Abstract
Infection with candidatus “Helicobacter heilmannii” is associated with gastritis and mucosa-associated lymphoid tissue lymphoma in people. Infection with “H. heilmannii” type 1 predominates (80%) and is thought to be acquired from dogs, cats, or pigs. We further examined the zoonotic potential of dogs and cats by amplifying gastric DNA from cats (n = 45) and dogs (n = 10) with primers against “H. heilmannii” ureB and 16S rRNA genes and sequencing the products. Fluorescence in situ hybridization (FISH) with eubacterial and “H. heilmannii”-specific probes was employed to directly visualize “H. heilmannii” types and their intragastric distribution. ureB sequences of “H. heilmannii” amplicons clustered with human and feline isolates of “H. heilmannii” and were distinct from the “H. heilmannii”-like organisms (HHLO) H. felis, H. salomonis, and H. bizzozeronii. 16S ribosomal DNA sequences in 20 “H. heilmannii”-infected cats and dogs were distinct from “H. heilmannii” type 1 and “H. suis” and clustered with “H. heilmannii” types 2 and 4. FISH confirmed the presence of “H. heilmannii” types 2 and 4 in dogs but failed to definitively characterize the “H. heilmannii” types present in cats. In infected dogs, “H. heilmannii” inhabited the gastric mucus and glands, and in dogs coinfected with other HHLO it shared the same gastric niche. The results indicate that dogs and cats are predominantly colonized by “H. heilmannii” bacteria that are distinct from type 1 and from “H. suis.” As “H. heilmannii” type 1 predominates in people, the zoonotic risk posed by dogs and cats is likely small.
Helicobacter heilmannii is the name proposed for a 4- to 10-μm-long, spiral-shaped, motile bacterium with three to eight coils, a wavelength of about 1 μm, up to 14 uni- or bipolar flagella, and no periplasmic filaments (1, 24) that is found in the stomachs of 0.2 to 4% of patients with gastritis (1, 2, 7, 27). The bacterium was first described as “Gastrospirillium hominis” but was reclassified following 16S ribosomal DNA (rDNA) sequencing (3, 22, 23) as “H. heilmannii.” “H. heilmannii,” like H. pylori, has been associated with gastritis, adenocarcinoma, and gastric mucosa-associated lymphoid tissue lymphoma (13).
Definitive culture of “H. heilmannii” has not been achieved to date (2) and diagnosis is usually made on the basis of its distinct spiral morphology, compared with H. pylori, on silver- stained tissue sections. However, as a variety of large gastric spiral organisms such as H. felis, H. salomonis, and H. bizzozeronii are indistinguishable from “H. heilmannii” on routine light microscopy, and as H. pylori grown in a broth culture can adopt a morphology identical to that of “H. heilmannii” (5), genetic techniques such as PCR, sequence analysis of cloned and uncloned PCR products and fluorescence in situ hybridization (FISH) with specific probes are required for more definitive identification (28). Using these approaches, Helicobacter heilmannii was initially subdivided into types 1 and 2 on the basis of 16S rDNA sequences obtained from gastric tissues of an infected person (7, 22) and further subdivided into four types on the basis of 16S rDNA sequences and FISH (28). The majority of humans (78.5%) are infected with “H. heilmannii” type 1, with types 2, 3, and 4 infecting 8, 1, and 10% of individuals, respectively (28). “H. heilmannii”-like organisms cultured from infected people are very similar to H. bizzozeronii, H. salomonis, or H. felis but can be distinguished from “H. heilmannii” by sequence analysis and FISH. Thus, candidatus “H. heilmannii” is currently considered a group of noncultivable gastric spiral organisms that lack periplasmic fibrils and are genetically distinct from H. felis, H. bizzozeronii, and H. salomonis (21).
While gastric infection with “H. heilmannii”-like organisms (HHLO) in people is rare, it is common in domestic animals, and contact with cats, dogs, and pigs (12, 25, 27, 30) has been correlated with increased risk of “H. heilmannii” infection in people; Stolte et al. found 70% of “H. heilmannii”-infected patients had contact with one or more animals compared with 37% in the healthy population (12, 25). The zoonotic potential of dogs and cats is further supported by the identification of an uncultivable bacterium with high homology to “H. heilmannii” (21) (using species-specific PCR) in 57 to 100% of HHLO-infected cats (14, 15, 18) and 20 to 25% of infected dogs (16, 17). “H. heilmannii” strains with identical urease B gene (ureB) restriction fragment length polymorphism analysis results have also been described for a patient suffering from acute gastric erosions and for one of his cats (4). The cultivable HHLO—H. bizzozeronii, H. salomonis, and H. felis (7, 21)—are also commonly identified in the stomachs of dogs and cats. These observations strongly suggest that cats and dogs infected with HHLO are a zoonotic risk. However, as humans are predominantly colonized by “H. heilmannii” type 1, and the subtypes of “H. heilmannii” present in dogs and cats have not been determined, a more informed estimate of zoonotic potential is not available.
The principal aim of this study was to further define the subtypes of candidatus “H. heilmannii” present in the stomachs of dogs and cats. Subsidiary aims were to examine genetic variation in candidatus “H. heilmannii” present in dogs and cats from different countries and to determine the pattern of gastric colonization in dogs and cats.
MATERIALS AND METHODS
Animals.
This study evaluated 55 archived DNA samples prepared from gastric biopsies of Helicobacter-infected cats from the United States (group 1, 17 cats: 15 clinically healthy young adults presented for spay or neuter [C1 to C15] and 2 adults with chronic vomiting [C16 and C17] [26]) and Germany (group 2, 28 cats: 12 clinically healthy [C18 to C29] and 16 with chronic gastrointestinal signs [C30 to C45]) and dogs from Denmark (10 dogs; 9 dogs (D1 to D9) with gastrointestinal disease and 1 healthy dog [D10]). DNA was prepared from gastric biopsies frozen at −80°C using the Qiamp tissue kit (QIAGEN Inc., Valencia, Calif.). DNA was stored frozen at −80°C.
