Abstract
Successful infection and replication of bacteriophages is indicative of the presence of viable bacteria. We describe here the development of a bacteriophage replication assay for the detection of Mycobacterium tuberculosis by using mycobacteriophage D29. Optimization of phage inoculate and incubation times allowed highly sensitive detection of M. bovis BCG. Fewer than 10 CFU (100 CFU/ml) were detected. No false-positive results were observed in negative samples. Application of the assay to 496 sputum specimens in the National Reference Laboratory of Zambia produced sensitivity, specificity, and positive and negative predictive values of 44.1, 92.6, 82.2, and 67.5%, respectively, compared to culture on Lowenstein-Jensen medium. The equivalent corresponding results for direct fluorescent smear microscopy were 42.3, 96.8, 91.2, and 67.6%. The small increase in sensitivity over that of direct microscopy does not justify the introduction of this technique for routine diagnosis of pulmonary tuberculosis at this time.
Tuberculosis remains a major threat to public health, particularly in regions such as sub-Saharan Africa with a high prevalence of human immunodeficiency virus infection. It is currently the leading cause of adult mortality in Zambia where, during 2001, this disease accounted for 13% of recorded deaths. Prompt diagnosis and access to treatment is a prerequisite to control of this disease. However, the diagnostic test currently used for pulmonary tuberculosis in developing countries—microscopy of sputum for acid-fast bacilli—lacks sensitivity.
A low-cost diagnostic technology currently under investigation is mycobacteriophage (phage) replication. Mycobacteriophages that exclusively infect mycobacteria can be used to detect bacilli extracted from clinical specimens. Replication of phages is indicative of bacterial infection since progeny phages will only develop within viable host bacteria. Detection of Mycobacterium tuberculosis infection has previously been reported with mycobacteriophage D29 (4, 14). D29 is a lytic, double-stranded DNA phage with a wide mycobacterial host range (7, 11). A suspension of D29 phages was used to inoculate specimens that had been previously treated to extract the bacilli. On incubation, samples containing viable M. tuberculosis bacilli supported phage replication, whereas no progeny phages were observed from uninfected samples. Replication of lytic phages may be detected by observing an increase in the numbers of viral particles present in a sample. More accurate detection may be achieved after differential chemical inactivation of phages that have not infected a host bacterium (5, 14). This treatment does not affect phage DNA that has previously entered a bacilli or the production of progeny phages within the bacilli. The elimination of exogenous phages means that all phages detected in a sample after treatment are progeny phages, thus demonstrating replication. Phages may be detected by plating samples in a lawn of fast-growing indicator bacteria (4). Chemical inactivation agent remaining in the sample is neutralized by dilution during addition to the lawn and on incubation at 37°C phage replication continues. Repeated cycles of infection and lysis cause clear areas (plaques) to form in the bacterial lawn which can be visualized after overnight incubation.
Diagnostic tools for detecting M. tuberculosis in clinical specimens need to be robust and sensitive. To maximize the sensitivity of bacteriophages for the detection of M. tuberculosis, it was necessary to optimize phage infection and replication. We describe here an investigation of infection of M. tuberculosis by mycobacteriophage D29. Initial studies were done to establish the optimum phage inoculation. A second objective was to establish the kinetics of the infection cycle to allow optimization of incubation times. It was necessary to investigate the time taken for the phage DNA to enter a host bacterium and the time at which lysis of the host bacteria commenced (latent phase of infection). Knowledge of the length of the latent period also permitted investigation of the numbers of plaques obtained if samples were plated postlysis and the effect of this on the sensitivity of the assay. The final objective of the study was to investigate the use of the optimized phage replication test for the diagnosis of tuberculosis from sputum specimens. The phage replication test was compared to fluorescent smear microscopy and culture on Lowenstein-Jensen medium. The present study was performed in the national reference laboratory of Zambia, a high-burden, low-income country with a high prevalence of human immunodeficiency virus infection and AIDS.
MATERIALS AND METHODS
Bacteriophages.
Suspensions of bacteriophage D29 (London School of Hygiene and Tropical Medicine) were prepared by cultivation on M. smegmatis as previously described (14). Stocks of between 5 × 109 and 1 × 1010/ml were stored at 4°C. Inoculation of bacterial suspensions was routinely performed by addition of an equal volume of phage suspension prepared at double the required concentration.
Bacteria.
M. smegmatis MC2155 (18) (William R. Jacobs, Jr., Howard Hughes Medical Institute, Albert Einstein College of Medicine, New York, N.Y.) were maintained on Middlebook 7H9 agar plates supplemented with 10% oleic acid-albumin-dextrose-catalase (OADC; Difco Laboratories, Detriot, Mich.). M. bovis BCG and M. tuberculosis strains were maintained on Lowenstein-Jensen slopes at 37°C or in Middlebook 7H9 broth supplemented with 10% OADC and 0.2% glycerol. All manipulations involving M. tuberculosis were performed under biohazard category 3 safety conditions by using a class 1 microbiological safety cabinet.
