Abstract
To study the immunological effects of nicotine, there are several rodent models for chronic nicotine administration. These models include subcutaneously implanted miniosmotic pumps, nicotine-spiked drinking water, and self-administration via jugular cannulae. Administration of nicotine via these routes affects the immune system. Smokers frequently use nicotine patches to quit smoking, and the immunological effects of nicotine patches are largely unknown. To determine whether the nicotine patch affects the immune system, nicotine patches were affixed daily onto the backs of Lewis rats for 3 to 4 weeks. The patches efficiently raised the levels of nicotine and cotinine in serum and strongly inhibited the antibody-forming cell response of spleen cells to sheep red blood cells. The nicotine patch also suppressed the concanavalin A-induced T-cell proliferation and mobilization of intracellular Ca2+ by spleen cells, as well as the fever response of animals to subcutaneous administration of turpentine. Moreover, immunosuppression was associated with chronic activation of protein tyrosine kinase and phospholipase C-γ1 activities. Thus, in this animal model of nicotine administration, the nicotine patch efficiently raises the levels of nicotine and cotinine in serum and impairs both the immune and inflammatory responses.
Cigarette smoke is a major health risk factor worldwide and significantly increases the incidence of several diseases (reviewed in reference 38). It is hypothesized that this increased disease susceptibility reflects cigarette smoke-induced changes in the immune system (11), and chronic exposure to cigarette smoke suppresses a wide range of immunological parameters in human and animal models (35, 38). Nicotine (NT), a major component of cigarette smoke, has been shown to suppress various parameters of the immune system (reviewed in references 36 and 38). Chronic NT administration of rats by subcutaneously or intracerebroventricularly implanted miniosmotic pumps or self-administration through indwelling jugular cannulae suppresses the T-cell-dependent antibody and T-cell mitogenic responses and inhibits the T-cell antigen receptor (TCR)-mediated cell signaling (8, 31). TCR ligation by anti-TCR antibodies is an accepted in vitro model for an antigen-induced T-cell activation that stimulates protein tyrosine kinase (PTK) and phospholipase C-γ1 (PLC-γ1) activities (22, 26) and increases the intracellular Ca2+ concentration ([Ca2+]i) (2, 4). Use of the NT patch (NTP) has been shown to significantly help human smokers quit smoking (6, 14, 23, 24, 29), and its use has increased dramatically in recent years. In addition, NTPs have been considered for therapeutic use in some diseases such as Parkinson's disease and ulcerative colitis. However, the immunological effects of NTPs are largely unknown. Therefore, in the present study we used Lewis rats to examine the effects of the NTP on the immune and inflammatory responses.
MATERIALS AND METHODS
Animals.
Pathogen-free male Lewis rats were purchased from Harlan Sprague-Dawley Farms (Indianapolis, Ind.). Food (Lab Blox; Tekland, Madison, Wis.) and water were provided ad libitum to the animals. Animals that were 6 to 12 weeks old were used in these studies.
NTP treatment.
Seven-milligram NTPs (Nicoderm CQ) were purchased locally from a Wal-Mart store. The backs of the rats were shaved, and one-eighth or one-fourth of the patch (i.e., ∼0.8 or 1.7 mg of NT, respectively) was applied to the skin and swathed with a Johnson & Johnson self-adhesive bandage. The patch was replaced every day for 3 to 4 weeks. The levels of NT and cotinine in serum of the one-fourth NTP-treated animals were 75 ± 25 and 850 ± 250 ng/ml, respectively; this approximates the concentrations of NT and cotinine in serum in humans that smoke two to four packs/day (7, 44).
Measurement of Tb.
To measure deep body temperature (Tb), rats were intraperitoneally implanted with biotelemeters (model VM-FH; Mini-Mitter Co., Sunriver, Oreg.) (17). Following the implantation, animals were housed individually in plastic cages in rooms with an ambient temperature of 25°C. Signals were collected by receiver boards (model RA1010; Mini-Mitter Co.) placed under each cage and stored on an IBM personal computer using a data acquisition system (Dataquest 111; Mini-Mitter Co.).
Turpentine-induced sterile abscess.
Sterile tissue damage (local inflammation) was induced using commercial-grade, steam-distilled Fir oil (turpentine) (Fluka Chemie GmbH, Buchs, Switzerland). Rats were injected subcutaneously in the left hind limb with 100 μl of turpentine or pyrogen-free saline (control [CON]) and sacrificed 48 h later.
Immunizations.
To measure antibody-forming cell (AFC) response, animals were injected intravenously with 5 × 108 sheep red blood cells (SRBC) 4 days prior to sacrifice as described previously (34).
Determination of NT and cotinine levels in serum.
