Abstract
The Campylobacter excretion patterns of 26 domestic pet dogs were described in a longitudinal study. The dogs entered the study between 3 and 8 months of age and were monitored until 2 years of age. They were tested monthly for Campylobacter carriage in stool samples that were cultured on the Campylobacter-selective media CAT and modified CCDA agar at 37 and 42°C. This study comprised 366 fecal swab samples, of which 278 (76.2%) were found to be Campylobacter positive, with the following distribution of species: 75.0% Campylobacter upsaliensis, 19.4% Campylobacter jejuni, 2.1% Campylobacter lari, 0.7% Campylobacter coli, and 2.8% Campylobacter spp. Isolates were typed by pulsed-field gel electrophoresis (PFGE) to elucidate the strain excretion pattern. All study dogs excreted Campylobacter spp. during the study period. At 3 months of age, 60% of the dogs carried Campylobacter, increasing to nearly 100% carriers at 1 year of age, whereafter the carriage rate decreased to 67% at 24 months of age. The PFGE types showed that individual dogs were often colonized by unique strains of C. upsaliensis for several months, up to 21 months or longer. These C. upsaliensis strains were either clonal (or underwent concurrent minor mutative changes) or independent strains. In contrast, the excreted C. jejuni isolates were much more diverse and, in most cases, only seen in one sample from each dog. A high degree of diversity among different dogs was seen. We conclude that young domestic pet dogs excreted Campylobacter spp. during the majority of their puppyhood and adolescent period. In general C. upsaliensis strains were excreted for months, with short-term interruptions by or cocolonization with other transitory Campylobacter spp., predominantly C. jejuni. C. jejuni was more prevalent in dogs between 3 months and 1 year of age than in dogs between 1 and 2 years of age.
The pathogenicity of Campylobacter spp. to humans is well recognized throughout the world. In surveys, Campylobacter spp. have been isolated from between 2.8 and 10.5% of gastroenteritis cases in Utah (17), Australia (19), Belgium (15), Sweden (24), and Denmark (10) and from 21.8% of children in South Africa (20). The majority of campylobacteriosis cases in the reported surveys are caused by Campylobacter jejuni or Campylobacter coli, whereas Campylobacter upsaliensis accounts for less than 1% of the total number of cases, except for the South African survey, in which C. upsaliensis was isolated from 4.9% of 20,458 children with gastroenteritis.
Having a pet dog has repeatedly been identified as a risk factor for human campylobacteriosis in the developed part of the world (41). Certainly, Campylobacter carriage in healthy pet dogs is common according to several studies in the last two decades, with reported carrier rates from 21 to 75% (4, 5, 12, 16, 27, 29, 35, 40, 42). The species distribution of isolates from dogs differs considerably between publications and years. In early studies, only C. jejuni and C. coli were reported. However, in 1983, a new species named C. upsaliensis was isolated for the first time from canine feces (37) and further characterized and described in both human feces in 1990 (15) and dog feces in 1991 (36). Since then, C. upsaliensis has been increasingly prevalent in dogs, according to published reports, and even more prevalent than C. jejuni, comprising 64 to 82% (4, 27, 35, 37) of the strains isolated from dogs. This links dogs to humans, particularly to children, as evident sources of infection with C. upsaliensis, as the only significant sources of C. upsaliensis, according to present knowledge, are dogs and cats. Actual transmission of C. jejuni or C. upsaliensis from pet to child has often been suspected (14, 19, 32) and was recently proven (45).
Previous studies of Campylobacter carriage of dogs have all been cross-sectional investigations yielding no information about the duration of the carrier period for each dog. The aim of the present study was to elucidate the Campylobacter excretion pattern of young dogs over time and, thus, their potential significance as sources of human campylobacteriosis. Study dogs were surveyed for fecal excretion of thermophilic Campylobacter spp. between entry of the study at 3 to 8 months of age until they reached 2 years of age. The clonality and epidemiology of the isolates were examined by pulsed-field gel electrophoresis (PFGE). To our knowledge, this investigation is the first reported longitudinal study with multiple sampling of individual dogs.
MATERIALS AND METHODS
Study design.
Healthy pet dogs (n = 26) from different parts of Denmark were recruited for the investigation and entered the study between April 2000 and April 2001. A short telephone interview was performed with the owner of the dog concerning data of the dog and its environment. The study comprised 26 dogs from the beginning, 20 dogs at 1 year of age, and 18 dogs at 2 years of age (closure of the study). The mean age of the puppies at entry was 4 months (range, 2.2 to 8.1 months). They were all pet dogs living in private homes in either rural (n = 14) or urban (n = 12) areas. The dogs were mixed breed (n = 8) or purebred (n = 18) of 10 different dog races. Gender distribution was 13 males and 13 females. Each dog was sampled once per month; fecal swab samples (Duo-Transwab; MW & E, Wiltshire, United Kingdom) were taken by the owner from freshly voided feces and forwarded to the laboratory by ordinary mail. One of the Duo-swabs was used for culture of Campylobacter. The other swab was used for culture of Salmonella during the first year of the study period. Information regarding the consistency of the fecal voiding as normal, semisoft, or diarrheic at the time of sampling was enclosed with each sample.
Culture of Campylobacter and species identification.
Swab samples (n = 366) were streaked on modified CCDA (mCCDA) (blood-free agar base with cefoperazone [32 mg/liter] and amphotericin B [10 mg/liter]) (CM739 plus SR155; Oxoid, Basingstoke, United Kingdom) and CAT (blood-free agar base with cefoperazone [8 mg/liter], teicoplanin [4 mg/liter], and amphotericin B [10 mg/liter]) (CM739 plus SR174; Oxoid) agar plates, incubated in a microaerobic atmosphere (6% O2, 6% CO2, 4% H2 in N2) at both 37 and 42°C, i.e., four inoculated plates per sample. However, the first 59 samples were only cultured on mCCDA at 42°C. Campylobacter-like colonies were detected visually and/or by microscopic observation of spiral-shaped bacteria. To obtain pure cultures, isolates were subcultured on blood agar containing 40 g of Oxoid CM 271/liter supplemented with 5% calf blood and incubated microaerobically at 37°C for 2 to 4 days. Isolates were identified to species level by the following phenotypic tests: catalase, indoxyl acetase, hippuricase, and resistance to nalidixic acid and cephalothin, as recommended by the Nordic Committee on Food Analysis (2). In an attempt to discriminate between C. upsaliensis, Campylobacter helveticus and Helicobacter canis, a subset of 69 isolates, phenotypically identified as C. upsaliensis by the above tests, were additionally tested for reduction of nitrate (8) and selenite (30) and microaerobic growth at 37°C on buffered charcoal-yeast extract agar (BCY) (11). The identity of these 69 isolates was subsequently tested by PCR as described below.