Liquid cultures were routinely left to stand for 5 min to allow aggregates of cells to settle prior to samples being removed for experimentation. To quantify the bacteria used in experimental work, the number of CFU were determined by plating aliquots of stock suspensions on supplemented Middlebrook 7H9 agar plates. For M. smegmatis the numbers of colonies were assessed at 3 days; for M. bovis BCG the numbers of colonies were assessed at 2 and 6 weeks.
Phage assay reagents.
The phages were maintained and experiments performed in Middlebook 7H9 broth supplemented with 10% OADC and 1 mM calcium chloride. Indicator agar used for detection of PFU was prepared by adding 10% (vol/vol) stationary-phase M. smegmatis culture to 1.5% agar in Luria broth (Difco) prior to pouring in triple-vented plastic petri dishes (14). Molten agar was cooled to ca. 45°C before addition of the bacteria or samples. Volumes of 10 and 5 ml were used with 90- and 55-mm plates, respectively. Plaque formation was routinely observed after overnight incubation at 37°C.
Inactivation of exogenous D29.
Differential chemical inactivation of D29 phages was achieved by the addition of 10 mM ferrous ammonium sulfate (FAS) (Sigma-Aldrich Co., Ltd., Poole, United Kingdom) as previously described (14). This was routinely achieved by the addition of 10% (vol/vol) 100 mM FAS in sterile water, followed by mixing. When necessary samples were diluted by the addition of 4 volumes of fresh assay broth to neutralize the FAS and avoid unwanted damage to progeny phages released after cell lysis.
Optimization of phage inoculation.
To investigate the optimal concentration of phages with which to inoculate the bacteria, preliminary investigations were undertaken with M. smegmatis. Aliquots of a suspension of M. smegmatis MC2 155 at 2 × 103 CFU/ml were inoculated with D29 at eight different phage concentrations ranging from 5 × 104 to 8 × 108 PFU/ml. Five replicate 0.1-ml aliquots of the bacteria were inoculated with an equal volume of D29 phages for 40 min at 37°C. After the addition of 10 mM FAS, each aliquot was plated in 5 ml of indicator agar, and plaque formation was observed overnight. The numbers of plaques detected were analyzed to determine the inoculation of phages that resulted in the maximum infection. Investigation of D29 infection in bacteria of the tuberculosis complex was undertaken with M. bovis BCG. To investigate the effect of high concentrations of phages, BCG bacteria in log-phase growth were diluted to ca. 5 × 105 CFU/ml, i.e., the concentration of bacteria that might be expected in sputum from a tuberculosis patient with a heavily positive smear result (3).
Five replicate 0.1-ml aliquots of bacteria were inoculated with equal volumes of D29 at five different concentrations to give phage inoculates of between 3.3 × 107 and 3.3 × 108 PFU/ml. After infection for 90 min at 37°C and the addition of 10 mM FAS, each aliquot was plated in 10 ml of indicator agar and plaque formation was observed overnight. To investigate the optimum phage inoculate when BCG was infected, a further experiment was performed with a range of 10 phage concentrations from 2 × 107 to 1.6 × 108 PFU/ml. Five aliquots of a suspension of bacteria at ca. 5 × 105 CFU/ml were infected under the conditions described above. The numbers of plaques observed were analyzed to determine the concentration of phages that produced the optimum rate of infection.
Investigation of phage infection times.
Investigation of the infective cycle of D29 was undertaken in Middlebrook 7H9 broth supplemented with 10% OADC, 0.2% glycerol, and 1 mM calcium chloride at 37°C. For technical and safety reasons initial studies where performed with M. bovis BCG in preference to M. tuberculosis. Seventy-two 1-ml aliquots of a suspension of BCG (∼103 CFU) were inoculated with D29 at 3 × 107 PFU/ml at 37°C. At 15-min time intervals, triplicate aliquots were treated with FAS and plated in 10 ml of indicator agar. Plaques were counted after overnight incubation at 37°C. After treatment with FAS all extracellular phages are inactivated, and any phages previously released during lysis of host bacteria have been killed. Thus, the plaques observed during this experiment derived from intact infected bacteria, the number of which were seen to decrease as lysis proceeded. The length of the latent period is related to the growth rate of the host bacteria. Thus, it was necessary to also investigate clinical strains of M. tuberculosis that may grow at different rates. To establish the minimum latent period in M. tuberculosis, 14 isolates were selected for inoculation with D29. After incubation for 1 h at 37°C the samples were treated with FAS, diluted with fresh broth (warmed to 37°C) to neutralize the FAS, and returned to the incubator. Aliquots were plated directly after dilution (time zero) and at hourly intervals thereafter. The number of plaques seen on the indicator plates were recorded next day. Plaques observed during this experiment derived both from infected bacteria and from progeny phages present in the supernatant. Increased numbers of plaques were observed as host bacteria were lysed, releasing the phages within them. Analysis of the PFU at each time point enabled the length of latent period of D29 infection in M. tuberculosis to be estimated.
