Abstract
The development of a serological test for foot-and-mouth disease virus (FMDV) which is quick and easy to use, which can identify all seven serotypes, and which can differentiate vaccinated from convalescing or potential virus carriers would be a major advance in the epidemiological toolkit for FMDV. The nonstructural polyprotein 3ABC has recently been proposed as such an antigen, and a number of diagnostic tests are being developed. This paper evaluates the performance of two FMDV tests for antibodies to nonstructural proteins in an unvaccinated cattle population from a region of Cameroon with endemic multiple-serotype FMD. The CHEKIT-FMD-3ABC bo-ov (CHEKIT) enzyme-linked immunosorbent assay (ELISA) (Bommeli Diagnostics/Intervet) is a commercially available test that was compared with a competitive 3ABC ELISA (C-ELISA) developed in Denmark. The tests were compared with the virus neutralization test as the “gold standard.” Diagnostic sensitivity and specificity were examined over a range of test cutoffs by using receiver operating characteristic curves, which allowed comparison of the overall performance of each test. The results indicated that the CHEKIT ELISA kit was 23% sensitive and 98% specific and the Danish C-ELISA was 71% sensitive and 90% specific at the recommended cutoff. These results have important implications if the tests are to be used to screen herds or individual cattle in surveillance programs, at border crossings for import-export clearance, or following emergency vaccination in an outbreak situation.
Foot-and-mouth disease (FMD) is a highly contagious viral disease of even-toed ungulates caused by Foot-and-mouth disease virus (FMDV), which is a member of the genus Aphthovirus and the family Picornaviridae (25). FMDV is a small nonenveloped virus with an ∼8.5-kbp genome which codes for structural as well as nonstructural proteins (NSPs) (11, 18). There are seven serotypes, known as serotypes O, A, C, SAT 1, SAT 2, SAT 3, and Asia 1, recognized worldwide. All of these occur in Africa except Asia 1 (43). These serotypes are clinically indistinguishable, and there may be considerable variation in the disease presentation depending on the strain within a serotype, the species affected, and previous exposure (26, 27, 29). It is one of the most important economic diseases of livestock owing to both the production losses caused by clinical disease and the disruption caused in international trade with disease-free countries.
The two principal control strategies for FMD are stamping out and vaccination, which may be used either prophylactically or as an emergency campaign during an outbreak. In May 2002 the Office International des Epizooties (OIE) updated its International Animal Health Code in light of advances in diagnostic tests, allowing countries that vaccinate in the face of an outbreak of FMD to regain disease-free status after 6 months if they can differentiate vaccinated from infected or convalescing animals. Differentiation of convalescing or infected animals is based on identifying antibodies to the NSPs of FMDV (2, 15, 39, 40).
The NSPs are expressed only by replicating viruses. Inactivated vaccines are purified to remove cellular proteins and NSPs, and therefore only animals that have been infected with live virus should develop antibodies to these proteins (2, 4, 35). Currently, the polyproteins 3ABC and 3AB appear to be most promising as diagnostic antigens (6, 15, 30, 37, 41). They have been expressed as fusion proteins in Escherichia coli (30) and baculovirus vectors in insect cells (34, 41) used in a variety of assays, including agar gel immunodiffusion (33), latex agglutination (42), immunoelectrotransfer blot analysis (3), and direct and blocking enzyme-linked immunosorbent assays (ELISAs) (30, 39).
As these tests will be used in the regulation of trade in live animals and their products, their evaluation in a range of populations is essential since diagnostic sensitivity (Se) and specificity (Sp) are parameters that describe test performance for a given reference population (21).
Here we describe a comparison of two NSP tests, the CHEKIT-FMD-3ABC bo-ov (CHEKIT) ELISA (Bommeli Diagnostics/Intervet), which is a commercially available test, and an experimental competitive ELISA (C-ELISA) developed in Denmark (39), in an unvaccinated cattle population and using a combined virus neutralization test (cVNT) result as the “gold standard.”
MATERIALS AND METHODS
Study population.