Culture of Salmonella.
A total of 179 fecal swab samples were cultured for Salmonella according to a modified International Standardization Organization method (3). Swabs were transferred to 10 ml of buffered peptone water (catalog no. 1.07228; Merck, Darmstadt, Germany) and subjected to preenrichment by incubation at 37°C for 16 to 20 h, whereafter 100 μl was transferred to 10 ml of Rappaport-Vassiliadis-soy peptone broth (CM866; Oxoid) and further incubated at 42°C for 18 to 24 h. One loopful, corresponding to 10 μl, of Rappaport-Vassiliadis-soy peptone broth was then streaked on Rambach agar (catalog no. 1.07500/0002; Merck). Typical Salmonella suspect colonies on Rambach agar were tested for agglutination with polyvalent Salmonella O-serum (Statens Serum Institut, Copenhagen, Denmark). If they reacted positively, they were subcultured and serotyped according to the Kauffmann-White scheme (33). Salmonella enterica subsp. enterica serovar Typhimurium was further phage typed according to the methods of Anderson et al. (1) and Callow (7).
PCR.
Lysates for PCR from the subset of the 69 C. upsaliensis isolates as well as 22 other catalase-negative yet phenotypically unidentified isolates were prepared by suspending culture material in 200 μl of lysis buffer (20 mM Tris-HCl [pH 8] and 0.1% sodium dodecyl sulfate). After thorough vortexing, 12 μl of proteinase K (20 mg/ml) was added and samples were incubated at 56°C for 30 min and then at 95°C for 10 min. DNA preparations were stored at −20°C until use in PCR assays. C. jejuni-C. coli duplex PCR was performed as described by van de Giessen et al. (43) with the following modifications: the PCR mixture contained 27 ng of bovine serum albumin per sample together with 50 and 5 pmol, respectively, of C. jejuni- and C. coli-specific primers. C. upsaliensis-C. helveticus duplex PCR was performed as described by Lawson et al. (21) with the following modifications: the PCR mixture contained 27 ng of bovine serum albumin and 0.5 U of TaqDNA polymerase in volumes of 25 μl per sample.
PFGE.
Selected Campylobacter isolates (n = 269) from 14 of the 26 dogs were subjected to PFGE to investigate their clonality and epidemiology. From each positive sample, one or two isolates, preferably one from CAT agar and one from CCDA agar were tested by PFGE, which was carried out essentially according to the protocol recommended by CAMPYNET (protocol by S. L. W. On, M.-L. Hänninen, and F. Thomson-Carter, available at http://campynet.vetinst.dk/PFGE.html). Preliminarily, a subset of isolates were PFGE typed by using SmaI (Invitrogen), BamHI (Invitrogen), SalI (Invitrogen), SpeI (New England Biolabs), XbaI (New England Biolabs), NotI (New England Biolabs), and KpnI (Invitrogen) as restriction enzymes. The best results were obtained with KpnI, and this enzyme was therefore applied on all 269 isolates. PFGE types were assigned arbitrary numbers. Photographs of PFGE gels were digitalized into TIFF files and loaded into the image analyzer program GelCompar (Applied Maths, Kortrijk, Belgium) for computer-assisted identification of banding patterns and clustering analysis. A maximum tolerance of 1.5% was used at identification of bands.
Data analysis.
The calculation of similarity between isolates on the basis of the PFGE profiles was done by using the Dice coefficient, whereafter clustering was performed by using the unweighted pair group method with arithmetic averages. These calculations were carried out in GelCompar. The performance of the two culture media, CAT and mCCDA, and the two temperatures, 37 and 42°C, for recovery of Campylobacter, irrespective of species, was evaluated by using McNemar's test and a 5% significance level. Differences in carrier rates between age groups, gender, and other data of the dogs and their environment were calculated by using Fischer's exact test. Calculations were done in SAS, version 8.2 (SAS Institute Inc., Cary, N.C.). The 69 catalase-negative isolates subjected to supplementary phenotypic tests together with 22 catalase-negative but phenotypically unidentified isolates were statistically considered random samples without replacement from a finite population. The hypergeometric distribution was iteratively used to calculate confidence limits (with at least 95% confidence) for the number of phenotypic misidentifications among all catalase-negative isolates (n = 216) (18).
RESULTS
Carrier state of the dogs.
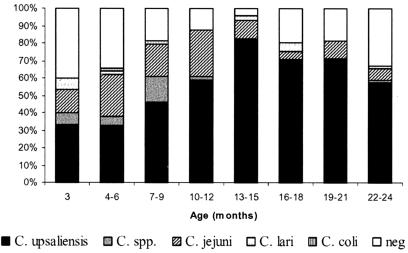
The prevalence of Campylobacter-positive dogs in different age groups is shown in Fig. 1. Between approximately 9 and 15 months of age, most dogs were excreting campylobacters constantly. Apparently, some of the dogs cleared out the infection between 15 months and 2 years of age. However, a considerable percentage (67%) of the dogs were still carrying campylobacters at 2 years of age. The further duration of the carrier periods remains undetermined by this study design. In total, 278 (76.2%) of the 366 samples taken were positive for Campylobacter spp. by culture. The negative samples (23.8%) were distributed among all dogs, particularly during the first and last months of their participation, but occasionally, negative samples were recovered for most of the dogs, also during the carrier period. No seasonal variation in the carrier rate or of the carrier period was noted, but a variation according to the age of the dog and the Campylobacter species isolated was seen, as C. jejuni was significantly more prevalent in dogs of less than 1 year (39 of 178) than in dogs between 1 and 2 years of age (9 of 188). Fisher's exact test for age below or above 1 year was highly significant (P = 0.00013). Dogs in cities were more likely to be infected (173 of 203) with Campylobacter than dogs living in rural areas (105 of 161) (P = 0.000025), and male dogs were more likely to be infected (146 of 179) than female dogs (132 of 187) (P = 0.015). There was no statistical difference between dogs living together with other pet animals in the home and dogs living as sole pets.
FIG. 1.
Prevalence of Campylobacter-positive samples and species distribution according to the age of the dogs.
Species distribution and diversity.
