2. V. Risk of recurrent disease following primary infection: modulation of T cell subsets and lymphokine (LIF) production. Diagn. Immunol. 1:246-256. [PubMed] [Google Scholar]
- 86.Sheridan, J. F., M. Beck, L. Aurelian, and M. Radowsky. 1985. Immunity to herpes simplex virus. Virus reactivation, modulates lymphokine (NK enhancing) activity. J. Infect. Dis. 152:449-456. [DOI] [PubMed] [Google Scholar]
- 87.Sheridan, J. F., M. Beck, C. C. Smith, and L. Aurelian. 1987. Reactivation of herpes simplex virus is associated with production of a low molecular weight factor that inhibits lymphokine activity in vitro. J. Immunol. 138:1234-1239. [PubMed] [Google Scholar]
- 88.Simms, J. R., A. W. Heath, and R. Jennings. 2000. Use of herpes simplex virus (HSV) type 1 ISCOMS 703 vaccine for prophylactic and therapeutic treatment of primary and recurrent HSV-2 infection in guinea pigs. J. Infect. Dis. 181:1240-1248. [DOI] [PubMed] [Google Scholar]
- 89.Simms, J. R., R. Jennings, V. J. Richardson, and A. W. Heath. 2002. Herpes simplex virus (HSV) type 2 glycoprotein D subunit vaccines and protection against genital HSV-1 or HSV-2 disease in guinea pigs. J. Med. Virol. 68:82-91.12210434 [Google Scholar]
- 90.Sin, J. I., J. J. Kim, R. L. Arnold, K. E. Shroff, D. McCallus, C. Pachuk, S. P. McElhiney, M. W. Wolf, S. J. Pompa-de Bruin, T. J. Higgins, R. B. Ciccarelli, and D. B. Weiner. 1999. IL-12 gene as a DNA vaccine adjuvant in a herpes mouse model: IL-12 enhances Th1-type CD4+ T cell-mediated protective immunity against herpes simplex virus-2 challenge. J. Immunol. 162:2912-2921. [PubMed] [Google Scholar]
- 91.Sin, J., J. J. Kim, C. Pachuk, C. Satishchandran, and D. B. Weiner. 2000. DNA vaccines encoding interleukin-8 and RANTES enhance antigen-specific Th1-type CD4+ T-cell-mediated protective immunity against herpes simplex virus type 2 in vivo. J. Virol. 74:11173-11180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Singh, R., A. Kumar, W. D. Creery, M. Ruben, A. Giulivi, and F. Diaz-Mitoma. 2003. Dysregulated expression of IFN-gamma and IL-10 and impaired IFN-gamma-mediated responses at different disease stages in patients with genital herpes simplex virus-2 infection. Clin. Exp. Immunol. 133:97-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Smith, C. C., T. Peng, M. Kulka, and L. Aurelian. 1998. The PK domain of the large subunit of herpes simplex virus type 2 ribonucleotide reductase (ICP10) is involved in IE gene transcription and virus growth. J. Virol. 72:9131-9141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Smith, J. S., R. Herrero, C. Bosetti, N. Munoz, F. X. Bosch, J. Eluf-Neto, X. Castellsague, C. J. Meijer, A. J. Van den Brule, S. Franceschi, R. Ashley, and International Agency for Research on Cancer (IARC) Multicentric Cervical Cancer Study Group. 2002. Herpes simplex virus-2 as a human papillomavirus cofactor in the etiology of invasive cervical cancer. J. Natl. Cancer Inst. 94:1604-1613. [DOI] [PubMed] [Google Scholar]
- 95.Spector, F. C., E. R. Kern, J. Palmer, E. Kaiwar, T.-A. Cha, P. Brown, and R. R. Spaete. 1998. Evaluation of a live attenuated recombinant virus RAV 9395 as a herpes simplex virus type 2 vaccine in guinea pigs. J. Infect. Dis. 177:1143-1154. [DOI] [PubMed] [Google Scholar]
- 96.Spruance, S. L., S. K. Tyring, M. H. Smith, and T. C. Meng. 2001. Application of a topical immune response modifier, resiquimod gel, to modify the recurrence rate of recurrent genital herpes: a pilot study. J. Infect. Dis. 184:196-200. [DOI] [PubMed] [Google Scholar]
- 97.Stanberry, L. R., D. I. Bernstein, R. L. Burke, C. Pachl, and M. G. Myers. 1987. Vaccination with recombinant herpes simplex virus glycoproteins: protection against initial and recurrent genital herpes. J. Infect. Dis. 155:914-920. [DOI] [PubMed] [Google Scholar]
- 98.Stanberry, L. R., S. L. Spruance, A. L. Cunningham, D. I. Bernstein, A. Mindel, S. Sacks, S. Tyring, F. Y. Aoki, M. Slaoui, M. Denis, P. Vandepapeliere, G. Dubin, and GlaxoSmithKline Herpes Vaccine Efficacy Study Group. 2002. Glycoprotein-D-adjuvant vaccine to prevent genital herpes. N. Engl. J. Med. 347:1652-1661. [DOI] [PubMed] [Google Scholar]
- 99.Strasser, J. E., R. L. Arnold, C. Pachuk, T. J. Higgins, and D. I. Bernstein. 2000. Herpes simplex virus DNA vaccine efficacy: effect of glycoprotein D plasmid constructs. J. Infect. Dis. 182:1304-1310. [DOI] [PubMed] [Google Scholar]
- 100.Straus, S. E., L. Corey, R. L. Burke, B. Savarese, G. Barnum, P. R. Krause, R. G. Kost, J. L. Meier, R. Sekulovich, S. F. Adair, et al. 1994. Placebo-controlled trial of vaccination with recombinant glycoprotein D of herpes simplex virus type 2 for immunotherapy of genital herpes. Lancet 343:1460-1463. [DOI] [PubMed] [Google Scholar]
