Abstract
Objective
Leukocyte persistence during chronic (quiescent) phases of asthma is a major hallmark of the disease. The mechanisms regulating these persistent leukocyte populations are not clearly understood. An alternative family of chemoattracting proteins, cyclophilins, has recently been shown to contribute to leukocyte recruitment in animal models of allergic asthma. The goal of this study was to determine if cyclophilins are present in asthma patients during the chronic phase of disease, and to investigate whether levels of cyclophilins associate with clinical parameters of disease severity.
Methods
Nasal wash samples from an urban cohort of 137 6- to 20-year olds with physician-diagnosed asthma were examined for the presence of cyclophilin A (CypA), cyclophilin B (CypB), as well as several other classical chemokines. Linear, logistic, or ordinal regressions were performed to identify associations between cyclophilins, chemokines, and clinical parameters of asthma. The asthma cohort was further divided into previously established phenotypic clusters (Cluster 1 n=55; cluster 2 n=31; and cluster 3 n=51), and examined for associations.
Results
Levels of CypB in the asthma group were highly elevated compared to non-asthmatic controls, while a slight increase in MCP-1 was also observed. CypA and MCP-1 were associated with levels of eosinophil cationic protein (ECP; a marker of eosinophil activation). Cluster-specific associations were found for CypA and CypB and clinical asthma parameters [e.g. forced expiratory volume in 1 second (FEV1) and ECP].
Conclusions
Cyclophilins are present in nasal wash samples of asthma patients and may be a novel biomarker for clinical parameters of asthma severity.
Keywords: asthma, cyclophilin, chemokine, cluster analysis, phenotype
Introduction
Cyclophilins (Cyps) are ubiquitous intracellular proteins commonly known as the target of the immunosuppressive drug cyclosporine A (1). In addition to their intracellular role, increasing evidence has suggested a role for Cyps in intercellular communication (2). We (3-6) and others (7-9) have shown that cyclophilin A (CypA) and/or cyclophilin B (CypB) are potent chemoattractants for a variety of human and mouse leukocyte subsets. In light of these findings, we have previously proposed that extracellular Cyps may be important alternative regulators of leukocyte migration (2, 10). Indeed, elevated levels of Cyps have been reported in several inflammatory diseases, including rheumatoid arthritis (11, 12), vascular smooth muscle disease (13, 14), and severe sepsis (15). Evidence that extracellular Cyps are not only present, but also contribute to leukocyte recruitment during inflammatory responses was demonstrated in several animal models of inflammation, including acute lung injury (3) and acute allergic asthma (4, 6).
A major hallmark of chronic allergic asthma is the persistence of pro-inflammatory leukocytes within the airways, sub-mucosa, and mucosal epithelium during these quiescent (or chronic) periods of disease. The factors that regulate leukocyte persistence in human asthma are not clearly understood. Levels of classical chemokines responsible for leukocyte influx into the lung, such as eotaxins 1-3, MIP-1α, and MCP-1, have been shown to increase rapidly in airways after acute allergen exposure, but return to baseline levels by 24h after exposure (16, 17). In other studies, levels of eotaxin were found to be equivalent between asthma patients in the chronic phase of disease and healthy controls, despite the former group having elevated airway eosinophil numbers (18). Such findings suggest that factors other than classical chemokines may contribute to the persistent recruitment of pro-inflammatory leukocytes during chronic asthma.
The presence of cytokines, chemokines, and other inflammatory proteins has been demonstrated in nasal wash samples from infants and children with several types of respiratory inflammation (19-22). It has also been shown that changes in these protein levels in nasal wash samples correlate well with changes seen in the lower airways (for review see (23)). Studies in our laboratory examining the role of Cyps in acute allergic asthma demonstrate that blocking the activity of extracellular Cyps reduced leukocyte influx into airways and tissues by up to 80% (4). Other recent studies in our laboratory using a new mouse model of chronic allergic asthma show elevated levels of extracellular Cyps present in airways, not only following acute allergen exposure, but also during the chronic phase of disease (24). Based on these observations, extracellular Cyps are potential candidates for regulating the persistent leukocyte infiltration observed during chronic phases of human asthma. Further, the association shown between Cyps and inflammation severity in other inflammatory diseases may suggest similar associations for Cyps in asthma. In the current study we investigated whether extracellular Cyps are present in nasal wash samples collected from asthma patients in the chronic phase of their disease, and whether those levels demonstrate any specific associations with parameters of disease phenotype and/or severity.
Methods
Study Cohort
Study subjects were enrolled in the Asthma Severity Modifying Polymorphisms (AsthMaP) Project, a study of asthma in urban children and adolescents based at the Children’s National Medical Center (Washington, DC). The AsthMaP Project is described in detail elsewhere (25-28). Briefly, 154 participants aged 6-20 years, with physician-diagnosed asthma for at least one year (with varying chronic asthma therapy), were recruited from the Children’s National Emergency Department. Participants then returned to the Clinical Research Center for a study visit at least 4 weeks after completion of their most recent oral steroid dose. If subjects arrived at the Clinical Research Center with current symptoms, they were deferred to return when asymptomatic. Informed consent and assent were obtained from participants and/or guardians. Healthy non-smoking, non-asthmatic subjects without seasonal nasal allergies (n=22) were recruited from adult volunteers to provide control nasal wash samples. These studies were approved by our Institutional Review Board.
Clinical Data Collection
Clinical characteristics were measured in each study participant. Parental interviews using the Integrated Therapeutics Group (ITG) Child Asthma Short Form (29), and the National Institutes of Health National Asthma Education and Prevention Program (NAEPP) 2007 criteria (30, 31), were conducted at least four weeks after most recent oral corticosteroid dose. Spirometry measurements were performed with a MedGraphics CPSF/D™ USB PC-based system (MedGraphics, St Paul, MN) before and after short-acting β-agonist treatment. Levels of serum immunoglobulin-E (IgE) were measured by Quest Diagnostics (Herndon, VA). Details of β-agonist, inhaled corticosteroid, and leukotriene agonist use were also recorded. Only limited clinical data were collected from the anonymous healthy control volunteers (n=22) in whom nasal washes were performed.
