Abstract
A duplex quantitative real-time PCR (qPCR) assay was designed to detect both the polymerase gene (pol) and the glycoprotein gene (gB) of cytomegalovirus (CMV). The detection limit of the qPCR was determined to be 1 to 3 copies/reaction and the linear measure interval was 103 to 108 copies/ml. The qPCR system was compared to the COBAS Amplicor CMV Monitor test (COBAS) by an analysis of 138 plasma samples. Both systems detected CMV in 71 cases and had negative results for 33 samples. In addition, 34 samples were positive by qPCR and negative by the COBAS assay, but in no case was the COBAS result positive and the qPCR result negative. Thus, qPCR detected 48% more positive cases than the COBAS method. For samples with ≥105 copies/ml by qPCR, a saturation effect was seen in the COBAS assay and quantification required dilution. Copy numbers for pol and gB by qPCR generally agreed. However, the reproducibility of qPCR assays and the need for an international standard are discussed. Discrepant copy numbers for pol and gB by qPCR were found for samples from two patients, and sequence analysis revealed that the corresponding CMV strains were mismatched at four nucleotide positions compared with the gB fragment primer sequences. In conclusion, a duplex qPCR assay in a real-time format facilitates quantitative measurements and minimizes the risk of false-negative results.
Human cytomegalovirus (CMV) is a common opportunistic pathogen found in immunocompromised patients, e.g., patients in a posttransplantation state or those infected with human immunodeficiency virus. Early detection and close monitoring of CMV infections are important for preventing the reactivation of endogenous CMV and for the surveillance of recipients of seropositive donor transplants. Appropriate antiviral therapy is based on a knowledge of the viral load as well as a quantification of the initial amount of CMV and the rate of increase between the last negative and the first positive PCR sample. These are independent risk factors for CMV disease (7).
Quantitative assays can be based on pp65 antigenemia (6, 9), PCR with detection by an enzyme immunoassay (2, 8, 18, 25), or other detection techniques (19, 20). The introduction of a commercial quantitative PCR assay, the COBAS Amplicor CMV Monitor test (Roche Molecular Systems, Pleasanton, Calif.), has provided a system that is easily used for clinical routine diagnostics and enables comparisons between laboratories (First EU-QCCA human cytomegalovirus proficiency panel, Quality Control for Molecular Diagnostics [http://www.qcmd.org]). In recent years, fluorogenic probes and real-time PCR have facilitated quantification and enabled multiplex systems with several target genes in the same assay.
At our laboratory, the COBAS method has been used for routine diagnostics for several years. With the aims of simplifying the workflow and testing a system that is easily adaptable for multiple target detection, we were interested in real-time PCR. In this study, we evaluate a duplex quantitative PCR (qPCR), which is an optimized combination of two recently described methods (23, 24), and compare it with the COBAS assay.
MATERIALS AND METHODS
Clinical specimens.
A total of 138 plasma samples were retrieved from 44 patients with suspected CMV infections during 2001. The group of patients consisted of 24 transplant patients (stem cell or renal transplantation), 6 with malignant diseases, 5 with fevers of unknown origin, 3 with suspected congenital CMV disease, and 6 with other disorders. The selection of samples for the comparison of the two test systems was performed by a random selection of 25 patients with negative COBAS test results. An additional 19 patients with CMV-positive tests in any of the samples analyzed by the COBAS assay were included in the study.
Duplex qPCR.
DNA was extracted from 200 μl of plasma, and after binding to a silica membrane, was eluted in 80 μl of elution buffer by use of a Qiagen DNA mini kit (Qiagen, Hilden, Germany). For the PCR, 10 μl of DNA eluate (corresponding to 25 μl of original plasma sample) was added to a 40-μl reaction mixture.