Comparison of PFU before and after lysis.
To investigate the increase in PFU due to release of progeny phages after cell lysis, samples of D29-infected BCG were examined at three time points during the infection cycle. Initial samples were taken during the latent period at 90 min postinoculation, a second set of samples were taken during the rise period at 240 min and a third set of samples was obtained later in the rise period at 420 min postinoculation. Twofold serial dilutions of a suspension of BCG in log-phase growth were prepared. To permit enumeration of the bacteria, 1 ml of each dilution was plated on 1.5% agar in Middlebrook 7H9 supplemented with OADC and colonies were counted at 2 and 6 weeks. Thirty replicate 0.1-ml aliquots of each bacterial dilution and thirty replicate 0.1-ml aliquots of assay broth alone were inoculated with D29 phages at 3 × 107/ml. After a 90-min incubation at 37°C, extracellular phages were inactivated by addition of 10 mM FAS. Ten tubes of each dilution were plated in 10 ml of indicator agar. One milliliter of fresh assay broth was added to each of the remaining tubes to neutralize the effect of FAS prior to incubation for a further 150 or 330 min before plating. The numbers of plaques observed were recorded next day.
Detection of M. tuberculosis in sputum.
To establish the sensitivity of the phage replication assay for diagnosis of pulmonary tuberculosis, the test was compared to smear microscopy and culture on Lowenstein-Jensen medium in a routine diagnostic laboratory in Zambia. Two sputum specimens were collected from each of 278 suspected new pulmonary tuberculosis cases. Informed consent was obtained from all subjects. A sample was taken for smear microscopy prior to treatment of the specimen in preparation for culturing. After inoculation of the Lowenstein-Jensen slopes, the phage assay was performed on the remaining specimen. Samples for smear microscopy were stained with auramine-phenol and examined by fluorescence microscopy (22) Microscopy results were recorded as negative (no bacilli per 100 fields), scanty (1 to 9 bacilli per 100 fields), or positive (>10 bacilli per 100 fields). In preparation for culture the specimens were decontaminated and digested in 20-ml universal containers using WHO recommended protocols (modified Petroff method) by adding 4% sodium hydroxide, followed by incubation for 15 min at room temperature (23). After centrifugation for 15 min at 3,000 × g, the sediment was resuspended in 20 ml of sterile water. After a second centrifugation, samples were taken for inoculation on two Lowenstein-Jensen slopes, one supplemented with glycerol and the other supplemented with pyruvate. Slopes were incubated at 37°C for up to 8 weeks. After inoculation of the Lowenstein-Jensen medium, the remaining sample was taken for analysis by the phage replication assay. Sediments were resuspended in 2 ml of Middlebrook 7H9 supplemented with 10% OADC, 0.2% glycerol, and 1 mM calcium chloride and placed at 37°C for between 20 and 24 h. The following day, 2 ml of D29 phages at ca. 6 × 107 PFU/ml was added to each sample. After 1 h of incubation at 37°C 10% (vol/vol), 100 mM FAS was added, and the tube contents were mixed by vortexing. Samples were left to stand for 5 min to allow aerosols formed during mixing to disperse. Then, 1 ml of each sample was removed for plating in 10 ml of indicator agar. Indicator plates were placed at 37°C and examined for plaques the next day. The presence of visible plaques was taken to indicate a positive specimen; plates containing no plaques were classified as negative. The results were available on the third day after receipt of the specimen. The technician performing the phage replication assay was blinded to the smear microscopy result.
RESULTS
Optimization of phage inoculation.
Initial investigations to investigate the optimal concentration for D29 inoculation were undertaken with M. smegmatis. Low numbers of plaques were observed when phage inoculates of 104 and 105 were used. The efficiency of infection was found to improve with increased concentrations of phages up to 107 PFU/ml, a multiplicity of infection (MOI) of 104 . However, decreased infection efficiency was observed at phage concentrations of >108 (MOI = 105), suggesting that abortive infection might be occurring at the higher concentration of phages. To investigate the effect of high phage concentration on D29 infection of M. bovis BCG, a range of phage concentrations between 3.3 × 107 and 3.3 × 108 PFU/ml were used representing MOIs between 1.3 × 102 and 1.3 × 103. The results are presented in Table 1. Decreased numbers of plaques were observed from inoculates of 1.6 × 108 and 3.3 × 108, suggesting that abortive infection occurs when phage concentrations exceed 108 PFU/ml. In a further experiment to investigate the optimum phage inoculate for BCG, 10 phage concentrations were tested. The results are shown in Table 2. No significant differences were observed in the number of plaques obtained from phage inoculations of between 2.2 × 107 and 8 × 107 PFU/ml (t test). However, significant reductions in the numbers of plaques were observed with inoculates of 1.6 × 108 PFU/ml. It was concluded that to achieve maximum infection rates inoculation with D29 should not exceed 108 PFU/ml.
