Abstract
Intraperitoneal injection of lipopolysaccharide (LPS; 100 μg) in mice resulted in the disappearance of almost all proteose peptone-induced polymorphonuclear neutrophils (PMNs) with high-level fluorescence for the cell surface marker Gr-1 (Gr-1high) at 15 min postinjection, followed by doubling of their proportion at 30 min postinjection. High staining levels of 3′-acetyl-2′-carboxyl-6′,7′-(dihyropyran-2′-one)-5 or 6-carboxyfluorescein diacethoxylmethyl ester-labeled PMNs injected into the peritoneal cavity were detected in mesenteric lymph nodes 15 min postinjection of LPS. Therefore, the time of decrease of Gr-1high PMNs coincided with that of the increase in cell accumulation in mesenteric lymph nodes. Since milk fat globule-EGF factor 8 (MFG-E8), which is secreted by macrophages, bound many PMNs exhibiting Gr-1high and Gr-1medium at 30 min postinjection of LPS, the staining level of annexin V on those cells was very low because its binding site is the same as the receptor for MFG-E8. At 60 min postinjection of LPS, the proportion of Gr-1high PMNs decreased, and almost all Gr-1medium PMNs tended to shift to the right compared with those at 30 min postinjection. The geomeans of Toll-like receptor 4 (TLR4) expression on PMNs at 15, 30, and 60 min postinjection of LPS were 63, 66, and 24%, respectively, compared with that on normal PMNs, indicating that the expression of TLR4 decreases in response to exposure to LPS. Our results suggest that LPS induced PMN death and that many PMNs expressing Gr-1high undergo apoptosis 180 min postinjection of LPS.
The toxic effects of lipopolysaccharide (LPS) are mediated by endogenous cytokines and other mediators released by LPS-activated host cells, such as macrophages. Recent studies suggest that the administration of LPS initiates the production and release of various cytokines, such as tumor necrosis factor alpha (TNF-α), interleukin 1 (IL-1), IL-6, and gamma interferon, in a complex cascade through which the lethal effects of LPS are mediated (2, 12, 16, 18, 29). On the other hand, the inhibitory effects of LPS on apoptosis may represent an autoprotective mechanism of the host to increase the defense response against invasion of microorganisms and their cell components (4, 5, 15). For example, many in vitro studies have indicated that inflammatory cytokines, such as TNF-α and IL-1β, at low concentrations prolong neutrophil survival (1, 4, 9, 20, 25).
Polymorphonuclear neutrophils (PMNs) play an important role in the inflammation process. As a first-line defense, PMNs leave the circulation upon stimulation and migrate into the inflamed site to kill the invading microorganisms. Once PMNs have completed their tasks, an orderly elimination of PMNs is essential for the resolution of inflammation. The death of PMNs by disintegration or necrosis can cause cellular damage through the release of granule contents, stimulating resident phagocyte populations and prolonging the inflammatory reaction (11). However, apoptotic PMNs are often recognized and phagocytosed intact by macrophages without the release of proinflammatory mediators, thus limiting tissue injury and promoting resolution of the inflammation (17, 26, 28).
Questions still remain about the action of LPS in infections by gram-negative bacteria. Although LPS at high and low doses has negative and positive effects, respectively, on host cells, as described previously (2, 4, 15, 16, 25, 29), there are no reports about the behavior of PMNs among peritoneal exudate cells (PECs) following intraperitoneal (i.p.) injection of LPS in mice. In preliminary experiments in mice, we noted, following i.p. administration of a bolus (medium) dose of 100 μg of LPS, a triphasic response of the peritoneal PMN count consisting of an initial decrease at the early stage followed by an increase and a final marked decrease at 180 min. The present study was designed to investigate in more detail this in vivo dynamic phenomenon by using FACStar flow cytometry. We also examined the site of PMN migration using labeled PMNs induced by i.p. injection of proteose peptone (PP).
MATERIALS AND METHODS
Mice.
The experimental protocol was approved by the Ethics Review Committee for Animal Experimentation of Toho University School of Medicine. Eight-week-old male BALB/c mice were obtained from Japanese Charles River (Atsugi, Kanagawa, Japan).
Preparation of PMNs.
Mice were injected i.p. with 3 ml of 3% PP (Difco Laboratories Inc., Detroit, Mich.). Three hours later, the mice were anesthetized with ketamine-xylazine and bled through the axillary artery, and PECs were harvested by peritoneal lavage with 3 ml of warm RPMI 1640 (Gibco, Grand Island, N.Y.) supplemented with 10% heat-inactivated fetal calf serum. The cell suspension was washed by centrifugation at 400 × g for 5 min at 4°C. The number of cells was counted using a hemocytometer. The suspension was placed on a slide and stained with May-Grunwald-Giemsa stain. Granulocytes were differentiated from mononuclear cells in counts of 200 cells. The number of PMNs represented the total number PECs multiplied by the proportion of PMNs. The PMNs were resuspended in the same medium described above and then used for experiments.