One milliliter of a serum sample from an NTP-treated or CON animal was extracted with 1 ml of sodium tetraborate (20 g/liter), 3 ml of 50:50 dichloromethane-dichloroethane (Sigma-Aldrich Corp., St. Louis, Mo.), and 100 ng each of deuterated NT and cotinine (Cerilliant, Austin, Tex.). The sample extract (lower layer of centrifuged solution) was decanted in a scintillation vial, evaporated under a gentle stream of nitrogen, and reconstituted in 1 ml of analytical-grade methanol (Fisher Scientific, Irvine, Calif.). The sample was analyzed by high-pressure liquid chromatography (Shimadzu SCL 10A system controller coupled to a triple quadrupole mass spectrometer [model API 365; Applied Biosystems] in the positive ionization mode using a turbo ion-spray ionization source). Parent and daughter ions for NT and deuterated NT were selectively monitored at 163 and 106 atomic mass units (amu) and 167 and 134 amu, while cotinine and deuterated cotinine were monitored at 177 and 80 amu and 180 and 80 amu, respectively. Analyte concentrations were determined as the ratio of the compound area to the area counts of the spiked deuterated analog. We compensated for differences in response between deuterated compounds and target analytes by creating calibration curves that spanned the range of the sample concentrations.
Preparation of spleen cell suspensions.
Spleen cell suspensions were prepared as described previously (34). Briefly, spleens were pressed through stainless steel mesh, and RBCs were lysed by treatment with NH4Cl solution. The cell preparations were washed with phosphate-buffered saline (PBS), counted, and suspended in complete medium (RPMI 1640 containing 10% fetal calf serum, 2 mM glutamine, 50 mM 2-mercaptoethanol, and 10 μg of gentamicin per ml).
Enumeration of lymphocyte subpopulations.
The number of cells and purity of lymphocyte subpopulations were determined by fluorescence-activated cell sorting analysis on an EPICS C flow cytometer (Coulter) as reported previously (7). Briefly, to ascertain the percentages of B cells and T cells, spleen cells were incubated with predetermined optimal amounts of fluorescein isothiocyanate-conjugated anti-rat CD45RA and anti-rat CD3 monoclonal antibodies (MAb) (PharMingen, San Diego, Calif.), respectively; to determine the number of cells in T-cell subsets, the cells were incubated with anti-rat CD4 and anti-rat CD8 MAb, respectively. Cells were treated with the appropriate isotype-matched immunoglobulins to control for nonspecific binding. The percentage of positive cells was determined by subtracting the nonspecific (isotype control) from the specific fluorescence profiles.
AFC assay.
The primary direct AFC response was determined by the method of Cunningham and Szenberg (5) as modified by Sopori et al. (34). Briefly, various concentrations of spleen cells were mixed with 2% SRBC and 20 μl of reconstituted Hemo-Lo guinea pig complement (Accurate Chemicals, Westbury, N.Y.) in a final volume of 140 μl of complete medium. The cell suspension was injected into Cunningham slides, sealed with petroleum wax, and incubated for 45 min at 37°C. Plaques were counted under a microscope, and counts were expressed as numbers of AFC/106 spleen cells.
Proliferative response to ConA.
The proliferative response of spleen cells to the T-cell mitogen concanavalin A (ConA) was determined by the method of Sopori et al. (33). Briefly, 5 × 105 cells were cultured in triplicate in 0.2 ml of complete medium in the presence of various concentrations of ConA (Sigma Chemical Co., St. Louis, Mo.) in microtiter plate wells. The cultures were incubated at 37°C in the presence of 5% CO2; after 3 days, they were pulsed with 0.5 μCi of [3H]thymidine for 18 h before harvesting. The cells were harvested onto filter paper by a Skatron cell harvester (Skatron, Inc., Sterling, Va.) and counted in a liquid scintillation counter.
Assay for [Ca2+]i.
[Ca2+]i levels were determined by the indo-1AM {1-[2-amino-5-(6-carboxy-2-indolyl)phenoxy]-2-(2-amino-5-methylphenoxy)ethane-N,N,N′,N′-tetraacetic acid, pentaacetoxymethyl ester} (Molecular Probes, Eugene, Oreg.) fluorescence method as described previously (25). Briefly, spleen cells (5 × 106/ml) were incubated at 37°C for 45 min with 5 μM indo-1AM in the loading medium (PBS containing 1% fetal calf serum). After the cells were washed, they were suspended in the loading medium and incubated at 37°C for 15 min in 5% CO2. Cells were kept in the dark on ice until the assay. Before each measurement, 2 ml of the cell suspension was quickly spun and resuspended in 2 ml of PBS containing 1 mM Ca2+. Measurement of [Ca2+]i was started 60 s before and after the addition of anti-αβ-TCR MAb and anti-mouse immunoglobulin G (IgG) (second Ab) by spectrofluorometry in a PTI Deltascan fluorometer (Photon Technology International, South Brunswick, N.J.) at 37°C with constant gentle stirring. [Ca2+]i was calculated as described previously (7).
Western blot analysis for PTK and PLC-γ1 activities.
