Abstract
We used the promoter of the human C-reactive protein (CRP) gene to drive inflammation-inducible overexpression of the cytokine granulocyte-macrophage colony-stimulating factor (GM-CSF) in transgenic mice. Transgenic mice carrying a CRP/GM-CSF fusion gene show a >150-fold increases in circulating levels of GM-CSF within 6 h of intraperitoneal inoculation with 25 μg of lipopolysaccharide. However, some of the transgenic mice also display relatively high basal levels of GM-CSF in the absence of any obvious inflammatory stimulus. Raised basal levels of GM-CSF are associated with a number of pathological changes, including enlarged and histologically abnormal livers and spleens and with increases in the number and activation state of macrophages and granulocytes in the peripheral blood. Despite problems associated with the expression of such a potent pleiotropic cytokine as GM-CSF, the principle of inflammation-inducible expression of chimeric constructs has been shown to be feasible. Inducible expression systems such as that described here could be of potential use in the study of the role of cytokines in health and disease and in the development of disease-resistant strains of livestock.
Overexpression of genes of interest in transgenic animals has been of great value in the study of the host immune response to infection. Of particular interest to us was the potential use of transgenic technology to enhance the innate resistance of animals to infectious disease. The need for this arises from the desire to reduce the use of antibiotics in intensively reared farm animals, particularly antibiotics also used in human medicine. Enhanced resistance to disease would enable a reduction in the use of antibiotics and could also be of particular value in developing countries where infections in farm animals are rife, but accurate diagnosis and appropriate treatments are often unavailable.
We have aimed for overexpression of a cytokine because these molecules are potent mediators of the immune responses to a wide range of different infectious diseases, inducing many effects including cellular proliferation, differentiation, activation, and the release of other cytokines. The uncontrolled overexpression of such biologically active proteins as cytokines is problematic, however. Of 38 transgenic animal lines reviewed by Taverne (30), each expressing cytokine genes under the control of a constitutive heterologous promoter, only two lines were free of abnormalities, which included alopecia, wasting, tumors, thromboses, and premature death. Therefore, a system conferring inducible expression is desirable in order to avoid damage to the host caused by the constitutive expression of cytokines.
In the present study, we have chosen to overexpress the cytokine granulocyte-macrophage colony-stimulating factor (GM-CSF). GM-CSF activates and enhances the production and survival of neutrophils, eosinophils, and macrophages, cells which have key roles in the innate immune response. Increased expression of this cytokine might therefore be expected to increase the ability of an animal to combat certain pathogens. A number of studies have indeed shown this to be the case. Tanaka et al. (27) showed that administration of recombinant GM-CSF significantly increased the number of mice surviving a normally lethal dose of Pseudomonas aeruginosa. Administration of GM-CSF has also been shown to significantly increase the survival of rats challenged with Staphylococcus aureus (8) and to reduce and delay the peak of parasitemia when administered to mice prior to a lethal challenge with Trypanosoma cruzi (7). Hebert and O'Reilly (13) showed that administration of GM-CSF could protect splenectomized mice from challenge with a lethal dose of pneumococci. The important role of GM-CSF in defense against infectious disease was further demonstrated by LeVine et al. (19), who showed that GM-CSF-deficient mice are unusually susceptible to pulmonary group B streptococcal infection.
Despite the evidence showing the protective effects of GM-CSF, administering it to farm animals on a large scale to enhance immunity is not economically feasible. Transgenic technology offers an alternative way of “administering” GM-CSF to animals. Transgenic mice showing constitutive overexpression of murine GM-CSF have already been described (18). These mice were found to have macrophages which were activated to a higher than normal level and which showed enhanced phagocytic activity in vitro. However, these mice had abnormalities, including accumulations of activated macrophages in the eyes, leading to blindness, and in the muscles, leading to progressive wasting, and showed greatly increased rates of premature death after 6 weeks of age.
Because of the side effects of constitutive overexpression of this and other cytokines, we have sought here to develop an inducible expression system by which highly active immunity-enhancing proteins such as GM-CSF could be inducibly overexpressed in transgenic animals only when they are actually required and are likely to be most beneficial, i.e., during an infection.
The promoter we chose to drive inducible gene expression was that of the human C-reactive protein (CRP) gene. CRP is one of the acute-phase proteins, a group of proteins produced by the liver that are rapidly and strongly induced in the early stages of infection, injury, or malignancy. The concentration of CRP in the blood can rapidly rise by up to 1,000-fold, returning to its low basal levels when inflammatory stimuli are no longer present (9). In addition, the human CRP promoter has been well characterized in transgenic mice and has been shown to be strongly induced in response to inflammatory stimuli such as bacterial lipopolysaccharide (LPS) (4, 23).
In the present study we made a transgenic mouse line that displays inducible overexpression of murine GM-CSF, under the control of the human CRP promoter, in response to stimulation with LPS. Inducible expression systems such as the one we describe here could be of use in the study of the role of cytokines in health and disease and in the development of disease-resistant livestock strains.
MATERIALS AND METHODS
Construction of CRP flanking DNA constructs.
Two CRP constructs, termed pC79-NotI and pBNB-NotI, that are based on different-size regions of DNA (6.7 and 4.47 kb, respectively) encompassing the human CRP gene were made. These constructs differ only in the amount of 3′ CRP-flanking DNA they contain. The constructs are equivalent to constructs 79 and 4, respectively, used to make CRP transgenic mice by Murphy et al. (23), except that we have removed the CRP promoter and protein coding region (from −114 to +1187 bp relative to the mRNA cap site) and replaced them with a single NotI site (linking what was base −122 to what was base +1195) by using inverse PCR. The sequences of the oligonucleotide primers used for this inverse PCR are shown in Table 1. Construct C79 (23) was kindly provided by U. Ruther (European Molecular Biology Laboratory, Heidelberg, Germany). Construct pBNB was derived from a 31-kb cosmid clone (pCosCRP1) containing the human CRP gene (4) by using PCR.
TABLE 1.
PCR primers used to produce CRP-GM-CSF fusion gene and to introduce NotI restriction sites into the CRP flanking DNA constructsa
| Primer | Sequence (5′-3′) | Purpose |
|---|---|---|
| Fu5PR | AAT AAT TTT GCG GCC GCT CTT CCC GAA GCT CTG ACA CC | 5′-Terminal primer for the production of fusion between CRP and GM-CSF; the NotI site introduced at the 5′ end is in boldface |
| Fu3PR | GGA TTA CGA GCG GCC GCA TCC TAT TAT TTT TGG ACT GG | 3′-Terminal primer for the production of fusion between CRP and GM-CSF; the NotI site introduced at the 3′ end is in boldface |
| TM8 | GCT TTT GGC CAG ACA ACGCGTAGCCCGATCACTGTC | Internal fusion primer for amplification of the GM-CSF half of the CRP/GM-CSF fusion; the underlined region is complementary to GM-CSF |
| TM7-2 | GAC AGT GAT CGG GCT ACG CGT TGTCTGGCCAAAAGC | Internal fusion primer for amplification of the CRP half of the CRP/GM-CSF fusion; the underlined region is complementary to CRP |
| CRP R1 | CTG AGG CCA GCG GCC GCT CCT GAA GGT ACC TCC CGG TT | Used in inverse PCR for the production of vectors carrying CRP flanking DNA; introduces a NotI restriction site (in boldface) at the 3′ end of the CRP gene |
| CRP L1 | TCG GGA AGA GCG GCC GCA AAA TTA TTT CAG ACC AGA GA | Used in inverse PCR for the production of vectors carrying CRP flanking DNA; introduces a NotI restriction site (in boldface) at the 5′ end of the CRP gene |
The sequences of oligonucleotides used in PCRs for the construction of CRP/GM-CSF, pGM-BNB, and pGM-C79 are presented.
