Abstract
This study examined the interleukin-8 (IL-8) response of the intestinal adenocarcinoma HCT-8 cell line to infection with enteroaggregative and enterotoxigenic Escherichia coli pathotypes isolated from patients with travelers' diarrhea. Individual diarrheagenic E. coli strains (enteroaggregative E. coli [EAEC]; n = 30), heat-stable enterotoxin (ST)-producing enterotoxigenic E. coli (ETEC ST; n = 11), heat-labile enterotoxin (LT)-producing enterotoxigenic E. coli (ETEC LT; n = 10), and ST- and LT-producing enterotoxigenic E. coli (ETEC ST:LT; n = 8) were coincubated with HCT-8 cells for 3 h. Tissue culture supernatants were assayed for IL-8 content by enzyme-linked immunosorbent assay. Fifty percent of EAEC (72% of those EAEC carrying the virulence factors aggR, aggA, and aspU and 40% of those EAEC not carrying virulence factors) and 64% of ETEC ST elicited IL-8 production. In contrast, 10% of ETEC LT elicited the production of IL-8 above baseline. These results suggest that (i) the HCT-8 cell line infection model can be used as a tool to differentiate proinflammatory E. coli from noninflammatory isolates; (ii) EAEC has a heterogeneous ability to induce the production of IL-8, and this may be associated with the presence of virulence factors; and (iii) ETEC ST can elicit an inflammatory response and helps explain our earlier findings of increased fecal IL-8 in patients with ETEC diarrhea.
Diarrheagenic Escherichia coli has been classified into at least five pathotypes based on clinical associations, serotyping, phenotypic assays, and virulence genes (19). These five pathotypes include enterotoxigenic E. coli (ETEC), enteroaggregative E. coli (EAEC), enteropathogenic E. coli (EPEC), enterohemorrhagic or Shiga toxin-producing E. coli (EHEC or STEC), and enteroinvasive E. coli. However, under this classification, there is clinical overlap between the diarrheagenic E. coli pathotypes isolated from patients with travelers' diarrhea. For example, ETEC, currently considered a cause of a secretory diarrhea, has been shown to be associated with inflammatory diarrhea in some cases (3). Previous studies have shown that ETEC diarrhea is associated with increased levels of fecal inflammatory cytokines (9). Also, EAEC, a common cause of an inflammatory diarrhea, has been shown to produce secretory noninflammatory diarrhea (9).
Interleukin-8 (IL-8) was selected for study because this proinflammatory chemokine is a potent chemoattractant for polymorphonuclear leukocytes and T lymphocytes and can be identified as an inflammatory marker in stools of subjects with diverse causes of diarrhea (9, 25). In the case of EAEC, flagellin (the major structural protein of E. coli flagella) (6), other virulence genes (11), and specific host genetic variants (13) are all associated with IL-8 secretion in humans. The secretion of IL-8 is mediated through toll-like receptors (TLRs), specifically TLR5 (6). TLRs play an important role in intestinal innate immunity by recognizing microbial particles.
We hypothesized that a differential IL-8 response of an intestinal adenocarcinoma HCT-8 cell line would occur to infection with enteroaggregative and enterotoxigenic E. coli, including ETEC isolates producing heat-stable enterotoxin (ETEC ST).
MATERIALS AND METHODS
Isolates studied.
EAEC (n = 30), ETEC ST (n = 11), heat-labile enterotoxin-producing ETEC (ETEC LT; n = 10), and ST- and LT-producing enterotoxigenic E. coli (ETEC ST:LT; n = 8) isolates were recovered from 59 patients experiencing acute travelers' diarrhea during short-term stays in Guadalajara, Mexico, in 2002 (11, 12, 27). Stool samples were collected and submitted to the field laboratory, where they were examined for the presence of enteroaggregative and enterotoxigenic E. coli. All samples were negative for all other conventional enteric pathogens tested as previously described (11, 12, 27).
This study was approved by the Institutional Review Board of the University of Texas—Houston Health Science Center.
Identification of enteroaggregative and enterotoxigenic E. coli isolates. (i) ETEC.
ETEC ST and ETEC LT isolates were identified by the use of oligonucleotide probes labeled by T4 polynucleotide kinase and [32P]ATP as previously described (17).
