Abstract
Proinflammatory cytokines mediate the toxic effect of superantigenic staphylococcal exotoxins (SE). A pan-caspase inhibitor suppressed SE-stimulated T-cell proliferation and the production of cytokines and chemokines by human peripheral blood mononuclear cells. These data suggest that caspase inhibitors may represent a novel therapeutic modality for treating SE-induced toxic shock.
Staphylococcal exotoxins (SE) have been implicated as major virulence factors responsible for toxic shock syndrome (2, 15, 19). This disease is characterized by fever, hypotension, desquamation of skin, and multiple-organ system failure. Staphylococcal toxic shock syndrome toxin 1 (TSST-1) and the structurally related staphylococcal enterotoxin B (SEB) are potent stimulators of the immune system, as they bind directly to the major histocompatibility complex class II molecules on antigen-presenting cells (9). These superantigens polyclonally activate T cells (4), resulting in proliferation and, eventually, apotosis. Multiple components of the host inflammatory response triggered by SE have been shown to contribute to toxic shock, including the cascade of inflammatory cytokines and chemokines (3, 4, 9, 16, 19). These cytokines include interleukin 1 (IL-1), tumor necrosis factor alpha (TNF-α), and gamma interferon (IFN-γ). These proinflammatory mediators have potent immunoenhancing effects and are known to be pathogenic at high levels (12). The massive production of inflammatory mediators from both T cells and monocytes appears early, within hours after the SE bind to these cells, while T-cell proliferation occurs later.
Recently, caspase inhibitors were used to prevent allergic airway inflammation (7) and to decrease the death rate from acute experimental pancreatitis (18). Caspase inhibitors also blocked murine liver injury (13) and attenuated bleomycin-induced pneumopathy (14). Other studies indicated that caspase activation is required for mitogen- or anti-CD3-stimulated T-cell proliferation (1, 8, 17). Caspases belong to a family of autocatalytic cysteine proteases which regulate many cellular processes, including apotosis, cell differentiation, and inflammation (5). They share sequence homologies and are grouped into three subfamilies based on their function and substrate specificity (5, 6, 18, 20). Group 1 caspases, which include caspase-1, -4, -5, and -14, mediate cytokine processing and inflammation. Caspase-1, originally known as the IL-1β-converting enzyme, is the first-discovered caspase and is essential in the proteolytic processing of cytokines IL-1 and IL-18. The other major groups of caspases, groups 2 and 3, function mostly in apotosis and in other cellular proteolytic cascades resulting in DNA fragmentation and degradation of multiple cellular substrates (5). To elucidate further the cellular mechanisms contributing to toxic shock, caspase activation in SE-stimulated human peripheral blood mononuclear cells (PBMC) was examined through the use of specific and pan-caspase inhibitors.