The target regions in the CMV AD169 genome (accession no. X17403) were pol, at nucleotides (nt) 78197 to 78262, and gB, at nt 81855 to 81916. Primers and probes were designed as previously described by Yun et al. (23, 24), except that the pol probe was labeled with a 6CR6G fluorophore (absorption maximum, 524 nm) (Scandinavian Gene Synthesis, Köping, Sweden) instead of FAM and dark quenchers replaced TAMRA at the 3′ ends of the two probes. To avoid degradation of the fluorogenic labeled probes, we routinely divided them into aliquots at a concentration of 45 μM in 30-μl volumes before storage at −20°C.
For an optimization of primer concentrations for the duplex qPCR, a range of final concentrations from 0.15 to 1.2 μM was evaluated. Probes were tested at concentrations between 0.10 and 0.60 μM. Annealing temperatures between 56 and 61°C were tested. The final amplification conditions are presented in Results. The assay was run on a RotorGene 2072 instrument (Corbett Research, Mortlake, Australia). Hereafter, the duplex qPCR method is referred to as the qPCR system to signify that the DNA extraction step is included in comparisons with other PCR systems.
qPCR standard.
The target DNA used for standard curves was purified genomic DNA from CMV strain AD169. The concentration of the quantification standard was established by parallel end-point titrations with a reference standard of AD169 DNA in a conventional PCR based on the pol gene (25), with the detection of PCR products in ethidium bromide-stained agarose gels.
The reference standard with AD169 DNA was prepared with infected lung fibroblasts as previously described at the Swedish Institute for Infectious Disease Control (3), and a defined copy number was calculated.
The stock solution of the prepared standard contained 1.5 × 106 genome copies per μl, and working solutions were divided into aliquots at −20° C until use. For qPCR analysis, duplicate samples of the AD169 genome were used at 1,000, 100, 30, and 10 copies per PCR. The standard curve was constructed by plotting the CMV DNA input against the corresponding PCR threshold cycle (Ct) values. For the qPCR analysis to be acceptable, a correlation coefficient of >0.95 was required for the slope of the standard curve.
Inhibition test for qPCR assay.
Seventy-one PCRs with clinical samples that were negative for CMV were spiked with 50 copies of genomic AD169 in order to measure the eventual inhibition of PCR amplification. The test aimed to measure the inhibition of amplification due to remaining constituents in the DNA extract, without including the effect of the extraction yield. Therefore, the PCR tubes were spiked with target copies instead of clinical samples before DNA extraction.
COBAS Amplicor CMV Monitor test.
For the COBAS system, the target was the fragment from nt 80133 to 80494 of the pol gene from the AD169 genome (accession no. X17403). The extraction of DNAs from plasma samples by isopropanol precipitation and the following PCR amplification and quantitative detection by enzyme immunoassay were performed according to the manufacturer's instructions. The volume of DNA eluate analyzed by the assay corresponded to 25 μl of original plasma.
DNA sequencing.
A gB fragment of 682 bp was amplified by using the primers 5′-TTCTGGGAAGCCTCGGAACG-3′ and 5′-GAGTAGCAGCGTCCTGGCGA-3′ in a PCR. Both strands were sequenced by using the same primers and a BigDye Terminator cycle sequencing ready reaction kit (Applied Biosystems, Foster City, Calif.). The reactions were loaded onto a 310 Genetic Analyzer (Applied Biosystems).
RESULTS
Optimization and performance of qPCR.
The optimization of the duplex qPCR resulted in optimal concentrations of 0.9 μM (each) for primers and 0.25 μM (each) for probes. Optimal thermal conditions were 50°C for 3 min to degrade contaminating amplicons by uracil glycosylase, 95°C for 10 min to activate the DNA polymerase, and then 45 cycles of 95°C for 15 s and 57°C for 60 s.
