Abstract
Eight hundred Erysipelothrix strains isolated between 1992 and 2002 from swine with erysipelas in Japan were serotyped. Thirty-seven, 47, 73, and 643 strains were isolated from animals with acute septicemia, urticaria, chronic endocarditis, and chronic arthritis, respectively, of which 381, 146, 254, and 19 isolates belonged to serotypes 1a, 1b, and 2b and other serotypes, respectively. All serotype 1a isolates were further examined for acriflavine resistance and their genotypes to discriminate them from the attenuated live vaccine strain, defined as serotype 1a, which is resistant to 0.02% acriflavine and which shows low levels of pathogenicity in mice. Of the serotype 1a isolates, 64.6% were acriflavine resistant, with 98.4% of these acriflavine-resistant strains having been isolated from animals with chronic arthritis. By randomly amplified polymorphic DNA (RAPD) analysis, almost all the acriflavine-resistant serotype 1a strains showed the 253-bp band characteristic of vaccine strains and were easily discriminated from all 113 strains of acriflavine-sensitive serotype 1a strains from animals with acute and subacute swine erysipelas. The incidence of acriflavine-resistant strains of the distinctive RAPD type 1-2 was markedly higher than that of the other RAPD types and serotypes. RAPD type 1-2 strains also included a specific group identifiable by restriction fragment length polymorphism DNA analysis. Furthermore, the pathogenicities of 29 isolates of RAPD type 1-2 for mice were lower than those of the 21 isolates of other RAPD types. Our results indicate that RAPD type 1-2 strains are live vaccine strains and that 37% of the cases of chronic swine erysipelas detected in the past 11 years in Japan have occurred as a side effect of live vaccine use.
Swine erysipelas is a disease caused by a small, gram-positive, rod-shaped bacterium, Erysipelothrix rhusiopathiae, and manifests as acute or subacute septicemia and chronic proliferative lesions (23). The disease is worldwide in distribution and is economically significant. E. rhusiopathiae was once thought to be the only member of the genus Erysipelothrix and was classified into 23 serotypes plus type N on the basis of the peptidoglycan antigens on the cell wall (23). At present, the genus Erysipelothrix contains two species, E. rhusiopathiae and E. tonsillarum, and two genetically distinct unclassified groups (18). Among the 15 serotypes of E. rhusiopathiae, serotype 1 (subdivided into serotypes 1a and 1b) and serotype 2 (subdivided into serotypes 2a and 2b) are the most important in the pig production industry (23). Species other than E. rhusiopathiae have low levels of virulence in swine (18).
Because of the importance of swine erysipelas, vaccines with killed organisms and attenuated live vaccines are used worldwide. In Japan, an intensive vaccination program with live vaccine has been in place since the occurrence of a major swine erysipelas outbreak in 1966 and 1967. However, in the last 15 years about 2,000 pigs annually have been shown to have acute and subacute septicemia; and about 2,000 pigs have been condemned by meat inspection authorities each year due to chronic arthritis, chronic endocarditis, and subacute urticaria. In Japan, unlike in other countries, only live vaccine has been used for a long time because the acriflavine-resistant attenuated live vaccine was first developed in Japan in 1932 (8). From 1997 to 2001, to avoid the interference of live vaccine with maternal antibody or the side effects of live vaccine, three kinds of inactivated vaccines, a formalin-inactivated aluminum-adsorbed vaccine and two kinds of NaOH-treated oil adjuvant vaccines, became commercially available. However, the rate of use of the inactivated vaccines is only 8% because of the higher vaccination cost. Although there is no experimental evidence that the strains used in attenuated live swine erysipelas vaccines can regain their virulence, live vaccine carries the risk of posing a hazard to susceptible swine (23). To identify the exact state of swine erysipelas, it is important to discriminate live vaccine strains from others. However, there is only one report on this subject, by Makino et al. (10), who unsuccessfully attempted to differentiate live vaccine strains by randomly amplified polymorphic DNA (RAPD) analysis-PCR, acriflavine resistance tests, and mouse pathogenicity tests.
In a preliminary study, we observed that the incidence of acriflavine-resistant strains of serotype 1a among strains isolated from animals with chronic swine erysipelas rapidly increased from 1992 to 1996 and found that acriflavine resistance is closely related to a specific RAPD type. In this study, we serotyped 800 strains of Erysipelothrix isolated from pigs with erysipelas between 1992 and 2002 and attempted to discriminate attenuated live vaccine strains from others not only by the acriflavine resistance test and mouse pathogenicity test but also by three genotyping methods: RAPD typing, ribotyping, and restriction fragment length polymorphism (RFLP) typing.
MATERIALS AND METHODS
Bacterial strains.
Three serotype 1a strains (strains Fujisawa, Koganei 60-0.15 [Koganei-NVAL], and Koganei-NIAH) were used as reference strains for the acriflavine resistance test, the mouse pathogenicity test, and genotyping. The Fujisawa strain is a virulent official challenge strain that is sensitive to acriflavine. Strain Koganei-NVAL is an attenuated live vaccine strain developed at the National Veterinary Assay Laboratory (NVAL) of Japan in 1971 (14) and has three markers: serotype 1a, resistance to 0.02% acriflavine, and low levels of virulence in mice. It does not kill mice but causes arthritis if 107 organisms are subcutaneously injected into 4-week-old ddY outbred female mice (14). All live vaccines in Japan have been produced by the seed-lot system with strain Koganei-NVAL. Strain Koganei-NIAH is also a live vaccine strain developed at the National Institute of Animal Health of Japan (NIAH) (3) and was used for commercial vaccine production before it was replaced with Koganei-NVAL from 1973 to 1983. It is also resistant to 0.02% acriflavine.