Of the 278 positive samples, C. upsaliensis was isolated in 194, C. jejuni was isolated in 56, Campylobacter lari was isolated in 6, and C. coli was isolated in 2. Twenty-two isolates were catalase negative but phenotypically unidentified due to resistance to nalidixic acid and/or cephalothin. All of these 22 isolates were identified as C. upsaliensis by PCR. Eight isolates that died before species identification could be completed were designated Campylobacter spp. The final species distribution after culture supplemented with PCR was then as follows: C. upsaliensis, 75.0% (n = 216); C. jejuni, 19.4% (n = 56); C. lari, 2.1% (n = 6); C. coli, 0.7% (n = 2); Campylobacter spp., 2.8% (n = 8). Coinfection was detected in 10 samples (3.4%) infected with C. upsaliensis and C. jejuni (n = 8) or C. upsaliensis and C. lari (n = 2). Significantly more samples were positive on CAT than on mCCDA agar (P = 3.40 × 10−5 in McNemar's test), with an agreement expressed with the kappa coefficient of 0.64. There was no difference in performance at 37 and 42°C (P = 0.80), and the kappa value here was 0.78.
Of the subset of 69 of 194 C. upsaliensis isolates subjected to supplementary phenotypical tests to discover C. helveticus strains or H. canis, 49 isolates were again identified as C. upsaliensis (reduced nitrate and selenite and grew on BCY), 10 isolates were identified as C. helveticus (reduced nitrate but not selenite), and 10 isolates were unidentified (reduced nitrate and selenite but did not grow on BCY). Yet, all of the 69 isolates were anyhow identified as C. upsaliensis strains by C. upsaliensis-C. helveticus duplex PCR, and therefore, they were finally considered true C. upsaliensis strains. In conclusion, neither C. helveticus nor H. canis strains were isolated by culture, and the supplementary phenotypical tests applied were furthermore found insufficient to discriminate C. upsaliensis from C. helveticus.
PFGE.
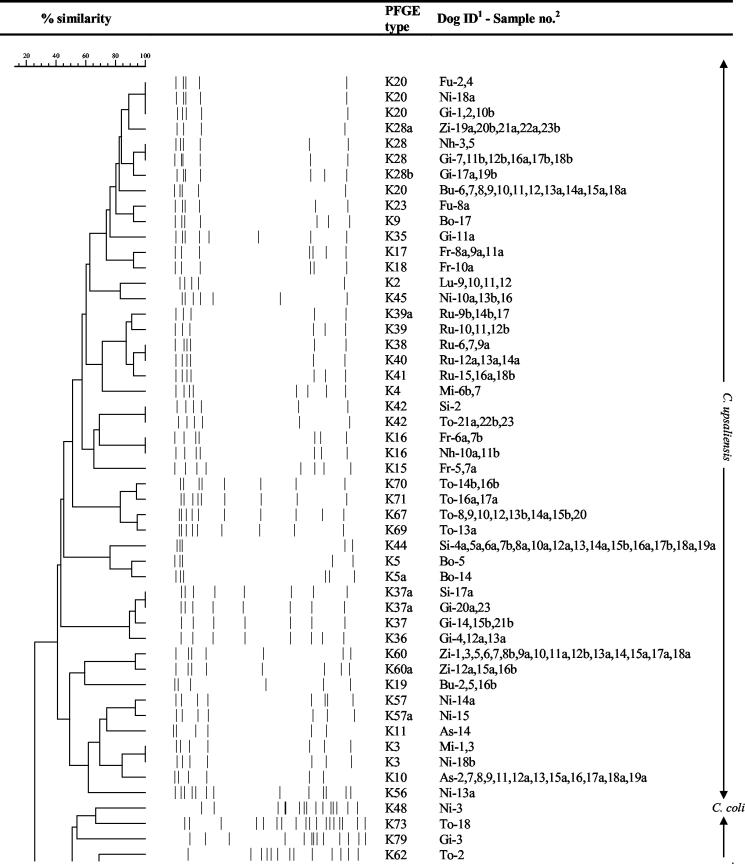
All PFGE profiles from the 14 dogs selected for pulsed-field profiling are displayed in a dendrogram (Fig. 2). If more than one PFGE type was identified in a sample, both types are shown. The excretion pattern for each of the 14 dogs is listed in chronological order in Table 1. Three main characteristic carrier patterns were found. Thus, 7 of 14 dogs were colonized with the same C. upsaliensis strain over 8 to 21 months. Ann Sophie was already colonized with C. upsaliensis PFGE type K10 at the second sampling, and this strain was still detected at the last sampling 21 months later. In between, three of the samplings had resulted in the culture of three different C. jejuni strains. Also Mille, Rufus, Sita, Tor, Zicka, and Buster had long-term persistent strains. The chronological excretion pattern of the PFGE profiles of Buster is displayed in Fig. 3. Another 4 of 14 dogs, Bonnie, Frigg, Lucca, and Nhala, had persistence of certain C. upsaliensis strains for 2 to 5 months only, whereafter a negative period or another strain took over. Nhala was first infected with PFGE type K28 in 2 samples, which was replaced by type K29 in 4 samples, which was again replaced by type K16 in 2 samples. The remaining 3 of 14 dogs, Nikki, Futte, and Gilli, had no persistent strain or succession of persistent strains but were constantly colonized with alternating Campylobacter strains. An example of this pattern, from Nikki, is displayed in Fig. 4. Coinfection with two different C. upsaliensis strains were detected in 10 of the 14 dogs in some of the samples. Thus, in Buster one strain, type K19, was found in samples at 9 and 12 months, whereafter another unrelated strain, PFGE type K20, was found in all of the samples at 13 to 24 months. At 22 to 23 months, however, coexistence of both PFGE types, K19 and K20, was demonstrated (Table 1).
FIG. 2.
Dendrogram showing PFGE types and relatedness of Campylobacter isolates from 14 dogs. The dog identification (ID) is the first two letters of the dog's name. The sample number is extended with an a or b where two isolates of a sample PFGE typed. Both profiles are shown if the PFGE profiles were different, otherwise either a or b is shown on the dendrogram. Marker, lambda ladder from New England Biolabs.
TABLE 1.