PTK activity was determined by immunoblotting using antiphosphotyrosine (anti-PY) MAb (8). Briefly, freshly prepared spleen cells (107) were suspended in complete medium and incubated with 2 μg of anti-αβ-TCR MAb per ml for 2 min at 37°C. The reaction was stopped by the addition of ice-cold PBS, and after gentle centrifugation, the cell pellet was extracted with lysis buffer (10 mM Tris-HCl [pH 7.8], 150 mM NaCl, 0.1 mM sodium vanadate, 50 mM NaF, 30 mM sodium pyrophosphate, 2 mM iodoacetate, 5 μM zinc chloride, 1.5 mM phenylmethylsulfonyl fluoride, 2 μg of leupeptin per ml, 10 μg of antipain per ml, 5 μg of aprotinin per ml, and 3 μg of pepstatin A per ml) containing 1% Nonidet P-40. Lysates were centrifuged at 12,000 × g for 15 min at 4°C to remove insoluble debris and boiled in Laemmli sample buffer (60 mM Tris-HCl [pH 6.8], 2% sodium dodecyl sulfate [SDS], 10% glycerol, 100 mM EDTA, 0.001% bromophenol blue) for 5 min. Protein concentration was determined by the bicinchoninic acid method (Pierce, Rockford, Ill.), and equal amounts of protein (10 to 20 μg) were electrophoresed on SDS-7.5% polyacrylamide gels and transferred to nitrocellulose membranes. The blots were blocked with 5% dry skim milk protein (Upstate Biotechnology, Lake Placid, N.Y.) in 10 mM Tris-HCl (pH 7.4) containing 150 mM NaCl for 1.5 h at room temperature. The blots were incubated with 1 μg of anti-PY MAb per ml for 1 h at room temperature, washed, and developed with horseradish peroxidase-conjugated goat anti-mouse IgG (Bio-Rad, Hercules, Calif.).
PLC-γ1 activity was determined by immunoprecipitation followed by immunoblotting (8). Briefly, after the detergent-soluble lysates were precleared with protein A-agarose, the lysates were incubated overnight at 4°C with anti-PY Ab agarose beads (Transduction Laboratories, Lexington, Ky.) with constant gentle rocking. Immunoprecipitates were washed three times with Nonidet P-40-containing lysis buffer and twice with lysis buffer without Nonidet P-40. Washed immunoprecipitates were boiled in sample buffer for 5 min, and proteins were separated on SDS-7.5% polyacrylamide gels. After the proteins were transferred and blocked, the blots were treated with anti-PLC-γ1 MAb and developed with alkaline phosphatase-conjugated goat anti-mouse IgG (Bio-Rad, Hercules, Calif.). Blots were analyzed with anti-PY MAb as described above.
Data analysis.
Excel software was used for the statistical evaluation of T-cell proliferation data. Data were expressed as means ± standard deviations of three replicate cultures. Comparisons between CON and treated groups were made by the unpaired t test, and a P value of ≤0.05 was considered statistically significant.
RESULTS
The NTP inhibits the AFC response in rats.
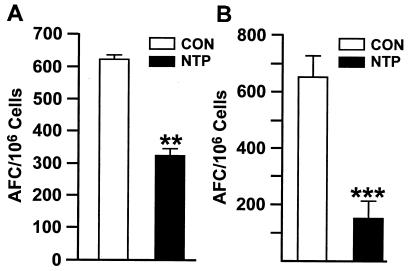
To examine whether NTPs are effective in modulating the immune response in rats, a commercially available 7-mg Nicoderm CQ NTP was cut into four and eight equal pieces, and one piece (equivalent to approximately 1.7 and 0.8 mg of NT, respectively) was applied daily to each animal for 3 to 4 weeks. Four days prior to sacrifice, animals were immunized with the T-cell-dependent antigen, SRBC. Figure 1 shows that the NTP strongly inhibited the anti-SRBC AFC responses of animals with one-eighth (Fig. 1A) and one-fourth (Fig. 1B) NTPs. Thus, in rats, the NTP raises the levels of NT and cotinine in blood and inhibits the T-cell-dependent AFC response.
FIG. 1.
The NTP inhibits the AFC response to SRBC. Five CON rats and five NTP-treated rats (with one-eighth [A] or one-fourth [B] section of a NTP) were immunized with SRBC and euthanized 4 days later. The anti-SRBC AFC response of spleen cells was determined as described in Materials and Methods. Values that were significantly different from the values for CON rats are indicated as follows: **, P ≤ 0.05; ***, P ≤ 0.001.
NTP inhibits T-cell proliferation.
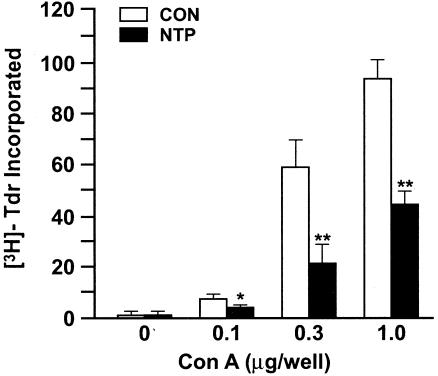
The response to the T-cell mitogen ConA is an established criterion for cell-mediated immunity. To determine whether the NTP affected the cell-mediated immune response, spleen cells were cultured with various concentrations of ConA for 3 days, and T-cell proliferation was determined by incorporation of [3H]thymidine into the cells. Figure 2 shows that rats treated daily with one-fourth NTPs exhibited significant reductions in ConA-induced T-cell proliferation at all tested concentrations. Similar changes in the ConA response were also observed with one-eighth NTP (not shown). Changes in T-cell proliferation were not due to significant changes in lymphocyte numbers or subset distribution in spleen cells, i.e., CD45RA (B cells), CD3+ (T cells) CD4+ (T-helper subset), or CD8+ (T cytotoxic or suppressor subset) as assayed by flow cytometry (data not shown). Thus, the NTP inhibits both the humoral and cell-mediated immune responses, and these effects are not associated with significant changes in the lymphocyte subset distribution.