A full description of the study area, the livestock population, and the study design is given elsewhere (9). In brief, a population-based sample of herds was selected from the Adamawa Province of Cameroon by use of a sample frame constructed from the government's rinderpest vaccination lists which were maintained at each of the 88 local veterinary centers. A total of 13,006 herds were included in the database, and a two-stage random sample of herds was selected assuming a herd-level prevalence of 50%. The first stage was a random sample of veterinary centers (with replacement), with the probability of selection proportional to the number of herds registered at the center, and the second stage was a random sample of three herds per center (without replacement). The herd sample size was calculated using the Survey Toolbox software (A. R. Cameron, Wentworth Falls, New South Wales, Australia), and a total of 162 herds in 54 veterinary centers were selected.
Within herds a stratified sample of five juvenile (8- to 24-month-old) and five adult (>24-month-old) cattle was randomly selected. This allowed 95% confidence of identifying at least one seropositive animal in a herd of 70 cattle with a seroprevalence of 50% (10).
Collection of samples.
Cattle were cast in lateral recumbency and examined for lesions, and a serum sample and an oropharyngeal fluid or probang (OP) sample were taken. Blood samples were allowed to clot and were then centrifuged at 1,100 × g for 10 min in the field by use of a Mobilespin 12-V field centrifuge (Vulcon Technologies) or a hand-cranked centrifuge (OFI Testing Equipment, Inc.). Approximately 3.5 ml of serum was aliquoted into two 1.8-ml cryovials (Nunc) and kept at 4°C in a portable gas refrigerator until they could be frozen and stored at −20°C. The OP samples were stored in liquid nitrogen at the end of each day's sampling. The serum and OP samples were transferred to the FMD World Reference Laboratory (WRL) on dry ice or liquid nitrogen, where they were stored at −20°C and −70°C, respectively. Data collected by questionnaire indicated that no herdsman reported using an FMDV vaccine and no government licenses had been issued for import of vaccines into the country; therefore, the population was believed to be unvaccinated. The study was conducted between April and October 2000, which encompasses the rainy season, when herds are close to their home areas.
Tissue culture of OP samples.
A 0.2-ml sample of each OP sample was inoculated onto five bovine thyroid cell monolayers (38) and incubated at 37°C for up to 72 h on rollers, following the OIE/WRL protocol (28). The cultures were examined daily for any signs of cytopathic effect. Cultures with a positive cytopathic effect were then typed using the WRL sandwich antigen ELISA (17, 28, 36). Of 38 samples that were positive, 20 were serotype A and 18 were serotype SAT 2. In a separate study of pigs, serotype O was isolated from clinical samples and may have been present in the cattle population, but no probang samples were positive during the sampling for the cross-sectional study.
VNT.
Virus isolation from probang samples indicated that the FMDV serotypes A and SAT 2 were actively circulating in the cattle population and that serotype O was actively circulating in the swine population at the time of the cross-sectional study. VNTs were carried out for these three serotypes following OIE/WRL protocol (20, 28). Serum samples were heat inactivated (56°C, 30 min), and neutralizing antibodies were assessed against FMDV type O Manisa and homologous Cameroon isolates of type A (P59/2000-VBM/153/09) and type SAT 2 (P26/2000-FDL/74/10). Duplicate, doubling dilutions of serum samples were tested. The end point for 100 50% tissue culture infective doses was estimated for each sample (24), and the standard OIE cutoff dilution, ≤1/101.56 (1:45), was used. Control dilutions of the virus and reference sera were carried out, and in cases where the control dilutions were out of range, the entire test was repeated. For each batch of VNTs, the viral dose used was calculated from control plates, and the batch was rejected if the control doses were outside the control range of 100 ± 100.5 50% tissue culture infective doses. Similarly, the working titer of the control sera was calculated for each batch, and if the titer differed by more than ±100.3 of the running mean, the batch was retested. The results of the three VNTs were combined such that an animal that was positive for one or more serotypes was classed as positive for the cVNT.
CHEKIT ELISA.
