Abstract
Using PCR, we screened 411 ticks from four genera collected in Russia and Kazakhstan for the presence of rickettsiae and ehrlichiae. In Russia, we detected “Rickettsia heilongjiangensis,” Rickettsia sp. strain RpA4, and Ehrlichia muris. In Kazakhstan, we detected Rickettsia sp. strain RpA4 and a rickettsia closely related to Rickettsia aeschlimannii. These agents should be considered in a differential diagnosis of tick-borne infections in these areas.
In Russia, two human tick-transmitted rickettsioses have been described: Siberian tick typhus caused by Rickettsia sibirica (9) and Astrakhan spotted fever due to the as-yet-unnamed Astrakhan fever rickettsia (13). The main vectors of R. sibirica are Dermacentor sp. and Haemaphysalis concinna ticks (9), while the etiological agent of Astrakhan spotted fever is transmitted by Rhipicephalus pumilio (13). Another five spotted fever group (SFG) rickettsiae have recently been found in Russia: Rickettsia slovaca, a species of recognized pathogenicity found in Dermacentor marginatus ticks (12), and four rickettsiae of unknown pathogenicity detected in ticks, namely, Rickettsia sp. strain RpA4, Rickettsia sp. strain DnS14, Rickettsia sp. strain DnS28 (10), and “Candidatus Rickettsia tarasevichiae” (11).
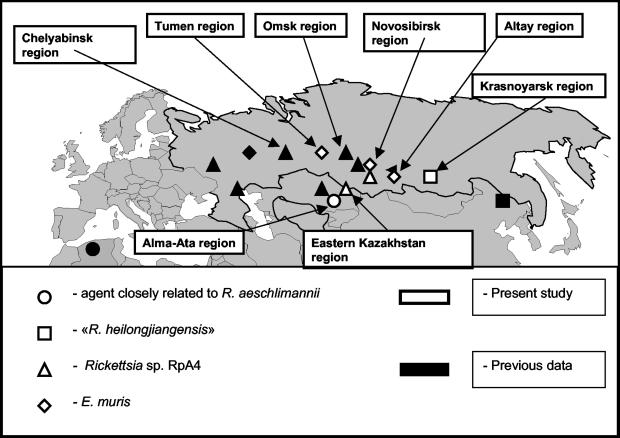
From April to June 2001, we collected 411 adult ticks from four genera (Dermacentor, Haemaphysalis, Hyalomma, and Ixodes) in Russia and Kazakhstan and tested them by PCR for SFG rickettsiae and ehrlichiae (Table 1). Ticks were collected from vegetation in the forest-steppe zone of Russia (the Chelyabinsk, Tumen, Omsk, Novosibirsk, Altay, and Krasnoyarsk regions) and in the steppe zone of Kazakhstan (the Alma-Ata and East Kazakhstan regions) (Fig. 1). Ticks were kept in 70% alcohol until they were further processed. Prior to individual DNA extraction with a QIAamp tissue kit (QIAGEN, Hilden, Germany), ticks were rinsed in distilled water and dried on sterile filter paper, according to the manufacturer's recommendations. In order to detect cross-contamination, sterile water was processed according to the instructions of the QIAamp tissue kit throughout the amplification process. For each tick, a 632-bp fragment of the ompA gene specific for SFG rickettsiae was amplified by PCR and sequenced with the Rr190.70p and Rr190.701n primers (7), and a 1,174-bp fragment of the gltA gene, present in most rickettsiae, was amplified with the CS1d and CS1273r primers (8). Ehrlichial DNA was also detected with the 16EHRD and 16EHRR primers, which amplify a 345-bp fragment of the 16S rRNA gene of all known ehrlichiae (3). PCRs were carried out in a Peltier model PTC-200 thermal cycler (MJ Research, Inc., Watertown, Mass.), as previously described (3, 7, 8). Each PCR included a negative control (distilled water) and a positive control (DNA from Rickettsia montanensis and Ehrlichia canis). All negative controls remained negative. Positive PCR products were purified using a QIAquick PCR purification kit (QIAGEN). Sequencing was performed with the ompA PCR primers, gltA primers CS409d and CS535d (8), and 16S ribosomal DNA-based ehrlichial primers with a dRhodamine terminator cycle sequencing ready reaction kit (Applied Biosystems, Warrington, United Kingdom) and an ABI 3100 PRISM automated sequencer (Applied Biosystems). Sequences were identified by comparison with GenBank entries. Among recognized Rickettsia species, gltA nucleotide similarity values range from 85.5% between Rickettsia canadensis (GenBank accession number U59713) and Rickettsia bellii (U59716) to 99.9% between Rickettsia parkeri (U59732) and R. sibirica (U59734). ompA nucleotide similarity values range from 55.4% between Rickettsia felis (AF210694) and Rickettsia aeschlimannii (U43800) to 98.8% between Rickettsia massiliae (U43799) and Rickettsia rhipicephali (U43803).
TABLE 1.