FIG. 2.
The NTP suppresses the ConA-induced proliferative response. Spleen cells from five CON rats and five NTP-treated rats were treated with the indicated concentration of ConA for 3 days. Cell proliferation was determined as described in Materials and Methods. The level of [3H]thymidine ([3H]-Tdr) incorporated into cells (103 cpm) is shown. Results are representative of two separate experiments. Values that were significantly different from the values for CON rats are indicated as follows: *, P ≤ 0.05; **, P ≤ 0.01.
The NTP inhibits the increase in [Ca2+]i after TCR ligation.
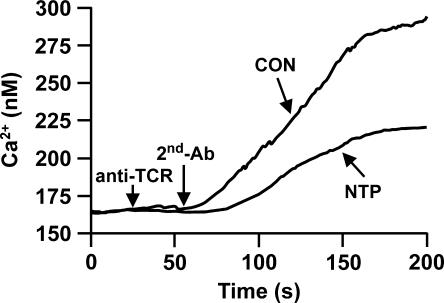
The proliferation of rat T cells is activated in response to TCR ligation with anti-CD3 or anti-αβ-TCR Abs (15), and an increase in the [Ca2+]i is an early event in the T-cell activation process (4). To investigate whether the NTP affected the antigen-mediated signaling in T cells, spleen cells were treated with mouse anti-rat αβ-TCR MAb followed by anti-mouse IgG. Figure 3 shows that the NTP inhibits the ability of T cells to mobilize [Ca2+]i. Compared to CON animals, T cells from rats treated with one-fourth NTP exhibit smaller increases in the [Ca2+]i (50 to 70% lower) after TCR ligation with the five different CON-NTP combinations studied. Thus, treatment with an NTP impairs the TCR-mediated Ca2+ mobilization in T cells.
FIG. 3.
The NTP decreases the TCR-induced increase in [Ca2+]i in T cells. Indo-1AM-labeled spleen cells were incubated with mouse anti-rat anti-αβ-TCR MAb followed by goat anti-mouse polyclonal Ab (second Ab). The [Ca2+]i was calculated as described in Materials and Methods. Compared to CON animals, T cells from rats treated with one-fourth NTPs exhibited a ca. 50% lower increase in the [Ca2+]i after TCR ligation. Results are representative of experiments testing four separate combinations of CON and NTP-treated animals.
The NTP causes activation of PTK and PLC-γ1 activities.
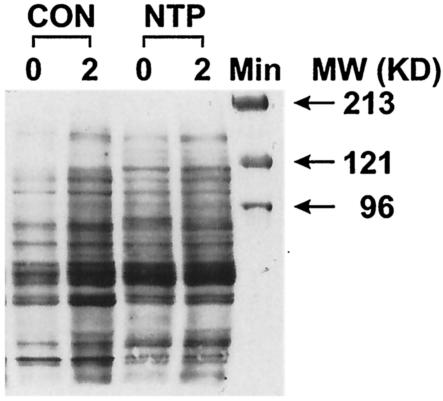
Increased PTK activity is one of the earliest steps in T-cell activation (44, 45); however, despite the decreased [Ca2+]i, the induction of T-cell anergy in cell culture models is associated with chronic activation of PTK activity (16, 32). In fact, chronic subcutaneous administration of NT via miniosmotic pumps causes constitutive activation of PTK activity (8). To ascertain whether NTP affects PTK activity, spleen cells from CON and NTP-treated animals were treated with anti-αβ-TCR MAb for 2 min or not treated with anti-αβ-TCR MAb (background), and PTK activity was analyzed by Western blotting. Figure 4 shows that, while CON animals showed significant increases in tyrosine phosphorylation of various substrates, PTK activity was elevated even before the addition of the anti-αβ-TCR MAb in NTP-treated animals. Indeed, in NTP-treated spleen cells, the magnitude of tyrosine phosphorylation did not change significantly after the addition of anti-αβ-TCR MAb. Thus, the NTP causes constitutive activation of PTK activity in T cells. Because the Western blots show tyrosine phosphorylation of multiple bands (substrates), it is likely that NTP causes a generalized activation of multiple PTK activities.
FIG. 4.
The NTP constitutively activates PTK activities. Western blot analysis of spleen cell lysates from CON and NTP-treated rats after incubation with anti-αβ-TCR MAb for 0 and 2 min. PTK activity was determined by immunoblotting using antiphosphotyrosine (anti-Pγ) MAb. The positions of molecular mass (MW) markers (in kilodaltons) are shown to the right of the gel.
One of the downstream effects of PTK activation in the TCR-mediated signaling pathway is an increase in PLC-γ1 activity that is regulated by tyrosine phosphorylation of the enzyme (i.e., the active enzyme is tyrosine phosphorylated). To examine whether NTP treatment affected activation stimulated by anti-αβ-TCR PLC-γ1, cell lysates from CON and NTP-treated spleen cells were immunoprecipitated with anti-PLC-γ1 MAb, and the immunoprecipitates were run on polyacrylamide gels. The blots were probed with anti-PY MAb. Data presented in Fig. 5 show that NTP caused constitutive activation (tyrosine phosphorylation) of PLC-γ1.