The CHEKIT ELISA was used according to the manufacturer's instructions. Briefly, the serum was diluted 1/100, added in duplicate to the wells of a 96-well microtiter plate precoated with the vector-expressed viral 3ABC antigen, and incubated for 60 min at 37°C in a humid chamber. Unbound antibody was washed away, and a horseradish peroxidase-labeled guinea pig anti-bovine immunoglobulin G conjugate was added. Unbound conjugate was removed by washing, and the chromogen substrate was added and incubated until the difference in the optical density (OD) reading between the negative and positive controls was ≥0.4 (after about 20 min). The OD was determined for each well at 405 nm with an automatic plate reader. The final OD value for the sample was expressed as a percentage of the OD of the positive control by using the mean OD of each pair of samples (ODsample) and the median OD of the four positive and negative controls (ODpositive and ODnegative, respectively) on each plate in the following formula: final OD = [(ODsample − ODnegative)/(ODpositive − ODnegative)] × 100.
The manufacturer's recommended interpretation was that a final OD value of <20% is negative, one of 20 to 30% is ambiguous, and one of >30% is positive.
C-ELISA.
The C-ELISA was performed as described previously (41), with modifications. The original samples were aliquoted, heat treated at 56°C for 2 h, and then shipped to Denmark for testing. Microtiter plates were prepared by capturing 3ABC protein produced in a baculovirus expression system with a monoclonal antibody (MabL74D5) coated on the plates. Dilutions (1:5) of the sera were added, the plates were incubated overnight at 37°C and washed, and the competing antibody, horseradish peroxidase-conjugated monoclonal antibody MabL74D5, was added; the plates were then incubated for another hour at 37°C. After washing the plates and adding chromogen substrate (tetramethylbenzidine and H2O2), the color development (OD) was measured after 15 min at 450 nm, and the results were expressed as a percentage of the negative control values determined by the following: OD = (ODsample/ODmean negative controls) × 100. The recommended cutoff is ≤50% for a positive result.
Statistical analysis.
The cVNT and ELISA results were first compared at the individual animal level in 2 × 2 tables. The Se and Sp of each test were calculated using the recommended cutoffs of >30% for the CHEKIT ELISA and ≤50% for the C-ELISA. The test characteristics were further investigated by use of the receiver operating characteristic (ROC) curves calculated with AccuROC version 2.4 (Accumetric). The ROC curve is drawn by calculating the Se and Sp for all possible cutoff values of the test and then plotting the Se against 1 minus the Sp (i.e., the probability of detecting a true positive result against the probability of a false positive result). The area under the curve (AUC) then represents the overall performance of the test with a maximum area of 1 for a test with perfect Se and Sp. The AUC was calculated for each test using the nonparametric method of DeLong et al. (16), and these values were then compared using a Z statistic and a two-tailed test which is available within the package. Based on the recommended cutoff values of the tests, the Se and Sp of the CHEKIT ELISA and the C-ELISA were estimated. Furthermore, kappa values were calculated to assess the agreement between the two tests (19).
The herd-level sensitivity (HSe; the conditional probability that a herd will test positive given that it is diseased) and the herd-level specificity (HSp; the conditional probability that a herd will test negative given that the herd is not diseased) of the two tests were examined over a range of possible within-herd prevalences by using Herdacc version 3.0 software (D. Jordan, University of Guelph, Guelph, Canada) with a theoretical herd of 100 animals. A herd would be categorized as positive if one animal in the sample from the herd was positive. The HSp and HSe are calculated from the formulae in equations 1 and 2 (32), which are shown below. It is important to note that the HSp is related only to the individual test Sp and the sample size, while the HSe is related to the individual test Se and Sp, the sample size, and importantly, the true prevalence of disease in the herd.
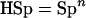 |
(1) |
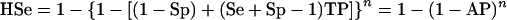 |
(2) |
where n is the sample size, TP is the true disease prevalence in the herd, and AP is the apparent prevalence, i.e., the proportion of test positives, in the population.
RESULTS
Comparison of the C-ELISA and CHEKIT ELISA with the cVNT in adults and juveniles.