(ii) EAEC.
EAEC was identified by the use of the HEp-2 cell adherence assay (7). EAEC strain O42 and nonpathogenic strain HS were used as a positive and negative control, respectively. An isolate was interpreted as positive for EAEC if it showed the characteristic “stacked-brick” aggregative appearance with cultured HEp-2 cells (20).
Identification of EAEC virulence factors.
EAEC virulence factors were studied in a subset of EAEC isolates by PCR. Specific primers and assays employed for the detection of EAEC virulence factors aggA, aggR, and aspU have been previously described (5, 21). Briefly, an amplification mix of 98 μl of PCR mix (10 mM Tris-HCl [pH 8.3], 50 mM KCl, 2 mM MgCl2, 100 μM [each] dATP, dCTP, dGTP, and dTTP, and 2.5 U of AmpliTaq polymerase [Perkin-Elmer, Norwalk, Conn.]), 25 pmol of each primer, and 2 μl of DNA template were heated for 5 min at 94°C. The reaction mixture was then subjected to 35 cycles (94°C for 30 s, 50°C for 1 min, and 72°C for 45 s) and then to a final extension at 72°C for 7 min in a DNA thermal cycler (Perkin-Elmer). Ten microliters of the amplified PCR product was then separated on a 1% agarose electrophoresis gel along with a 1-kb DNA molecular weight marker (Gibco BRL, Gaithersburg, Md.). A positive result was determined by the presence of a PCR product of expected size.
Culture conditions.
Four groups of diarrheagenic E. coli and reference strains were studied. They are listed in Table 1. E. coli was grown in Luria broth at 37°C overnight with shaking. EAEC 042 served as a positive IL-8 control, since this strain is known to elicit high amounts of IL-8 from intestinal epithelial cells in vitro (25, 26). Nonpathogenic E. coli strain HS served as a negative control. All bacteria were harvested by centrifugation after 3 h as previously described (8).
TABLE 1.
Characteristics of the diarrheagenic E. coli and control strains studied
| Isolate | No. tested | Characteristics | References |
|---|---|---|---|
| EAEC O42 | 1 | An EAEC clinical isolate from the stool of a child with diarrhea in Peru; in published studies, EAEC O42 stimulates IL-8 release | 19, 26, 27 |
| ETEC ST | 11 | ST probe-positive isolates from U.S. travelers to Guadalajara, Mexico | 12, 18 |
| ETEC LT | 10 | LT probe-positive isolates from U.S. travelers to Guadalajara, Mexico | 12, 18 |
| ETECT ST:LT | 8 | Both ST and LT probe-positive isolates from U.S. travelers to Guadalajara, Mexico | 12, 18 |
| EAEC | 30 | E. coli strains, negative for ST or LT probes, that adhere to HEp-2 cells in an aggregative pattern, from U.S. travelers to Guadalajara, Mexico | 12, 18 |
| HS | 1 | Commensal reference strain obtained from an asymptomatic adult that is nonpathogenic; it is not EAEC, ETEC, or EPEC |
HCT-8 cell culture assay.
The HCT-8 cell line, a known human intestinal adenocarcinoma cell line (4), has been shown to be of use as a model of enteric pathogen infectivity (4, 24) and is easily adapted as a laboratory assay (10, 16, 22). Monolayers of the HCT-8 cells (ATCC CCL-244; American Type Culture Collection, Rockville, Md.) were grown in a 24-well culture plate (Corning Costar, Corning, N.Y.). Cells were cultured in RPMI 1640 medium (Sigma Chemical Co., St. Louis, Mo.) supplemented with 100 U of penicillin per ml, 100 μg of streptomycin per ml, and 0.25 μg of amphotericin B per ml. Cell culture growth medium was supplemented with 10% fetal bovine serum (HyClone, Logan, Utah) for maintenance of uninfected cells and growth until confluency with approximately 4 × 105 cells/well was achieved.
Bacterial infection.
