Abstract
Objective
The aim of this meta-analysis was to compare the efficacy and safety of infliximab-biosimilar and other available biologicals for the treatment of rheumatoid arthritis (RA), namely abatacept, adalimumab, certolizumab pegol, etanercept, golimumab, infliximab, rituximab and tocilizumab.
Methods
A systematic literature review of MEDLINE database until August 2013 was carried out to identify relevant randomized controlled trials (RCTs). Bayesian mixed treatment comparison method was applied for the pairwise comparison of treatments. Improvement rates by the American College of Rheumatology criteria (ACR20 and ACR50) at week 24 were used as efficacy endpoints, and the occurrence of serious adverse events was considered to assess the safety of the biologicals.
Results
Thirty-six RCTs were included in the meta-analysis. All the biological agents proved to be superior to placebo. For ACR20 response, certolizumab pegol showed the highest odds ratio (OR) compared to placebo, OR 7.69 [95 % CI 3.69–14.26], followed by abatacept OR 3.7 [95 % CI 2.17–6.06], tocilizumab OR 3.69 [95 % CI 1.87–6.62] and infliximab-biosimilar OR 3.47 [95 % CI 0.85–9.7]. For ACR50 response, certolizumab pegol showed the highest OR compared to placebo OR 8.46 [3.74–16.82], followed by tocilizumab OR 5.57 [95 % CI 2.77–10.09], and infliximab-biosimilar OR 4.06 [95 % CI 1.01–11.54]. Regarding the occurrence of serious adverse events, the results show no statistically significant difference between infliximab-biosimilar and placebo, OR 1.87 [95 % CI 0.74–3.84]. No significant difference regarding efficacy and safety was found between infliximab-biosimilar and the other biological treatments.
Conclusion
This is the first indirect meta-analysis in RA that compares the efficacy and safety of biosimilar-infliximab to the other biologicals indicated in RA. We found no significant difference between infliximab-biosimilar and other biological agents in terms of clinical efficacy and safety.
Keywords: Arthritis, Rheumatoid, Biosimilar pharmaceuticals, Meta-analysis, Mixed treatment comparison
Introduction
Currently eight biological medicines—namely, abatacept, adalimumab, certolizumab pegol, etanercept, golimumab, infliximab, rituximab and tocilizumab—are registered by the European Medicines Agency (EMA) for the treatment of rheumatoid arthritis (RA). These biologicals are indicated for the treatment of adult patients with active disease when “the response to disease-modifying antirheumatic drugs (DMARDs), including methotrexate (MTX), has been inadequate.” Adalimumab, etanercept, golimumab, infliximab are also indicated for “adult patients with severe, active and progressive disease not previously treated with MTX or other DMARDs” as a ‘first-line therapy’.1,2
In September 2013, infliximab-biosimilar therapy (CT-P13, Trade names: Remsima and Inflectra) was also licensed in the EU for the treatment of RA. According to the EMA, Remsima and Inflectra are ‘biosimilar’3 medicines of infliximab.
The results of the randomized controlled trial (RCT) with biosimilar-infliximab treatment in RA were published in May 2013 [1]. PLANETRA was a double-blind, non-inferiority study, and aimed to prove the similar efficacy and safety of infliximab-biosimilar in combination with MTX and the originator infliximab combined with MTX. The primary endpoint of the trial was the therapeutic equivalence of clinical response according to ACR20 criteria at week 30 (See the definition of ACR20 in the “Methods” section). The study was performed between November 2010 and November 2011 at 100 centers across 19 countries in Europe, Asia, Latin America and the Middle East. Altogether, 606 patients with active RA despite MTX treatment were enrolled in the study. According to the results, at week 30, ACR20 and ACR50 responses were 60.9 and 35.1 %, respectively, on the infliximab-biosimilar arm, and 58.6 and 34.2 % on the originator infliximab arm in the intention-to-treat population. The difference not statistically significant at these two efficacy endpoints. Nor was a significant difference found in other efficacy and safety endpoints.
The aim of this study is to compare the efficacy and safety of the new infliximab-biosimilar treatment to the available originator biological drugs. We carry out systematic literature review and meta-analysis of published RCTs with infliximab-biosimilar and other biological treatments in the recommended doses defined by EMA’s product characteristic information in RA, applying mixed treatment comparison (MTC). This method allows us to carry out pairwise comparison of treatments with different comparators. In our case, infliximab-biosimilar is only compared to the originator infliximab, while other biologicals are compared to placebo in most of the studies. According to our knowledge, no indirect meta-analyses have yet been published that involve the infliximab-biosimilar treatment in the comparison.
Methods
Treatments
The analysis compared the recommended doses of biological DMARDs indicated in RA:4 abatacept (10 mg/kg at days at weeks 0, 2 and 4, and every 4 weeks thereafter, or by patient groups based on patient weight <60 kg, 500 mg; 60–100 kg, 750 mg; >100 kg, 1,000 mg, administered as a 30-min intravenous infusion); adalimumab (40 mg every other week as subcutaneous injection); certolizumab pegol (400 mg at 0, 2, 4 weeks and then 200 mg at every 2 weeks or 400 mg at every 4 weeks as subcutaneous injection); etanercept (25 mg twice weekly or 50 mg once weekly as subcutaneous injection); golimumab (50 mg once a month as subcutaneous injection); infliximab (3 mg/kg at 0, 2, 6 weeks and then every 8 weeks as intravenous infusions over a 2-h period) rituximab (1,000 mg on weeks 0, 2 as intravenous infusions); tocilizumab (8 mg/kg every 4 weeks as intravenous infusions) and infliximab-biosimilar (CT-P13) (3 mg/kg at 0, 2, 6 weeks and then every 8 weeks as intravenous infusions over a 2-h period).
In RA, infliximab-biosimilar (as well as the originator infliximab) must be administered concomitantly with MTX. Thus, we only included studies evaluating combination therapy with biologicals and conventional synthetic DMARD (csDMARD)5 In this way, we expected to increase the comparability of the results.
Endpoints
The rates of patients who achieved ACR20 and ACR50 response at week 24 were used as efficacy endpoints in the meta-analysis of RA trials. The American College of Rheumatology (ACR) response core set consists of a tender joint count, swollen joint count, patient’s assessment of pain, patient’s and physician’s global assessments of disease activity, patient’s assessment of physical function (HAQ), and laboratory evaluation of one acute-phase reactant [2]. ACR criteria are indicated as ACR20, ACR50, and ACR70, reflecting 20, 50, or 70 % relative improvement compared to baseline. Most of the RA clinical trials with biologicals use ACR20 as primary endpoint, but also report the percentage of study participants who achieve ACR50 response as a secondary endpoint.
The safety of biological therapies was also evaluated. The occurrence of serious adverse events at week 24 of the treatment was used as safety endpoint in the analysis.
Search strategy
Electronic databases (MEDLINE and Cochrane Library) as well as references of retrieved articles were searched. The Cochrane Highly Sensitive Search Strategy [3] was applied to identify randomized controlled publications and was combined with the disease MeSH terms ‘arthritis, rheumatoid’ and the drug names.6
The search dates were 1 November 2009 to 20 August 2013. References of RCTs from earlier time periods were taken from our previous systematic review [4].
A separate search was carried out to identify RCTs with a biosimilar agent with the generic name (CT-P13), without any restrictions.
Exclusion and inclusion criteria
We have applied the following inclusion criteria:
Double-blind, parallel RCTs with full paper obtainable (studies with only abstracts available were excluded). Non-randomized or uncontrolled studies, observational studies, case series, letters to the editor, studies with no abstracts or with conference abstracts only were not included.
The patients of interest are adults with moderate-to-severe RA. (Trials in diseases other than RA were not included.)
Head-to-head trials of combined biological therapies or studies with MTX or other csDMARD therapy control.
RA patients in at least one arm of the trial must receive one of the following treatments: abatacept, adalimumab, certolizumab pegol, etanercept, golimumab, infliximab, rituximab, tocilizumab or infliximab-biosimilar treatment in the licensed dose combined with csDMARDs.
We have applied the following exclusion criteria:
Off-label doses.
Monotherapy in RA (infliximab-biosimilar can be administered only in combination with MTX; therefore, only combination therapies were compared).
Studies reporting solely on laboratory measures aimed at investigating disease, or treatment mechanisms and which do not report relevant clinical outcomes.
Studies involving patients with age <18 years.
Pilot studies.
Studies shorter than 20 weeks, or studies that do not report ACR50 response at month 6.
Studies where all the patients enrolled previously failed biological therapy.