Construction of CRP/GM-CSF gene fusions.
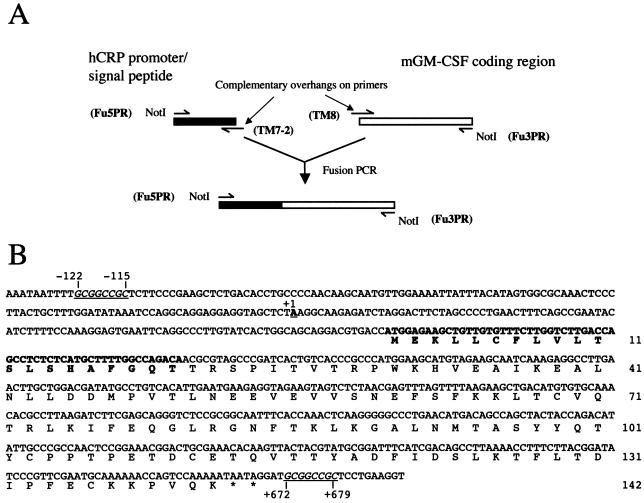
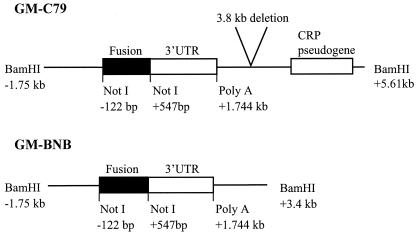
PCR was used to create a CRP/GM-CSF gene fusion, flanked by NotI restriction sites, consisting of the promoter (from −114 bp), signal peptide, and the first two amino acids of human CRP, linked to the cDNA sequence encoding mature (i.e., lacking the signal peptide) murine GM-CSF (mGM-CSF). The CRP portion was amplified from pCosCRP1 (described above). The murine GM-CSF portion was amplified from a cDNA clone obtained from R&D Systems. Three separate PCRs were involved in the production of the fusion (Fig. 1A). The first two amplified the desired parts of the two genes to be fused. The primers at the 3′ end of the CRP product (TM7-2) and the 5′ end of the GM-CSF product (TM8) are completely complementary to each other. Each consists of a region complementary to the sequence to be amplified and an overhang region complementary to the end of the other gene (Table 1). The PCR products of these two separate PCRs were purified by elution from agarose gels to remove the primers. In the third PCR, the two PCR products were mixed, denatured, allowed to anneal to each other via the complementary sequences introduced by using the primers, and amplified by using the 5′ primer from the CRP PCR (Fu5Pr) and the 3′ primer from the GM-CSF PCR (Fu3Pr), which results in the linkage of the two initial PCR products to form a CRP/GM-CSF fusion flanked at both ends by NotI restriction sites to facilitate cloning (Fig. 1A). The fusion was sequenced to check for errors introduced during PCR; none were present. The fusion was cloned into the NotI site of pBluescript II to create a minimal construct, which lacks CRP flanking DNA, termed pFusion. The NotI sites flanking the fusion were then used to clone it into the two CRP flanking DNA plasmids, pC79-NotI and pBNB-NotI, described above. The sequence of the fusion after it was cloned into the pC79-NotI construct is shown in Fig. 1B. The NotI sites at the 5′ and 3′ ends of the inserted fusion are at bases −122 to −115 and at bases +672 to +679, respectively, relative to the transcription start site. The resultant constructs, termed pGM-C79 and pGM-BNB, respectively, contain 1.745 kb of DNA from the 5′ flanking region of the CRP gene. These constructs include the entire CRP promoter, the CRP signal peptide, the first two amino acids of mature CRP, the complete amino acid sequence of mature (i.e., lacking its signal peptide) murine GM-CSF, and the 1.2-kb 3′ untranslated region of human CRP, including the transcription termination and polyadenylation sequences, plus further 3′ CRP flanking DNA. pGM-C79 and pGM-BNB differ only in the amount of 3′ CRP flanking DNA they contain (Fig. 2).
FIG. 1.
(A) Schematic of the three-PCR strategy used to produce a fusion between the promoter, signal peptide, and first two amino acids of human CRP and the open reading frame of mature (post-signal peptide cleavage) murine GM-CSF. NotI sites were introduced to facilitate cloning. Primer names are shown in boldface. Primer sequences are shown in Table 1. (B) Sequence of the human CRP/mGM-CSF fusion gene being cloned into the NotI sites of the pC79 construct, which contains flanking DNA from the human CRP gene. NotI sites introduced to facilitate cloning are underlined and in italics. Ten bases lying outside the fusion in the pC79 construct, 5′ and 3′ of the NotI sites, are also shown. The CRP cap site (1), taken as base +1, is underlined and in boldface. The human CRP signal peptide sequence and the first two amino acids of human CRP are in boldface. In-frame termination codons are indicated with asterisks. Amino acids are numbered on the right.
FIG. 2.
Schematic diagram of the two CRP/GM-CSF vectors used: pGM-C79 and pGM-BNB. Restriction enzyme sites and the Poly A site (+1.744 kb) are shown relative to the mRNA cap site (position +1). The pGM-C79 and pGM-BNB constructs differ only in their 3′ end. pGM-C79 lacks a HindIII-BamHI fragment present in GM-BNB but also has additional 3′ sequences including the CRP pseudogene. The construction of C79 is described by Murphy et al. (23). UTR, untranslated region.
Cell culture and transfections.
All cell culture reagents were obtained from Gibco-BRL. The Hep3B human hepatoma cell line was obtained from the European Collection of Cell Cultures and grown in RPMI 1640 medium supplemented with 10% fetal calf serum, penicillin (100 U/ml), streptomycin (100 μg/ml), and 4 mM l-glutamine. Next, 80 to 90% confluent monolayers of cells in 60-mm-diameter dishes (Corning) were transfected with 10 μg of plasmid DNA. Transfections were carried out in Dulbecco modified Eagle medium by using the Profectin kit (Promega) according to the manufacturer's instructions. The medium containing the DNA precipitate was removed after 16 h, and the cells were stimulated with interleukins as described below.
In vitro interleukin stimulation.
Human recombinant interleukin-1β (IL-1β) was obtained from R&D Systems and human recombinant IL-6 was from Sigma. Stocks were made up in phosphate-buffered saline (PBS)-0.1% bovine serum albumin and frozen in single use aliquots at −70°C. After transfection or mock transfection of cells, medium was replaced with fresh medium containing dexamethasone (1 μM) and the desired concentrations of IL-1β and IL-6. The cells were incubated for a further 24 h since this had been shown previously to be the time at which peak expression of CRP occurs (1). After incubation, the medium was removed and stored at −70°C for later analysis. Cell monolayers were then lysed by using Profectin cell lysis buffer (Promega), cell debris was pelleted by centrifugation at 13,000 × g for 10 min, and the supernatant was stored at −70°C for later analysis.