Strains ATCC 19414T of E. rhusiopathiae, ATCC 43339T of E. tonsillarum, Pécs 56 (serotype 13) of Erysipelothrix sp. strain 1, and 715 (serotype 18) of Erysipelothrix sp. strain 2 (18) were used as reference strains for species-specific PCR and microplate hybridization.
Erysipelothrix strains isolated from pigs with swine erysipelas.
A total of 800 strains isolated from pigs with swine erysipelas in Japan between 1992 and 2002 were used in this study. Thirty-seven strains were isolated at animal hygiene centers in 9 prefectures, mainly from animals with acute septicemia; and 761 strains were isolated at 17 meat inspection centers in 16 prefectures and one city from animals with chronic arthritis, chronic endocarditis, and subacute urticaria. At each institute they were identified as members of the genus Erysipelothrix by the appropriate biological tests. They were then submitted to the National Institute of Animal Health of Japan for further examination, e.g., serotyping, genotyping, acriflavine resistance testing, and mouse pathogenicity testing. To avoid excessive passage, isolates were cultivated on agar plates, suspended in broth medium containing 15% glycerol, and kept at −80°C. All cultivations were done from the same stock.
Serotyping.
Serotyping was carried out by agar gel precipitation tests with autoclaved cell extracts and rabbit antisera against formalin-killed cells of reference strains (9, 17). Briefly, rabbits were immunized by five successive intravenous injections of formalin-killed whole-cell suspensions of international reference strains of serotypes 1 to 23. Isolates were cultivated in brain heart infusion (Difco) supplemented with 0.3% Tris-0.1% Tween 80 (pH 7.8) at 37°C overnight. Cells were collected by centrifugation at 7,000 × g for 5 min, resuspended in 1/30 volume of distilled water, and autoclaved at 121°C for 60 min. After centrifugation, the supernatant was probed with rabbit antisera against serotypes 1a, 1b, and 2a in 1% Noble agar with 150 mM sodium chloride-10 mM phosphate buffer (pH 7.2) containing 0.1% sodium azide. Serotype 2b was distinguished from serotype 2a by examining whether the precipitation line between the sample and anti-serotype 2a fused completely with the homologous precipitation line for serotype 2a. If fusion was incomplete, the strain was designated serotype 2b. Isolates showing no precipitation lines with these three antisera were further tested with all other antisera, and isolates that gave no precipitation line with any antisera were described for convenience as “untypeable.” For the exact identification of serotype N, it is necessary to prepare antiserum against test strains and to confirm that the antiserum cannot produce any precipitation lines with the homologous antigen.
Identification of the genus and species of isolates other than those of serotypes 1 and 2 by PCR.
The genus and species of all 20 isolates other than those of serotypes 1 and 2 were determined by genus-specific and species-specific PCRs. An Erysipelothrix genus-specific PCR was reported by Makino et al. (11), and a PCR specific for four species was reported by Takeshi et al. (19). Both PCRs were performed in 25-μl reaction mixtures containing 10 ng of genomic DNA; 1.5 mM MgCl2; 12.5 pmol of each primer; 0.625 U of AmpliTaq DNA polymerase (Applied Biosystems); 200 μM each dCTP, dGTP, dATP, and dTTP in 10 mM Tris-HCl (pH 8.3); and 50 mM KCl under 1 drop of mineral oil. The cycling program of the genus-specific PCR was 30 cycles of 94°C for 1 min, 54°C for 2 min, and 72°C for 2 min; and that of the species-specific PCR was 30 cycles of 94°C for 1 min, 58°C for 1 min, and 72°C for 1 min. The amount of template DNA in the species-specific PCR was 10 ng per reaction mixture, and the number of cycles was limited to 30 to avoid cross-reactions among the four species. A total of 10 μl of each of the PCR products was analyzed by 1% agarose gel electrophoresis.
Identification of the species of isolates other than E. rhusiopathiae by microplate hybridization.
The species of two isolates other than E. rhusiopathiae were also identified by the microplate hybridization test, with slight modifications (5). Briefly, 10 μg of DNA from each of four reference strains (E. rhusiopathiae, E. tonsillarum, Erysipelothrix sp. strain 1, Erysipelothrix sp. strain 2) and two clinical isolates was dissolved in 100 μl of distilled water and heat denatured. The DNA solutions were diluted with 900 μl of 0.1 M MgCl2 in 0.01 M phosphate-buffered saline at pH 7.2 and dispensed into the wells of a high-adsorption, flat-bottom enzyme-linked immunosorbent assay plate (Immulon 600; Greiner) at 100 μl per well. After incubation at 37°C for 4 h, the DNA solution was discarded and the plates were then dried at 37°C. Another 2.5 μg of DNA from each strain was labeled with 2.5 μg of photobiotin (Vector Laboratories), according to the instruction manual provided by the manufacturer, and diluted with 1 ml of prehybridization buffer containing 50% formamide and 0.025% dextran sulfate. The microplate wells sensitized with the respective DNAs were prehybridized with 200 μl of prehybridization buffer at 30°C for 30 min and then incubated with 100 μl of biotin-labeled DNA at 30°C overnight. After the plate was washed with 1× SSC (0.15 M NaCl plus 0.015 M sodium citrate), 100 μl of horseradish peroxidase-labeled streptavidin diluted 1:1,000 was added and the wells were incubated at 30°C for 30 min. After the plate was washed, 100 μl of a substrate solution containing 0.4 mg of tetramethylbenzidine per ml, 0.02% H2O2 solution in 0.05 M citric acid, and 0.1 M sodium phosphate buffer (pH 4.5) was dispensed into each well and the reaction was allowed to proceed for 30 min at room temperature. The reaction was stopped by the addition of 100 μl of 2 N H2SO4, and the absorbance at 450 nm was read.
Acriflavine resistance test.