Nasal washes were performed by instilling 3mL of sterile isotonic saline into each nare, holding for 10 seconds, and then collecting in a specimen collection container. For comparison, nasal washes were also performed on 22 control individuals without asthma. The collected material was then centrifuged to separate cells and soluble factors. Analyses were conducted on individual samples. Nasal washes were performed as a minimally invasive alternative to bronchoalveolar lavage. Other studies have shown that nasal samples correlate well with samples collected from the lower airways (23).
Eosinophil and neutrophil numbers were counted from nasal wash samples on slides stained with Wright’s stain. P-selectin, MIP-1α (CCL3), MCP-1 (CCL2), and RANTES (CCL5) were measured in the soluble fraction (previously frozen) using a FlowCytomix Simplex Kit, and analyzed using FlowCytomix Pro software v2.3 (eBioscience, San Diego CA). Eotaxin-1 (CCL11), eotaxin-2 (CCL24) and eosinophil cationic protein (ECP) were measured by ELISA (Eotaxins: R&D Systems, Minneapolis, MN; ECP: Medical & Biological Laboratories Co, Ltd, Nagoya, Japan). Levels of extracellular cyclophilins A (CypA) and B (CypB) were measured by Western blot analysis: several concentrations of recombinant CypA and B were used to establish a standard curve of cyclophilin concentrations, which was then used to quantify the amount of cyclophilin present in each sample.
Asthma Phenotypic Clustering
The 154 AsthMaP participants were grouped into four phenotypic clusters based on groupings of clinical variables as we previously described (26). One cluster consisted of six subjects displaying a mild asthma phenotype, and was excluded from further analyses. An additional 11 participants lacked nasal wash samples and were also excluded from analyses. The remaining 137 participants were grouped into the clusters summarized in Table 1. The first cluster (n=55) was predominantly male (80%), with a preponderance of neutrophils in their nasal wash. Cluster 2 (n=31) was predominantly female (67.7%) with a high mean BMI percentile (86), as well as later onset asthma. The third cluster (n=51) is a typical allergic asthma phenotype characterized by elevated eosinophils in nasal washes (Table 1). The phenotype of cluster 2 is important because the combination of high BMI and late age of onset (8.0 years old, compared to 2.0 and 3.3 for clusters 1 and 3, respectively) of asthma is frequently seen in the clinic. Further, studies have suggested a connection between obesity and asthma, particularly in females during adolescence (32, 33). β-agonist, inhaled corticosteroid, and leukotriene agonist use were similar among the three clusters (Table 1). There were no significant differences in the variables summarized in Table 1 between the clustered participants and those lacking nasal wash samples.
Table 1.
| (neutrophilic) | (female late onset) | (allergic asthma) | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| Variable | Healthy Controls (n = 22) |
All Asthma (n=137) |
Cluster 1 (n=55) | Cluster 2 (n=31) | Cluster 3 (n=51) | p-value† |
| Sex, % Male | 41 | 58 | 80 | 32 | 51 | <0.001 |
| Age, years (SE) | 31.6 (2.4) | 11.5 (0.3) | 11.2 (0.6) | 13.5 (0.6) | 10.0 (1.0) | 0.002 |
| Age of onset, years (SE) | - | 3.9 (0.3) | 3.3 (0.4) | 8.0 (0.7) | 2.0 (0.4) | <0.001 |
| Body Mass Percentile, % (SE) | - | 72 (2) | 50 (3) | 86 (3) | 87 (2) | <0.001 |
| Prebronchodilator FEV1 % predicted | - | 87 (2) | 86 (3) | 99 (3) | 82 (2) | <0.001 |
| FEV1 change with bronchodilator (SE) | - | 8 (1) | 7 (2) | 6 (1) | 10 (2) | 0.38 |
| NAEPP severity classification (IQR) | - | 3 (2, 4) | 3 (2, 3) | 3 (2, 4) | 3 (2, 4) | 0.28 |
| ITG-composite score (IQR) | - | 81 (67, 92) | 83 (67, 94) | 81.0 (67, 92) | 79 (65, 88) | 0.26 |
| Nasal eosinophils, % (IQR) | - | 52 (3, 92) | 10 (0, 63) | 56 (14, 91) | 82 (45, 96) | <0.001 |
| Nasal neutrophils, % (IQR) | - | 33 (5, 71) | 50 (8, 95) | 38 (3, 49) | 13 (3, 50) | 0.014 |
| Nasal Wash ECP, ng/mL (IQR) | - | 9.0 (1.3, 47.2) | 27.0 (1.3, 51.8) | 3.6 (0.6, 38.1) | 6.9 (1.3, 37.5) | 0.06 |
| Nasal Wash P-selectin, ng/mL (SE) | - | 1.4 (0.2) | 1.3 (0.2) | 1.4 (0.13) | 1.0 (0.2) | 0.31 |
| Nasal Wash CypA, ng/mL (IQR) | 0 (0,0) | 0 (0,0) | 0 (0,0) | 0 (0,0) | 0 (0,0) | 0.14 |
| Nasal Wash CypB, ng/mL (IQR) | 1.2 (0.9, 3.9) | 7.9 (5.3, 14.4) * | 8.6 (5.1, 14.5) | 8.9 (5.3, 15.5) | 7.6 (5.6, 12.0) | 0.75 |
| Nasal Wash MCP-1, pg/mL (IQR) | 16.3 (0, 104.5) | 58.9 (0, 149.8) * | 80.4 (20.6, 172.2) | 51.9 (0, 105.0) | 48.7 (0, 113.7) | 0.16 |
| Total Serum IgE, IU/mL (IQR) | - | 248 (70, 512) | 210 (59, 202) | 265 (28, 531) | 248 (95, 577) | 0.55 |
| Current β-agonist use, % | - | 69 | 62 | 74 | 75 | 0.29 |
| Current inhaled steroid use, % | - | 34 | 24 | 36 | 45 | 0.07 |
| Current leukotriene antagonist use, % | - | 4 | 4 | 7 | 4 | 0.81 |
Notes: SE = Standard Error; FEV1 = Forced expiratory volume in 1 second; NAEPP = National Asthma Education and Prevention Program, 2007 criteria; IQR = Interquartile Range; ITG = Integrated Therapeutics Group’s Child Asthma Short Form; ECP = Eosinophil Cationic Protein
Indicates significant difference (p-value <0.05) compared to control group.