Purified TSST-1 and SEB were obtained from Toxin Technology (Sarasota, Fla.). The endotoxin content of these preparations was <1 ng of endotoxin per mg of protein, as determined by the Limulus amoebocyte lysate gelation test (BioWhittaker, Walkersville, Md.). Human recombinant TNF-α (hrTNFα), antibodies against hrTNFα, peroxidase-conjugated anti-rabbit immunoglobulin G, and peroxidase-conjugated anti-goat immunoglobulin G were obtained from Roche (Indianapolis, Ind.). hrIL-1β was kindly provided by J. Oppenheim (National Cancer Institute, Frederick, Md.). hrIFN-γ and hrIL-6 were obtained from Collaborative Research (Boston, Mass.). Antibodies against IFN-γ and MCP-1 were obtained from BD PharMingen (San Diego, Calif.). hrMCP-1, hrMIP-1α, and hrMIP-1β, and antibodies against IL-1β, IL-6, MIP-1α, and MIP-1β were purchased from R&D Systems (Minneapolis, Minn.). The caspase inhibitors benzyloxycarbonyl-Val-Ala-Asp-fluoromethylketone (Z-VAD-fmk), acetyl-Tyr-Val-Ala-Asp-chloromethylketone (Ac-YVAD-cmk), Z-Asp-(2,6-dichlorobenzoyl)oxymethane (Z-D-CH2-DCB), Z-Asp-Glu-Val-Asp-fmk (Z-DEVD-fmk), and Z-Ile-Glu-Thr-Asp-fmk (Z-IETD-fmk) were obtained from Calbiochem (San Diego, Calif.) and dissolved in dimethyl sulfoxide. All other common reagents were obtained from Sigma (St. Louis, Mo.). Human PBMC were isolated by Ficoll-Hypaque density gradient centrifugation of heparinized blood from normal human donors. PBMC were cultured in 24-well plates at 37°C in RPMI 1640 supplemented with 10% fetal bovine serum at a concentration of 106 cells/ml. Cells were stimulated with either TSST-1 (200 ng/ml) or SEB (200 ng/ml) for 16 h. The exotoxins and the various concentrations of caspase inhibitors were added simultaneously. Supernatants were harvested and analyzed for IL-1β, TNF-α, IL-6, IFN-γ, MCP-1, MIP-1α, and MIP-1β. Cytokines and chemokines were measured by an enzyme-linked immunosorbent assay with cytokine- or chemokine-specific antibodies, as previously described (10, 11). Human recombinant cytokines and chemokines (20 to 1,000 pg/ml) were used as calibration standards for each plate. The detection limit of each assay was 20 pg/ml. Cytotoxicity was measured by the release of lactate dehydrogenase (LDH) from the cytosol into the culture supernatant. LDH was measured by using a cytotoxicity kit (Roche) in accordance with the manufacturer's instructions. T-cell proliferation was assayed with PBMC (105 cells/well), which were plated in triplicate with TSST-1 or SEB (200 ng/ml), with or without caspase inhibitors, for 48 h at 37°C in 96-well microtiter plates. Cells were pulsed with 1 μCi of [3H]thymidine (New England Nuclear, Boston, Mass.) per well during the last 5 h of culture (10). Cells were harvested onto glass fiber filters, and the incorporated [3H]thymidine was measured by liquid scintillation. Caspase-1, -3, and -8 activities were determined using, respectively, caspase -1, -3, and -8 assay kits obtained from R&D Systems. Cell lysate was prepared in triplicate from cells stimulated with SE for 48 h, and the specific caspase was measured according to the manufacturer's instructions. DNA fragmentation was detected by the presence of oligonucleosomes by using a cell death detection ELISA PLUS kit (Roche) according to the manufacturer's instructions. All data were analyzed for significant difference by Student's t test with Stata Corp. (College Station, Tex.) software. Differences between treated and untreated control groups were considered significant if P was <0.05.