Production of transgenic mice.
DNA of the GM-C79 construct was prepared by BamHI restriction digestion to remove noneukaryotic plasmid DNA and isolated from agarose gels by using the Sephaglass Bandprep kit (Pharmacia Biotech). The DNA was further purified and concentrated by binding, washing, and elution from a NACS column (Gibco-BRL) according to the manufacturer's instructions. The DNA was quantified, diluted to 5 ng/μl, and passed through a Costar spin column to remove particulate matter. DNA was microinjected into the male pronuclei of fertilized F2 mouse eggs (produced by crossing (C57Black6/CBA F1 mice), and surviving eggs were surgically transferred to the Fallopian tubes of pseudopregnant C57Black6/CBA F1 females to allow development of the embryos. Mice were reared in accordance with, and all experimental procedures complied with, relevant legislation and institutional policies.
LPS inoculation of mice.
Mice were inoculated intraperitoneally with 25 μg of LPS from Escherichia coli serotype O127:B8 (Sigma) in a volume of 200 μl of sterile PBS. Control animals were inoculated with 200 μl of sterile PBS. Blood samples were taken from tail veins prior to inoculation and at intervals after inoculation. Moribund animals were cardiac bled under terminal halothane anesthesia. Blood was allowed to coagulate at room temperature for 2 h, and samples were centrifuged at 13,000 × g for 5 min. Serum was removed and stored at −70°C prior to analysis.
ELISA for murine GM-CSF and IL-6.
GM-CSF and IL-6 enzyme-linked immunosorbent assays (ELISAs) were carried out by using the appropriate Quantikine M Murine ELISA kit (R&D Systems) according to the manufacturer's instructions.
Flow cytometry and cell counts.
Whole blood was treated with heparin to prevent coagulation. Cells were pelleted by centrifugation at 350 × g for 5 min at room temperature and then resuspended in 50 μl of fluorescence-activated cell sorting (FACS) buffer (PBS with 1% fetal calf serum and 0.1% sodium azide). To prevent nonspecific binding of antibody to Fc receptors, 5 μl of Fc Block (anti-CD16/CD32 antibody; Becton Dickinson) was added to the cell suspension, followed by incubation at room temperature for 5 min. The fluorescence-labeled antibodies were then added to the cell suspensions and incubated at room temperature, in the dark, for 15 min. Then, 2 ml of FACSLYSE (Becton Dickinson) was added to the cell suspension and incubated at room temperature, in the dark, for 10 min to lyse red blood cells. The cells were then washed twice by centrifugation (350 × g for 5 min), followed by resuspension in 2 ml of FACS buffer. The cells were washed again by centrifugation and resuspension in 2 ml of FACSFLOW (Becton Dickinson). The cells were then prepared for analysis by centrifugation (350 × g for 5 min) and resuspension in 100 μl of FACSFLOW and then analyzed by using a FACScan flow cytometer (Becton Dickinson). The data analysis was performed by using PC-Lysis software (Becton Dickinson). Fluorescence-labeled isotype control antibodies were used in each analysis to control for the specificity of antibody binding. The following antibodies, obtained from Pharmingen, were used: F4/80, which labels macrophages; mac3, which labels activated macrophages; CD11b, which labels monocytes/macrophages and granulocytes; and Ly6g, which labels neutrophils. Total white blood cell counts in the blood of transgenic and nontransgenic mice were determined with a hemocytometer.
Sequencing.
Sequencing of the CRP/GM-CSF fusion and its flanking human DNA within the pGM-C79 construct was carried out by using an automated sequencer and primer walking. Transcription factor binding site searches were carried out by using the MatInspector website (http://transfac.gbf.de). The sequence has been submitted to GenBank under accession number AF474411.
Statistics.
The Mann-Whitney U test was used to assess the statistical significance of experimental data.
RESULTS
Investigation of the functionality and inducibility of CRP/GM-CSF fusion constructs in vitro.
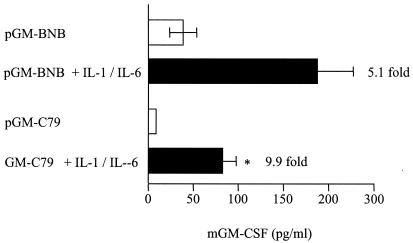
In order to analyze the pattern of expression of GM-CSF produced by the different CRP/GM-CSF fusion constructs, they were transfected into Hep3B cells, and the cells were incubated for 24 h in the presence or absence of IL-1β and IL-6. Transfected cells were initially treated with these two cytokines separately, but GM-CSF induction was poor (data not shown). However, the use of both interleukins together (500 U of IL-6 and 200 U of IL-1β/ml) gave marked inductions (Fig. 3), thus confirming the previously reported synergy between IL-1β and IL-6 in upregulating expression from the CRP gene (10, 33). The pGM-BNB construct, containing ca. 4.47 kb of CRP flanking DNA, showed high constitutive levels of expression (38.8 pg/ml) and also high levels of expression after interleukin stimulation (196 pg/ml), an induction of 5.1-fold. The pGM-C79 construct, with ca. 6.7 kb of CRP flanking DNA, gave lower basal levels of expression (8.9 pg/ml) than the pGM-BNB construct and was more inducible, increasing 9.9-fold to 87.8 pg/ml after stimulation with interleukins, a significant increase (5% > P > 1%). The pGM-C79 construct gave a pattern of expression most closely resembling an acute-phase response, i.e., low constitutive levels of expression in the absence of stimulation and high expression after stimulation with interleukins. Although there is only a 9.9-fold induction in cell culture, it has previously been shown that induction of the C79 construct in transgenic mice is much higher, with an average of 69-fold across 11 different mouse lines (23), and this construct was therefore used to make transgenic mice.
FIG. 3.
Interleukin-stimulated murine GM-CSF release from Hep3B cells transfected with CRP/GM-CSF fusion constructs pGM-BNB, or pGM-C79, with or without stimulation with IL-1β and IL-6. Murine GM-CSF was quantified by ELISA in cell lysates. The combined results from either three (pGM-BNB) or four (pGM-C79) separate experiments are shown. Error bars represent the standard error of the mean. Fold induction (stimulated compared to unstimulated) is indicated at the end of each stimulated bar. ✽, Significant increase in murine GM-CSF expression after stimulation with IL-1β and IL-6 (5% > P > 1%).
An important reason for testing the CRP/GM-CSF fusion constructs in vitro prior to using them to make a transgenic mouse line was to ascertain whether the GM-CSF produced from the fusion would be efficiently secreted. This was a concern because of the use of a heterologous signal peptide from both a different protein and a different species (human CRP) to export GM-CSF. Also, the amino acid sequence at the junction between the signal peptide and the start of the mature GM-CSF protein was not identical to that found in either native protein, a factor which could potentially have affected signal peptide cleavage and protein export. However, GM-CSF was detectable at relatively high levels in the cell culture medium of transfected monolayers after stimulation (35 pg/ml for pGM-C79 and 95 pg/ml for pGM-BNB) and showed the same fold inductions as cell lysates (data not shown), implying that GM-CSF was being released into the medium in proportion to its production rather than remaining associated with the cells. Calculations based on the relative volumes of the cell lysate and culture medium showed that there was on average six times more GM-CSF in the medium as in the cell lysate at the end of the 24-h interleukin stimulation period (data not shown). Microscopic examination at the time of removal of the medium showed an apparently healthy monolayer with no evidence of widespread cell lysis, leading us to conclude that secretion of GM-CSF into the medium seemed to be occurring efficiently.