Infection was previously confirmed by a positive urease test, modified Steiner stain and Helicobacter genus-specific PCR. Inclusion in this study was determined by demonstration of infection with “H. heilmannii” using species-specific PCR primers directed against “H. heilmannii” (14). All samples had been previously demonstrated to be H. pylori negative by species-specific PCR (26). The presence of H. felis (14) and H. bizzozeronii had also been determined by species-specific PCR, and many animals were coinfected with a variety of these “H. heilmannii”-like organisms (see Table 2). The H. bizzozeronii ureB primers were designed by the authors based on the sequence of H. bizzozeronii urease determined in our laboratories (31). PCR amplification of H. bizzozeronii ureB was performed using primers F, 5′-GAAGTCGAACATGACTGCAC-3′, and R, 5′-GGTCGCATTAGTCCCATCAG-3′, under the following conditions: 94°C for 1 min, 57°C for 1 min, and 72°C for 1 min (35 cycles) with a final extension at 72°C for 15 min.
TABLE 2.
Infection status of “H. heilmannii”-infected cats and dogs
| Animal group | No. (%) with pattern | Identification of species by specific PCRa
|
||
|---|---|---|---|---|
| “H. heilmannii” | H. bizzozeronii | H. felis | ||
| Cats 1 (C1-C17) | 13 (76) | + | − | − |
| (n = 17) | 3 (18) | + | − | + |
| 1 (6) | + | + | + | |
| Cats 2 (C18-C45) | 16 (57) | + | − | − |
| (n = 28) | 7 (25) | + | + | − |
| 3 (11) | + | + | + | |
| 2 (7) | + | − | + | |
| Dogs (D1-D10) | 6 (60) | + | + | − |
| (n = 10) | 4 (40) | + | − | − |
Helicobacter spp. were determined by use of species-specific PCR primers (see Materials and Methods). All samples were negative for H. pylori.
None of the animals had been treated with antibiotics, steroids, or antacids within 3 to 4 weeks prior to examination.
PCR amplification and sequencing.
All DNA samples from dogs and cats were subject to amplification with primers directed against “H. heilmannii” ureB. DNA samples that were PCR positive for “H. heilmannii” but negative for H. felis, H. pylori, and H. bizzozeronii were amplified with 16S rRNA gene primers against Helicobacter species.
ureB.
DNA extracts were thawed on ice and 2 μl was added to a 50-μl reaction volume containing 25 pmol of each primer (Table 1) and 25 μl of TaqPCR Master Mix (QIAGEN Inc.). Amplification (94°C for 3 min, 57°C for 2 min, and 72°C for 3 min; 35 cycles of 94°C for 30 s, 57°C for 30 s, 72°C for 1 min; and 72°C for 5 min) was performed as described by Neiger et al. (14), except 35 cycles were used. A hot-start method was employed, using the Personal Cycler thermocycler (Biometra Inc, Tampa, Fla.). Negative controls in which the DNA extract was replaced by sterile distilled water were included with each reaction and carried through as negative controls for the agarose gel DNA extraction process.
TABLE 1.
Sequences of specific primers and probes for PCR and FISH
| Primer or probe name | Gene | Nucleotide sequencea | Specificity(ies) | Position |
|---|---|---|---|---|
| Heil 1F | ureB | 5′-GGG-CGA-TAA-AGT-GCG-CTT-G-3′ | “H. heilmannii,” PCR | 980-997b |
| Heil 2R | ureB | 5′-CTG-GTC-AAT-GAG-AGC-AGG-3′ | “H. heilmannii,” PCR | 1542-1559b |
| Hel Fc | 16S rRNA | 5′-CGT-GGA-GGA-TGA-AGG-TTT-TA-3′ | Helicobacter genus, PCR | 402-421 |
| Hel R1c | 16S rRNA | 5′-TAC-ACC-AAG-AAT-TCC-ACC-TA-3′ | Helicobacter genus, PCR | 667-686 |
| Hel R2c | 16S rRNA | 5′-AAT-TCC-ACC-TAC-CTC-TCC-C-3′ | Helicobacter genus, PCR | 659-677 |
| Heihei 1+2c,d | 16S rRNA | 5′-CCC-ACA-CTC-CAG-AAG-RAT-AG-3′ | “H. heilmannii” type 1, H. suis | 642-661 |
| Heifel 1+2c,d | 16S rRNA | 5′-CCC-ACA-CTC-TAG-GGT-KGC-AG-3′ | “H. heilmannii” types 2 and 4 | 642-661 |
| Hhe-3c | 16S rRNA | 5′-CCC-ACA-CTC-TAG-AAA-GAT-AG-3′ | “H. heilmannii” type 3 | 642-661 |
| Heinov2 1+2c,d | 16S rRNA | 5′-CAC-ATC-TGA-CTT-GCC-ACT-CCG-3′ | “H. heilmannii” type 4 | 586-606 |
| Heibiz Sonde 7c,d | 16S rRNA | 5′-CCC-ACA-CTC-CAG-AGT-TGT-AG-3′ | H. felis, H. bizzozeronii, H. salomonis | 642-661 |
| Heibiz Sonde 7Cc | 16S rRNA | 5′-CCC-ACA-CTC-CAG-AGT-TGT-AG-3′ | H. felis, H. bizzozeronii, H. salomonis | 642-661 |
| Eub-338c | 16S rRNA | 5′-GCT-GCC-TCC-CGT-3′ | Bacteria | 338-349 |
16S rRNA gene.