To evaluate the influence of LPS on PP-induced PMNs, mice injected with the latter also received a bolus i.p. injection of 100 μg of LPS prepared from Pseudomonas aeruginosa (Sigma Chemical, St. Louis, Mo.) at 15 (165 min postinjection of PP), 30 (150 min postinjection), 60 (120 min postinjection), or 180 (simultaneous injection) min before the harvest of peritoneal PMNs. The method of preparation of PMNs was similar to that described above. In addition, necrosis of PMNs was assessed at all time points with trypan blue.
Flow cytometry.
Freshly isolated PMNs were first blocked with the supernatant of 1G10 hybridoma cells for 15 min at 4°C and then stained with fluorescein isothiocyanate (FITC)-conjugated Gr-1 monoclonal antibody (MAb) (BD PharMingen, San Diego, Calif.) for 30 min at 4°C. Cells stained with appropriate isotype-matched immunoglobulin (BD PharMingen) were used as negative controls. After being washed, the cells were stained with phycoerythrin (PE)-conjugated annexin V (BD PharMingen), PE-conjugated anti-mouse Toll-like receptor 4 (TLR4) (eBioscience, San Diego, Calif.), or tricolor-conjugated anti-milk fat globule-EGF factor 8 (MFG-E8) MAb for 30 min at 4°C. The method of preparation of MFG-E8 was described previously (10). and tricolor-conjugated goat F(ab′)2 anti-hamster immunoglobulin G was from Caltag Laboratories (Burlingame, Calif.). The stained cells were analyzed on a FACStar flow cytometer (Becton Dickinson Immunocytometry Systems USA, Mountain View, Calif.).
PMNs (107/ml) were labeled with 3 μM 3′-acetyl-2′-carboxyl-6′,7′-(dihyropyran-2′-one)-5 or 6-carboxyfluorescein diacethoxylmethyl ester (BCECF-AM) (Dojindo Laboratories, Kumamoto, Japan) at 37°C for 1 min in Hanks' balanced salt solution containing 5% fetal calf serum. After three washes, the labeled cells (107 cells) were injected, along with LPS (100 μg/mouse) and PP solution. BCECF-AM-labeled PMNs obtained from the peritoneal cavity and mesenteric lymph nodes were analyzed with a FACStar flow cytometer.
Cytokine assay.
At 120 min after the injection of LPS, blood samples were obtained as described previously (19) and assayed for TNF-α, IL-6, and IL-10 using an enzyme-linked immunosorbent assay kit specific to each murine cytokine (R&D Systems, Minneapolis, Minn.) according to the instructions provided by the manufacturer. The sensitivities of the different assays were 5 to 10 pg/ml. In preliminary experiments, the concentration of TNF-α in serum at 120 min postinjection of LPS was apparently higher than at 180 min postinjection.
Effect of cytokines on PMNs harvested from the peritoneal cavity.
Recombinant murine TNF-α and recombinant murine IL-1β were purchased from Pepro Tech, Inc. (Rocky Hill, N.J.). Solutions of these cytokines at various concentrations were added to the PMN suspension and kept in a CO2 incubator at 35°C. After 1 h, the cells were counted using a hemocytometer. Preliminary experiments indicated that the number of cells remaining at 60 min postincubation was similar to that at 120 min postincubation.
Statistical analysis.
All data were expressed as means ± standard deviations (SD). Differences between in vitro and in vivo numbers of PMNs and the geomean of TLR4 expression were examined for statistical significance by a Dunnett multiple test and a Mann-Whitney U test, respectively. All data were expressed as means ± standard errors of the mean. A P value of <0.05 indicated a statistically significant difference.
RESULTS
Effect of LPS on PMN count.
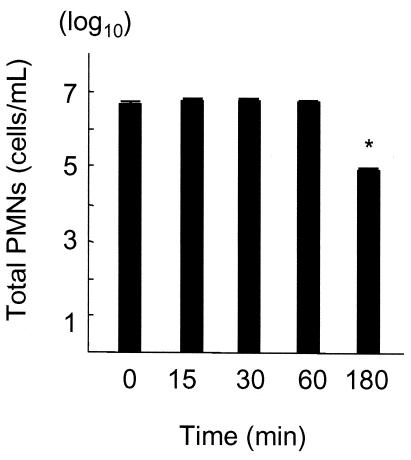
The numbers of PMNs induced by i.p. injection of PP were counted at various time intervals. The proportion of PMNs relative to PECs of mice not treated with LPS was constant (90 ± 4%) in all samples. The number of PMNs in the exudate at 180 min post-PP injection was similar to that at 60 min postinjection (data not shown). Simultaneous i.p. injection of LPS (100 μg/animal) and PP resulted in a significant decrease in the number of PMNs at 180 min postinjection of LPS (P < 0.05) compared with other samples (Fig. 1). However, the number did not decrease until 60 min postinjection of LPS.