FIG. 5.
The NTP increases tyrosine phosphorylation of PLC-γ1. Western blot analysis was performed on spleen cell lysates from CON and NTP-treated rats after incubation with anti-TCR MAb for 0 and 2 min. Densitometric analysis indicates increases of more than twofold over the basal levels (unstimulated [0 min]) of PLC-γ by NTP treatment. The position of the 135-kDa molecular mass marker is shown to the left of the blot.
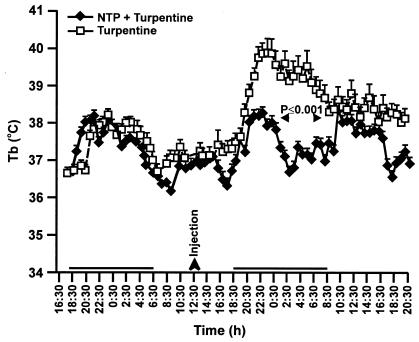
The NTP suppresses turpentine-induced inflammation.
One of the earliest responses to turpentine-induced inflammation (sterile abscess) is an increased Tb (9, 18), and fever is a recognized manifestation of inflammation and activation of innate immune responses (28). To determine whether the NTP modulated the inflammatory response, NTP-treated and CON animals were injected with turpentine in the hind leg, and their Tbs were recorded. Figure 6 shows that the turpentine-induced fever was significantly attenuated in NTP-treated rats. Thus, NTP treatment suppresses inflammatory responses.
FIG. 6.
The NTP attenuates turpentine-induced increase in Tb. Five CON rats and five NTP-treated rats were injected with 100 μl of turpentine, and the Tb of each was recorded for 32 h after the injection. NTP treatment attenuates the increase in Tb by turpentine.
DISCUSSION
Tobacco smoking suppresses the immune system, and smokers have a higher incidence of diseases, such as lung cancer, cardiovascular disease, chronic obstructive pulmonary disease, and respiratory infections (38). Interestingly, smokers have a lower incidence of some diseases, including ulcerative colitis, sarcoidosis, endometriosis, uterine fibroids, farmers' lung, pigeon breeders' disease, Parkinson's disease, and Sjögren's syndrome (38). Many of these diseases are inflammatory in nature or have an inflammatory component. In animal studies, NT, a major constituent of cigarette smoke, suppresses the immune system when provided by subcutaneously implanted miniosmotic pumps (38). NT may help in human cutaneous inflammation (19) and ulcerative colitis (13, 30, 39). Transdermal NTPs have been used in humans primarily to aid in smoking cessation (6, 14, 23, 24, 29); however, the efficacy of the NTP to modulate immune and inflammatory responses has not been ascertained in animal models. Results presented herein show that a section (one-quarter to one-eighth) of a 7-mg NTP applied to the back of an adult rat raises the levels of NT and cotinine in serum to levels comparable to those of a two- to four-pack/day human smoker. NTP treatment modulated various parameters of the immune response. Thus, animals treated with one-eighth or one-fourth of an NTP exhibited a significant drop in the anti-SRBC AFC and ConA-induced proliferative responses, indicating that NTP suppresses both the humoral and cell-mediated immunities. It is possible that the decreased T-cell responses in NTP-treated splenocytes reflected NTP-induced changes in the composition of lymphocyte populations (e.g., decreased T-cell numbers or altered subset distribution). However, flow cytometric analysis of splenocytes did not show significant differences in lymphocyte numbers or subset distribution in CON and NTP-treated animals. Thus, transdermal NTPs effectively release NT and suppress the immunological responses in rodent models.
We have previously reported that chronic subcutaneous NT administration is associated with impaired TCR-mediated signaling (8). Spleen cells from animals treated for 3 to 4 weeks with NTP showed constitutive activation of PTKs and PLC-γ1 in T cells. In T cells, activation of PLC-γ1 is dependent on the TCR-mediated stimulation of PTK activity (22), leading to the production of inositol-1,4,5-trisphosphate (IP3). The latter releases Ca2+ from the IP3-sensitive intracellular Ca2+ stores that trigger Ca2+ influx and an increase in [Ca2+]i in the cell. The increase in the [Ca2+]i after anti-αβ-TCR MAb treatment was significantly reduced in animals treated with one-quarter NTPs. However, several animals treated with one-eighth NTPs did not show reproducible significant reductions in the anti-TCR-induced increase in the [Ca2+]i. Our preliminary results with T-cell lines show that compared to activation of PTK and PLC-γ1, changes in the [Ca2+]i require much higher concentrations of NT (S. Razani-Boroujerdi, R. Kalra, C. Knall, F. F. Hahn, S. P. Singh, R. J. Langley, and M. L. Sopori, submitted for publication). Thus, in addition to PTK, the NT-induced [Ca2+]i might be regulated by several factors or pathways. Constitutive PTK activation coupled with decreased TCR-mediated Ca2+ response is a hallmark of in vitro anergized T cells (16, 32). Thus, chronic NT treatment with NTP might produce T-cell anergy.