Production of GM-CSF transgenic mice.
Transgenic mice, carrying construct GM-C79, were produced as described in Materials and Methods. A total of 171 mouse pups were born and screened by PCR analysis and Southern hybridization of DNA extracted from tail biopsies (data not shown). Two male transgenic mice were identified. One of these mice failed to breed. The other mouse was used to produce the GM-CSF transgenic line used in the present study. This line bred normally, giving 50% transgenic versus 50% nontransgenic offspring. The male-female split among the transgenic mice was also 50:50.
Expression of GM-CSF in transgenic mice.
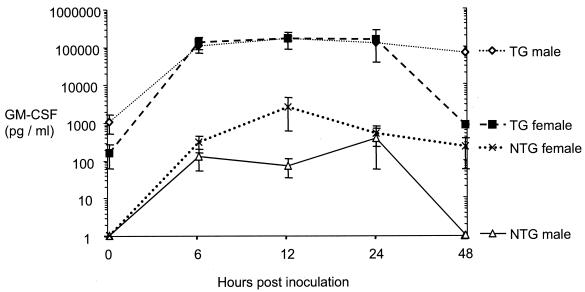
Transgenic and nontransgenic sibling mice of between 9 and 18 weeks of age were administered intraperitoneally with 25 μg of LPS to induce the acute-phase response. Sera from blood samples taken immediately prior to inoculation with LPS (time zero) and at 6-, 12-, 24-, and 48-h time points thereafter were tested for the presence of murine GM-CSF by ELISA (Fig. 4). Levels of GM-CSF in serum were below the level of detection (160 pg/ml) in all nontransgenic animals at time zero. In contrast, many transgenic animals (80% of males and 40% of females) had detectable levels ranging from 240 to >5,400 pg/ml, with a mean of 814 pg/ml. Mean basal levels of GM-CSF at time zero were higher among transgenic males (1,102 ± 581 pg/ml) than among transgenic females (166 ± 103 pg/ml). The basal GM-CSF levels within the male transgenic group showed a remarkably wide spread, varying by >30-fold from undetectable (<160 pg/ml) to >5,400 pg/ml. In contrast, the levels of GM-CSF in transgenic females at time zero ranged from undetectable to 420 pg/ml.
FIG. 4.
Murine GM-CSF levels in serum in GM-C79 transgenic and nontransgenic littermate mice after intraperitoneal administration of 25 μg of LPS (transgenic animals, 9 males and 4 females; nontransgenic animals, 10 males and 9 females). Blood was taken from the tail vein just prior to LPS administration and at 6, 12, 24, and 48 h afterward for quantification of murine GM-CSF by ELISA. Pooled data from two independent experiments are shown.
After the administration of LPS, very marked increases of GM-CSF in serum were observed in transgenic mice by 6 h postinoculation, with a mean value of 124,484 pg/ml, an induction of 153-fold over the mean basal level. The levels in individual transgenic mice at 6 h varied markedly, with a minimum level of 15,694 pg/ml and a maximum of 258,000 pg/ml, the highest level observed at any time point during the experiments. The levels of GM-CSF increased with a similar trend in nontransgenic animals, but the mean level at 6 h was 215 pg/ml (minimum, <160 pg/ml; maximum, 1,224 pg/ml), ∼500-fold lower than in transgenic mice (Fig. 4).
Despite the higher basal levels detected in male transgenic mice, the peak GM-CSF levels observed after LPS inoculation were very similar at ∼250,000 pg/ml for male and female transgenic animals. Similarly, in the transgenic group as a whole, which showed a >30-fold variation in basal GM-CSF level, the basal level was not an indicator of likely peak postinoculation GM-CSF level or of survival time. A high mortality rate was observed in transgenic mice after LPS inoculation: 8% of animals were moribund or dead by 6 h, 38% were moribund or dead by 12 h, 77% were moribund or dead by 24 h, and 85% were moribund or dead by 48 h. Deaths occurred more rapidly among male mice, beginning at 6 h and reaching 55% by 12 h, when all female mice were still alive. Deaths of female mice were first observed at 24 h. No deaths occurred among nontransgenic animals given LPS. Among transgenic and nontransgenic control animals given PBS only, no marked increases in GM-CSF or deaths occurred at any stage of the experiments.
Altered production of IL-6 by transgenic mice.
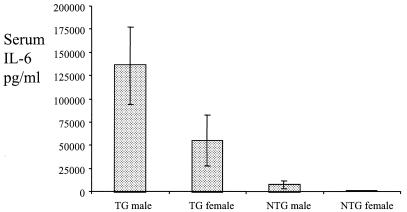
The levels of the acute-phase cytokine IL-6 were examined in the same LPS-inoculated transgenic and nontransgenic mice for which data are shown in Fig. 4. Limitations on the amount of sera available meant that only samples taken immediately prior to inoculation and at 6 h postinoculation could be tested for the presence of IL-6 by ELISA. IL-6 was not detected in either transgenic or nontransgenic animals at time zero (data not shown), but by 6 h postinoculation it was detected in all of the transgenic animals, with a mean level of 115,017 pg/ml (means of 137,156 pg/ml for males and 55,981 pg/ml for females), but in only 42% of nontransgenic animals, with a mean level of 4,578 pg/ml (means of 8,058 pg/ml for males and 711 pg/ml for females; Fig. 5). Interestingly, higher mean levels were observed in males than in females for both transgenic and nontransgenic mice.
FIG. 5.
Measurement by ELISA of IL-6 levels in serum in transgenic and nontransgenic littermate mice after intraperitoneal administration of 25 μg of LPS (transgenic animals, 9 males and 4 females; nontransgenic animals, 10 males and 9 females). Error bars represent the standard error of the mean. Blood was taken from the tail vein just prior to LPS administration, and 6 h afterward for quantification of IL-6. The mean values were 137,156 pg/ml for male transgenic mice, 55,981 pg/ml for female transgenic mice, 8058 pg/ml for male nontransgenic mice, and 711 pg/ml for female nontransgenic mice. Pooled data from two independent experiments are shown. GM-CSF data for the same mice are shown in Fig. 4.
Quantification of circulating leukocytes in unstimulated transgenic and nontransgenic mice.
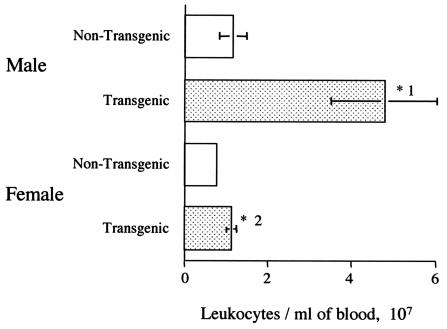
The mean blood leukocyte count for the male transgenic mice (4.8 × 107 ± 1.3 × 107 cells/ml) was significantly higher (P < 1%) than the mean counts for the other three groups (1.2 × 107 ± 3.2 × 106 cells/ml for male nontransgenic mice, 1.1 × 107 ± 1.1 × 106 cells/ml for female transgenic mice, and 8 × 106 ± 8.2 × 105 cells/ml for female nontransgenic mice; Fig. 6). The finding of higher leukocyte numbers in transgenic males compared to transgenic females accords well with our finding of higher mean basal GM-CSF levels in male mice. It is also noticeable that the counts from the male transgenic mice are very variable, which is probably related to the finding that the basal GM-CSF levels were highly variable in transgenic males, as described above. There was also a significant difference between the cell counts from the female transgenic mice compared to the female nontransgenic mice (5% > P > 1%).