FIG. 1.
PMN counts in exudates obtained at various time intervals after PP and LPS injections. The data are means ± SD of five experiments. *, P < 0.05 compared with other samples.
Cytokine concentrations in blood and abdominal-lavage fluid of mice treated with LPS.
The above results suggested that i.p. injection of LPS resulted in apoptosis and/or elimination of PMNs. LPS induces a variety of cytokines, and high concentrations of TNF-α and IL-1β exert proapoptotic actions on PMNs (23, 30, 33). The concentrations of TNF-α, IL-1β, IL-6, and IL-10 in murine serum and abdominal-lavage fluid were assayed 120 min after i.p. injection of 100 μg of LPS. The serum TNF-α, IL-1β, IL-6, and IL-10 concentrations were 1,205 ± 311, 239 ± 131, 456 ± 103, and 89 ± 40 pg/ml, respectively. The concentrations of the same cytokines in abdominal-lavage fluid were 2,205 ± 351, 339 ± 151, 556 ± 83, and 189 ± 20 pg/ml, respectively. On the other hand, the level of each cytokine was below the detection limit in the samples obtained from mice not treated with LPS.
Effects of TNF-α and IL-1β on apoptosis of PMNs.
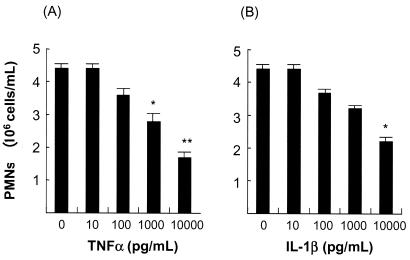
It is possible that the high concentrations of TNF-α were involved in the induction of apoptosis of PMNs. Treatment of PMNs with TNF-α at a concentration of 1,000 pg/ml reduced their number to 63% of the baseline value, which was significantly different from that of the control and PMNs treated with a lower concentration (10 pg of the same cytokine/ml) (Fig. 2A). IL-1β also showed a similar apoptotic activity against PMNs, although the activity was weaker than that of TNF-α, since the number of cells remaining after treatment with 1,000 pg of IL-1β/ml was not significantly different from the control (Fig. 2B).
FIG. 2.
Effects of TNF-α and IL-1β on PP-induced PMNs. (A) TNF-α treatment. (B) IL-1β treatment. The data are means plus SD of five experiments. *, P < 0.05; **, P < 0.01 compared with other samples.
Amount of Gr-1 on PMNs.
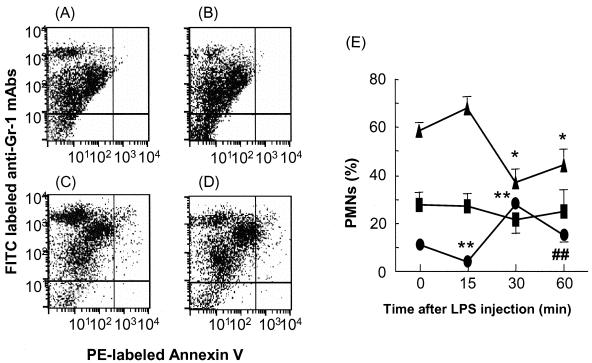
The cell surface antigen Gr-1 is expressed on myeloid cells, and the expression level increases with granulocyte maturation (13). Annexin V is a Ca2+-dependent phospholipid-binding protein with high affinity for phosphatidylserine. The annexin V assay allows the detection of early phases of apoptosis before the loss of cell membrane integrity (31). To analyze apoptosis of PMNs obtained 15, 30, 60, and 180 min postinjection of LPS in more detail, PMNs were assayed by flow cytometry using anti-Gr-1 antibody for neutrophils and annexin V for apoptosis. PMNs identified in the abdominal-lavage fluid of normal mice were divided into three groups: cells displaying Gr-1 expression at high, medium, and low fluorescence levels (Gr-1high, Gr-1medium, and Gr-1low, respectively) (Fig. 3A-D). Relative to untreated PMNs (11%), the proportion of Gr-1high PMNs decreased to one-third at 15 min but more than doubled (27.6%) at 30 min postinjection of LPS and then decreased again to 14.5% at 60 min postinjection of LPS (Fig. 3E). The proportion of Gr-1medium PMNs was roughly constant after injection of LPS. However, 30 min postadministration of LPS, many PMNs were apparently alive, as indicated by the annexin V assay. At 60 min after the administration of LPS, Gr-1high PMNs decreased again, whereas the percentage of apoptotic PMNs was almost the same as at 30 min postinjection of LPS. However, the proportion of Gr-1medium PMNs tended to shift to the right compared with data obtained 30 min postinjection of LPS.