The acriflavine resistance of all 381 strains of serotype 1a and 169 strains of serotypes 1b, 2b, and N (54, 105, and 10 strains, respectively) was examined by streaking them onto agar plates containing 0.02, 0.01, 0.005, 0.0025, or 0% acriflavine (Wako Pure Chemical Industries, Osaka, Japan). The acriflavine agar plates were prepared by the addition of 1% acriflavine solution in distilled water to the sterilized basal medium, which consisted of brain heart infusion agar (Difco) supplemented with 0.3% Tris-0.1% Tween 80 and adjusted to pH 7.8. The isolates were preliminarily cultivated on basal agar plates at 37°C overnight and were then thickly streaked onto the agar plates. Strains Koganei-NVAL and Fujisawa were also streaked onto each plate as positive and negative controls, respectively. The growth on the plates was observed after incubation in a 10% CO2 incubator at 37°C for 4 days. The acriflavine concentration to which the strains were considered resistant was the highest concentration in which strains showed almost the same growth as they did on 0% acriflavine agar. Strains Koganei-NVAL and Koganei-NIAH were resistant to 0.01 and 0.005% acriflavine, respectively, but not to 0.02% acriflavine, even though they are defined as resistant. In this study, isolates resistant to 0.0025 to 0.01% acriflavine were defined as acriflavine resistant. Strain Koganei-NVAL showed acriflavine resistance at the same level only when the reagents were supplied by Wako Pure Chemical Industries and Tokyo Kasei Kogyo (Tokyo, Japan) but not when the reagents were supplied by other manufacturers, e.g., Aldrich Chemical (Milwaukee, Wis.), Sigma Chemical (St. Louis, Mo.), Acros Organics, and Nacalai Tesque (Kyoto, Japan).
DNA preparation.
E. rhusiopathiae DNA was prepared for RAPD typing, ribotyping and RFLP typing as follows. Bacteria were grown at 37°C in brain heart infusion (Difco) supplemented with 0.3% Tris-0.1% Tween 80 at pH 7.8. Three milliliters of overnight broth cultures was collected by centrifugation and resuspended in 200 μl of GTE buffer (50 mM glucose, 50 mM Tris-HCl [pH 8.0], 50 mM EDTA) containing 10 mg of lysozyme per ml and 10 mg of N-acetylmuramidase (mutanolysin; Difco) per ml, and the mixture was incubated for 30 min at 37°C by the methods of Galan and Timoney (6) and Makino et al. (11). Then, 250 μl of lysis buffer (50 mM Tris-HCl [pH 8.0] and 50 mM EDTA containing 1% sodium dodecyl sulfate and 50 mg of proteinase K per ml) was added, and the solution was incubated for 1 h at 55°C. The solution was extracted twice with equal volumes of phenol-chloroform-isoamyl alcohol, and the DNA was precipitated with 1/10 volume of 3 M sodium acetate and 2 volumes of ethanol. The precipitates were dissolved in 200 μl of TE buffer (10 mM Tris-HCl, 1 mM disodium EDTA [pH 8.0]) and were treated with 20 μg of RNase per ml for 30 min at 37°C. After extraction with an equal volume of chloroform, the DNA was precipitated with 1/10 volume of 3 M sodium acetate and 2 volumes of ethanol, washed in 70% ethanol, and dissolved in 100 μl of TE buffer. The DNA concentration was adjusted to 100 ng/μl for ribotyping and RFLP typing and to 20 ng/μl for RAPD typing.
RAPD typing.
RAPD typing was performed by the method by Akopyanz et al. (2) for the genotyping of Helicobacter pylori. Ten primers were examined for their abilities to discriminate between the Koganei-NVAL, Koganei-NIAH, and Fujisawa strains. Nine primers (primers 1254, 1283, 1247, 1281, 1290, 1252, D8635, D9355, and D14307) were reported by Akopyanz et al. (2); and one primer (primer 5′-TCACGATGCA-3′) was reported by Williams et al. (21). In the preliminary study, only primer D9355 (5′-CCGGATCCGTGATGCGGTGCG-3′) was able to distinguish Koganei-NVAL from Koganei-NIAH and Fujisawa. RAPD PCR was carried out with a 25-μl reaction mixture containing 20 ng of purified genomic DNA; 3 mM MgCl2; 20 pmol of primer D9355; 1 U of AmpliTaq DNA polymerase (Applied Biosystems); 250 μM each dCTP, dGTP, dATP, and dTTP in 10 mM Tris-HCl (pH 8.3); and 50 mM KCl under a drop of mineral oil. The thermal cycling conditions were 4 cycles of 94°C for 5 min, 40°C for 5 min, and 72°C for 5 min; 30 cycles of 94°C for 1 min, 55°C for 1 min, and 72°C for 2 min; and a final incubation at 72°C for 10 min. After PCR, 10 μl of each of the PCR products was electrophoresed in a 1% agarose gel (Mupid). The agarose gels were stained with 0.5 μg of ethidium bromide per ml for 15 min and photographed under UV light. A 1-kb DNA ladder (Gibco-BRL) was used as a size marker in all gels.
Ribotyping.