RA trials range widely in design regarding patient population. Some of them include patients not responding to csDMARD therapy, while others involve csDMARD-naïve patients. The authorization of infliximab-biosimilar also allows the application of the drug for RA patients previously not treated with MTX or other csDMARDs, in the case of severe, active and progressive disease. Thus, we included studies with MTX-naïve (or csDMARD-naïve) patients in the analysis as well.
However, we excluded studies that only involved patients who failed previous biological therapy.
Data extraction and quality assessment
Data were extracted by two independent researchers and checked by a third reviewer. Any disagreement was resolved through discussion until consensus was reached. For each selected study, details regarding study design, patients’ demographic and morbidity characteristics, treatment interventions, end-points and duration of follow-up were subtracted.
The quality of selected studies was evaluated using the Jadad score [5]. This is the most frequently used scale in quality assessment of clinical trials [6]. The Jadad scale assesses the quality of published clinical trials through methods of random assignment, double blinding, and the withdrawals and dropout of patients. Jadad score ranges from zero to five.
Meta-analysis: mixed treatment comparison
We have conducted a meta-analysis to compare the efficacy and safety of the biologicals included in the studies. An indirect comparison of study outcomes for biological therapies was carried out. In this paper, we examine the relative effectiveness of each individual treatment using the Lu method for combining direct and indirect evidence in mixed treatment comparisons (MTC), a Bayesian approach [7, 8]. Statistical models developed by NICE Decision Support Unit (DSU) were used. We estimated the posterior densities for all unknown parameters using MCMC (Markov chain Monte Carlo) for each model in WinBUGS version 1.4.3 (MRC Biostatistics Unit, Cambridge, UK). Each outcome measure was analyzed using random effects models.
All MTC models used the odds ratio (OR) as the measure of relative treatment effect, and assumed that treatment effects on the odds-ratio scale were multiplicative and exchangeable between trials. We also present the 95 % credibility intervals (CI) containing the true value of OR with 95 % probability.
Results
Literature review
The search in MEDLINE yielded 354 potential citations for RCTs examining the biological treatment of RA (search period: 01.11.2009–20.08.2013). In RA, 15 RCTs identified by our search met our inclusion criteria. Furthermore, out of 32 RCTs identified by Brodszky et al. [4], 21 were taken and included in our analysis. The rest were not included because they were either monotherapy studies, or examined biologicals after previous failure with biologicals [9–12].
The search for infliximab-biosimilar did not identify RCTs other than the PLANETRA trial [1].
Altogether, we included 36 RCTs (RA patients with combination therapy of MTX or other DMARDs). Most of the studies compared biologicals with placebo: five abatacept [13–17], seven adalimumab [18–24], three certolizumab pegol [25–27], four etanercept [28–31],7 four golimumab [32–35], three infliximab [36–38], four rituximab [39–42] and three tocilizumab [43–45]. One study compared abatacept versus adalimumab [46], one infliximab versus abatacept versus placebo [47] and one infliximab versus infliximab-biosimilar [1]. The number of trials in given comparisons might be different (e.g., on efficacy or safety endpoints) because of the specific inclusion criteria for each comparison and the distinct endpoints reporting across trials.
The main characteristics of the trials, i.e., number of patients, treatment arms, and JADAD score, are presented in Table 1.
Table 1.
Characteristics of the RCTs in RA included in the analysis
| Study | Treatment arms | Study duration (weeks) | N (ITT) | Age, years | HAQ score | Disease duration, years | JADAD score |
|---|---|---|---|---|---|---|---|
| Kremer [13] | ABT(2 mg/kg) + MTX | 26 | 105 | 54.4 | 1 | 9.7 | 5 |
| ABT(10 mg/kg) + MTX | 115 | 55.8 | 1 | 9.7 | |||
| Placebo + MTX | 119 | 54.7 | 1 | 8.9 | |||
| Kremer [14] | ABT(10 mg/kg) + MTX | 26 | 433 | 51.5 | 1.7 | 8.5 | 5 |
| AIM | Placebo + MTX | 219 | 50.4 | 1.7 | 8.9 | ||
| Weinblatt [15] | ABT(10 mg/kg) + DMARD | 52 | 959 | 52.5 | 1.5 | 10.4 | 5 |
| ASSURE | Placebo + DMARD | 482 | 52.1 | 1.6 | 10.4 | ||
| Westhovens [16]* | ABT(10 mg/kg) + MTX | 104 | 256 | NR | 1.7 | 0.56 | 5 |
| Placebo + MTX | 253 | 1.7 | 0.56 | ||||
| Takeutchi [17] | ABA(10 mg/kg) + MTX | 61 | 53.4 | 1.33 | 7.4 | 5 | |
| ABA(2 mg/kg) + MTX | 67 | 52.5 | 1.24 | 8.5 | |||
| Placebo + MTX | 66 | 53.4 | 1.5 | 7.3 | |||
| Furst [18] | ADL(40 mg/eow) + DMARD | 24 | 318 | 55 | 1.4 | 9.3 | 3 |
| STAR | Placebo + DMARD | 318 | 55.8 | 1.4 | 11.5 | ||
| Keystone [19] | ADL(20 mg/ew) + MTX | 24 | 212 | 57.3 | 1.4 | 11 | 3 |
| ADL(40 mg/eow) + MTX | 207 | 56.1 | 1.5 | 11 | |||
| Placebo + MTX | 200 | 56.1 | 1.5 | 10.9 | |||
| Weinblatt [20] ARMADA |
ADL(20 mg/eow) +MTX | 24 | 69 | 53.5 | 1.52 | 13.1 | 3 |
| ADL(40 mg/eow) + MTX | 67 | 57.2 | 1.55 | 12.2 | |||
| ADL(80 mg/eow) + MTX | 73 | 55.5 | 1.55 | 12.8 | |||
| Placebo + MTX | 62 | 56 | 1.64 | 11.1 | |||
| Breedveld [21] PREMIER* |
ADL(40 mg/eow) | 52 | 274 | 52.1 | 0.7 | 1.6 | 5 |
| ADL(40 mg/eow) + MTX | 268 | 51.9 | 0.7 | 1.5 | |||
| Placebo + MTX | 257 | 52 | 0.7 | 1.5 | |||
| Kim [22] | ADL(40 mg/eow) + MTX | 24 | 65 | 48.5 | 1.4 | 6.8 | 1 |
| Placebo + MTX | 63 | 49.8 | 1.3 | 6.9 | |||
| Detert [23] HIT HARD* |
ADL(40 mg/eow) + MTX | 24 | 87 | 47.2 | 1.4 | 1.8 | 5 |
| Placebo + MTX | 85 | 52.5 | 1.3 | 1.6 | |||
| Kavanaugh [24] | ADL(40 mg/eow) + MTX | 26 | 515 | 50.7 | 1.61 | 0.3 | 4 |
| OPTIMA* | Placebo + MTX | 517 | 50.4 | 1.6 | 0.4 | ||
| Keystone [25] RAPID1 |
CRT(200 mg) + MTX | 52 | 393 | 51.4 | 1.7 | 6.1 | 5 |
| CRT(400 mg) + MTX | 390 | 52.4 | 1.7 | 6.2 | |||