HCT-8 cells were infected with enteroaggregative and enterotoxigenic E. coli isolates in triplicate at a 100:1 ratio, as described before (8, 25), in antibiotic- and serum-free RPMI 1640 medium. Briefly, cells were incubated in a humidified incubator at 37°C in an atmosphere containing 5% CO2. After 3 h at 37°C in the 5% CO2 chamber, supernatants were collected and frozen at −70°C. The IL-8 supernatant concentration for each well was determined by using a commercially available enzyme-linked immunosorbent assay system for human IL-8 (Quantikine; R&D Systems, Minneapolis, Minn.) as previously described (9). Results of cytokine concentration were expressed as picograms per milliliter of culture medium. Each study run included wells with no bacteria in the HCT-8 cell culture assay as a control as well as a negative and a positive control (HS and EAEC O42, respectively). All samples were analyzed in triplicate.
Statistical analysis.
Mean elicited IL-8 levels from HCT-8 cells to infection were reported. Significant amounts of IL-8 elicited in the tissue culture model were defined as IL-8 levels of >100 pg/ml (mean IL-8 production of HCT-8 cells to infection with the nonpathogenic HS strain plus 2 standard deviations). Comparisons between groups and levels of IL-8 secretion were done by analysis of variance.
RESULTS
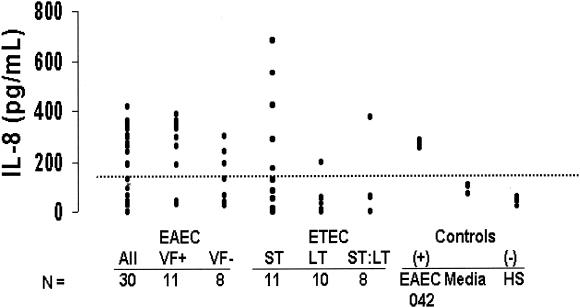
The inflammatory chemokine IL-8 response from HCT-8 intestinal epithelial cells infected with diarrheagenic E. coli pathotypes is shown in Fig. 1. HCT-8 cells produced variable levels of IL-8 in response to infection within and between the different pathotypes studied when compared to E. coli strain HS (negative control). Fifty percent of EAEC isolates caused HCT-8 cells to produce IL-8 levels of >100 pg/ml (mean, 264.32 pg/ml; standard deviation, 89), which were significantly higher than levels seen in response to infection with the HS reference strain (P < 0.01). A subgroup of EAEC was studied for the presence of the virulence factors aggR, aggA, and aspU. Seventy-two percent of EAEC isolates possessing these genes and 40% of EAEC isolates not possessing these genes elicited an IL-8 response of >100 pg/ml. Eleven of 29 (38%) ETEC isolates (7 of which were ETEC ST) also caused HCT-8 colonic epithelial cells to produce IL-8 at levels that were comparable to those seen in response to the EAEC strains. In contrast, ETEC LT and ETEC ST:LT were less likely to cause HCT-8 colonic epithelial cells to produce increased levels of IL-8, at 41.13 pg/ml (±61) and 61.88 pg/ml (±129), respectively (P < 0.05 compared to ETEC ST or EAEC).
FIG. 1.
IL-8 response from intestinal epithelial HCT-8 cell line infected with diarrheagenic E. coli pathotypes. Abbreviations: VF+, virulence factor positive for aggR, aggA, and aspU by PCR; VF-, virulence factor negative; (+), positive control (EAEC strain O42); (-), negative control (HS commensal reference E. coli strain). Note: Data points do not appear to coincide with the number of strains tested due to overlapping values.
DISCUSSION
The intestinal epithelial cell likely acts as a sensor for bacterial invasion and provides early signals of the initiation of the mucosal inflammatory response (15, 23). For instance, HCT-8 cells infected with Cryptosporidium parvum have been shown to elicit C-X-C chemokines, including IL-8 and growth-related oncogene α. Both these chemokines play an important role in the attraction of neutrophils to sites of inflammation and in the activation of cells in the underlying intestinal mucosa (2). Once IL-8 is released, this leads to the accumulation of neutrophils in the intestinal mucosa, leading to tissue disruption and fluid secretion. Upregulation of this proinflammatory response by infected intestinal epithelial cells has also been reported with gram-negative and gram-positive bacteria (8, 14, 23). Intestinal epithelial cells infected with EAEC strain O42 are known to elicit increased amounts of IL-8 release in vitro (25, 26).