The linear measure interval of the qPCR was determined to be between 103 and 108 copies/ml by repeated dilution experiments with an in-house CMV standard. For both target genes, the detection sensitivity was 1 to 3 copies/reaction (40 to 120 copies/ml of plasma). The reproducibility of the assay was evaluated by including both DNA extraction and PCR amplification of a monitor (a run control with a defined copy number per microliter) that was used in 39 consecutive runs during the 6 months when the method was in routine operation. Two runs were excluded, since by mistake no monitor was added into the reaction tubes. The mean values for the remaining 37 runs were 1.81 ± 0.44 log10 copies/reaction (mean ± standard deviation [SD]) for gB and 1.89 ± 0.32 log10 copies/reaction for pol. During the same 6-month period, the correlation coefficients of the standard curve slopes were 0.98 ± 0.01 (mean ± SD) for gB and 0.98 ± 0.02 for pol.
The inhibition of PCR amplification was tested with 71 CMV-negative clinical samples by adding 50 copies of the monitor to each reaction. CMV was detected in all samples, and the quantity was within 0.5 log10 of the expected copy number, i.e., between 16 and 158.
Comparison between COBAS Amplicor method and qPCR method.
A comparison was performed with 138 plasma samples from 44 patients with suspected CMV infections. Both methods detected CMV in 71 samples and had negative results in 33 cases, which led to a concordance of 75.4% for the two tests. In addition, the qPCR assay was positive for 34 samples that were negative by the COBAS method, but no sample was negative by qPCR and positive by COBAS. Thus, the qPCR assay detected 48% more positive cases than the COBAS method. The results for 39 consecutive samples from a CMV-infected leukemic girl undergoing periods of antiviral treatment were monitored during a study period of 246 days (Fig. 1). The patient suffered from severe CMV retinitis and CMV myelitis and was treated with cidofovir at day 1. Due to the progression of the myelitis, the therapy was switched to ganciclovir and foscarnet at day 18. The clinical symptoms and viral load diminished, and the foscarnet was discontinued at day 86. During the following month, repeated PCRs (by the COBAS method) were negative, while in retrospective we found a significant level of CMV positivity by the qPCR method. At day 124, the patient presented with severe hemolytic anemia due to a reactivated CMV infection, and therefore foscarnet and immunoglobulin were administered. The patient improved and ganciclovir was discontinued at day 175. Due to another CMV reactivation with hemolytic anemia, ganciclovir was administered at day 191, with a successful outcome. In general, the qPCR assay resulted in higher copy numbers than the COBAS assay. Of the 39 samples, 15 were positive by qPCR and negative by the COBAS assay, 1 was negative by both assays, and the remaining 23 samples were positive by both assays. This indicates that the COBAS system has a lower capacity for CMV detection than the qPCR assay.
FIG. 1.
CMV load in a leukemic patient during 246 days as determined by duplex qPCR (pol [] and gB [▴]) and the COBAS test (○). Antiviral treatment periods and the administration of immunoglobulin are indicated at the top.
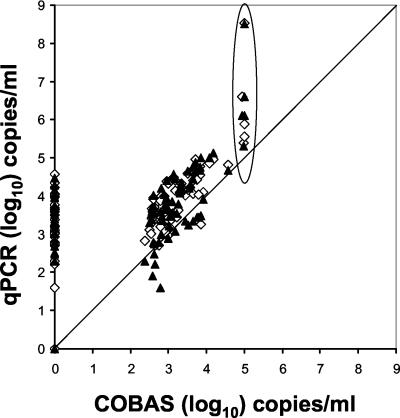
The 71 samples that were positive by both assays resulted in an average (±95% confidence interval) of 103.28 ± 100.14 copies/ml for the COBAS assay and corresponding values of 103.96 ± 100.16 for pol and 103.78 ± 100.19 for gB for the qPCR assay. The lowest level for a quantitative result by the COBAS method was set at 400 copies/ml (10 copies/reaction). In a scatter plot analysis, the COBAS method showed a saturation effect at 105 copies/ml, while the qPCR assay showed a linear distribution that was at least 2 orders of magnitude higher (Fig. 2). The quantification results for pol and gB were mostly very similar, as indicated by the presented mean values and as shown for individual samples in Fig. 1. If the obtained copy numbers for pol and gB differed >0.5 log10, we interpreted this as a sign of true discrepancy and retesting was performed.