Detection and identification of SFG Rickettsia sp. and Ehrlichia sp. in ticks collected from Russia and Kazakhstan
| Tick species | Region | No. of rickettsia-positive ticks/no. of ticks tested (%) | Bacterial species identified |
|---|---|---|---|
| H. concinna | Krasnoyarsk, Russia | 1/38 (2.6) | “R. heilongjiangensis” |
| D. marginatus | Novosibirsk, Russia | 3/7 (42.8) | Rickettsia sp. strain RpA4 |
| D. reticulatus | Novosibirsk, Russia | 28/56 (50) | Rickettsia sp. strain RpA4 |
| H. punctata | Alma-Ata, Kazahkstan | 2/36 (5.5) | Rickettsia sp. closely related to R. aeschlimannii |
| Hyalomma asiaticum | Alma-Ata, Kazahkstan | 0/47 | |
| D. marginatus | Eastern Kazahkstan, Kazahkstan | 6/18 (33.3) | Rickettsia sp. strain RpA4 |
| I. persulcatus | Chelyabinsk, Russia | 0/39 | |
| Tumen, Russia | 1/19 (5.3) | E. muris | |
| Omsk, Russia | 0/69 | ||
| Novosibirsk, Russia | 1/23 (4.3) | E. muris | |
| Altay, Russia | 1/39 (2.6) | E. muris | |
| Krasnoyarsk, Russia | 0/20 |
FIG. 1.
Geographic distribution of an agent closely related to R. aeschlimannii, “R. heilongjiangensis,” Rickettsia sp. strain RpA4, and E. muris in Russia and Kazakhstan.
Rickettsial DNA was detected in 64 (15.6%) of the 411 studied ticks. Among these, we found a rickettsia closely related to R. aeschlimannii (98.5 and 99.6% similarity with R. aeschlimannii for the gltA and ompA genes, respectively) in 5.5% of Haemaphysalis punctata ticks collected in the Alma-Ata region of Kazakhstan (Fig. 1). A rickettsia exhibiting 100% gltA and ompA similarity to “R. heilongjiangensis” (2) was identified in 1 of 38 (2.6%) H. concinna ticks collected in the Krasnoyarsk region of Russia. A rickettsia 100% similar to Rickettsia sp. strain RpA4 in both gltA and ompA sequences was detected in 3 of 7 (42.8%) and 6 of 18 (33.3%) Dermacentor marginatus ticks from the Novosibirsk area and eastern Kazakhstan, respectively, and in 28 of 56 (50%) Dermacentor reticulatus ticks from the Novosibirsk area. Ehrlichia muris was identified in 3 of 209 (1.4%) Ixodes persulcatus ticks from the Russian territories of Tumen (1 of 19), Novosibirsk (1 of 23), and Altay (1 of 39).
We detected for the first time a rickettsia closely related to R. aeschlimannii in Eurasia. Given the degree of its gltA (98.5%) and ompA (99.6%) similarities with R. aeschlimannii, this rickettsia may be considered an R. aeschlimannii variant. R. aeschlimannii was described to occur in Hyalomma marginatum ticks collected in Morocco in 1997 (1) and was found in this tick species in Zimbabwe, Mali, and Niger (4). The first documented infections caused by R. aeschlimannii were described for a patient returning to France from Morocco (6) and for a hunter in South Africa (5) in 2000. Cases of mild diseases following tick bites have been reported in the Alma-Ata region since 1949 and have been attributed to R. sibirica despite the absence of biological evidence of such an infection. Since R. aeschlimannii is a proven pathogen and H. punctata may bite humans, some of the cases in Kazakhstan may have been caused by R. aeschlimannii.
“R. heilongjiangensis” was detected in one H. concinna tick. This recently described Rickettsia species (2) has been implicated as a human pathogen in China (15) and in the Russian Far East, where H. concinna ticks are also prevalent (O. Mediannikov, unpublished data). Therefore, “R. heilongjiangensis” may also cause human infection in the Krasnoyarsk region, possibly transmitted by H. concinna bites.
Rickettsia sp. strain RpA4 was found in various territories of Russia and Kazakhstan. This rickettsia was detected in D. reticulatus ticks collected in the territories of Voronezh (the European part of Russia), Omsk, and Novosibirsk (western Siberia) and in Dermacentor niveus from the Karaganda region (central Kazakhstan) (12). The pathogenic role of this rickettsia has not yet been proven. However, we recently observed a patient with a clinical picture identical to that of a patient with an R. slovaca infection, and the only organism found in the tick was Rickettsia sp. strain RpA4 (D. Raoult, unpublished data).
Additionally, we detected E. muris in I. persulcatus for the first time in the Asiatic part of Russia. This bacterium was isolated for the first time from a wild mouse in Japan. E. muris is closely related to Ehrlichia chaffeensis, an agent of human monocytic ehrlichiosis. The pathogenic role of E. muris for humans is as yet unknown (14).