Many explanations have been offered for the decreased incidence of some inflammatory and neurological diseases in smokers (1, 3, 12, 20, 27, 42). It is possible that the anti-inflammatory property of NT is, at least in part, responsible for the decreased incidence of these diseases in tobacco smokers (37). To ascertain whether the NTP affects inflammation, we used the turpentine-induced sterile abscess model for inflammatory responses. Administration of turpentine in CON rats caused an increase in body temperature within 1 or 2 h after the administration (18); NTP treatment, however, essentially eliminated this response. Attenuation of the inflammatory response in NTP-treated rats might result from decreased migration of leukocytes to the site of inflammation (Razani-Boroujerdi et al., submitted). In addition, our preliminary results indicate that chronic NT administration decreases the inducible expression of proinflammatory cytokines, such as interleukin 1β and tumor necrosis factor alpha (data not shown). Similarly, it has been reported that the nicotinic acetylcholine receptor alpha 7 subunit is required for acetylcholine inhibition of the release of tumor necrosis factor from macrophages(43). Thus, NTP-induced changes in inflammation might explain some of the “beneficial” effects of smoking. The Lewis rat has been used as an experimental animal model for many inflammatory and autoimmune diseases (10, 21, 40, 41, 46, 47), and the transdermal NTP could be used to test the therapeutic potential of NT for various inflammatory diseases.
Acknowledgments
These studies were supported in part by grants from the National Institutes of Health (DAO4208-14 and DAO4208-07) and Lovelace Respiratory Research Institute (SOPO-02 and SOPO-03). In conducting research using animals, the investigators adhered to the Guide for the Care and Use of Laboratory Animals (4a). These studies were conducted at the Lovelace Respiratory Research Institute, a facility fully accredited by the Association for the Assessment and Accreditation of Laboratory Animal Care International.
We thank Sandra McKay for critically reading the manuscript.
REFERENCES
- 1.Arneric, S. P., J. P. Sullivan, M. W. Decker, J. D. Brioni, A. W. Bannon, C. A. Briggs, D. Donnelly-Roberts, R. J. Radek, K. C. Marsh, and J. Kyncl. 1995. Potential treatment of Alzheimer disease using cholinergic channel activators (ChCAs) with cognitive enhancement, anxiolytic-like, and cytoprotective properties. Alzheimer Dis. Assoc. Disord. 9(Suppl. 2):50-61. [DOI] [PubMed] [Google Scholar]
- 2.Berridge, M. J. 1993. Cell signalling. A tale of two messengers. Nature 365:388-389. [DOI] [PubMed]
- 3.Birtwistle, J., and K. Hall. 1996. Does nicotine have beneficial effects in the treatment of certain diseases? Br. J. Nurs. 5:1195-1202. [DOI] [PubMed] [Google Scholar]
- 4.Clapham, D. E. 1995. Calcium signaling. Cell 80:258-268. [DOI] [PubMed] [Google Scholar]
- 4a.Committee on Care and Use of Laboratory Animals. 1985. Guide for the care and use of laboratory animals. Institute of Laboratory Animal Resources, Commission on Life Sciences, National Research Council, National Academy Press, Washington, D.C.
- 5.Cunningham, A. J., and A. Szenberg. 1968. Further improvements in the plaque technique for detecting single antibody-forming cells. Immunology 14:599-600. [PMC free article] [PubMed] [Google Scholar]
- 6.Davidson, M., M. Epstein, R. Burt, C. Schaefer, G. Whitworth, and A. McDonald. 1998. Efficacy and safety of an over-the-counter transdermal nicotine patch as an aid for smoking cessation. Arch. Fam. Med. 7:569-574. [DOI] [PubMed] [Google Scholar]
- 7.Geng, Y., S. M. Savage, L. J. Johnson, J. C. Seagrave, and M. L. Sopori. 1995. Effects of nicotine on the immune response. I. Chronic exposure to nicotine impairs antigen receptor-mediated signal transduction in lymphocytes. Toxicol. Appl. Pharmacol. 135:268-278. [DOI] [PubMed] [Google Scholar]
- 8.Geng, Y., S. M. Savage, S. Razani-Boroujerdi, and M. L. Sopori. 1996. Effects of nicotine on the immune response. II. Chronic nicotine treatment induces T cell anergy. J. Immunol. 156:2384-2390. [PubMed] [Google Scholar]
- 9.Gershenwald, J. E., Y. M. Fong, T. J. Fahey III, S. E. Calvano, R. Chizzonite, P. L. Kilian, S. F. Lowry, and L. L. Moldawer. 1990. Interleukin 1 receptor blockade attenuates the host inflammatory response. Proc. Natl. Acad. Sci. USA 87:4966-4970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hasegawa, Y., H. Kaneoka, T. Tanaka, S. Ogahara, T. Matsumae, R. Noda, K. Yoshitake, T. Murata, and S. Naito. 2001. Suppression of experimental membranous glomerulonephritis in rats by an anti-MHC class II antibody. Nephron 88:233-240. [DOI] [PubMed] [Google Scholar]
- 11.Holt, P. G., and D. Keast. 1977. Environmentally induced changes in immunological function: acute and chronic effects of inhalation of tobacco smoke and other atmospheric contaminants in man and experimental animals. Bacteriol. Rev. 41:205-216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.James, J. R., and A. Nordberg. 1995. Genetic and environmental aspects of the role of nicotinic receptors in neurodegenerative disorders: emphasis on Alzheimer's disease and Parkinson's disease. Behav. Genet. 25:149-159. [DOI] [PubMed] [Google Scholar]
