Abstract
Staphylococcus epidermidis is an important cause of bloodstream infections in patients with hematological malignancies. Knowledge of the long-term epidemiology of these infections is limited. We surveyed all S. epidermidis blood culture isolates from patients treated for hematological malignancies at the University Hospital of Örebro, Sweden from 1980 to 2009. A total of 373 S. epidermidis isolates were identified and multilocus sequence typing, staphylococcal chromosome cassette mec (SCCmec) typing and standard antibiotic susceptibility testing were employed to characterize these isolates. The majority of the isolates 361/373 (97%) belonged to clonal complex 2, and the 373 isolates were divided into 45 sequence types (STs); Simpson's Diversity Index was 0.56. The most prevalent STs were ST2 (243/373, 65%) and ST215 (28/373, 8%). Ninety three percent (226/243) of the ST2 isolates displayed either SCCmec type III or IV. ST2 and 215 were isolated during the entire study period, and together these STs caused temporal peaks in the number of positive blood cultures of S. epidermidis. Methicillin resistance was detected in 213/273 (78%) of all isolates. In the two predominating STs, ST2 and ST215, methicillin resistance was detected in 256/271 isolates (95%), compared with 34/100 (34%) in other STs (p<0.001). In conclusion, in this long-term study of patients with hematological malignancies, we demonstrate a predominance of methicillin-resistant ST2 among S. epidermidis blood culture isolates.
Introduction
Staphylococcus epidermidis is a common colonizer of the human skin and mucous membranes that rarely causes disease in healthy individuals. However, it has been increasingly recognized as an important etiology of bloodstream infections (BSIs) in immunocompromised hosts and in patients with indwelling medical devices [1]. S. epidermidis is now one of the most prevalent causes of BSIs in patients with hematological malignancies [2]. The pathogenic potential of S. epidermidis in this population attributes to a major extent to its capacity to form biofilm, which interferes with phagocytosis and antimicrobial peptides[3] as well as reduces the efficacy of antimicrobials [4]. An additional factor in the establishment of S. epidermidis as a nosocomial pathogen is its widespread resistance to various antimicrobial agents. Currently, approximately 60–70% of healthcare-associated S. epidermidis isolates are resistant to the first-line anti-staphylococcal penicillins [5]–[7]. This resistance is mediated by the mecA gene that is carried by a mobile genetic element called staphylococcal cassette chromosome mec (SCCmec). Currently there are 11 different SCCmec structures identified in staphylococci (http://www.sccmec.org).
An essential issue is whether S. epidermidis BSIs are caused by nosocomial clones of S. epidermidis or by sporadically occurring strains from patients' commensal flora. Previous studies on this topic have demonstrated that 1) the S. epidermidis population is composed of hospital-associated clonal types, community-associated clonal types and clonal types that are able to survive in both environments[8], 2) S. epidermidis populations are highly diverse in the community [9], [10] as opposed to in clinical settings, and 3) clonal outbreaks of S. epidermidis can be identified in clinical settings [11]–[14]. These data are in support of that S. epidermidis to a major extent is a nosocomial pathogen that is spread in hospital environments.
At a local hospital level, previous reports using pulsed-field gel electrophoresis (PFGE) have demonstrated the occurrence, persistence and spread of closely related S. epidermidis strains [15] even during long term-follow up[16].
The improved multilocus sequence typing (MLST) scheme for S. epidermidis [17] is a genetic typing method based on the sequence polymorphism of fragments of seven housekeeping genes. It has proven to be highly discriminatory and can be used to elucidate relationships between strains and to identify ancestral genotypes [18]. MLST is now considered the method of choice to investigate long-term genetic relatedness within S. epidermidis populations [19]. Using MLST, it has been shown that the population structure of S. epidermidis is composed of a major and highly diverse lineage, clonal complex 2 (CC2), which can further be divided into two subdivisions or clusters: CC2-I and CC2-II [20]. MLST has been used to investigate S. epidermidis clonality within and between hospitals, both nationally and internationally [21]–[26]. These data indicate that a single sequence type (ST), ST2 belonging to CC2 cluster CC2-I, represents the majority of methicillin-resistant S. epidermidis that causes healthcare-associated infections.
In a previous study from our institution, we observed marked temporal variation in the number of positive blood cultures of S. epidermidis in patients with hematological malignancies, with peak incidence during the mid-1990s [27]. In this report, we analyzed all stored S. epidermidis blood culture isolates from patients with hematological malignancies during 30 years (from 1980 to 2009) using MLST, SCCmec typing, and standard antibiotic susceptibility testing. The aim was to describe the long-term molecular epidemiology of S. epidermidis and to elucidate whether the observed variation in incidence could be explained by the temporary occurrence of specific S. epidermidis genotypes.