| Placebo + MTX | 199 | 52.2 | 1.7 | 6.2 | |||
| Smolen [26] RAPID2 |
CRT(200 mg) + MTX | 24 | 246 | 51.9 | 1.6 | 6.1 | 3 |
| CRT(400 mg) + MTX | 246 | 52.2 | 1.6 | 6.5 | |||
| Placebo + MTX | 127 | 51.5 | 1.6 | 5.6 | |||
| Choy [27] | CRT(400meg/4 week) + MTX | 24 | 126 | 53 | 1.4 | 9.4 | 5 |
| Placebo + MTX | 121 | 55.6 | 1.5 | 9.9 | |||
| Weinblatt [28] | ETN(2 × 25 mg/ew) + MTX Placebo + MTX | 24 | 59 | 48 | 1.5 | 13 | 3 |
| 30 | 53 | 1.5 | 13 | ||||
| Emery [30] COMET* |
ETN(50 mg/ew) + MTX Placebo + MTX | 52 | 274 | 50.5 | 1.7 | 0.7 | 5 |
| 268 | 52.3 | 1.6 | 0.8 | ||||
| Klareskog [29] TEMPO |
ETN(2 × 25 mg/ew) | 24 | 223 | 53.2 | 1.7 | 6.3 | 5 |
| ETN(2 × 25 mg/ew) + MTX | 231 | 52.5 | 1.8 | 6.8 | |||
| Placebo + MTX | 228 | 53 | 1.7 | 6.8 | |||
| Moreland [31] (till week 24)* | ETN(50 mg/ew) + MTX | 102 | 244 | 50.7 | NR | 3.5 | 5 |
| Placebo + MTX | 379 | 48.8 | 3.4 | ||||
| Keystone [32] GO-FORWARD |
GOL(100 mg) + placebo | 24 | 133 | 51 | 1.4 | 5.9 | 5 |
| GOL(50 mg) + MTX | 89 | 52 | 1.4 | 4.5 | |||
| GOL(100 mg) + MTX | 89 | 50 | 1.4 | 6.7 | |||
| Placebo + MTX | 133 | 52 | 1.3 | 6.5 | |||
| Emery [33]* | GOL(100 mg) + PLACEBO | 24 | 159 | 48.2 | 1.6 | 4.1 | 5 |
| GOL(50 mg) + MTX | 159 | 50.9 | 1.5 | 3.5 | |||
| GOL(100 mg) + MTX | 159 | 50.2 | 1.5 | 3.6 | |||
| Placebo + MTX | 160 | 48.6 | 1.5 | 2.9 | |||
| Kremer [34] | GOL(50 mg) | 24 | 128 | NR | 1.6 | 7.4 | 5 |
| GOL(50 mg) + MTX | 129 | 1.5 | 8.1 | ||||
| GOL(100 mg) | 129 | 1.5 | 8.4 | ||||
| GOL(100 mg) + MTX | 128 | 1.5 | 9.4 | ||||
| Placebo + MTX | 129 | 1.5 | 7.4 | ||||
| Tanaka [35] | GOL(50 mg) + MTX | 86 | 50.4 | 1 | 8.8 | 5 | |
| GOL(100 mg) + MTX | 87 | 50 | 0.9 | 8.1 | |||
| Placebo + MTX | 88 | 51.1 | 1 | 8.7 | |||
| Maini [36] | INF(3 mg/kg/4 weeks) + MTX | 30 | 86 | 56 | 1.8 | 8.4 | 5 |
| ATTRACT | INF(3 mg/kg/4 weeks) + MTX | 86 | 51 | 1.8 | 7.2 | ||
| INF(10 mg/kg/8 weeks) + MTX | 81 | 55 | 1.8 | 9 | |||
| INF(10 mg/kg/4 weeks) + MTX | 81 | 52 | 1.5 | 8.7 | |||
| Placebo + MTX | 88 | 51 | 1.8 | 8.9 | |||
| Clair [37] ASPIRE* |
INF(3 mg/kg) + MTX | 54 | 373 | 51 | 1.5 | 0.8 | 3 |
| INF(6 mg/kg) + MTX | 378 | 50 | 1.5 | 0.9 | |||
| Placebo + MTX | 298 | 50 | 1.5 | 0.9 | |||
| Westhovens [38] START |
INF(3 mg/kg) + MTX | 22 | 361 | 53 | 1.5 | 7.8 | 5 |
| INF(10 mg/kg) + MTX | 360 | 52 | 1.5 | 6.3 | |||
| Placebo + MTX | 363 | 52 | 1.5 | 8.4 | |||
| Edwards [39] | RTX(2 × 500 mg) | 24 | 40 | 54 | 1.8 | 12 | 3 |
| RTX(2 × 500 mg) + cyclo. | 41 | 53 | 9 | ||||
| RTX(2 × 500 mg) + MTX | 40 | 54 | 10 | ||||
| Placebo + MTX | 40 | 54 | 11 | ||||
| Emery [40] DANCER |
RTX(2 × 500 mg) + MTX | 24 | 123 | 51.4 | 1.7 | 11.1 | 5 |
| RTX(2 × 1,000 mg) + MTX | 122 | 51.1 | 1.8 | 10.8 | |||
| Placebo + MTX | 123 | 51.1 | 1.7 | 9.3 | |||
| Emery [41] SERRENE |
RTX(2 × 500 mg) + MTX | 168 | NR | NR | 7.1 | 3 | |
| RTX(2 × 1,000 mg) + MTX | 172 | 6.61 | |||||
| Placebo + MTX | 172 | 7.48 | |||||
| Tak [42] IMAGE* |
RTX(2 × 500 mg) + MTX | 249 | NR | NR | 0.99 | 5 | |
| RTX(2 × 1,000 mg) + MTX | 250 | 0.92 | |||||
| Placebo + MTX | 249 | 0.91 | |||||
| Smolen [43] OPTION |
TCL(8 mg/kg) + MTX | 24 | 205 | 50.8 | 1.6 | 7.5 | 5 |
| TCL(4 mg/kg) +MTX | 214 | 51.4 | 1.6 | 7.4 | |||
| Placebo + MTX | 204 | 50.6 | 1.5 | 7.8 | |||
| Genovese [44] TOWARD |
TCL(8 mg/kg) + DMARD | 24 | 805 | 53 | 1.5 | 9.8 | 5 |
| Placebo + DMARD | 415 | 54 | 1.5 | 9.8 | |||
| Yazici [45] | TCL(8 mg/kg) + DMARD | 409 | 55.2 | NA | 8.62 | 3 | |
| ROSE | Placebo + DMARD | 207 | 55.8 | NA | 8.52 | ||
| Weinblatt [46] | ABT(10 mg/kg) + MTX | 318 | 51.4 | 1.5 | 1.9 | 5 | |
| ADL + MTX | 328 | 51 | 1.5 | 1.7 | |||
| Schiff [47] ATTEST |
ABT(10 mg/kg) + MTX | 52 | 156 | 49 | 1.8 | 7.9 | 5 |
| INF (3 mg/kg) + MTX | 165 | 49.1 | 1.7 | 7.3 | |||
| Placebo + MTX | 110 | 49.4 | 1.8 | 8.4 | |||
| Yoo [1] | INF-biosimilar(3 mg) + MTX | 30 | 304 | 50 | 1.6 | NR | |
| PLANETRA | INF(3 mg) + MTX | 302 | 50 | 1.6 |
ABT abatacept, ADA adalimumab, CRT certolizumab pegol, ETN etanercept, GOL golimumab, INF infliximab, RTX rituximab, TCL tocilizumab. Bold letters indicate the treatment arms included in the meta-analysis. * Studies with MTX-naïve or csDMARD-naïve patients
Out of the 36 RA trials included in this analysis, eight studies applied study drugs to MTX-naïve patients [16, 21, 24, 30, 31, 33, 37, 42], and one study on csDMARD-naïve patients [23]. The rest of the studies involved patients with prior inadequate response to csDMARDs.
In some abatacept, rituximab, tocilizumab and golimumab studies [13, 15, 34, 40, 45], patients were not excluded if previously treated with biologicals prior to the study. Since the share of patients who were treated with biologicals before was relatively low in these studies, we included them in the meta-analysis. However, studies where all patients were previously treated with biologicals [12] or all patients gave prior inadequate response to biologicals [9–11] were not included in our meta-analysis.
Most of the RCTs reported ACR20 and ACR50 response at week 24. In contrast, the infliximab-biosimilar RCT reported results at week 30. However, patients in the infliximab-biosimilar study received the same number of infusions as patients in the infliximab trials.
Mixed treatment comparison: efficacy and safety
Efficacy
Out of the 36 RA trials identified by our search, 34 reported results for ACR20 response at week 24, and 35 reported ACR50 response at week 24. Weinblatt et al. [15] reported study results only on safety and Westhovens et al. [16] did not report ACR20 response. Data for 15,044 patients for ACR20 response and 14,535 for ACR50 response were included in the analysis.
All biological drugs were found to be superior to placebo regarding ACR20 and ACR50 responses. The results are presented in Table 2. On the ACR20 endpoint, certolizumab pegol showed the highest odds ratio compared to placebo, OR 7.69 [95 % CI 3.69–14.26], followed by abatacept OR 3.7 [95 % CI 2.17–6.06], tocilizumab OR 3.69 [1.87–6.62], and infliximab-biosimilar OR 3.47 [95 % CI 0.85–9.7].
Table 2.