TABLE 1.
PFU resulting from infection of five replicate aliquots of a suspension of M. bovis BCG at approximately 2.5 × 105 CFU/ml with bacteriophage D29 over a range of inoculation concentrations
| Inoculum (PFU/ml) | No. of plaques observed in expt:
|
Mean no. of Plaques | ||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | ||
| 3.3 × 108 | 88 | 93 | 107 | 103 | 109 | 100 |
| 1.6 × 108 | 144 | 238 | 148 | 118 | 153 | 160 |
| 8 × 107 | 306 | 302 | 150 | 373 | 189 | 264 |
| 6.6 × 107 | 311 | 243 | 196 | 325 | 197 | 254 |
| 3.3 × 107 | 214 | 219 | 251 | 219 | 229 | 226 |
TABLE 2.
PFU resulting from infection of five replicate aliquots of a suspension of M. bovis BCG at approximately 2.5 × 105 CFU/ml with bacteriophage D29 over a range of inoculation concentrations
| Inoculum (PFU/ml) | No. of plaques observed in expt:
|
Mean no. of plaques | ||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | ||
| 1.6 × 108 | 107 | 119 | 153 | 117 | 139 | 127 |
| 8 × 107 | 279 | 174 | 172 | 178 | 208 | 202 |
| 6.5 × 107 | 196 | 250 | 208 | 200 | 217 | 214 |
| 5.5 × 10 | 199 | 216 | 203 | 207 | 210 | 205 |
| 4.7 × 107 | 249 | 214 | 226 | 195 | 204 | 218 |
| 4.1 × 107 | 272 | 189 | 263 | 243 | 217 | 237 |
| 3.6 × 107 | 174 | 186 | 217 | 186 | 209 | 188 |
| 3.3 × 107 | 209 | 213 | 188 | 186 | 209 | 201 |
| 3 × 107 | 216 | 201 | 224 | 211 | 225 | 215 |
| 2.2 × 107 | 307 | 198 | 212 | 239 | NAa | 239 |
NA, result not available due to damaged petri dish.
Determination of infection cycle times of D29.
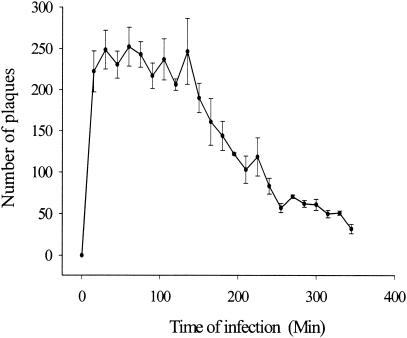
To determine the length of the infection cycle, aliquots of a suspension of BCG were inoculated with D29 phages and incubated at 37°C. At intervals of 15 min, triplicate aliquots were treated with FAS to inactivate exogenous phages and were then plated in indicator agar. The plaques observed the next day are presented in Fig. 1. After treatment with FAS all extracellular phages were inactivated, and the plaques observed in this experiment reflect the number of intact infected bacteria in the sample. Maximum numbers of infected CFU were observed within 15 to 30 min of inoculation, suggesting that infection occurred rapidly. The level of infection remained constant until 150 min when the number of plaques were seen to decline over the remaining 3 h of observation.
FIG. 1.
Infection cycle of D29 infection in M. bovis BCG. Plaques were obtained from plating triplicate samples at 15-min time intervals. Each plaque represents an intact D29-infected CFU of M. bovis BCG.
To investigate the length of latent period in M. tuberculosis, 14 clinical isolates were inoculated with D29 and monitored for a rise in the number of PFU. Samples were treated with FAS to inactivate extracellular phages, diluted, and maintained at 37°C. Aliquots were plated directly after FAS treatment, at 1 h postinoculation, and at hourly intervals. Plaques observed during this experiment derived both from infected bacteria and from any progeny phages released after the lysis of host cells. Increased numbers of PFU were observed in one strain at 2 h, in seven strains at 3 h, and in two strains at 5 h postinoculation. Three strains showed no increase in the numbers of PFU over the 6 h of monitoring, and one strain did not support sufficient infection to permit assessment.
Comparison of PFU before and after lysis.
