Helicobacter pylori infects one-half of the world's population, leading to peptic ulcer in up to 15% of adult cases, and increases the risk of gastric adenocarcinoma by 2.3- to 9-fold (11). Colonization induces an intense immune response in the human host which rarely leads to clearance of the organism but which is linked to disease expression (3, 4).
Childhood H. pylori infection is common in developing countries. In The Gambia, 85% of children are colonized by 30 months of age (12). Despite this, gastric cancer is uncommon in West Africa (8). It has been suggested that the host immune response to H. pylori infection in an African population differs from that observed in subjects from developed countries, although it is unclear whether this reflects changes in host response at all stages of infection (10). We investigated whether circulating H. pylori-specific immunoglobulin G2 (IgG2) and IgG1 levels, as markers of mucosal Th1 and Th2 responses, respectively (1), were similar between Gambian and United Kingdom adults and children. Sustained differences in mucosal Th1/Th2 bias against H. pylori might explain a low prevalence of adult disease in a population with a very high early prevalence of colonization.
Four groups of subjects were recruited after appropriate ethical approval and consent, comprising adults and children with H. pylori infection from the United Kingdom and from The Gambia. Thirty-four United Kingdom children 4 to 14 years of age (mean age, 11 years) with H. pylori infection confirmed by endoscopy (hematoxylin and eosin [H+E] stain and Giemsa stain; urease positive) were studied. H. pylori was successfully cultured from all subjects in this group. A group of 56 Gambian children (2 to 5 years of age), of whom 19 had H. pylori infection confirmed by endoscopy (H+E and Giemsa, but cultures not undertaken) were recruited, while the remaining 37 did not undergo endoscopy but were IgG seropositive. One hundred sixty-three seropositive United Kingdom adults aged 50 years and 30 adult Gambians (25 to 70 years) with severe dyspepsia and chronic gastritis found to be H. pylori colonized at endoscopy made up the remaining subjects. A single blood sample was taken from each subject, and H. pylori-specific IgG2 and IgG1 levels were measured by enzyme-linked immunosorbent assay (ELISA).
An in-house ELISA utilized whole-cell antigen, as previously described from five isolates of H. pylori (13). In brief, antigen was prepared by culturing gastric biopsies (five individuals, two United Kingdom adults with duodenal ulcer and three Gambian adults). H. pylori strains were antigenically distinct on the basis of genotype analysis (two cag+ and one cag−) and are described elsewhere. All five strains produced different patterns of response when immunoblotted against a single control serum (9). All subjects had histologically proven gastritis and were dyspeptic. After 48 h of incubation on Oxoid Columbia base, 10% laked blood agar plates, organisms were harvested, suspended in phosphate-buffered saline (PBS), and lysed by sonication. Particulate matter was removed by centrifugation at 7,000 × g for 10 min. Supernatant was used as an antigen suspension as a soluble protein at a concentration of 20 ng/ml. Multiwell ELISA plates (Nalgene, Immunosorb, Rochester, N.Y.) were coated with this antigen and incubated with patient sera. Bound patient antibody was incubated with biotinylated monoclonal mouse anti-human IgG2 and IgG1 (Sigma, Poole, Dorset, United Kingdom) and identified by further incubation with streptavidin-conjugated peroxidase and enzyme substrate (Sigma). Five controls (positive and negative for H. pylori colonization, determined by endoscopy and biopsy, covering the linear portion of the assay) were included in all plates. The linear portion of the calibration curve was determined to lie between 0.006 and 1.63 (IgG1) and 1.8 and 27 (IgG2). Endpoint titration was determined for both IgG1 and IgG2 as two standard deviations above the mean relative concentration for 12 adults uninfected with H. pylori. Concentrations below this value were regarded as zero.
Table 1 shows differences in H. pylori-specific subclass responses among the four subject groups. Assay specificities are demonstrated by titers of IgG1 and IgG2 being 10,000- and 1,000-fold lower in 35 subjects (age range, 7 to 16 years) free of H. pylori (biopsy negative and total IgG negative). The most striking finding was a 10-fold increase in relative concentration of IgG2 in United Kingdom adults compared to United Kingdom children and Gambian children and adults. There were no other differences in any H. pylori IgG subclasses. IgG2 levels were at the limit of detection in 51 of 86 (59%) Gambian subjects and 22 of 30 (61%) United Kingdom children compared to 5 of 161 (3%) of the United Kingdom adults (chi-square: 119.3, 3df, P < 0.0001).