Significance values among the three clusters were derived using one-way ANOVA for normally distributed continuous variables, or Kruskal-Wallis for nonparametric continuous variables, and chi-square for nominal variables.
Statistical analyses
Associations between the variables summarized in Table 1 were investigated between the asthma and healthy non-asthmatic control groups as a whole, as well as between the clusters within the asthma group. Data were log10 transformed when not normally distributed. T-tests were used to compare cyclophilin and chemokine levels between the asthma and control groups. To identify associations between the variables and cluster groups we used logistic, linear, or ordinal regression. All beta coefficients and P values were corrected for age, race, gender, and body mass index (BMI) percentile during analysis. Statistical tests were performed using SPSS Statistics 18 (SPSS, Chicago IL).
Results
Associations between Cyclophilins, classical chemokines, and Asthma
This study examined nasal wash samples from 137 participants in the AsthMaP project for whom nasal wash samples were available (Table 1). In the asthma cohort as a whole, 58.4% were male and the average age was 11.5 (SE, 0.3) years. The mean body mass index (BMI) percentile was 72 (2.0). 90% of the participants self-identified as African American, and 89% had persistent asthma as defined by NAEPP 2007 criteria.
We first measured levels of extracellular CypA and CypB, as well as the classical chemokines MCP-1, MIP-1α, RANTES, eotaxin-1, and eotaxin-2 in nasal wash samples collected from the AsthMaP cohort and 22 healthy non-asthmatic control participants. As shown in Figure 1, levels of both CypA and CypB were elevated in asthma patients, relative to control donors. In the case of CypB, this difference was significant (P<0.001), however in the case of CypA, levels of the protein were undetectable in many samples. Levels of MCP-1 were also significantly increased in the asthma group (P<0.05). RANTES, MIP-1α, and eotaxin-2 were not different between the two groups (Fig 1) and levels of eotaxin-1 were below the sensitivity threshold of the assay in all samples (data not shown).
Figure 1. CypB and MCP-1 Increased in Asthma Patients During the Chronic Phase of Disease.
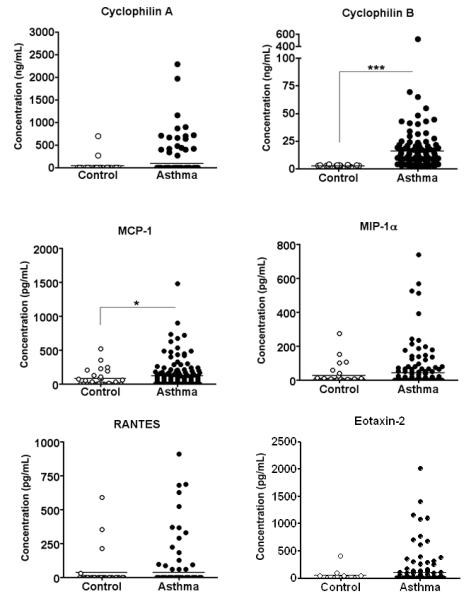
Levels of extracellular CypA, CypB, and other classical chemokines were assessed in nasal wash samples from asthma patients and healthy control volunteers. CypA and CypB were measured by Western Blot analysis, and classical chemokines were measured by cytometric bead array or ELISA (eotaxin-2). Asthma group n = 137; control group n = 22. For analysis data were log10 transformed, and a t-test was used to identify significant differences from the control group. *p<0.05; ***p<0.0001.
We next compared levels of CypA, CypB, and MCP-1 between the asthma and control group in the context of the phenotypic clusters of asthma. We observed no significant associations between any of these three chemotactic agents and the AsthMaP clusters, nor were there any significant increases in these variables in the cluster groups (Fig 2).
Figure 2. Levels of CypA, CypB, and MCP-1 by Asthma Cluster.
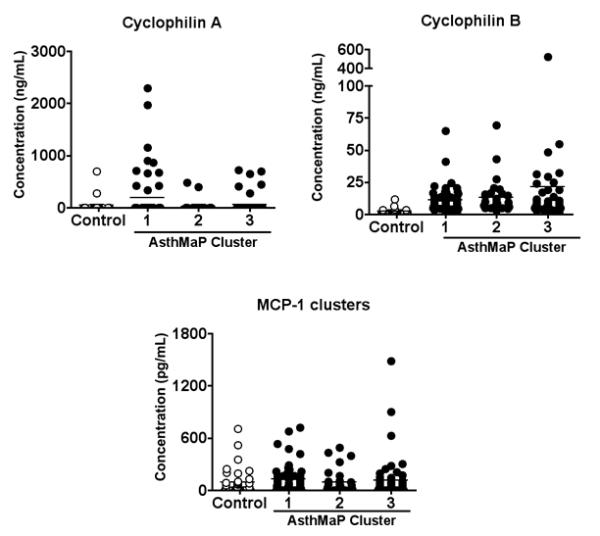
Levels of extracellular CypA, CypB, and MCP-1 were measured in nasal wash samples as described above, and then grouped according to asthma phenotype (Table 1). Asthma cluster 1 n = 55; cluster 2 n = 31; cluster 3 n = 51; control group n = 22. Data were log10 transformed, and t-tests were used to identify significant differences from the control group. No significant differences were observed.