Materials and Methods
Bacterial isolates
We surveyed positive blood cultures isolated from patients with hematological malignancies treated in 1980–2009 at the Division of Hematology of the Örebro University Hospital. The unit provides full hematological care except for allogeneic stem cell transplantation for the residents of Örebro County (population 285,000). After the exclusion of multiple positive cultures with the same microorganism and the same antibiogram isolated within 48 hours, we identified 567 positive blood cultures with coagulase-negative staphylococci determined by DNAse and coagulase. Of these isolates, 460 had been stored at −70°C and were possible to subculture. Coagulase-negative staphylococci were primarily determined to species level by API/ID32 Staph (BioMérieux, Marcy l'Etoile, France). In addition, nine isolates that yielded ambiguous MLST results were reevaluated to a different species level using MALDI-TOF; as a result, a total of 373 S. epidermidis isolates were included in the study. Fifty-five of these isolated were collected during the first, 217 during the second and 101 during the third decade of the study period.
S. epidermidis were isolated from 241 individual patients of whom 120 had Acute myeloid leukemia (AML), 44 Multiple myeloma, 33 Acute lymphoblastic leukemia (ALL), 18 Lymphoma, 15 Chronic lymphocytic leukemia (CLL) and 11 other hematological malignancies. Among the study isolates 234/373 (63%) grew in 2 or more blood culture sets, 120/373 (32%) grew in 1 of 2 sets, 7/373 (2%) grew in 1 of 1 set and for 10/373 (3%) data were missing. For a subset of the cohort (S. epidermidis isolated from 1996–2001) additional clinical data have previously been published [28]. Among these patients 83% had a central venous access and the median neutrophil count was 0.1×109/L.
Antimicrobial resistance profiles
Antibiotic susceptibility testing was performed for cefoxitin, fusidic acid, clindamycin, erythromycin, gentamicin, rifampicin, trimethoprim/sulfamethoxazole, and norfloxacin on Mueller-Hinton agar (Mueller Hinton II Agar, BD Diagnostics, Sparks, MD, USA) using the disc diffusion method (Oxoid, Cambridge, UK). Breakpoints for antibiotic resistance were according to EUCAST (http://www.eucast.org). Multidrug resistance (MDR) was defined as resistance to ≥3 antibiotic classes.
Isolation of genomic DNA
Genomic DNA was isolated using the NorDiag Bullet with BUGS'n BEADS STI-fast-kit (DiaSorin, Dublin, Ireland). DNA preparations were stored at +4°C prior to PCR.
Multilocus sequence typing
Amplification of the seven housekeeping genes (arC, aroE, gtr, mutS, pyrR, tpiA, and yqiL) was initially performed using the primer sequences described by Thomas et al. [17]. For isolates in which no PCR products were detected for aroE and tpiA, alternative primers described by Wang [29] and Wisplinghoff [30] were used. Fragments of the seven genes were amplified using a conventional PCR system (GeneAmp PCR System 9700, Applied Biosystem, Foster City, CA, USA). Nucleotide sequences were determined for both strands using the same primers on an ABI Prism 3130 Genetic Analyzer (Applied BioSystems, Biosystem, Foster City, CA, USA). Nucleotide sequences were compared with the MLST database via the MLST website (http://www.mlst.net) as of 1 August 2013. Individual STs were assigned to clonal complexes (CCs) using the eBURST algorithm (http://eburst.mlst.net).
Detection of mecA gene
The mecA gene was detected using real-time PCR as previously described [31].
SCCmec typing
All PCRs were run separately with specific primers. Amplification of the ccr gene complex (ccr1, 2, 3, and ccrC) and the class A mec complex were performed by real-time PCR using a LightCycler system (Roche Diagnostics, Mannheim, Germany) as previously described [31]. Amplification of the class B mec complex, and ccr4 gene complex were performed in a conventional PCR system (GeneAmp PCR System 9700, Applied Biosystem, Foster City, CA, USA) [31]. The amplified products were identified by agarose gel (1%, High Strength Analytic Grade Agarose, Bio-Rad Laboratories, Hercules, CA, USA) electrophoresis. The S. epidermidis reference strain ATCC35984 was used as control. Isolates were assigned a SCCmec type according to the nomenclature of S. aureus [32]. The SCCmec analyses were made exclusively; first mecA positive isolates were analyzed for class A or B mec complex. Class A isolates were analyzed for ccr3, positive isolates were considered SSCmec type III and in remaining isolates no ccr gene complex were detected and these were considered non typeable. For class B isolates, ccr2 positive isolates were considered SSCmec IV and remaining isolates where ccr1 positive and considered SCCmec type I.
Data analysis
The chi-squared test was used to analyze the association of categorical outcomes. Diversity among strains was evaluated using Simpson's Diversity Index, where the index equals the probability that two randomly selected strains will belong to different STs. Diversity comparisons were obtained using bootstrapping. A p-value of <0.05 (two-sided) was considered statistically significant.
Results
Multilocus sequence typing
The sequences of the seven housekeeping genes resulted in a MLST profile in 371 out of 373 isolates. For two isolates it was not possible to obtain PCR products for the aroE gene, even with the alternative PCR primers. These two isolates were considered untypeable by MLST (Table 1).