The efficacy and safety of biological and biosimilar treatment of RA compared to placebo, the results of the mixed treatment comparison
| Treatment | ACR20 at week 24 OR [95 % CI] | ACR50 at week 24 OR [95 % CI] | Serious AEs OR [95 % CI] |
|---|---|---|---|
| Abatacept vs placebo | 3.7 [2.17–6.06] | 3.64 [2.25–5.76] | 0.91 [0.64–1.18] |
| Adalimumab vs placebo | 2.92 [1.9–4.36] | 3.48 [2.27–5.22] | 0.85 [0.57–1.19] |
| Certolizumab pegol vs placebo | 7.69 [3.69–14.26] | 8.46 [3.74–16.82] | 2.02 [1.16–3.3] |
| Etanercept vs placebo | 2.72 [1.47–4.71] | 3.07 [1.68–5.38] | 0.84 [0.48–1.34] |
| Golimumab vs placebo | 2.8 [1.5–4.83] | 2.83 [1.48–4.98] | 1.63 [0.74–3.14] |
| Infliximab vs placebo | 2.71 [1.51–4.54] | 3.3 [1.82–5.66] | 1.15 [0.77–1.64] |
| Rituximab vs placebo | 2.81 [1.5–4.86] | 3.19 [1.66–5.62] | 1.18 [0.7–1.87] |
| Tocilizumab vs placebo | 3.69 [1.87–6.62] | 5.57 [2.77–10.09] | 1.46 [0.89–2.27] |
| Infliximab-biosimilar vs placebo | 3.47 [0.85–9.7] | 4.06 [1.01–11.54] | 1.87 [0.74–3.84] |
For ACR50 response, certolizumab pegol showed the highest OR compared to placebo OR 8.46 [3.74–16.82], followed by tocilizumab OR 5.57 [95 % CI 2.77–10.09], and infliximab-biosimilar OR 4.06 [95 % CI 1.01–11.54].
The results of pairwise comparison did not show significant differences between the efficacy of infliximab-biosimilar and the other biologicals in terms of ACR20 or ACR50 response at week 24 (see Fig. 1).
Fig. 1.
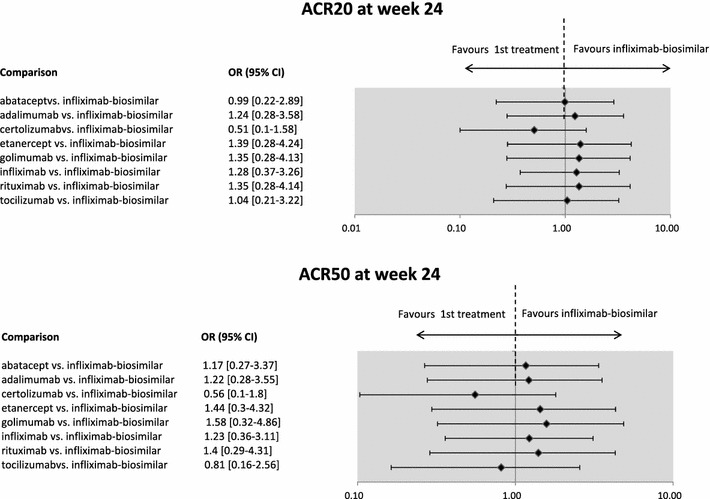
Efficacy results of the mixed treatment comparison of infliximab-biosimilar versus other biologicals in RA–ACR20 and ACR50 response at week 24. The infliximab-biosimilar study presented results for week 30. The figure presents odds ratios (OR) between treatments. If the point estimate is greater than 1, then the biosimilar treatment is more effective (although not necessarily statistically significantly more effective) compared to the originator biologicals. Credibility intervals provide information on whether the difference between treatments is statistically significant. If the CI contains the value 1, the difference is not statistically significant
Safety and tolerability
Thirty studies reported the occurrence of serious adverse events at week 24. Data for 14,708 patients were included in the analysis.
Etanercept had the lowest OR 0.84 [95 % CI 0.48–1.34], followed by adalimumab OR 0.85 [95 % CI 0.57–1.19] and abatacept 0.91 [95 % CI 0.64–1.18]. For infliximab-biosimilar the OR was 1.87 [95 % CI 0.74–3.84], while for the originator infliximab the OR was 1.15 [0.77–1.64]. In this endpoint, the lower ORs are in favor of biologicals, as the lower OR, the lower the chance of the occurrence of serious adverse events (AEs) compared to placebo. Certoluzimab pegol was found to have significantly worse safety profile than placebo OR 2.02 [1.16–3.3]. For the rest of the treatments, the difference between placebo and biological treatments was not significant.
Regarding the pairwise comparison of the treatments, we found no significant difference in the safety of infliximab-biosimilar and other biological treatments (see Fig. 2).
Fig. 2.
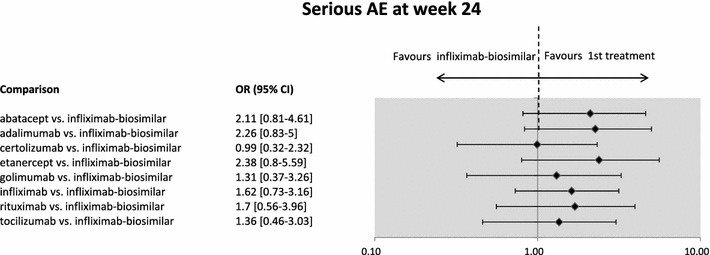
Safety: Serious adverse events (AEs) in RA infliximab-biosimilar versus other biologicals at week 24. The infliximab-biosimilar study presented results for week 30. The figure presents odds ratios (OR) between treatments. If the point estimate is lower than 1, then the biosimilar treatment is safer (although not necessarily statistically significantly safer). Credibility intervals provide information on whether the difference between treatments is statistically significant. If the CI contains the value 1, the difference is not statistically significant
Discussion
The efficacy and safety of infliximab-biosimilar was compared only to infliximab in a non-inferiority RCT. This study was aimed to carry out an indirect meta-analysis and compare the efficacy and safety of infliximab-biosimilar to each biological available for the treatment of RA. We used mixed-treatment comparison, which in contrast to traditional methods allows the pairwise comparison of the treatments with different comparators. This was necessary, as infliximab-biosimilar was only compared to the originator infliximab treatment, while the other biologicals were usually compared to placebo, and moreover, head-to-head comparisons were rare.
Our study, involving altogether 15,044 RA patients, has demonstrated that there is no significant difference in the efficacy and safety of infliximab-biosimilar and other biological drugs in RA.
Thus far, several reviews have been published synthesizing the findings on direct comparisons of a single biological agent combined with sDMARDs and sDMARDs alone [48–57]. These studies come to the same conclusion as ours, that biologicals (including the originator infliximab) are significantly more efficient treatments compared to csDMARDs. Only the latest by Nam et al. [48] involved infliximab-biosimilar in the review; however, its efficacy and safety was not compared to other biologicals [48].
Indirect comparisons published previously have not considered infliximab-biosimilar, but the originator infliximab.
Some studies that carried out indirect comparison of the efficacy of different biologicals in RA found that the difference between infliximab and other biologicals were not statistically significant [4, 58–61], which supports our results for infliximab and infliximab-biosimilar.
However, some studies found significant differences between infliximab and certolizumab pegol in favor of certolizumab pegol [62–64]. Schmitz et al.'s study [63] involving 16 RCTs with patients who produced an inadequate response to MTX, and Turkstra et al.'s study [64] involving 27 RCTs with patients who produced an inadequate response to MTX found certolizumab pegol to be superior to infliximab in ACR20 and ACR50 responses at month 6. In our study, we also found that certolizumab pegol gave the highest ORs in terms of ACR20 and ACR50 response, but the difference between cetrolizumab pegol and infliximab and infliximab-biosimilar therapies were not statistically significant. In contrast to our study, the meta-analyses of Turkstra et al. [64] and Schmitz et al. [63] did not include the results of a recently published RCT with certolizumab pegol [27]. Furthermore, they also involved studies where patients were enrolled after MTX or other csDMARD failure, and studies where biologicals were administered in monotherapy. Turkstra et al. [64] included only two RCTs for infliximab (one small and one large), while we included five. These differences might partly explain the contradictory results.
Nevertheless, the outstanding result of certolizumab pegol deserves further considerations. In two of the certolizumab pegol RCTs (RAPID 1 and RAPID 2), patients who did not show an ACR20 response at both weeks 12 and 14 were to be withdrawn from the study [25, 26]. This design differs from the first biological RCTs in RA, and the early evaluation of efficacy reflects the EMA guideline (2003), which suggests to consider the principle as follows: “since it would be unethical to retain a patient with rheumatoid arthritis on placebo treatment indefinitely, the duration of placebo control must necessarily be limited. Depending on the severity and the activity of the disease, 3–6 months is acceptable” [65]. As a consequence, we observe an extremely high rate of early withdrawal in the placebo group in these certolizumab pegol trials [66]. The high withdrawal rates resulted in the high OR rate of certolizumab pegol compared to placebo.
