Abstract
We report the synthesis, in vitro antiprotozoal (against Plasmodium and Leishmania), antimicrobial, cytotoxicity (Vero and MetHb-producing properties) and in vivo antimalarial activities of two series of 8-quinolinamines. N1-{4-[2-(tert-Butyl)-6-methoxy-8-quinolylamino]pentyl}-(2S/2R)-2-aminosubstitutedamides (21–33) and N1-[4-(4-ethyl-6-methoxy-5-pentyloxy-8-quinolylamino)pentyl]-(2S/2R)-2-aminosubstitutedamides (51–63) were synthesized in six steps from 6-methoxy-8-nitroquinoline and 4-methoxy-2-nitro-5-pentyloxyaniline, respectively. Several analogs displayed promising antimalarial activity in vitro against P. falciparum D6 (chloroquine-sensitive) and W2 (chloroquine-resistant) clones with high selectivity indices vs. mammalian cells. The most promising analogs (21–24) also displayed potent antimalarial activity in vivo in a P. berghei-infected mouse model. Most interestingly, many analogs exhibited promising in vitro antileishmanial activity against L. donovani promastigotes, and antimicrobial activities against a panel of pathogenic bacteria and fungi. Several analogs, notably 21–24, 26–32 and 60, showed less MetHb formation compared to primaquine indicating the potential of these compounds in 8-quinolinamine-based antimalarial drug development.
1. Introduction
Protozoan infections remain a major threat to public health especially in the tropical parts of the world. More than a third of world's population is at risk of contracting malaria. It is estimated that approximately 7000 people, mainly children under the age of 5 years and pregnant women in Africa and other parts of world, die of malaria every day.1 Malaria is one of the main obstacles to socio-economic development in sub-Saharan Africa and other tropical regions of the world.
Leishmaniases is a group of diseases caused by infection with intracellular species of the parasitic protozoan of the genus Leishmania with different clinical forms ranging from cutaneous leishmaniasis (CL) with skin lesions to visceral leishmaniasis (VL) with enlargement of liver, spleen and bone marrow dysfunctions.2 The disease is endemic worldwide with estimated 12 million cases, which are mostly centered in Asia, Mediterranean regions of Europe, Africa, Central America and South America. VL caused due to infection with Leishmania donovani is fatal if left untreated.
In the early 1970s, it was believed that virtually any microbial infection could be treated, as a wide range of antimicrobial agents (antibiotics) were available. The belief proved short-lived, when pathogens resistant to the conventional antibiotics routinely used to treat microbial infections emerged. The widespread use of antibiotics allowed many microbial strains to evolve ways to adapt or become resistant to the currently available treatment regimens, resulting in an urgent need for new antimicrobial drugs.3, 4
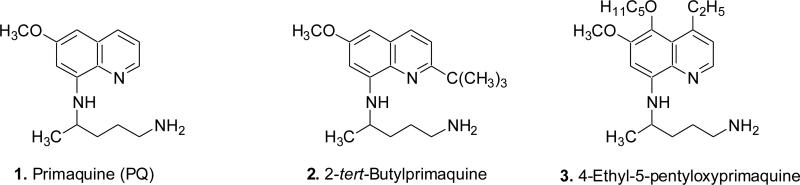
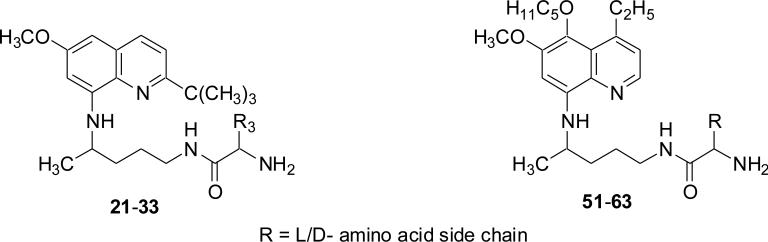
The emergence of Plasmodium falciparum strains resistant to almost all the antimalarials currently in use has prompted researchers around the world to search for its effective replacement. This occurrence revitalized research efforts to discover an entirely new structural class of compounds or revisit the existing antimalarials which were earlier considered inactive against P. falciparum.5 8-Quinolinamines, including primaquine (1, PQ, Fig. 1), constitute an interesting and versatile class of drugs,5 which in addition to antimalarial activity against P. vivax and P. ovale, also exhibit antileishmanial6, 7 and anticoccidial activities.8 PQ has a limited role in current malaria chemotherapy due to its limited activity against asexual blood stages of the malaria parasite, severe hematotoxicities in patients with the glucose-6-phosphate dehydrogenase (G-6-PD) deficiency,9 and a short half-life due to its rapid metabolism to inactive and toxic metabolites.10–13 Despite of these negative attributes, PQ is deemed a worthy candidate for additional structural optimization because it is the only antimalarial drug which exhibit a certain degree of activity against almost all the stages in the life cycle of the human malaria parasite.5 We have already reported two new 8-quinolinamines (2 and 3, Fig. 1) with promising blood schizontocidal antimalarial activities. 2-tert-Butylprimaquine 2 (suppressive at 10 mg/kg, in vivo against P. berghei) was synthesized to eliminate a putative oxidative metabolic pathway known for several quinoline ring-containing antimalarial drugs including quinine by the placement of a bulky metabolically stable tert-butyl group at C-2 of the heterocycle.14 While, 4-ethyl-5-pentyloxyprimaquine 3 (suppressive at 5 mg/kg, in vivo against P. berghei) was synthesized to optimize substitution at the C-4 and C-5 position of PQ, known sites of transformation to inactive/toxic metabolites.15 It is known that approximately 35-83% of PQ is metabolized to the inactive 4-(6-methoxy-quinolin-8-ylamino)pentanoic acid in a primate model.11 We have reported antimalarial activities of several L-amino acid conjugates of PQ in which the amino acid residue possibly protects the side-chain amino group of PQ from oxidation to the abovementioned carboxylic acid.16–18 In continuation of our efforts on the development of 8-quinolinamines as a versatile bioactive class of compounds, we report herein synthesis, of two series of the amino acid conjugated 8-quinolinamines 21–33 and 51–63 (Fig. 2), in which side-chain amino group of the most promising 8-quinolinamines 2 and 3 was derivatized,19 and their in vitro and in vivo antimalarial activity, Vero cell cytotoxicity, in vitro MetHb-inducing properties, and in vitro antileishmanial and antimicrobial activity.
Figure 1.
Structures of primaquine (1) and promising 8-quinolinamines 2–3
Figure 2.
General structure of the newly synthesized 8-quinolinamines
2. Chemistry
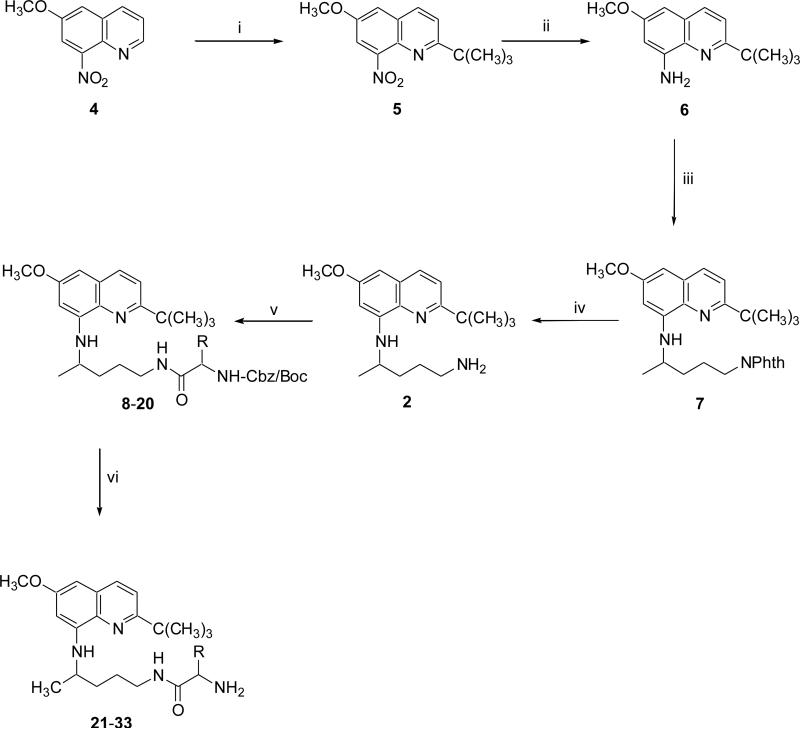
Commercially available 6-methoxy-8-nitroquinoline 4 upon direct ring-alkylation via a silver catalyzed radical oxidative decarboxylation of trimethylacetic acid by ammonium persulfate in CH3CN and 10% H2SO4 at 70-80 °C for 15 min produced 2-tert-butyl-6-methoxy-8-nitroquinoline 5 (Scheme 1).14 The reaction is highly regiospecific and provides an efficient method for direct ring-alkyl of electron deficient quinoline ring under acidic reaction conditions. Catalytic hydrogenation of the latter compound 5 in 95% ethyl alcohol with wet raney-nickel catalyst (T1 grade) at 45 psi in a Parr hydrogenator for 45 min gave the highly hygroscopic and light-sensitive 2-tert-butyl-6-methoxy-8-quinolinamine 6 which was subjected to the next step without purification. Condensation of 6 with 2-(4-bromopentyl)-1,3-isoindolinedione20 in the presence of Et3N at 120 °C for 24 h provided the 2-[4-(2-tert-butyl-6-methoxy-8-quinolylamino)pentyl]-1,3-isoindolinedione 7, which upon hydrazinolysis with hydrazine hydrate in 95% ethyl alcohol at 80 °C for 8 h afforded the N8-(4-amino-1-methylbutyl)-2-(tert-butyl)-6-methoxy-8-quinolinamine 2 (Scheme 1).14
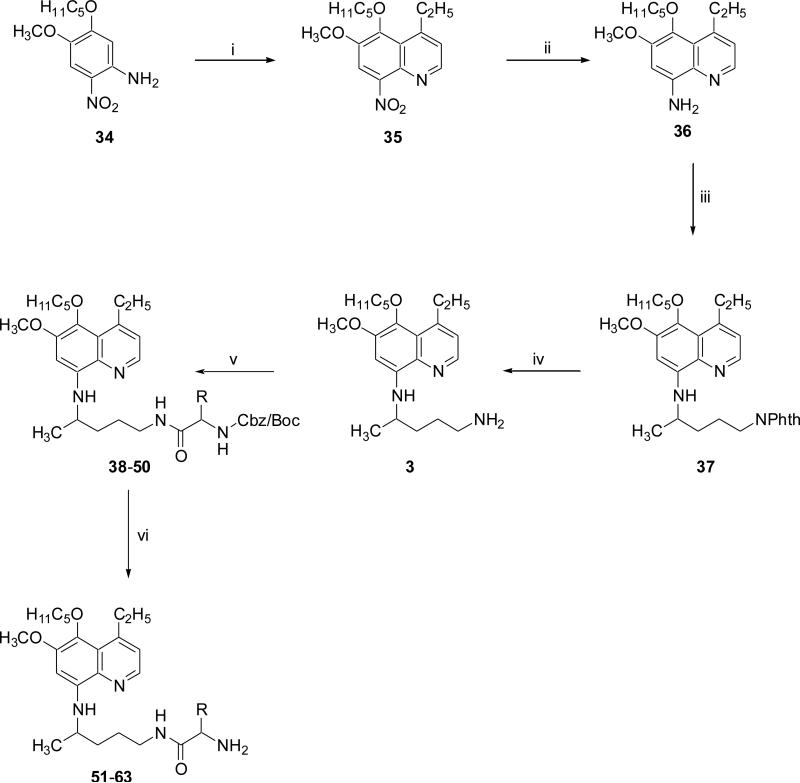
Reaction of 1-chloro-3-pentanone by its addition in two equal portions to the well stirred homogenous mixture of 4-methoxy-2-nitro-5-pentyloxyaniline 34 and o-phosphoric acid at 80 °C followed by the addition of As2O5 produced the 4-ethyl-6-methoxy-8-nitro-5-pentyloxyquinoline 35. The latter compound 35 was converted to N8-(4-amino-1-methylbutyl)-4-ethyl-6-methoxy-5-pentyloxy-8-quinolinamine 3 in three steps following aforementioned procedure (Scheme 2).17
Compounds 2 and 3 upon reaction with suitably side-chain protected Cbz/Boc-L/D- amino acid in the presence of DCC in DCM for 6 h at ambient temperature gave the (S/R)-{alkoxycarbonylamino-1-[4-(2-tert-butyl-6-methoxy-8-quinolylamino)pentylcarbamoyl]-alkyl} carbamic acid benzyl/tert-butyl esters 8–20 and (S/R)-{alkoxycarbonylamino-1-[4-(4-ethyl-6-methoxy-5-pentyloxy-8-quinolylamino)pentylcarbamoyl]alkyl}carbamic acid benzyl/tert-butyl esters 38–50, respectively (Scheme 1-2).
Finally, (S/R)-{alkoxycarbonylamino-1-[4-(2-tert-butyl-6-methoxy-8-quinolylamino)-pentylcarbamoyl]alkyl}carbamic acid benzyl/tert-butyl esters 8–20 and (S/R)-{alkoxycarbonylamino-1-[4-(4-ethyl-6-methoxy-5-pentyloxy-8-quinolylamino)pentylcarbamoyl]alkyl}carbamic acid benzyl/tert-butyl esters 38–50 were deprotected using the procedure(s) described in the experimental section (5.3) to provide N1-{4-[2-(tert-butyl)-6-methoxy-8-quinolylamino]pentyl}-(2S/2R)-2-aminosubstitutedamides 21–33 and N1-[4-(4-ethyl-6-methoxy-5-pentyloxy-8-quinolylamino)pentyl]-(2S/2R)-2-aminosubstitutedamides 51–63, respectively (Scheme 1-2).
3. Biological Activities
3.1.1. Antimalarial, cytotoxicity and MetHb Activities
Determination of in vitro antimalarial activity was based on the plasmodial LDH activity.21 As shown in Table 1-2, antimalarial activities of analogs 21–33 and 51–63 are reported as IC50 values versus chloroquine-sensitive (D6) and chloroquine-resistant (W2) strains of P. falciparum. Analogs 21–24 (series 1) were most potent with IC50 values in the range of 180 – 300 ng/mL for D6 and 300 – 450 ng/mL for W2 compared to IC50 of 2000 and 2800 ng/mL for standard drug primaquine (Table 1). While, remaining analogs 25–32 produced modest IC50 in the range between 770 – 2800 ng/mL for D6 strain and 600–2300 ng/mL for drug-resistant W2 strain of P. falciparum. Analog 33 was found to be inactive. In contrast, analogs 51–63 were less active (Table 2). The most active compound 51 of this series exhibited IC50 of 580 ng/mL for D6 strain and 730 ng/mL for W2 strain of P. falciparum. The remaining analogs produced modest IC50 in the range between 700 – 4760 ng/mL for D6 clone and 950 – 3600 ng/mL for W2 clone (Table 2).