Table 1. MLST and antibiotic susceptibility results of 373 S. epidermidis blood culture isolates from patients with hematological malignancies.
| ST | CC | N (%) | MLST profile | MRSE % | MDR % |
| 2 | 2-I | 243 (65) | 7-1-2-2-4-1-1 | 95 | 86 |
| 215 | 2-II | 28 (7.5) | 1-6-2-1-1-16-1 | 93 | 89 |
| 73 | 2-II | 11 (2.9) | 1-5-2-6-2-1-6 | 0 | 0 |
| 23 | 2-II | 9 (2.4) | 7-1-2-1-3-3-1 | 89 | 100 |
| 5 | 2-II | 7 (1.9) | 1-1-1-2-2-1-1 | 57 | 71 |
| 22 | 2-I | 6 (1.6) | 7-1-2-2-4-7-1 | 100 | 67 |
| 38 | 2-II | 6 (1.6) | 1-2-2-5-1-1-10 | 33 | 17 |
| 57 | 2-II | 6 (1.6) | 1-1-1-1-2-1-1 | 0 | 17 |
| 59 | 2-II | 6 (1.6) | 2-1-1-1-2-1-1 | 67 | 67 |
| 6 | 2-II | 3 (0.8) | 1-1-2-2-2-1-1 | 67 | 67 |
| 25 | 2-II | 3 (0.8) | 1-6-2-1-1-1-3 | 100 | 100 |
| 295 | 2-II | 3 (0.8) | 1-1-3-6-2-1-1 | 0 | 0 |
| 17 | 2-II | 2 (0.5) | 1-1-6-2-2-1-1 | 0 | 17 |
| 19 | Undefined | 2 (0.5) | 8-7-12-4-12-2-2 | 0 | 0 |
| 32 | Singleton | 2 (0.5) | 1-1-7-1-3-5-14 | 0 | 0 |
| 173 | 2-II | 2 (0.5) | 1-6-6-1-2-1-10 | 0 | 100 |
| 225 | 2-II | 2 (0.5) | 1-13-7-2-2-1-29 | 0 | 0 |
| 520 | 2-II | 2 (0.5) | 1-1-2-1-1-1-33 | 0 | 0 |
| 524 | 2-II | 2 (0.5) | 1-1-2-2-4-16-1 | 0 | 0 |
| 1 | 2-II | 1 (0.3) | 1-2-2-2-1-1-10 | 100 | 100 |
| 4 | 2-II | 1 (0.3) | 1-1-6-6-2-1-1 | 100 | 100 |
| 7 | 2-II | 1 (0.3) | 1-1-1-2-4-1-1 | 0 | 0 |
| 10 | 2-II | 1 (0.3) | 1-1-1-1-3-1-1 | 100 | 100 |
| 40 | 2-II | 1 (0.3) | 1-1-2-1-3-1-1 | 100 | 100 |
| 53 | 365 | 1 (0.3) | 3-1-5-5-11-4-11 | 0 | 0 |
| 85 | 2-II | 1 (0.3) | 1-1-2-2-1-1-1 | 0 | 0 |
| 86 | 2-II | 1 (0.3) | 1-2-2-1-1-1-1 | 0 | 0 |
| 88 | 2-II | 1 (0.3) | 1-1-2-1-2-1-7 | 0 | 0 |
| 136 | 2-II | 1 (0.3) | 1-1-7-2-2-1-1 | 0 | 0 |
| 152 | 2-II | 1 (0.3) | 1-1-2-6-2-1-1 | 0 | 0 |
| 190 | 2-II | 1 (0.3) | 1-1-1-2-5-1-1 | 0 | 0 |
| 210 | 2-II | 1 (0.3) | 1-1-1-2-2-1-25 | 0 | 0 |
| 218 | 2-II | 1 (0.3) | 1-1-2-6-2-16-1 | 0 | 0 |
| 327 | 2-II | 1 (0.3) | 1-1-2-1-4-1-1 | 0 | 0 |
| 337 | Singleton | 1 (0.3) | 32-10-10-9-10-16-31 | 0 | 0 |
| 372 | 365 | 1 (0.3) | 12-25-5-5-3-4-11 | 0 | 0 |
| 414 | 2-II | 1 (0.3) | 1-1-1-1-2-1-4 | 0 | 0 |
| 457 | Singleton | 1 (0.3) | 3-16-16-5-3-19-31 | 0 | 0 |
| 521 | 2-II | 1 (0.3) | 1-23-3-6-2-1-10 | 0 | 0 |
| 522 | Singleton | 1 (0.3) | 32-1-10-2-10-1-1 | 0 | 0 |
| 523 | 2-II | 1 (0.3) | 1-6-2-5-1-16-1 | 100 | 100 |
| 525 | 2-II | 1 (0.3) | 1-2-1-1-2-1-1 | 0 | 100 |
| 526 | Singleton | 1 (0.3) | 8-24-17-4-12-6-9 | 0 | 0 |
| 527 | 2-II | 1 (0.3) | 43-6-6-1-2-1-10 | 0 | 100 |
| 528 | 2-II | 1 (0.3) | 1-1-7-2-2-1-29 | 0 | 100 |
| Untypeable | - | 2 (0.5) | - | 50 | 0 |
| Total | 373 | 78 | 73 |
ST, sequence type, CC, clonal complex, MLST, multi locus sequence typing, MRSE, methicillin resistant S. epidermidis, MDR, multidrug resistant (≥3 antibiotic groups)
The most prevalent single ST was ST2, which constituted 243 of all 373 (65%) isolates, and this ST dominated during the entire study period. The second most prevalent ST was ST215, which comprised 28/373 (8%) of the isolates, followed by ST73 with 11/373 (3%) (Table 1).