FIG. 6.
Total leukocyte counts in the blood of nontransgenic mice and GM-CSF transgenic littermates (for each group, n = 22). Error bars represent the standard error of the mean. *1, A significant difference was observed between the counts from the male transgenic mice and all of the other groups (P < 1% for all comparisons); *2, a significant difference was observed between the counts from transgenic and nontransgenic females (5% > P > 1%).
Flow cytometry of leukocytes in peripheral blood, the peritoneal cavity, and the spleen.
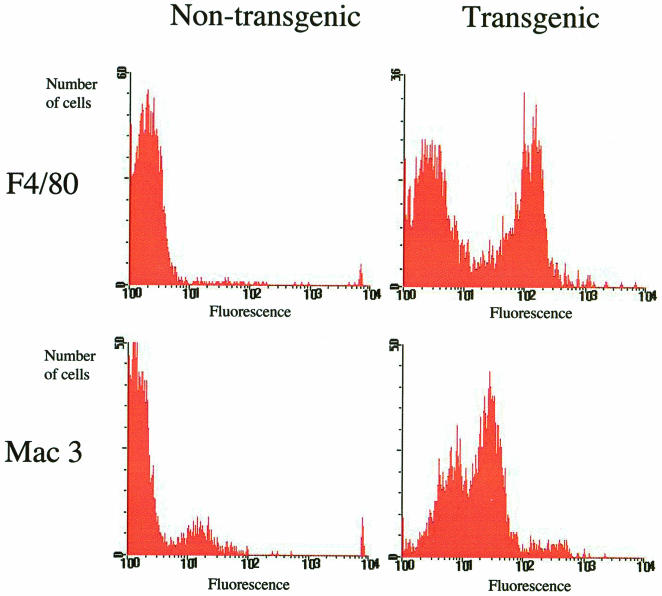
FACS analysis of the blood of male and female transgenic and nontransgenic mice was carried out by using the antibodies F4/80, which labels mature monocytes/macrophages and is unreactive with immature blood monocyte precursors, and mac3, which is a marker for activation of macrophages. Both the male and the female transgenic mice had a high proportion of mature monocytes in their blood; typically, ca. 50% of monocytes were positive for F4/80. In contrast, the nontransgenic mice had very few F4/80-positive monocytes, usually <10% (Fig. 7). In transgenic mice, a large proportion of monocytes (ca. 60%) were positive for the mac3 activation marker, with some cells being very highly positive. A much smaller proportion (ca. 20%) of cells from nontransgenic mice were positive for this marker, with few or no cells being very highly labeled (Fig. 7). These results indicate that transgenic mice differ from nontransgenic mice in that they have large numbers of relatively mature monocytes, many of which appear be activated (as indicated by high mac3 reactivity), in their blood. Similar results were obtained with spleen cells, with a clear increase in the proportion of F4/80 positive macrophages observed in transgenic spleens (data not shown). Analysis of peritoneal lavage cells showed no consistent differences between transgenic and nontransgenic mice; large numbers of F4/80-positive cells were observed in both, as expected (data not shown).
FIG. 7.
Representative FACS analyses of blood from GM-CSF transgenic and nontransgenic mice. Fluorescence graphs shown are those of monocytes/macrophages only, defined as cells falling within the region of the side-scatter-forward-scatter plot known to contain monocytes/macrophages. Fluorescence-labeled antibodies raised against the macrophage maturation marker F4/80 and the activation marker mac3 were used. An arbitrary fluorescence cutoff point of 10 was used to designate cells as either positive or negative. The percentages of positive cells were as follows: F4/80, 55% transgenic and 8% nontransgenic; and mac3, 63% transgenic and 19% nontransgenic.
Analysis of neutrophils by using an antibody to Ly6g and an antibody to CD11b, a marker shared by granulocytes and monocytes, also showed differences between transgenic and nontransgenic mice, if somewhat less pronounced than those observed with the macrophage markers F4/80 and mac3. Neutrophil populations from transgenic mice were more diverse than those from nontransgenic mice, in terms of the intensity with which they were labeled with antibodies to Ly6g and CD11b, and more variable in terms of forward scatter-side scatter than those from nontransgenic animals and in general were marginally larger and denser (data not shown), possibly indicating changed activation states.
Characterization of pathologies apparent in GM-CSF transgenic mice.
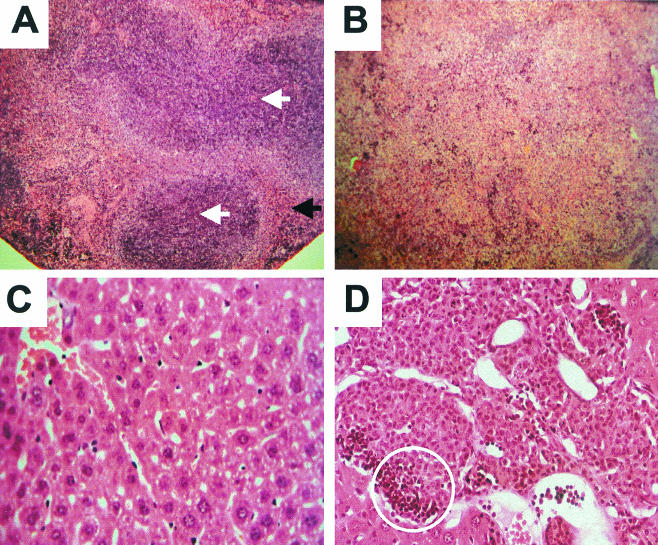
In contrast to the transgenic mice produced by Lang et al. (18), all of our female transgenic and 69% of our male transgenic mice survived to at least 10 months of age. The survival rate for male nontransgenic mice was 82%. However, ca. 60% of transgenic mice exhibited pathological abnormalities compared to their nontransgenic littermates. These abnormalities were more pronounced in males. Gross abnormalities typically observed included an enlarged and mottled liver and a massively enlarged spleen and occasionally general muscle wasting. Postmortem analysis of male transgenic mice that died prematurely revealed consistent pathological changes, most noticeably enlarged spleens and livers. Histological comparison of the spleens of transgenic mice selected for having high basal levels of GM-CSF and of nontransgenic littermates (Fig. 8) revealed that transgenic animals had a normal white pulp and a massively increased red pulp containing many precursor cells of different cell lineages, including megakaryocytes, erythroid precursors, and myeloid elements. Another abnormality noticed, particularly in older transgenic mice, was the presence of areas of ossification in the spleen. Extramedullary hemopoiesis was also seen in the livers of transgenic mice, although to a lesser extent than in the spleen.
FIG. 8.
(A) Spleen from nontransgenic male showing normal white pulp (white arrows) and red pulp (black arrow), which appears normal although occasional megakaryocytes are identified, indicating a low degree of extramedullary hemopoiesis. (B) Spleen from a transgenic male. The red pulp is greatly expanded compared to the nontransgenic spleen in panel A and has largely replaced the white pulp. The red pulp contains a large amount of extramedullary hemopoiesis, including erythroid and myeloid elements and megakaryocytes. Magnification (A and B), ×100. (C) Liver from a nontransgenic male. Magnification, ×800. (D) Liver from a transgenic male (magnification, ×400), showing collections of monocytic cells, one of which is circled.