FIG. 3.
Effects of LPS on PP-induced PMNs. LPS (100 μg/animal) was injected i.p. into mice pretreated with a PP solution. PMNs were stained with PE-labeled annexin V and FITC-labeled anti-Gr-1 MAbs and finally analyzed by FACScan. (A to D) PP-induced PMNs were obtained 0 (A), 15 (B), 30 (C), and 60 (D) min postinjection of LPS. (E) Proportions of Gr-1high, Gr-1medum, and Gr-1low PMNs. The percentages of the three groups (•, Gr-1high; ▪, Gr-1medium; ▴, Gr-1low) at different intervals after injection of LPS are shown. The data are means ± SD of five experiments. *, P < 0.01 compared with the proportions of Gr-1low PMNs at 15 min postinjection and at baseline (no LPS); **, P < 0.01 compared with the proportion of Gr-1high PMNs at 15 min postinjection of LPS; ##, P < 0.0115 compared with the proportion of Gr-1high PMNs at 30 min postinjection.
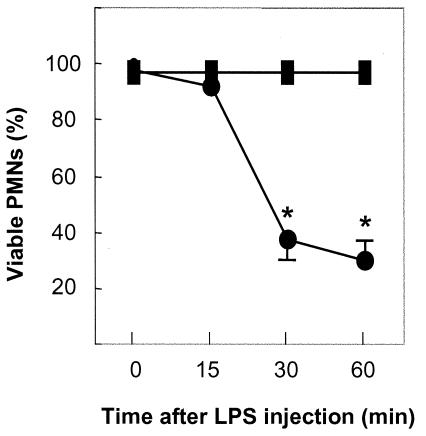
To determine whether the increased proportion of Gr-1high PMNs were live cells, PMNs in the peritoneal cavity were assessed by trypan blue exclusion. Almost all PMNs of normal mice at 15 min postinjection of LPS were alive (Fig. 4). In contrast, the mean proportions of live PMNs at 30 and 60 min postinjection of LPS had decreased to 37.1 and 29.9%, respectively.
FIG. 4.
Viability of PMNs after i.p. injection of LPS. PMNs were obtained from mice not treated with LPS (▪) or treated with LPS (•). The data are means ± SD of five experiments. *, P < 0.01 compared with the proportion of viable PMNs at 15 min postinjection and the control (no LPS).
Effect of LPS on expression of TLR4 on PMNs.
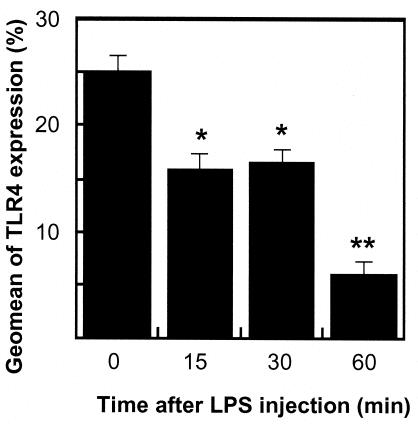
Although only ∼25% of normal peritoneal PMNs induced by PP injection expressed TLR4, the proportion of such cells decreased to ∼15, 16, and 5% at 15, 30, and 60 min postinjection of 100 μg of LPS, respectively (Fig. 5). These results indicate that LPS-induced decrease of TLR4 expression on PMNs was exposure time dependent.
FIG. 5.
Effects of LPS on the expression of TLR4 on PMNs. The data are means plus SD of five experiments. *, P < 0.01; **, P < 0.01 compared with the expression at baseline.
Migration of viable PMNs from the abdominal cavity.