FIG. 2.
Scatter plot analysis of the COBAS Amplicor CMV test compared to the duplex qPCR assay with pol (◊) and gB (▴) target genes. A line for equivalent copy number determination is denoted and deviating results in the upper range are encircled.
Discrepant results between gB and pol assays.
With samples from two patients with CMV reactivation, obvious differences in the numbers of copies per milliliter of plasma were noted for gB and pol (Table 1). For patient A, two samples collected 113 days apart were amplified by a PCR including the target region of the gB assay and were sequenced thereafter. The sequences obtained were compared with published sequences in GenBank and an alignment was created (Fig. 3). This revealed one mismatch in the reverse primer and three mismatches in the forward primer when the sequences from both patients were compared. The same sequence variation was detected in two samples collected 28 days apart from patient B. The mismatch in the reverse primer was also noted in three other sequences from GenBank. Between one and three mismatches in the forward primer region were found in GenBank sequences. The gB sequences found in patients A and B were of type 3 (5).
TABLE 1.
Discrepant quantitative results for detection of gB and pol genes in a duplex qPCR assay for CMV
| Patient identification | Day | Result for gB (copies/ml) | Result for pol (copies/ml) | Ratio of pol/gB |
|---|---|---|---|---|
| A | 1 | 0 | 0 | |
| 8 | 0 | 0 | ||
| 15 | 40 | 800 | 20 | |
| 22 | 200 | 4,600 | 23 | |
| 29 | 3,800a | 191,000 | 50 | |
| 37 | 7,000 | 370,000 | 53 | |
| 40 | 34,000 | 1,200,000 | 35 | |
| 44 | 62,000 | 2,100,000 | 34 | |
| 51 | 35,000 | 200,000 | 5.7 | |
| 58 | 1,300 | 10,600 | 8.1 | |
| 61 | 5,400 | 10,000 | 1.8 | |
| 65 | 4,000 | 34,600 | 8.6 | |
| 72 | 4,400 | 20,000 | 4.5 | |
| 79 | 800 | 13,000 | 16 | |
| 87 | 2,000 | 12,800 | 6.4 | |
| 93 | 3,000 | 19,000 | 6.3 | |
| 96 | 1,200 | 13,000 | 10 | |
| 110 | 240 | 7,900 | 33 | |
| 115 | 3,800 | 37,400 | 10 | |
| 117 | 2,500 | 35,400 | 14 | |
| 122 | 200 | 1,800 | 9.0 | |
| 128 | 80 | 700 | 8.8 | |
| 139 | 16,000 | 300,000 | 19 | |
| 142 | 12,400a | 280,000 | 23 | |
| B | 1 | 0 | 0 | |
| 7 | 0 | 200 | ||
| 15 | 80 | 200 | 2.5 | |
| 21 | 340 | 2,100 | 6.2 | |
| 28 | 900a | 5,200 | 5.8 | |
| 35 | 2,300 | 8,400 | 3.7 | |
| 42 | 360 | 640 | 1.8 | |
| 47 | 0 | 800 | ||
| 53 | 880 | 5,000 | 5.7 | |
| 56 | 1,400a | 6,800 | 4.9 |
Sequence analysis was performed on this sample.
FIG. 3.
Sequences of gB region from two samples from a CMV-infected patient compared to sequences in GenBank. Arrows indicate nucleotide positions at which the patient strain sequence differs from the primer sequences in the qPCR assay.
DISCUSSION
Since there is no international standard available for CMV PCR, the definitions of the number of copies in all CMV DNA standards are to some extent arbitrary. The determination of the standard may be influenced by errors in measuring the DNA concentration, pipetting, storage conditions, and other factors related to instrumentation or manual performance. The detection limit of our duplex qPCR assay was found to be one to three copies per reaction for both gB and pol. Our standard was defined by the end-point titration of genomic AD169 DNA together with a standard obtained from another laboratory, and calculated end-point concentrations coincided with the zone of stochastic positivity. Thus, the concentration is probably correct. Furthermore, our qPCR was compared with the same PCR assays in a single PCR format for which the standard consisted of a plasmid containing the target sequences for gB and pol (23, 24). Irrespective of whether DNA preparations from a complete genome or from plasmids were used and independent of the mono or duplex format, the detection limit was about five copies per reaction. These findings support our definition of the detection limit.