TABLE 1.
Titers of H. pylori IgG1 and IgG2a
| Immuno- globulin | Median titer (interquartile range)
|
||||
|---|---|---|---|---|---|
| UK uninfected children (n = 35) | UK children (n = 35) | Gambian children (n = 56) | UK adults (n = 163) | Gambian adults (n = 30) | |
| IgG1 | ‡4 × 10−5 (8 × 10−6-2 × 10−4) | 3.9 (1.5-11.8) | 4.37 (1.6-11.9) | 4.07 (1.7-32.5) | 6.5 (2.4-13.1) |
| IgG2 | ‡1 × 10−3 (7.3 × 10−4-2 × 10−3) | 1.3 (0.8-10.9) | 1.95 (0.9-16.7) | †9.46 (5.0-17.9) | 1.3 (0.9-10.2) |
Mann-Whitney U test. Symbols: †, P < 0.0001 for all between-group comparisons; ‡, P < 0.0001 for all between-group comparisons. UK, United Kingdom.
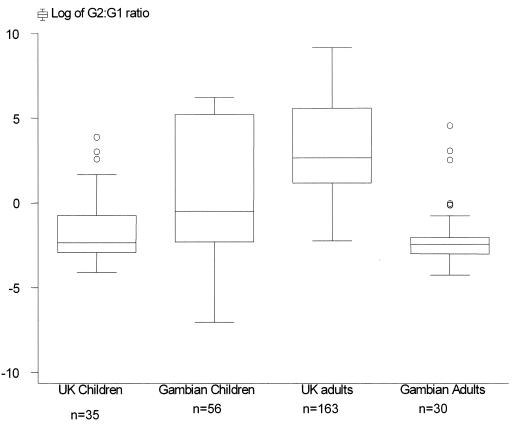
Individual H. pylori IgG2/IgG1 ratios emphasize the difference in IgG subclass production between groups. A Kruskal-Wallis test suggested significant variation in the responses in the four populations (P < 0.0001). The response in the United Kingdom adult population was significantly higher than in the other three populations (P < 0.0001). The median IgG2/IgG1 ratio for United Kingdom and Gambian children and Gambian adults demonstrated an IgG1-biased response, whereas United Kingdom adults demonstrated a log-fold increase in the same ratio with a predominantly IgG2-biased response (Fig. 1).
FIG. 1.
Box plot of log10 IgG2/IgG1 titer ratios in United Kingdom (UK) and Gambian adults and children.
We have observed that United Kingdom adults produce high H. pylori-specific IgG2 levels (indicating a Th1 reaction to H. pylori), whereas Gambian adults and children and United Kingdom children produce predominantly IgG1 (indicating a Th2 response). These data suggest that Gambians, who are challenged with H. pylori infection in childhood, maintain the Th2 response throughout life, whereas United Kingdom adults appear to convert to a Th1 response.
European children may mount a gastric mucosal Th1 response to H. pylori (7), similar to adults (2). However, we have measured an apparent systemic Th2 response. This discrepancy between the mucosal and systemic responses may be explained by local regulatory responses, such as bystander suppression, mediated by transforming growth factor beta. Such a mechanism can selectively suppress local cell-mediated inflammation (6), encouraging a Th2 bias. This process, however, requires a continual mucosal challenge, which is not sustained in United Kingdom children, allowing a Th1 phenotype to develop. This Th1 bias may be associated with gastric carcinogenesis (3).
The bias in IgG2 production in United Kingdom adults is consistent with the hypothesis that a shift in polarity of T-helper-cell responses in chronic H. pylori infection could provide an explanation for the apparent difference in prevalence of H. pylori-associated gastric carcinoma between West African and European populations. These results require confirmation with direct studies of gastric mucosa in these four groups of subjects. The factors involved in the reversal of T-helper-cell polarity in United Kingdom children to one that leads to gastric cancer are unknown but are likely to include bacterial factors, host genetics, and coexistent enteropathy (5).