Table 1.
In vitro antimalarial activity (P. falciparum), cytotoxicity, and MetHb formation activities of the N1-{4-[2-(tert-butyl)-6-methoxy-8-quinolylamino]pentyl}-(2S/2R)-2-aminosubstitutedamides 21–33 (series 1).
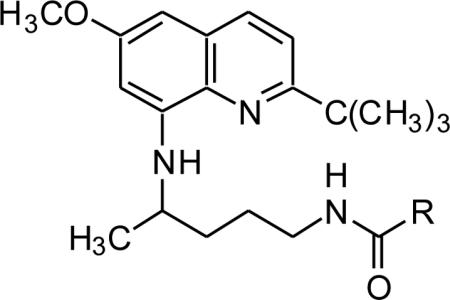
| |||||||
|---|---|---|---|---|---|---|---|
| P. falciparum (D6 clone) | P. falciparum (W2 clone) | Vero cell cytotoxicity | MetHb Toxicity | ||||
| S. No. | R | IC50 (ng/mL) | Sel. Index* | IC50 (ng/mL) | Sel. Index* | IC50 (ng/mL) | % MetHb formation (20 μg/mL) |
| 21 | (R)-Lys | 180 | >132.2 | 300 | >79.3 | NC | 40.1 |
| 22 | (R)-Orn | 190 | >125.3 | 370 | >64.3 | NC | 34.6 |
| 23 | (S)-Arg | 300 | >79.3 | 450 | >52.9 | NC | 34.2 |
| 24 | (R)-Arg | 280 | >85.0 | 400 | >59.5 | NC | 39.2 |
| 25 | (S)-His | 1200 | >19.8 | 1200 | >19.8 | NC | 26.8 |
| 26 | (R)-His | 2200 | >10.8 | 2200 | >10.8 | NC | 40.6 |
| 27 | (S)-Trp | 2800 | >8.5 | 2300 | >10.3 | NC | 28.2 |
| 28 | (S)-Phe | 770 | >30.9 | 920 | >25.9 | NC | 35.7 |
| 29 | (S)-Tyr | 870 | >27.4 | 650 | >36.6 | NC | 34.3 |
| 30 | (S)-Met | 870 | >27.4 | 600 | >39.7 | NC | 34.5 |
| 31 | (S)-Ser | 1000 | >23.8 | 670 | >35.5 | NC | 36.5 |
| 32 | (R)-(Bzl)-Cys | 880 | >27.0 | 760 | >31.3 | NC | 35.0 |
| 33 | (R)-Cys | NA | – | NA | – | NC | NT |
| Primaquine (PQ) | 2000 | >11.9 | 2800 | >8.5 | NC | 47.9 | |
NC=not cytotoxic upto 23800 ng/mL (23.8 μg/mL)
NT = not tested
NA = Not active
Selectivity index is the ratio of IC50 in Vero cells to IC50 in P. falciparum (D6 or W2).
Chloroquine. IC50 = 14 ng/mL, Sel. Index = 1700 (D6 clone); IC50 = 100 ng/mL, Sel. Index = 238 (W6 clone).
Artemisinin. IC50 = 15.2 ng/mL, Sel. Index = 1565 (D6 clone); IC50 = 9 ng/mL, Sel. Index = 2644 (W6 clone).
Table 2.
In vitro antimalarial activity (P. falciparum), cytotoxicity and MetHb formation activity of the N1-[4-(4-ethyl-6-methoxy-5-pentyloxy-8-quinolylamino)pentyl]-(2S/2R)-2-aminosubstitutedamides 51–63 (series 2).
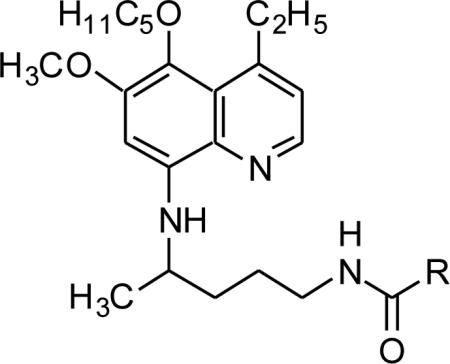
| |||||||
|---|---|---|---|---|---|---|---|
| P. falciparum (D6 clone) | P. falciparum (W2 clone) | Vero cell cytotoxicity | MetHb toxicity | ||||
| S. No. | R | IC50 (ng/mL) | Sel. Index* | IC50 (ng/mL) | Sel. Index* | IC50 (ng/mL) | % MetHb formation (20 μg/mL) |
| 51 | (R)-Lys | 580 | >41.0 | 730 | >32.6 | NC | 48.7 |
| 52 | (R)-Orn | 700 | >34.0 | 1300 | >18.3 | NC | 43.6 |
| 53 | (S)-Arg | 1300 | >18.3 | 1400 | >3.4 | NC | 39.2 |
| 54 | (R)-Arg | 1400 | >17.0 | 1700 | >2.8 | NC | 46.1 |
| 55 | (S)-His | NA | – | NA | – | NC | NT |
| 56 | (R)-His | NA | – | NA | – | NC | NT |
| 57 | (S)-Trp | 860 | >20.9 | 950 | >18.9 | NC | 39.1 |
| 58 | (S)-Phe | 3700 | >6.4 | 2200 | >10.8 | NC | NT |
| 59 | (S)-Tyr | 3800 | >6.3 | 3300 | >7.2 | NC | NT |
| 60 | (S)-Met | 1900 | >12.5 | 1800 | >13.2 | NC | 34.2 |
| 61 | (S)-Ser | 4760 | >5.0 | 3600 | >6.6 | NC | NT |
| 62 | (R)-(Bzl)-Cys | 2000 | >11.9 | 1200 | >19.8 | NC | NT |
| 63 | (R)-Cys | NA | – | NA | – | NC | 47.5 |
| Primaquine (PQ) | 2000 | >11.9 | 2800 | >8.5 | NC | 47.9 | |
NC=not cytotoxic upto 23800 ng/mL (23.8 μg/mL)
NT = not tested
NA = Not active
Selectivity index is the ratio of IC50 in Vero cells to IC50 in P. falciparum (D6 or W2).
Chloroquine. IC50 = 14 ng/mL, Sel. Index = 1700 (D6 clone); IC50 = 100 ng/mL, Sel. Index = 238 (W6 clone).
Artemisinin. IC50 = 15.2 ng/mL, Sel. Index = 1565 (D6 clone); IC50 = 9 ng/mL, Sel. Index = 2644 (W6 clone).
The in vitro cytotoxicity of analogs (series 1-2) was determined against mammalian kidney fibroblast (Vero) cell line (obtained from ATCC) up to a highest concentration of 23.8 μg/mL by neutral red assay.22, 23 None of the compounds were cytotoxic up to the highest test concentration. The selectivity index (ratio of IC50 in Vero cells to IC50 in P. falciparum strain) for all compounds was calculated (Table 1-2). Analogs 21–24 (series 1) with highest antimalarial activity were found to exhibit a very high selectivity index to plasmodial strains (>132 – >79.33 for D6 clone and >79 – 53 for W2 clone) compared to 11.9 (D6 clone) and 8.5 (W2 clone) for PQ indicating their better safety profile.
Hematotoxicity by 8-quinolinamines is caused due to their metabolism to the toxic metabolites, which are unstable and difficult to isolate.24 The analogs of both series were also tested for metabolism-linked methemoglobin toxicity in vitro and % MetHb formation was calculated at 20 μg/mL in comparison to vehicle control.25 All the analogs of series 1 (21–33) induced substantially less % MetHb formation (26.8 – 40.6%) compared to PQ (47.9%) as shown in Table 1. All the tested analogs of series 2 produced almost similar MetHb formation as that of PQ except analog 60 which showed significantly lower 34.2% MetHb formation (Table 2).
The most active analogs 21–24 (series 1) were selected for in vivo evaluation of the blood-schizontocidal antimalarial activity against P. berghei (sensitive strain) in a rodent malaria model as described (Table 3).14 Briefly; mice (6 mice per group) were dosed orally at 100, 50, 25 and 10 mg/kg/day×4 (oral). Chloroquine was used as a positive control at a suppressive dose of 10 mg/kg/day×4 (oral). The negative consisted of untreated (vehicle only) mice in which 100% mortality was observed within 6–8 days, with a mean survival time of 6.2 days. The compounds/vehicle were administered on days 0 – 3 post infection. All compounds produced 100% cure at the primary test dose of 100 mg/kg. Analogs 21–22 and 24 were also curative at the lower test dose of 50 mg/kg, while 23 was found to show suppressive activity. The most potent compound 21 also produced a 100% cure rate at the lower test dose of 25 mg/kg and was suppressive at the lowest test dose of 10 mg/kg (2/6 cures) (Table 3). These results are in agreement with our earlier observation that attachment of a cationic side-chain amino acid led to a considerable increase in antimalarial activity of 8-quinolinamines.15 It can be presumed that these side-chain modified ring-substituted PQ analogs have substantially improved therapeutic index (higher blood-schizontocidal antimalarial activity and reduced MetHb toxicity) possibly due to their reduced penetration into the red cells because of steric hindrance that does not allow destabilization of the red cell membrane, inducing hemolysis, which is the source of main toxicity. At the same time, attachment of an amino acid residue may serve to protect the PQ's primary side-chain amino function against metabolic processes discussed earlier.
Table 3.
In vivo (P. berghei) antimalarial activity of the 8-quinolinamine analogs 21–24
| S. No. | P. berghei (mg/kg/day×4, oral) | |||
|---|---|---|---|---|
| 10 | 25 | 50 | 100 | |
| 21 | (2/6) Active | (6/6) Curative | (6/6) Curative | (6/6) Curative |
| 22 | – | (0/6) Inactive | (6/6) Curative | (6/6) Curative |
| 23 | – | – | (3/6) Active | (6/6) Curative |
| 24 | (0/6) Inactive | (6/6) Curative | (6/6) Curative | |
| Primaquine (PQ) | – | – | – | (0/6) Inactive |
The term “curative” indicates complete elimination of malaria parasites from the body, and animals survive up to day D+60. The term “active” or “suppressive” indicates that all of the treated animals show negative parasitaemia up to D+7. However, by D+60, some mice die, and some survive with complete elimination of parasitaemia as indicated by numbers given in parentheses. The term “inactive” indicates that the treated animals show positive parasitaemia either on D+4 or D+7 and usually die by D+14.
3.1.2. Antileishmanial Activities
Antileishmanial activity of the compounds was tested in vitro against a culture of L. donovani promastigotes by Alamar Blue assay.26, 27 It was interesting to note that 8-quinolinamine analogs 25, 27–29, 32, 57–59, and 62 exhibited stronger antileishmanial activities with IC50 values ranging between 2.7 – 4.6 μg/mL (Table 4) in comparison to the activity of PQ (IC50 = 19.9 μg/mL). The activity was comparable to the standard drug pentamidine (IC50 = 1 μg/mL) used as positive control. Their IC90 values ranged from 6.5 – 18 μg/mL as compared to IC90 of 3.8 μg/mL for pentamidine. However, they were much less potent than amphotericin B (IC50 = 0.19 and IC90 = 0.35 μg/mL).
Table 4.
In vitro antileishmenial (L. donovani) activity of the 8-quinolinamine analogs 21–33 and 51–63
| Leishmania donovani | ||
|---|---|---|
| S. No. | IC50 (μg/mL) | IC90 (μg/mL) |
| 21 | 12 | 37 |
| 22 | 4.6 | 26 |
| 23 | 7.5 | 30 |
| 24 | 16 | 38 |
| 25 | 4 | 18 |
| 26 | 6.5 | 30 |
| 27 | 2.9 | 6.5 |
| 28 | 2.7 | 6.6 |
| 29 | 3 | 6.6 |
| 30 | 9.5 | 32 |
| 31 | 4.5 | 24 |
| 32 | 4 | 18 |
| 33 | 15 | 33 |
| 51 | 17 | 39 |
| 52 | 17 | 37 |
| 53 | 18 | >40 |
| 54 | 19 | >40 |
| 55 | NA | NA |
| 56 | NA | NA |
| 57 | 3.3 | 6.7 |
| 58 | 4.2 | 26 |
| 59 | 4.4 | 26 |
| 60 | 19 | 37 |
| 61 | 19 | 38 |
| 62 | 4.6 | 23 |
| 63 | 6 | 29 |
| Primaquine (PQ) | 19.9 | NA |
IC50 and IC90 are the sample conc that kill 50% and 90% cells compared to vehicle control
NA= no activity
Pentamidine. IC50 = 1 μg/mL, IC90 = 3.8 μg/mL
Amphotericin B. IC50 = 0.19 μg/mL, IC90 = 0.35 μg/mL
3.1.3. Antimicrobial Activities
The antibacterial activities of the 8-quinolinamines 21–33 and 51–63 against methicillin-resistant S. aureus (MRS) and Mycobacterium intracellulare are reported in Table 5 including the positive control Ciprofloxacin. Most of the analogs (e.g. 21–32, 57–59) exhibited promising antibacterial activity against MRS (IC50 = 3 – 15 μg/mL and MIC = 5 – 20 μg/mL). Compounds 57 and 27 were bactericidal at 5 and 10 μg/mL, respectively (Table 5). Analogs 22–24, 27–29 and 32 also showed activity against M. intracellulare (IC50 = 4.5 – 15 μg/mL and MIC = 10 – 20 μg/mL). Analogs 27 and 29 were the most potent against both MRS (IC50 = 3 μg/mL, MIC = 5 μg/mL, MBC = 10 μg/mL) and M. intracellulare (IC50 = 7 μg/mL, MIC = 10 μg/mL, MBC = 20 μg/mL) and were bactericidal to both organisms. Analog 57 was the most bactericidal against MRS with an IC50 of 3.0 μg/mL, MIC and MBC of 5 μg/mL but was inactive against M. intracellulare at the highest test concentration of 20 μg/mL (Table 5).
Table 5.