Associations between Cyclophilins, MCP-1, and parameters of asthma severity
We next examined whether the observed levels of CypA, CypB, or MCP-1 associate with parameters associated with disease severity (Table 2). After correcting for age, gender, BMI, and race, we found significant associations with several clinical parameters. Despite a failure to observe a statistically significant difference in levels between asthma and control groups, we observed associations between levels of CypA and several different parameters of disease. For every log10 unit increase in CypA there was a 0.204 unit increase in log10 ECP (β=0.204 [95% C.I. 0.089, 0.319], adjusted P=0.001), a marker of eosinophil activation. CypA levels were also negatively associated with a spirometry measurement: forced expiratory volume in 1 second (FEV1), change with bronchodilator. For every log10 unit increase in CypA there was a 2.973 unit decrease in log10 FEV1 change with bronchodilator (−2.973 [−4.887, −1.059]; adjusted P=0.003), indicating a worse response to the bronchodilator drug. Based on recently published observations that platelet activation correlates with airway eosinophilia in asthma (25), we also examined whether levels of CypA or CypB might be associated with platelet activation in asthmatic airways. Both Cyps were associated with levels of P-selectin, which is a measurement of platelet activation (CypA: 0.418 [0.153, 0.683], adjusted P=0.002; CypB: 0.842 [0.018, 1.665]; adjusted P=0.045). For every log10 unit increase in Cyp, there was a corresponding increase in P-selectin (0.418 for CypA; 0.842 for CypB), indicating that greater levels of cyclophilins are associated with more platelet activation. In the case of CypB, this was the only significant association in the asthma cohort as a whole.
Table 2.
| Associations between CypA levels and Asthma Parameters | ||||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| n=137 |
Cluster 1 (n=55) |
Cluster 2 (n=31) |
Cluster 3 (n=51) |
|||||
| Continuous Variables | (95% CI) | p value | (95% CI) | p value | (95% CI) | p value | (95% CI) | p value |
| Log ECP | 0.204 (0.089, 0.319) | 0.001 | 0.123 (−0.027, 0.277) | 0.106 | 0.333 (−0.075, 0.741) | 0.101 | 0.274 (0.07, 0.478) | 0.01 |
| P-selectin | 0.418 (0.153, 0.683) | 0.002 | 0.553 (0.142, 0.965) | 0.01 | 0.346 (−0.363, 1.054) | 0.298 | 0.224 (−0.140, 0.588) | 0.214 |
| Log FEV1 ,prebronchodilator |
2.157 (−0.212, 4.526) | 0.074 | 3.749 (0.218, 7.28) | 0.038 | 1.882 (−4.432, 8.196) | 0.544 | 0.13 (−3.631, 3.892) | 0.945 |
| Log FEV1 change with bronchodilator |
−2.973 (−4.887, −1.059) | 0.003 | −4.009 (−7.007, −1.012) | 0.01 | −1.937 (−5.410, 1.535) | 0.261 | −1.823 (−5.336, 1.69) | 0.31 |
| NAEPP Severity Classification |
0.148 (−0.093, 0.389) | 0.229 | 0.270 (−0.079, 0.619) | 0.129 | −0.72 (−0.812, 0.668) | 0.849 | 0.018 (−0.410, 0.446) | 0.935 |
| Associations between CypB levels and Asthma Parameters | ||||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| n=137 |
Cluster 1 (n=55) |
Cluster 2 (n=31) |
Cluster 3 (n=51) |
|||||
| Continuous Variables | (95% CI) | p value | (95% CI) | p value | (95% CI) | p value | (95% CI) | p value |
| Log ECP | 0.31 (−0.152, 0.772) | 0.186 | −0.21 (−0.833, 0.791) | 0.958 | 0.638 (−0.709, 1.984) | 0.325 | 0.562 (−0.107, 1.232) | 0.096 |
| P-selectin | 0.842 (0.018, 1.665) | 0.045 | 2.685 (0.497, 4.874) | 0.018 | −0.239 (−2.558, 2.081) | 0.821 | 0.590 (−0.527, 1.707) | 0.284 |
| Log FEV1, prebronchodilator |
3.668 (−5.45, 12.786) | 0.428 | −0.689 (−19.982, 18.604) | 0.943 | 12.889 (−5.959, 31.736) | 0.171 | 0.576 (−10.856, 12.008) | 0.92 |
| Log FEV1 change with bronchodilator |
−0.268 (−0.7828, 0.7292) | 0.944 | 2.124 (−14.672, 18.919) | 0.8 | −0.364 (−11.356, 10.628) | 0.946 | 0.02 (−10.792, 10.831) | 0.997 |
| NAEPP Severity Classification |
0.462 (−1.367, 0.444) | 0.318 | 0.370 (−1.321, 2.061) | 0.668 | −1.548 (−3.973, 8.77) | 0.211 | −0.279 (−1.562, 1.003) | 0.669 |
| Associations between MCP-1 levels and Asthma Parameters | ||||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| n=137 |
Cluster 1 (n=55) |
Cluster 2 (n=31) |
Cluster 3 (n=51) |
|||||
| Continuous Variables | (95% CI) | p value | (95% CI) | p value | (95% CI) | p value | (95% CI) | p value |
| Log ECP | 0.209 (0.10, 0.318) | <0.001 | 0.215 (0.051, 0.380) | 0.011 | 0.322 (0.033, 0.612) | 0.032 | 0.066 (−0.169, 0.302 | 0.566 |
| P-selectin | 0.349 (0.113, 0.585) | 0.004 | 0.463 (−0.034, 0.961) | 0.067 | 0.340 (−0.178, 0.858) | 0.172 | 0.170 (−0.208, 0.547) | 0.36 |
| Log FEV1, prebronchodilator |
−0.1906 (−4.171, 0.360) | 0.098 | −2.404 (−6.566, 1.757) | 0.251 | −0.484 (−5.356, 4.387) | 0.839 | −2.458 (−6.215, 1.299) | 0.194 |
| Log FEV1 change with bronchodilator |
0.54 (−1.352, 2.431) | 0.573 | 0.672 (−2.997, 4.342) | 0.714 | −0.335 (−3.064, 2.394) | 0.802 | 0.188 (−3.434, 3.810) | 0.917 |
| NAEPP Severity Classification |
0.232 (0, 0.464) | 0.05 | −0.042 (−0.409, 0.325) | 0.823 | 0.239 (−0.442, 0.919) | 0.492 | 0.498 (0.045, 0.095) | 0.031 |
Notes: ECP = Eosinophil Cationic Protein;
FEV1 = Forced expiratory volume in 1 second; NAEPP = National Asthma Education and Prevention program, 2007 Criteria.