Each 1 μg of DNA was digested with 10 U of HindIII restriction enzyme in 15 μl of reaction buffer at 37°C overnight and electrophoresed in a 1% agarose gel. The gel was gently shaken in 0.1 N HCl for 4 min, and the DNA fragments were transferred to nylon membranes (Hybond N; Amersham) by the vacuum transfer method with alkaline transfer buffer (0.25 N NaOH, 0.5 M NaCl). After a brief wash with 2× SSC, the blot was fixed to the membrane by drying overnight at room temperature. DNA probes were prepared with PCR digoxigenin (DIG) labeling mix (Roche), universal primers for the bacterial 16S rRNA gene (primers 5′-AGTTTGATCCTGGCTC-3′ and 5′-AAGGAGGTGATCCAGCC-3′), and genomic DNA of the Fujisawa strain, according to the instruction manual provided by the manufacturer. Briefly, PCR was done with a 100-μl reaction mixture containing 400 ng of purified DNA, 1.5 mM MgCl2, 50 pmol of each primer, 2.5 U of AmpliTaq DNA polymerase (Applied Biosystems), and 10 μl of PCR DIG labeling mix (2 mM each dATP, dCTP, and dGTP; 1.9 mM dTTP; and 0.1 mM DIG-11dUTP) in 10 mM Tris-HCl (pH 8.3)-50 mM KCl under 2 drops of mineral oil. The thermal cycling conditions were 94°C for 2 min and then 30 cycles of 94°C for 1 min, 55°C for 2 min, and 72°C for 2 min, with a final incubation at 72°C for7 min. The PCR product was mixed with 10 ml of prehybridization buffer, heat denatured, and then hybridized with the prehybridized Southern-blotted membrane at 68°C overnight. After two washes with 0.1× SSC-0.1% sodium dodecyl sulfate at 68°C for 15 min, the blot was blocked with 1% blocking reagent (Roche) and reacted for 30 min with alkaline phosphatase-labeled anti-DIG (Roche) diluted 1:5,000. The blot was then washed and reacted with substrate solution containing 0.34 mg of nitroblue tetrazolium per ml and 0.175 mg of 5-bromo-4-chloro-3-indolylphosphate per ml in 0.1 M Tris-HCl-0.1 M NaCl-0.05 M MgCl2 (pH 9.5) for an additional 30 min or until the band pattern became clear. In preliminary tests, of seven restriction enzymes examined, only HindIII could discriminate strains Koganei-NVAL, Fujisawa, and Koganei-NIAH from each other; the other restriction enzymes examined showed similar patterns for all three strains.
RFLP typing.
The 253-bp RAPD band specific to strain Koganei-NVAL obtained with primer D9355 was excised from the agarose gel, purified by use of a QIAEX II gel extraction kit (Qiagen), and cloned into plasmid pCRII with a TA cloning kit (Stratagene). As a result, a recombinant plasmid, pKR2, was obtained and the insert was labeled with DIG by using the DIG-PCR labeling kit, as described above, with primer D9355 and 10 pg of plasmid pKR2 DNA. Southern hybridization and the detection of the hybridized probe were performed by the same method described above for ribotyping. All five restriction enzymes (BanIII, EcoRI, EcoRV, HaeIII, and HindIII) clearly discriminated Koganei-NVAL from Fujisawa and Koganei-NIAH. Fifty isolates examined for their pathogenicities for mice were further analyzed by RFLP typing with EcoRV or HindIII.
Mouse pathogenicity test.
Four female ddY mice (age, 4 weeks; SLC, Shizuoka, Japan) received subcutaneous injections of 0.1 ml containing 2 × 104 to 4 × 104 CFU of isolates. The symptoms of the mice were observed for 2 weeks, after which they were euthanized and autopsied. The hearts and spleens were stamped on selective brain heart infusion agar supplemented with 0.1% Tween 80-0.3% Tris-500 μg of kanamycin per ml-25 μg of gentamicin per ml at pH 7.8. They were then cut into small pieces and cultivated in brain heart infusion supplemented as described above. The Japanese standards for animal vaccines and biological diagnostics indicate that 107 CFU of strain Koganei-NVAL should not kill mice when it is injected subcutaneously but that more than 80% of mice should develop acute arthritis. On the other hand, at less than 101 CFU the virulent Fujisawa strain kills 100% of mice, and 100 50% lethal doses (4 × 102 CFU) is usually used for challenge exposure in mice. Because the pathogenicities of the isolates were considered to have been enhanced by passage though the pig body, a pathogenicity test was carried out with 104 CFU, an intermediate value between 107 and 101 CFU.
Nucleotide sequence accession number.
The nucleotide sequence of the 253-bp insert of pKR2 will appear in the DDBJ/EMBL/GenBank nucleotide sequence databases under accession no. AB159679.
RESULTS
Serotypes of the 800 Erysipelothrix isolates.
The isolates belonged to serotypes 1a, 1b, 2b, 4, 6, and 16, with only 16 (2%) isolates being untypeable. Table 1 shows the relationship between the serotypes and disease. Among the 800 isolates, 781 (97.6%) belonged to serotypes 1 and 2 and 798 (99.8%) were E. rhusiopathiae. This result was similar to the serotyping results seen by Takahashi et al. (17) for 1,046 strains isolated from swine with erysipelas from 1983 to 1993 in Japan, except that the proportion of untypeable strains was five times higher in their study.
TABLE 1.
Serotypes of the 800 Erysipelothrix strains from pigs with erysipelas isolated from 1992 to 2002
| Disease | No. of strains of serotype:
|
|||||||
|---|---|---|---|---|---|---|---|---|
| 1a | 1b | 2b | 4 | 6 | 16 | Untypeablea | Total | |
| Septicemia | 33 | 0 | 6 | 0 | 0 | 0 | 0 | 39 |
| Urticaria | 11 | 4 | 31 | 0 | 0 | 0 | 1 | 47 |
| Endocarditis | 28 | 6 | 34 | 0 | 1 | 0 | 2 | 71 |
| Arthritis | 309 | 136 | 183 | 1 | 0 | 1b | 13c | 643 |
| Total | 381 | 146 | 254 | 1 | 1 | 1 | 16 | 800 |
Strains untypeable with antisera against serotypes 1 to 23.
One strain of serotype 16 was identified as E. tonsillarum by species-specific PCR.
One untypeable strain was identified as a novel Erysipelothrix sp. by microplate hybridization.