In humans, bacterial diarrhea can be associated with measurable increases in fecal IL-8. This has been observed in persons infected with diarrheagenic EAEC possessing the virulence factors EAST-1, a homologue of ST, a secreted protease toxin called plasmid-encoded toxin (Pet), and aggR, a plasmid-borne virulence factor (11).
The results of this study suggest that the HCT-8 cell line allows for the detection of a differential IL-8 response to infection with enteroaggregative and enterotoxigenic E. coli strains and that the HCT-8 cell line can assist in the identification of inflammatory E. coli pathotypes. An area of interest to our lab is the replicability of this response using other intestinal epithelial cell lines. These studies are currently ongoing.
In the case of EAEC, a heterogeneous response was seen to the different isolates. Fifty percent of isolates elicited an IL-8 response. Isolates possessing virulence factors were more likely to be associated with IL-8 production. This is in concordance with our group's previous studies that have shown high levels of fecal IL-8 in subjects with diarrhea due to EAEC that possess specific virulence factors (11). However, in that study as well as in the present study, a proportion of isolates without virulence factors were associated with elicited IL-8, suggesting that additional virulence genes are involved in the epithelial IL-8 response. This is also consistent with the heterologous virulence of EAEC seen in patients with diarrhea (1, 21).
ETEC are considered the most common cause of diarrheal illness in international travelers. These pathogens are thought to be noninvasive without damage to the intestinal epithelial cells and have traditionally been thought to cause noninflammatory diarrhea. ETEC are defined by the detection of ST or LT enterotoxin. The ST enterotoxin is a methanol-soluble peptide that binds and then activates a transmembrane guanylate cyclase and increases intracellular levels of cyclic GMP. In this study, purified ETEC ST was tested as a potential cause of IL-8 release, since 38% (11 of 29) of ETEC ST isolates caused HCT-8 cells to produce increased levels of IL-8. We did not, however, find IL-8 release from HCT-8 cells in response to the addition of purified ETEC ST to HCT-8 cells (data not shown). The LT enterotoxin, which is closely related to cholera toxin, is a peptide that binds to a GM1 ganglioside receptor that activates an adenylate cyclase and increases intracellular levels of cyclic AMP. Both ST and LT enterotoxins cause fluid and electrolyte secretion in the intestinal epithelium, which contributes to the clinical features of ETEC infections. To the authors' knowledge, there are no previous reports on the ability of ETEC to elicit IL-8 production in vitro. In our group's earlier studies, we found that in naturally occurring ETEC ST diarrhea, adult travelers often had high levels of fecal IL-8 (9). It is unclear why ETEC isolates that produce ST enterotoxin would elicit increased amounts of IL-8 and ETEC isolates that possess both ST and LT enterotoxin do not. It is possible that specific colonization factor antigens of ETEC associated with ST-only or ST:LT strains may correspond to increased IL-8 production. Infection with ST-producing ETEC has traditionally been thought of as causing secretory diarrhea with little or no inflammatory response. However, in our experience, a subset of individuals infected with ETEC ST have demonstrated inflammatory markers in stools, such as the presence of fecal leukocytes, lactoferrin, and occult blood (3). The mechanisms responsible for this inflammatory response to ETEC ST have not been well characterized. In our study, 7 of 11 (64%) ETEC ST isolates elicited significant amounts of IL-8 in the tissue culture model.
As mentioned, flagellin is a major determinant of IL-8 release. In our study, the differences in IL-8 release by HCT-8 cells may have been due to the studied virulence factors of EAEC but may have also been due to differences in release of flagellin or in other virulence genes not studied among the different strains of EAEC and ETEC ST.
The present research describes an in vitro study of bacterial enteropathogenesis. HCT-8 cells infected with various diarrheagenic E. coli strains elicit differential IL-8 responses and may help us determine which bacterial enteropathogens produce an intestinal IL-8 response in natural illness (9). Use of the HCT-8 cell line may also assist in the identification of E. coli pathotypes that elicit an intestinal inflammatory response and that currently do not fit the known pathotype definition. It is likely, within each pathotype, that there is an existing spectrum of ability to elicit an intestinal inflammatory response. Future work is needed to identify mechanisms responsible for this proinflammatory effect and to determine the mechanisms that can distinguish the proinflammatory isolates from those that do not elicit IL-8.