Excretion of Campylobacter PFGE types of 14 dogs in order of the age of the dogsa
| Age (mo) | PFGE type(s) for dog:
|
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ann Sophie | Bonnie | Buster | Frigg | Futte | Gilli | Lucca | Mille | Nhala | Nikki | Rufus | Sita | Tor | Zicka | |
| 3 | — | — | / | / | — | K20 | / | K3 | / | / | / | K74 | K61 | K60, K58 |
| 4 | K10 | — | / | / | / | K20 | — | K3 | / | / | / | K42 | K62 | K60 |
| 5 | — | K5 | / | NT | K20 | K36, K79 | — | — | — | / | — | K43 | K63 | K59 |
| 6 | — | K6 | / | / | K20 | — | — | — | K28 | K47 | NT | / | K63 | K60 |
| 7 | K12 | K7 | / | K8 | / | K31 | — | / | NT | NT | — | / | K64 | K60 |
| 8 | K13 | K7 | — | K8 | K24 | K28 | / | / | K28 | K48 | NT | K44, K44 | NT | K60 |
| 9 | K10 | — | K19 | / | K25 | K33 | NT | / | / | K75 | K38 | K44, K44 | K65, K66 | K60, K60 |
| 10 | K10 | K7 | NT | K15 | / | K34, K34 | — | / | / | K26 | K38 | K44, K44 | K67 | K60 |
| 11 | K10 | K1, K1 | / | / | / | K20, K20 | K2 | / | K29 | K50 | / | K44, K45 | K67, K67 | K60 |
| 12 | K14 | — | K19 | / | — | K28, K35 | K2 | / | K29, K29 | K51 | K38, K39a | K44, K44 | K68 | K60, K60a |
| 13 | K10 | K5a | K20 | K16, K16 | / | K28, K36 | K2 | / | K29 | K52 | K39 | K46, K46 | K67 | K60, K60 |
| 14 | K10, K10 | — | K20 | K15, K16 | K23 | K37 | K2 | / | K29, K29 | K53 | K39 | K44, K44 | K67, K69 | K60, K60 |
| 15 | / | NT | K20 | K17, K17 | / | K37, K37 | — | / | K16, K16 | K45, K45 | K39, K40 | K44, K44 | K67, K70 | K60a, K60a |
| 16 | K10 | K9 | K20 | K17, K17 | — | K28, K28 | — | / | / | K54, K54 | K40, K40 | K44, K44 | K67, K67 | K60, K60 |
| 17 | K11 | NT | K20 | K17, K18 | — | K28, K28b | — | / | K16, K16 | K55, K55 | K39a, K40 | K44 | K70, K71 | K60a, K60a |
| 18 | K10, K10 | — | K20 | / | / | / | K27, K27 | / | NT | K45, K56 | K41 | / | K71, K71 | K60, K60 |
| 19 | K10 | — | K20, K20 | K17, K17 | — | K28a, K28 | — | K3, K4 | / | K57, K57 | K41, K41 | K44, K44 | K73 | K60, K60 |
| 20 | K10, K10 | NT | K20, K20 | / | — | K28a, K28b | — | K4 | / | K57a | K39a | K44 | K72, K72 | K28a |
| 21 | / | / | K20, K20 | / | — | K37a, K47 | — | NT | / | K45 | K41, K41 | K44, K44 | K67 | K28a, K28a |
| 22 | K10, K29 | NT | K19, K20 | / | / | K37, K77 | — | — | NT | — | K74, K74 | K44, K37a | K42, K42 | K28a, K28a |
| 23 | / | — | K19, K20 | / | — | K47 | — | — | / | K20, K3 | K74, K74 | K44, K44 | K42, K42 | K28a, K74 |
| 24 | K10, K10 | NT | K20, K20 | / | — | K37a | — | — | / | — | / | K44, K44 | K42 | K28a |
For samples from which two culture plates were typed, both results are shown. C. upsaliensis PFGE types are shown in boldface type. /, no sample obtained; —, Campylobacter-negative sample; NT, isolate(s) obtained were not PFGE typed due to death or other reason.
FIG. 3.
Chronological excretion of PFGE types of Campylobacter isolates from the dog Buster (dog identification [ID], Bu). The figure shows an example of a dog with a persistent infection with a C. upsaliensis (C. ups) strain over several months.
FIG. 4.
Chronological excretion of PFGE types of Campylobacter isolates from the dog Nikki (dog identification [ID], Ni). The figure shows an example of a dog with no persistent infection but the presence of several different Campylobacter strains (C. jejuni [C. jej], C. coli [C. col], and C. upsaliensis [C. ups]).
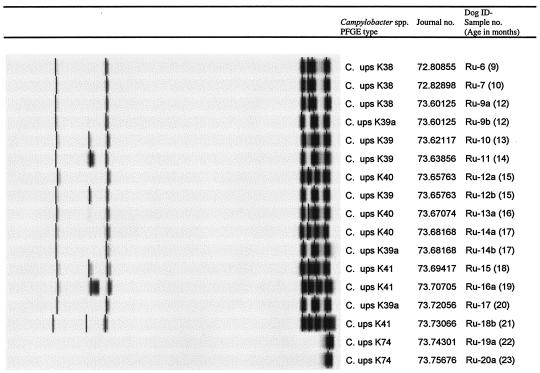
Closely related but different PFGE types were occasionally found, primarily in the dogs colonized with long-term persistent C. upsaliensis strains. For example, in Ann Sophie, where the C. upsaliensis strain recovered from several samples had PFGE type K10 and the strain from the sample at 17 months had the closely related type K11 (Fig. 2; Table 1). Another example was Rufus, where the five different but closely related PFGE types, K38, K39, K39a, K40, and K41, were found (Fig. 5; Table 1). In samples at 9 and 10 months, K38 was found alone, at 12 months, K38 and K39a were both present, and at 13 and 14 months, K39 was found alone. Then at 15 months, K39 and K40 were detected as a coinfection, at 16 months, K40 was found, at 17 months, both types K39a and K40 were found, and at 18, 19, and 21 months, K41 alone was found, and at 20 months, K39a alone was found. Also, Tor was colonized with different but closely related PFGE types, K67, K69, K70, and K71, in various combinations (Fig. 2; Table 1).
FIG. 5.
Chronological excretion of PFGE types of Campylobacter isolates from the dog Rufus (dog identification [ID], Ru). The figure shows an example of a dog with a persistent infection with C. upsaliensis (C. ups). The closely related types indicate infection with several closely related strains or, more likely, the occurrence of in vivo mutations in a single strain.
The PFGE results revealed considerable diversity among Campylobacter isolates. Within the same dog, the diversity was lower but differed from one dog to another. Most C. upsaliensis PFGE types were restricted to one dog, but some types (K28, K42, K16, K37a, and K3) were found in two dogs and one type (K20) was found in four dogs. K20 was the only type isolated simultaneously in different dogs, in Buster, Futte, and Gilli, between June 2000 and May 2001. Concerning C. jejuni, all PFGE types except one, K47, were found in one dog only, and most PFGE types were found only once in the same dog but occasionally twice (K8 in Futte) or three times (K7 in Bonnie).