A heminested PCR system was used (28) with primers HelF and HelR1 (25 pmol) and 25 μl of Taq Master Mix (QIAGEN Inc.) in a total volume of 50 μl (94°C for 10 min, followed by 30 cycles of 30 s at 94°C, 30 s at 58°C, and 30 s at 72°C) for the first stage. A 5-μl aliquot of the PCR product was transferred to a new tube containing the second-stage primers, HelF and HelR2 (25 pmol), and the other reagents, and cycle conditions were the same as those in the first round. Negative controls in which the DNA extract was replaced by sterile distilled water were included with each reaction and carried through as negative controls for the agarose gel DNA extraction process.
The PCR products were visualized by agarose gel electrophoresis and purified from the gel using a Perfectprep Gel Cleanup kit (Eppendorf Scientific Inc., Westbury, N.Y.). The yield of DNA from the gel extraction was determined using a Biophotometer spectrophotometer (Eppendorf Scientific Inc.).
Both forward and reverse strands were sequenced completely using an ABI 3700 automated DNA sequencer and ABI PRISM BigDye Terminator Sequencing kits with AmpliTaq DNA polymerase (Applied Biosystems, Foster City, Calif.). The sequencing was performed by DNA Services (Biotechnology Resource Center, Cornell University, Ithaca, N.Y.). Primers for sequencing were the same as those for PCR amplification (Table 1).
Sequence analysis.
Forward and reverse DNA sequences were used to generate a contiguous sequence in SeqMan (DNASTAR Inc., Madison, Wis.), which was then used to create alignments using MegAlign (DNASTAR Inc.). Other Helicobacter species from the GenBank database were also included in the alignments. The aligned sequences were entered into MEGA (9) and a phylogenetic tree was constructed using the neighbor-joining method (20) and the Jukes-Cantor model (8)
FISH.
The FISH protocol employed and reagents used were based on the CreaFAST “H. heilmannii” kit (Creatogen AG, Augsburg, Germany) and Trebesius et al. (28). Paraffin embedded gastric biopsy specimens were sectioned at 4.0 μm and mounted on ProbeOn Plus slides (Fisher Scientific, Pittsburgh, Pa.). The sections were deparaffinized by passage through xylene (three times, 20 min each) and then 100% alcohol (20 min), 95% ethanol (20 min), and finally 70% ethanol (20 min). The slides were allowed to air dry. The DNA probes within the kit (Table 1) were reconstituted with DNA buffer and then diluted to a working concentration of 5 ng μl−1 with hybridization buffer (Creatogen AG) according to the protocol. The other probes (Table 1) were reconstituted with ultrapure sterile water to a concentration of 5 ng μl−1. The sections were allowed to prehybridize with 20 μl of hybridization buffer under a coverslip for 60 min at 46°C in a humid chamber. For hybridization, the coverslip was removed and 20 μl of DNA probe mix was added and the slides were then replaced in the hybridization chamber at 46°C for 4 h. Washing of the slides was performed in wash buffer (Creatogen AG) at 48°C for 5 min. Hybridized samples were washed in PBS, allowed to air dry and mounted with a ProLong Antifade kit (Molecular Probes Inc., Eugene, Oreg.).
The sections were examined with an Axioskop 2 plus epifluorescence microscope and images were captured with AxioCam and AxioVision (Carl Zeiss Inc., Thornwood, N.Y.).
Nucleotide sequence accession numbers.
Representative samples of the ureB (C3, C4, C5, C6, and C7) and 16S rDNA (D1, D2, C1, C2, and C3) sequences reported here have been deposited with GenBank under accession no. AY139170, AY139171, AY139172, AY139173, and AY139174 (ureB) and accession no. AY139175, AY139176, AY139177, AY139178, and AY139179 (16S rDNA).
RESULTS
PCR-based amplification and sequencing from gastric biopsy specimens. (i) ureB.
A 580-bp PCR product was obtained using “H. heilmannii” ureB primers from 45 cats and 10 dogs (Fig. 1). The majority of cats (76% of those from the United States and 57% of those from Germany) were infected solely with “H. heilmannii,” as determined by species-specific PCR (Table 2). In animals infected with “H. heilmannii” and one other HHLO, coinfection with H. bizzozeronii was more common in German cats (25%) than in American cats (6%), while American cats had a higher prevalence of H. felis coinfection (18%) than German cats (7%). In contrast to cats, the majority of dogs (60%) were coinfected with H. bizzozeronii, with only 40% solely infected with “H. heilmannii”; no H. felis was found in the samples from dogs.
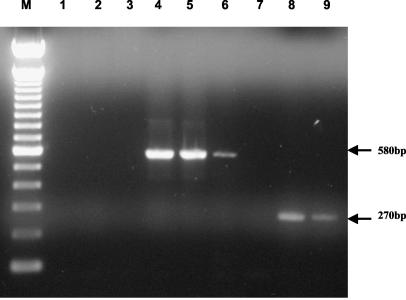
FIG. 1.
Detection of Helicobacter DNA in gastric biopsy specimens by PCR. Lanes: M, 100-bp DNA ladder; 1 to 6, “H. heilmannii”-specific ureB primers (lane 1, water control; lane 2, H. pylori control; lane 3, H. bizzozeronii control; lane 4, C8; lane 5, C20; lane 6, D5); lanes 7 to 9, Helicobacter genus 16S rRNA primers (lane 7, water control; lane 8, C8; lane 9, D5).