Acknowledgments
Financial support for this study was provide by the Public Health Service (grant DK56338), which funds the Texas Gulf Coast Digestive Diseases Center; the National Institute of Allergy and Infectious Diseases (grant NO1-AI-25465); and the Clinical Research Center (grant MOIRR02558), which funds the University of Texas General Clinical Research Center.
REFERENCES
- 1.Adachi, J. A., C. D. Ericsson, Z. D. Jiang, M. W. DuPont, S. R. Pallegar, and H. L. DuPont. 2002. Natural history of enteroaggregative and enterotoxigenic Escherichia coli infection among US travelers to Guadalajara, Mexico. J. Infect. Dis. 185:1681-1683. [DOI] [PubMed] [Google Scholar]
- 2.Baggiolini, M., B. Dewald, and B. Moser. 1994. Interleukin-8 and related chemotactic cytokines—CXC and CC chemokines. Adv. Immunol. 55:97-179. [PubMed] [Google Scholar]
- 3.Bouckenooghe, A. R., H. L. Dupont, Z. D. Jiang, J. Adachi, J. J. Mathewson, M. P. Verenkar, S. Rodrigues, and R. Steffen. 2000. Markers of enteric inflammation in enteroaggregative Escherichia coli diarrhea in travelers. Am. J. Trop. Med. Hyg. 62:711-713. [DOI] [PubMed] [Google Scholar]
- 4.Current, W. L., and T. B. Haynes. 1984. Complete development of Cryptosporidium in cell culture. Science 224:603-605. [DOI] [PubMed] [Google Scholar]
- 5.Czeczulin, J. R., T. S. Whittam, I. R. Henderson, F. Navarro-Garcia, and J. P. Nataro. 1999. Phylogenetic analysis of enteroaggregative and diffusely adherent Escherichia coli. Infect. Immun. 67:2692-2699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Donnelly, M. A., and T. S. Steiner. 2002. Two nonadjacent regions in enteroaggregative Escherichia coli flagellin are required for activation of toll-like receptor 5. J. Biol. Chem. 277:40456-40461. [DOI] [PubMed] [Google Scholar]
- 7.Donnenberg, M. S., and J. P. Nataro. 1995. Methods for studying adhesion of diarrheagenic Escherichia coli. Methods Enzymol. 253:324-336. [DOI] [PubMed] [Google Scholar]
- 8.Eckmann, L., M. F. Kagnoff, and J. Fierer. 1993. Epithelial cells secrete the chemokine interleukin-8 in response to bacterial entry. Infect. Immun. 61:4569-4574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Greenberg, D. E., Z. D. Jiang, R. Steffen, M. P. Verenker, and H. L. DuPont. 2002. Markers of inflammation in bacterial diarrhea among travelers, with a focus on enteroaggregative Escherichia coli pathogenicity. J. Infect. Dis. 185:944-949. [DOI] [PubMed] [Google Scholar]
- 10.Hijjawi, N. S., B. P. Meloni, U. M. Morgan, and R. C. Thompson. 2001. Complete development and long-term maintenance of Cryptosporidium parvum human and cattle genotypes in cell culture. Int. J. Parasitol. 31:1048-1055. [DOI] [PubMed] [Google Scholar]
- 11.Jiang, Z. D., D. Greenberg, J. P. Nataro, R. Steffen, and H. L. DuPont. 2002. Rate of occurrence and pathogenic effect of enteroaggregative Escherichia coli virulence factors in international travelers. J. Clin. Microbiol. 40:4185-4190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jiang, Z. D., B. Lowe, M. P. Verenkar, D. Ashley, R. Steffen, N. Tornieporth, F. von Sonnenburg, P. Waiyaki, and H. L. DuPont. 2002. Prevalence of enteric pathogens among international travelers with diarrhea acquired in Kenya (Mombasa), India (Goa), or Jamaica (Montego Bay). J. Infect. Dis. 185:497-502. [DOI] [PubMed] [Google Scholar]
- 13.Jiang, Z. D., P. C. Okhuysen, D. C. Guo, R. He, T. M. King, H. L. DuPont, and D. M. Milewicz. 2003. Genetic susceptibility to enteroaggregative Escherichia coli diarrhea: polymorphism in the interleukin-8 promoter region. J. Infect. Dis. 188:506-511. [DOI] [PubMed] [Google Scholar]