To investigate the numbers of PFU released into the assay broth during the rise period of infection, 30 replicate samples of a serial dilution of BCG were inoculated with D29. Extracellular phages were inactivated by treatment with FAS at 90 min postinoculation, this being during the latent period of infection. To assess the numbers of PFU present prior to lysis, 10 replicate samples of each of the bacterial dilutions being tested were plated in indicator agar. The remaining samples were incubated for a longer period to permit the infection to continue to cell lysis and release of progeny phages. To assess the PFU present in samples during the rise period, samples were plated 240 or 420 min postinoculation. With samples plated prelysis (90 min), each plaque represents an infected CFU, whereas in samples plated postlysis released progeny phages may form individual plaques. The results are presented in Table 3 and demonstrate a high sensitivity for the detection of BCG. In samples plated at 90 min, plaques were observed in all samples estimated to contain BCG at 11 CFU (117 CFU/ml) or higher. With the 10 replicate samples estimated to contain 5 and 3 CFU, plaques were seen in seven and eight of the samples, respectively. No plaques were observed from bacterial suspensions estimated to contain 6 CFU/ml or those containing no bacteria. The amplification effect of delaying plating until lysis of host bacteria was clearly demonstrated with increased numbers of plaques observed at 240 min and a further increase at 420 min. Large variations in the number of PFU were observed postlysis. Whereas plaques observed in the samples plated prelysis reflected the numbers of CFU present, this was not the case in samples plated postlysis, where large numbers of plaques could be observed from some of the samples containing few bacteria. Although an increase was observed in mean numbers of PFU following lysis, the limit of detection of BCG was not improved by delaying plating.
TABLE 3.
PFU detected during D29 infection of 0.1-ml portions of a serial dilution of BCG samples plated prelysis (90 min postinoculation) and during the rise period (240 and 420 min)a
| CFU/ ml | No. of PFU at:
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 90 min (n = 10)
|
240 min (n = 10)
|
420 min (n = 10)
|
||||||||||
| Mean | Min | Max | SD | Mean | Min | Max | SD | Mean | Min | Max | SD | |
| 506 | 17.5 | 13 | 20 | 2.7 | 200.9 | 86 | 474 | 116.2 | 240.8 | 9 | 744 | 249.5 |
| 242 | 6.3 | 3 | 10 | 2.7 | 62.2 | 7 | 107 | 35.5 | 186.7 | 7 | 366 | 155.4 |
| 117 | 3.6 | 1 | 9 | 2.4 | 48.8 | 7 | 165 | 55.8 | 116.6 | 0 | 339 | 144.3 |
| 51 | 1.3 | 0 | 3 | 1.06 | 4.5 | 0 | 26 | 8.2 | 80.3 | 0 | 436 | 145.3 |
| 30 | 1.5 | 0 | 4 | 1.35 | 103.1 | 1 | 379 | 157.6 | 118.5 | 0 | 496 | 171.6 |
| 6 | 0 | 0 | 0 | 0 | 0 | 0 | 21.5 | 0 | 215 | 68 | ||
| 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |||
The minimum (Min), maximum (Max), and mean numbers of plaques observed per sample are recorded. n, the number of replicate portions tested.
Detection of M. tuberculosis in sputum.
A total of 513 sputum specimens were examined by direct fluorescence microscopy, culture on Lowenstein-Jensen slopes, and phage replication assay. The results for the phage assay were available on the third day compared to the same day with direct microscopy and up to 8 weeks for culture. No mycobacteria other than tuberculosis were identified. Microscopy results were recorded as negative (no bacilli per 100 fields), scanty (1 to 9 bacilli per 100 fields) or positive (>10 bacilli per 100 fields). Seventeen samples (3.3%) had no culture result due to contamination and were excluded from the study. The results obtained for the remaining 496 specimens are shown in Table 4. When compared to culture, the sensitivity, specificity, positive and negative predictive values of the phage assay were 44.1, 92.4, 82.2, and 67.5%, respectively. In this setting, scanty smear microscopy results are routinely reported with a request for a further specimen. For the purposes of the present study, duplicate analysis of results was undertaken classing the scanty results as either negative or positive. With scanty results classed as negative, the sensitivity, specificity, and positive and negative predictive values of smear microscopy were 42.3, 96.7, 91.2, and 67.6%, respectively, and for microscopy with scanty results classed as positive, these values were 59.5, 87.3, 78.9, and 73.1%, respectively. Analysis of discrepant results revealed nine specimens that were found smear positive but culture negative. Of these, seven specimens were found to be from patients where a second specimen was found to be culture positive, thus suggesting the patient was positive for tuberculosis. Of 21 phage-positive, culture-negative specimens 10 were found to be from patients for whom a second specimen yielded a positive culture result.
TABLE 4.