- 13.Jarvik, M. E. 1991. Beneficial effects of nicotine. Br. J. Addict. 86:571-575. [DOI] [PubMed] [Google Scholar]
- 14.Jolicoeur, D. G., K. P. Richter, J. S. Ahluwalia, M. C. Mosier, and K. Resnicow. 2003. Smoking cessation, smoking reduction, and delayed quitting among smokers given nicotine patches and a self-help pamphlet. Subst. Abuse 24:101-106. [DOI] [PubMed] [Google Scholar]
- 15.Kalra, R., S. P. Singh, S. M. Savage, G. L. Finch, and M. L. Sopori. 2000. Effects of cigarette smoke on the immune response: chronic exposure to cigarette smoke impairs antigen-mediated signaling in T cells and depletes IP3-sensitive calcium stores. J. Pharmacol. Exp. Ther. 293:166-171. [PubMed] [Google Scholar]
- 16.Kimura, M., M. Yamashita, M. Kubo, M. Iwashima, C. Shimizu, K. Tokoyoda, J. Chiba, M. Taniguchi, M. Katsumata, and T. Nakayama. 2000. Impaired Ca/calcineurin pathway in in vivo anergized CD4 T cells. Int. Immunol. 12:817-824. [DOI] [PubMed] [Google Scholar]
- 17.Kozak, W., H. Zheng, C. A. Conn, D. Soszynski, L. H. van der Ploeg, and M. J. Kluger. 1995. Thermal and behavioral effects of lipopolysaccharide and influenza in interleukin-1 beta-deficient mice. Am. J. Physiol. 269:R969-R977. [DOI] [PubMed] [Google Scholar]
- 18.Kozak, W., J. J. Klir, C. A. Conn, and M. J. Kluger. 1997. Attenuation of lipopolysaccharide fever in rats by protein kinase C inhibitors. Am. J. Physiol. 273:R873-R879. [DOI] [PubMed] [Google Scholar]
- 19.Mills, C. M., T. J. Peters, and A. Y. Finlay. 1993. Does smoking influence acne? Clin. Exp. Dermatol. 18:100-101. [DOI] [PubMed] [Google Scholar]
- 20.Newhouse, P. A., A. Potter, and E. D. Levin. 1997. Nicotinic system involvement in Alzheimer's and Parkinson's diseases. Implications for therapeutics. Drugs Aging 11:206-228. [DOI] [PubMed] [Google Scholar]
- 21.Nicot, A., P. V. Ratnakar, Y. Ron, C. C. Chen, and S. Elkabes. 2003. Regulation of gene expression in experimental autoimmune encephalomyelitis indicates early neuronal dysfunction. Brain 126:398-412. [DOI] [PubMed] [Google Scholar]
- 22.Nishibe, S., M. Wahl, S. M. T. Hernandez-Sotomayor, N. K. Tonks, S. G. Rhee, and G. Carpenter. 1990. Increase of the catalytic activity of phospholipase C-γ1 by tyrosine phosphorylation. Science 250:1253-1256. [DOI] [PubMed] [Google Scholar]
- 23.Perng, R. P., W. C. Hsieh, Y. M. Chen, C. C. Lu, and S. J. Chiang. 1998. Randomized, double-blind, placebo-controlled study of transdermal nicotine patch for smoking cessation. J. Formos. Med. Assoc. 97:547-551. [PubMed] [Google Scholar]
- 24.Prochazka, A. V. 2000. New developments in smoking cessation. Chest 117:169S-175S. [DOI] [PubMed]
- 25.Razani-Boroujerdi, S., L. D. Partridge, and M. L. Sopori. 1994. Intracellular calcium signaling induced by thapsigargin in excitable and inexcitable cells. Cell Calcium 16:467-474. [DOI] [PubMed] [Google Scholar]
- 26.Robey, E., and J. P. Allison. 1995. T-cell activation: integration of signals from the antigen receptor and costimulatory molecules. Immunol. Today 16:306-310. [DOI] [PubMed] [Google Scholar]
- 27.Salomon, A. R., K. J. Marcinowski, R. P. Friedland, and M. G. Zagorski. 1996. Nicotine inhibits amyloid formation by the beta-peptide. Biochemistry 35:13568-13578. [DOI] [PubMed] [Google Scholar]
- 28.Saper, C. B. 1998. Neurobiological basis of fever. Ann. N. Y. Acad. Sci. 856:90-94. [DOI] [PubMed] [Google Scholar]
- 29.Silagy, C., D. Mant, G. Fowler, and T. Lancaster. 2000. Nicotine replacement therapy for smoking cessation. Cochrane Database Syst. Rev. 2000(3):CD000146. [Online.] [DOI] [PubMed]
- 30.Silverstein, M. D., B. A. Lashner, and S. B. Hanauer. 1994. Cigarette smoking and ulcerative colitis: a case-control study. Mayo Clin. Proc. 69:425-429. [DOI] [PubMed] [Google Scholar]
- 31.Singh, S. P., R. Kalra, P. Puttfarcken, A. Kozak, J. Tesfaigzi, and M. L. Sopori. 2000. Acute and chronic nicotine exposures modulate the immune system through different pathways. Toxicol. Appl. Pharmacol. 164:65-72. [DOI] [PubMed] [Google Scholar]
- 32.Sloan-Lancaster, J., and P. M. Allen. 1996. Altered peptide ligand-induced partial T cell activation: molecular mechanisms and role in T cell biology. Annu. Rev. Immunol. 14:1-27. [DOI] [PubMed] [Google Scholar]
- 33.Sopori, M. L., Y. L. Hurt, S. Cherian, A. M. Kaplan, and T. Diamantstein. 1987. Differential requirement for accessory cells in polyclonal T cell activation. Cell. Immunol. 105:174-186. [DOI] [PubMed] [Google Scholar]
- 34.Sopori, M. L., S. Cherian, R. Chilukuri, and G. M. Shopp. 1989. Cigarette smoke causes inhibition of the immune response to intratracheally administered antigens. Toxicol. Appl. Pharmacol. 97:489-499. [DOI] [PubMed] [Google Scholar]
- 35.Sopori, M. L., N. Goud, and A. M. Kaplan. 1994. Effects of tobacco smoke on the immune system, p. 413-434. In D. Luster, A. Munson, and I. Kimber (ed.), Immunotoxicology and immunopharmacology. Raven Press, New York, N.Y.