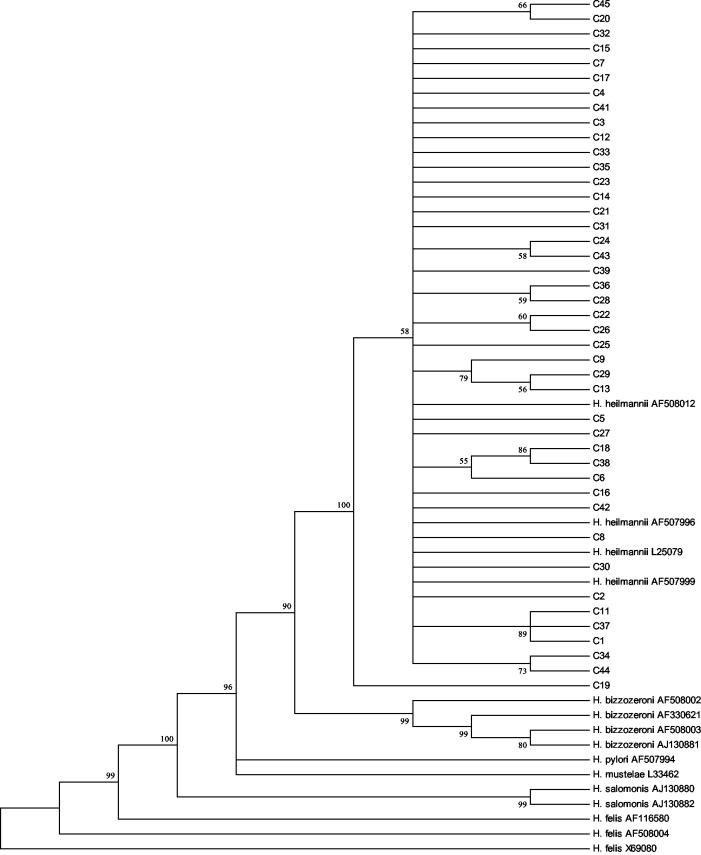
Sequenced ureB amplicons were aligned, both with themselves and with other published Helicobacter sequence data from GenBank, and a phylogenetic tree was constructed (Fig. 2). The tree (Fig. 2) shows the cat isolates to be similar to previously sequenced “H. heilmannii” isolates but clearly distinct from other Helicobacter species. No consistent differences were apparent between sequences obtained from cats from different countries.
FIG. 2.
Phylogenetic consensus tree showing the genetic relationship of “H. heilmannii” ureB sequences amplified from feline (C) gastric mucosa (C1 to C17, United States; C18 to C45, Germany) to other Helicobacter spp. The numbers in boldface type at the nodes are the bootstrap percentages (1,000 replications; 50% cutoff). Vertical distance has no meaning. GenBank accession numbers are given after each isolate name.
Sequencing of dog ureB PCR products repeatedly produced short runs of contiguous sequences that precluded meaningful alignment with the cat sequences and inclusion of the sequences in the phylogenetic tree; however, BLAST searches with the sequences from dogs D5, D6, and D7 showed strong homologies with three published “H. heilmannii” ureB sequences (GenBank accession no. AF508012, AF507996, and L25079: D5, 99% identity, score of 331 and 2e−88; D6, 97% identity, score of 283 and e−74; and D7, 99% identity, score of 379 and e−103).
(ii) 16S rDNA.
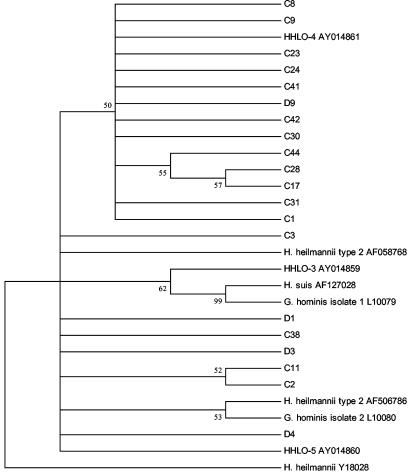
16S rRNA gene amplification was performed on 20 DNA samples (16 cats and 4 dogs) determined by species-specific PCR to be positive for “H. heilmannii,” but negative for H. bizzozeronii, H. felis, and H. pylori, to produce a 275-bp product (Fig. 1). Following sequencing, the aligned data were used to construct a phylogenetic tree also containing other published Helicobacter 16S rDNA sequences (Fig. 3). Sequences from cats and dogs were clearly separated from “G. hominis” isolate 1 (L10079) and “H. suis” (AF127028). Two main clusters of isolates were observed, with 13 of 20 sequences (65%) (12 cats and 1 dog) around “H. heilmannii” type 4 (HHLO-4 AY014861) and 7 of 20 sequences (35%) (4 cats and 3 dogs) around “H. heilmannii” type 2 (AF506786) and “H. heilmannii”-like organisms (HHLO-5).
FIG. 3.
Phylogenetic consensus tree showing the genetic relationship of Helicobacter sp. 16S rRNA gene sequences amplified from cats (C) and dogs (D) with “H. heilmannii” infection (C1 to C17, United States; C18 to C45, Germany; D1 to D10, Denmark) to archival “H. heilmannii,” “G. hominis,” and “H. suis” and 16S rRNA gene sequences. The numbers in boldface type at the nodes are the bootstrap percentages (1,000 replications; 50% cutoff). Vertical distance has no meaning. GenBank accession numbers are given after each isolate name.
FISH.
As a prelude to FISH the 16S sequences obtained from DNA samples determined by species-specific PCR to be positive for “H. heilmannii” but negative for H. bizzozeronii, H. felis, and H. pylori were evaluated for the presence of the binding sites for the “H. heilmannii” type-specific probes described by Trebesius et al. (28) (Table 1). The presence of the probe sequence was in agreement with the “H. heilmannii” subtype indicated by analysis of the aligned 225-bp sequences (Fig. 3) in 15 of 16 cats and four of four dogs.
Sixteen of 20 sequences contained the 16S rRNA bp 642 to 661 probe sequence (CCCACACTCTAGGGTGGCAG) common to “H. heilmannii” types 2 and 4, and 12 of 16 contained the 16S rRNA bp 586 to 606 probe sequence (CACATCTGACTTGCCACTCCG) described as specific for type 4 (C8, C9, C17, C23, C24, C28, C30, C31, C41, C42, C44, and D9).