Levels of MCP-1 were associated with ECP (0.209 [0.10, 0.318]; adjusted P<0.001) and P-selectin (0.349 [0.113, 0.585]; adjusted P=0.004). Additionally, MCP-1 was associated with increases in NAEPP Step level. For every log10 unit increase in MCP-1 there was a 0.232 unit increase in NAEPP step level ([0, 0.64] adjusted P=0.05).
We next looked within each phenotypic cluster for associations between levels of CypA, CypB, MCP-1 and disease parameters associated with asthma severity (Table 2). In Cluster 1 we observed associations between CypA and P-selectin (0.553 [0.142, 0.965], adjusted P=0.01), and between CypB and P-selectin (2.685 [0.497, 4.874], adjusted P=0.018); indicating that for each log10 unit increase in Cyp there was a corresponding increase in P-selectin (0.553 for CypA, 2.685 for CypB). Also in cluster 1, for every log10 unit increase in MCP-1 there was a 0.215 log10 unit increase in ECP ([0.051, 0.380], adjusted P=0.011). In Cluster 2 we also observed a similar association between MCP-1 and nasal wash ECP (0.322 [0.033, 0.612], adjusted P=0.032). The only significant association in Cluster 3 was a 0.274 log10 increase in ECP for every log10 unit increase in CypA ([0.0.07, 0.478], adjusted P=0.01).
Discussion
Asthma is characterized by recurrent periods of acute airway constriction, mucus hypersecretion, and lung inflammation in response to inhaled allergen or other environmental stimuli (34). This inflammation is driven by specific subsets of leukocytes, such as antigen-specific T-cells and IgE-switched B-cells, mast cells, and eosinophils (35). CD4+ T-cells expressing the activation markers CD25 and MHC class II have been reported to be increased in the airways of asthmatic patients, and have also been shown to correlate with disease severity (36, 37). These activated CD4+ T-cells promote and perpetuate the asthmatic response by secreting TH2-associated cytokines such as IL-4, IL-5, and IL-13, which drive isotype switching in B-cells and help recruit activated eosinophils into airways (35, 38, 39).
However, asthma is also a chronic disease in that the inflammation observed during acute responses never completely resolves, even in the absence of allergen stimulation. A major hallmark of chronic allergic asthma is the persistence of pro-inflammatory leukocytes within the submucosa and mucosal epithelium during these chronic periods of disease. Indeed, bronchial biopsies in asthma patients sampled during the chronic phase show elevated numbers of eosinophils, activated lymphocytes, and mast cells, compared to biopsies of healthy control subjects (18). These persistent inflammatory cells are thought to contribute to the tissue injury and remodeling that mediate the pathology of chronic asthma (40). Importantly, activated effector leukocytes recruited to the lung have a limited lifespan (41-43) and do not proliferate at sites of inflammation (44). Therefore the persistent inflammation seen during chronic phases of asthma likely requires recruitment stimuli to replenish these populations of leukocytes.
The specific factors regulating leukocyte persistence in the absence of allergen stimulation are not known. Obvious candidates are chemokines typically associated with allergic responses, including eotaxins 1-3, RANTES, MIP-1α, and MCP-1. However, while levels of these chemokines are greatly increased after acute allergen challenge, they have been shown to return to baseline levels by 24h after exposure (16, 17). In other studies, levels of eotaxin were found to be equivalent between asthma patients in clinical remission and healthy controls, despite the former group having elevated airway eosinophil numbers (18). Such findings suggest that factors other than classical chemokines may instead contribute to the persistent recruitment of pro-inflammatory leukocytes during chronic asthma
Our investigations of extracellular Cyps in nasal wash samples from human asthma patients revealed significant increases in CypB compared to classical chemokines in the asthma group, relative to healthy controls (Fig. 1). In fact, the levels of most classical chemokines measured were either comparable between control and asthmatic groups, or below the sensitivity threshold of the assay used for detection. One interesting finding was that although levels of CypA were not significantly elevated in the asthma group compared to the control group, CypA showed significant associations with several parameters of disease (Table 2). Of particular interest is that levels of CypA were negatively associated with a parameter of lung function: as levels of CypA increased, patients’ response to bronchodilator drugs decreased. It should be noted that while interesting, the biological and clinical relevance of associations with CypA remain unclear since there was no difference in CypA between the control group and the asthma groups as a whole. Nonetheless, in light of our recent findings in animal models of asthma (24, 45), we believe these interesting associations warrant further investigation.