Sequence analysis of the C79-GM construct.
The higher basal levels of GM-CSF that were seen in the male transgenic mice were of some concern. However, this observation was not totally unexpected, since it has previously been reported that the human CRP promoter has higher basal activity in male than in female transgenic mice (25, 26). This phenomenon may be linked to the effects of testosterone on growth hormone, which has a sexually dimorphic pattern of secretion in rodents (28). Several studies have implicated STAT5 in transducing the sexually dimorphic pattern of growth hormone secretion into sex-specific patterns of liver gene expression (14, 24, 32).
With these findings in mind, the CRP/GM-CSF fusion and its flanking human DNA within the pGM-C79 construct was sequenced and searched for putative growth hormone responsive elements. Four putative STAT5 binding sites were identified within pGM-C79, three upstream of the CRP transcription start site and one 1,281 bp downstream (Table 2).
TABLE 2.
Putative STAT 5 binding sites present in GM-C79a
| Sequence of STAT 5 site (5′ to 3′) | Sequence present in GM-C79 (5′ to 3′) | Base position | Refer- ence |
|---|---|---|---|
| TTC TCA GAA | TTC TCA TAA | −1100 to −1092 (−) | 31 |
| TTC TCA GAA | TTC TCT GAA | −794 to −786 (−) | 31 |
| TTC TGA GAA | TTC AGA GAA | −794 to −786 (+) | 21 |
| TTC CCA GAA | TTC CCA GAA | +1281 to +1296 (−) | 31 |
The putative STAT5 binding sites present in the human CRP gene DNA contained within pGM-C79, identified by using the MatInspector website (http://transfac.gbf.de). “+” or “−” refer to the coding (+) or noncoding (−) strand.
DISCUSSION
The aim of the present study was to design an inflammation-inducible expression system that would allow the overexpression of cytokine genes in transgenic mice. We reasoned that such a system would avoid some of the problems expected with the constitutive expression of such highly biologically active molecules. The promoter and flanking sequences of the human CRP gene were chosen as the backbone for the expression system due to the kinetics of human CRP expression in transgenic mice: rapid induction following stimulation and a decrease to preinduction levels within 48 h (4). We decided to use GM-CSF as the transgene for two reasons. First, administration of GM-CSF to animals has been shown to enhance their resistance to infectious challenge with several organisms (7, 8, 13, 19, 27). Second, the biological consequences of constitutive expression of this gene are known (18), allowing assessment of how controlled the expression system is.
We produced two constructs that differed only in the amount of flanking DNA from the human CRP gene: pGM-BNB had ca. 4.47 kb, and pGM-C79 had ca. 6.7 kb (Fig. 2). The constructs were assessed for functionality in vitro in a liver cell line by costimulation with IL-1β and IL-6. Although pGM-C79 gave lower levels of GM-CSF than pGM-BNB, it had a higher induction level after cytokine stimulation (9.9-fold) and so was chosen for gene transfer into mice. The data from the in vitro experiments were consistent with previous findings that the inducibility of the CRP gene is dependent on the flanking sequences present in the construct (1, 10, 23).
To induce expression of the transgene, mice were administered intraperitoneally with LPS. Rapid and marked increases (mean induction of 153-fold within 6 h) of GM-CSF levels in serum were observed in transgenic mice. Nontransgenic littermates responded by increasing their levels of GM-CSF with similar kinetics but with levels 100 to 500 times lower (Fig. 4). The rapid induction and overexpression of GM-CSF seen in the transgenic mice compared to nontransgenic mice suggests that the inducible expression system is functional in vivo, in line with our in vitro data, and previous studies of the human CRP promoter in transgenic mice (23). The very high levels of GM-CSF (Fig. 4) and IL-6 (Fig. 5) observed in transgenic mice after LPS administration are likely to account for the high mortality rate in these animals due to endotoxic shock. It is worth noting that the mean post-LPS levels of GM-CSF are similar in transgenic males and females, but the mean levels of IL-6 after 6 h are considerably higher in males (137,156 versus 55,981 pg/ml). This trend is also found in the nontransgenic littermates (8,058 versus 711 pg/ml for females) and is in accordance with previous findings that estrogen downregulates IL-6 in humans (16) and mice (20) and that male mice produce higher levels of IL-6 in bronchoalveolar lavage fluid in response to LPS than females (31). The higher levels of IL-6 in transgenic males could explain the more rapid deaths observed among them than among transgenic females after LPS administration.
In contrast to our experimental model, which involved a single relatively large dose of LPS, it is possible that a natural infection, in which endotoxin release from invading pathogenic bacteria is likely to increase gradually, might lead to less dramatic increases in GM-CSF levels in transgenic mice, allowing them to mount a more proportionate and effective response to the challenge rather than a sudden overwhelming one.
The higher-than-normal levels of serum GM-CSF seen in some transgenic mice prior to LPS administration are likely to be the cause of the abnormalities observed in ca. 60% of the mice. These abnormalities included high numbers of circulating mature (F4/80 positive) macrophages (Fig. 7), enlargement and histological abnormalities in the liver and spleen (Fig. 8), and wasting. These findings are all consistent with those previously observed in transgenic mice constitutively expressing GM-CSF (18). However, a number of differences were observed between the abnormalities observed in our study and those of Lang et al. (18). For example, we observed significant increases in the number of peripheral blood leukocytes in both male and female transgenic mice compared to nontransgenic mice (Fig. 6), in contrast to Lang et al., who found that leukocyte numbers in most transgenic mice aged 8 to 12 weeks were not different from littermate controls. However, the most obvious difference is that our transgenic mice do not exhibit the pronounced bilateral opacity of the eye and retinal damage observed in all of the transgenic mice made by Lang et al. The differences in the pathologies observed in our studies are likely to be due to the fact that the retroviral promoter Lang et al. used was constitutive and is also active in macrophages. Macrophages are present in the eye during organ development (2), and autocrine activation due to GM-CSF overexpression is thought to lead to increases in activation status and phagocytic activity (5). The majority of our transgenic animals survived to at least 10 months of age, whereas in the study by Lang et al. (18) premature deaths began after only 6 weeks, with the majority of the animals being dead by 6 months. Thus, our use of a promoter that is inducible and that is not expressed by macrophages, eliminating the problem of autocrine activation and accumulation in nondiseased sites, is an improvement on the system used by Lang et al.
The high basal levels of GM-CSF observed in some of the transgenic animals, and hence the various pathologies related to increases in macrophage numbers and chronic activation, may be related to possible interactions between the human CRP promoter we used and GM-CSF. It is known that GM-CSF increases the rate of proliferation of monocytes/macrophages and enhances their survival, activation, and cytokine production (29). Activated macrophages are capable of releasing the cytokines IL-1β and IL-6, which we have shown in vitro are sufficient to increase expression from the human CRP promoter. In vivo studies of the human CRP gene in transgenic mice have shown that IL-6 is the main inducer of the gene (26). Thus, a positive feedback loop could become established in which a “leaky” constitutive expression of GM-CSF or GM-CSF released in response to minor wounds or infections would result in increased numbers of monocytes/macrophages and in higher levels of activation. These activated macrophages are in turn capable of releasing IL-6 in response to any subsequent inflammatory stimulus, leading to further increases in GM-CSF levels and macrophage numbers, leading to development of pathological abnormalities.