Reproducibility is fundamental for quantitative calculations, but due to the multistep process of PCR assays (sample preparation, amplification, and detection) and the high sensitivity of the technique, a considerable interassay variability is inevitable. In a proficiency panel distribution for CMV (range, 2.0 to 3.8 log10 copies/ml), an SD between 0.48 and 0.71 log10 was found for 59 data sets (about 80% of the sets were from real-time PCR) (QCMD 2002 cytomegalovirus proficiency programme, Quality Control for Molecular Diagnostics [http://www.qcmd.org]). A variation of 0.5 log10 is often considered to be acceptable for determinations of genome equivalents. We calculated the reproducibility of the duplex qPCR assay in clinical routine work during a 6-month period and found that for monitors with average values of 1.81 log10 (65) and 1.89 log10 (78) copies/reaction, the SD was between 0.32 and 0.44 log10. This deviation is acceptable, but it is considerably higher than a previously reported value of 0.13 log10 (23). This difference in reproducibility is probably explained by the difference in copy number of the monitors, i.e., when using a monitor of a higher concentration, such as the 3.18-log10 concentration used in the paper by Yun (23), a lower SD value is obtained. Such a decrease in SD at increasing genome numbers is statistically expected and has been shown for another qPCR assay (11). A run control at a low concentration facilitates the monitoring of the detection capacity of the PCR but has a higher uncertainty in measurement. At present, there are insufficient data to define a clinically significant CMV concentration in plasma; it differs by type of patient group (7) and may vary over time in the same individual due to a changing immune status (16). It has been stated that copy numbers below 103 to 104 per ml of blood are rarely associated with CMV disease (1, 11, 12, 21), but the increased use of preemptive antiviral treatment (10, 15) has complicated the understanding of what is significant. Therefore, it is not evident what an optimal concentration of monitor control means.
According to our definition, a linear standard curve should have a correlation coefficient of at least 0.95 to accept a qPCR analysis. The correlation coefficient of >0.98 during the study period indicated the high reproducibility of the linear standard curve down to the detection limit. The reproducibility of quantification in the lower part of the linear measure interval is limited mainly due to factors that are independent of the instrument. Thus, although the dynamic range goes down to ≤10 copies/reaction, reliable quantification is not obtained at that level. For clinical routine analyses, we do not give quantitative results when the concentration is <1,000 copies/ml. With the COBAS Amplicor CMV test, the quantitative lower level has been set to 400 copies/ml, but it has been reported that below 1,000 copies/ml the variation increases (4), and in the latest issue of the manual for the test the lower level of the linear range is not defined.
The inhibition of amplification has been reported as a significant difficulty (13, 22). If DNA extraction is performed properly, however, our experience is that inhibition in plasma is an almost negligible issue. For this study, we found no qualitative inhibition in 71 samples. In order to detect low levels of inhibition, we added only 50 copies/reaction, but the quantitative inhibition was difficult to assess since the uncertainty in measurement is high at this concentration.
A comparison between the COBAS Amplicor method and the qPCR method showed a poor level of concordance. The qPCR system detected 48% more positive cases. In 19 of the 34 COBAS-negative and qPCR-positive cases, the copy number was <3,000 copies/ml, indicating that the difference in detection capacity was mainly due to the higher sensitivity of the qPCR. This superior sensitivity for qPCR facilitates the management of CMV-infected patients and enables faster preemptive antiviral therapy. Our results are in agreement with two recent reports (14, 15) but contrast with a previous evaluation of the quantitative COBAS test in which it had a similar sensitivity to a real-time PCR assay with fluorescence resonance energy transfer probes (17). An alternative explanation for the discrepancy between the methods would be mismatching nucleotides in the primer and/or probe regions used in the COBAS assay. A GenBank search showed that nucleotide variation occurs in one primer region. Therefore, a sequence analysis was performed with five CMV-positive samples, but no variation was noted (data not shown) in the actual primer regions. The protocol for the COBAS test prescribes that plasma, not sera, be used and this was followed in our testing.