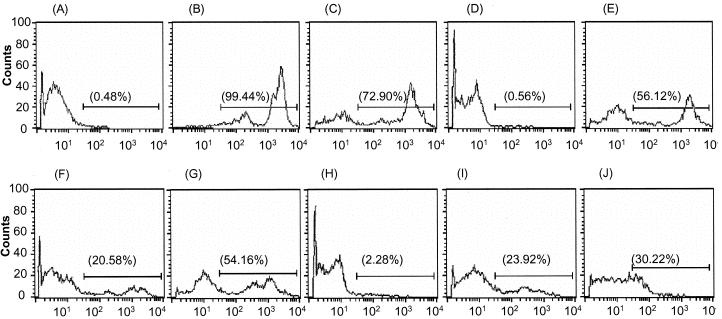
LPS-activated TLR4 signaling regulates PMN migration by modulating the expression of chemokine receptors in a GRK2- and GRK5-dependent manner (8). To investigate this, we studied the destinations of migrating PMNs by simultaneous i.p. injection of BCECF-AM-labeled PMNs and LPS (Fig. 6). At 15 min postinjection of BCECF-AM-stained PMNs, PMNs with high expression of BCECF-AM were detected in the abdominal-lavage fluid samples of mice treated with LPS or phosphate-buffered saline (PBS) (Fig. 6C and E). Moreover, BCECF-AM-labeled PMNs were found in the mesenteric lymph nodes of mice treated with LPS, and their fluorescence level was similar to that of cells from abdominal-lavage fluid (Fig. 6F). On the other hand, at 180 min postinjection of BCECF-AM-labeled PMNs, the staining of BCECF-AM-labeled PMNs in the abdominal-lavage fluid of mice simultaneously injected with LPS was lower than in mice injected with PBS as a control (Fig. 6G and I). At that time, BCECF-AM-stained PMNs were detected in the mesenteric lymph nodes of mice treated with LPS, but the staining intensity was less than that of their counterparts in the abdominal cavities of the same mice and in the mesenteric lymph nodes of mice treated with PBS (Fig. 6J). However, no BCECF-AM-labeled PMNs were detected in other lymph nodes, such as Peyer's patches or popliteal, inguinal, or renal lymph nodes, 15 and 180 min after injection of LPS (data not shown).
FIG. 6.
Migration of PP-induced PMNs following i.p. injection of LPS. PMNs stained with BCECF-AM and PBS or LPS were i.p. injected into mice simultaneously and analyzed by FACScan at various time intervals. PMNs exhibiting BCECF-AM staining were counted. (A to D) Unlabeled PMNs (A), BCECF-AM-labeled PMNs (B), peritoneal exudates (C), and mesenteric lymph nodes (D) 15 min after simultaneous injection of labeled PMNs and PBS. (E and F) Peritoneal exudates (E) and mesenteric lymph nodes (F) 15 min after simultaneous injection of both labeled PMNs and LPS. (G and H) Peritoneal exudates (G) and mesenteric lymph nodes (H) 180 min after simultaneous injection of both labeled PMNs and PBS. (I and J) Peritoneal exudates (I) and mesenteric lymph nodes (J) 180 min after simultaneous injection of both labeled PMNs and LPS.
MFG-E8 links apoptotic cells to phagocytes.
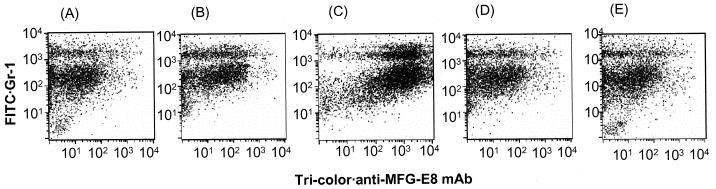
In the present study, the results of the annexin V assay conflicted with those of trypan blue exclusion. Recently, Hanayama et al. (10) demonstrated that MFG-E8 secreted by macrophages bound to apoptotic thymocytes, which were then engulfed by macrophages. Therefore, we studied MFG-E8 bound to PMNs present in the peritoneal cavity. MFG-E8 bound to many PMNs displaying not only Gr-1high but also Gr-1medium at 30 min postinjection of LPS (Fig. 7C). This result was in conflict with annexin V staining; the annexin V staining on Gr-1medium PMNs after the same time interval hardly shifted to the right compared with that at 60 min postinjection. On the other hand, MFG-E8 bound a few Gr-1high and Gr-1medium PMNs at 0, 60, and 180 min postinjection of LPS.
FIG. 7.
Binding of MFG-E8 to PP-induced PMNs. LPS (100 μg/animal) was injected i.p. into mice pretreated with PP, and PP-induced PMNs were obtained 0 (A), 15 (B), 30 (C), 60 (D), and 180 (E) min postinjection of LPS, stained with tricolor-labeled anti-MFG-E8 and FITC-labeled anti-Gr-1 MAbs, and then analyzed by FACScan.
DISCUSSION
In the present in vivo and in vitro studies, only PP-induced PMNs were used because we were unable to obtain sufficient numbers of PMNs in the blood to assay apoptosis and the expression level of Gr-1 after addition of LPS. Parker et al. (24) reported that PMN infiltration into the peritoneal exudate reached a maximum 240 min after LPS injection.