In vitro antibacterial activities of the 8-quinolinamine analogs 21–33 and 51–63
| S. No. | Methicillin-resistant S. aureus (MRS) | M. intracellulare | ||||
|---|---|---|---|---|---|---|
| IC50 (μg/mL) | MIC (μg/mL) | MBC (μg/mL) | IC50 (μg/mL) | MIC (μg/mL) | MBC (μg/mL) | |
| 21 | 15 | 20 | NA | NA | NA | NA |
| 22 | 8 | 20 | NA | 9 | 20 | NA |
| 23 | 7 | 20 | 20 | 15 | 20 | NA |
| 24 | 9 | 20 | 20 | 7 | 10 | NA |
| 25 | 7 | 10 | 20 | NA | NA | NA |
| 26 | 6 | 10 | 20 | NA | NA | NA |
| 27 | 3 | 5 | 10 | 7 | 10 | 20 |
| 28 | 7 | 10 | NA | 10 | 20 | NA |
| 29 | 3 | 5 | 20 | 4.5 | 10 | 20 |
| 30 | 10 | 20 | NA | NA | NA | NA |
| 31 | 6.5 | 10 | NA | NA | NA | NA |
| 32 | 4 | 10 | NA | 10 | 20 | NA |
| 33 | 15 | NA | NA | NA | NA | NA |
| 51 | 15 | NA | NA | NA | NA | NA |
| 52 | 15 | 20 | NA | NA | NA | NA |
| 53 | 15 | NA | NA | NA | NA | NA |
| 54 | NA | NA | NA | NA | NA | NA |
| 55 | NA | NA | NA | NA | NA | NA |
| 56 | NA | NA | NA | NA | NA | NA |
| 57 | 3 | 5 | 5 | NA | NA | NA |
| 58 | 6.5 | 10 | NA | NA | NA | NA |
| 59 | 6 | 10 | NA | NA | NA | NA |
| 60 | NA | NA | NA | NA | NA | NA |
| 61 | NA | NA | NA | NA | NA | NA |
| 62 | 6.5 | NA | NA | NA | NA | NA |
| 63 | NA | NA | NA | NA | NA | NA |
IC50 = the concentration (μg/mL) that affords 50% growth inhibition
MIC = Minimum Inhibitory Concentration (the lowest concentration in μg/mL that allows no detectable growth)
MBC = Minimum Bactericidal Concentration (the lowest concentration in μg/mL that kills the organism)
NA= no activity at the highest test concentration of 20μg/mL
Ciprofloxacin. IC50 = 0.09 μg/mL, MIC = 0.31 μg/mL, MBC = 2.5 μg/mL (MRS); IC50 = 0.3 μg/mL, MIC = 0.63 μg/mL, MBC = 2.5 μg/mL (Mi).
The antifungal of the 8-quinolinamines 21–33 and 51–63 against the opportunistic yeast Candida albicans, C. glabrata, C. krusei, and Cryptococcus neoformans, along with the positive control Amphotericin B, are summarized in Table 6. All compounds were inactive at 20 μg/mL against the filamentous fungus Aspergillus fumigatus (data not shown).
Table 6.
In vitro antifungal activities of the 8-quinolinamine analogs 21–33 and 51–63
| S. NO. | Candida albicans | Candida glabrata | Candida krusei | Cryptococcus neoformans | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IC50 (μg/mL) | MIC (μg/mL) | MFC (μg/mL) | IC50 (μg/mL) | MIC (μg/mL) | MFC (μg/mL) | IC50 (μg/mL) | MIC (μg/mL) | MFC (μg/mL) | IC50 (μg/mL) | MIC (μg/mL) | MFC (μg/mL) | |
| 21 | NA | NA | NA | NA | NA | NA | 15 | 20 | 20 | 3.5 | 5 | 5 |
| 22 | NA | NA | NA | 15 | NA | NA | 8 | 20 | 20 | 3.5 | 5 | 5 |
| 23 | NA | NA | NA | 15 | 20 | NA | 8.5 | 20 | 20 | 3.5 | 5 | 5 |
| 24 | NA | NA | NA | NA | NA | NA | 15 | 20 | 20 | 3.5 | 10 | 10 |
| 25 | NA | NA | NA | NA | NA | NA | 9.5 | 20 | 20 | 3 | 5 | 5 |
| 26 | 15 | 20 | NA | 15 | NA | NA | 7.5 | 10 | 20 | 3.5 | 5 | 10 |
| 27 | 15 | 20 | NA | 15 | 20 | NA | 10 | 20 | 20 | 3.5 | 5 | 5 |
| 28 | NA | NA | NA | NA | NA | NA | 15 | NA | NA | 3.5 | 10 | 10 |
| 29 | 15 | 20 | NA | 7 | 20 | 20 | 7.5 | 10 | 20 | 3 | 5 | 10 |
| 30 | NA | NA | NA | NA | NA | NA | NA | NA | NA | 10 | 20 | 20 |
| 31 | 15 | 20 | NA | 15 | NA | NA | 15 | 20 | 20 | 6.5 | 10 | 10 |
| 32 | NA | NA | NA | 7.5 | 20 | 20 | 20 | NA | NA | 3.5 | 10 | 10 |
| 33 | NA | NA | NA | NA | NA | NA | NA | NA | NA | 6.5 | 10 | 10 |
| 51 | NA | NA | NA | NA | NA | NA | NA | NA | NA | 6 | 10 | 20 |
| 52 | NA | NA | NA | NA | NA | NA | 15 | NA | NA | 4.5 | 20 | 20 |
| 53 | NA | NA | NA | NA | NA | NA | NA | NA | NA | 6 | 10 | 10 |
| 54 | NA | NA | NA | NA | NA | NA | NA | NA | NA | 15 | 20 | 20 |
| 55 | NA | NA | NA | NA | NA | NA | NA | NA | NA | 10 | 20 | 20 |
| 56 | NA | NA | NA | NA | NA | NA | NA | NA | NA | 10 | 20 | 20 |
| 57 | 10 | 20 | 20 | 4 | 10 | 10 | 7 | 10 | 10 | 3.5 | 5 | 5 |
| 58 | NA | NA | NA | 15 | NA | NA | 15 | 20 | 20 | 3.5 | 5 | 5 |
| 59 | NA | NA | NA | 10 | 20 | 20 | 8 | 20 | 20 | 3.5 | 5 | 10 |
| 60 | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| 61 | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| 62 | NA | NA | NA | 15 | NA | NA | NA | NA | NA | 3.5 | 5 | 5 |
| 63 | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
IC50 = the concentration (μg/mL) that affords 50% growth inhibition
MIC = Minimum Inhibitory Concentration (the lowest concentration in μg/mL that allows no detectable growth)
MFC = Minimum Fungicidal Concentration (the lowest concentration in μg/mL that kills the organism)
NA= no activity at the highest test concentration of 20μg/mL
Amphotericin B. IC50 = 0.25 μg/mL, MIC = 0.63 μg/mL, MFC = 1.25 μg/mL (Ca); IC50 = 0.07 μg/mL, MIC = 0.31 μg/mL, MFC = 0.625 μg/mL (Cg); IC50 = 0.6 μg/mL, MIC = 1.25 μg/mL, MFC = 1.25 μg/mL (Ck); IC50 = 0.75 μg/mL, MIC = 1.25 μg/mL, MFC = 1.5 μg/mL (Cn).
Analog 57 was the most potent of all 8-quinolinamines tested and produced fungicidal activities against C. albicans (IC50 = 10 μg/mL, MIC = 20 μg/mL, MFC = 20 μg/mL), C. glabrata (IC50 = 4 μg/mL, MIC = 10 μg/mL, MFC = 10 μg/mL), C. krusei (IC50 = 7 μg/mL, MIC = 10 μg/mL, MFC = 10 μg/mL) and C. neoformans (IC50 = 3.5 μg/mL, MIC = 5 μg/mL, MFC = 5 μg/mL). All analogs (excluding 60, 61 and 63) exhibited promising activity against C. neoformans with MFCs as low as 5 μg/mL (analogs 21–23, 25, 27, 57–58, 62) (Table 6).
4. Conclusions
We have reported synthesis and biological activities of two new series of 8-quinolinamines. Several of the reported analogs have exhibited potent in vitro activity against drug-sensitive and drug-resistant malaria parasites. Analogs 21–24 have also displayed promising antimalarial activity in vivo in a P. berghei-mouse malaria model. The most potent compound 21 was found curative at 25 mg/kg and suppressive at 10 mg/kg. The potent 8-quinolinamines were also found to exhibit high selectivity index and a significantly reduced methemoglobin toxicity, indicating their better safety profiles than primaquine. Several analogs also displayed high antileishmanial activities comparable to standard drug pentamidine and superior to that of primaquine. The compounds were also evaluated against a panel of pathogenic bacteria and fungi and displayed promising activities. In conclusion, the results of this study confirm that 8-quinolinamines are a versatile class of compounds that exhibit broad-spectrum of activities against parasitic and infectious diseases. The careful structural optimization of this class of compounds could lead to promising agents with utility in treatment of malaria, leishmaniasis and opportunistic infections.
5. Experimental
5.1. Synthesis
Melting points were recorded on Mettler DSC 851 or capillary melting point apparatus and are uncorrected. 1H spectra were recorded on 300 MHz Bruker FT-NMR (Avance DPX300) spectrometer using tetramethylsilane as internal standard and the chemical shifts are reported in δ units. Mass spectra were recorded on HRMS (Finnigan Mat LCQ spectrometer) (APCI/ESI). Elemental analyses were recorded on Elementar Vario EL spectrometer. All chromatographic purification was performed with silica gel 60 (230–400 mesh), whereas all TLC (silica gel) development was performed on silica gel coated (Merck Kiesel 60 F254, 0.2 mm thickness) sheets. All chemicals were purchased from Aldrich Chemical Ltd. (Milwaukee, WI, USA). Solvents used for the chemical synthesis acquired from commercial sources were of analytical grade, and were used without further purification unless otherwise stated.
5.2. General method for the synthesis of S/R)-{alkoxycarbonylamino-1-[4-(2-tert-butyl-6-methoxy-8-quinolylamino)pentylcarbamoyl]alkyl}carbamic acid benzyl/tert-butyl esters (8–20) and (S/R-{alkoxycarbonylamino-1-[4-(4-ethyl-6-methoxy-5-pentyloxy-8-quinolylamino)pentylcarbamoyl]alkyl}carbamic acid benzyl/tert-butyl esters (38–50)
To an ice cooled stirred solution of N8-(4-amino-1-methylbutyl)-2-(tert-butyl)-6-methoxy-8-quinolinamine14 (2, 1 mmol) or N8-(4-amino-1-methylbutyl)-4-ethyl-6-methoxy-5-pentyloxy-8-quinolinamine17 (3, 1 mmol) and suitably side-chain protected Cbz/Boc-L/D-amino acid (1.1 mmol) in CH2Cl2 (15 mL), DCC (1.1 mmol) was added. Reaction mixture was allowed to attain ambient temperature and stirring was continued for another 6 h. The solvent was removed under reduced pressure and ethyl acetate (20 mL) was added to the residue. The reaction mixture was kept in refrigerator overnight and the separated 1,3-dicyclohexylurea (DCU) was filtered. Filtrate was washed with saturated sodium bicarbonate solution (3 × 5 mL) followed by water (2 × 5 mL), and dried over Na2SO4. The solvent was removed under reduced pressure to afford the crude product, which was purified by flash column chromatography on silica gel using EtOAc/hexanes (30:70) to afford the (S/R)-{alkoxycarbonylamino-1-[4-(2-tert-butyl-6-methoxy-8-quinolylamino)pentylcarbamoyl]-alkyl}carbamic acid benzyl/tert-butyl esters (8–20) and (S/R)-{alkoxycarbonylamino-1-[4-(4-ethyl-6-methoxy-5-pentyloxy-8-quinolylamino)pentylcarbamoyl]alkyl}carbamic acid benzyl/tert-butyl esters (38–50).
5.2.1. (R)-{5-Benzyloxycarbonylamino-1-[4-(2-tert-butyl-6-methoxy-8-quinolyl-amino)pentylcarbamoyl]pentyl}carbamic acid benzyl ester (8)
Yield: 85%; oil; IR (neat): 3431, 3019, 2855, 1713, 1675 cm−1; 1H NMR (CDCl3): δ 7.86 (d, 1H, J = 8.60 Hz), 7.40 (d, 1H, J = 8.55 Hz), 7.31 (m, 10H), 6.30 (d, 1H, J = 2.40 Hz), 6.24 (d, 1H, J = 2.40 Hz), 5.04 (m, 4H), 4.10 (m, 1H), 3.84 (s, 3H), 3.58 (m, 2H), 3.27 (m, 2H), 3.12 (m, 2H), 1.41 (m, 10H), 1.40 (s, 9H), 0.89 (d, 3H, J = 6.50 Hz); APCIMS: m/z 712 (M+1); Anal. Calcd for C41H53N5O6 (711.9): C, 69.17; H, 7.50; N, 9.84. Found: C, 69.34; H, 7.34; N, 9.99.
5.2.2. (R)-{4-tert-Butoxycarbonylamino-1-[4-(2-tert-butyl-6-methoxy-8-quinolyl-amino)pentylcarbamoyl]butyl}carbamic acid tert-butyl ester (9)
Yield: 75%; oil; IR (neat): 3362, 2965, 2863, 1715, 1694 cm−1; 1H NMR (CDCl3): δ 7.86 (d, 1H, J = 8.60 Hz), 7.40 (d, 1H, J = 8.58 Hz), 6.30 (d, 1H, J = 2.30 Hz), 6.24 (s, 1H, J = 2.30 Hz), 5.14 (brs, 1H), 4.64 (brs, 1H), 4.10 (m, 1H), 3.87 (s, 3H), 3.32 (t, 2H, J = 6.60 Hz), 3.05 (m, 1H), 1.65 (m, 4H), 1.59 (m, 6H), 1.42 (m, 18H), 1.40 (s, 9H), 1.29 (d, 3H, J = 6.40 Hz); APCIMS: m/z 630 (M+1); Anal. Calcd for C34H55N5O6 (629.8): C, 64.84; H, 8.80; N, 11.12. Found: C, 64.54; H, 8.99; N, 10.99.