Sequences from three samples (D1, D3, and C38) that clustered with HHLO-5 and “H. heilmannii” type 2 had DNA sequences in the 16S rRNA 642 to 661 region that had ambiguous FISH probe binding sites. Sample C38 contained a sequence from bp 642 to 661, CCCACACTCTAGGGTTGTAG, that appeared to be a combination of probe types 2 or 4 and 5 and samples D1 and D3 had sequences where the 5′ end was consistent with the type 2/4 probe and the 3′ end was consistent with Hhe-5 probe (this probe recognizes H. felis, H. bizzozeronii, and H. salomonis) (D1, CCCACACTTTAGGGTTGTAG; D3, CCCACACTTCAGAGTTGTAG). It is possible that these three samples are chimeric, potentially reflecting the presence of undetermined coinfecting Helicobacter spp.
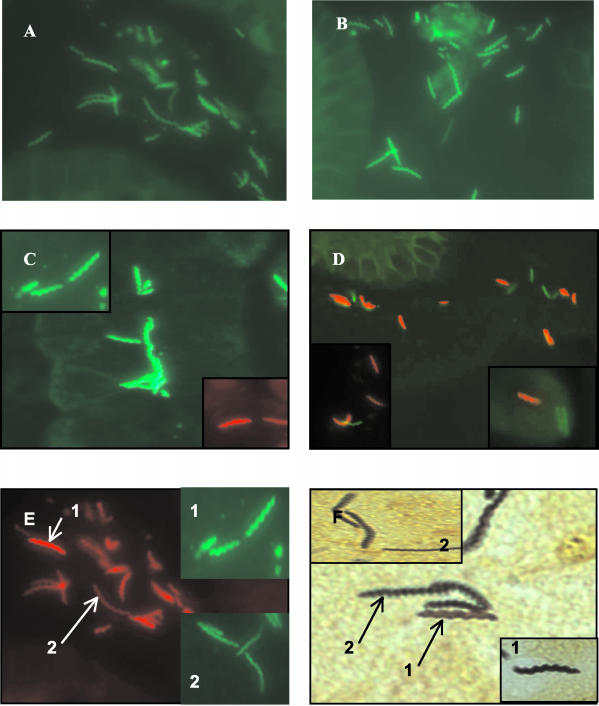
After determining the presence of the “H. heilmannii” probe binding sites in DNA samples from dogs and cats, a probe that hybridizes with a conserved 16S rRNA region of eubacteria was used (Eub-338) to confirm the presence and viability of rRNA in tissue sections from seven dogs and 17 American cats. Tissue blocks were not available for dogs D3, D9, and D10 and the German cats (C18 to 45) (Table 1). A positive result, indicated by spiral red organisms (Fig. 4E) was obtained in 23 of 25 sections. Sections from cats C14 and C17 failed to hybridize with the eubacterial probe. Hybridization with specific “H. heilmannii” probes was subsequently performed on the 23 eubacterial positive sections.
FIG. 4.
Formalin-fixed paraffin-embedded gastric sections from animals with “H. heilmannii” detected by FISH using the oligonucleotide probes summarized in Table 1. Probes were labeled with fluorescein isothiocyanate (green) or Cy-3 (red). (A) “H. heilmannii” type 2 from dog (D1); (B) “H. heilmannii” type 4 from dog (D5); (C) HHLO from cat (C15); (D) cocolonization with “H. heilmannii” type 2 (green) and other HHLO (red); (E) spiral bacteria detected with eubacterial probe Eub-338 (red) and HHLO probe (green) (inset 1, short loosely helical rods resembling H. salomonis; inset 2, elongated thin, tightly spiraled bacteria resembling “H. heilmannii” or H. bizzozeronii; (F) same as panel E but stained with Steiner stain.
In 14 of 15 cats probes against “H. heilmannii” types 1 to 4 failed to bind, despite positive eubacterial FISH, precluding definitive characterization of the “H. heilmannii” subtypes present. In a single cat (C16) that was coinfected with H. felis and “H. heilmannii,” hybridization was observed with probes against “H. heilmannii” type 2 and HHLO (Table 3).
TABLE 3.
Summary of FISH with “H. heilmannii” probes
| Animal | Infection statusa |
Helicobacter probe hybridization
|
||||
|---|---|---|---|---|---|---|
| “H. heilmannii” type
|
HHLOb | |||||
| 1 | 2 | 3 | 4 | |||
| D1 | “H. heilmannii” | − | + | − | − | − |
| D2 | “H. heilmannii,” H. bizzozeronii | − | + | − | − | + |
| D4 | “H. heilmannii” | − | − | − | − | − |
| D5 | “H. heilmannii,” H. bizzozeronii | − | − | − | + | + |
| D6 | “H. heilmannii,” H. bizzozeronii | + | + | − | − | − |
| D7 | “H. heilmannii,” H. bizzozeronii | − | − | − | + | + |
| D8 | “H. heilmannii,” H. bizzozeronii | − | − | − | − | + |
| C16 | “H. heilmanii,” H. felis | − | + | − | − | + |
Determined by species-specific PCR with primers to “H. heilmannii,” H. felis, H. bizzozeronii, and H. pylori.
This probe hybridizes with the HHLO, H. bizzozeronii, H. felis, and H. salomonis.
In sections from two dogs with a mono-infection of “H. heilmannii” binding of the “H. heilmannii” probes but not the HHLO probe was observed (Table 3). In sections from five dogs coinfected with “H. heilmannii” and H. bizzozeronii hybridization was observed with probes against “H. heilmannii” in four sections and against HHLO (which detects H. bizzozeronii, H. felis, and H. salomonis) in four sections (Table 3). Sections from one dog coinfected with “H. heilmannii” and H. bizzozeronii were positive for “H. heilmannii” types 1 and 2. The results of FISH and species-specific PCR were in agreement in five of the eight animals evaluated.