Conventional vaccines have failed to eradicate H. pylori, but this study highlights differences in immune responses associated with populations with high and low risks of gastric cancer. Identifying those factors that lead to a reversal of the African phenotype to the cancer-prone United Kingdom phenotype could allow immunomodulation to achieve the elusive goal of preventing H. pylori-associated gastric cancer.
Acknowledgments
This work was funded by the Medical Research Council (United Kingdom) MRC Keneba, Special Trustees of the Royal Victoria Infirmary, Newcastle-upon-Tyne, and The Children's Foundation, Newcastle-upon-Tyne.
REFERENCES
- 1.Bontkes, H. J., R. A. Veenendaal, A. S. Pena, J. G. Goedhard, W. van Duijn, J. Kuiper, et al. 1992. IgG subclass response to Helicobacter pylori in patients with chronic active gastritis and duodenal ulcer. Scand. J. Gastroenterol. 27:129-133. [DOI] [PubMed] [Google Scholar]
- 2.D'Elios, M. M., M. Manghetti, M. De Carli, F. Costa, C. T. Baldari, D. Burroni, et al. 1997. T helper 1 effector cells specific for Helicobacter pylori in gastric antrum of patients with peptic ulcer disease. J. Immunol. 158:962-967. [PubMed] [Google Scholar]
- 3.El-Omar, E. M., M. Carrington, and W. H. Chow. 2000. Interleukin-1 polymorphisms associated with increased risk of gastric cancer. Nature 404:398-402. [DOI] [PubMed] [Google Scholar]
- 4.Ernst, P., S. Crowe, and V. Reyes. 1997. How does Helicobacter pylori cause mucosal damage? The inflammatory response. Gastroenterology 113(Suppl. 6):s35-s42. [DOI] [PubMed] [Google Scholar]
- 5.Fox, J. G., P. Beck, C. A. Dangler, M. T. Whary, T. C. Wang, H. N. Shi, et al. 2000. Concurrent enteric helminth infection modulates inflammation and gastric immune responses and reduces helicobacter-induced gastric atrophy. Nat. Med. 6:536-542. [DOI] [PubMed] [Google Scholar]
- 6.Fukaura, H., S. C. Kent, M. J. Peitrusewicz, S. J. Khoury, H. L. Weiner, and D. A. Hafler. 1996. Antigen-specific TGF-beta 1 secretion with bovine myelin oral tolerization in multiple sclerosis. Ann. N. Y. Acad. Sci. 778:251-257. [DOI] [PubMed] [Google Scholar]
- 7.Guiraldes, E., I. Duarte, A. Pena, A. Godoy, M. N. Espinosa, R. Bravo, et al. 2001. Proinflammatory cytokine expression in gastric tissue from children with Helicobacter pylori associated gastritis. J. Pediatr. Gastroenterol. Nutr. 33:127-132. [DOI] [PubMed] [Google Scholar]
- 8.Holcombe, C. 1992. Helicobacter pylori: The African enigma. Gut 33:429-431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kersulyte, D., A. K. Mukhopadhyay, B. Velapatino, W. Su, Z. Pan, C. Garcia,et al. 2000. Differences in genotype of Helicobacter pylori from different human populations. J. Bacteriol. 182:3210-3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mitchell, H. M., R. Ally, A. Wadee, M. Wiseman, and I. Segal. 2002. Major differences in the IgG subclass response to Helicobacter pylori in the first and third worlds. Scand. J. Gastroenterol. 37:517-522. [DOI] [PubMed] [Google Scholar]
- 11.Parsonnet, J., G. Friedman, and D. Vandersteen. 1991. Helicobacter pylori infection and the risk of gastric adenocarcinoma. N. Engl. J. Med. 325:1127-1131. [DOI] [PubMed] [Google Scholar]
- 12.Thomas, J. E., A. Dale, M. Harding, L. T. Weaver, and W. A. Coward. 1999. Helicobacter colonisation during early life in West Africa. Pediatr. Res. 45:218-223. [DOI] [PubMed] [Google Scholar]
- 13.Thomas, J. E., A. M. Whatmore, M. B. Barer, E. J. Eastham, and M. A. Kehoe. 1990. Serodiagnosis of Helicobacter pylori infection in childhood. J. Clin. Microbiol. 28:2641-2646. [DOI] [PMC free article] [PubMed] [Google Scholar]