- 101.Straus, S. E., A. Wald, R. G. Kost, R. McKenzie, A. G. M. Langerberg, P. Hohman, J. Lekstrom, E. Cox, M. Nakamura, R. Sekulovich, A. Izu, C. Dekker, and L. Corey. 1997. Immunotherapy of recurrent genital herpes with recombinant herpes simplex virus type 2 glycoprotein D and B: results of placebo-controlled vaccine trial. J. Infect. Dis. 176:1129-1134. [DOI] [PubMed] [Google Scholar]
- 102.Suter, M., A. M. Lew, P. Grob, G. J. Adema, M. Ackermann, K. Shortman, and C. Fraefel. 1999. BAC-VAC, a novel generation of (DNA) vaccines: a bacterial artificial chromosome (BAC) containing a replication-competent, packaging-defective virus genome induces protective immunity against herpes simplex virus 1. Proc. Natl. Acad. Sci. USA 96:12697-12702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Taboulet, F., B. Halioua, and J. E. Malkin. 1999. Quality of life and use of health care among people with genital herpes in France. Acta Dermatol. Venereol. 79:380-384. [DOI] [PubMed] [Google Scholar]
- 104.Tomazin, R., N. E. van Schoot, K. Goldsmith, P. Jugovic, P. Sempe, K. Fruh, and D. C. Johnson. 1998. Herpes simplex virus type 2 ICP47 inhibits human TAP but not mouse TAP. J. Virol. 72:2560-2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ugozzoli, M., D. T. O'Hagan, and G. S. Ott. 1998. Intranasal immunization of mice with herpes simplex virus type 2 recombinant gD2: the effect of adjuvants on mucosal and serum antibody responses. Immunology 93:563-571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Valyi-Nagy, T., S. Deshmane, A. Dillner, and N. W. Fraser. 1991. Induction of cellular transcription factors in trigeminal ganglia of mice by corneal scarification, herpes simplex virus type 1 infection, and explantation of trigeminal ganglia. J. Virol. 65:4142-4152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wachsman, M., J. H. Luo, L. Aurelian, M. E. Perkus, and E. Paoletti. 1989. Antigen-presenting capacity of epidermal cells infected with vaccinia virus recombinants containing the herpes simplex virus glycoprotein D, and protective immunity. J. Gen. Virol. 70:2513-2520. [DOI] [PubMed] [Google Scholar]
- 108.Wachsman, M., L. Aurelian, C. C. Smith, M. E. Perkus, and E. Paoletti. 1989. Regulation of expression of herpes simplex virus (HSV) glycoprotein D in vaccinia recombinants affects their ability to protect from cutaneous HSV-2 disease. J. Infect. Dis. 159:625-634. [DOI] [PubMed] [Google Scholar]
- 109.Wachsman, M., J. H. Luo, L. Aurelian, and E. Paoletti. 1992. Protection from herpes simplex virus type 2 is associated with T cells involved in delayed type hypersensitivity that recognize glycosylation-related epitopes on glycoprotein D. Vaccine 10:447-454. [DOI] [PubMed] [Google Scholar]
- 110.Wachsman, M., M. Kulka, C. C. Smith, and L. Aurelian. 2001. A growth and latency defective herpes simplex virus type 2 mutant (ICP10ΔPK) has prophylactic and therapeutic protective activity in guinea pigs. Vaccine 19:1879-1890. [DOI] [PubMed] [Google Scholar]
- 111.Wang, K., L. Pesnicak, E. Guancial, P. R. Krause, and S. E. Straus. 2001. The 2.2-kilobase latency-associated transcript of herpes simplex virus type 2 does not modulate viral replication, reactivation, or establishment of latency in transgenic mice. J. Virol. 75:8166-8172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Wymer, J. P., T. D. Chung, Y. N. Chang, G. S. Hayward, and L. Aurelian. 1989. Identification of immediate-early type cis response elements in the promoter for the ribonucleotide reductase large subunit from herpes simplex virus type 2. J. Virol. 63:2773-2784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Xu, F., J. A. Schillinger, M. R. Sternberg, R. E. Johnson, F. K. Lee, A. J. Nahmias, and L. E. Markowitz. 2002. Seroprevalence and coinfection with herpes simplex virus type 1 and type 2 in the United States, 1988-1994. J. Infect. Dis. 185:1019-1024. [DOI] [PubMed] [Google Scholar]
- 114.Yesliwska, J., P. Trzonkowski, E. Bryl, K. Lukaszuk, and A. Myesliwski. 2000. Lower interleukin-2 and higher serum tumor necrosis factor α levels are associated with perimenstrual, recurrent, facial herpes simplex infection in young women. Eur. Cytokine Netw. 11:397-406. [PubMed] [Google Scholar]
- 115.Zhu, J., and L. Aurelian. 1997. AP-1 cis-response elements are involved in basal expression and Vmw110 transactivation of the large subunit of herpes simplex virus type 2 ribonucleotide reductase (ICP0). Virology 231:301-312. [DOI] [PubMed] [Google Scholar]
- 116.Zou, W., J. Borvak, F. Marches, S. Wei, P. Galanaud, D. Emilie, and T. J. Curiel. 2000. Macrophage-derived dendritic cells have strong Th1-polarizing potential mediated by beta-chemokines rather than IL-12. J. Immunol. 165:4388-4396. [DOI] [PubMed] [Google Scholar]