In dogs coinfected with “H. heilmannii” and H. bizzozeronii FISH clearly demonstrated that different HHLO cohabit the same region of gastric mucosa in the canine stomach (Fig. 4D). Morphologically two distinct spiral forms were observed using Steiner's silver stain and confirmed with FISH (Fig. 4E and F). Short, loosely spiraled rods (Fig. 4E1 and F1) were observed in sections from D2, D5, and D7; these were approximately half the length of another distinct, tightly spiraled, elongated form of Helicobacter (Fig. 4E, inset 2, and F, inset 2) within the same sections. Using a eubacterial FISH probe, the two forms were observed to be located in mixed populations within the gastric mucus. The shorter spirals (Fig. 4E, inset 1) hybridized with an HHLO probe (Table 1 [Heibiz Sonde 7]) and not with any “H. heilmannii”-specific probes. The longer spirals (Fig. 4E, inset 2) hybridized with an “H. heilmannii”-specific probe (Table 1 [Heifel 1 + 2]) and with an HHLO probe detecting H. felis, H. bizzozeronii, and H. salomonis. Additionally, using “H. heilmannii”-specific probes, two morphological forms (a long and a short spiral) were visible in the same specimens (Fig. 4A and B).
DISCUSSION
Infection with candidatus “Helicobacter heilmannii” in humans is associated with gastritis and mucosa-associated lymphoid tissue lymphoma and is thought to be acquired by zoonotic transmission from dogs, cats or pigs, which are commonly infected with HHLO (12, 25, 27, 30). While “H. heilmannii” type 1 is the predominant organism in people and pigs (19, 28), the types colonizing dogs and cats have not been defined. The present study demonstrates that “H. heilmannii” ureB sequences generated from gastric biopsies of 45 cats and 10 dogs are similar to “H. heilmannii” sequences deposited in GenBank and clearly distinct from the “Helicobacter heilmannii”-like organisms H. felis, H. salomonis, and H. bizzozeronii. These results confirm the specificity of ureB-based PCR for the identification of “H. heilmannii” (14) and should be useful for discriminating “H. heilmannii” from other large spiral organisms in tissues from infected people.
As ureB sequencing does not enable discrimination between “H. heilmannii” subtypes we employed 16S rDNA amplification and sequence analysis of PCR products without cloning, which has been previously used to characterize “H. heilmannii” infections in people (28). To avoid producing a composite sequence of multiple Helicobacter spp. we sequenced only DNA samples determined by species-specific PCR to be positive for “H. heilmannii” but negative for H. bizzozeronii, H. felis, and H. pylori. DNA sequence traces were also carefully scrutinized for underlying peaks that would indicate the presence of a different species. The agreement between the “H. heilmannii” subtype designated for aligned 225-bp 16S DNA sequences and 16S rRNA bp 642 to 661 FISH probe binding sequences in 19 of 20 DNA samples further supports the presence of a single strain. However, in 3 of 20 samples (D1, D3, and C38), which clustered with HHLO-5 and “H. heilmannii” type 2, we observed DNA sequences in the 16S rRNA 642 to 661 region that had ambiguous FISH probe binding sites. It is possible that these three sequences indicate novel “H. heilmannii” subtypes, or perhaps they are chimeras resulting from the presence of undetermined coinfecting Helicobacter spp. Despite the possible limitations of direct sequencing of PCR products our results indicate that infected dogs and cats harbor “H. heilmannii” bacteria that are clearly distinct from “G. hominis” isolate 1 and “H. suis” (bootstrap value of 98%). A total of 65% (13 of 20) of sequences clustered with “H. heilmannii” type 4 and 35% of sequences (7 of 20) clustered with “H. heilmannii” type 2 and HHLO-5. Isolates clustering with “H. heilmannii” type 4 were more prevalent in cats than dogs; three out of four dog 16S sequences obtained from mono-infected dogs clustered with type 2. Our results suggest that other species such as pigs, which are known to harbor “H. heilmannii” type 1, pose a greater zoonotic threat than dogs and cats. This is consistent with the increased relative risk of infection reported by Stolte et al. (25) for humans exposed to pigs.
It was interesting that dogs and cats differed in their patterns of cocolonization by different Helicobacter species. The majority of cats were colonized by “H. heilmannii,” whereas dogs were more likely to be coinfected with H. bizzozeronii and “H. heilmannii.” Cats from different countries also showed different patterns of cocolonization: American cats were more frequently cocolonized with H. felis (18%) than H. bizzozeronii (6%), whereas German cats were predominantly cocolonized with H. bizzozeronii (25%) rather than H. felis (7%). These results are in broad agreement with a previous PCR-based study of Swiss cats where “H. heilmannii” was the predominant species (78% of Swiss cats). However, Swiss cats were more commonly cocolonized with unclassified Helicobacter spp., and H. bizzozeronii or H. felis was not detected (14). The reasons for these differences and the source of different Helicobacter spp. in cats have not been defined. The findings for dogs are also in broad agreement with previous PCR-based studies that showed 25% of dogs colonized by “H. heilmannii” and frequent cocolonization with “H. heilmannii” and H. bizzozeronii (16, 17).
We employed FISH with eubacterial and “H. heilmannii”-specific probes that have been previously evaluated in human infections (28) to provide simultaneous evaluation of infecting Helicobacter species by genotype and morphology in a subset of infected cats and dogs. The present study was not constructed to provide a direct comparison of species-specific PCR, 16S rDNA sequence analysis and FISH. Our results complement and extend previous electron microscopic and PCR-based studies of infected dogs and cats (10, 18, 24, 29) that indicated that the stomachs of cats and dogs are colonized by a diverse population of morphologically and genetically distinct spiral organisms.