Species of 19 isolates other than serotypes 1 and 2.
By PCR, all 19 isolates other than those of serotypes 1 and 2 were identified as members of the genus Erysipelothrix, of which 17 strains were E. rhusiopathiae (1 serotype 4 strain, 1 serotype 6 strain, and 15 untypeable strains) (data not shown). One serotype 16 strain was identified as E. tonsillarum by the 87% homology of its DNA with that of the E. tonsillarum DNA sequence and by a positive reaction by an E. tonsillarum-specific PCR. One untypeable strain was revealed to be a novel species of the genus Erysipelothrix, since it showed less than 40% homology with the DNAs of four known species and had negative results by PCRs specific for known species.
RAPD types of 381, 56, and 83 serotype 1a, 1b, and 2b isolates, respectively.
The serotype 1a isolates were separated into four RAPD types (Fig. 1). A total of 266 (69.8%) serotype 1a isolates showed the same RAPD pattern (pattern 1-2) as strain Koganei-NVAL (Table 2). Strains of serotypes 1b and 2b belonged to three and eight RAPD types, respectively, and most of them were different from those of the serotype 1a strains. These results indicate that strains of serotypes 1a, 1b, and 2b are genetically different from each other and that strains of serotype 2b are more genetically diverse than strains of serotypes 1a and 1b.
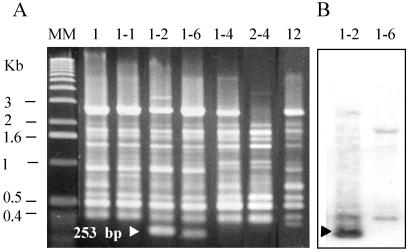
FIG. 1.
Seven major RAPD types shown by the 492 isolates of serotypes 1 and 2 primer with D9355 (A). Strain Koganei-NVAL belonged to RAPD type 1-2. Strains Fujisawa and Koganei-NIAH belonged to RAPD type 1. Strains of serotype 1a belonged to four RAPD types: types 1, 1-1, 1-2, and 1-6 (n = 2, isolated from one pig). Lane MM, molecular size marker. Although the patterns of RAPD types 1-2 and 1-6 strains resembled each other, Southern hybridization with a 253-bp RAPD band probe (B) revealed that they are completely different.
TABLE 2.
RAPD types of the 524 E. rhusiopathiae strains of serotypes 1a, 1b, and 2b
| Serotypea | No. of strains of RAPD type:
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 1-1 | 1-2 | 1-6 | 1-4 | 2-4 | 12 | Otherb | Total | |
| 1a R | 2 | 0 | 244 | 0 | 0 | 0 | 0 | 0 | 246 |
| 1a S | 73 | 38 | 22 | 2 | 0 | 0 | 0 | 0 | 135 |
| 1b | 4 | 0 | 0 | 0 | 39 | 0 | 13 | 0 | 56 |
| 2b | 0 | 0 | 0 | 0 | 42 | 13 | 0 | 32 | 87 |
R, resistant to 0.0025 to 0.01% acriflavine; S, sensitive to 0.0025% acriflavine.
Eleven other RAPD types: 1-5, 2-3, 2-5, 2-6, 2-7, 3, 4, 4-1, 5, 6, and 10.
Acriflavine resistance of all 381 serotype 1a isolates.
The incidence of acriflavine resistance among strains of RAPD type 1-2 was 91.7%, which is extremely high compared to that among other strains of serotype 1a (1.7%) as well as those among strains of serotype 1b (3.7%) and serotype 2b (5.7%). Furthermore, 98.1% of RAPD type 1-2 strains were isolated from pigs with chronic arthritis but not from pigs with septicemia (Table 3). Although 8.7% of the RAPD type 1-2 strains were sensitive to acriflavine, the evidence strongly suggests that all strains of RAPD type 1-2 were live vaccine strains, since many strains of RAPD type 1-2 showed acriflavine resistance at different levels, ranging from less than 0.0025 to 0.01%. These results suggest that they were live vaccine strains and were losing acriflavine resistance by passage through the pig body.
TABLE 3.
Acriflavine resistance and RAPD types of the 381 E. rhusiopathiae serotype 1a strains
| Disease | No. of strains of the following serotype and the indicated RAPD typea
|
||||
|---|---|---|---|---|---|
| 1a R
|
1a S
|
Total | |||
| 1-2 | Otherb | 1-2 | Other | ||
| Septicemia | 0 | 0 | 0 | 33 | 33 |
| Urticaria | 1 | 0 | 0 | 10 | 11 |
| Endocarditis | 4 | 0 | 0 | 24 | 28 |
| Arthritis | 239 | 2 | 22 | 46 | 309 |
R, resistant to 0.0025 to 0.01% acriflavine; S, sensitive to 0.0025% acriflavine.
Three other 3 RAPD types: 1, 1-1, and 1-6.
Murine pathogenicities of the 50 serotype 1a isolates.
Regardless of their acriflavine resistance, almost all isolates of RAPD type 1-2 showed low levels of virulence in mice; in contrast, almost all isolates of other RAPD types had high levels of virulence (Table 4). This result supports the hypotheses that all isolates of RAPD type 1-2 were the live vaccine strain and that the acriflavine-sensitive strains of RAPD type 1-2 were strains that had lost their acriflavine resistance.
TABLE 4.
RAPD types and mouse pathogenicities of the 50 E. rhusiopathiae isolates of serotype 1a
| RAPD type | Acriflavine resistancea | No. of strains for which mouse pathogenicity was as followsb
|
|||
|---|---|---|---|---|---|
| Low | Medium | High | Total | ||
| 1-2 | R | 19 | 3 | 1 | 23 |
| S | 6 | 0 | 0 | 6 | |
| Otherc | R | 0 | 0 | 1 | 1 |
| S | 0 | 1 | 19 | 20 | |
R, resistant to 0.0025 to 0.01% acriflavine; S, sensitive to 0.0025% acriflavine.