5.2.3. (S)-{4-(N,N’-Bisbenzyloxycarbonylguanidino)-1-[4-(2-tert-butyl-6-methoxy-8-quinolylamino)pentylcarbamoyl]butyl}carbamic acid tert-butyl ester (10)
Yield: 67%; mp. 113-115 °C; IR (KBr): 3384, 2965, 2843, 1718 cm−1; 1H NMR (CDCl3): δ 9.40 (brs, 2H), 7.86 (d, 1H, J = 8.60 Hz), 7.40 (d, 1H, J = 8.60 Hz), 7.35 (m, 10H), 6.55 (brs, 1H), 6.30 (s, 1H), 6.21 (s, 1H), 6.08 (brs, 1H), 5.21 (m, 2H), 5.09 (m, 2H), 4.96 (m, 1H), 4.29 (m, 2H), 3.86 (s, 3H), 3.65 (m, 2H), 3.47 (t, 2H, J = 6.70 Hz), 2.97 (m, 1H), 1.71 (m, 6H), 1.42 (s, 9H), 1.38 (s, 9H), 1.26 (d, 3H, J = 6.0 Hz); APCIMS: m/z 841 (M+1); Anal. Calcd for C46H61N7O8 (840.0): C, 65.77; H, 7.32; N, 11.67. Found: C, 66.03; H, 6.99; N, 11.57.
5.2.4. (R)-{4-(N,N’-Bisbenzyloxycarbonylguanidino)-1-[4-(2-tert-butyl-6-methoxy-8-quinolylamino)pentylcarbamoyl]butyl}carbamic acid tert-butyl ester (11)
Yield: 70%; mp. 114-116 °C; IR (KBr): 3390, 2970, 2840, 1688, 1727 cm−1; 1H NMR (CDCl3): δ 9.43 (brs, 1H), 9.40 (brs, 1H), 7.84 (d, 1H, J = 8.70 Hz), 7.42 (d, 1H, J = 8.70 Hz), 7.33 (m, 10H), 6.51 (brs, 1H), 6.32 (s, 1H), 6.25 (s, 1H), 6.10 (brs, 1H), 5.20 (m, 2H), 5.14 (m, 2H), 4.93 (m, 1H), 4.23 (m, 2H), 3.80 (s, 3H), 3.67 (m, 2H), 3.44 (t, 2H, J = 6.70 Hz), 2.99 (m, 1H), 1.73 (m, 6H), 1.42 (s, 9H), 1.40 (s, 9H), 1.25 (d, 3H, J = 6.0 Hz); APCIMS: m/z 841 (M+1); Anal. Calcd for C46H61N7O8 (840.0): C, 65.77; H, 7.32; N, 11.67. Found: C, 65.70; H, 7.55; N, 11.41.
5.2.5. (S)-{2-(3-Benzyloxymethyl-1H-imidazol-4-yl)-1-[4-(2-tert-butyl-6-methoxy-8-quinolylamino)pentylcarbamoyl]ethyl}carbamic acid tert-butyl ester (12)
Yield: 85%; oil; IR (neat): 3476, 2965, 2863, 1698, 1091 cm−1; 1H NMR (CDCl3): δ 7.86 (d, 1H, J = 8.60 Hz), 7.48 (s, 1H), 7.44 (d, 1H, J = 8.60 Hz), 7.29 (m, 5H), 6.88 (s, 1H), 6.30 (s, 1H), 6.23 (s, 1H), 6.10 (brs, 1H), 5.98 (m, 2H), 5.33 (brs, 1H), 5.21 (m, 2H), 4.47 (m, 1H), 3.85 (s, 3H), 3.13 (m, 2H), 3.05 (m, 3H), 1.65 (m, 4H), 1.42 (s, 9H), 1.38 (s, 9H), 1.28 (d, 3H, J = 6.50 Hz); APCIMS: m/z 673 (M+1); Anal. Calcd for C38H52N6O5 (672.9): C, 67.83; H, 7.79; N, 12.49. Found: C, 67.96; H, 7.51; N, 12.31.
5.2.6. (R)-{2-(3-Benzyloxymethyl-1H-imidazol-4-yl)-1-[4-(2-tert-butyl-6-methoxy-8-quinolylamino)pentylcarbamoyl]ethyl}carbamic acid tert-butyl ester (13)
Yield: 90%; oil; IR (neat): 3426, 2965, 2870, 1726 cm−1; 1H NMR (CDCl3): δ 7.90 (d, 1H, J = 8.60 Hz), 7.50 (s, 1H), 7.44 (d, 1H, J = 8.60 Hz), 7.30 (m, 5H), 6.90 (s, 1H), 6.32 (s, 1H), 6.24 (s, 1H), 6.12 (brs, 1H), 5.95 (m, 2H), 5.34 (brs, 1H), 5.22 (m, 2H), 4.50 (m, 1H), 3.84 (s, 3H), 3.17 (m, 2H), 3.08 (m, 3H), 1.67 (m, 4H), 1.40 (s, 9H), 1.40 (s, 9H), 1.30 (d, 3H, J = 6.30 Hz); APCIMS: m/z 673 (M+1); Anal. Calcd for C38H52N6O5 (672.9): C, 67.83; H, 7.79; N, 12.49. Found: C, 67.53; H, 7.88; N, 12.20.
5.2.7. (S)-[1-[4-(2-tert-Butyl-6-methoxy-8-quinolylamino)pentylcarbamoyl]-2-(1H-indol-3-yl)ethyl]carbamic acid tert-butyl ester (14)
Yield: 96%; oil; IR (neat): 3384, 2927, 2854, 1694 cm−1; 1H NMR (CDCl3): δ 7.91 (d, 1H, J = 8.60 Hz), 7.47 (d, 1H, J = 8.60 Hz), 7.10 (m, 5H), 6.89 (d, 1H, J = 5.70 Hz), 6.35 (s, 1H), 6.22 (s, 1H), 6.05 (brs, 1H), 5.39 (brs, 1H), 4.36 (m, 1H), 3.90 (s, 3H), 3.46 (m, 2H), 3.28 (m, 2H), 3.03 (m, 1H), 1.55 (m, 4H), 1.42 (s, 9H), 1.25 (s, 9H), 0.88 (d, 3H, J = 6.50 Hz); APCIMS: m/z 602 (M+1); Anal. Calcd for C35H47N5O4 (601.8): C, 69.86; H, 7.87; N, 11.64. Found: C, 69.55; H, 7.63; N, 12.03.
5.2.8. (S)-{1-[4-(2-tert-Butyl-6-methoxy-8-quinolylamino)pentylcarbamoyl]-3-methyl-sulfanylpropyl}carbamic acid tert-butyl ester (15)
Yield: 90%; oil; IR (neat): 3345, 2965, 2870, 1694 cm−1; 1H NMR (CDCl3): δ 7.86 (d, 1H, J = 8.60 Hz), 7.40 (d, 1H, J = 8.60 Hz), 6.30 (d, 1H, J = 2.40 Hz), 6.24 (d, 1H, J = 2.40 Hz), 6.14 (brs, 1H), 5.18 (brs, 1H), 4.17 (m, 1H), 3.86 (s, 3H), 3.60 (m, 1H), 3.29 (m, 2H), 2.55 (m, 2H), 2.06 (m, 2H), 1.65 (m, 7H), 1.42 (s, 9H), 1.40 (s, 9H), 1.22 (d, 3H, J = 6.40 Hz); APCIMS: m/z 547 (M+1); Anal. Calcd for C29H46N4O4S (546.8): C, 63.70; H, 8.48; N, 10.25. Found: C, 63.31; H, 8.78; N, 10.12.
5.2.9. (S)-{1-[4-(2-tert-Butyl-6-methoxy-8-quinolylamino)pentylcarbamoyl]-2-phenyl-ethyl}carbamic acid tert-butyl ester (16)
Yield: 92%; oil; IR (neat): 3383, 2965, 2875, 1724, 1678 cm−1; 1H NMR (CDCl3): δ 7.86 (d, 1H, J = 8.60 Hz), 7.40 (d, 1H, J = 8.60 Hz), 7.22 (m, 5H), 6.30 (s, 1H), 6.22 (s, 1H), 6.09 (brs, 1H), 5.68 (brs, 1H,), 4.23 (m, 1H), 3.86 (s, 3H), 3.53 (m, 1H), 3.20 (m, 2H), 2.98 (m, 2H), 1.64 (m, 4H), 1.42 (s, 9H), 1.37 (s, 9H), 1.25 (d, 3H, J = 6.50 Hz); APCIMS: m/z 563 (M+1); Anal. Calcd for C33H46N4O4 (562.7): C, 70.43; H, 8.24; N, 9.96. Found: C, 70.79; H, 8.18; N, 10.24.
5.2.10. (S)-{2-(4-Benzyloxyphenyl)-1-[4-(2-tert-butyl-6-methoxy-8-quinolylamino)-pentylcarbamoyl]ethyl}carbamic acid tert-butyl ester (17)
Yield: 97%; oil; IR (neat): 3324, 2929, 2854, 1690, 1124 cm−1; 1H NMR (CDCl3): δ 7.86 (d, 1H, J = 8.60 Hz), 7.40 (d, 1H, J = 8.60 Hz), 7.24 (m, 5H), 7.10 (d, 2H, J = 7.50 Hz), 6.86 (d, 2H, J = 7.50 Hz), 6.30 (d, 1H, J = 2.30 Hz), 6.22 (d, 1H, J = 2.30 Hz), 6.09 (brs, 1H), 5.73 (brs, 1H), 5.03 (m, 1H), 4.93 (m, 2H), 3.86 (s, 3H), 3.55 (m, 2H), 3.20 (m, 2H), 2.98 (m, 1H), 1.64(m, 4H), 1.42 (s, 9H), 1.37 (s, 9H), 1.26 (d, 3H, J = 6.48 Hz); APCIMS: m/z 669 (M+1); Anal. Calcd for C40H52N4O5 (668.9): C, 71.83; H, 7.84; N, 8.38. Found: C, 71.66; H, 7.98; N, 8.14.
5.2.11. (S)-{2-Benzyloxy-1-[4-(2-tert-butyl-6-methoxy-8-quinolylamino)pentyl-carbamoyl]ethyl}carbamic acid tert-butyl ester (18)
Yield: 80%; oil; IR (neat): 3372, 2966, 2848, 1707 cm−1; 1H NMR (CDCl3): δ 7.86 (d, 1H, J = 8.58 Hz), 7.40 (d, 1H, J = 8.56 Hz), 7.33 (m, 5H), 6.44 (brs, 1H), 6.30 (d, 1H, J = 2.20 Hz), 6.24 (s, 1H), 6.12 (brs, 1H), 5.40 (brs, 1H), 4.52 (m, 2H), 4.21 (m, 1H), 3.86 (s, 3H), 3.52 (m, 2H), 3.33 (m, 1H), 3.29 (d, 2H, J = 6.36 Hz), 1.64 (m, 4H), 1.42 (s, 9H), 1.39 (s, 9H), 1.26 (d, 3H, J = 6.48Hz); APCIMS: m/z 593 (M+1); Anal. Calcd for C34H48N4O5 (592.8): C, 68.89; H, 8.16; N, 9.45. Found: C, 69.12; H, 7.99; N, 9.17.
5.2.12. (R)-{2-Benzylsulfanyl-1-[4-(2-tert-butyl-6-methoxy-8-quinolylamino)pentyl-carbamoyl]ethyl}carbamic acid tert-butyl ester (19)
Yield: 95%; oil; IR (neat): 3324, 2959, 2856, 1659 cm−1; 1H NMR (CDCl3): δ 7.86 (d, 1H, J = 8.58 Hz), 7.43 (d, 1H, J = 8.58 Hz), 7.30 (m, 5H), 6.30 (d, 1H, J = 2.28 Hz), 6.25 (d, 1H, J = 2.21 Hz), 6.14 (m, 2H), 5.26 (brs, 1H), 4.15 (m, 1H), 3.86 (s, 3H), 3.59 (m, 2H), 3.30 (m, 2H), 2.78 (m, 1H), 1.64 (m, 4H), 1.41 (s, 9H), 1.37 (s, 9H), 1.25 (d, 3H, J = 6.48 Hz); APCIMS: m/z 609 (M+1); Anal. Calcd for C34H48N4O4S (608.9): C, 67.07; H, 7.95; N, 9.20. Found: C, 69.12; H, 7.99; N, 9.17.
5.2.13. (R)-{1-[4-(2-tert-Butyl-6-methoxy-8-quinolylamino)pentylcarbamoyl]-2-trityl-sulfanylethyl}carbamic acid tert-butyl ester (20)
Yield: 90%; oil; IR (neat): 3324, 2929, 2856, 1662 cm−1; 1H NMR (CDCl3): δ 7.86 (d, 1H, J = 8.37 Hz), 7.34 (m, 15H), 7.21 (d, 1H, J = 8.41 Hz), 6.30 (s, 1H), 6.22 (s, 1H), 6.09 (brs, 1H), 5.98 (brs, 1H), 4.81 (m, 1H), 3.86 (s, 3H), 3.52 (m, 2H), 3.22 (m, 2H), 2.50 (m, 1H), 1.61 (m, 4H), 1.41 (s, 9H), 1.37 (s, 9H), 1.25 (d, 3H, J = 5.13 Hz); APCIMS: m/z 762 (M+1); Anal. Calcd for C46H56N4O4S (761.0): C, 72.60; H, 7.42; N, 7.36. Found: C, 72.51; H, 7.64; N, 7.57.
5.2.14. (R)-{5-Benzyloxycarbonylamino-1-[4-(4-ethyl-6-methoxy-5-pentyloxy-8-quinolyl-amino)pentylcarbamoyl]pentyl}carbamic acid benzyl ester (38)
Yield: 84%; oil; IR (neat): 3303, 1684 cm−1; 1H NMR (CDCl3): δ 8.38 (m, 1H), 7.31 (m, 10H), 7.13 (m, 1H), 6.48 (s, 1H), 5.07 (m, 4H), 4.13 (m, 1H), 3.97 (s, 3H), 3.86 (t, 2H, J = 7.0 Hz), 3.64 (m, 1H), 3.20 (m, 6H), 1.56 (m, 15H), 1.27 (m, 6H), 0.92 (t, 3H, J = 7.2 Hz); APCIMS: m/z 770.4 (M+1); Anal. Calcd for C44H59N5O7 (769.7): C, 68.64; H, 7.72; N, 9.10. Found: C, 68.95; H, 7.39; N, 9.47.