Neutrophils are terminally differentiated cells and are produced in the bone marrow from myeloid stem cells by a process called phagocytopoiesis. While they have a very short half-life in the circulation (8 to 20 h), this can increase severalfold once they enter infected or inflamed tissues (3). Aged neutrophils undergo spontaneous apoptosis (programmed cell death) in the absence of cytokines or other proinflammatory agents prior to their removal by macrophages (26). Neutrophils lose their functional properties during apoptosis, and they display morphological and biochemical characteristics of apoptotic cells, including cell shrinkage, compaction of chromatin, and loss of the multilobed shape of the nucleus (20, 27).
Phagocytosis by macrophages or other cells is the final event in the lives of many cells undergoing apoptosis. Apoptotic cells express cell surface antigens that allow their recognition and removal. Various substances, such as vitronectin and phosphatidylserine, have been reported as recognition sites of neutrophils undergoing apoptosis (6, 7, 26, 28). Phosphatidylserine, a molecular marker of apoptotic cells, also appears on the surfaces of apoptotic neutrophils once the symmetry of their plasma membrane phospholipids is altered (14, 22). The externalization of this molecule facilitates the recognition of apoptotic neutrophils by macrophages and is also a convenient indicator of apoptotic neutrophils, as phosphatidylserine can bind annexin V (6, 7). Hanayama et al. (10) demonstrated that MFG-E8 secreted by macrophages bound to apoptotic thymocytes, which were then engulfed by macrophages. They also showed that both MFG-E8 and annexin V bind to phosphatidylserine on apoptotic cells. Thus, since LPS-induced apoptotic PMNs were already bound to MFG-E8, there were fewer free receptors for annexin V than on those free of MFG-E8. The present study clarified that the proportion of MFG-E8-bound PMNs displaying Gr-1high and Gr-1medium reached a peak level at 30 min postinjection of LPS, but many of them appeared not to be apoptotic, according to the level of annexin V. At 0, 60, and 180 min postinjection of LPS, only some PP-induced PMNs were bound with MFG-E8. There were some Gr-1high and Gr-1medium PMNs showing high levels of annexin V at 30 and 60 min postinjection of LPS, although the levels in both types of cells were similar. However, 60 min postinjection of LPS, the staining level of annexin V on PMNs displaying Gr-1medium shifted to the right, which means that cells tended to become apoptotic, compared with the findings at 30 min postinjection. In addition, a marked decrease of TLR4 expression on PMNs was observed 60 min postinjection. These data support the notion that almost all MFG-E8 produced by macrophages bound to Gr-1high and Gr-1medium PMNs until 30 min postinjection and that little free MFG-E8 remained 60 min postinjection because MFG-E8-producing macrophages might have undergone apoptosis by i.p. injection of LPS.
TNF-α strongly activates p38 mitogen-activated protein kinase, which might generate the death signal in a subpopulation of neutrophils susceptible to this agent (32). It must also be pointed out that ligation of TNF-α to its receptor can activate caspase 8, which in turn activates the caspase cascade, which can also generate strong death signals (23). Intravenous injection of LPS resulted in an early rise in serum TNF-α to a peak level and its disappearance after a few hours, even in the presence of persistent bacteremia (24, 33). Based on those results and our preliminary results, we assayed the cytokine concentrations at 120 min postinjection of LPS. In the present study, the concentration of TNF-α was 1,200 pg/ml, which was sufficient to induce apoptosis of PMNs because TNF-α at a concentration of 1,000 pg/ml could induce apoptosis. Murray et al. (21) also reported that TNF-α at a dose of 2,800 pg/ml induced apoptosis of human neutrophils. In the present study, IL-1β also decreased the proportion of LPS-induced PMN, but it was less potent than TNF-α. In addition, the concentration of IL-1β was apparently lower than that of TNF-α in the sera and abdominal-lavage fluid of mice injected with LPS. These results indicate that one of the factors causing the decrease in PMNs in the abdominal-lavage fluid of mice injected with LPS 180 min postinjection of LPS was apoptosis induced by TNF-α.
Acknowledgments
We thank Isamu Sato for excellent technical assistance.