These associations and findings of elevated levels of Cyps in clinical patients provide the first evidence that Cyps may play a role in human asthma, particularly in the chronic phase of disease. This may extend beyond leukocyte recruitment to other mediators of asthma such as airway remodeling. For example, we observed that increases in CypA and CypB associated with increased levels of eosinophil (measured by ECP levels) and platelet (measured by P-selectin levels) activation. Studies have demonstrated not only that CypA can be released from activated platelets (46), but also that CypB increases platelet adhesion to collagen (47). Further, platelet activation has also been shown to be important for leukocyte activation and recruitment into tissues (25, 48). Thus, Cyps have the potential to be involved in many aspects of asthma pathology, from leukocyte activation and recruitment, to airway remodeling. This could also extend to lung function, since CypA was also associated with a worse response to bronchodilator drugs. An important caveat to these studies is the relatively small sample size available for our analyses, particularly after subdividing the asthma group into phenotypic clusters. Studies of larger cohorts will allow a more powerful analysis of clinically relevant asthma parameters and could further investigate the role of Cyps in human disease.
Conclusions/key findings
Extracellular cyclophilins have been shown to associate with inflammation in a variety of human diseases. Further, recent studies have demonstrated a potential role for cyclophilins in leukocyte recruitment in animal models of acute (45) and chronic (24) allergic asthma. This study provides the first evidence that cyclophilins are elevated in human asthma patients, and that increases in levels of cyclophilins associate with several clinical parameters of disease severity.
Acknowledgments
This project was conducted through the General Clinical Research Center under the CReFF program at Children’s National Medical Center and supported by the National Institutes of Health National Center for Research Resources, Grant M01RR020359 with additional funding support from: Sheldon C. Siegel Investigator Award Grant from the Asthma and Allergy Foundation of America (RJF), National Institutes of Health grants K23RR020069 (RJF), P20MD000198 (RJF), and American Heart Association Pre-doctoral Award 0815226E (EJS).
Footnotes
Declaration of Interest
The authors have no conflicts of interest associated with the current studies.
References
- 1.Handschumacher RE, Harding MW, Rice J, Drugge RJ, Speicher DW. Cyclophilin: a specific cytosolic binding protein for cyclosporin A. Science. 1984 Nov 2;226(4674):544–7. doi: 10.1126/science.6238408. [DOI] [PubMed] [Google Scholar]
- 2.Bukrinsky MI. Cyclophilins: unexpected messengers in intercellular communications. Trends Immunol. 2002 Jul;23(7):323–5. doi: 10.1016/s1471-4906(02)02237-8. [DOI] [PubMed] [Google Scholar]
- 3.Arora K, Gwinn WM, Bower MA, Watson A, Okwumabua I, MacDonald HR, Bukrinsky MI, Constant SL. Extracellular cyclophilins contribute to the regulation of inflammatory responses. J Immunol. 2005 Jul 1;175(1):517–22. doi: 10.4049/jimmunol.175.1.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Balsley MA, Malesevic M, Stemmy EJ, Gigley J, Jurjus RA, Herzog D, Bukrinsky MI, Fischer G, Constant SL. A cell-impermeable cyclosporine a derivative reduces pathology in a mouse model of allergic lung inflammation. J Immunol. Dec 15;185(12):7663–70. doi: 10.4049/jimmunol.1001707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Damsker JM, Bukrinsky MI, Constant SL. Preferential chemotaxis of activated human CD4+ T cells by extracellular cyclophilin A. J Leukoc Biol. 2007 Sep;82(3):613–8. doi: 10.1189/jlb.0506317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gwinn WM, Damsker JM, Falahati R, Okwumabua I, Kelly-Welch A, Keegan AD, Vanpouille C, Lee JJ, Dent LA, Leitenberg D, Bukrinsky MI, Constant SL. Novel approach to inhibit asthma-mediated lung inflammation using anti-CD147 intervention. J Immunol. 2006 Oct 1;177(7):4870–9. doi: 10.4049/jimmunol.177.7.4870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sherry B, Yarlett N, Strupp A, Cerami A. Identification of cyclophilin as a proinflammatory secretory product of lipopolysaccharide-activated macrophages. Proc Natl Acad Sci U S A. 1992 Apr 15;89(8):3511–5. doi: 10.1073/pnas.89.8.3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xu Q, Leiva MC, Fischkoff SA, Handschumacher RE, Lyttle CR. Leukocyte chemotactic activity of cyclophilin. J Biol Chem. 1992 Jun 15;267(17):11968–71. [PubMed] [Google Scholar]
- 9.Yurchenko V, Zybarth G, O’Connor M, Dai WW, Franchin G, Hao T, Guo H, Hung HC, Toole B, Gallay P, Sherry B, Bukrinsky M. Active site residues of cyclophilin A are crucial for its signaling activity via CD147. J Biol Chem. 2002 Jun 21;277(25):22959–65. doi: 10.1074/jbc.M201593200. [DOI] [PubMed] [Google Scholar]