- 14.Jung, H. C., L. Eckmann, S. K. Yang, A. Panja, J. Fierer, E. Morzycka-Wroblewska, and M. F. Kagnoff. 1995. A distinct array of proinflammatory cytokines is expressed in human colon epithelial cells in response to bacterial invasion. J. Clin. Investig. 95:55-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kagnoff, M. F., and L. Eckmann. 1997. Epithelial cells as sensors for microbial infection. J. Clin. Investig. 100:6-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Meloni, B. P., and R. C. Thompson. 1996. Simplified methods for obtaining purified oocysts from mice and for growing Cryptosporidium parvum in vitro. J. Parasitol. 82:757-762. [PubMed] [Google Scholar]
- 17.Murray, B. E., J. J. Mathewson, H. L. DuPont, and W. E. Hill. 1987. Utility of oligodeoxyribonucleotide probes for detecting enterotoxigenic Escherichia coli. J. Infect. Dis. 155:809-811. [DOI] [PubMed] [Google Scholar]
- 18.Nataro, J. P., S. Hicks, A. D. Phillips, P. A. Vial, and C. L. Sears. 1996. T84 cells in culture as a model for enteroaggregative Escherichia coli pathogenesis. Infect. Immun. 64:4761-4768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nataro, J. P., and J. B. Kaper. 1998. Diarrheagenic Escherichia coli. Clin. Microbiol. Rev. 11:142-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nataro, J. P., I. C. Scaletsky, J. B. Kaper, M. M. Levine, and L. R. Trabulsi. 1985. Plasmid-mediated factors conferring diffuse and localized adherence of enteropathogenic Escherichia coli. Infect. Immun. 48:378-383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Okeke, I. N., A. Lamikanra, J. Czeczulin, F. Dubovsky, J. B. Kaper, and J. P. Nataro. 2000. Heterogeneous virulence of enteroaggregative Escherichia coli strains isolated from children in southwest Nigeria. J. Infect. Dis. 181:252-260. [DOI] [PubMed] [Google Scholar]
- 22.Rochelle, P. A., M. M. Marshall, J. R. Mead, A. M. Johnson, D. G. Korich, J. S. Rosen, and R. De Leon. 2002. Comparison of in vitro cell culture and a mouse assay for measuring infectivity of Cryptosporidium parvum. Appl. Environ. Microbiol. 68:3809-3817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schulte, R., P. Wattiau, E. L. Hartland, R. M. Robins-Browne, and G. R. Cornelis. 1996. Differential secretion of interleukin-8 by human epithelial cell lines upon entry of virulent or nonvirulent Yersinia enterocolitica. Infect. Immun. 64:2106-2113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Slifko, T. R., D. Friedman, J. B. Rose, and W. Jakubowski. 1997. An in vitro method for detecting infectious Cryptosporidium oocysts with cell culture. Appl. Environ. Microbiol. 63:3669-3675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Steiner, T. S., A. A. Lima, J. P. Nataro, and R. L. Guerrant. 1998. Enteroaggregative Escherichia coli produce intestinal inflammation and growth impairment and cause interleukin-8 release from intestinal epithelial cells. J. Infect. Dis. 177:88-96. [DOI] [PubMed] [Google Scholar]
- 26.Steiner, T. S., J. P. Nataro, C. E. Poteet-Smith, J. A. Smith, and R. L. Guerrant. 2000. Enteroaggregative Escherichia coli expresses a novel flagellin that causes IL-8 release from intestinal epithelial cells. J. Clin. Investig. 105:1769-1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.von Sonnenburg, F., N. Tornieporth, P. Waiyaki, B. Lowe, L. F. Peruski, Jr., H. L. DuPont, J. J. Mathewson, and R. Steffen. 2000. Risk and aetiology of diarrhoea at various tourist destinations. Lancet 356:133-134. [DOI] [PubMed] [Google Scholar]