As the majority of criteria used to determine the significance of positive blood cultures of S. epidermidis involve the number of positive blood culture sets of S. epidermidis [33]the predominant STs were evaluated in this respect. Among ST2 234/354 (66%), ST215 14/27 (52%) and ST73 7/11 (64%), grew in ≥2 blood culture sets. There were no significant differences comparing growth in ≥2 blood culture sets and growth in 1of 2 sets for these STs.
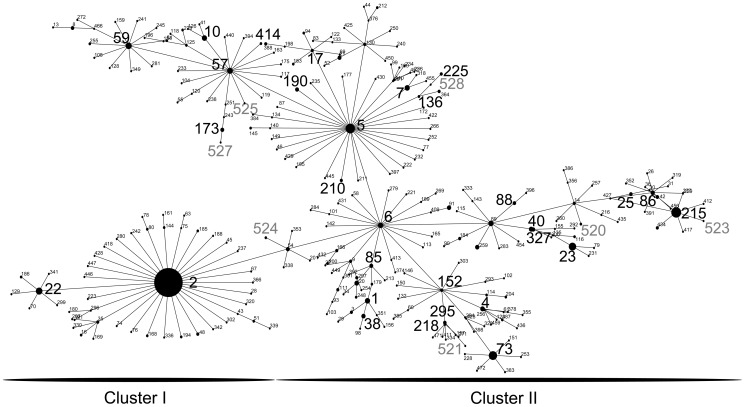
Regarding CCs, 361/373 (97%) of the isolates belonged to CC2; within CC2, 247/361 (68%) of the isolates clustered in CC2-I and 32% clustered in CC2-II (Fig. 1). Non-CC2 isolates were singletons, clustered in CC365, or in a CC without a known ST founder (Table 1). Nine STs not previously described in the MLST database were found and these were allocated ST numbers 520 to 528 in the MLST database. Of new STs, seven clustered in CC2-II (Fig. 1) and two were singletons. Simpson's Diversity Index was 0.56 for all isolates genotyped by MLST.
Figure 1. eBURST analysis of the S. epidermidis sequence types (STs) belonging to clonal complex 2 observed in this study compared with STs in the multilocus sequence typing (MLST) database via the MLST website (http://www.mlst.net) as of 1 August 2013.
STs in large digits are STs found in this study. Not previously described STs are depicted in gray (ST520-528).
SCCmec typing
All ST2 isolates were further characterized by SCCmec typing. One-hundred eighty-two isolates of 243 (75%) carried SCCmec type III, 44/243 (18%) SCCmec type IV, 9/243 (4%) were SCCmec type I, 2/243 (1%) were untypeable and 6/243 (2%) were mecA negative.
Antibiotic susceptibility
Methicillin resistance, evaluated by cefoxitin disc diffusion, was detected in 291/373 (78%) of all isolates, and MDR was present in 272/373 (73%) isolates. There was a marked disparity in resistance profiles when comparing different STs (Table 1). In the two predominant STs, ST2 and ST215, methicillin resistance was detected in 256/271 isolates (95%), compared with 34/100 (34%) in other STs (p<0.001). Correspondingly, ST2 and ST215 displayed an MDR phenotype in 234/271 (86%), compared with 38/100 (38%) in other STs (p<0.001). Simpson's Diversity Index was 0.36 in methicillin-resistant isolates, compared with 0.94 in methicillin-sensitive isolates (p = 0.001).
There was a gradual increase in antibiotic resistance over time. During the first decade of the study 24/55 (44%) of the isolates were MDR and during the second and third decade 164/217 (76%) and 84/101 (83%) respectively were MDR, p<0.001.