Our results from consecutive samples from a CMV-infected leukemic girl undergoing episodic treatment during a study period of 246 days demonstrated the lower capacity to detect CMV of the COBAS test than the qPCR assay. Since the qPCR assay was run retrospectively, we conclude that antiviral treatment would have been started earlier at one stage if qPCR results had been available. Therefore, the detection capacity of the methods used has a clinical impact. Moreover, viral DNA concentrations in the results from the COBAS test were lower than those from the qPCR assay. Variations in viral concentrations were approximately correlated to periods of antiviral treatment. In a scatter plot analysis, the COBAS method showed a saturation effect at about 105 copies/ml, while the qPCR assay showed a linear distribution up to 108 copies/ml. Our results are in agreement with reports of lower CMV DNA concentrations indicated by the COBAS method than by other detection methods when measuring viral loads of over 100,000 copies/ml (4, 17). In practice, this leads to reanalysis of a diluted sample. The viral loads for 71 CMV-positive samples had an about 0.6-log10 higher average value by the qPCR assay than by the COBAS system. An explanation for this could be different yields of DNA extraction. Although the extraction procedures of the two PCR systems were not compared formally, this explanation is less likely, since several parallel runs with the two extraction methods followed by the COBAS PCR yielded very similar results (data not shown). Another explanation could be a different accuracy in definitions of the number of genomes in the two standards. Notably, the phenomenon of higher values by the qPCR assay than by the COBAS system has even been reported in previous evaluations (4, 14).
Sequence variation in target genes for CMV detection is well known and may be driven by immunological pressure as for gB or by antiviral treatment as for pol. Of three commonly used target genes, pol is the most conserved, followed by gB, while the major immediate-early gene is more variable (25). Therefore, we designed a duplex qPCR with the simultaneous detection of both pol and gB. Some may question whether this concern about incorrect test results is justified or is merely a theoretical problem. In this study, we found two obvious cases in which the detected copy number was considerably lower for gB than for pol. Sequence analyses of both cases revealed mismatches in the primer regions, which explains the poor outcome for gB detection. The ratio in copy number between pol and gB increased in samples with high viral loads, which is suggested to be an effect of a lower amplification efficiency of gB. As mentioned above, a search in GenBank also showed sequence variation in the primer region of the pol gene used for the COBAS method. A single target gene may cause suboptimal patient management in cases of sequence mismatches in primer or probe regions, resulting in falsely low copy numbers. A duplex qPCR based on two target genes minimizes the risk of false-negative results or an underestimation of the viral load and therefore facilitates proper antiviral treatment.
In summary, we have shown that quantitative detection by our duplex real-time PCR system has a higher sensitivity than the COBAS Amplicor CMV Monitor test system and that the linear measure interval is at least 3 orders of magnitude higher. The reproducibility of both test systems is acceptable, but imperfect, and an international reference standard is needed. Although sequence variation is a limited problem for the detection of CMV, it exists irrespective of the target gene used, and a duplex PCR minimizes this concern.
Acknowledgments
We thank Andy Metcalfe for linguistic revisions.