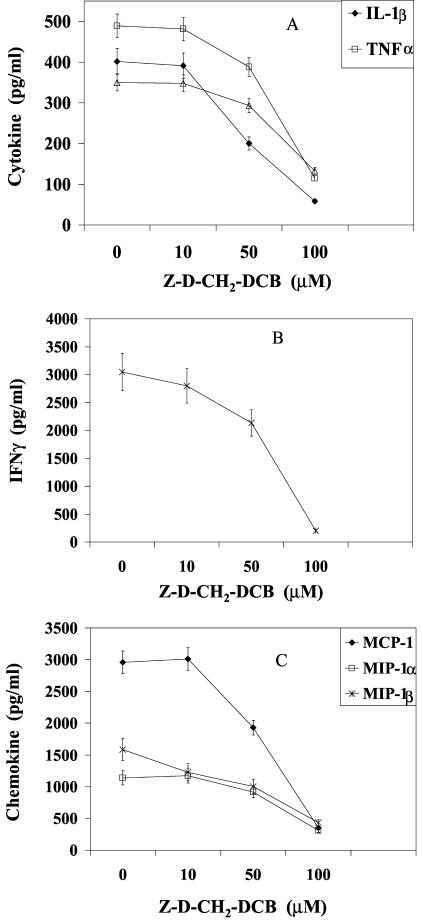
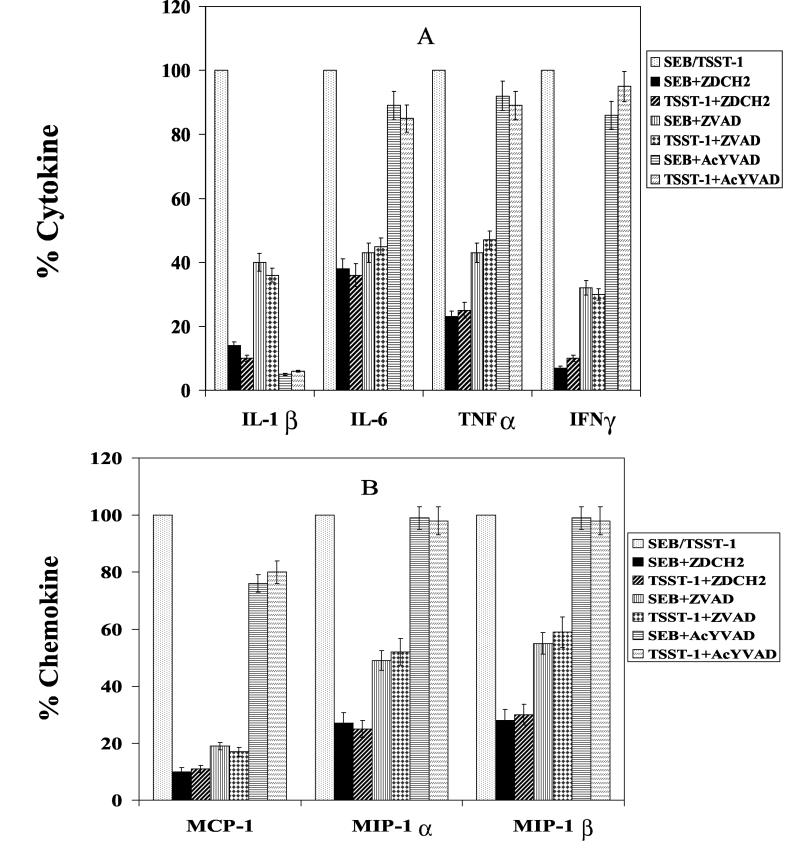
Caspases have been implicated in the pathogenesis of many inflammatory diseases (5, 13, 14, 21). The stimulatory role of caspases in superantigen-stimulated PBMC was examined by observing the effect of caspase inhibitors on cytokine and chemokine production. Figure 1 shows that the pan-caspase inhibitor Z-D-CH2-DCB blocked the production of IL-1β, TNF-α, IL-6, and IFN-γ in SEB-stimulated PBMC in a dose-dependent manner. The production of the chemokines MCP-1, MIP-1α, and MIP-1β was also suppressed (Fig. 1C). The inhibitory effect of Z-D-CH2-DCB on TSST-1-activated PBMC was similar, reducing IL-1β, IL-6, TNF-α, IFN-γ, MCP-1, MIP-1α, and MIP-1β to 10, 36, 25, 10, 11, 25, and 30%, respectively, of levels in untreated cells (Fig. 2A and B). In contrast, another broad-spectrum caspase inhibitor, Z-VAD-fmk, inhibited the SEB- and TSST-induced cytokines and chemokines less effectively (Fig. 2A and B).
FIG. 1.
Dose-response inhibition of IL-1β, IL-6, and TNF-α (A); IFN-γ (B); and MCP-1, MIP-1α, and MIP-1β (C) production by PBMC stimulated with 200 ng of SEB/ml in the presence of various concentrations of Z-D-CH2-DCB. Values are the means ± standard deviations (SD) of values for duplicate samples from three experiments.
FIG. 2.
Inhibition of IL-1β, IL-6, TNF-α, and IFN-γ (A) and MCP-1, MIP-1α, and MIP-1β (B) production by PBMC stimulated with 200 ng of SEB or TSST-1 per ml in the presence of a 100 μM concentration of the caspase inhibitors indicated. Values are the means ± SD of results from PBMC cultures from six blood donors. ZDCH2, Z-D-CH2-DCB; ZVAD, Z-VAD-fmk; AcYVAD Ac-YVAD-cmk.
More-specific caspase inhibitors were used to further determine which enzymes were involved in SE-stimulated PBMC. The caspase 1 specific inhibitor, Ac-YVAD-cmk, attenuated IL-1β and monocyte chemotactic protein production in PBMC stimulated by SEB and TSST-1 to 95 and 18%, respectively, but had minimal effects on the other cytokines (Fig. 2A and B). The caspase 3-specific inhibitor, Z-DEVD-fmk, and the caspase 8 inhibitor, Z-IETD-fmk, did not significantly affect the levels of cytokines or chemokines from SE-stimulated cells (data not shown). Neither the caspase inhibitors nor dimethyl sulfoxide (0.26%), the solvent used in preparations of these inhibitors, affected the viability of the cells at the concentrations used, as confirmed by a trypan blue dye exclusion test and the release of cytosol LDH. The two pan-caspase inhibitors did not inhibit [35S]Met incorporation into proteins in PBMC cultures (data not shown) and therefore are not general inhibitors of protein synthesis.