Incidence variations
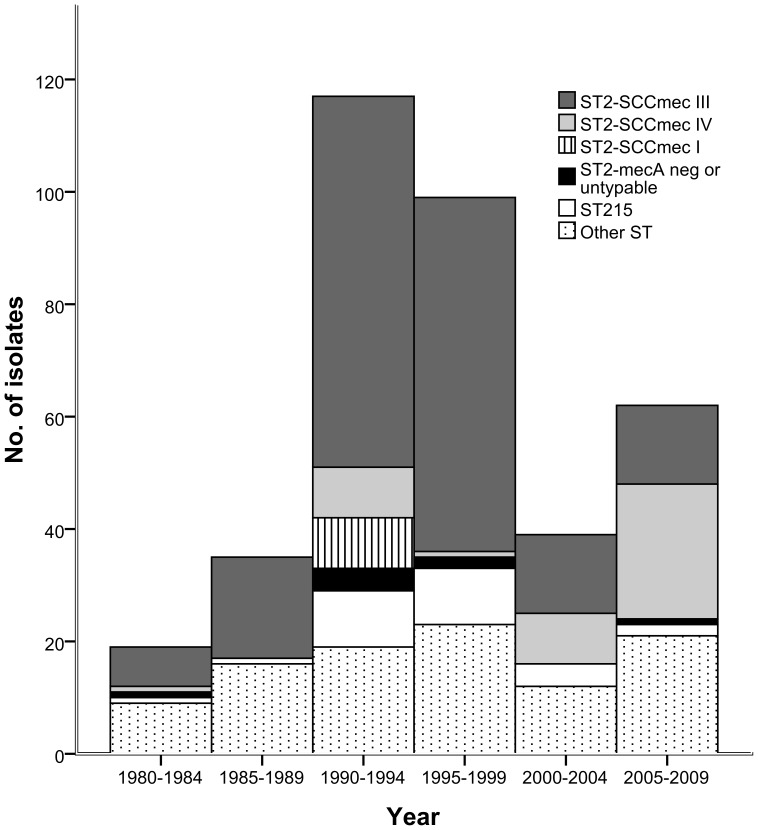
STs other than the two most prevalent STs (ST2 and ST215) were isolated at a relatively constant level during the study period (Fig. 2). In contrast, the number of isolated ST2 and ST215 exhibited marked variations, and together these two STs accounted for the major proportion of the peak of the number of positive blood cultures of S. epidermidis during the 1990s (Fig. 2).
Figure 2. The number of positive blood cultures of S. epidermidis in patients with hematological malignancies.
Each bar represents a 5-year period and is divided in ST2-SCCmec I, ST2-SCCmec III, ST2-SCCmec IV, ST2 with nontypable SCCmec or mecA negative, ST215 and other sequence types.
Among ST2 isolates, SCCmec type III was predominating. However, during the last decade of the study period there was a trend towards increasing numbers of isolates carrying SCCmec type IV (Fig. 2). ST2 with SCCmec type I was only present during 1990–1994 (Fig. 2).
Discussion
In the present study, we describe the molecular epidemiology of S. epidermidis blood culture isolates from a single hematological unit during a 30-year period. Such long-term data has not previously been presented.
The main finding is that ST2, which belongs to CC2-I, was the dominating ST of S. epidermidis in blood cultures at our institution during the entire study period. ST2 is a well-recognized genotype that causes nosocomial infections worldwide. It has been the most prevalent genotype reported in several studies [18], [21], [23]–[25], [34], especially in BSIs and catheter-related infections [24]. To our knowledge only one study reported ST5 more common than ST2 [26]. ST2 has only rarely been described to colonize healthy individuals in Swedish studies [9], [35], and ST2 was not found at all in Chinese commensal isolates [25]. Furthermore, ST2 has been associated with many of the currently known virulence factors, such as biofilm production and antibiotic resistance [24], [25], which is in accordance with the association between ST and methicillin resistance, as well as the MDR demonstrated in this study. However, no single virulence factor has been linked to the success of ST2 as a nosocomial pathogen.
In our study almost all (93%) ST2 isolates carried either SCCmec type III or IV, a finding that is in accordance with previous studies in the field [24], [25], [36].
The second most prevalent ST in this study, ST215, has previously been described as the cause of a wide range of healthcare-associated infections in Sweden and Norway [22], [35]. Interestingly, ST215 has not been described among commensal isolates [9], [35].
ST73 was the third most isolated ST. ST73 has been described as a colonizer of healthy subjects in Sweden and Portugal [9], [18], which is consistent with the antibiotic-sensitive phenotype of ST73 in this study.
Although the investigated S. epidermidis isolates were either causative agents of bacteremia or contaminants, the genetic diversity according to STs was considerably lower than in global [18] and multicenter studies [26]. In contrast, a Finnish study found ST2 alone among 60 S. epidermidis isolates from bacteremic patients in three units at the same hospital [23]. These differences underscore that S. epidermidis infections in general are hospital acquired and caused by genetically related strains that persist in the hospital environment (i.e., endemic clones).