REFERENCES
- 1.Akgul, C., D. A. Moulding, and S. W. Edwards. 2001. Molecular control of neutrophil apoptosis. FEBS Lett. 487:318-322. [DOI] [PubMed] [Google Scholar]
- 2.Alexander, H. R., G. M. Doherty, C. M. Buresh, S. J. Venzon, and D. J. Norton. 1991. A recombinant human receptor antagonist interleukin 1 improves survival after lethal endotoxemia in mice. J. Exp. Med. 173:1029-1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brach, M., S. deVos, H. J. Gruss, and F. Hermann. 1992. Prolongation of survival of human polymorphonuclear neutrophils by granulocyte-macrophage colony-stimulating factor is caused by inhibition of programmed cell death. Blood 80:2920-2924. [PubMed] [Google Scholar]
- 4.Colotta, F., F. Re, N. Polentarutti, S. Sozzani, and A. Mantovani. 1992. Modulation of granulocyte survival and programmed cell death by cytokines and bacterial products. Blood 80:2012-2020. [PubMed] [Google Scholar]
- 5.Cox, G. 1996. IL-10 enhances resolution of pulmonary inflammation in vivo by promoting apoptosis of neutrophils. Am. J. Physiol. 27:L566-L571. [DOI] [PubMed] [Google Scholar]
- 6.Fadok, V. A., D. R. Voelker, P. A. Cambell, J. J. Cohen, D. L. Batton, and P. M. Henson. 1992. Exposure of phosphatidylserine on the surface of apoptotic lymphocytes triggers specific recognition and removal by macrophages. J. Immunol. 148:2207-2216. [PubMed] [Google Scholar]
- 7.Fadok, V. A., M. L. Warmer, D. L. Bratton, and P. M. Henson. 1998. CD36 is required for phagocytosis of apoptotic cells by human macrophages that use either a phosphatidylserine receptor or the vitronectin receptor (alpha v beta 3). J. Immunol. 161:6250-6257. [PubMed] [Google Scholar]
- 8.Fan, J., and A. B. Malik. 2003. Toll-like receptor-4 (TLR4) signaling augments chemokine-induced neutrophil migration by modulating cell surface expression of chemokine receptors. Nat. Med. 9:315-321. [DOI] [PubMed] [Google Scholar]
- 9.Girard, D., M. E. Paquet, R. Paquin, and A. D. Beaulieu. 1996. Differential effects of interleukin-15 (IL-15) and IL-2 on human neutrophils: modulation of phagocytosis, cytoskeleton rearrangement, gene expression, and apoptosis by IL-15. Blood 88:3176-3184. [PubMed] [Google Scholar]
- 10.Hanayama, R., M. Yanaka, K. Miwa, A. Shiohara, A. Iwamatsu, and S. Nagata. 2002. Identification of a factor that links apoptotic cells to phagocytes. Nature 417:182-187. [DOI] [PubMed] [Google Scholar]
- 11.Henson, P. M., and R. B. Johnston, Jr. 1987. Tissue injury in inflammation. Oxidants, proteinases and cationic proteins. J. Clin. Investig. 79:669-674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hesse, D. G., K. J. Tracey, Y. Fong, K. R. Manogue, M. A. Palladino, Jr., A. Cerami, G. T. Shires, and S. F. Lowry. 1988. Cytokine appearance in human endotoxemia and primate bacteremia. Surg. Gynecol. Obstet. 166:147-153. [PubMed] [Google Scholar]
- 13.Hestdal, K., F. W. Ruscetti, J. N. Ihle, S. E. Jacobsen, C. M. Dubois, W. C. Kopp, and S. D. L. Longo. 1991. Characterization and regulation of RB6-8C5 antigen expression on murine bone marrow cells. J. Immunol. 147:22-28. [PubMed] [Google Scholar]
- 14.Homburg, C. H., M. de Haas, A. E. von dem Borne, A. J. Veroeven, C. P. Reutelingspeger, and D. Roos. 1995. Human neutrophils lose their surface Fcγ RIII and acquire annexin V binding sites during apoptosis in vitro. Blood 85:532-540. [PubMed] [Google Scholar]
- 15.Lee, A., M. K. Whyte, and C. Haslett. 1993. Inhibition of apoptosis and prolongation of neutrophil functional longevity by inflammatory mediators. J. Leukoc. Biol. 54:283-288. [PubMed] [Google Scholar]
- 16.Mathison, J. C., E. Wolfson, and R. J. Ulevitch. 1988. Participation of tumor necrosis factor in the mediation of Gram-negative bacterial lipopolysaccharide-induced injury in rabbits. J. Clin. Investig. 81:1925-1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Meagherm, L. C., J. S. Savill, A. Baker, R. W. Fuller, and C. Haslett. 1992. Phagocytosis of apoptotic neutrophils does not induce macrophage release of thromboxane B2. J. Leukoc. Biol. 52:269-273. [PubMed] [Google Scholar]