- 10.Yurchenko V, Constant S, Bukrinsky M. Dealing with the family: CD147 interactions with cyclophilins. Immunology. 2006 Mar;117(3):301–9. doi: 10.1111/j.1365-2567.2005.02316.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Billich A, Winkler G, Aschauer H, Rot A, Peichl P. Presence of cyclophilin A in synovial fluids of patients with rheumatoid arthritis. J Exp Med. 1997 Mar 3;185(5):975–80. doi: 10.1084/jem.185.5.975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim H, Kim WJ, Jeon ST, Koh EM, Cha HS, Ahn KS, Lee WH. Cyclophilin A may contribute to the inflammatory processes in rheumatoid arthritis through induction of matrix degrading enzymes and inflammatory cytokines from macrophages. Clin Immunol. 2005 Sep;116(3):217–24. doi: 10.1016/j.clim.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 13.Jin ZG, Melaragno MG, Liao DF, Yan C, Haendeler J, Suh YA, Lambeth JD, Berk BC. Cyclophilin A is a secreted growth factor induced by oxidative stress. Circ Res. 2000 Oct 27;87(9):789–96. doi: 10.1161/01.res.87.9.789. [DOI] [PubMed] [Google Scholar]
- 14.Suzuki J, Jin ZG, Meoli DF, Matoba T, Berk BC. Cyclophilin A is secreted by a vesicular pathway in vascular smooth muscle cells. Circ Res. 2006 Mar 31;98(6):811–7. doi: 10.1161/01.RES.0000216405.85080.a6. [DOI] [PubMed] [Google Scholar]
- 15.Tegeder I, Schumacher A, John S, Geiger H, Geisslinger G, Bang H, Brune K. Elevated serum cyclophilin levels in patients with severe sepsis. J Clin Immunol. 1997 Sep;17(5):380–6. doi: 10.1023/a:1027364207544. [DOI] [PubMed] [Google Scholar]
- 16.Brown JR, Kleimberg J, Marini M, Sun G, Bellini A, Mattoli S. Kinetics of eotaxin expression and its relationship to eosinophil accumulation and activation in bronchial biopsies and bronchoalveolar lavage (BAL) of asthmatic patients after allergen inhalation. Clin Exp Immunol. 1998 Nov;114(2):137–46. doi: 10.1046/j.1365-2249.1998.00688.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Holgate ST, Bodey KS, Janezic A, Frew AJ, Kaplan AP, Teran LM. Release of RANTES, MIP-1 alpha, and MCP-1 into asthmatic airways following endobronchial allergen challenge. Am J Respir Crit Care Med. 1997 Nov;156(5):1377–83. doi: 10.1164/ajrccm.156.5.9610064. [DOI] [PubMed] [Google Scholar]
- 18.van den Toorn LM, Overbeek SE, de Jongste JC, Leman K, Hoogsteden HC, Prins JB. Airway inflammation is present during clinical remission of atopic asthma. Am J Respir Crit Care Med. 2001 Dec 1;164(11):2107–13. doi: 10.1164/ajrccm.164.11.2006165. [DOI] [PubMed] [Google Scholar]
- 19.Gern JE, Martin MS, Anklam KA, Shen K, Roberg KA, Carlson-Dakes KT, Adler K, Gilbertson-White S, Hamilton R, Shult PA, Kirk CJ, Da Silva DF, Sund SA, Kosorok MR, Lemanske RF., Jr. Relationships among specific viral pathogens, virus-induced interleukin-8, and respiratory symptoms in infancy. Pediatr Allergy Immunol. 2002 Dec;13(6):386–93. doi: 10.1034/j.1399-3038.2002.01093.x. [DOI] [PubMed] [Google Scholar]
- 20.Hurst JR, Perera WR, Wilkinson TM, Donaldson GC, Wedzicha JA. Systemic and upper and lower airway inflammation at exacerbation of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2006 Jan 1;173(1):71–8. doi: 10.1164/rccm.200505-704OC. [DOI] [PubMed] [Google Scholar]
- 21.Legg JP, Hussain IR, Warner JA, Johnston SL, Warner JO. Type 1 and type 2 cytokine imbalance in acute respiratory syncytial virus bronchiolitis. Am J Respir Crit Care Med. 2003 Sep 15;168(6):633–9. doi: 10.1164/rccm.200210-1148OC. [DOI] [PubMed] [Google Scholar]
- 22.Midulla F, Tromba V, Lo Russo L, Mileto F, Sabatino G, Sgarrella M, Panuska JR, Manganozzi L, Korn D, Moretti C. Cytokines in the nasal washes of children with respiratory syncytial virus bronchiolitis. Int J Immunopathol Pharmacol. 2006 Jan-Mar;19(1) [PubMed] [Google Scholar]
- 23.Frischer T, Baraldi E. Upper airway sampling. Am J Respir Crit Care Med. 2000 Aug;162(2 Pt 2):S28–30. doi: 10.1164/ajrccm.162.supplement_1.maic-7. [DOI] [PubMed] [Google Scholar]
- 24.Stemmy EJ, Balsley MA, Jurjus RA, Damsker JM, Bukrinsky MI, Constant SL. Blocking Cyclophilins in Chronic Phase of Asthma Reduces Leukocyte Persistence and Disease Reactivation. American Journal of Respiratory Cell and Molecular Biology. 2011 Apr 14; doi: 10.1165/rcmb.2011-0007OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Benton AS, Kumar N, Lerner J, Wiles AA, Foerster M, Teach SJ, Freishtat RJ. Airway platelet activation is associated with airway eosinophilic inflammation in asthma. J Investig Med. 2010 Dec;58(8):987–90. doi: 10.231/JIM.0b013e3181fa02f7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Benton AS, Wang Z, Lerner J, Foerster M, Teach SJ, Freishtat RJ. Overcoming heterogeneity in pediatric asthma: tobacco smoke and asthma characteristics within phenotypic clusters in an African American cohort. J Asthma. 2010 Sep;47(7):728–34. doi: 10.3109/02770903.2010.491142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Freishtat RJ, Iqbal SF, Pillai DK, Klein CJ, Ryan LM, Benton AS, Teach SJ. High prevalence of vitamin D deficiency among inner-city African American youth with asthma in Washington, DC. J Pediatr. 2010 Jun;156(6):948–52. doi: 10.1016/j.jpeds.2009.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Watson AM, Ngor WM, Gordish-Dressman H, Freishtat RJ, Rose MC. MUC7 polymorphisms are associated with a decreased risk of a diagnosis of asthma in an African American population. J Investig Med. 2009 Dec;57(8):882–6. doi: 10.231/JIM.0b013e3181c0466d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bukstein DA, McGrath MM, Buchner DA, Landgraf J, Goss TF. Evaluation of a short form for measuring health-related quality of life among pediatric asthma patients. J Allergy Clin Immunol. 2000 Feb;105(2 Pt 1):245–51. doi: 10.1016/s0091-6749(00)90072-1. [DOI] [PubMed] [Google Scholar]