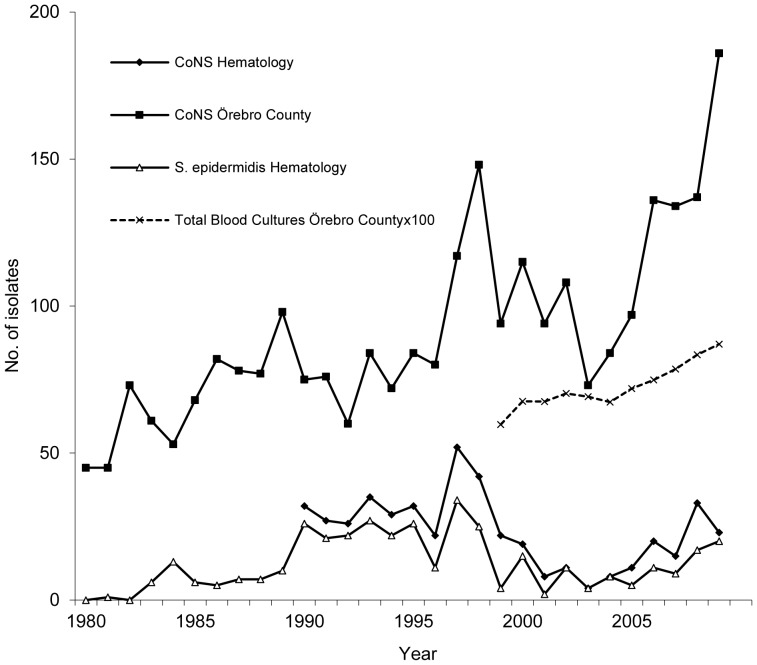
In our institution, we observed a marked variation in the number of positive blood cultures of S. epidermidis during 1980–2009 (Fig. 3). This variation corresponded to a variation in the total number of positive blood cultures of coagulase-negative staphylococci in the entire county of Örebro (Fig. 3). We have no plausible explanation for this phenomenon since we do not know of any significant changes in the patient population or in patient care (e.g., antimicrobial prophylaxis policies and blood culture methodology) that could have influenced these fluctuations. Our data do not support the hypothesis that these variations are caused by transient occurring MLST genotypes, because the main part of the incidence peaks can be explained by increased number of positive cultures of the predominating STs, ST2 and ST215. We report temporal variations of different SCCmec types of ST2 isolates during the study period. During 1990–1994 ST2 carrying SCCmec type I were temporarily isolated and during the last decade of the study there was a gradual increase in the number of ST2 carrying SCCmec type IV. Interestingly, this increase coincides with the emergence of community acquired methicillin resistant S. aureus (MRSA) even though the study was performed in an area with a low incidence of MRSA, (approximately 1% of clinical isolates). In the time period with the higher incidence of S. epidermidis during the 1990ies isolates of ST2 carrying SCCmec type III predominate which may indicate that SCCmec type III provides a fitness advantage in the hospital environment. In summary, these observations may reflect that health care-associated S. epidermidis infections at least partly are caused by temporally occurring genotypes.
Figure 3. The number of positive blood cultures of coagulase-negative staphylococci (CoNS) in Örebro County, Sweden (filled squares); CoNS at the Division of Hematology, Örebro University Hospital (filled diamonds); and S. epidermidis in patients with hematological malignancies, Örebro University Hospital (study population, open triangles).
The total number of blood cultures performed in Örebro County from 1999 to 2009 is also shown (crosses).
One strength of the present report is the restriction of the study population to a limited geographic area with solely one microbiological laboratory service. Thereby it was possible to include the vast majority of S. epidermidis blood culture isolates from patients with hematological malignancies. However, a limitation is that S. epidermidis isolates that were not stored at the time of diagnosis had to be excluded. Isolates not stored predominantly represented multiple positive cultures from the same infection episode or isolates with growth in only one blood culture set that were interpreted as contamination at the time of diagnosis. Few isolates were identified during the 1980s, possibly because less intensive chemotherapy schedules were used in 1980s, but it might also indicate a selection bias in that a smaller proportion of S. epidermidis isolates were stored during this time. Another limitation is that it was not possible to assess whether the analyzed S. epidermidis isolates had caused true BSIs or were blood culture contaminants. However, such distinction is not easily made since a uniform gold standard to make this important distinction is lacking. The present study population represents a high risk population for S. epidermidis BSIs and most likely a great proportion of the included isolates represented true BSIs. PFGE may have provided additional discriminatory power as well as a different SCCmec typing strategy allowing the identification of potential multiple SCCmec types.
In conclusion, although S. epidermidis is known to possess a highly dynamic and plastic genome, MLST has made it possible to investigate the distribution of various genotypes at a single unit during a 30-year period, which underscores the importance of MLST in studying the long-term relatedness of S. epidermidis infections. The predominant STs of S. epidermidis blood culture isolates obtained from patients with hematological malignancies from 1980 to 2009 were ST2 and ST215. The findings in the study add to the comprehension that healthcare-associated blood culture isolates of S. epidermidis in immunocompromised patients belong to the same genetic background.