- 18.Michie, H. R., K. R. Manogue, D. R. Spriggs, et al. 1988. Detection of circulating tumor necrosis factor after endotoxin administration. N. Engl. J. Med. 318:1481-1486. [DOI] [PubMed] [Google Scholar]
- 19.Miyazaki, S., T. Nunoya, T. Matsumoto, N. Furuya, K. Tateda, and K. Yamaguchi. 2001. Lipopolysaccharide indirectly enhances inflammatory lesions in lungs as a primary infection site by non-encapsulated and type b Haemophilus influenzae through production of cytokines. Cytokine 15:171-174. [DOI] [PubMed] [Google Scholar]
- 20.Moulding, D. A., J. A. Quayle, C. A. Hart, and S. W. Edwards. 1998. Mcl-1 expression in human neutrophils: regulation by cytokines and correlation with cell survival. Blood 92:2492-2502. [PubMed] [Google Scholar]
- 21.Murray, J., J. A. Barbara, S. A. Dunkley, et al. 1997. Regulation of neutrophil apoptosis by tumor necrosis factor-alpha: requirement for TNFR55 and TNFR75 for induction of apoptosis in vitro. Blood 90:2772-2783. [PubMed] [Google Scholar]
- 22.Naito, M., K. Nagashima, T. Mashima, and T. Tsuruo. 1997. Phosphatidylserine externalization is a downstream event of interleukin-1β-converting enzyme family protease activation during apoptosis. Blood 89:2060-2066. [PubMed] [Google Scholar]
- 23.Ohlsson, K., P. Bjork, M. Bergenfeldt, R. Hageman, and R. C. Thompson. 1990. Interleukin-1 receptor antagonist reduces mortality from endotoxin shock. Nature 348:550-552. [DOI] [PubMed] [Google Scholar]
- 24.Parker, K. P., W. R. Bejamin, K. L. Kaffka, and P. L. Kilian. 1989. Presence of IL-1 receptors on human and murine neutrophils. Relevance to IL-1-mediated effects in inflammation. J. Immunol. 142:537-542. [PubMed] [Google Scholar]
- 25.Pericle, F., J. H. Liu, J. L. Diaz, D. K. Blanchard, S. Wei, G. Formi, and J. Y. Djeu. 1994. Interleukin-2 prevention of apoptosis in human neutrophils. Eur. J. Immunol. 24:440-444. [DOI] [PubMed] [Google Scholar]
- 26.Sasvill, J. S., A. H. Wyllie, J. E. Henson, M. J. Walport, P. M. Henson, and C. Haslett. 1989. Macrophage phagocytosis of aging neutrophils in inflammation. Programmed cell death in the neutrophil leads to its recognition by macrophages. J. Clin. Investig. 83: 865-875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Savill, J., V. Fadok, P. Henson, and C. Haslett. 1993. Phagocyte recognition of cells undergoing apoptosis. Immunol. Today 14:131-136. [DOI] [PubMed] [Google Scholar]
- 28.Savill, J., I. Dransfield, N. Hogg, and C. Haslett. 1990. Vitronectin receptor-mediated phagocytosis of cells undergoing apoptosis. Nature 343:170-173. [DOI] [PubMed] [Google Scholar]
- 29.Tracey, K. J., Y. Fong, D. G. Hesse, K. R. Manogue, A. T. Lee, G. C. Kuo, S. F. Lowry, and A. Gerami. 1987. Anti-cachectin/TNF monoclonal antibodies prevent septic shock during lethal bacteraemia. Nature 330:662-664. [DOI] [PubMed] [Google Scholar]
- 30.Tsuchida, H., Y. Takeda, H. Takei, H. Shinzawa, T. Takahashi, and F. Sendo. 1995. In vivo regulation of rat neutrophil apoptosis occurring spontaneously or induced with TNF-α or cyclohexamide. J. Immunol. 154:2403-2412. [PubMed] [Google Scholar]
- 31.Vermes, I., C. Haanen, H. Sreffens-Nakken, and C. Reutelingsperger. 1995. A novel assay for apoptosis. Flow cytometric detection of phosphatidylserine expression on early apoptotic cells using fluorescein labeled Annexin V. J. Immunol. Methods 184:39-51. [DOI] [PubMed] [Google Scholar]
- 32.Wakabayashi, G., J. A. Gelfand, J. F. Burke, R. C. Thompson, and C. A. Dinarello. 1991. A specific receptor antagonist for interleukin 1 prevents Escherichia coli-induced shock in rabbits. FASEB J. 5:338-342. [DOI] [PubMed] [Google Scholar]
- 33.Zanetti, G., D. Heumann, J. Gerain, J. Kohler, et al. 1992. Cytokine production after intravenous or peritoneal gram-negative bacterial challenge in mice. Comparative protective efficacy of antibodies to tumor necrosis factor α and to lipopolysaccharide. J. Immunol. 148:1890-1897. [PubMed] [Google Scholar]