Four mice each received a subcutaneously injection of 104 CFU of virulent strain Fujisawa. High, all mice died 3 to 4 days after challenge; medium, some mice died later; low, no or a few symptoms.
Three other RAPD types: 1, 1-1, and 1-6.
RFLP types of the 50 serotype 1a isolates examined for mouse pathogenicity.
Fifty isolates examined for mouse pathogenicity were separated into three RFLP types with restriction enzymes EcoRV and HindIII (Fig. 2). This result shows that the RAPD type 1-2 strains, including strain Koganei-NVAL, belong to a distinct group that is genetically different from strains of the other RAPD types.
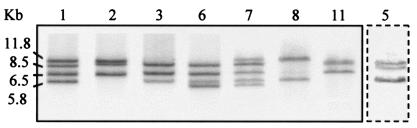
FIG. 2.
Seven major ribotypes shown by the 499 isolates of serotypes 1 and 2 with restriction enzyme HindIII. Strains Koganei-NVAL and Fujisawa belonged to ribotypes 1 and 6, respectively. Strain Koganei-NIAH belonged to minor ribotype 5 (lane boxed with dashed lines).
Ribotypes of all 381 serotype 1a isolates.
Most strains of serotype 1a belonged to five ribotypes, as shown in Fig. 3 and Table 5. Among the RAPD type 1-2 isolates, 71% belonged to ribotype 1, which is the same ribotype as Koganei-NVAL, and 21% belonged to ribotype 2. Conversely, of the isolates of other RAPD types, 24, 34, and 23% belonged to ribotypes 1, 3, and 6, respectively. Although ribotyping could not clearly discriminate isolates of RAPD type 1-2 from the other isolates, the incidence of each ribotype was different between the two groups. The discriminatory ability of ribotyping was greater than that of RAPD typing.
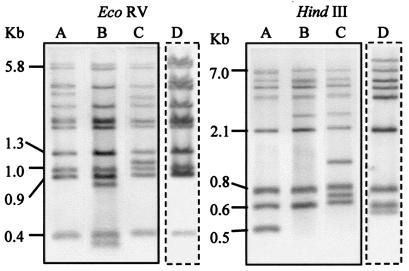
FIG. 3.
Three RFLP types, types A to C (lanes A, B, and C, respectively), shown by the 50 isolates of serotype 1a examined for mouse pathogenicity and another RFLP type, type D (lane D), shown by strain Koganei-NIAH. DNA was digested with EcoRV or HindIII and was hybridized with a probe specific for the 253-bp RAPD band. Strains Koganei-NVAL and Fujisawa belonged to RFLP types A and B, respectively. All 29 strains of RAPD type 1-2 belonged to RFLP type A. All 21 strains of other RAPD types belonged to RFLP type B or C.
TABLE 5.
Ribotypes of the 524 E. rhusiopathiae isolates of serotypes 1a, 1b, and 2b
| Serotype (RAPD type) | No. of isolates of ribotype:
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 6 | 7 | 8 | 11 | Othera | Total | |
| 1a (1-2) | 189 | 55 | 0 | 1 | 6 | 9 | 0 | 6 | 266 |
| 1a (otherb) | 28 | 7 | 39 | 27 | 5 | 1 | 5 | 3 | 115 |
| 1b | 10 | 3 | 6 | 23 | 2 | 0 | 3 | 9 | 56 |
| 2b | 13 | 8 | 11 | 30 | 13 | 1 | 4 | 7 | 87 |
Twelve other ribotypes: 4, 5, 9, 12, 14, 15, 16, 24, 25, 27, 28, and 29.
Three other RAPD types: 1, 1-1, and 1-6.
Serotypes of 622 strains isolated from pigs with chronic arthritis in 10 major prefectures.
Strains of RAPD type 1-2 were isolated from pigs with chronic arthritis in all 10 prefectures at an incidence of 25 to 100% (mean incidence, 45%) and from 72% of farms where chronic swine erysipelas was detected (data not shown). In contrast, serotype 1a strains of other RAPD types were isolated from only 6% of farms in two prefectures. Serotype 1b and 2b strains were isolated from 11 and 34% of farms in four and nine prefectures, respectively.
Annual incidence of strains of the respective serotypes and RAPD type 1-2 from pigs with chronic arthritis.
The incidence of RAPD type 1-2 increased rapidly from 10 to 75% between 1993 and 1996 and then decreased to 25 to 45%. From 1995 to 2002, almost all of the serotype 1a strains isolated from pigs with chronic arthritis were RAPD type 1-2.
DISCUSSION
For a long time epidemiological studies of swine erysipelas have been carried out by simply serotyping the causative agent, E. rhusiopathiae (4, 9, 17, 22). Although strains of the genus Erysipelothrix are classified into 23 serotypes plus type N, only serotypes 1 and 2 are of importance in swine (23). Therefore, effective methods of discrimination between serotype 1 and 2 strains are urgently required for epidemiological studies of swine erysipelas. These days, a variety of genomic methods, e.g. RFLP analysis, RAPD analysis, and amplified fragment length polymorphism analysis, have been successfully applied to the typing of both prokaryotes and eukaryotes (2, 7, 20, 21). Although two reports have described the possibility of ribotyping (1) and RAPD typing (13) of isolates of the genus Erysipelothrix, they did not focus on serotypes 1 and 2 and did not constitute epidemiological studies of swine erysipelas. In this study, we evaluated the epidemiological value of genomic typing, especially for the discrimination of a live vaccine strain, using a number of serotype 1 and 2 isolates, the most important in the pig production industry.