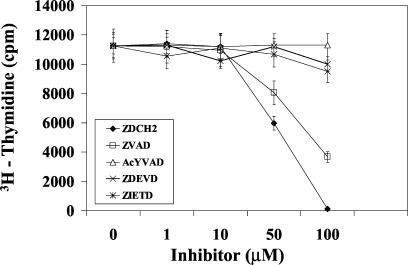
Because superantigens also cause T-cell proliferation, the effect of these caspase inhibitors on SE-induced T-cell proliferation was investigated next. Figure 3 shows that Z-D-CH2-DCB was also the most potent inhibitor and reduced SEB-1-stimulated T-cell proliferation in a dose-dependent manner, achieving 98% inhibition at 100 μM. The pan-caspase inhibitor Z-VAD-fmk blocked SEB-activated cells by 32%, whereas inhibitors specific for caspase-1, -3, and -8 failed to have an effect on SEB-induced T-cell proliferation.
FIG. 3.
Inhibition of T-cell proliferation in PBMC stimulated with 200 ng of SEB/ml by various concentrations of caspase inhibitors. Values are the mean counts ± SD of results from triplicate cultures from three experiments.
The presence of three different caspases, representing the three major groups of caspases, was examined next. Caspase-1 was found in SEB- and TSST-1-stimulated cell lysate (80 ± 6 and 68 ± 6 pg/ml, respectively, versus 14 ± 4 pg/ml in the unstimulated control). Insignificant amounts of caspase-8 were present in the SEB- and the TSST-1-treated cells (23 ± 7 and 28 ± 6 pg/ml versus 12 ± 5 pg/ml in the control lysate), whereas caspase-3 was completely absent in these cells. Thus, in agreement with previous reports, caspase-1 was required for the proteolytic processing of the cytokine IL-1β, as caspase-1 inhibitor suppressed its release (5, 6, 21). The presence of oligonucleosomes in cell lysate was also examined, because some caspases promote apotosis. SEB- and TSST-1-stimulated cells were not apoptotic, as oligonucleosomes were absent in these cells (data not shown).
The novel observations presented here indicate that the broad-spectrum caspase inhibitor Z-D-CH2-DCB suppressed multiple proinflammatory cytokines and chemokines in SEB- or TSST-1-stimulated human mononuclear cells. T-cell proliferation was also blocked by this pan-caspase inhibitor but not by specific inhibitors of caspase-1, -3, or -8. Moderate caspase-1 activity was found in SE-stimulated PBMC, and a specific caspase-1 inhibitor blocked SE-induced IL-1β but not other cytokines or chemokines. While the inhibition mechanism has not been identified, it is clear that multiple caspases aside from caspase 1, perhaps unidentified, are required in the activation of T cells and the production of these inflammatory cytokines and chemokines. These results suggest that the inactivation of caspases represents a previously unrecognized modality for blocking superantigen activation of cellular responses and treatment of toxic shock.
Acknowledgments
I thank Marilyn Buckley for excellent technical assistance.