5.2.15.(R)-{4-tert-Butoxycarbonylamino-1-[4-(4-ethyl-6-methoxy-5-pentyloxy-8-quinolylamino)pentylcarbamoyl]butyl}carbamic acid tert-butyl ester (39)
Yield: 67%; oil; IR (neat): 3333, 1694 cm−1; 1H NMR (CDCl3): δ 8.38 (d, 1H, J = 4.4 Hz), 7.12 (d, 1H, J = 4.3 Hz), ), 6.51 (brs, 1H), 6.43 (s, 1H), 6.06 (brs, 1H), 5.15 (brs, 1H), 4.67 (brs, 1H), 4.12 (m, 1H), 3.96 (s, 3H), 3.87 (t, 2H, J = 6.9 Hz), 3.63 (m, 1H), 3.27-3.20 (m, 6H), 1.70 (m, 14H), 1.39 (s, 18H), 1.28 (m, 6H), 0.94 (t, 3H, J = 7.0 Hz); APCIMS: m/z 688 (M+1); Anal. Calcd for C37H61N5O7 (687.9): C, 64.60; H, 8.94; N, 10.18. Found: C, 64.31; H, 8.55; N, 10.37.
5.2.16. (S)-{4-(N,N’-Bisbenzyloxycarbonylguanidino)-1-[4-(4-ethyl-6-methoxy-5-pentyloxy-8-quinolylamino)pentylcarbamoyl]butyl}carbamic acid benzyl ester (40)
Yield: 77%; mp. 120-122 °C; IR (KBr): 3384, 3292, 1704, 1642 cm−1; 1H NMR (CDCl3): δ 9.42 (brs, 1H), 9.29 (brs, 1H), 8.38 (d, 1H, J = 4.0 Hz), 7.34 (m, 15H), 7.11 (d, 1H, J = 3.60 Hz), 6.57 (brs, 1H), 6.38 (s, 1H), 6.27 (brs, 1H), 6.01 (brs, 1H), 5.20 (s, 2H), 5.11 (m, 4H), 4.31 (m, 1H), 3.95 (s, 3H), 3.86 (t, 2H, J = 6.70 Hz), 3.51 (m, 1H), 3.37 (m, 2H), 2.92 (m, 2H), 1.74 (m, 6H), 1.38 (m, 8H), 1.30 (m, 6H), 1.19 (d, 2H, J = 6.0 Hz), 0.92 (t, 3H, J = 6.80 Hz); APCIMS: m/z 933 (M+1); Anal. Calcd for C52H65N7O9 (932.1): C, 67.00; H, 7.03; N, 10.50. Found: C, 67.19; H, 7.30; N, 10.32.
5.2.17. (R)-{4-(N,N’-Bisbenzyloxycarbonylguanidino)-1-[4-(4-ethyl-6-methoxy-5-pentyloxy-8-quinolylamino)pentylcarbamoyl]butyl}carbamic acid benzyl ester (41)
Yield: 75%; mp. 120-122 °C; IR (KBr): 3385, 3292, 1716, 1623 cm−1; 1H NMR (CDCl3): δ 9.41 (brs, 1H), 9.29 (brs, 1H), 8.39 (d, 1H, J = 4.30 Hz), 7.35 (m, 15H), 7.11 (d, 1H, J = 4.20 Hz), 6.58 (brs, 1H), 6.39 (s, 1H), 6.27 (brs, 1H), 6.02 (brs, 1H), 5.20 (s, 2H), 5.11 (m, 4H), 4.31 (m, 1H), 3.95 (s, 3H), 3.86 (t, 2H, J = 7.10 Hz), 3.52 (m, 1H), 3.25 (m, 2H), 2.92 (m, 2H), 1.77 (m, 6H), 1.44-1.36 (m, 8H), 1.31 (m, 6H), 1.19 (d, 2H, J = 6.20 Hz), 0.94 (t, 3H, J = 7.0 Hz); APCIMS: m/z 933 (M+1); Anal. Calcd for C52H65N7O9 (932.1): C, 67.00; H, 7.03; N, 10.50. Found: C, 66.77; H, 6.88; N, 10.89.
5.2.18. (S)-{2-(1-Benzyloxymethyl-1H-imidazol-4-yl)-1-[4-(4-ethyl-6-methoxy-5-pentyloxy-8-quinolylamino)-pentylcarbamoyl]ethyl}carbamic acid tert-butyl ester (42)
Yield: 73%; oil; IR (neat): 3332, 1662 cm-1; 1H NMR (CDCl3): δ 8.39 (d, 1H, J = 4.0 Hz), 7.50 (d, 1H, J = 5.0 Hz), 7.30 (m, 5H), 7.12 (d, 1H, J = 4.4 Hz), 6.89 (s, 1H), 6.42 (s, 1H), 6.04 (brs, 1H), 5.31 (m, 3H), 4.49 (s, 2H), 4.34 (m, 1H), 3.97 (s, 3H), 3.87 (t, 2H, J = 7.0 Hz), 3.57 (m, 1H), 3.22 (m, 2H), 3.14 (m, 2H), 3.06 (d, 2H, J = 7.0 Hz), 1.83 (m, 2H), 1.45 (m, 17H), 1.28 (m, 6H), 0.94 (t, 3H, J = 7.0 Hz); APCIMS: m/z 731 (M+1); Anal. Calcd for C 41H58N6O6 (730.9): C, 67.37; H, 8.00; N, 11.50. Found: C, 67.76; H, 7.77; N, 11.21.
5.2.19. (R)-{2-(1-Benzyloxymethyl-1H-imidazol-4-yl)-1-[4-(4-ethyl-6-methoxy-5-pentyloxy-8-quinolylamino)pentylcarbamoyl]ethyl}carbamic acid tert-butyl ester (43)
Yield: 67%; oil; IR (neat): 3316, 1675 cm−1; 1H NMR (CDCl3): δ 8.38 (d, 1H, J = 4.0 Hz), 7.48 (d, 1H, J = 5.3 Hz), 7.29 (m, 5H), 7.10 (d, 1H, J = 4.2 Hz), 6.89 (s, 1H), 6.42 (s, 1H), 6.06 (brs, 1H), 5.29 (m, 3H), 4.48 (s, 2H), 4.32 (m, 1H), 3.96 (s, 3H), 3.87 (t, 2H, J = 7.0 Hz), 3.57 (m, 1H), 3.23 (m, 2H), 3.13 (m, 2H), 3.05 (d, 2H, J = 7.1 Hz), 1.83 (t, 2H), 1.46 (m, 17H), 1.27 (m, 6H), 0.94 (t, 3H, J = 6.9 Hz); APCIMS: m/z 731 (M+1); Anal. Calcd for C41H58N6O6 (730.9): C, 67.37; H, 8.00; N, 11.50. Found: C, 68.00; H, 8.34; N, 11.75.
5.2.20. (S)-[1-[4-(4-Ethyl-6-methoxy-5-pentyloxy-8-quinolylamino)pentylcarbamoyl]-2-(1H-indol-3-yl)ethyl]carbamic acid tert-butyl ester (44)
Yield: 76%; mp. 46-48 °C; IR (KBr): 3320, 1701, 1659 cm−1; 1H NMR (CDCl3): δ 9.53 (brs, 1H), 9.05 (brs, 1H), 8.48 (d, 1H, J = 4.5 Hz), 7.70 (m, 2H), 7.30-6.98 (m, 5H), 6.42 (s, 1H), 5.85 (s, 1H), 5.53 (brs, 1H), 4.44 (m, 1H), 3.97 (s, 3H), 3.91 (t, 2H, J = 6.9 Hz), 3.51 (m, 1H), 3.30 (m, 4H), 3.06 (m, 2H), 1.85 (m, 7H), 1.65 (m, 6H), 1.40 (s, 9H), 1.32 (m, 6H), 0.95 (t, 3H, J = 7.0 Hz); APCIMS: m/z 660 (M+1); Anal. Calcd for C38H53N5O5 (659.9): C, 69.17; H, 8.10; N, 10.61. Found: C, 69.45; H, 8.02; N, 10.31.
5.2.21. (S)-1-[4-(4-Ethyl-6-methoxy-5-pentyloxy-8-quinolylamino)pentylcarbamoyl]-2-phenylethyl}carbamic acid tert-butyl ester (45)
Yield 73%; oil; IR (neat): 3392, 1718, 1668 cm−1; 1H NMR (CDCl3): δ 8.39 (d, 1H, J = 3.4 Hz), 7.18 (m, 6H), 6.41 (s, 1H), 6.03 (brs, 1H), 5.87 (brs, 1H), 5.11 (brs, 1H), 4.25 (m, 1H), 3.96 (s, 3H), 3.87 (t, 2H, J = 6.7 Hz), 3.58 (m, 1H), 3.23 (m, 4H), 3.02 (m, 2H), 1.83 (m, 2H), 1.53 (m, 8H), 1.38 (s, 9H), 1.30 (m, 6H), 0.94 (t, 3H, J = 6.7 Hz); APCIMS: m/z 621 (M+1); Anal. Calcd for C36H52N4O5 (620.8): C, 69.65; H, 8.44; N, 9.02. Found: C, 69.78; H, 8.23; N, 9.34.
5.2.22. (S-{2-(4-Benzyloxyphenyl)-1-[4-(4-ethyl-6-methoxy-5-pentyloxy-8-quinolyl-amino)pentylcarbamoyl]ethyl}carbamic acid tert-butyl ester (46)
Yield 97%; mp. 60-62 °C; IR (KBr): 3335, 1684, 1654 cm−1; 1H NMR (CDCl3): δ 8.38 (d, 1H, J = 4.2 Hz), 7.35 (m, 5H), 7.09 (m, 3H), 6.87 (m, 2H), 6.42 (s, 1H), 6.02 (brs, 1H), 5.74 (brs, 1H), 5.03 (brs, 1H), 4.96 (m, 2H), 4.19 (m, 1H), 3.95 (s, 3H), 3.86 (t, 2H, J = 6.8 Hz), 3.59 (m, 1H), 3.22 (m, 4H), 2.99 (m, 2H), 1.77 (m, 2H), 1.60 (m, 8H), 1.39 (s, 9H), 1.27 (m, 6H), 0.94 (t, 3H, J = 7.0 Hz); APCIMS: m/z 727 (M+1); Anal. Calcd for C43H58N4O6 (726.9): C, 71.05; H, 8.04; N, 7.71. Found: C, 71.41; H, 8.21; N, 7.48.
5.2.23. (S)-{1-[4-(4-Ethyl-6-methoxy-5-pentyloxy-8-quinolylamino)pentylcarbamoyl]-3-methylsulfanylpropyl}carbamic acid tert-butyl ester (47)
Yield 85%; mp. 46-48 °C; IR (KBr): 3320, 1693, 1660 cm−1; 1H NMR (CDCl3): δ 8.40 (d, 1H, J = 4.3 Hz), 7.13 (m, 1H), 6.44 (s, 1H), 6.28 (brs, 1H), 5.17 (brs, 1H), 4.20 (m, 2H), 3.96 (s, 3H), 3.88 (t, 2H, J = 7.0 Hz), 3.64 (m, 1H), 3.26 (m, 4H), 2.53 (m, 2H), 2.07 (m, 5H), 1.85 (m, 4H), 1.68 (m, 6H), 1.41 (s, 9H), 1.26 (m, 6H), 0.94 (t, 3H, J = 7.0 Hz); APCIMS: m/z 605 (M+1); Anal. Calcd for C32H52N4O5S (604.8): C, 63.54; H, 8.67; N, 9.26. Found: C, 63.24; H, 8.44; N, 8.91.
5.2.24. (S)-{2-Benzyloxy-1-[4-(4-ethyl-6-methoxy-5-pentyloxy-8-quinolylamino)pentyl-carbamoyl]ethyl}carbamic acid tert-butyl ester (48)
Yield 77%; oil; IR (neat): 3401, 1742, 1664 cm−1; 1H NMR (CDCl3): δ 8.38 (d, 1H, J = 4.0 Hz), 7.27 (m, 5H), 7.11 (d, 1H, J = 4.2 Hz), 6.47 (brs, 1H), 6.42 (s, 1H), 6.07 (brs, 1H), 5.39 (brs, 1H), 4.50 (m, 2H), 4.25 (m, 1H), 3.96 (s, 3H), 3.87 (t, 2H, J = 7.18 Hz), 3.60 (m, 1H), 3.55 (m, 2H), 3.26 (m, 4H), 1.80 (m, 2H), 1.65 (m, 8H), 1.39 (s, 9H), 1.27(m, 6H), 0.94 (t, 3H, J = 6.9 Hz); APCIMS: m/z 651 (M+1); Anal. Calcd for C37H54N4O6 (650.8): C, 68.28; H, 8.36; N, 8.61. Found: C, 68.56; H, 8.09; N, 8.21.
5.2.25. (R)-{2-Benzylsulfanyl-1-[4-(4-ethyl-6-methoxy-5-pentyloxy-8-quinolylamino)-pentylcarbamoyl]ethyl}carbamic acid tert-butyl ester (49)
Yield 97%; oil; IR (neat): 3324, 1652 cm−1; 1H NMR (CDCl3): δ 8.39 (d, 1H, J = 4.5 Hz), 7.32-7.21 (m, 5H), 7.12 (d, 1H, J = 4.3 Hz), 6.43 (s, 1H), 6.24 (brs, 1H), 6.06 (brs, 1H), 5.25 (brs, 1H), 4.15 (m, 1H), 4.05 (s, 3H), 3.87 (t, 2H, J = 6.9 Hz), 3.71 (d, 2H, J = 2.9 Hz), 3.63 (m, 1H), 3.25 (m, 4H), 2.82 (m, 2H), 1.80 (m, 2H), 1.64 (m, 8H), 1.37 (s, 9H), 1.29-1.25 (m, 6H), 0.94 (t, 3H, J = 7.0 Hz); APCIMS: m/z 667 (M+1); Anal. Calcd for C37H54N4O5S (666.9): C, 66.63; H, 8.16; N, 8.40. Found: C, 66.33; H, 8.44; N, 8.21.
5.2.26. (R)-{{1-[4-(4-Ethyl-6-methoxy-5-pentyloxy-8-quinolylamino)pentylcarbamoyl]-2-tritylsulfanylethyl}carbamic acid tert-butyl ester (50)
Yield 98%; oil; IR (neat): 3322, 1666 cm−1; 1H NMR (CDCl3): δ 8.37 (m, 1H), 7.40 (m, 5H), 7.24(m, 10H), 7.11 (m, 1H), 6.41 (s, 1H), 6.04 (brs, 2H), 4.80 (brs, 1H), 4.12 (m, 1H), 3.95 (s, 3H), 3.89 (t, 2H, J = 6.7 Hz), 3.60 (m, 1H), 3.22 (m, 4H), 2.04 (m, 2H), 1.82 (m, 2H), 1.63 (m, 8H), 1.38 (s, 9H), 1.27 (m, 6H), 0.94 (t, 3H, J = 6.70 Hz); APCIMS: m/z 820 (M+1); Anal. Calcd for C49H62N4O5S (819.1): C, 71.85; H, 7.63; N, 6.84. Found: C, 72.11; H, 7.39; N, 7.02.