Funding Statement
The study was supported by grants from the Örebro county council research committee at the Örebro University Hospital, Sweden. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Rogers KL, Fey PD, Rupp ME (2009) Coagulase-negative staphylococcal infections. Infectious Disease Clinics of North America 23: 73–98. [DOI] [PubMed] [Google Scholar]
- 2. Wisplinghoff H, Seifert H, Wenzel RP, Edmond MB (2003) Current trends in the epidemiology of nosocomial bloodstream infections in patients with hematological malignancies and solid neoplasms in hospitals in the United States. Clinical Infectious Diseases 36: 1103–1110. [DOI] [PubMed] [Google Scholar]
- 3. Vuong C, Voyich JM, Fischer ER, Braughton KR, Whitney AR, et al. (2004) Polysaccharide intercellular adhesin (PIA) protects Staphylococcus epidermidis against major components of the human innate immune system. Cell Microbiol 6: 269–275. [DOI] [PubMed] [Google Scholar]
- 4. Otto M (2009) Staphylococcus epidermidis–the ‘accidental’ pathogen. Nat Rev Microbiol 7: 555–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Diekema DJ, Pfaller MA, Schmitz FJ, Smayevsky J, Bell J, et al. (2001) Survey of infections due to Staphylococcus species: frequency of occurrence and antimicrobial susceptibility of isolates collected in the United States, Canada, Latin America, Europe, and the Western Pacific region for the SENTRY Antimicrobial Surveillance Program, 1997–1999. Clinical Infectious Diseases 32 Suppl 2 S114–132. [DOI] [PubMed] [Google Scholar]
- 6. Santos Sanches I, Mato R, de Lencastre H, Tomasz A (2000) Patterns of multidrug resistance among methicillin-resistant hospital isolates of coagulase-positive and coagulase-negative staphylococci collected in the international multicenter study RESIST in 1997 and 1998. Microb Drug Resist 6: 199–211. [DOI] [PubMed] [Google Scholar]
- 7. Claesson C, Hallgren A, Nilsson M, Svensson E, Hanberger H, et al. (2007) Susceptibility of staphylococci and enterococci to antimicrobial agents at different ward levels in four north European countries. Scandinavian Journal of Infectious Diseases 39: 1002–1012. [DOI] [PubMed] [Google Scholar]
- 8. Rolo J, de Lencastre H, Miragaia M (2012) Strategies of adaptation of Staphylococcus epidermidis to hospital and community: amplification and diversification of SCCmec. J Antimicrob Chemother 67: 1333–1341. [DOI] [PubMed] [Google Scholar]
- 9. Widerstrom M, Wistrom J, Ek E, Edebro H, Monsen T (2011) Near absence of methicillin-resistance and pronounced genetic diversity among Staphylococcus epidermidis isolated from healthy persons in northern Sweden. APMIS 119: 505–512. [DOI] [PubMed] [Google Scholar]
- 10. Jamaluddin TZ, Kuwahara-Arai K, Hisata K, Terasawa M, Cui L, et al. (2008) Extreme genetic diversity of methicillin-resistant Staphylococcus epidermidis strains disseminated among healthy Japanese children. Journal of Clinical Microbiology 46: 3778–3783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kelly S, Collins J, Maguire M, Gowing C, Flanagan M, et al. (2008) An outbreak of colonization with linezolid-resistant Staphylococcus epidermidis in an intensive therapy unit. Journal of Antimicrobial Chemotherapy 61: 901–907. [DOI] [PubMed] [Google Scholar]
- 12. Nouwen JL, van Belkum A, de Marie S, Sluijs J, Wielenga JJ, et al. (1998) Clonal expansion of Staphylococcus epidermidis strains causing Hickman catheter-related infections in a hemato-oncologic department. Journal of Clinical Microbiology 36: 2696–2702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Burnie JP, Naderi-Nasab M, Loudon KW, Matthews RC (1997) An epidemiological study of blood culture isolates of coagulase-negative staphylococci demonstrating hospital-acquired infection. Journal of Clinical Microbiology 35: 1746–1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Muldrew KL, Tang YW, Li H, Stratton CW (2008) Clonal dissemination of Staphylococcus epidermidis in an oncology ward. Journal of Clinical Microbiology 46: 3391–3396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Widerstrom M, Monsen T, Karlsson C, Wistrom J (2006) Molecular epidemiology of meticillin-resistant coagulase-negative staphylococci in a Swedish county hospital: evidence of intra- and interhospital clonal spread. Journal of Hospital Infection 64: 177–183. [DOI] [PubMed] [Google Scholar]
- 16. Krediet TG, Mascini EM, van Rooij E, Vlooswijk J, Paauw A, et al. (2004) Molecular epidemiology of coagulase-negative staphylococci causing sepsis in a neonatal intensive care unit over an 11-year period. Journal of Clinical Microbiology 42: 992–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Thomas JC, Vargas MR, Miragaia M, Peacock SJ, Archer GL, et al. (2007) Improved multilocus sequence typing scheme for Staphylococcus epidermidis. Journal of Clinical Microbiology 45: 616–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Miragaia M, Thomas JC, Couto I, Enright MC, de Lencastre H (2007) Inferring a population structure for Staphylococcus epidermidis from multilocus sequence typing data. Journal of Bacteriology 189: 2540–2552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Widerstrom M, Wistrom J, Sjostedt A, Monsen T (2011) Coagulase-negative staphylococci: update on the molecular epidemiology and clinical presentation, with a focus on Staphylococcus epidermidis and Staphylococcus saprophyticus. European Journal of Clinical Microbiology and Infectious Diseases. [DOI] [PubMed]