Launois et al. [66] also doubt the comparability of the certolizumab pegol studies, due to the low ACR responses to placebo mentioned as a limitation in their study. However, they do not discuss that the low placebo response rate (as well as high ORs) and the extremely high rate of early withdrawal in the placebo group are in correlation.
Regarding safety and tolerability, some of the studies found significant differences between treatments, e.g., in favor of etanercept [58] or in favor of abatacept compared to a combined group of biologicals [61]. However, these studies examined a tolerability endpoint, namely the withdrawal of therapy due to adverse events, while we examined safety in terms of the occurrence of serious adverse events. The unfavorable safety results for certolizumab pegol can be also explained by the different study design and the extremely high withdrawal rates in the placebo group (see above).
We have to acknowledge some limitations of our study. Due to the diversity of study designs regarding patient population, we pooled the evidence from studies with DMARD-naïve patients (i.e., early aggressive treatment) and patients with inadequate response to DMARDs. Yet, Brodszky et al. [4] found a significant positive association with the disease duration efficacy of the drug. Our reasons to pool evidence from studies with different study populations were twofold: (1) some of the biologicals are also indicated for patients not previously treated with MTX or other csDMARDs, in the case of severe, active and progressive disease; (2) excluding studies with DMARD-naïve patients would have resulted in the exclusion of studies with high number of patients, which would result in biased results. For example, three of the four etanercept studies involved DMARD-naïve patients (N = 1,624), while only one with low sample population studied the efficacy of etanercept on patients who did not respond to previous treatments with csDMARD (N = 89).
Also, only combination therapy with csDMARDs was examined in this study. Furthermore, it is to be highlighted that the infliximab-biosimilar study reports efficacy and safety results at week 30, 6 weeks later than most of the studies included in the analysis, which report the results at week 24. However, patients in the infliximab-biosimilar study received the same number of injections as patients in the infliximab studies.
We acknowledge that estimated ORs might vary depending on the designs of the mixed treatment comparison (e.g., whether monotherapy studies, or studies with DMARD-naïve patients, are included); however, we found that the main conclusions, i.e., the similar efficacy and safety of biologicals, did not change. Also, the analysis was limited to the endpoints selected; however, the examination of other safety and efficacy endpoints may be of interest as well.
To conclude, according to our knowledge, this is the first study in RA that includes infliximab-biosimilar in a meta-analysis, and compares it to the originator biologicals that are approved for use in European clinical practice. Our study, involving data for 15,044 RA patients, has demonstrated the similar efficacy and safety profile of infliximab-biosimilar treatment compared to other biologicals. The results might support clinical as well as financial decision making, providing evidence on the similar efficacy and safety of infliximab-biosimilar and other biologicals indicated in RA.
Acknowledgments
The study was supported by an unrestricted grant from EGIS Pharmaceuticals and the Center for Public Affairs Studies Foundation.
Footnotes
The product information details can be found at http://www.ema.europa.eu/ema/.
Also when the patient is intolerant to previous therapy with tumour-necrosis-factor (TNF) antagonists.
According to the definition of EMA, “A biosimilar medicine is a medicine which is similar to a biological medicine that has already been authorized (the ‘biological reference medicine’). The active substance of a biosimilar medicine is similar to that of the biological reference medicine. Biosimilar and biological reference medicines are used in general at the same dose to treat the same disease.”
Anakinra is also registered for the treatment of RA by the EMA; however, its utilization has not spread in the clinical practice of CEE countries where infliximab-biosimilar is marketed. Thus, anakinra was not included in the meta-analysis.
Adalimuab, certolizumab, etanercept and tocilizumab can be administered as monotherapy, in the case of intolerance to methotrexate.
(arthritis. rheumatoid"[MeSH Terms]) AND (abatacept OR adalimumab OR certolizumab pegol OR golimumab OR infliximab OR etanercept OR rituximab OR tocilizumab) AND ((randomized controlled trial[pt] OR controlled clinical trial[pt] OR randomized[tiab] OR placebo[tiab] OR "clinical trials as topic"[MeSH Terms:noexp] OR randomly[tiab] OR trial[ti]) NOT ("animals"[MeSH Terms] NOT "humans"[MeSH Terms])) AND ("2009/11/01"[PDAT]: "2013/08/20"[PDAT]).
Moreland et al. [31] was a 2-year, randomized, double-blind trial with four treatment arms: immediate treatment with MTX plus etanercept, immediate oral triple therapy (MTX plus sulfasalazine plus hydroxychloroquine), or step-up from MTX monotherapy to one of the combination therapies (MTX plus etanercept or MTX plus sulfasalazine plus hydroxychloroquine) at week 24. Since before week 24, treatment arms with MTX + etanercept and MTX alone were selected to be included in this meta-analysis.
References
- 1.Yoo, D.H., Hrycaj, P., Miranda, P., Ramiterre, E., Piotrowski, M., Shevchuk, S., Kovalenko, V., Prodanovic, N., Abello-Banfi, M., Gutierrez-Urena, S., Morales-Olazabal, L., Tee, M., Jimenez, R., Zamani, O., Lee, S.J., Kim, H., Park, W., Muller-Ladner, U.: A randomised, double-blind, parallel-group study to demonstrate equivalence in efficacy and safety of CT-P13 compared with innovator infliximab when coadministered with methotrexate in patients with active rheumatoid arthritis: the PLANETRA study. Ann Rheum Dis(ARD Online First, published on May 21, 2013 as doi:10.1136/annrheumdis-2012-203090. Downloaded from ard.bmj.com on May 23, 2013–Published by group.bmj.com) (2013) [DOI] [PMC free article] [PubMed]
- 2.Felson DT, Anderson JJ, Boers M, Bombardier C, Chernoff M, Fried B, Furst D, Goldsmith C, Kieszak S, Lightfoot R, et al. The American College of Rheumatology preliminary core set of disease activity measures for rheumatoid arthritis clinical trials. The Committee on Outcome Measures in Rheumatoid Arthritis Clinical Trials. Arthritis Rheum. 1993;36(6):729–740. doi: 10.1002/art.1780360601. [DOI] [PubMed] [Google Scholar]
- 3.Higgins, J.P.T., Green, S.: Cochrane handbook for systematic reviews of interventions Version 5.0.2 [updated September 2009]. Cochrane Collab (2009)
- 4.Brodszky V. Effectiveness of biological treatments based on ACR70 response in rheumatoid arthritis: indirect comparison and meta-regression using Bayes-model. Orv. Hetil. 2011;152(23):919–928. doi: 10.1556/OH.2011.29138. [DOI] [PubMed] [Google Scholar]
- 5.Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, McQuay HJ. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control. Clin. Trials. 1996;17(1):1–12. doi: 10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]
- 6.Olivo SA, Macedo LG, Gadotti IC, Fuentes J, Stanton T, Magee DJ. Scales to assess the quality of randomized controlled trials: a systematic review. Phys. Ther. 2008;88(2):156–175. doi: 10.2522/ptj.20070147. [DOI] [PubMed] [Google Scholar]