In Japan, the attenuated live vaccine for swine erysipelas was the only vaccine used for a long time and even now is most widely used because of the following advantages: one subcutaneous injection can induce complete protection in pigs, immunity persists for 6 months from only 2 weeks after vaccination, and vaccination costs are lower than those for inactivated vaccines. However, live vaccines also have several disadvantages: both maternal antibodies and drugs to which the vaccine strain is sensitive easily interfere with the effectiveness of the vaccine. Moreover, attenuated vaccines carry the risk of causing disease in susceptible swine if the attenuated vaccine strains regain their virulence (23).
The attenuated live vaccine strain used in Japan at present, Koganei-NVAL, was developed 1971 at the National Veterinary Assay Laboratory. The former vaccine strain, Koganei-NIAH, was gradually replaced by Koganei-NVAL over a period of 15 years. Strain Koganei-NVAL has three markers: serotype 1a; resistance to 0.02% acriflavine; and low virulence in mice, which survive inoculation. However, the ability to cause arthritis in more than 80% of mice is necessary for the induction of complete immunity in pigs (14). The directions for the use of live vaccine include a caution that the vaccine might induce systemic urticarial lesions in highly susceptible pigs, such as specific-pathogen-free (SPF) pigs. Moreover, it has been reported that the pathogenicity of strain Koganei-NVAL can rapidly increase and that the strain can kill 100% of mice after only five passages on medium containing Tween 80 and meat extract (12). It is thus probable that strain Koganei-NVAL has the potential to cause chronic arthritis in highly susceptible SPF pigs or pigs under stress, since even subclinical infection can cause chronic swine erysipelas (23).
We serotyped 800 Erysipelothrix strains isolated from pigs with acute and chronic cases of swine erysipelas from 1992 to 2002 and discriminated strain Koganei-NVAL by using the acriflavine resistance test, three genotyping methods (RAPD analysis, ribotyping, and RFLP analysis), and mouse pathogenicity testing. Almost all acriflavine-resistant strains were isolated from pigs with chronic arthritis and were closely related to RAPD type 1-2. Kondô et al. (8) reported that the acriflavine resistance of attenuated E. rhusiopathiae strains decreased as a result of passage on agar medium containing no acriflavine.
The hypothesis that the RAPD type 1-2 strains were live vaccine strains was also supported by the following facts: (i) they did not cause septicemia, which requires a high degree of virulence; (ii) they were isolated in all prefectures from which isolates were taken; and (iii) in 10 major prefectures, they were isolated from 72% of herds, although serotype 1a strains of other RAPD types and serotype 1b and 2b strains were isolated from only 6, 11, and 34% of herds, respectively. The incidence of acriflavine resistance among serotype 1a strains increased rapidly from 1993 to 1996. It was speculated that this was caused by synergy among the following factors: increased population of sensitive SPF herds, an increased vaccination rate as a result of combining the live attenuated vaccine for erysipelas with a live attenuated vaccine for swine fever, and increases in inducing factors such as porcine reproductive and respiratory syndrome virus infection. In 1992 the live vaccine for swine erysipelas was combined with the vaccine for swine fever, after which the vaccine was used intensively to eradicate swine fever. However, the vaccination rate decreased by half, according to the cessation of vaccination for swine fever in 2000.
In this study, we found that live vaccine strains have caused about half of the cases of chronic arthritis-type swine erysipelas that have appeared as a side effect of the use of the live vaccine for the past 11 years in Japan. Typing by RAPD analysis is a simpler and more effective method than the acriflavine resistance test for identifying the live vaccine strain. Although RFLP typing with the pKR2 probe for serotype 1a gave clearer results than RAPD typing, it is laborious and the results obtained were the same as those obtained by RAPD typing. Although ribotyping classified the serotype 1a strains into 12 types and its discriminatory ability was greater than that of RAPD typing, its epidemiological value is unknown because it differentiated the strains of RAPD 1-2 into two major types and six minor types.
Vaccination plays a key role in the control of swine erysipelas, especially for the prevention of sudden death from acute septicemia. However, these results indicate that the live vaccine should be used more carefully than the inactivated vaccine, with full knowledge of its merits and demerits, because most live vaccine strains are attenuated and are not necessarily avirulent. Although many live vaccine strains have been developed by passage through rabbits or chicken embryos, air drying, or growth in media containing acridine dyes (23), the attenuation mechanism is unknown. In the future, new types of live vaccine strains, such as a mutant strain from which the capsule gene is deleted (15, 16), may be necessary for the production of a safer live vaccine.
Acknowledgments
We thank the many veterinarians at meat inspection centers and at livestock hygiene service centers of the prefectures and the city who isolated and provided us with so many strains and so much information.