Increases in the number of activated macrophages would thus prime the mice to over-respond to inflammatory stimuli, and this is likely to be at least partly responsible for the high mortality rate we observed in GM-CSF transgenic mice after the administration of LPS (Fig. 4). Our data show that the level of IL-6 is rapidly elevated to superphysiological levels in GM-CSF transgenic mice (>10-fold higher than nontransgenic littermates at 6 h postinoculation) in response to LPS. The increased levels are particularly extreme in male transgenic mice (Fig. 5). These observations are in agreement with the previous finding that subcutaneous administration of GM-CSF primes mice for enhanced cytokine production after administration of LPS and lowers the lethal dose of LPS (3). Macrophages stimulated by GM-CSF have been shown to upregulate CD14, the receptor for LPS, and to express increased levels of the proinflammatory cytokine TNF and reduced levels of the anti-inflammatory IL-10 (17).
Clearly, the use of the CRP promoter to drive inflammation-inducible overexpression of cytokines such as IL-1β or IL-6, which act directly on the CRP promoter, or genes such as GM-CSF that act indirectly to increase the release of these cytokines from macrophages is not a viable approach to increasing the disease resistance of animals. However, the use of the CRP promoter to overexpress other genes that could enhance the disease resistance of animals, for example, mammalian peptide antibiotics such as defensins and cathelins (15) would be unlikely to suffer from the positive feedback and consequent hypersensitivity to infectious challenge that appear to have affected our GM-CSF transgenic mice and might therefore be more suitable for inflammation-inducible overexpression. Another possible application of an inflammation or infection-inducible construct would be the use of a reporter gene which could give early warning of infection in individual animals within a group, allowing treatment to be administered in a timely manner and targeted where it is needed, thus helping to prevent the spread of disease.
We observed marked male/female differences in the basal level of GM-CSF in transgenic mice (mean basal levels of 1,102 pg/ml in males versus 166 pg/ml in females). This is likely be a consequence of using the CRP promoter as the backbone to the expression system. Szalai et al. (25) showed that male transgenic mice carrying the human CRP gene expressed higher levels of CRP both prior to and after an inflammatory stimulus and that this was due to the action of testosterone. Intriguingly, however, the human CRP gene is not sexually dimorphic in humans. A possible answer to this paradox lies in the fact that testosterone produces pulses of high growth hormone release in male mice (12), resulting in mean levels in serum twice those observed in females (22). Growth hormone has been shown to be able to upregulate the human CRP gene in transgenic mice (26). Since that study, the transcription factor STAT5 has emerged as an important transducer of the pulsatile growth hormone secretion pattern seen in male rodents into sex-specific patterns of liver gene expression (11, 14, 24). Also, binding of STAT5 to the promoter of the murine Sex-limited protein (slp) gene, which is known to be regulated indirectly by testosterone, via its effects on growth hormone (12), results in male-specific transcription of the gene (32). In addition, Feldman et al. (6) have shown that GM-CSF is involved in the activation of STAT5, thereby providing another mechanism whereby GM-CSF overexpression in male GM-CSF transgenic mice might lead to the generation of a positive feedback loop, upregulating the CRP promoter and producing more GM-CSF.
In the light of these previous findings, we sequenced the pGM-CSF construct to look for the presence of STAT5 binding sites. This revealed three putative binding sites for STAT5 upstream of the CRP transcription start site and one 1.3 kb downstream. One of these sites is identical to one of the STAT5 binding sites in the slp promoter (32), whereas the others differ only by a single base. It is therefore possible that the high basal levels of GM-CSF seen in our male transgenic mice may be at least in part a consequence of the STAT5 sites present in the construct. This opens up the possibility of removing or mutating these sites in future CRP-based constructs in an attempt to abolish the sex differences in expression levels that we and others have observed. We are currently doing experiments to test this hypothesis.
A second possible explanation exists for the sex difference in activity of the human CRP promoter in mice. As mentioned previously, estrogen downregulates IL-6 in humans (16) and mice (20), and male mice produce higher levels of IL-6 in response to LPS than female mice (31). Since IL-6 is one of the key cytokines responsible for upregulating the CRP promoter, this could explain part of the observed GM-CSF sex difference.
The primary aim of the present study was to produce transgenic mice showing inflammation-inducible overexpression of GM-CSF and to assess the responses of these animals to inflammatory stimuli. The construct we developed, although highly inducible, appears not to be suitable for the overexpression of GM-CSF because the ability of this cytokine to raise the levels of other cytokines means that positive feedback on the CRP promoter can occur. However, proof of principal has been obtained, and the selection of a transgene that is not capable of affecting transcription from the CRP promoter could deliver the desired on/off inducible expression. The data we present here could be of future use in the development of genetically modified animals showing infection-inducible expression of a gene of interest, whether it might be immunity-enhancing or a reporter gene capable of signaling infection or inflammation in individual animals within a group, allowing treatment to be targeted where it is needed.
Acknowledgments
We thank Ulrich Ruther for kindly providing construct C79.
This research was funded by the Wellcome Trust.