REFERENCES
- 1.Boeckh, M., and G. Boivin. 1998. Quantitation of cytomegalovirus: methodologic aspects and clinical applications. Clin. Microbiol. Rev. 11:533-554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boivin, G., C. A. Olson, M. R. Quirk, S. M. St.-Cyr, and M. C. Jordan. 1995. Quantitation of human cytomegalovirus glycoprotein H gene in cells using competitive PCR and a rapid fluorescence-based detection system. J. Virol. Methods 51:329-342. [DOI] [PubMed] [Google Scholar]
- 3.Brytting, M., V. A. Sundqvist, P. Stalhandske, A. Linde, and B. Wahren. 1991. Cytomegalovirus DNA detection of an immediate early protein gene with nested primer oligonucleotides. J. Virol. Methods 32:127-138. [DOI] [PubMed] [Google Scholar]
- 4.Caliendo, A. M., R. Schuurman, B. Yen-Lieberman, S. A. Spector, J. Andersen, R. Manjiry, C. Crumpacker, N. S. Lurain, and A. Erice. 2001. Comparison of quantitative and qualitative PCR assays for cytomegalovirus DNA in plasma. J. Clin. Microbiol. 39:1334-1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chou, S. 1992. Comparative analysis of sequence variation in gp116 and gp55 components of glycoprotein B of human cytomegalovirus. Virology 188:388-390. [DOI] [PubMed] [Google Scholar]
- 6.Egan, J., L. Barber, J. Lomax, A. Fox, N. Yonan, A. Rahman, C. Campbell, A. Deiraniya, K. Carroll, J. Craske, et al. 1995. Detection of human cytomegalovirus antigenaemia: a rapid diagnostic technique for predicting cytomegalovirus infection/pneumonitis in lung and heart transplant recipients. Thorax 50:9-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Emery, V. C., C. A. Sabin, A. V. Cope, D. Gor, A. F. Hassan-Walker, and P. D. Griffiths. 2000. Application of viral-load kinetics to identify patients who develop cytomegalovirus disease after transplantation. Lancet 355:2032-2036. [DOI] [PubMed] [Google Scholar]
- 8.Fox, J. C., P. D. Griffiths, and V. C. Emery. 1992. Quantification of human cytomegalovirus DNA using the polymerase chain reaction. J. Gen. Virol. 73:2405-2408. [DOI] [PubMed] [Google Scholar]
- 9.Gerna, G., D. Zipeto, M. Parea, M. G. Revello, E. Silini, E. Percivalle, M. Zavattoni, P. Grossi, and G. Milanesi. 1991. Monitoring of human cytomegalovirus infections and ganciclovir treatment in heart transplant recipients by determination of viremia, antigenemia, and DNAemia. J. Infect. Dis. 164:488-498. [DOI] [PubMed] [Google Scholar]
- 10.Goodrich, J. M., M. Mori, C. A. Gleaves, C. Du Mond, M. Cays, D. F. Ebeling, W. C. Buhles, B. DeArmond, and J. D. Meyers. 1991. Early treatment with ganciclovir to prevent cytomegalovirus disease after allogeneic bone marrow transplantation. N. Engl. J. Med. 325:1601-1607. [DOI] [PubMed] [Google Scholar]
- 11.Guiver, M., A. J. Fox, K. Mutton, N. Mogulkoc, and J. Egan. 2001. Evaluation of CMV viral load using TaqMan CMV quantitative PCR and comparison with CMV antigenemia in heart and lung transplant recipients. Transplantation 71:1609-1615. [DOI] [PubMed] [Google Scholar]
- 12.Hassan-Walker, A. F., I. M. Kidd, C. Sabin, P. Sweny, P. D. Griffiths, and V. C. Emery. 1999. Quantity of human cytomegalovirus (CMV) DNAemia as a risk factor for CMV disease in renal allograft recipients: relationship with donor/recipient CMV serostatus, receipt of augmented methylprednisolone and antithymocyte globulin (ATG). J. Med. Virol. 58:182-187. [PubMed] [Google Scholar]
- 13.Lantz, P. G., W. Abu al-Soud, R. Knutsson, B. Hahn-Hagerdal, and P. Radstrom. 2000. Biotechnical use of polymerase chain reaction for microbiological analysis of biological samples. Biotechnol. Annu. Rev. 5:87-130. [DOI] [PubMed] [Google Scholar]