Results obtained from testing 496 sputum samples for tuberculosis by phage replication assay and smear microscopy compared to culture on Lowenstein-Jensen medium
| Test result | No. of samples found to be culture:
|
Total no. of samples | |
|---|---|---|---|
| Positive | Negative | ||
| Phage positive | 97 | 21 | 118 |
| Phage negative | 123 | 255 | 378 |
| Smear positive | 93 | 9 | 102 |
| Smear scanty | 38 | 26 | 64 |
| Smear negative | 89 | 241 | 330 |
| Total | 220 | 276 | 496 |
DISCUSSION
We have investigated detection of tuberculosis by using phage replication. Work was undertaken to optimize infection and replication of bacteriophage D29 in M. tuberculosis . Initial studies demonstrated that the efficiency of phage infection is improved by using a high MOI. The numbers of M. tuberculosis bacilli found in the sputum from infected patients varies widely and may range from less than 10 to more than 105. Thus, to maximize the sensitivity of the assay a high concentration of D29 is required. However, reduced infection rates were observed when bacterial suspensions were inoculated at phage concentrations of >108 PFU/ml. This is probably due to abortive killing of bacilli prior to the production of progeny phages. It has previously been speculated that cell wall damage resulting from infection with large numbers of phage particles may cause abortive killing (1).
Once the optimum phage inoculate had been established, investigation of the infection cycle was undertaken. The time taken from the initial infection to the commencement of lysis (length of the latent period) and the time taken to complete release of progeny phages (rise period) are related to host generation times and environmental factors such as temperature (9, 14). To ensure optimal detection of bacteria by the phage assay, inactivation of extracellular phages should take place after maximum infection has been achieved but before lysis of host bacilli and the release of progeny phages. It was observed that, in supplemented Middlebrook 7H9 broth at 37°C, D29 infection of the BCG bacteria occurred within 30 min and that lysis of bacteria had commenced between 135 and 150 min after inoculation. Investigation of clinical isolates of M. tuberculosis demonstrated that lysis may commerce 120 min after inoculation. Thus, FAS inactivation treatment should take place after 30 min and not more than 2 h postinoculation. These findings are similar to those of David et al. (6), who observed that when M. tuberculosis H37Ra was infected with D29 the majority of the phages adsorb during the first 20 min of infection. The same group also reported a latent period of ca. 120 min (7). The rise period (release of progeny phages) was observed to continue for at least 180 min. Rise periods of up to 600 min have been reported with infection of M. tuberculosis H37Rv (4). The kinetics of infection and subsequent release of phages is likely to be affected by the presence of aggregates of bacteria, and it is possible that a second round of infection may occur within clumps, thus extending the rise period (2, 20). The highly hydrophobic nature of the M. tuberculosis cell wall results in the aggregation of the bacteria. Large clumps were removed by sedimentation with cultures left to stand for 5 min before samples were taken for experimentation. Unfortunately, it was not possible to prevent aggregation by the addition of polyoxyethylene-sorbitan (Tween), which is commonly added to liquid cultures of mycobacteria since it inhibits adsorption of the phage to its receptor on the cell surface and thus prevents infection (19). Three of the clinical isolates of tuberculosis examined did not provide an increase in the number of PFU during 6 h of observation, suggesting that lysis of infected bacteria had not occurred, whereas a further strain provided too few plaques to assess. Subsequent investigation found that these isolates were not resistant to D29 phages and suggested that the failure to complete the infective cycle may have been due to an inactive metabolic state at the time of testing.
Delaying plating of samples until after lysis and release of progeny phages resulted in an amplification of the numbers of plaques observed. However, the limit of detection of dilute suspensions of BCG was not improved. Accurate quantification of this amplification effect was not possible due to the large variation in the numbers of plaques observed with dilute suspensions of BCG. The numbers of progeny phages released from individual bacteria may vary widely (8). For D29 infection in M. smegmatis , Sellers et al. reported a range of 34 to 156 with an average burst size of 98 (17), whereas David et al. observed an average release of 120 for infection in both M. smegmatis and M. tuberculosis (4). Burst sizes may be influenced by culture conditions and the phase of growth of the bacteria (17). Phage infection of mycobacteria will be influenced by the presence of aggregates of cells and, although the distribution of CFU may be predicted to follow a Poisson distribution, variations in the size of clumps may exacerbate variation in the number of progeny phages produced.