5.3. General method for the synthesis of N1-{4-[2-(tert-butyl)-6-methoxy-8-quinolylamino]pentyl}-(2S/2R)-2-aminosubstitutedamides (21 –33) and N1-[4-(4-ethyl-6-methoxy-5-pentyloxy-8-quinolylamino)pentyl]-(2 S/2 R)-2-aminosubstitutedamides (51–63)
In cases where α-amino group was protected with N-α-tert-butoxycarbonyl (tert-Boc) group following protocol was used.
To a solution of tert-Boc protected quinoline derivatives (0.5 mmol) was added 4N methanolic HCl solution (5 mL). Reaction mixture was allowed to stir at ambient temperature for 1 h. Solvent was removed under reduced pressure to afford oil, which upon crystallization with anhydrous diethyl ether gave product.
In cases where α-amino group was protected with N-α-benzyloxycarbonyl (Cbz) group, following protocol was used.
To a mixture of Cbz protected quinoline derivatives (0.5 mmol), glacial acetic acid (1 mL) and 10% Pd/C (0.1 g) in methanol (20 mL) was bubbled a slow stream of hydrogen gas for 1 h. The catalyst was removed by filtration, and filtrate was concentrated under reduce pressure to afford oil, which upon treatment with a solution of ethereal hydrogen chloride produced product. Recrystallized from diethyl ether.
Alternatively, in cases where amino acid was protected with benzyl (Bzl) and tert-Boc groups, following protocol was used.
A mixture of fully protected amino acid conjugates of 8-quinolinamine (0.5 mmol) and 30% HBr in acetic acid (5 ml) was stirred at ambient temperature for 30 min. Solvent was removed under reduced pressure to afford oil, which upon recrystallization with diethyl ether gave desired product.
5.3.1.N1-{4-[2-(tert-Butyl)-6-methoxy-8-quinolylamino]pentyl}-(2 R)-2,6-diaminohexan- amide.3HCl (21)
Yield: 95%; mp. (salt) 108-110 °C; IR (KBr): 3392, 3019, 2856, 1690 cm−1; 1H NMR (CD3OD): δ 7.72 (m, 2H), 7.60 (m, 2H), 4.22 (brs, 2H), 4.10 (m, 1H), 4.06 (m, 4H), 3.85 (s, 3H), 3.81 (m, 1H), 1.68 (m, 4H), 1.35 (m, 6H), 0.97 (m, 12H); APCIMS: m/z 444 (M+1); Anal. Calcd for C25H44Cl3N5O2 (553.0): C, 54.30; H, 8.02; N, 12.66. Found: C, 53.94; H, 7.77; N, 12.95.
5.3.2.N1-{4-[2-(tert-Butyl)-6-methoxy-8-quinolylamino]pentyl}-(2 R)-2,6-diaminopentan- amide.3HCl (22)
Yield: 96%; mp. (salt) 100-102 °C; IR (KBr): 3390, 2962, 1673 cm−1; 1H NMR (D2O): δ 8.22 (d, 1H, J=8.81 Hz), 7.72 (m, 2H), 7.26 (s, 1H), 4.71 (m, 1H), 3.90 (s, 3H), 3.18 (t, 2H, J = 6.28 Hz), 2.97 (m, 3H), 1.85 (m, 4H), 1.80 (m, 4H), 1.38 (s, 9H), 1.27 (d, 3H, J = 6.48 Hz); APCIMS: m/z 430 (M+1); Anal. Calcd for C24H42Cl3N5O2 (539.0): C, 53.48; H, 7.85; N, 12.99. Found: C, 53.23; H, 7.47; N, 12.76.
5.3.3.N1-{4-[2-(tert-Butyl)-6-methoxy-8-quinolylamino]pentyl}-(2 S)-2-amino-5-amino-(imino)methylaminopentanamide.3HCl (23)
Yield: 67%; mp. (salt) 112-114 °C; IR (KBr): 3407, 2963, 1666 cm−1; 1H NMR (D2O): δ 8.01 (d, 1H, J = 8.80 Hz), 7.49 (d, 1H, J = 8.80 Hz), 7.21 (s, 1H), 7.04 (s, 1H), 4.55 (m, 1H), 3.63 (s, 3H), 3.30 (m, 2H), 2.79 (m, 5H), 1.50 (m, 6H), 1.31 (s, 9H), 1.04 (d, 3H, J = 6.52 Hz); APCIMS: m/z 472 (M+1); Anal. Calcd for C25H44Cl3N7O2 (581.0): C, 51.68; H, 7.63; N, 16.87. Found: C, 51.21; H, 7.44; N, 17.09.
5.3.4.N1-{4-[2-(tert-Butyl)-6-methoxy-8-quinolylamino]pentyl}-(2 R)-2-amino-5-amino-(imino)methylaminopentanamide.3HCl (24)
Yield: 83%; mp. (salt) 111-112 °C; IR (KBr): 3355, 2929, 1667 cm−1; 1H NMR (D2O): δ 8.10 (d, 1H, J = 8.70 Hz), 7.53 (d, 1H, J = 8.70 Hz), 7.20 (s, 1H), 7.02 (s, 1H), 4.51 (m, 1H), 3.65 (s, 3H), 3.32 (m, 2H), 2.81 (m, 5H), 1.55 (m, 6H), 1.30 (s, 9H), 1.04 (d, 3H, J = 6.52 Hz); APCIMS: m/z 472 (M+1); Anal. Calcd for C25H44Cl3N7O2 (581.0): C, 51.68; H, 7.63; N, 16.87. Found: C, 51.89; H, 7.96; N, 16.68.
5.3.5.N1-{4-[2-(tert-Butyl)-6-methoxy-8-quinolylamino]pentyl}-(2S)-2-amino-3-(1H-imidazol-4-yl)propanamide.3HCl (25)
Yield: 70%; mp. (salt) 121-123 °C; IR (KBr): 3418, 2929, 2870, 1679 cm−1; 1H NMR (D2O): δ 8.62 (s, 1H), 8.39 (d, 1H, J = 8.52 Hz), 7.56 (d, 1H, J = 8.52 Hz), 7.45 (s, 1H), 7.32 (m, 2H), 4.13 (m, 1H), 3.47 (s, 3H), 3.40 (t, 2H, J = 6.28 Hz), 3.23 (m, 1H), 3.04 (m, 2H) 1.50 (m, 4H), 1.24 (s, 9H), 1.12 (d, 3H, J = 5.20 Hz); APCIMS: m/z 453 (M+1); Anal. Calcd for C25H39Cl3N6O2 (562.0): C, 53.43; H, 6.99; N, 14.95. Found: C, 53.72; H, 7.21; N, 14.68.
5.3.6.N1-{4-[2-(tert-Butyl)-6-methoxy-8-quinolylamino]pentyl}-(2R)-2-amino-3-(1H-imidazol-4-yl)propanamide.3HCl (26)
Yield: 79%; mp. (salt) 120-122 °C; IR (KBr): 3402, 2931, 2856, 1682 cm−1; 1H NMR (CD3OD): δ 8.82 (s, 1H), 8.73 (d, 1H, , J = 8.76 Hz), 8.49 (brs, 1H), 7.79 (d, 1H, J = 8.80 Hz), 7.75 (s, 1H), 7.52 (s, 1H), 7.28 (s, 1H), 4.29 (m, 1H), 4.06 (brs, 1H), 3.88 (s, 3H), 3.48 (m, 2H), 3.34 (m, 2H), 3.24 (m, 1H), 1.45 (m, 4H), 1.31 (s, 9H), 1.14 (d, 3H, J = 6.48 Hz); APCI: m/z 453 (M+1); Anal. Calcd for C25H39Cl3N6O2 (562.0): C, 53.43; H, 6.99; N, 14.95. Found: C, 53.21; H, 6.88; N, 14.88.
5.3.7.N1-{4-[2-(tert-Butyl)-6-methoxy-8-quinolylamino]pentyl}-(2S)-2-amino-3-(1H-indol-3-yl)propanamide.2HCl (27)
Yield: 85%; mp. (salt) 117-119 °; IR (KBr): 3387, 2926, 1675 cm−1; 1H NMR (DMSO-d6): δ 8.26 (brs, 1H), 8.18 (d, 1H, J = 8.40 Hz), 7.69 (m, 2H), 7.40 (d, 1H, J = 8.0 Hz), 7.25 (s, 1H), 7.11 (m, 1H), 7.00 (m, 1H), 6.86 (m, 2H), 4.27 (m, 1H), 3.94 (s, 3H), 3.22 (m, 2H), 3.04 (m, 2H), 2.54 (m, 1H), 1.64 (m, 4H), 1.43 (s, 9H), 1.26 (d, 3H, J = 6.52 Hz); APCIMS: m/z 502 (M+1); Anal. Calcd for C30H41Cl2N5O2 (574.6): C, 62.71; H, 7.19; N, 12.19. Found: C, 62.98; H, 7.43; N, 12.99.
5.3.8.N1-{4-[2-(tert-Butyl)-6-methoxy-8-quinolylamino]pentyl}-(2S)-2-amino-3-phenyl-propanamide.2HCl (28)
Yield: 84%; mp. (salt) 105-107 °C; IR (KBr): 3413, 2948, 1674 cm−1; 1H NMR (D2O): δ 8.47 (d, 1H, J = 8.70 Hz), 7.93 (d, 1H, J = 8.70 Hz), 7.36 (m, 7H), 4.25 (m, 1H), 4.10 (s, 3H), 3.55 (t, 2H, J = 7.02 Hz), 3.33 (m, 1H), 3.18 (m, 2H), 1.81 (m, 4H), 1.61 (s, 9H), 1.27 (d, 3H, J = 6.52 Hz); APCIMS: m/z 463 (M+1); Anal. Calcd for C28H40Cl2N4O2 (535.6): C, 62.80; H, 7.53; N, 10.46. Found: C, 62.60; H, 7.26; N, 10.19.
5.3.9.N1-{4-[2-(tert-Butyl)-6-methoxy-8-quinolylamino]pentyl}-(2S)-2-amino-3-(4-hydroxyphenyl)propanamide.2HCl (29)
Yield: 79%; mp. (salt) 85-87 °C; IR (KBr): 3247, 2922, 2856, 1671 cm−1; 1H NMR (CD3OD): δ 8.30 (d, 1H, J = 8.90 Hz), 7.80 (d, 1H, J = 8.90 Hz), 7.10 (m, 3H), 6.78 (m, 3H), 6.09 (brs, 1H), 4.01 (m, 1H), 3.95 (s, 3H), 3.49 (t, 2H, J = 6.62 Hz), 3.31 (m, 2H), 2.98 (m, 1H), 1.52 (m, 4H), 1.42 (s, 9H), 1.20 (m, 3H); APCIMS: m/z 479 (M+1); Anal. Calcd for C28H40Cl2N4O3 (551.6): C, 60.97; H, 7.31; N, 10.16. Found: C, 61.28; H, 7.44; N, 10.34.
5.3.10.N1-{4-[2-(tert-Butyl)-6-methoxy-8-quinolylamino]pentyl}-(2S)-2-amino-4-methylsulfanylbutanamide.2HCl (30)
Yield: 62%; mp. (salt) 70-72 °C; IR (KBr): 3402, 2929, 2856, 1679cm−1; 1H NMR (DMSO-d6): δ 8.69 (brs, 1H), 8.36 (brs, 1H), 8.16 (d, 1H, J = 8.60 Hz), 7.63 (d, 1H, J = 8.60 Hz), 7.66 (m, 2H), 3.84 (s, 3H), 3.78 (m, 1H), 3.15 (m, 2H), 2.99 (m, 1H), 2.50 (m, 2H), 2.04 (s, 2H), 1.55 (m, 7H), 1.39 (s, 9H), 1.22 (d, 3H, J = 6.52 Hz); APCIMS: m/z 447 (M+1); Anal. Calcd for C24H40Cl2N4O2S (519.6): C, 55.48; H, 7.76; N, 10.78. Found: C, 55.24; H, 7.97; N, 10.67.
5.3.11.N1-{4-[2-(tert-Butyl)-6-methoxy-8-quinolylamino]pentyl}-(2S)-2-amino-3-hydroxypropanamide.2HBr (31)
Yield: 65%; mp. (salt) 78-80 °C; IR (KBr): 3400, 2890, 1685 cm−1; 1H NMR (DMSO-d6): δ 8.64 (brs, 1H), 8.27 (d, 1H, J = 8.20 Hz), 8.19 (brs, 1H), 7.68 (d, 1H, J = 8.60 Hz), 6.83 (s, 1H), 6.71 (s, 1H), 4.01 (m, 1H), 3.86 (s, 3H), 3.84 (m, 2H), 3.70 (m, 1H), 3.20 (m, 2H), 1.75 (brs, 1H), 1.63 (m, 4H), 1.45 (s, 9H), 1.27 (d, 3H, J = 5.30 Hz); APCIMS: m/z 403 (M+1); Anal. Calcd for C22H36Br2N4O3 (564.4): C, 46.82; H, 6.43; N, 9.93. Found: C, 47.09; H, 6.33; N, 9.78.
5.3.12.N1-{4-[2-(tert-Butyl)-6-methoxy-8-quinolylamino]pentyl}-(2R)-2-amino-3-benzylsulfanylpropanamide.2HCl (32)
Yield: 61%; mp. (salt) 94-96 °C; IR (KBr): 3402, 2930, 2870, 1674 cm−1; 1H NMR (D2O): δ 8.11 (d, 1H, J = 8.90 Hz), 7.56 (d, 1H, J = 8.90 Hz), 7.01 (m, 7H), 3.86 (m, 1H), 3.74 (s, 3H), 3.51 (m, 2H), 3.12 (m, 2H), 2.97 (m, 1H), 2.67 (d, 2H, J = 6.30 Hz), 1.54 (m, 4H), 1.24 (s, 9H), 1.16 (d, 3H, J = 6.48 Hz); APCIMS: m/z 509 (M+1); Anal. Calcd for C29H42Cl2N4O2S (581.7): C, 59.88; H, 7.28; N, 9.63. Found: C, 60.15; H, 7.33; N, 9.88.