- 20. Miragaia M, Carrico JA, Thomas JC, Couto I, Enright MC, et al. (2008) Comparison of molecular typing methods for characterization of Staphylococcus epidermidis: proposal for clone definition. Journal of Clinical Microbiology 46: 118–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Widerstrom M, McCullough CA, Coombs GW, Monsen T, Christiansen KJ (2012) A multidrug-resistant Staphylococcus epidermidis clone (ST2) is an ongoing cause of hospital-acquired infection in a Western Australian hospital. Journal of Clinical Microbiology 50: 2147–2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Widerstrom M, Monsen T, Karlsson C, Edebro H, Johansson A, et al. (2009) Clonality among multidrug-resistant hospital-associated Staphylococcus epidermidis in northern Europe. Scandinavian Journal of Infectious Diseases 41: 642–649. [DOI] [PubMed] [Google Scholar]
- 23. Ibrahem S, Salmenlinna S, Lyytikainen O, Vaara M, Vuopio-Varkila J (2008) Molecular characterization of methicillin-resistant Staphylococcus epidermidis strains from bacteraemic patients. Clin Microbiol Infect 14: 1020–1027. [DOI] [PubMed] [Google Scholar]
- 24. Li M, Wang X, Gao Q, Lu Y (2009) Molecular characterization of Staphylococcus epidermidis strains isolated from a teaching hospital in Shanghai, China. Journal of Medical Microbiology 58: 456–461. [DOI] [PubMed] [Google Scholar]
- 25. Du X, Zhu Y, Song Y, Li T, Luo T, et al. (2013) Molecular Analysis of Staphylococcus epidermidis Strains Isolated from Community and Hospital Environments in China. PLoS One 8: e62742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mendes RE, Deshpande LM, Costello AJ, Farrell DJ (2012) Molecular epidemiology of Staphylococcus epidermidis clinical isolates from U.S. hospitals. Antimicrobial Agents and Chemotherapy 56: 4656–4661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ahlstrand E, Svensson K, Persson L, Tidefelt U, Soderquist B (2011) Glycopeptide resistance in coagulase-negative staphylococci isolated in blood cultures from patients with hematological malignancies during three decades. European Journal of Clinical Microbiology and Infectious Diseases 30: 1349–1354. [DOI] [PubMed] [Google Scholar]
- 28. Persson L, Strid H, Tidefelt U, Soderquist B (2006) Phenotypic and genotypic characterization of coagulase-negative staphylococci isolated in blood cultures from patients with haematological malignancies. European Journal of Clinical Microbiology and Infectious Diseases 25: 299–309. [DOI] [PubMed] [Google Scholar]
- 29. Wang XM, Noble L, Kreiswirth BN, Eisner W, McClements W, et al. (2003) Evaluation of a multilocus sequence typing system for Staphylococcus epidermidis. Journal of Medical Microbiology 52: 989–998. [DOI] [PubMed] [Google Scholar]
- 30. Wisplinghoff H, Rosato AE, Enright MC, Noto M, Craig W, et al. (2003) Related clones containing SCCmec type IV predominate among clinically significant Staphylococcus epidermidis isolates. Antimicrobial Agents and Chemotherapy 47: 3574–3579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Berglund C, Molling P, Sjoberg L, Soderquist B (2005) Predominance of staphylococcal cassette chromosome mec (SCCmec) type IV among methicillin-resistant Staphylococcus aureus (MRSA) in a Swedish county and presence of unknown SCCmec types with Panton-Valentine leukocidin genes. Clin Microbiol Infect 11: 447–456. [DOI] [PubMed] [Google Scholar]
- 32. Classification of staphylococcal cassette chromosome mec (SCCmec): guidelines for reporting novel SCCmec elements. Antimicrob Agents Chemother 53: 4961–4967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Beekmann SE, Diekema DJ, Doern GV (2005) Determining the clinical significance of coagulase-negative staphylococci isolated from blood cultures. Infection Control and Hospital Epidemiology 26: 559–566. [DOI] [PubMed] [Google Scholar]
- 34. Gordon RJ, Miragaia M, Weinberg AD, Lee CJ, Rolo J, et al. (2012) Staphylococcus epidermidis Colonization Is Highly Clonal Across US Cardiac Centers. Journal of Infectious Diseases 205: 1391–1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hellmark B, Soderquist B, Unemo M, Nilsdotter-Augustinsson A (2013) Comparison of Staphylococcus epidermidis isolated from prosthetic joint infections and commensal isolates in regard to antibiotic susceptibility, agr type, biofilm production, and epidemiology. International Journal of Medical Microbiology 303: 32–39. [DOI] [PubMed] [Google Scholar]
- 36. Miragaia M, de Lencastre H, Perdreau-Remington F, Chambers HF, Higashi J, et al. (2009) Genetic diversity of arginine catabolic mobile element in Staphylococcus epidermidis. PLoS One 4: e7722. [DOI] [PMC free article] [PubMed] [Google Scholar]