Phage replication demonstrated high sensitivity for detection of M. bovis BCG. Samples tested containing 100 CFU/ml were consistently found to be positive, suggesting a sensitivity 100-fold greater than that of microscopy (25). The high degree of correlation between CFU and PFU in samples plated prior to lysis would suggest that the method might be used in situations in which the comparative detection of viable bacteria is required, such as in drug susceptibility testing. The number of plaques observed ranged from one-fifth to half of the estimated numbers of CFU in the samples, suggesting that successful infection did not occur in a substantial proportion of bacteria. Failure to infect may be due to a number of factors. Phage replication is dependent on mechanisms of replication of the host bacteria and will not proceed in bacteria that are dormant or where replication has been disrupted. It may be assumed that the availability of a phage binding site (receptor) is a determining factor for infection. Electron microscopy has demonstrated that within populations of mycobacteria some cells adsorb large numbers of phages to the cell wall, whereas other bacteria have no phage particles attached (13). David et al. observed that adsorption to actively growing bacteria improved after washing and resuspension in fresh broth for one to two division times (4). Abortive infection, where infection is not followed by production of viable progeny phages may also be a contributory factor (20). Low levels of available calcium have been reported to increase the incidence of abortive infection with D29 (21); however, increasing the calcium supplement did not increase the rate of successful infection (results not shown).
Having established an assay that was sensitive for the detection of mycobacteria in laboratory cultures, the technology was transferred to a routine diagnostic setting in which it was compared to culture on Lowenstein-Jensen medium for the diagnosis of pulmonary tuberculosis. It has previously been demonstrated that higher rates of D29 infection may be obtained from suspensions of bacteria left in fresh culture broth for between one and two cell cycles (4). To maximize infection rates the bacteria extracted from clinical specimens samples were cultured in broth for between 20 to 24 h prior to inoculation with D29. The phage replication assay demonstrated sensitivity slightly greater than that of direct smear microscopy but was less specific. In this setting, no false-positive results were observed that were due to detection of mycobacteria other than tuberculosis. With the notable exception of bacteria belonging to the M. avium complex, D29 will replicate in a broad range of mycobacteria (10, 11). Thus, the specificity of both the phage assay and microscopy for acid-fast organisms is likely to decrease in settings in which infections with mycobacteria other than tuberculosis are more common. The high proportion of specimens found to be culture positive for tuberculosis in the present study is a reflection of the serious nature of the tuberculosis epidemic in Zambia. The phage replication assay failed to provide significant improvement over smear microscopy for the diagnosis of pulmonary tuberculosis. We suggest that the current sensitivity and specificity of the assay does not justify its application to the routine diagnosis of tuberculosis at this time. Its lack of sensitivity may reflect damage to bacilli sustained during the treatment with sodium hydroxide to decontaminate the specimens. It has been suggested that less-harsh treatments might be applied (15). However, failure to adequately decontaminate in this setting is likely to result in high levels of contamination from other microorganisms and thus prevent isolation and culture for subsequent identification and susceptibility testing. An alternative strategy might be to culture the bacteria for a few days prior to phage testing to allow new generations of undamaged bacteria to emerge. However, this would decrease the rapidity of the test. A third strategy to increase sensitivity might be to increase the volume of sample tested and to combine multiple specimens from the same patient, thus increasing the probability of a positive result. We conclude that further study is required to identify the factors affecting phage infection of mycobacteria. This would enable optimization of detection of M. tuberculosis in clinical specimens and may lead to the development of a more sensitive diagnostic test.
Acknowledgments
This study was funded by the United Kingdom Department for International Development.
We thank the staff of the Chest Clinic of University Teaching Hospital and Matero Urban Health Clinic for assistance with the recruitment of patients; the technical staff at the Chest Diseases Laboratory in Lusaka, Zambia, for expert assistance with the laboratory studies; and G. Kahenya and the Central Board of Health for facilitating this study.
REFERENCES
- 1.Adams, M. H. (ed.). 1959. Bacteriophages. Interscience Publishers, Inc., New York, N.Y.
- 2.Bowman, B. U. 1958. Quantitative studies on some mycobacterial phage-host systems. J. Bacteriol. 76:52-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.David, H. L. 1976. The bacteriology of the mycobacterioses. Centers for Disease Control and Prevention, Atlanta, Ga.
- 4.David, H. L., S. Clavel, and F. Clement. 1980. Adsorption and growth of the bacteriophage D29 in selected mycobacteria. Ann. Virol. 131:167-184. [Google Scholar]
- 5.David, H. L., N. Rastogi, S. Clavel Seres, and F. Clement. 1986. Action of colistin (polymyxin E) on the lytic cycle of the mycobacteriophage D29 in Mycobacterium tuberculosis. Zentbl. Bakteriol. Mikrobiol. Hyg. A 262:321-334. [DOI] [PubMed] [Google Scholar]
- 6.David, H. L., S. Seres Clavel, F. Clement, and N. Rastogi. 1984. Further observations on the mycobacteriophage D29-mycobacterial interactions. Acta Leprol. 2:359-367. [PubMed] [Google Scholar]
- 7.David, H. L., F. Clement, S. Seres Clavel, and N. Rastogi. 1984. Abortive infection of Mycobacterium leprae by the mycobacteriophage D29. Int. J. Lepr. Mycobact. Dis. 52:515-523. [PubMed] [Google Scholar]
- 8.Delbruck, M. 1945. The burst size distribution in the growth of bacterial viruses. J. Bacteriol. 50:131-135. [DOI] [PubMed] [Google Scholar]
- 9.Ellis, E. L., and M. Delbruck. 1939. The growth of bacteriophage. J. Gen. Physiol. 22:365-384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Froman, S., D. W. Will, and E. Bogen. 1954. Bacteriophage active against virulent Mycobacterium tuberculosis. I. Isolation and activity. Am. J. Public Health 44:1326-1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hatfull, G. F. 2000. Molecular genetics of mycobacteriophages, p. 37-54. In G. F. Hatfull and W. R. Jacobs (ed.), Molecular genetics of mycobacteria. ASM Press, Washington, D.C.