5.3.13.N1-{4-[2-(tert-Butyl)-6-methoxy-8-quinolylamino]pentyl}-(2R)-2-amino-3-sulfanylpropanamide.2HBr (33)
Yield: 76%; mp. (salt) 77-79 °C; IR (KBr): 3402, 2930, 1668 cm−1; 1H NMR (DMSO-d6): δ 8.57 (brs, 1H), 8.31 (brs, 1H), 8.03 (d, 1H, J = 8.80 Hz), 7.56 (d, 1H, J = 8.80 Hz), 6.50 (s, 1H), 6.50 (s, 1H), 3.99 (m, 1H), 3.78 (s, 3H), 3.68 (m, 2H), 3.20 (m, 2H), 3.02 (m, 1H), 1.55 (m, 4H), 1.34 (s, 9H), 1.16 (d, 3H, J = 6.48 Hz) ; APCIMS: m/z 419 (M+1); Anal. Calcd for C22H36Br2N4O2S (580.4): C, 45.52; H, 6.25; N, 9.65. Found: C, 45.33; H, 6.33; N, 9.47.
5.3.14.N1-[4-(4-Ethyl-6-methoxy-pentyloxy-8-quinolylamino)pentyl]-(2R)-2,6-diamino-hexanamide.3HCl (51)
Yield: 86%; mp. (salt) 119-122 °C (dec.); IR (neat, free base): 3382, 1576 cm−1; 1H NMR (free base, CD3OD): δ 8.36 (d, 1H, J = 4.40), 7.19 (d, 1H, J = 4.40), 6.58 (s, 1H), 3.96 (s, 3H), 3.89 (t, 2H, J = 6.80 Hz), 3.71 (m, 2H), 3.30 (m, 4H), 2.87 (m, 2H), 1.50 (m, 18H), 1.30 (m, 6H), 0.96 (t, 3H, J = 7.10 Hz); APCIMS: m/z 502 (M+1); Anal. Calcd for C28H50Cl3N5O3 (611.1): C, 55.03; H, 8.25; N, 11.46. Found: C, 54.77; H, 8.48; N, 11.83.
5.3.15.N1[4-(4-Ethyl-6-methoxy-5-pentyloxy-8-quinolylamino)pentyl]-(2R)-2,5-diaminopentanamide.3HCl (52)
Yield: 79%; mp. (salt) 117-120 °C (dec.); IR (KBr): 3402, 1668 cm−1; 1H NMR (CD3OD): δ 8.70 (d, 1H, J = 5.0 Hz), 7.59 (s, 1H), 7.58 (d, 1H, J = 5.0 Hz), 4.13 (t, 2H, J = 6.60 Hz), 4.08 (s, 3H) 3.96 (m, 2H), 3.48 (m, 2H), 3.36 (m, 2H), 3.02 (m, 2H), 1.90 (m, 12H), 1.50 (m, 4H), 1.40 (m, 6H), 0.99 (t, 3H, J = 7.0 Hz); APCIMS: m/z 488 (M+1); Anal. Calcd for C27H48Cl3N5O3 (597.1): C, 54.31; H, 8.10; N, 11.73. Found: C, 54.77; H, 8.48; N, 11.83.
5.3.16.N1[4-(4-Ethyl-6-methoxy-5-pentyloxy-8-quinolylamino)pentyl]-(2S)-2-amino-5-amino(imino)methylaminopentanamide.3HCl (53)
Yield: 67%; mp. (salt) 101-104 °C (dec.); IR (neat, free base): 3374, 1680 cm−1; 1H NMR (free base, CD3OD): δ 8.36 (d, 1H, J = 4.30 Hz), 7.19 (d, 1H, J = 4.40 Hz), 6.58 (s, 1H), 3.96 (s, 3H), 3.89 (t, 2H, J = 6.80 Hz), 3.65 (m, 2H), 3.27 (m, 4H), 3.15 (m, 2H), 1.60 (m, 16H), 1.31-1.26 (m, 6H), 0.96 (t, 3H, J = 7.0 Hz); APCIMS: m/z 530 (M+1); Anal. Calcd for C28H50Cl3N7O3 (639.1): C, 52.62; H, 7.89; N, 15.34. Found: C, 52.86; H, 8.07; N, 15.63.
5.3.17.N1[4-(4-Ethyl-6-methoxy-5-pentyloxy-8-quinolylamino)pentyl]-(2R)-2-amino-5-amino(imino)methylaminopentanamide.3HCl (54)
Yield: 84%; mp. (salt) 100-103 °C (dec.); IR (neat, free base): 3369, 1716.1 cm−1; 1H NMR (free base, CDCl3): δ 8.38 (d, 1H, J = 4.30 Hz), 7.11 (d, 1H, J = 4.30 Hz), 6.44 (s, 1H), 3.96 (s, 3H), 3.87 (t, 2H, J = 6.90 Hz), 3.63 (m, 2H), 3.24 (m, 2H), 2.78 (m, 2H), 2.03 (brs, 1H), 1.83 (m, 2H), 1.47 (m, 16H), 1.26 (m, 6H), 0.94 (t, 3H, J = 6.9 Hz); APCIMS: m/z 530 (M+1); Anal. Calcd for C28H50Cl3N7O3 (639.1): C, 52.62; H, 7.89; N, 15.34. Found: C, 52.34; H, 7.65; N, 15.12.
5.3.18.N1-[4-(4-Ethyl-6-methoxy-5-pentyloxy-8-quinolylamino)pentyl]-(2S)-2-amino-3-(1H-imidazol-4-yl)propanamide.3HCl (55)
Yield: 96%; mp. (salt) 122-124 °C (dec.); IR (KBr): 3402, 1679 cm−1; 1H NMR (CD3OD): δ 9.25 (s, 1H), 8.71 (d, 1H, J = 4.90 Hz), 7.60 (m, 3H), 4.53 (m, 1H), 4.08 (m, 5H), 3.97 (m, 1H), 3.42 (m, 6H), 1.85 (m, 2H), 1.63 (m, 10H), 1.36 (m, 6H), 0.97 (t, 3H, J = 6.90 Hz); APCIMS: m/z 511 (M+1); Anal. Calcd for C28H45Cl3N6O3 (620.1): C, 54.24; H, 7.32; N, 13.55. Found: C, 53.90; H, 7.03; N, 13.47.
5.3.19.N1-[4-(4-Ethyl-6-methoxy-5-pentyloxy-8-quinolylamino)pentyl]-(2R)-2-amino-3-(1H-imidazol-4-yl)propanamide.3HCl (56)
Yield: 87%; mp. (salt) 124-126 °C (dec.); IR (KBr): 3387, 1679 cm−1; 1H NMR (CD3OD): δ 9.15 (s, 1H), 8.61 (d, 1H, J = 5.0 Hz), 7.50 (m, 3H), 4.35 (m, 1H), 3.99 (m, 5H), 3.85 (m, 1H), 3.38 (m, 6H), 1.77 (m, 8H), 1.43 (m, 4H), 1.30 (m, 6H), 0.88 (t, 3H, J = 6.90 Hz); APCIMS: m/z 511 (M+1); Anal. Calcd for C28H45Cl3N6O3 (620.1): C, 54.24; H, 7.32; N, 13.55. Found: C, 54.55; H, 7.09; N, 13.33.
5.3.20.N1-[4-(4-Ethyl-6-methoxy-5-pentyloxy-8-quinolylamino)pentyl]-(2S)-2-amino-3-(1H-indol-3-yl)propanamide.2HCl (57)
Yield: 63%; mp. (salt) 114-116 °C (dec.); IR (KBr): 3370, 1668 cm−1; 1H NMR (CD3OD): δ 8.66 (d, 1H, J = 4.60 Hz), 7.59 (m, 2H), 7.51 (s, 1H), 7.33 (m, 1H), 7.20 (s, 1H), 7.03 (m, 2H), 4.06 (m, 5H), 3.82 (m, 2H), 3.35 (d, 2H, J = 6.30 Hz), 3.19 (m, 4H), 1.81 (m, 4H), 1.45 (m, 8H), 1.30 (m, 6H), 0.96 (t, 3H, J = 6.80 Hz); APCIMS: m/z 560 (M+1); Anal. Calcd for C33H47Cl2N5O3 (632.7): C, 62.65; H, 7.49; N, 11.07. Found: C, 62.88; H, 7.75; N, 13.33.
5.3.21.N1-[4-(4-Ethyl-6-methoxy-5-pentyloxy-8-quinolylamino)pentyl]-(2S)-2-amino-3-phenylpropanamide.2HCl (58)
Yield: 76%; mp. (salt) 104-106 °C (dec.); IR (KBr): 3414, 1664 cm−1; 1H NMR (CD3OD): δ 8.75 (m, 1H), 7.64 (m, 1H), 7.53 (d, 1H, J = 6.10 Hz), 7.23 (m, 3H), 7.13 (d, 2H, J = 8.10 Hz), 4.11 (s, 3H), 3.93 (m, 2H), 3.87 (m, 2H), 3.37 (m, 2H), 3.00 (m, 4H), 1.85 (m, 2H), 1.52 (m, 10H), 1.30 (m, 6H), 0.97 (t, 3H, J = 6.20 Hz); APCIMS: m/z 521 (M+1); Anal. Calcd for C31H46Cl2N4O3 (593.6): C, 62.72; H, 7.81; N, 9.44. Found: C, 62.97; H, 7.58; N, 9.68.
5.3.22.N1-[4-(4-Ethyl-6-methoxy-5-pentyloxy-8-quinolylamino)pentyl]-(2S)-2-amino-3-(4-hydroxyphenyl)propanamide.2HBr (59)
Yield: 80 %; mp. (salt) 130-132 °C (dec.); IR (KBr): 3408, 1667 cm−1; 1H NMR (CD3OD): δ 8.69 (d, 1H, J = 4.30 Hz), 7.60 (s, 1H), 7.55 (m, 1H), 7.34 (s, 1H), 7.11 (m, 2H), 6.86 (m, 1H), 4.27 (m, 1H), 4.15 (m, 5H), 3.45 (m, 2H), 3.13 (m, 2H), 2.97 (m, 2H), 1.83 (m, 2H), 1.54 (m, 10H), 1.32 (m, 6H), 0.97 (t, 3H, J = 7.0 Hz); APCIMS: m/z 537 (M+1); Anal. Calcd for C31H46Cl2N4O4 (609.7): C, 61.08; H, 7.61; N, 9.19. Found: C, 61.37; H, 7.88; N, 9.23.
5.3.23.N1-[4-(4-Ethyl-6-methoxy-5-pentyloxy-8-quinolylamino)pentyl]-(2S)-2-amino-4-methylsulfanylbutanamide.2HCl (60)
Yield: 91 %; mp. (salt) 72-74 °C (dec.); IR (KBr): 3355, 1668 cm−1; 1H NMR (CD3OD): δ 8.68 (d, 1H, J = 4.90 Hz), 7.56 (s, 1H), 7.55 (d, 1H, J = 4.30 Hz), 4.11 (t, 2H, J = 6.50 Hz), 4.06 (s, 3H), 3.98 (m, 2H), 3.47 (m, 2H), 3.27 (m, 2H), 2.54 (t, 2H, J = 7.40 Hz), 2.06 (m, 5H), 1.87 (m, 2H), 1.77 (m, 2H), 1.50 (m, 8H), 1.36 (m, 6H), 0.97 (t, 3H, J = 7.10 Hz); APCIMS: m/z 505 (M+1); Anal. Calcd for C27H46Cl2N4O3S (577.8): C, 56.14; H, 8.03; N, 9.70. Found: C, 56.45; H, 7.87; N, 10.06.
5.3.24.N1-[4-(4-Ethyl-6-methoxy-5-pentyloxy-8-quinolylamino)pentyl]-(2S)-2-amino-3-hydroxypropionamide.2HBr(61)
Yield: 96 %; mp. (salt) 88-90 °C (dec.); IR (KBr): 3402, 1672 cm−1; 1H NMR (CD3OD): δ 8.61 (d, 1H, J = 4.80 Hz), 7.54 (s, 1H), 7.46 (d, 1H, J = 4.80 Hz), 4.15 (m, 1H), 4.02 (t, 2H, J = 6.10 Hz), 3.98 (s, 3H), 3.84 (m, 2H), 3.37 (m, 2H), 3.21 (m, 2H), 1.97 (m, 2H), 1.78 (m, 4H), 1.65 (m, 4H), 1.44 (m, 4H), 1.28 (m, 6H), 0.88 (t, 3H, J = 7.20 Hz); APCIMS: m/z 461 (M+1); Anal. Calcd for C25H42Cl2N4O4 (533.5): C, 56.28; H, 7.93; N, 10.50. Found: C, 56.55; H, 8.08; N, 10.23.
5.3.25.N1-[4-(4-Ethyl-6-methoxy-5-pentyloxy-8-quinolylamino)pentyl]-(2R)-2-amino-3-benzylsulfanylpropanamide.2HCl (62)
Yield 79 %; mp. (salt) 94-96 °C (dec.); IR (KBr): 3321, 1682 cm−1; 1H NMR (CD3OD): δ 8.57 (d, 1H, J = 4.10 Hz), 7.44 (d, 1H, J = 4.70 Hz), 7.43 (s, 1H), 7.18 (m, 5H), 4.00 (t, 2H, J = 6.40 Hz), 3.96 (s, 3H), 3.84 (m, 1H), 3.82 (m, 1H), 3.70 (d, 2H, J = 4.70 Hz), 3.34 (m, 2H), 3.21 (m, 2H), 2.78 (m, 2H), 1.75 (m, 4H), 1.65 (m, 4H), 1.44 (m, 4H), 1.27 (m, 6H), 0.88 (t, 3H, J = 7.0 Hz); APCIMS: m/z 567 (M+1); Anal. Calcd for C32H48Cl2N4O3S (639.7): C, 60.08; H, 7.56; N, 8.76. Found: C, 60.35; H, 7.84; N, 8.95.
5.3.26.N1-[4-(4-Ethyl-6-methoxy-5-pentyloxy-8-quinolylamino)-pentyl]-(2R)-2-amino-3-sulfanylpropanamide.2HBr (63)
Yield 83 %; mp. (salt) 102-104 °C (dec.); IR (KBr): 3402, 1672 cm−1; 1H NMR (CD3OD): δ 8.59 (d, 1H, J = 4.90 Hz), 7.52 (s, 1H), 7.43 (d, 1H J = 4.80 Hz), 4.00 (m, 5H), 3.83 (m, 1H), 3.58 (m, 1H), 3.31 (m, 4H), 3.03 (m, 2H), 1.68 (m, 9H), 1.38 (m, 4H), 1.23 (m, 6H), 0.88 (t, 3H, J = 7.0 Hz); APCIMS: m/z 477 (M+1); Anal. Calcd for C25H42Br2N4O3S (638.5): C, 47.03; H, 6.63; N, 8.77. Found: C, 47.36; H, 6.47; N, 8.52.