- 30.Expert Panel Report 3 (EPR-3): Guidelines for the Diagnosis and Management of Asthma-Summary Report 2007. J Allergy Clin Immunol. 2007 Nov;120(5 Suppl):S94–138. doi: 10.1016/j.jaci.2007.09.043. [DOI] [PubMed] [Google Scholar]
- 31.Gorelick MH, Brousseau DC, Stevens MW. Validity and responsiveness of a brief, asthma-specific quality-of-life instrument in children with acute asthma. Ann Allergy Asthma Immunol. 2004 Jan;92(1):47–51. doi: 10.1016/S1081-1206(10)61709-7. [DOI] [PubMed] [Google Scholar]
- 32.Castro-Rodriguez JA, Holberg CJ, Morgan WJ, Wright AL, Martinez FD. Increased incidence of asthmalike symptoms in girls who become overweight or obese during the school years. Am J Respir Crit Care Med. 2001 May;163(6):1344–9. doi: 10.1164/ajrccm.163.6.2006140. [DOI] [PubMed] [Google Scholar]
- 33.Guerra S, Wright AL, Morgan WJ, Sherrill DL, Holberg CJ, Martinez FD. Persistence of asthma symptoms during adolescence: role of obesity and age at the onset of puberty. Am J Respir Crit Care Med. 2004 Jul 1;170(1):78–85. doi: 10.1164/rccm.200309-1224OC. [DOI] [PubMed] [Google Scholar]
- 34.Cookson W. The alliance of genes and environment in asthma and allergy. Nature. 1999 Nov 25;402(6760 Suppl):B5–11. doi: 10.1038/35037002. [DOI] [PubMed] [Google Scholar]
- 35.Cohn L, Elias JA, Chupp GL. Asthma: mechanisms of disease persistence and progression. Annu Rev Immunol. 2004;22:789–815. doi: 10.1146/annurev.immunol.22.012703.104716. [DOI] [PubMed] [Google Scholar]
- 36.Corrigan CJ, Hartnell A, Kay AB. T lymphocyte activation in acute severe asthma. Lancet. 1988 May 21;1(8595):1129–32. doi: 10.1016/s0140-6736(88)91951-4. [DOI] [PubMed] [Google Scholar]
- 37.Walker C, Kaegi MK, Braun P, Blaser K. Activated T cells and eosinophilia in bronchoalveolar lavages from subjects with asthma correlated with disease severity. J Allergy Clin Immunol. 1991 Dec;88(6):935–42. doi: 10.1016/0091-6749(91)90251-i. [DOI] [PubMed] [Google Scholar]
- 38.Larche M, Robinson DS, Kay AB. The role of T lymphocytes in the pathogenesis of asthma. J Allergy Clin Immunol. 2003 Mar;111(3):450–63. doi: 10.1067/mai.2003.169. quiz 64. [DOI] [PubMed] [Google Scholar]
- 39.Wills-Karp M. Immunologic basis of antigen-induced airway hyperresponsiveness. Annu Rev Immunol. 1999;17:255–81. doi: 10.1146/annurev.immunol.17.1.255. [DOI] [PubMed] [Google Scholar]
- 40.Homer RJ, Elias JA. Consequences of long-term inflammation. Airway remodeling. Clin Chest Med. 2000 Jun;21(2):331–43. doi: 10.1016/s0272-5231(05)70270-7. ix. [DOI] [PubMed] [Google Scholar]
- 41.Medoff BD, Thomas SY, Luster AD. T cell trafficking in allergic asthma: the ins and outs. Annu Rev Immunol. 2008;26:205–32. doi: 10.1146/annurev.immunol.26.021607.090312. [DOI] [PubMed] [Google Scholar]
- 42.Robertson JM, MacLeod M, Marsden VS, Kappler JW, Marrack P. Not all CD4+ memory T cells are long lived. Immunol Rev. 2006 Jun;211:49–57. doi: 10.1111/j.0105-2896.2006.00383.x. [DOI] [PubMed] [Google Scholar]
- 43.Rothenberg ME, Hogan SP. The eosinophil. Annu Rev Immunol. 2006;24:147–74. doi: 10.1146/annurev.immunol.24.021605.090720. [DOI] [PubMed] [Google Scholar]
- 44.Harris NL, Watt V, Ronchese F, Le Gros G. Differential T cell function and fate in lymph node and nonlymphoid tissues. J Exp Med. 2002 Feb 4;195(3):317–26. doi: 10.1084/jem.20011558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Balsley MA, Malesevic M, Stemmy EJ, Gigley J, Jurjus RA, Herzog D, Bukrinsky MI, Fischer G, Constant SL. A cell-impermeable cyclosporine A derivative reduces pathology in a mouse model of allergic lung inflammation. J Immunol. 2010 Dec 15;185(12):7663–70. doi: 10.4049/jimmunol.1001707. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Coppinger JA, Cagney G, Toomey S, Kislinger T, Belton O, McRedmond JP, Cahill DJ, Emili A, Fitzgerald DJ, Maguire PB. Characterization of the proteins released from activated platelets leads to localization of novel platelet proteins in human atherosclerotic lesions. Blood. 2004 Mar 15;103(6):2096–104. doi: 10.1182/blood-2003-08-2804. [DOI] [PubMed] [Google Scholar]
- 47.Allain F, Durieux S, Denys A, Carpentier M, Spik G. Cyclophilin B binding to platelets supports calcium-dependent adhesion to collagen. Blood. 1999 Aug 1;94(3):976–83. [PubMed] [Google Scholar]
- 48.Pitchford SC, Momi S, Giannini S, Casali L, Spina D, Page CP, Gresele P. Platelet P-selectin is required for pulmonary eosinophil and lymphocyte recruitment in a murine model of allergic inflammation. Blood. 2005 Mar 1;105(5):2074–81. doi: 10.1182/blood-2004-06-2282. [DOI] [PubMed] [Google Scholar]