REFERENCES
- 1.Ahrné, S., I. Stenström, N. E. Jensen, B. Pettersson, M. Uhlén, and G. Molin. 1995. Classification of Erysipelothrix strains on the basis of restriction fragment length polymorphisms. Int. J. Syst. Bacteriol. 45:382-385. [DOI] [PubMed] [Google Scholar]
- 2.Akopyanz, N., N. O. Bukanov, T. U. Westblom, S. Kresovich, and D. E. Berg. DNA diversity among clinical isolates of Helicobacter pylori detected by PCR-based RAPD fingerprinting. Nucleic Acids Res. 20:5137-5142. [DOI] [PMC free article] [PubMed]
- 3.Andoh, K., and H. Nakamura. 1958. Research on the live vaccine for swine erysipelas. II. Freeze-drying conditions for the acriflavine resistant avirulent strain. Bull. Natl. Inst. Anim. Health 34:1-10. (In Japanese.) [Google Scholar]
- 4.Eamens, G. J., M. J. Turner, and R. E. Catt. 1988. Serotypes of Erysipelothrix rhusiopathiae in Australian pigs, small ruminants, poultry, and captive wild birds and animals. Aust. Vet. J. 65:249-252. [DOI] [PubMed] [Google Scholar]
- 5.Ezaki, T., Y. Hashimoto, and E. Yabuuchi. 1989. Fluorometric deoxyribonucleic acid-deoxyribonucleic acid hybridization in microdilution wells as an alternative to membrane filter hybridization in which radioisotopes are used to determine genetic relatedness among bacterial strains. Int. J. Syst. Bacteriol. 39:224-229. [Google Scholar]
- 6.Galan, J. E., and J. F. Timoney. 1990. Cloning and expression in Escherichia coli of a protective antigen of Erysipelothrix rhusiopathiae. Infect. Immun. 58:3116-3121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gürtler, V., and B. C. Mayall. 2001. Genomic approaches to typing, taxonomy and evolution of bacterial isolates. Int. J. Syst. E vol. Microbiol. 51:3-16. [DOI] [PubMed] [Google Scholar]
- 8.Kondô, S., S. Yamada, and K. Sugimura. 1932. Research on live vaccine for swine erysipelas. I. Acrydine dye resistance, pathogenicity and antigenicity of strains after serial passage on medium containing acrydine dye. Bull. Natl. Inst. Anim. Health 13:131-151. (In Japanese.) [Google Scholar]
- 9.Kucsera, G. 1979. Serological typing of Erysipelothrix rhusiopathiae strains and the epizootiological significance of the typing. Acta Vet. Hung. 27:19-28. [PubMed] [Google Scholar]
- 10.Makino, S., H. Ishizaki, T. Shirahata, S. Fujiwara, and T. Sawada. 1998. Isolation of acriflavine-resistant Erysipelothrix rhusiopathiae from slaughter pigs in Japan. J. Vet. Med. Sci. 60:1017-1019. [DOI] [PubMed] [Google Scholar]
- 11.Makino, S., Y. Okada, T. Maruyama, K. Ishikawa, T. Takahashi, M. Nakamura, T. Ezaki, and H. Morita. 1994. Direct and rapid detection of Erysipelothrix rhusiopathiae DNA in animals by PCR. J. Clin. Microbiol. 32:1526-1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Muramatsu, M., T. Sawada, and K. Seto. 1975. Recovery of virulence of attenuated strain Koganei by passage in broth media containing tween 80. Ann. Rep. Natl. Vet. Assay Lab. 12:3-8. (In Japanese.) [Google Scholar]
- 13.Okatani, A. T., H. Hayashidani, T. Takahashi, T. Taniguchi, M. Ogawa, and K. Kaneko. 2000. Randomly amplified polymorphic DNA analysis of Erysipelothrix spp. J. Clin. Microbiol. 38:4332-4336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Seto, K., Y. Nishimura, M. Fujiki, H. Azechi, and K. Suzuki. 1971. Studies on acryflavin-fast attenuated Erysipelothrix insidiosa. Comparison on pathogenicity and immunogenicity between mice and pigs. Jpn. J. Vet. Sci. 33:161-171. (In Japanese.) [DOI] [PubMed] [Google Scholar]
- 15.Shimoji, Y., E. Oishi, R. Kitajima, Y. Muneta, S. Shimizu, and Y. Mori. 2002. Erysipelothrix rhusiopathiae YS-1 as a live vaccine vehicle for heterologous protein expression and intranasal immunization of pigs. Infect. Immun. 70:226-232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shimoji, Y., Y. Mori, T. Sekizaki, T. Shibahara, and Y. Yokomizo. 1998. Construction and vaccine potential of acapsular mutants of Erysipelothrix rhusiopathiae: use of excision of Tn916 to inactivate a target gene. Infect. Immun. 66:3250-3254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Takahashi, T., N. Nagamine, M. Kijima, S. Suzuki, M. Takagi, Y. Tamura, M. Nakamura, M. Muramatsu, and T. Sawada. 1996. Serovars of Erysipelothrix strains isolated from pigs affected with erysipelas in Japan. J. Vet. Med. Sci. 58:587-589. [DOI] [PubMed] [Google Scholar]
- 18.Takahashi, T., T. Fujisawa, Y. Tamura, S. Suzuki, M. Muramatsu, T. Sawada, Y. Benno, and T. Mitsuoka. 1992. DNA relatedness among Erysipelothrix rhusiopathiae strains representing all twenty-three serovars and Erysipelothrix tonsillarum. Int. J. Syst. Bacteriol. 42:469-473. [DOI] [PubMed] [Google Scholar]
- 19.Takeshi, K., S. Makino, T. Ikeda, N. Takada, A. Nakashiro, K. Nakanishi, K. Oguma, Y. Katoh, H. Sunagawa, and T. Ohyama. 1999. Direct and rapid detection by PCR of Erysipelothrix sp. DNAs prepared from bacterial strains and animal tissues. J. Clin. Microbiol. 37:4093-4098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wassenaar, T. M., and D. G. Newell. 2000. Genotyping of Campylobater spp. Appl. Environ. Microbiol. 66:1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Williams, J. G. K., A. R. Kubelik, K. J. Livak, J. A. Rasfalski, and S. V. Tingey. 1990. DNA polymorphisms amplified by arbitrary primers are useful as genetic markers. Nucleic Acids Res. 18:6531-6535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wood, R. L., and R. Harrington, Jr. 1978. Serotypes of Erysipelothrix rhusiopathiae isolated from swine and from soil and manure of swine pens in the United States. Am. J. Vet. Res. 39:1833-1840. [PubMed] [Google Scholar]
- 23.Wood, R., L. 1992. Erysipelas, p. 475-486. In A. D. Leman et al. (ed.), Diseases of swine. Iowa State University Press, Ames.