- 7.Ades AE, Sculpher M, Sutton A, Abrams K, Cooper N, Welton N, Lu G. Bayesian methods for evidence synthesis in cost-effectiveness analysis. Pharmacoeconomics. 2006;24(1):1–19. doi: 10.2165/00019053-200624010-00001. [DOI] [PubMed] [Google Scholar]
- 8.Lu G, Ades AE. Combination of direct and indirect evidence in mixed treatment comparisons. Stat. Med. 2004;23(20):3105–3124. doi: 10.1002/sim.1875. [DOI] [PubMed] [Google Scholar]
- 9.Cohen SB, Emery P, Greenwald MW, Dougados M, Furie RA, Genovese MC, Keystone EC, Loveless JE, Burmester GR, Cravets MW, Hessey EW, Shaw T, Totoritis MC. Rituximab for rheumatoid arthritis refractory to anti-tumor necrosis factor therapy: results of a multicenter, randomized, double-blind, placebo-controlled, phase III trial evaluating primary efficacy and safety at twenty-four weeks. Arthritis Rheum. 2006;54(9):2793–2806. doi: 10.1002/art.22025. [DOI] [PubMed] [Google Scholar]
- 10.Emery P, Keystone E, Tony HP, Cantagrel A, van Vollenhoven R, Sanchez A, Alecock E, Lee J, Kremer J. IL-6 receptor inhibition with tocilizumab improves treatment outcomes in patients with rheumatoid arthritis refractory to anti-tumour necrosis factor biologicals: results from a 24-week multicentre randomised placebo-controlled trial. Ann. Rheum. Dis. 2008;67(11):1516–1523. doi: 10.1136/ard.2008.092932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Genovese MC, Becker JC, Schiff M, Luggen M, Sherrer Y, Kremer J, Birbara C, Box J, Natarajan K, Nuamah I, Li T, Aranda R, Hagerty DT, Dougados M. Abatacept for rheumatoid arthritis refractory to tumor necrosis factor alpha inhibition. N. Engl. J. Med. 2005;353(11):1114–1123. doi: 10.1056/NEJMoa050524. [DOI] [PubMed] [Google Scholar]
- 12.Smolen JS, Kay J, Doyle MK, Landewe R, Matteson EL, Wollenhaupt J, Gaylis N, Murphy FT, Neal JS, Zhou Y, Visvanathan S, Hsia EC, Rahman MU. Golimumab in patients with active rheumatoid arthritis after treatment with tumour necrosis factor alpha inhibitors (GO-AFTER study): a multicentre, randomised, double-blind, placebo-controlled, phase III trial. Lancet. 2009;374(9685):210–221. doi: 10.1016/S0140-6736(09)60506-7. [DOI] [PubMed] [Google Scholar]
- 13.Kremer JM, Westhovens R, Leon M, Di Giorgio E, Alten R, Steinfeld S, Russell A, Dougados M, Emery P, Nuamah IF, Williams GR, Becker JC, Hagerty DT, Moreland LW. Treatment of rheumatoid arthritis by selective inhibition of T-cell activation with fusion protein CTLA4Ig. N. Engl. J. Med. 2003;349(20):1907–1915. doi: 10.1056/NEJMoa035075. [DOI] [PubMed] [Google Scholar]
- 14.Kremer JM, Genant HK, Moreland LW, Russell AS, Emery P, Abud-Mendoza C, Szechinski J, Li T, Ge Z, Becker JC, Westhovens R. Effects of abatacept in patients with methotrexate-resistant active rheumatoid arthritis: a randomized trial. Ann. Intern. Med. 2006;144(12):865–876. doi: 10.7326/0003-4819-144-12-200606200-00003. [DOI] [PubMed] [Google Scholar]
- 15.Weinblatt M, Combe B, Covucci A, Aranda R, Becker JC, Keystone E. Safety of the selective costimulation modulator abatacept in rheumatoid arthritis patients receiving background biologic and nonbiologic disease-modifying antirheumatic drugs: a one-year randomized, placebo-controlled study. Arthritis Rheum. 2006;54(9):2807–2816. doi: 10.1002/art.22070. [DOI] [PubMed] [Google Scholar]
- 16.Westhovens R, Robles M, Ximenes AC, Nayiager S, Wollenhaupt J, Durez P, Gomez-Reino J, Grassi W, Haraoui B, Shergy W, Park SH, Genant H, Peterfy C, Becker JC, Covucci A, Helfrick R, Bathon J. Clinical efficacy and safety of abatacept in methotrexate-naive patients with early rheumatoid arthritis and poor prognostic factors. Ann. Rheum. Dis. 2009;68(12):1870–1877. doi: 10.1136/ard.2008.101121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Takeuchi T, Matsubara T, Nitobe T, Suematsu E, Ohta S, Honjo S, Abe T, Yamamoto A, Miyasaka N. Phase II dose-response study of abatacept in Japanese patients with active rheumatoid arthritis with an inadequate response to methotrexate. Mod. Rheumatol. 2013;23(2):226–235. doi: 10.3109/s10165-012-0668-z. [DOI] [PubMed] [Google Scholar]
- 18.Furst DE, Schiff MH, Fleischmann RM, Strand V, Birbara CA, Compagnone D, Fischkoff SA, Chartash EK. Adalimumab, a fully human anti tumor necrosis factor-alpha monoclonal antibody, and concomitant standard antirheumatic therapy for the treatment of rheumatoid arthritis: results of STAR (Safety Trial of Adalimumab in Rheumatoid Arthritis) J. Rheumatol. 2003;30(12):2563–2571. [PubMed] [Google Scholar]
- 19.Keystone EC, Kavanaugh AF, Sharp JT, Tannenbaum H, Hua Y, Teoh LS, Fischkoff SA, Chartash EK. Radiographic, clinical, and functional outcomes of treatment with adalimumab (a human anti-tumor necrosis factor monoclonal antibody) in patients with active rheumatoid arthritis receiving concomitant methotrexate therapy: a randomized, placebo-controlled, 52-week trial. Arthritis Rheum. 2004;50(5):1400–1411. doi: 10.1002/art.20217. [DOI] [PubMed] [Google Scholar]
- 20.Weinblatt ME, Keystone EC, Furst DE, Moreland LW, Weisman MH, Birbara CA, Teoh LA, Fischkoff SA, Chartash EK. Adalimumab, a fully human anti-tumor necrosis factor alpha monoclonal antibody, for the treatment of rheumatoid arthritis in patients taking concomitant methotrexate: the ARMADA trial. Arthritis Rheum. 2003;48(1):35–45. doi: 10.1002/art.10697. [DOI] [PubMed] [Google Scholar]
- 21.Breedveld FC, Weisman MH, Kavanaugh AF, Cohen SB, Pavelka K, van Vollenhoven R, Sharp J, Perez JL, Spencer-Green GT. The PREMIER study: a multicenter, randomized, double-blind clinical trial of combination therapy with adalimumab plus methotrexate versus methotrexate alone or adalimumab alone in patients with early, aggressive rheumatoid arthritis who had not had previous methotrexate treatment. Arthritis Rheum. 2006;54(1):26–37. doi: 10.1002/art.21519. [DOI] [PubMed] [Google Scholar]
- 22.Kim H, Lee S, Song Y, Yoo D, Koh E, Yoo B, Luo A. A randomized, double-blind, placebo-controlled, phase III study of the human anti-tumor necrosis factor antibody adalimumab administered as subcutaneous injections in Korean rheumatoid arthritis patients treated with methotrexate. APLAR Journal of Rheumatology. 2007;10(1):9–16. doi: 10.1111/j.1479-8077.2007.00248.x. [DOI] [Google Scholar]
- 23.Detert J, Bastian H, Listing J, Weiss A, Wassenberg S, Liebhaber A, Rockwitz K, Alten R, Kruger K, Rau R, Simon C, Gremmelsbacher E, Braun T, Marsmann B, Hohne-Zimmer V, Egerer K, Buttgereit F, Burmester GR. Induction therapy with adalimumab plus methotrexate for 24 weeks followed by methotrexate monotherapy up to week 48 versus methotrexate therapy alone for DMARD-naive patients with early rheumatoid arthritis: HIT HARD, an investigator-initiated study. Ann. Rheum. Dis. 2013;72(6):844–850. doi: 10.1136/annrheumdis-2012-201612. [DOI] [PubMed] [Google Scholar]
- 24.Kavanaugh A, Fleischmann RM, Emery P, Kupper H, Redden L, Guerette B, Santra S, Smolen JS. Clinical, functional and radiographic consequences of achieving stable low disease activity and remission with adalimumab plus methotrexate or methotrexate alone in early rheumatoid arthritis: 26-week results from the randomised, controlled OPTIMA study. Ann. Rheum. Dis. 2013;72(1):64–71. doi: 10.1136/annrheumdis-2011-201247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Keystone E, Heijde D, Mason D, Jr, Landewe R, Vollenhoven RV, Combe B, Emery P, Strand V, Mease P, Desai C, Pavelka K. Certolizumab pegol plus methotrexate is significantly more effective than placebo plus methotrexate in active rheumatoid arthritis: findings of a fifty-two-week, phase III, multicenter, randomized, double-blind, placebo-controlled, parallel-group study. Arthritis Rheum. 2008;58(11):3319–3329. doi: 10.1002/art.23964. [DOI] [PubMed] [Google Scholar]