Ten of the C. upsaliensis isolates displayed only one or two bands when digested with KpnI, i.e., PFGE types K29, K74, and K75 (Fig. 2). However, when these isolates were digested with another enzyme, EagI, five different types could be distinguished (data not shown). The four K74 isolates could be divided into two EagI types, where the two isolates from Rufus (Ru-19a and Ru-20a) (Fig. 2) were identical and the two isolates from Sita and Zicka (Si-1 and Zi-22b) (Fig. 2) were identical. The K75 isolate, Ni-4, had a unique EagI type, and among the five K29 isolates, the four isolates from Nhala, Nh-6, Nh-7a, Nh-8, and Nh-9a, had identical EagI types, whereas the isolate from Ann Sophie, As-18b, had a unique EagI type.
Clinical impact.
Among Campylobacter spp.-positive samples, 21 of 278 were soft or diarrheic feces, whereas 5 of 88 Campylobacter spp.-negative samples were soft or diarrheic. The difference was not statistically significant (P = 0.55), irrespective of the Campylobacter species present in the feces. So the 26 dogs in this study were colonized with campylobacters over several months, without significant impact on gastrointestinal health.
Salmonella results.
Of 179 samples tested, one was found to be positive for S. enterica subsp. enterica serovar Typhimurium phage type DT 104 and one was positive for Salmonella enterica subsp. enterica serovar Newport.
DISCUSSION
This study showed that the carrier period of puppies and young dogs commonly was long, often of a duration of more than 1 year. Others (27) have found a similar frequency of Campylobacter spp. in dogs <1 year of age (75%) but a lower frequency in dogs >1 year (32.7%), indicating that a further reduction in prevalence may take place for the dogs in this study after 2 years of age. The clear predominance of the proportion of C. upsaliensis strains over C. jejuni strains, 75.0 and 19.4%, respectively, that was found in this study compares well to other recent investigations of healthy dogs. Published ratios of C. upsaliensis to C. jejuni have been either high, 63 to 22% (5), 79 to 16% (4), and 80 to 19% (27), or low, 15 to 82% (35) and 19 to 76% (16). In the studies with high ratios of C. upsaliensis to C. jejuni, we note that each sample was treated according to two or three different detection methods and the final results were a summing up of the findings. In the latter two studies with low C. upsaliensis to C. jejuni ratios, only one method, with one inoculated plate (35) or a short incubation period (16), was applied. Furthermore, we found that isolation of C. jejuni was correlated with the age of the dogs, as the majority of C. jejuni strains were found in dogs under 1 year of age. Similar findings have been published in other studies of healthy dogs by Burnens et al. (5) and by Moser et al. (27). In humans, Lastovica and Engel (20) also found that C. jejuni predominated in children younger than a year, whereas C. upsaliensis was more common in older children.
In a former study (16) of 3- to 4-month-old puppies, 29% were found to be Campylobacter positive. In the present study, the prevalence of Campylobacter positive dogs within the same age group (12 to 16 weeks) was 58%. Also, the species distribution differed between the two studies, as the proportion of C. upsaliensis and C. jejuni found in the former study was 19 and 76%, respectively, compared to 75.0 and 19.4%, respectively, in the present study. We ascribe this discrepancy to an improved and more comprehensive culture method in the recent study, in particular, regarding the detection of C. upsaliensis. First, the prolongation of the incubation period from 2 to 4 days was found to be crucial, and second, each sample was incubated on four culture plates in this study, compared to two in the former study. Many of the C. upsaliensis strains were sparsely and slowly growing and required at least 3 to 4 days to develop visible growth. Moreno et al. (26) have found that an even longer incubation time, up to 8 days, was beneficial for detection of C. upsaliensis in fecal samples. In that study, no C. upsaliensis at all was isolated within the first 48 h of incubation. These observations stress the importance of a long incubation period for maximum recovery of C. upsaliensis, perhaps even longer than 4 days. Others have found that a filtration technique was important for sufficient isolation of C. upsaliensis (15, 23). Goossens (13) found a pronounced difference between selective media and the filtration technique in favor of the filtration, but the two selective media that were used contained 30 and 32 mg of cefoperazone/liter, which probably could have been inhibitory to the growth of C. upsaliensis. Our study showed that a filtration technique was not required to isolate C. upsaliensis as long as an incubation period of 4 days and a CAT plate were used. Similar findings have been published by Byrne et al. (6) and Corry and Atabay (9). Both papers state the superiority of CAT agar, with its lower content of cefoperazone than mCCDA agar for the isolation of C. upsaliensis. The discrimination of C. upsaliensis from other Campylobacter species, and C. helveticus in particular, constituted a problem, as C. upsaliensis could not be distinguished from C. helveticus by selenite reduction and microaerobic growth on BCY. Hence, only 49 of 69 isolates identified as C. upsaliensis by the tests recommended by the Nordic Committee on Food Analysis (2) and verified to be C. upsaliensis strains by PCR were indeed identified as C. upsaliensis by the supplementary tests. Extrapolation of this misclassification to all 216 catalase-negative isolates yields a 95% confidence interval for the misclassification rate of 22 to 36% by these tests. However, discrimination of C. upsaliensis from other Campylobacter spp. by the tests recommended by the Nordic Committee on Food Analysis also failed in some cases, as all of the 22 isolates identified as atypical due to resistance to nalidixic acid and/or cephalothin were demonstrated to be C. upsaliensis strains by PCR, giving a misclassification rate of 10 to 15% for all 216 catalase-negative isolates. However, since no C. helveticus or H. canis strains were found among any of the 91 catalase-negative isolates investigated by PCR, we identified all 216 catalase-negative isolates as C. upsaliensis strains based on their catalase-negative feature only. Assuming that the 125 C. upsaliensis isolates not tested by PCR were correctly identified, a misclassification rate of 0 to 4% of the 216 catalase-negative isolates was thus achieved.
Cocolonization with more than one species was only detected in 3.4% of the samples by culture. However, the PFGE excretion patterns revealed evidence of a substantially higher degree of coinfection than discovered by the bacteriological culture technique alone. Cocolonization of more than one species or strain of campylobacters has not previously been reported in studies of dogs. In studies of human clinical cases, however, cocolonization has been found by Lastovica and Engel (20), who showed that of 20,458 South African pediatric patients with gastroenteritis, 21.8% were Campylobacter positive and 16.2% were cocolonized with 2 to 5 different Campylobacter species; C. upsaliensis was frequently coisolated with C. jejuni. Likewise, Richardson et al. (34) found that by culture and typing of 10 single colonies from each of 53 Campylobacter-positive human fecal samples, 7.5% of the samples contained two strains of C. jejuni. Lawson et al. (22) detected two species of Campylobacter in 20 of 543 Campylobacter-positive human gastroenteritis samples by PCR enzyme-linked immunosorbent assay. In the present study, we only PFGE typed one or two isolates from each positive sample. It is therefore likely that, if we had tested more isolates from each positive sample, we would have found more dogs cocolonized by two or more strains.