REFERENCES
- 1.Arcone, R., G. Gualandi, and G. Ciliberto. 1988. Identification of sequences responsible for acute-phase induction of human C-reactive protein. Nucleic Acids Res. 16:3195-3207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Balazs, E. A., L. Z. Toth, and V. Ozanics. 1980. Cytological studies of the developing vitreous as related to the hyaloid vessel system. Albrecht Graefes Arch. Klin. Exp. Opthal. 213:71-85. [DOI] [PubMed] [Google Scholar]
- 3.Brissette, W. H., D. A. Baker, E. J. Stam, J. P. Umland, and R. J. Griffiths. 1995. GM-CSF rapidly primes mice for enhanced cytokine production in response to LPS and TNF. Cytokine 7:291-295. [DOI] [PubMed] [Google Scholar]
- 4.Ciliberto, G., R. Arcone, E. F. Wagner, and U. Ruther. 1987. Inducible and tissue-specific expression of human C-reactive protein in transgenic mice. EMBO J. 6:4017-4022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cuthbertson, R. A., and R. A. Lang. 1989. Developmental ocular disease in GM-CSF transgenic mice is mediated by autostimulated macrophages. Dev. Biol. 134:119-129. [DOI] [PubMed] [Google Scholar]
- 6.Feldman, G. M., L. A. Rosenthal, X. W. Liu, M. P. Hayes, B. A. Wynshaw, W. J. Leonard, L. Hennighausen, and D. S. Finbloom. 1997. STAT5a-deficient mice demonstrate a defect in granulocyte-macrophage colony-stimulating factor-induced proliferation and gene expression. Blood 90:1768-1776. [PubMed] [Google Scholar]
- 7.Fontt, E. O., C. Heirman, K. Thielemans, and B. Vray. 1996. Granulocyte-macrophage colony-stimulating factor: involvement in control of Trypanosoma cruzi infection in mice. Infect. Immun. 64:3429-3434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Frenck, R. W., G. Sarman, T. E. Harper, and E. S. Buescher. 1990. The ability of recombinant murine granulocyte-macrophage colony-stimulating factor to protect neonatal rats from septic death due to Staphylococcus aureus. J. Infect. Dis. 162:109-114. [DOI] [PubMed] [Google Scholar]
- 9.Gabay, C., and I. Kushner. 1999. Mechanisms of disease: acute-phase proteins and other systemic responses to inflammation. N. Engl. J. Med. 340:448-454. [DOI] [PubMed] [Google Scholar]
- 10.Ganter, U., R. Arcone, C. Toniatti, G. Morrone, and G. Ciliberto. 1989. Dual control of C-reactive protein gene-expression by interleukin-1 and interleukin-6. EMBO J. 8:3773-3779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gebert, C. A., S. H. Park, and D. J. Waxman. 1999. Down-regulation of Liver JAK2-STAT5b signaling by the female plasma pattern of continuous growth hormone stimulation. Mol. Endocrinol. 13:213-227. [DOI] [PubMed] [Google Scholar]
- 12.Georgatsou, E., P. Bourgarel, and T. Meo. 1993. Male-specific expression of mouse sex-limited protein requires growth-hormone, not testosterone. Proc. Natl. Acad. Sci. USA 90:3626-3630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hebert, J. C., and M. O'Reilly. 1996. Granulocyte-macrophage colony stimulating factor (GM-CSF) enhances pulmonary defenses against pneumococcal infections after splenectomy. J. Trauma Injury Infect. Crit. Care 41:663-666. [DOI] [PubMed] [Google Scholar]
- 14.Herrington, J., L. S. Smit, J. Schwartz, and C. Carter-Su. 2000. The role of STAT proteins in growth hormone signaling. Oncogene 19:2585-2597. [DOI] [PubMed] [Google Scholar]
- 15.Huttner, K. M., and C. L. Bevins. 1999. Antimicrobial peptides as mediators of epithelial host defense. Pediatr. Res. 45:785-794. [DOI] [PubMed] [Google Scholar]
- 16.Kassem, M., S. A. Harris, T. C. Spelsberg, and B. L. Riggs. 1996. Estrogen inhibits interleukin-6 production and gene expression in a human osteoblastic cell line with high levels of estrogen receptors. J. Bone Miner. Res. 11:193-199. [DOI] [PubMed] [Google Scholar]
- 17.Kreutz, M., B. Hennemann, U. Ackermann, E. Grage-Griebenow, S. W. Krause, and R. Andreesen. 1999. Granulocyte-macrophage colony-stimulating factor modulates lipopolysaccharide (LPS)-binding and LPS-response of human macrophages: inverse regulation of tumour necrosis factor-alpha and interleukin-10. Immunology 98:491-496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lang, R. A., D. Metcalf, R. A. Cuthbertson, I. Lyons, E. Stanley, A. Kelso, G. Kannourakis, D. J. Williamson, G. K. Klintworth, T. J. Gonda, and A. R. Dunn. 1987. Transgenic mice expressing a hemopoietic growth factor gene (GM-CSF) develop accumulations of macrophages, blindness, and a fatal syndrome of tissue damage. Cell 51:675-686. [DOI] [PubMed] [Google Scholar]
- 19.LeVine, A. M., J. A. Reed, K. E. Kurak, E. Cianciolo, and J. A. Whitsett. 1999. GM-CSF-deficient mice are susceptible to pulmonary group B streptococcal infection. J. Clin. Investig. 103:563-569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Messingham, K. A, S. A. Heinrich, and E. J. Kovacs. 2001. Estrogen restores cellular immunity in injured male mice via suppression of interleukin-6 production. J. Leukoc. Biol. 70:887-895. [PubMed] [Google Scholar]
- 21.Meyer, W. K.-H., P. Reichenbach, U. Schindler, E. Soldani, and M. Nabholz. 1997. Interaction of STAT5 dimers on two low-affinity binding sites mediates interleukin-2 (IL-2) stimulation of IL-2 receptor α gene transcription. J. Biol. Chem. 272:31821-31828. [DOI] [PubMed] [Google Scholar]
- 22.Millard, W. J., D. M. O'Sullivan, T. O. Fox, and B. M. Joseph. 1987. Sexually dimorphic patterns of growth hormone secretion. p. 287-304. In W. F. Crowley and J. G. Hoffer (ed.), The episodic secretion of hormones. Wiley, New York, N.Y.
- 23.Murphy, C., J. Beckers, and U. Ruther. 1995. Regulation of the human C-reactive protein gene in transgenic mice. J. Biol. Chem. 270:704-708. [DOI] [PubMed] [Google Scholar]
- 24.Subramanian, A., J. Teixeira, J. Wang, and G. Gil. 1995. A STAT factor mediates the sexually dimorphic regulation of hepatic cytochrome P450 3A10/lithocholic acid 6β-hydroxylase gene expression by growth hormone. Mol. Cell. Biol. 15:4672-4682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Szalai, A. J., D. E. Briles,. and J. E. Volanakis. 1995. Human C-reactive protein is protective against fatal Streptococcus pneumoniae infection in transgenic mice. J. Immunol. 155: 2557-2563. [PubMed] [Google Scholar]
- 26.Szalai, A. J., F. W. van Ginkel, S. A. Dalrymple, R. Murray, J. R. McGhee, and J. E. Volanakis. 1998. Testosterone and IL-6 requirements for human C-reactive protein gene expression in transgenic mice. J. Immunol. 160:5294-5299. [PubMed] [Google Scholar]
- 27.Tanaka, T., S. Okamura, K. Okada, A. Suga, N. Shimono, N. Ohhara, Y. Hirota, Y. Sawae, and Y. Niho. 1989. Protective effect of recombinant murine granulocyte-macrophage colony-stimulating factor against Pseudomonas aeruginosa infection in leukocytopenic mice. Infect. Immun. 57:1792-1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tannenbaum, G. S., and J. B. Martin. 1976. Evidence for an endogenous ultradian rhythm governing growth hormone secretion in the rat. Endocrinology 98:562-570. [DOI] [PubMed] [Google Scholar]
- 29.Tarr, P. E. 1996. Granulocyte-macrophage colony-stimulating factor and the immune system. Med. Oncol. 13:133-140. [DOI] [PubMed] [Google Scholar]
- 30.Taverne, J. 1993. Transgenic mice in the study of cytokine function. Int. J. Exp. Pathol. 74:525-546. [PMC free article] [PubMed] [Google Scholar]
- 31.Tesfaigzi, Y., K. Rudolph, M. J. Fischer, and C. A. Conn. 2001. Bcl-2 mediates sex-specific differences in recovery of mice from LPS-induced signs of sickness independent of IL-6. J. Appl. Physiol. 91:2182-2189. [DOI] [PubMed] [Google Scholar]
- 32.Varin-Blank, N., E. Dondi, M. Tosi, C. Hernandez, L. Boucontet, H. Gotoh, T. Shiroishi, K. Moriwaki, and T. Meo. 1998. Male-specific transcription of the C4-Slp gene in mouse liver follows activation of STAT5. Proc. Natl. Acad. Sci. USA 95:8750-8755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang, D. X., S. L. Jiang, D. Rzewinicki, D. Samols, and I. Kushner. 1995. The effect of interleukin-1 on C-reactive protein expression in Hep3B cells is exerted at the transcriptional level. Biochem. J. 310:143-148. [DOI] [PMC free article] [PubMed] [Google Scholar]