- 26.Smolen J, Landewe RB, Mease P, Brzezicki J, Mason D, Luijtens K, van Vollenhoven RF, Kavanaugh A, Schiff M, Burmester GR, Strand V, Vencovsky J, van der Heijde D. Efficacy and safety of certolizumab pegol plus methotrexate in active rheumatoid arthritis: the RAPID 2 study. A randomised controlled trial. Ann. Rheum. Dis. 2009;68(6):797–804. doi: 10.1136/ard.2008.101659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Choy E, McKenna F, Vencovsky J, Valente R, Goel N, Vanlunen B, Davies O, Stahl HD, Alten R. Certolizumab pegol plus MTX administered every 4 weeks is effective in patients with RA who are partial responders to MTX. Rheumatology (Oxford) 2012;51(7):1226–1234. doi: 10.1093/rheumatology/ker519. [DOI] [PubMed] [Google Scholar]
- 28.Weinblatt ME, Kremer JM, Bankhurst AD, Bulpitt KJ, Fleischmann RM, Fox RI, Jackson CG, Lange M, Burge DJ. A trial of etanercept, a recombinant tumor necrosis factor receptor:Fc fusion protein, in patients with rheumatoid arthritis receiving methotrexate. N. Engl. J. Med. 1999;340(4):253–259. doi: 10.1056/NEJM199901283400401. [DOI] [PubMed] [Google Scholar]
- 29.Klareskog L, van der Heijde D, de Jager JP, Gough A, Kalden J, Malaise M, Martin Mola E, Pavelka K, Sany J, Settas L, Wajdula J, Pedersen R, Fatenejad S, Sanda M. Therapeutic effect of the combination of etanercept and methotrexate compared with each treatment alone in patients with rheumatoid arthritis: double-blind randomised controlled trial. Lancet. 2004;363(9410):675–681. doi: 10.1016/S0140-6736(04)15640-7. [DOI] [PubMed] [Google Scholar]
- 30.Emery P, Breedveld FC, Hall S, Durez P, Chang DJ, Robertson D, Singh A, Pedersen RD, Koenig AS, Freundlich B. Comparison of methotrexate monotherapy with a combination of methotrexate and etanercept in active, early, moderate to severe rheumatoid arthritis (COMET): a randomised, double-blind, parallel treatment trial. Lancet. 2008;372(9636):375–382. doi: 10.1016/S0140-6736(08)61000-4. [DOI] [PubMed] [Google Scholar]
- 31.Moreland LW, O’Dell JR, Paulus HE, Curtis JR, Bathon JM, St Clair EW, Bridges SL, Jr, Zhang J, McVie T, Howard G, van der Heijde D, Cofield SS. A randomized comparative effectiveness study of oral triple therapy versus etanercept plus methotrexate in early aggressive rheumatoid arthritis: the treatment of Early Aggressive Rheumatoid Arthritis Trial. Arthritis Rheum. 2012;64(9):2824–2835. doi: 10.1002/art.34498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Keystone EC, Genovese MC, Klareskog L, Hsia EC, Hall ST, Miranda PC, Pazdur J, Bae SC, Palmer W, Zrubek J, Wiekowski M, Visvanathan S, Wu Z, Rahman MU. Golimumab, a human antibody to tumour necrosis factor alpha given by monthly subcutaneous injections, in active rheumatoid arthritis despite methotrexate therapy: the GO-FORWARD Study. Ann. Rheum. Dis. 2009;68(6):789–796. doi: 10.1136/ard.2008.099010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Emery P, Fleischmann RM, Moreland LW, Hsia EC, Strusberg I, Durez P, Nash P, Amante EJ, Churchill M, Park W, Pons-Estel BA, Doyle MK, Visvanathan S, Xu W, Rahman MU. Golimumab, a human anti-tumor necrosis factor alpha monoclonal antibody, injected subcutaneously every four weeks in methotrexate-naive patients with active rheumatoid arthritis: twenty-four-week results of a phase III, multicenter, randomized, double-blind, placebo-controlled study of golimumab before methotrexate as first-line therapy for early-onset rheumatoid arthritis. Arthritis Rheum. 2009;60(8):2272–2283. doi: 10.1002/art.24638. [DOI] [PubMed] [Google Scholar]
- 34.Kremer J, Ritchlin C, Mendelsohn A, Baker D, Kim L, Xu Z, Han J, Taylor P. Golimumab, a new human anti-tumor necrosis factor alpha antibody, administered intravenously in patients with active rheumatoid arthritis: forty-eight-week efficacy and safety results of a phase III randomized, double-blind, placebo-controlled study. Arthritis Rheum. 2010;62(4):917–928. doi: 10.1002/art.27348. [DOI] [PubMed] [Google Scholar]
- 35.Tanaka Y, Harigai M, Takeuchi T, Yamanaka H, Ishiguro N, Yamamoto K, Miyasaka N, Koike T, Kanazawa M, Oba T, Yoshinari T, Baker D. Golimumab in combination with methotrexate in Japanese patients with active rheumatoid arthritis: results of the GO-FORTH study. Ann. Rheum. Dis. 2012;71(6):817–824. doi: 10.1136/ard.2011.200317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Maini R, St Clair EW, Breedveld F, Furst D, Kalden J, Weisman M, Smolen J, Emery P, Harriman G, Feldmann M, Lipsky P. Infliximab (chimeric anti-tumour necrosis factor alpha monoclonal antibody) versus placebo in rheumatoid arthritis patients receiving concomitant methotrexate: a randomised phase III trial. ATTRACT Study Group. Lancet. 1999;354(9194):1932–1939. doi: 10.1016/S0140-6736(99)05246-0. [DOI] [PubMed] [Google Scholar]
- 37.St Clair EW, van der Heijde DM, Smolen JS, Maini RN, Bathon JM, Emery P, Keystone E, Schiff M, Kalden JR, Wang B, DeWoody K, Weiss R, Baker D. Combination of infliximab and methotrexate therapy for early rheumatoid arthritis: a randomized, controlled trial. Arthritis Rheum. 2004;50(11):3432–3443. doi: 10.1002/art.20568. [DOI] [PubMed] [Google Scholar]
- 38.Westhovens R, Yocum D, Han J, Berman A, Strusberg I, Geusens P, Rahman MU. The safety of infliximab, combined with background treatments, among patients with rheumatoid arthritis and various comorbidities: a large, randomized, placebo-controlled trial. Arthritis Rheum. 2006;54(4):1075–1086. doi: 10.1002/art.21734. [DOI] [PubMed] [Google Scholar]
- 39.Edwards JC, Szczepanski L, Szechinski J, Filipowicz-Sosnowska A, Emery P, Close DR, Stevens RM, Shaw T. Efficacy of B-cell-targeted therapy with rituximab in patients with rheumatoid arthritis. N. Engl. J. Med. 2004;350(25):2572–2581. doi: 10.1056/NEJMoa032534. [DOI] [PubMed] [Google Scholar]
- 40.Emery P, Fleischmann R, Filipowicz-Sosnowska A, Schechtman J, Szczepanski L, Kavanaugh A, Racewicz AJ, van Vollenhoven RF, Li NF, Agarwal S, Hessey EW, Shaw TM. The efficacy and safety of rituximab in patients with active rheumatoid arthritis despite methotrexate treatment: results of a phase IIB randomized, double-blind, placebo-controlled, dose-ranging trial. Arthritis Rheum. 2006;54(5):1390–1400. doi: 10.1002/art.21778. [DOI] [PubMed] [Google Scholar]
- 41.Emery P, Deodhar A, Rigby WF, Isaacs JD, Combe B, Racewicz AJ, Latinis K, Abud-Mendoza C, Szczepanski LJ, Roschmann RA, Chen A, Armstrong GK, Douglass W, Tyrrell H. Efficacy and safety of different doses and retreatment of rituximab: a randomised, placebo-controlled trial in patients who are biological naive with active rheumatoid arthritis and an inadequate response to methotrexate (Study Evaluating Rituximab’s Efficacy in MTX iNadequate rEsponders (SERENE)) Ann. Rheum. Dis. 2010;69(9):1629–1635. doi: 10.1136/ard.2009.119933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tak PP, Rigby WF, Rubbert-Roth A, Peterfy CG, van Vollenhoven RF, Stohl W, Hessey E, Chen A, Tyrrell H, Shaw TM. Inhibition of joint damage and improved clinical outcomes with rituximab plus methotrexate in early active rheumatoid arthritis: the IMAGE trial. Ann. Rheum. Dis. 2011;70(1):39–46. doi: 10.1136/ard.2010.137703. [DOI] [PubMed] [Google Scholar]