5.4. Assay for methemoglobin (MetHb) toxicity
An in vitro biotransformation-linked assay was used to evaluate methemoglobin toxicity of the analogs at 20 μg/mL. The method involves simultaneous incubation of the test compounds with the mouse liver microsomes and human erythrocytes followed by determination of methemoglobin formation.25 This allows the unstable metabolites generated in situ to react with the human erythrocytes. The assay mixture (500 μL) contained 100 μL erythrocytes suspension (50% hemtocrit), 100 μL NADPH regenerating co-factors and enzyme mix (0.8 μM NADP, 5 μM Glucose-6-phosphate, 3 μM MgCl2 and 0.2 units Glucose-6-phosphate dehydrogenase), 100 μL KCl (31 μM), 25 μl microsomes (equivalent to 500 μg protein), 5 μL test compound and 170 μL potassium phosphate buffer (100 mM, pH 7.4). The reaction tubes were loosely covered with aluminum foil and maintained at 37 °C in a metabolic water bath with constant shaking at 80 rpm for 1 h. The tubes were chilled on ice and centrifuged at 1000 rpm for 10 min. The supernatants were discarded and the erythrocyte pellets were hemolyzed with 2 mL of the lysis buffer (0.277% potassium dihydrogen phosphate, 0.289% disodium hydrogen phosphate, and 0.05% Triton X-100; pH 7.8). The methemoglobin levels relative to total hemoglobin in the lysates were measured by the spectrophotometric technique of Evelyn and Malloy adapted to 96 well plates.28
5.5. Assay for antimicrobial activity
All organisms are obtained from the American Type Culture Collection (Manassas, VA). They include the fungi C. albicans ATCC 90028, C. glabrata ATCC 90030, C. krusei ATCC 6258, C. neoformans ATCC 90113, and A. fumigatus ATCC 90906 and the bacteria methicillin-resistant S. aureus ATCC 43300 (MRS) and M. intracellulare ATCC 23068. Susceptibility testing is performed using a modified version of the CLSI (formerly NCCLS) methods.29–32M. intracellulare is tested using a modified method of Franzblau, et al.33 Samples are serially-diluted in 20% DMSO/saline and transferred in duplicate to 96 well flat bottom microplates. Inocula are prepared by correcting the OD630 of microbe suspensions in incubation broth [RPMI 1640/2% dextrose/0.03% glutamine/MOPS @ pH 6.0 (Cellgro) for C. albicans, Sabouraud Dextrose for C. neoformans, cation-adjusted Mueller-Hinton (Difco) @ pH 7.3 for MRS, 5% Alamar Blue™ (BioSource International, Camarillo, CA) in Middlebrook 7H9 broth with OADC enrichment, pH = 7.3 for M. intracellulare, and 5% Alamar Blue™/RPMI 1640 broth (2% dextrose, 0.03% glutamine, buffered with 0.165M MOPS at pH 7.3) for A. fumigatus to afford final target inocula of: C. albicans: 1.0 × 104, C. neoformans: 1.0 × 105, M. intracellulare: 2.0 × 106, MRS, C. glabrata, C. krusei: 5.0 × 105 CFU/mL, and A. fumigatus: 3.0 × 104 CFU/mL. Drug controls [Ciprofloxacin (ICN Biomedicals, Ohio) for bacteria and Amphotericin B (ICN Biomedicals, Ohio) for fungi] are included in each assay. All organisms are read at either 630 nm using the EL-340 Biokinetics Reader (Bio-Tek Instruments, Vermont) or 544ex/590em, (M. intracellulare, A. fumigatus)using the Polarstar Galaxy Plate Reader (BMG LabTechnologies, Germany) prior to and after incubation: C. albicans, MRS, C. glabrata and C. krusei at 37 °C for 18 – 24 h, C. neoformans and A. fumigatus at 30 °C for 68 – 72 h, and M. intracellulare at 37 °C and 10% CO2 for 68 – 72 h. The MIC is defined as the lowest test concentration that allows no detectable growth (for M. intracellulare and A. fumigatus, no color change from blue to pink). Minimum fungicidal or bactericidal concentrations are determined by removing 5 μL from each clear (or blue) well, transferring to agar and incubating as previously mentioned. The MFC/MBC is defined as the lowest test concentration that kills the organism (allows no growth on agar).
5.6. Assay for in vitro antimalarial activity
A suspension of red blood cells infected with D6 or W2 strains of P. falciparum (200 μL, with 2% parasitemia and 2% hematocrit in RPMI 1640 medium supplemented with 10% human serum and 60 μg/mL amikacin) is added to the wells of a 96-well plate containing 10 μL of test samples diluted in medium at various concentrations. The plate is placed in a modular incubation chamber (Billups-Rothenberg, CA) and flushed with a gas mixture of 90% N2, 5% O2, and 5% CO2 and incubated at 37 °C for 72 h. Parasitic LDH activity, as a measure of growth, is determined by using Malstat™ reagent (Flow Inc., Portland, OR) according to the procedure of Makler, et al.34 Briefly, 20 μL of the incubation mixture is mixed with 100 μl of the Malstat™ reagent and incubated at room temperature for 30 min. 20 μL of a 1:1 mixture of NBT/PES (Sigma, St. Louis, MO) is then added, and the plate is further incubated in the dark for 1 h. The reaction is then stopped by the addition of 100 μL of a 5% acetic acid solution. The plate is read at 650 nm using the EL-340 Biokinetics Reader (Bio-Tek Instruments, Vermont). IC50 values are computed from the dose response curves. Artemisinin and chloroquine are included in each assay as the drug controls. DMSO (0.25%) is used as vehicle control.
Scheme 1. Reagents and conditions.
(i) (CH3)3CCO2H, AgNO3, (NH4)2S2O8, 10% H2SO4, CH3CN, 70 °C; (ii) raney Ni, EtOH, H2, 45 psi, 45 min; (iii) 2-(4-bromopentyl)-1,3-isoindolinedione, Et3, 120 °C, 24 h; (iv) NH2NH2.H2O, EtOH, reflux, 8 h; (v) suitably N- and side-chain protected L/D-amino acid, DCC, DCM, rt, 6 h; (vi) H2/Pd-C, MeOH, 1h, rt or 4N HCl-MeOH, 1h, rt or 30% HBr-AcOH, 45 min, rt.
Scheme 2. Reagents and conditions.
(i) 1-chloro-3-pentanone, 85% o-H3PO4, As2O5, 80 °C, 3 h; (ii) raney nickel, EtOH, H2, 45 psi, 45 min; (iii) 2-(4-bromopentyl)-1,3-isoindolinedione, Et3N, 120 °C, 24 h; (iv) NH2NH2.H2O, EtOH, reflux, 8 h; (v) suitably N- and side-chain protected L/D-amino acid, DCC, DCM, rt, 6 h; (vi) H2/Pd-C, MeOH, 1h, rt or 4N HCl-MeOH, 1h, rt or 30% HBr-AcOH, 45 min, rt.
Acknowledgements
This research project was supported by the Council of Scientific and Industrial Research, New Delhi, India (Grant no. 01(1555)/98/EMR-II). Kirandeep Kaur thanks the Council of Scientific and Industrial Research (CSIR), India for the award of Junior Research Fellowship. The authors would like to thank Marsha Wright for conducting the antifungal and antibacterial testing and John Trott for conducting antimalarial testing. The biological testing was supported by the NIH, NIAID, Division of AIDS, Grant No. AI 27094, and the USDA Agricultural Research Service Specific Cooperative Agreement No. 58-6408-2-0009.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and Notes
- 1.World Health Organization Malaria Fact Sheet, No. 94, 2006. please see: http://www.who.int/mediacentre/factsheets/fs094/en/
- 2.World Health Organization Leishmaniases Fact Sheet, No. 116, 2000. please see: http://www.who.int/mediacentre/factsheets/fs116/en/
- 3.Overbye KM, Barrett JF. Drug Discov. Today. 2005;10:45. doi: 10.1016/S1359-6446(04)03285-4. [DOI] [PubMed] [Google Scholar]
- 4.Neu HC. Science. 1992;257:1064. doi: 10.1126/science.257.5073.1064. [DOI] [PubMed] [Google Scholar]
- 5.Vangapandu S, Jain M, Kaur K, Patil P, Patel SR, Jain R. Med. Res. Rev. 2006 doi: 10.1002/med.20062. DOI: 10.1002/med.20062. [DOI] [PubMed] [Google Scholar]
- 6.Yeates C. Curr. Opin. Investig. Drugs. 2002;3:1446. [PubMed] [Google Scholar]
- 7.Jain M, Khan SI, Tekwani BL, Jacob MR, Singh S, Singh PP, Jain R. Bioorg. Med. Chem. 2005;13:4458. doi: 10.1016/j.bmc.2005.04.034. [DOI] [PubMed] [Google Scholar]
- 8.Armer RE, Barlow JS, Dutton CJ, Greenway DHJ, Greenwood SDW, Lad N, Tommasini I. Bioorg. Med. Chem. Lett. 1997;7:2585. doi: 10.1016/s0960-894x(98)00243-1. [DOI] [PubMed] [Google Scholar]
- 9.Nodiff EA. Prog. Med. Chem. 1991;28:1. doi: 10.1016/s0079-6468(08)70362-x. [DOI] [PubMed] [Google Scholar]
- 10.Strother A, Allahyari R, Buchholz J, Fraser IM, Tilton BE. Drug Metab. Dispos. 1983;12:35. [PubMed] [Google Scholar]
- 11.Baker JK, Clark AM, McChesney JD. J. Pharm. Res. 1984;73:502. doi: 10.1002/jps.2600730418. [DOI] [PubMed] [Google Scholar]
- 12.Allahyari R, Strother A, Fraser IM, Verbiscar AJ. J. Med. Chem. 1984;27:407. doi: 10.1021/jm00369a031. [DOI] [PubMed] [Google Scholar]
- 13.Jain R, Gupta RC, Anand N. Ind. J. Chem. 1994;33B:792. [Google Scholar]
- 14.Jain M, Vangapandu S, Sachdeva S, Singh S, Singh PP, Jena GB, Tikoo K, Ramarao P, Kaul CL, Jain R. J. Med. Chem. 2004;47:285. doi: 10.1021/jm0304562. [DOI] [PubMed] [Google Scholar]
- 15.Vangapandu S, Sachdeva S, Jain M, Singh S, Singh PP, Kaul CL, Jain R. Bioorg. Med. Chem. 2003;11:4557. doi: 10.1016/j.bmc.2003.07.003. [DOI] [PubMed] [Google Scholar]
- 16.Jain R, Jain S, Gupta RC, Anand N, Dutta GP, Puri SK. Indian J. Chem. 1994;33B:251. [Google Scholar]
- 17.Vangapandu S, Sachdeva S, Jain M, Singh S, Singh PP, Kaul CL, Jain R. Bioorg. Med. Chem. 2004;12:239. doi: 10.1016/j.bmc.2003.10.028. [DOI] [PubMed] [Google Scholar]
- 18.Jain M, Vangapandu S, Sachdeva S, Jain R. Bioorg. Med. Chem. 2004;12:1003. doi: 10.1016/j.bmc.2003.12.029. [DOI] [PubMed] [Google Scholar]
- 19.The l- and d-primaquine exhibit similar antimalarial activities (Carroll FI, Berrang B, Linn CP. J. Med. Chem. 1978;21:326. doi: 10.1021/jm00202a002.) and drug is used clinically as racemate. In this study, racemic 2 and 3 were used, and attempts were not made to separate diastereoisomers of synthesized 8-quinolinamines 21–33 and 51–63.
- 20.Chu THL, C. K., Yang CS, Lu XT, Chang CC. Yao Hsueh Hsueh Pao. 1956;4:197. [Google Scholar]
- 21.Makler MT, Hinrichs DJ. Am. J. Trop. Med. Hyg. 1993;48:205. doi: 10.4269/ajtmh.1993.48.205. [DOI] [PubMed] [Google Scholar]
- 22.Borenfreund E, Babich H, Martin-Alguacil N. In Vitro Cell Dev. Biol. 1990;26:1030. doi: 10.1007/BF02624436. [DOI] [PubMed] [Google Scholar]
- 23.Mustafa J, Khan SI, Ma G, Walker LA, Khan IA. Lipids. 2004;39:167. doi: 10.1007/s11745-004-1215-5. [DOI] [PubMed] [Google Scholar]
- 24.Fasanmade AA, Jusko WJ. Drug Metab. Dispos. 1995;23:573. [PubMed] [Google Scholar]
- 25.Harrison JH, Jollow DJ. Mol. Pharmacol. 1987;32:423. [PubMed] [Google Scholar]
- 26.Mikus J, Steverding D. Parasitol. Int. 2000;48:265. doi: 10.1016/s1383-5769(99)00020-3. [DOI] [PubMed] [Google Scholar]
- 27.Ma G, Khan SI, Jacob MR, Tekwani BL, Li Z, Pasco DS, Walker LA, Khan IA. Antimicrob. Agents Chemother. 2004;48:4450. doi: 10.1128/AAC.48.11.4450-4452.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Evelyn KA, Malloy HT. J. Biol. Chem. 1938;126:655. [Google Scholar]
- 29.NCCLS . Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically M7-A5. 2. Vol. 20. National Committee on Clinical Laboratory Standards; 2000. [Google Scholar]
- 30.NCCLS . Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts; Approved Standard M27-A2. 15. Vol. 22. National Committee on Clinical Laboratory Standards; 2002. [Google Scholar]
- 31.NCCLS . Reference method for broth dilution antifungal susceptibility testing of filamentous fungi; approved standard, M38-A. 16. Vol. 22. National Committee on Clinical Laboratory Standards; 2002. [Google Scholar]
- 32.NCCLS . Susceptibility Testing of Mycobacteria, Nocardia, and Other Aerobic Actinomycetes; Tentative Standard—Second Edition, M24-T2. 26. Vol. 20. National Committee on Clinical Laboratory Standards; 2000. [PubMed] [Google Scholar]
- 33.Franzblau SG, Witzig RS, McLaughlin JC, Torres P, G. Madico G, Hernandez A, Degnan MT, Cook MB, Quenzer VK, Ferguson RM, Gilman RH. J. Clin. Microbiol. 1998;36:362. doi: 10.1128/jcm.36.2.362-366.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Makler MT, Hinrichs DJ. Am. J. Trop. Med. Hyg. 1993;48:205. doi: 10.4269/ajtmh.1993.48.205. [DOI] [PubMed] [Google Scholar]