- 12.Kambashi, B., G. Mbulo, R. McNerney, R. Tembwe, A. Kambashi, V. Tihon, and P. Godfrey-Fausset. 2001. Utility of nucleic acid amplification techniques for diagnosis of pulmonary tuberculosis in sub-Saharan Africa. Int. J. Tuberc. Lung Dis. 5:364-369. [PubMed] [Google Scholar]
- 13.Kolbel, H. 1970. Phage multiplication in mycobacterium as a function of time and multiplicity of infection: an electron microscopic study, p. 29-38. In S. E. Juhasz and G. Plummer (ed.), Host-virus relationships in Mycobacterium, Nocardia, and Actinomyces. Charles C Thomas, Springfield, Ill.
- 14.McNerney, R., S. M. Wilson, A. M. Sidhu, V. S. Harley, Z. al Suwaidi, P. M. Nye, T. Parish, and N. G. Stoker. 1998. Inactivation of mycobacteriophage D29 using ferrous ammonium sulphate as a tool for the detection of viable Mycobacterium smegmatis and M. tuberculosis. Res. Microbiol. 149:487-495. [DOI] [PubMed] [Google Scholar]
- 15.Park, D. J., F. A. Drobniewski, A. Meyer, and S. M. Wilson. 2003. Use of a phage-based assay for phenotypic detection of mycobacteria directly from sputum. J. Clin. Microbiol. 41:680-688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Parry, C. M., O. Kamoto, A. D. Harries, J. J. Wirima, C. M. Nyirenda, D. S. Nyangulu, and C. A. Hart. 1995. The use of sputum induction for establishing a diagnosis in patients with suspected pulmonary tuberculosis in Malawi. Tuberc. Lung Dis. 76:72-76. [DOI] [PubMed] [Google Scholar]
- 17.Sellers, M. I., W. L. Baxter, and H. R. Runnals. 1962. Growth characteristics of mycobacteriophages D28 and D29. Can. J. Microbiol. 8:389-399. [DOI] [PubMed] [Google Scholar]
- 18.Snapper, S. B., R. E. Melton, S. Mustafa, T. Kieser, and W. R. Jacobs, Jr. 1990. Isolation and characterization of efficient plasmid transformation mutants of Mycobacterium smegmatis. Mol. Microbiol. 4:1911-1919. [DOI] [PubMed] [Google Scholar]
- 19.Tokunaga, T., M. I. Sellers, and A. Furuchi. 1970. Phage receptor of Mycobacterium smegmatis, p. 119-133. In P. G. Juhasz and G. Plummer (ed.), Host-virus relationships in Mycobacterium, Nocardia, and Actinomyces. Charles C Thomas, Springfield, Ill.
- 20.Tokunaga, T., Y. Mizuguchi, and T. Murohashi. 1964. Abortive Infection and host killing of mycobacteriophage. Am. Rev. Respir. Dis. 89:929-932. [DOI] [PubMed] [Google Scholar]
- 21.Tokunaga, T., and M. I. Sellers. 1964. Infection of Mycobacterium smegmatis with D29 phage DNAJ. Exp. Med. 119:139-149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.World Health Organization. 1998. Services in tuberculosis control. Part II. Microscopy. World Health Organization, Geneva, Switzerland.
- 23.World Health Organization. 1998. Services in tuberculosis control. Part III. Culture. World Health Organization, Geneva, Switzerland.
- 24.Wilson, S. M., Z. Al-Suwaidi, R. McNerney, J. Porter, and F. Drobniewski. 1997. Evaluation of a new rapid bacteriophage-based method for the drug susceptibility testing of Mycobacterium tuberculosis. Nat. Med. 34:465-468. [DOI] [PubMed] [Google Scholar]
- 25.Yeager, H., Jr., J. Lacy, L. R. Smith, and C. A. LeMaistre. 1967. Quantitative studies of mycobacterial populations in sputum and saliva. Am. Rev. Respir. Dis. 95:998-1004. [DOI] [PubMed] [Google Scholar]