- 43.Smolen JS, Beaulieu A, Rubbert-Roth A, Ramos-Remus C, Rovensky J, Alecock E, Woodworth T, Alten R. Effect of interleukin-6 receptor inhibition with tocilizumab in patients with rheumatoid arthritis (OPTION study): a double-blind, placebo-controlled, randomised trial. Lancet. 2008;371(9617):987–997. doi: 10.1016/S0140-6736(08)60453-5. [DOI] [PubMed] [Google Scholar]
- 44.Genovese MC, McKay JD, Nasonov EL, Mysler EF, da Silva NA, Alecock E, Woodworth T, Gomez-Reino JJ. Interleukin-6 receptor inhibition with tocilizumab reduces disease activity in rheumatoid arthritis with inadequate response to disease-modifying antirheumatic drugs: the tocilizumab in combination with traditional disease-modifying antirheumatic drug therapy study. Arthritis Rheum. 2008;58(10):2968–2980. doi: 10.1002/art.23940. [DOI] [PubMed] [Google Scholar]
- 45.Yazici Y, Curtis JR, Ince A, Baraf H, Malamet RL, Teng LL, Kavanaugh A. Efficacy of tocilizumab in patients with moderate to severe active rheumatoid arthritis and a previous inadequate response to disease-modifying antirheumatic drugs: the ROSE study. Ann. Rheum. Dis. 2012;71(2):198–205. doi: 10.1136/ard.2010.148700. [DOI] [PubMed] [Google Scholar]
- 46.Weinblatt ME, Schiff M, Valente R, van der Heijde D, Citera G, Zhao C, Maldonado M, Fleischmann R. Head-to-head comparison of subcutaneous abatacept versus adalimumab for rheumatoid arthritis: findings of a phase IIIb, multinational, prospective, randomized study. Arthritis Rheum. 2013;65(1):28–38. doi: 10.1002/art.37711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schiff M, Keiserman M, Codding C, Songcharoen S, Berman A, Nayiager S, Saldate C, Li T, Aranda R, Becker JC, Lin C, Cornet PL, Dougados M. Efficacy and safety of abatacept or infliximab vs placebo in ATTEST: a phase III, multi-centre, randomised, double-blind, placebo-controlled study in patients with rheumatoid arthritis and an inadequate response to methotrexate. Ann. Rheum. Dis. 2008;67(8):1096–1103. doi: 10.1136/ard.2007.080002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nam, J.L., Ramiro, S., Gaujoux-Viala, C., Takase, K., Leon-Garcia, M., Emery, P., Gossec, L., Landewe, R., Smolen, J.S., Buch, M.H.: Efficacy of biological disease-modifying antirheumatic drugs: a systematic literature review informing the 2013 update of the EULAR recommendations for the management of rheumatoid arthritis. Ann. Rheum. Dis. 73(3), 516–528 (2014). doi:10.1136/annrheumdis-2013-204577 [DOI] [PubMed]
- 49.Aaltonen KJ, Virkki LM, Malmivaara A, Konttinen YT, Nordstrom DC, Blom M. Systematic review and meta-analysis of the efficacy and safety of existing TNF blocking agents in treatment of rheumatoid arthritis. PLoS ONE. 2012;7(1):e30275. doi: 10.1371/journal.pone.0030275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Blumenauer, B., Judd, M., Cranney, A., Burls, A., Coyle, D., Hochberg, M., Tugwell, P., Wells, G.: Etanercept for the treatment of rheumatoid arthritis. Cochrane Database Syst. Rev. (4), CD004525 (2003). http://www.ncbi.nlm.nih.gov/pubmed/14584021 [DOI] [PubMed]
- 51.Blumenauer, B., Judd, M., Wells, G., Burls, A., Cranney, A., Hochberg, M., Tugwell, P.: Infliximab for the treatment of rheumatoid arthritis. Cochrane Database Syst. Rev. (3), CD003785 (2002). http://www.ncbi.nlm.nih.gov/pubmed/12137712 [DOI] [PMC free article] [PubMed]
- 52.Maxwell, L., Singh, J.A.: Abatacept for rheumatoid arthritis. Cochrane Database Syst. Rev. (4), CD007277 (2009). doi:10.1002/14651858.CD007277.pub2 [DOI] [PMC free article] [PubMed]
- 53.Navarro-Sarabia, F., Ariza-Ariza, R., Hernandez-Cruz, B., Villanueva, I.: Adalimumab for treating rheumatoid arthritis. Cochrane Database Syst. Rev. (3), CD005113 (2005). http://www.ncbi.nlm.nih.gov/pubmed/16034967 [DOI] [PubMed]
- 54.Ruiz Garcia, V., Jobanputra, P., Burls, A., Cabello, J.B., Galvez Munoz, J.G., Saiz Cuenca, E.S., Fry-Smith, A.: Certolizumab pegol (CDP870) for rheumatoid arthritis in adults. Cochrane Database Syst. Rev. (2), CD007649 (2011). doi:10.1002/14651858.CD007649.pub2 [DOI] [PubMed]
- 55.Singh, J.A., Beg, S., Lopez-Olivo, M.A.: Tocilizumab for rheumatoid arthritis. Cochrane Database Syst. Rev. (7), CD008331 (2010). doi:10.1002/14651858.CD008331.pub2 [DOI] [PubMed]
- 56.Singh, J.A., Noorbaloochi, S., Singh, G.: Golimumab for rheumatoid arthritis. Cochrane Database Syst. Rev. (1), CD008341 (2010). doi:10.1002/14651858.CD008341 [DOI] [PMC free article] [PubMed]
- 57.Alonso-Ruiz A, Pijoan JI, Ansuategui E, Urkaregi A, Calabozo M, Quintana A. Tumor necrosis factor alpha drugs in rheumatoid arthritis: systematic review and metaanalysis of efficacy and safety. BMC Musculoskelet Disord. 2008;9:52. doi: 10.1186/1471-2474-9-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Singh JA, Christensen R, Wells GA, Suarez-Almazor ME, Buchbinder R, Lopez-Olivo MA, Ghogomu ET, Tugwell P. A network meta-analysis of randomized controlled trials of biologics for rheumatoid arthritis: a Cochrane overview. CMAJ. 2009;181(11):787–796. doi: 10.1503/cmaj.091391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Devine EB, Alfonso-Cristancho R, Sullivan SD. Effectiveness of biologic therapies for rheumatoid arthritis: an indirect comparisons approach. Pharmacotherapy. 2011;31(1):39–51. doi: 10.1592/phco.31.1.39. [DOI] [PubMed] [Google Scholar]
- 60.Guyot P, Taylor P, Christensen R, Pericleous L, Poncet C, Lebmeier M, Drost P, Bergman G. Abatacept with methotrexate versus other biologic agents in treatment of patients with active rheumatoid arthritis despite methotrexate: a network meta-analysis. Arthritis Res Ther. 2011;13(6):R204. doi: 10.1186/ar3537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hochberg MC, Berry S, Broglio K, Rosenblatt L, Nadkarni A, Trivedi D, Hebden T. Mixed treatment comparison of efficacy and tolerability of biologic agents in patients with rheumatoid arthritis. Curr. Med. Res. Opin. 2013;29(10):1213–1222. doi: 10.1185/03007995.2013.813839. [DOI] [PubMed] [Google Scholar]
- 62.Launois R, Avouac B, Berenbaum F, Blin O, Bru I, Fautrel B, Joubert JM, Sibilia J, Combe B. Comparison of certolizumab pegol with other anticytokine agents for treatment of rheumatoid arthritis: a multiple-treatment Bayesian metaanalysis. J. Rheumatol. 2011;38(5):835–845. doi: 10.3899/jrheum.100665. [DOI] [PubMed] [Google Scholar]
- 63.Schmitz S, Adams R, Walsh CD, Barry M, FitzGerald O. A mixed treatment comparison of the efficacy of anti-TNF agents in rheumatoid arthritis for methotrexate non-responders demonstrates differences between treatments: a Bayesian approach. Ann. Rheum. Dis. 2012;71(2):225–230. doi: 10.1136/annrheumdis-2011-200228. [DOI] [PubMed] [Google Scholar]
- 64.Turkstra E, Ng SK, Scuffham PA. A mixed treatment comparison of the short-term efficacy of biologic disease modifying anti-rheumatic drugs in established rheumatoid arthritis. Curr. Med. Res. Opin. 2011;27(10):1885–1897. doi: 10.1185/03007995.2011.608655. [DOI] [PubMed] [Google Scholar]
- 65.Points to consider on clinical investigation of medicinal products other than NSAIDS for treatment of rheumatoid arthritis. In: (CPMP), C.f.P.M.P. (ed). EMEA, (2003)
- 66.Gulácsi, L., Érsek, K., Péntek, M., Brodszky, V.: A certolizumab pegol hatásossága és biztonságossága rheumatoid arthritisben. [The efficacy and safety of certolizumab pegol in rheumatoid arthritis.] In: Brodszky V. (szerk). A certolizumab pegol kezelés rheumatoid arthritisben, irodalmi áttekintés és egészség-gazdaságtani elemzés. [Certolizumab pegol treatment in rheumatoid arthritis, literature review and health economic evaluation.] Budapest 2011, Budapesti Corvinus Egyetem Egészség-gazdaságtani és Egészségügyi Technológiaelemzési Kutatóközpont, 36–61. (ISBN: 978-963-503-448-2)