- 36.Sopori, M. L. 1998. Immunosuppressive and anti-inflammatory properties of nicotine, p. 197-209. In S. Americ and J. D. Brioni (ed.), Neuronal nicotinic receptors: pharmacology and therapeutic opportunities. John Wiley & Sons Press, New York, N.Y.
- 37.Sopori, M. L., W. Kozak, S. M. Savage, Y. Geng, D. Soszynski, M. J. Kluger, E. K. Perryman, and G. E. Snow. 1998. Effect of nicotine on the immune system: possible regulation of immune responses by central and peripheral mechanisms. Psychoneuroendocrinology 23:189-204. [DOI] [PubMed] [Google Scholar]
- 38.Sopori, M. L. 2002. Effects of cigarette smoke on the immune system. Nat. Rev. Immunol. 2:372-377. [DOI] [PubMed] [Google Scholar]
- 39.Srivastava, E. D., M. A. Russell, C. Feyerabend, and J. Rhodes. 1990. Effect of ulcerative colitis and smoking on rectal blood flow. Gut 31:1021-1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stanislaus, R., A. G. Gilg, A. K. Singh, and I. Singh. 2002. Immunomodulation of experimental autoimmune encephalomyelitis in the Lewis rats by lovastatin. Neurosci. Lett. 333:167-170. [DOI] [PubMed] [Google Scholar]
- 41.Stephan, M., R. H. Straub, T. Breivik, R. Pabst, and S. von Horsten. 2002. Postnatal maternal deprivation aggravates experimental autoimmune encephalomyelitis in adult Lewis rats: reversal by chronic imipramine treatment. Int. J. Dev. Neurosci. 20:125-132. [DOI] [PubMed] [Google Scholar]
- 42.Trauth, J. A., F. J. Seidler, E. C. McCook, and T. A. Slotkin. 1999. Adolescent nicotine exposure causes persistent upregulation of nicotinic cholinergic receptors in rat brain regions. Brain Res. 851:9-19. [DOI] [PubMed] [Google Scholar]
- 43.Wang, H., M. Yu, M. Ochani, C. A. Amella, M. Tanovic, S. Susarla, J. H. Li, H. Wang, H. Yang, L. Ulloa, Y. Al-Abed, C. J. Czura, and K. J. Tracey. 2003. Nicotinic acetylcholine receptor alpha 7 subunit is an essential regulator of inflammation. Nature 421:328-329. [DOI] [PubMed] [Google Scholar]
- 44.Weiss, A., G. Koretzky, R. C. Schatzman, and T. Kadlecek. 1991. Functional activation of the T-cell antigen receptor induces tyrosine phosphorylation of phospholipase C-γ1. Proc. Natl. Acad. Sci. USA 8:5484-5488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Weiss, A., and D. R. Littman. 1994. Signal transduction by lymphocyte antigen receptors. Cell 76:263-274. [DOI] [PubMed] [Google Scholar]
- 46.Yu, S., Y. Zhu, Z. Chen, M. Alheim, A. Ljungberg, and J. Zhu. 2002. Initiation and development of experimental autoimmune neuritis in Lewis rats is independent of the cytotoxic capacity of NKR-P1A+ cells. J. Neurosci. Res. 67:823-828. [DOI] [PubMed] [Google Scholar]
- 47.Zou, L. P., N. Abbas, I. Volkmann, I. Nennesmo, M. Levi, B. Wahren, B. Winblad, G. Hedlund, and J. Zhu. 2002. Suppression of experimental autoimmune neuritis by ABR-215062 is associated with altered Th1/Th2 balance and inhibited migration of inflammatory cells into the peripheral nerve tissue. Neuropharmacology 42:731-739. [DOI] [PubMed] [Google Scholar]