In infected dogs, FISH enabled the site of colonization to be examined. “H. heilmannii” types 2 and 4 inhabited the gastric mucus and gastric glands and in animals coinfected with other HHLO shared the same gastric niche. On occasion organisms appeared to be within parietal cells but were generally in the mucus and were not obviously adherent to cells. There was no clear evidence to support tropism of species to different parts of the stomach, suggesting that dogs and cats are colonized by a free mixing population of different Helicobacter spp. Such intermingling would potentially enable the transfer of DNA from one species to the other.
Two distinct forms of three Helicobacter spp. were recognized in dog samples—a short, loosely spiraled form which hybridized only with a HHLO probe reported to be specific for H. felis, H. bizzozeronii, and H. salomonis and two longer, tightly spiraled forms, one of which hybridized only with an “H. heilmannii” probe (Heifel 1+2) and the other only with a HHLO probe. The shorter forms of Helicobacter appeared to be similar to the Lockard and Boler type 2 organisms, now known to be H. felis; however, since PCR had revealed all dog specimens to be free from H. felis, we concluded that these organisms were most likely the morphologically similar H. salomonis (6, 10, 24). The two longer spiral forms appeared to be identical to the Lockard and Boler type 3 organisms (10) observed by electron microscopy in the canine stomach and now known to be “H. heilmannii.” However, since one longer form was observed only with an HHLO probe, this is likely to be H. bizzozeronii (6, 24) and the identical form with “H. heilmannii”-specific probes is likely to be “H. heilmannii” (6, 10, 24). Different morphologies observed with a specific “H. heilmannii” probe were likely due to the presence of different forms of the same species within an animal, as observed for “H. heilmannii” in cats (18).
FISH failed to definitively characterize “H. heilmannii” subtypes in 14 of 15 cats. The reasons for the failure of the “H. heilmannii” probes to hybridize to cat tissues, despite successful hybridization with the eubacterial probe, the presence of the probe binding sites in 16S sequences from these cats, and appropriate hybridization controls is not clear. It may reflect tissue processing, as these samples were obtained from clinical cases and may have been formalin fixed for more than the recommended 24 h. Perhaps the smaller size of the eubacterial probe (12 versus 20 bp) compared to the “H. heilmannii” probes enabled it to hybridize to the archival feline tissue samples.
In conclusion, our results indicate that dogs and cats are colonized by “H. heilmannii” organisms that are distinct from “G. hominis” and “H. suis” and more similar in genotype to “H. heilmannii” types 2 and 4. As “H. heilmannii” type 1 is the predominant species in infected people (80%), the zoonotic risk posed by dogs and cats is likely small.
Acknowledgments
This study was supported by grants from the U.S. Public Health Service (DK 002938) (K. Simpson), The Wellcome Trust (067808) (S. Priestnall), The Comparative Gastroenterology Society, and Waltham.
We thank Francis Davis for technical support.
REFERENCES
- 1.Andersen, L. P., K. Boye, J. Blom, S. Holck, A. Norgaard, and L. Elsborg. 1999. Characterization of a culturable “Gastrospirillum hominis” (Helicobacter heilmannii) strain isolated from human gastric mucosa. J. Clin. Microbiol. 37:1069-1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andersen, L. P., A. Norgaard, S. Holck, J. Blom, and L. Elsborg. 1996. Isolation of a “Helicobacter heilmannii”-like organism from the human stomach. Eur. J. Clin. Microbiol. Infect. Dis. 15:95-96. [DOI] [PubMed] [Google Scholar]
- 3.Dent, J. C., C. A. M. McNulty, J. C. Uff, S. P. Wilkinson, and M. W. Gear. 1987. Spiral organisms in the gastric antrum. Lancet ii:96. [DOI] [PubMed] [Google Scholar]
- 4.Dieterich, C., P. Wiesel, R. Neiger, A. Blum, and I. Corthesy-Theulaz. 1998. Presence of multiple “Helicobacter heilmannii” strains in an individual suffering from ulcers and in his two cats. J. Clin. Microbiol. 36:1366-1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fawcett, P. T., K. M. Gibney, and K. M. Vinette. 1999. Helicobacter pylori can be induced to assume the morphology of Helicobacter heilmannii. J. Clin. Microbiol. 37:1045-1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fox, J. G., and A. Lee. 1997. The role of Helicobacter species in newly recognized gastrointestinal tract diseases of animals. Lab. Anim. Sci. 47:222-255. [PubMed] [Google Scholar]
- 7.Jalava, K., S. L. On, C. S. Harrington, L. P. Andersen, M. L. Hanninen, and P. Vandamme. 2001. A cultured strain of “Helicobacter heilmannii,” a human gastric pathogen, identified as H. bizzozeronii: evidence for zoonotic potential of Helicobacter. Emerg. Infect. Dis. 7:1036-1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jukes, T. H., and C. R. Cantor. 1969. Evolution of protein molecules, vol. 3. Mammalian protein metabolism, p. 21-132. Academic Press, Inc., New York, N.Y.