REFERENCES
- 1.Alam, A., L. Y. Cohen, S. Aouad, and R. P. Sekaly. 1999. Early activation of caspases during T lymphocyte stimulation results in selective substrate cleavage in nonapoptotic cells. J. Exp. Med. 190:1879-1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bergdoll, M. S., and P. M. Schlievert. 1984. Toxic shock syndrome toxin. Lancet ii:691. [Google Scholar]
- 3.Cameron, S. B., M. C. Nawijn, W. W. Kum, H. F. Savelkoul, and A. W. Chow. 2001. Regulation of helper T cell responses to staphylococcal superantigens. Eur. Cytokine Netw. 12:210-222. [PubMed] [Google Scholar]
- 4.Choi, Y., B. Kotzin, L. Hernon, J. Callahan, P. Marrack, and J. Kappler. 1989. Interaction of Staphylococcus aureus toxin “superantigens” with human T cells. Proc. Natl. Acad. Sci. USA 86:8941-8945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cohen, G. M. 1997. Caspases: the executioners of apotosis. Biochem. J. 326:1-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Garcia-Calvo, M., E. P. Peterson, B. Leiting, R. Ruel, D. W. Nicholson, and N. A. Thornberry. 1998. Inhibition of human caspases by peptide-based and macromolecular inhibitors. J. Biol. Chem. 273:32608-32613. [DOI] [PubMed] [Google Scholar]
- 7.Iwata, A., K. Nishio, R. K. Winn, E. Y. Chi, W. R. Henderson, Jr., and J. M. Harlan. 2003. A broad-spectrum caspase inhibitor attenuates allergic airway inflammation in murine asthma model. J. Immunol. 170:3386-3391. [DOI] [PubMed] [Google Scholar]
- 8.Kennedy, N. J., T. Kataoka, J. Tschopp, and R. C. Budd. 1999. Caspase activation is required for T cell proliferation. J. Exp. Med. 190:1891-1896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kotzin, B. L., D. Y. M. Leung, J. Kappler, and P. A. Marrack. 1993. Superantigens and their potential role in human disease. Adv. Immunol. 54:99-166. [DOI] [PubMed] [Google Scholar]
- 10.Krakauer, T. 1994. Inhibition of toxic shock syndrome toxin-1-induced cytokine production and T cell activation by interleukin-10, interleukin-4, and dexamethasone. J. Infect. Dis. 172: 988-992. [DOI] [PubMed] [Google Scholar]
- 11.Krakauer, T. 1999. Induction of CC chemokines in human peripheral blood mononuclear cells by staphylococcal exotoxins and its prevention by pentoxifylline. J. Leukoc. Biol. 66:158-164. [DOI] [PubMed] [Google Scholar]
- 12.Krakauer, T., J. Vilcek, and J. J. Oppenheim. 1998. Proinflammatory cytokines: TNF and IL-1 families, chemokines, TGFβ and others, p. 775-811. In W. Paul (ed.), Fundamental immunology, 4th ed. Lippincott-Raven Press, Philadelphia, Pa.
- 13.Kunstle, G., M. Leist, S. Uhlig, L. Revesz, R. Feifel, A. MacKenzie, and A. Wendel. 1997. ICE-protease inhibitors block murine liver injury and apoptosis caused by CD95 or by TNF-alpha. Immunol. Lett. 55:5-10. [DOI] [PubMed] [Google Scholar]
- 14.Kuwano, K., R. Kunitake, T. Maeyama, N. Hagimoto, M. Kawasaki, T. Matsuba, M. Yoshimi, I. Inoshima, K. Yoshida, and N. Hara. 2001. Attenuation of bleomycin-induced pneumopathy in mice by a caspase inhibitor. Am. J. Physiol. Lung Cell. Mol. Physiol. 280:L316-L325. [DOI] [PubMed] [Google Scholar]
- 15.McCormick, J. K., J. M. Yarwood, and P. M. Schlievert. 2001. Toxic shock syndrome and bacterial superantigens: an update. Annu. Rev. Microbiol. 55:77-104. [DOI] [PubMed] [Google Scholar]
- 16.Miethke, T., C. Wahl, K. Heeg, B. Echtenacher, P. H. Krammer, and H. Wagner. 1992. T cell-mediated lethal shock triggered in mice by the superantigen SEB: critical role of TNF. J. Exp. Med. 175:91-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mukerjee, N., K. M. McGinnis, M. E. Gnegy, and K. K. Wang. 2001. Caspase-mediated calcineurin activation contributes to IL-2 release during T cell activation. Biochem. Biophys. Res. Commun. 285:1192-1199. [DOI] [PubMed] [Google Scholar]
- 18.Paszkowski, A. S., B. Rau, J. M. Mayer, P. Moller, and H. G. Beger. 2002. Therapeutic application of caspase 1/interleukin 1β-converting enzyme inhibitor decreases the death rate in severe acute experimental pancreatitis. Ann. Surg. 235:68-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stevens, D. L. 1996. The toxic shock syndromes. Infect. Dis. Clin. N. Am. 10:727-746. [DOI] [PubMed] [Google Scholar]
- 20.Talanian, R. V., C. Quinlan, S. Trautz, M. C. Hackett, J. A. Mankovich, D. Banach, T. Ghayur, K. D. Brady, and W. W. Wong. 1997. Substrate specificities of caspase family proteases. J. Biol. Chem. 272:9677-9682. [DOI] [PubMed] [Google Scholar]
- 21.Zeuner, A., A. Eramo, C. Peschle, and R. De Maria. 1999. Caspase activation without death. Cell Death Differ. 6:1075-1080. [DOI] [PubMed] [Google Scholar]