In some dogs, there was a remarkable stability in the colonization of specific C. upsaliensis strains, whereas in others, several different strains were found. Whether this persistence or succession of strains reflects differences in the ability to colonize the intestinal tract of dogs or differences between dogs in their exposure to different strains remains unclear. Exposure may depend on both diet and behavior. A dog living mainly indoors is likely to be less exposed than a dog that spends much time outdoors or has much contact with other dogs. This is also reflected by the fact that dogs in cities were more often colonized than dogs from the countryside. In cities, the density of dogs and contact with dog stools are generally higher than in rural areas. In contrast to C. upsaliensis, colonization with C. jejuni was almost always of short duration; in most cases, only in a single sample. Occasionally, the same C. jejuni strain was demonstrated in two successive samples, and on one occasion, a strain was found three times in the same dog over a 4- to 5-month period, which indicates that C. jejuni is also able to colonize some dogs for longer periods. An infection with a C. jejuni strain usually did not eliminate the already present C. upsaliensis strain, as the same C. upsaliensis PFGE type was often found both before and after a C. jejuni infection. The infection dynamics of the two species are therefore very different, for dogs at least. In some cases, a sample was found to be negative for Campylobacter, whereas the same clone had been recovered from both the following and the previous sample. This might indicate that the negative sample had also contained Campylobacter but that it either was present in numbers below the detection limit or had been lost during transport to the laboratory. A high level of diversity was found among C. upsaliensis as well as C. jejuni PFGE types. Anyhow, seven C. upsaliensis PFGE types (K16, K20, K28, K42, K37a, K3, and K74) and one C. jejuni type (K47) were found in more than one dog, indicating that certain strains, particularly those of C. upsaliensis, may be more common than others in dogs.
Special attention should be given to the animals where different but closely related PFGE types were found, in particular, Ann Sophie (types K10 and K11), Rufus (types K38, K39, K39a, K40, and K41) (Fig. 5), and Tor (types K67, K69, K70, and K71). The different PFGE types were found either as a solo infection or as a coinfection. It is possible that the small variations in PFGE banding patterns were caused by alterations due to laboratory handling. However, we consider it much more likely that the phenomenon must be ascribed to the occurrence of in cane mutations in particular strains. The mutant had then, in some cases, outnumbered the parent strain, and in other cases, it had existed concurrently with the parent strain. This is to our knowledge the first reported indication of in vivo mutations in C. upsaliensis.
Mixed colonizations were found on several occasions, either with closely related PFGE types as described above or with unrelated PFGE types. In the latter cases, the new type must be considered the result of an infection with a new C. upsaliensis strain. In, e.g., Buster, the PFGE type K19 strain existed concurrently with the PFGE type K20 strain for 2 months at 22 to 23 months of age but was then seemingly eradicated or outnumbered by the type K20 strain (Table 1; Fig. 3). Strangely enough, the type K19 strain had been found already at 9 and 12 months. Whether it had in fact persisted in low numbers concurrent with the type 20 strain for so many months or it had reinfected is not known. In other cases, new strains were able to outnumber already colonizing strains. This was the case for Nhala, which was first infected with PFGE type K28, then K29, and finally K16, and all three strains were found in more than one sample, indicating that all strains had colonized and were not merely passants.
Most PFGE studies on Campylobacter have been carried out on C. jejuni and C. coli. For these species, SmaI has been reported to give good results, and most studies have used this enzyme (28). To the authors' knowledge, no PFGE study has so far been published on C. upsaliensis. We tested seven different enzymes on a subset of isolates. SmaI, as well as XbaI, SpeI, and BamHI, yielded an excessive amount of bands which invalidated evaluation of the patterns. In contrast, both NotI and SalI yielded only very few bands. Like SmaI, SalI has been reported to give good results with C. jejuni (31). With KpnI, we obtained a suitable number of well-separated bands for most isolates, usually 4 to 13 bands, and this enzyme was therefore applied to all isolates. A small group of isolates yielded only one or two bands with KpnI, but we noticed that EagI was able to cut DNA from these isolates into three to five fragments, which allowed us to distinguish five different banding patterns among these isolates.
According to some investigations, a highly significant risk factor for Campylobacter carriage and fecal shedding of Campylobacter in healthy dogs is close contact with other dogs or their feces (4, 42). The exchange of C. upsaliensis strains between dogs under high-density housing conditions in shelters has been reported (39). Experimental infection experiments have shown that both C. jejuni and C. upsaliensis can cause diarrhea in dogs (25, 29), and some studies have found a higher prevalence of campylobacters among diarrheic than healthy dogs of <1 year (5, 44), whereas others did not (27, 35). The present study did not discover any statistically significant clinical impact on dog health that could be attributed to C. upsaliensis or C. jejuni.
This study showed that when dogs became colonized with campylobacters, the colonization often continued for an extended period of time. Between 9 and 15 months of age, all dogs excreted campylobacters, although occasional negative samples were found. However, it is likely that some of these negative samples were really positive, but the campylobacters were present in numbers below the detection limit or had died during transportation. This is supported by the fact that the strain isolated before and after a single negative sample was often identical in PFGE pattern.
The results from this study indicate that the exposure of humans to C. upsaliensis must be more intense than the exposure to C. jejuni from dogs, as many dogs were permanently colonized with one or more C. upsaliensis strains and colonization with C. jejuni was less frequent and of short duration. On a worldwide basis, according to available reports, C. upsaliensis accounts for an estimate of approximately 0.5 to 1% of registered cases of diarrhea in developed countries, whereas C. jejuni accounts for 5 to 10% of all cases. However, the ratio of isolated C. upsaliensis strains to other Campylobacter species in human gastroenteritis varies considerably between published studies, from 0.0% in the United States (17) and Denmark (10) and 2.5% in Belgium (15) and 2.9% in Australia (19) to a high proportion of 18.0% in Sweden (24) and 23.2% in South Africa (20). For the estimation of the role of dogs in this context, it is important to note that no significant sources of C. upsaliensis other than dogs and cats are known at present. Although 0.5 to 1.0% is a minor part of the cases of diarrhea registered, it is still a considerable number of cases on an international scale, which presumably have been transmitted from pet dogs or cats. It is also noteworthy that Lastovica and Engel (20) published a rate as high as 4.8% of pediatric gastroenteritis caused by C. upsaliensis in South Africa. A recent amplified fragment length polymorphism study of animal sources of human campylobacteriosis conducted by Siemer et al. (38) has shown similarity between a human amplified fragment length polymorphism profile of a C. jejuni serotype 2 strain and a dog profile from a previous study of healthy puppies (16). Although such studies do not indicate the direction of transmission, the most likely transmission route is from pet to human, as healthy human Campylobacter carriers are uncommon (19).