- 9.Kumar, S., K. Tamura, I. B. Jakobsen, and M. Nei. 2001. MEGA2: Molecular Evolutionary Genetics Analysis software, 2.1 ed. Arizona State University, Tempe. [DOI] [PubMed]
- 10.Lockard, V. G., and R. K. Boler. 1970. Ultrastructure of a spiraled microorganism in the gastric mucosa of dogs. Am. J. Vet. Res. 31:1453-1462. [PubMed] [Google Scholar]
- 11.McNulty, C. A., J. C. Dent, A. Curry, J. S. Uff, G. A. Ford, M. W. Gear, and S. P. Wilkinson. 1989. New spiral bacterium in gastric mucosa. J. Clin. Pathol. 42:585-591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Meining, A., G. Kroher, and M. Stolte. 1998. Animal reservoirs in the transmission of Helicobacter heilmannii. Results of a questionnaire-based study. Scand. J. Gastroenterol. 33:795-798. [DOI] [PubMed] [Google Scholar]
- 13.Morgner, A., E. Bayerdorffer, A. Meining, M. Stolte, and G. Kroher. 1995. Helicobacter heilmannii and gastric cancer. Lancet 346:511-512. [DOI] [PubMed] [Google Scholar]
- 14.Neiger, R., C. Dieterich, A. Burnens, A. Waldvogel, I. Corthesy-Theulaz, F. Halter, B. Lauterburg, and A. Schmassmann. 1998. Detection and prevalence of Helicobacter infection in pet cats. J. Clin. Microbiol. 36:634-637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Neiger, R., G. Seiler, and A. Schmassmann. 1999. Use of a urea breath test to evaluate short-term treatments for cats naturally infected with Helicobacter heilmannii. Am. J. Vet. Res. 60:880-883. [PubMed] [Google Scholar]
- 16.Neiger, R., and K. W. Simpson. 2000. Helicobacter infection in dogs and cats: facts and fiction. J. Vet. Intern. Med. 14:125-133. [DOI] [PubMed] [Google Scholar]
- 17.Neiger, R., M. E. Tschudi, A. Burnens, B. Goke, and A. Schmassmann. 1999. Diagnosis and identification of gastric helicobacter species by polymerase chain reaction in dogs. Microb. Ecol. Health Dis. 11:234-240. [Google Scholar]
- 18.Norris, C. R., S. L. Marks, K. A. Eaton, S. Z. Torabian, R. J. Munn, and J. V. Solnick. 1999. Healthy cats are commonly colonized with “Helicobacter heilmannii” that is associated with minimal gastritis. J. Clin. Microbiol. 37:189-194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Queiroz, D. M., G. A. Rocha, E. N. Mendes, S. B. De Moura, A. M. De Oliveira, and D. Miranda. 1996. Association between Helicobacter and gastric ulcer disease of the pars esophagea in swine. Gastroenterology 111:19-27. [DOI] [PubMed] [Google Scholar]
- 20.Saitou, N., and M. Nei. 1987. The neighbor-joining method: a new method for constructing phylogenetic trees. Mol. Biol. Evol. 4:406-425. [DOI] [PubMed] [Google Scholar]
- 21.Scanziani, E., K. W. Simpson, S. Monestiroli, S. Soldati, D. Strauss-Ayali, and F. Del Piero. 2001. Histological and immunohistochemical detection of different Helicobacter species in the gastric mucosa of cats. J. Vet. Diagn. Investig. 13:3-12. [DOI] [PubMed] [Google Scholar]
- 22.Solnick, J. V., J. O'Rourke, A. Lee, B. J. Paster, F. E. Dewhirst, and L. S. Tompkins. 1993. An uncultured gastric spiral organism is a newly identified Helicobacter in humans. J. Infect. Dis. 168:379-385. [DOI] [PubMed] [Google Scholar]
- 23.Solnick, J. V., J. O'Rourke, A. Lee, and L. S. Tompkins. 1994. Molecular analysis of urease genes from a newly identified uncultured species of Helicobacter. Infect. Immun. 62:1631-1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stoffel, M. H., A. E. Friess, A. Burnens, A. Schmassmann, and R. Neiger. 2000. Distinction of gastric Helicobacter spp. in humans and domestic pets by scanning electron microscopy. Helicobacter 5:232-239. [DOI] [PubMed] [Google Scholar]
- 25.Stolte, M., E. Wellens, B. Bethke, M. Ritter, and H. Eidt. 1994. Helicobacter heilmannii (formerly Gastrospirillum hominis) gastritis: an infection transmitted by animals? Scand. J. Gastroenterol. 29:1061-1064. [DOI] [PubMed] [Google Scholar]
- 26.Strauss-Ayali, D., E. Scanziani, D. Deng, and K. W. Simpson. 2001. Helicobacter spp. infection in cats: evaluation of the humoral immune response and prevalence of gastric Helicobacter spp. Vet. Microbiol. 79:253-265. [DOI] [PubMed] [Google Scholar]
- 27.Svec, A., P. Kordas, Z. Pavlis, and J. Novotny. 2000. High prevalence of Helicobacter heilmannii-associated gastritis in a small, predominantly rural area: further evidence in support of a zoonosis? Scand. J. Gastroenterol. 35:925-928. [DOI] [PubMed] [Google Scholar]
- 28.Trebesius, K., K. Adler, M. Vieth, M. Stolte, and R. Haas. 2001. Specific detection and prevalence of Helicobacter heilmannii-like organisms in the human gastric mucosa by fluorescent in situ hybridization and partial 16S ribosomal DNA sequencing. J. Clin. Microbiol. 39:1510-1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Weber, A. F., and E. F. Schmittdiel. 1962. Electron microscopic and bacteriological studies of spirilla isolated from the fundic stomachs of cats and dogs. Am. J. Vet. Res. 23:422-427. [PubMed] [Google Scholar]
- 30.Yeomans, N. D., and S. D. Kolt. 1996. Helicobacter heilmannii (formerly Gastrospirillum): association with pig and human gastric pathology. Gastroenterology 111:244-247. [DOI] [PubMed] [Google Scholar]
- 31.Zhu, J., C. H. Teng, C. F. Chang, C. D. Chang, K. W. Simpson, C. Wei, P. McDonough, S. McDonough, and Y. F. Chang. 2002. Cloning and characterization of a Helicobacter bizzozeronii urease gene cluster. DNA Seq. 13:321-331. [DOI] [PubMed] [Google Scholar]