In conclusion, clinicians have to be aware of emerging tick-borne diseases in Russia and Kazakhstan, particularly infections due to R. aeschlimannii and “R. heilongjiangensis.”
Nucleotide sequence accession numbers.
The GenBank accession numbers for the gltA and ompA sequences of the rickettsia closely related to R. aeschlimannii found in the Alma-Ata region are AY259084 and AY259083, respectively.
REFERENCES
- 1.Beati, L., M. Meskini, B. Thiers, and D. Raoult. 1997. Rickettsia aeschlimannii sp. nov., a new spotted fever group rickettsia associated with Hyalomma marginatum ticks. Int. J. Syst. Bacteriol. 47:548-554. [DOI] [PubMed] [Google Scholar]
- 2.Fournier, P.-E., J. S. Dumler, G. Greub, J. Zhang, Y. Wu, and D. Raoult. 2003. Gene sequence-based criteria for the identification of new Rickettsia isolates and description of Rickettsia heilongjiangensis sp. nov. J. Clin. Microbiol. 41:5456-5465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Inokuma, H., K. Ohno, T. Onishi, D. Raoult, and P. Brouqui. 2001. Detection of ehrlichial infection by PCR in dogs from Yamaguchi and Okinawa Prefectures, Japan. J. Vet. Med. Sci. 63:815-817. [DOI] [PubMed] [Google Scholar]
- 4.Parola, P., H. Inokuma, J. L. Camicas, P. Brouqui, and D. Raoult. 2001. Detection and identification of spotted fever group Rickettsiae and Ehrlichiae in African ticks. Emerg. Infect. Dis. 7:1014-1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pretorius, A. M., and R. J. Birtles. 2002. Rickettsia aeschlimannii: a new pathogenic spotted fever group rickettsia, South Africa. Emerg. Infect. Dis. 8:874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Raoult, D., P. E. Fournier, P. Abboud, and F. Caron. 2002. First documented human Rickettsia aeschlimannii infection. Emerg. Infect. Dis. 8:748-749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roux, V., P.-E. Fournier, and D. Raoult. 1996. Differentiation of spotted fever group rickettsiae by sequencing and analysis of restriction fragment length polymorphism of PCR-amplified DNA of the gene encoding the protein rOmpA. J. Clin. Microbiol. 34:2058-2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Roux, V., E. Rydkina, M. Eremeeva, and D. Raoult. 1997. Citrate synthase gene comparison, a new tool for phylogenetic analysis, and its application for the rickettsiae. Int. J. Syst. Bacteriol. 47:252-261. [DOI] [PubMed] [Google Scholar]
- 9.Rudakov, N. V. 1996. Tick-borne rickettsiosis in Russia: epidemiology and current conditions of natural foci, p. 216-220. In J. Kazar and R. Toman (ed.), Proceedings of the 5th International Symposium on Rickettsiae and Rickettsial Diseases. Veda, Bratislava, Slovak Republic.
- 10.Rydkina, E., V. Roux, N. Fetisova, N. Rudakov, M. Gafarova, I. Tarasevich, and D. Raoult. 1999. New rickettsiae in ticks collected in territories of the former Soviet Union. Emerg. Infect. Dis. 5:811-814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shpynov, S., P. E. Fournier, N. Rudakov, and D. Raoult. 2003. “Candidatus Rickettsia tarasevichiae” in Ixodes persulcatus ticks collected in Russia. Ann. N. Y. Acad. Sci. 990:162-172. [DOI] [PubMed] [Google Scholar]
- 12.Shpynov, S., P. Parola, N. Rudakov, I. Samoilenko, M. Tankibaev, I. Tarasevich, and D. Raoult. 2001. Detection and identification of spotted fever group rickettsiae in Dermatocentor ticks from Russia and central Kazakhstan. Eur. J. Clin. Microbiol. Infect. Dis. 20:903-905. [DOI] [PubMed] [Google Scholar]
- 13.Tarasevich, I. V., V. Makarova, N. F. Fetisova, A. Stepanov, E. Mistkarova, N. M. Balayeva, and D. Raoult. 1991. Astrakhan fever: a new spotted fever group rickettsiosis. Lancet 337:172-173. [DOI] [PubMed] [Google Scholar]
- 14.Wen, B., Y. Rikihisa, J. Mott, P. A. Fuerst, M. Kawahara, and C. Suto. 1995. Ehrlichia muris sp. nov., identified on the basis of 16S rRNA base sequences and serological, morphological, and biological characteristics. Int. J. Syst. Bacteriol. 45:250-254. [DOI] [PubMed] [Google Scholar]
- 15.Zhang, J. Z., M. Y. Fan, Y. M. Wu, P. E. Fournier, V. Roux, and D. Raoult. 2000. Genetic classification of “Rickettsia heilongjiangii” and “Rickettsia hulinii,” two Chinese spotted fever group rickettsiae. J. Clin. Microbiol. 38:3498-3501. [DOI] [PMC free article] [PubMed] [Google Scholar]