In conclusion, young dogs were found to be healthy carriers of C. upsaliensis and C. jejuni during the first 2 years of life. Persistent colonization with one or more strains of C. upsaliensis over several months, with intermittent sporadic excretion of C. jejuni, was common. In long-term colonizing strains of C. upsaliensis there was a clear indication of in vivo mutations. C. jejuni was correlated to the age of the dog, as C. jejuni was isolated more frequently in dogs under 1 year of age than in the older dogs. Although dogs are considered to be one of the minor risk factors for human campylobacteriosis, they may still account for a considerable number of cases worldwide.
Acknowledgments
The invaluable cooperation of the owners of the dogs is gratefully acknowledged. We also thank Lis Nielsen, Anita Fogh Hansen, Lotte Christensen, Grethe Stitz Berwald, and Susanne Obsen for skillful technical assistance.
The investigation was supported by a grant from The Danish Ministry of Food, Agriculture, and Fisheries.
REFERENCES
- 1.Anderson, E. S., L. R. Ward, M. J. Saxe, and J. D. de Sa. 1977. Bacteriophage-typing designations of Salmonella typhimurium. J. Hyg. (London) 78:297-300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anonymous. 1990. Nordic Committé on Food Analysis, 2nd ed. Methodic no. 119: Campylobacter jejuni/coli detection in foods. Statens Tekniska Forskningscentral, Esbo, Finland.
- 3.Anonymous. 1993. Microbiology—general guidance on methods for the detection of Salmonella (ISO 6579, 3rd ed.). International Organization for Standardization, Geneva, Switzerland.
- 4.Baker, J., M. D. Barton, and J. Lanser. 1999. Campylobacter species in cats and dogs in South Australia. Aust. Vet. J. 77:662-666. [DOI] [PubMed] [Google Scholar]
- 5.Burnens, A. P., B. Angeloz-Wick, and J. Nicolet. 1992. Comparison of Campylobacter carriage rates in diarrheic and healthy pet animals. J. Vet. Med. 39:175-180. [DOI] [PubMed] [Google Scholar]
- 6.Byrne, C., D. Doherty, A. Mooney, M. Byrne, D. Woodward, W. Johnson, F. Rodgers, and B. Bourke. 2001. Basis of the superiority of cefoperazone amphotericin teicoplanin for isolating Campylobacter upsaliensis from stools. J. Clin. Microbiol. 39:2713-2716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Callow, B. R. 1959. A new phage-typing scheme for Salmonella typhimurium. J. Hyg. (Cambridge) 57:346-359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cook, G. T. 1950. A plate test for nitrate reduction. J. Clin. Pathol. 3:359-362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Corry, J. E., and H. I. Atabay. 1997. Comparison of the productivity of cefoperazone amphotericin teicoplanin (CAT) agar and modified charcoal cefoperazone deoxycholate (mCCD) agar for various strains of Campylobacter, Arcobacter and Helicobacter pullorum. Int. J. Food Microbiol. 38:201-209. [DOI] [PubMed] [Google Scholar]
- 10.Engberg, J., S. L. On, C. S. Harrington, and P. Gerner-Smidt. 2000. Prevalence of Campylobacter, Arcobacter, Helicobacter, and Sutterella spp. in human fecal samples as estimated by a reevaluation of isolation methods for campylobacters. J. Clin. Microbiol. 38:286-291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Feeley, J. C., R. J. Gibson, G. W. Gorman, N. C. Langford, J. K. Rasheed, D. C. Mackel, and W. B. Baine. 1979. Charcoal-yeast extract agar: primary isolation medium for Legionella pneumophila. J. Clin. Microbiol. 10:437-441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gondrosen, B., T. Knævelsrud, and K. Dommarsnes. 1985. Isolation of thermophilic campylobacters from Norwegian dogs and cats. Acta Vet. Scand. 26:81-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goossens, H. 1996. Characterization and description of “Campylobacter upsaliensis” isolated from human feces. J. Clin. Microbiol. 28:1039-1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goossens, H., L. Vlaes, and J.-P. Butzler. 1991. Campylobacter upsaliensis enteritis associated with canine infections. Lancet 337:1486-1487. [DOI] [PubMed] [Google Scholar]
- 15.Goossens, H., L. Vlaes, M. de Boeck, B. Pot, K. Kersters, J. Levy, P. de Mol, J. P. Butzler, and P. Vandamme. 1990. Is “Campylobacter upsaliensis” an unrecognised cause of human diarrhoea? Lancet 335:584-586. [DOI] [PubMed] [Google Scholar]
- 16.Hald, B., and M. Madsen. 1997. Healthy puppies and kittens as carriers of Campylobacter spp., with special reference to Campylobacter upsaliensis. J. Clin. Microbiol. 35:3351-3352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hindiyeh, M., S. Jense, S. Hohmann, H. Benett, C. Edwards, W. Aldeen, A. Croft, J. Daly, S. Mottice, and K. C. Carroll. 2000. Rapid detection of Campylobacter jejuni in stool specimens by an enzyme immunoassay and surveillance for Campylobacter upsaliensis in the greater Salt Lake City area. J. Clin. Microbiol. 38:3076-3079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hoel, P., S. Port, and C. Stone. 1971. Introduction to probability theory. Houghton-Mifflin, Boston, Mass.
- 19.Jimenez, S. G., R. G. Heine, P. B. Ward, and R. M. Robins-Browne. 1999. Campylobacter upsaliensis gastroenteritis in childhood. Pediatr. Infect. Dis. J. 18:988-992. [DOI] [PubMed] [Google Scholar]
- 20.Lastovica, A. J., and M. E. Engel. 2001. Epidemiology of other Campylobacter species. In The increasing incidence of human campylobacteriosis. Report and proceedings of a consultation of experts, 21 to 25 November 2000, Copenhagen, Denmark. W.H.O./CDS/CSR/APH 2001.7. Department of Communicable Disease Surveillance and Response, World Health Organization, Geneva, Switzerland.