- 14.Pang, X. L., L. Chui, J. Fenton, B. LeBlanc, and J. K. Preiksaitis. 2003. Comparison of LightCycler-based PCR, COBAS Amplicor CMV monitor, and pp65 antigenemia assays for quantitative measurement of cytomegalovirus viral load in peripheral blood specimens from patients after solid organ transplantation. J. Clin. Microbiol. 41:3167-3174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Preiser, W., S. Brauninger, R. Schwerdtfeger, U. Ayliffe, J. A. Garson, N. S. Brink, S. Franck, H. W. Doerr, and H. F. Rabenau. 2001. Evaluation of diagnostic methods for the detection of cytomegalovirus in recipients of allogeneic stem cell transplants. J. Clin. Virol. 20:59-70. [DOI] [PubMed] [Google Scholar]
- 16.Roberts, T. C., D. C. Brennan, R. S. Buller, M. Gaudreault-Keener, M. A. Schnitzler, K. E. Sternhell, K. A. Garlock, G. G. Singer, and G. A. Storch. 1998. Quantitative polymerase chain reaction to predict occurrence of symptomatic cytomegalovirus infection and assess response to ganciclovir therapy in renal transplant recipients. J. Infect. Dis. 178:626-635. [DOI] [PubMed] [Google Scholar]
- 17.Schaade, L., P. Kockelkorn, K. Ritter, and M. Kleines. 2000. Detection of cytomegalovirus DNA in human specimens by LightCycler PCR. J. Clin. Microbiol. 38:4006-4009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shinkai, M., and S. A. Spector. 1995. Quantitation of human cytomegalovirus (HCMV) DNA in cerebrospinal fluid by competitive PCR in AIDS patients with different HCMV central nervous system diseases. Scand. J. Infect. Dis. 27:559-561. [DOI] [PubMed] [Google Scholar]
- 19.Spector, S. A., R. Wong, K. Hsia, M. Pilcher, and M. J. Stempien. 1998. Plasma cytomegalovirus (CMV) DNA load predicts CMV disease and survival in AIDS patients. J. Clin. Investig. 101:497-502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Toyoda, M., J. B. Carlos, O. A. Galera, K. Galfayan, X. Zhang, Z. Sun, L. S. Czer, and S. C. Jordan. 1997. Correlation of cytomegalovirus DNA levels with response to antiviral therapy in cardiac and renal allograft recipients. Transplantation 63:957-963. [DOI] [PubMed] [Google Scholar]
- 21.Weinberg, A., T. N. Hodges, S. Li, G. Cai, and M. R. Zamora. 2000. Comparison of PCR, antigenemia assay, and rapid blood culture for detection and prevention of cytomegalovirus disease after lung transplantation. J. Clin. Microbiol. 38:768-772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wilson, I. 1997. Inhibition and facilitation of nucleic acid amplification. Appl. Environ. Microbiol. 63:3741-3751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yun, Z., I. Lewensohn-Fuchs, P. Ljungman, L. Ringholm, J. Jonsson, and J. Albert. 2003. A real-time TaqMan PCR for routine quantitation of cytomegalovirus DNA in crude leukocyte lysates from stem cell transplant patients. J. Virol. Methods 110:73-79. [DOI] [PubMed] [Google Scholar]
- 24.Yun, Z., I. Lewensohn-Fuchs, P. Ljungman, and A. Vahlne. 2000. Real-time monitoring of cytomegalovirus infections after stem cell transplantation using the TaqMan polymerase chain reaction assays. Transplantation 69:1733-1736. [DOI] [PubMed] [Google Scholar]
- 25.Zweygberg Wirgart, B., M. Brytting, A. Linde, B. Wahren, and L. Grillner. 1998. Sequence variation within three important cytomegalovirus gene regions in isolates from four different patient populations. J. Clin. Microbiol. 36:3662-3669. [DOI] [PMC free article] [PubMed] [Google Scholar]