- 21.Lawson, A. J., D. Linton, J. Stanley, and R. J. Owen. 1997. Polymerase chain reaction detection and specification of Campylobacter upsaliensis and C. helveticus in human faeces and comparison with culture techniques. J. Appl. Microbiol. 83:375-380. [DOI] [PubMed] [Google Scholar]
- 22.Lawson, A. J., J. M. Logan, G. L. O'Neill, M. Desai, and J. Stanley. 1999. Large-scale survey of Campylobacter species in human gastroenteritis by PCR and PCR-enzyme-linked immunosorbent assay. J. Clin. Microbiol. 37:3860-3864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Le Roux, E., and A. J. Lastovica. 1998. The Cape Town protocol: how to isolate the most campylobacters for your dollar, pound, franc, yen, etc., p. 31-33. In Ninth International Workshop on Campylobacter, Helicobacter, and Related Organisms. Institute of Child Health, Red Cross Children’s Hospital, Cape Town, South Africa.
- 24.Lindblom, G. B., E. Sjögren, J. Hansson-Westerberg, and B. Kaijser. 1995. Campylobacter upsaliensis, C. sputorum sputorum and C. concisus as common causes of diarrhoea in Swedish children. Scand. J. Infect. Dis. 27:187-188. [DOI] [PubMed] [Google Scholar]
- 25.Macartney, L., R. R. Al Mashat, D. J. Taylor, and I. A. McCandlish. 1988. Experimental infection of dogs with Campylobacter jejuni. Vet. Rec. 122:245-249. [DOI] [PubMed] [Google Scholar]
- 26.Moreno, G. S., P. L. Griffiths, I. F. Connerton, and R. W. Park. 1993. Occurrence of campylobacters in small domestic and laboratory animals. J. Appl. Bacteriol. 75:49-54. [DOI] [PubMed] [Google Scholar]
- 27.Moser, I., B. Rieksneuwohner, P. Lentzsch, P. Schwerk, and L. H. Wieler. 2001. Genomic heterogeneity and O-antigenic diversity of Campylobacter upsaliensis and Campylobacter helveticus strains isolated from dogs and cats in Germany. J. Clin. Microbiol. 39:2548-2557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nielsen, E. M., J. Engberg, and V. Fussing. 2001. Genotypic and serotypic stability of Campylobacter jejuni strains during in vitro and in vivo passage. Int. J. Med. Microbiol. 291:379-385. [DOI] [PubMed] [Google Scholar]
- 29.Olson, P., and K. Sandstedt. 1987. Campylobacter in the dog: a clinical and experimental study. Vet. Rec. 121:99-101. [DOI] [PubMed] [Google Scholar]
- 30.On, S. L. W., and B. Holmes. 1991. Effect of inoculum size on the phenotypic characterization of Campylobacter species. J. Clin. Microbiol. 29:923-926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.On, S. L. W., E. M. Nielsen, J. Engberg, and M. Madsen. 1998. Validity of SmaI-defined genotypes of Campylobacter jejuni examined by SalI, KpnI, and BamHI polymorphisms: evidence of identical clones infecting humans, poultry, and cattle. Epidemiol. Infect. 120:231-237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Patton, C. M. 1989. Human disease associated with “Campylobacter upsaliensis” (catalase-negative or weakly positive Campylobacter species) in the United States. J. Clin. Microbiol. 27:66-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Popoff, M. Y., and L. Le Minor. 1997. Antigenic formulas of the Salmonella serovars. World Health Organization Collaborating Centre for Reference and Research on Salmonella, Paris, France.
- 34.Richardson, J. F., J. A. Frost, J. M. Kramer, R. T. Thwaites, F. J. Bolton, D. R. Wareing, and J. A. Gordon. 2001. Coinfection with Campylobacter species: an epidemiological problem? J. Appl. Microbiol. 91:206-211. [DOI] [PubMed] [Google Scholar]
- 35.Sandberg, M., B. Bergsjø, M. Hofshagen, E. Skjerve, and H. Kruse. 2002. Risk factors for Campylobacter infection in Norwegian cats and dogs. Prev. Vet. Med. 55:241-253. [DOI] [PubMed] [Google Scholar]
- 36.Sandstedt, K., and J. Ursing. 1991. Description of Campylobacter upsaliensis sp. nov. previously known as the CNW Group System. Appl. Microbiol. 14:39-45. [Google Scholar]
- 37.Sandstedt, K., J. Ursing, and M. Walder. 1983. Thermotolerant Campylobacter with no or weak catalase activity isolated from dogs. Curr. Microbiol. 8:209-213. [Google Scholar]
- 38.Siemer, B. L., C. S. Harrington, B. Borck, E. M. Nielsen, J. Engberg, N. L. Nielsen, and S. L. On. 2001. Application of AFLP fingerprinting to investigate the relationships between Campylobacter jejuni strains from humans, animals and foods. Int. J. Med. Microbiol. 291(Suppl. 31):68. [Google Scholar]
- 39.Stanley, J., C. Jones, A. Burnens, and R. J. Owen. 1994. Distinct genotypes of human and canine isolates of Campylobacter upsaliensis determined by 16S rRNA gene typing and plasmid profiling. J. Clin. Microbiol. 32:1788-1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Steinhauserova, I., K. Fojtikova, and J. Klimes. 2000. The incidence and PCR detection of Campylobacter upsaliensis in dogs and cats. Lett. Appl. Microbiol. 31:209-212. [DOI] [PubMed] [Google Scholar]
- 41.Tenkate, T. D., and R. J. Stafford. 2001. Risk factors for campylobacter infection in infants and young children: a matched case-control study. Epidemiol. Infect. 127:399-404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Torre, E., and M. Tello. 1993. Factors influencing fecal shedding of Campylobacter jejuni in dogs without diarrhea. Am. J. Vet. Res. 54:260-262. [PubMed] [Google Scholar]
- 43.van de Giessen, A. W., J. J. Tilburg, W. S. Ritmeester, and J. van der Plas. 1998. Reduction of campylobacter infections in broiler flocks by application of hygiene measures. Epidemiol. Infect. 121:57-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vandenberghe, J., S. Lauwers, P. Plehier, and J. Hoorens. 1982. Campylobacter jejuni related with diarrhoea in dogs. Br. Vet. J. 138:356-361. [DOI] [PubMed] [Google Scholar]
- 45.Wolfs, T. F., B. Duim, S. P. Geelen, A. Rigter, F. Thomson-Carter, A. Fleer, and J. A. Wagenaar. 2001. Neonatal sepsis by Campylobacter jejuni: genetically proven transmission from a household puppy. Clin. Infect. Dis. 32:97-99. [DOI] [PubMed] [Google Scholar]