The estimates of the Se and Sp of the C-ELISA and CHEKIT ELISA for all animals are given in Table 1. The results show that the C-ELISA has low relative Se (71%) and Sp (90%) at the recommended cutoff. In comparison, the Se of the CHEKIT ELISA is extremely low (23%) at the recommended cutoff of 30%, although the Sp is very high (98%).
TABLE 1.
Estimates of Se and Sp for the CHEKIT ELISA and the C-ELISA compared to the cVNT as a gold standarda
| cVNT result | CHEKIT result | No. of animals with C-ELISA result that was:
|
|
|---|---|---|---|
| + | − | ||
| Seropositiveb | + | 205 | 7 |
| − | 443 | 252 | |
| Seronegativec | + | 6 | 4 |
| − | 41 | 419 | |
Total number of animals tested, 1,377. The recommended cutoffs of 30% for the CHEKIT ELISA and 50% for the C-ELISA were used.
Se of CHEKIT ELISA = 0.234; Se of C-ELISA = 0.714; kappa value of 0.192 between CHEKIT and C-ELISA results.
Sp of CHEKIT ELISA = 0.979; Sp of C-ELISA = 0.900; kappa value of 0.182 between CHEKIT and C-ELISA results.
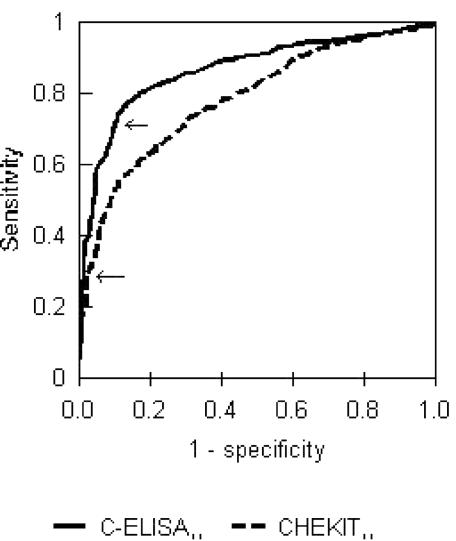
The test cutoffs were examined by using the ROC curves shown in Fig. 1. It is clear from Fig. 1 that the C-ELISA performs better overall and that this result was statistically significant (P < 0.01). The CHEKIT ELISA, though not performing as well overall, is not optimized at the cutoff of 30%, which lies in the vertical part of the curve in the lower left of the plot. The cutoff could be lowered significantly with very little loss of Sp.
FIG. 1.
ROC curves for 3ABC CHEKIT ELISA (AUC = 0.782) and C-ELISA (AUC = 0.865) for adult and juvenile animals (n = 1,377). Two-tailed test of AUC Z statistic = 5.217; P < 0.01. Arrows mark the recommended cutoffs for each test.
Comparison of the C-ELISA and CHEKIT ELISA with the VNT in juvenile animals.
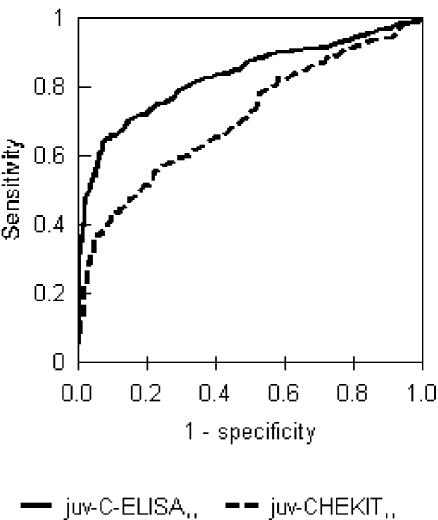
The analysis of Se and Sp of the C-ELISA and the CHEKIT ELISA was repeated for the subset of juvenile animals (8 to 24 months old) in an effort to investigate whether using animals that had been infected only in the last 2 years would improve the estimates, as suggested by Bergmann et al. (3). As shown in Table 2, there was no improvement in the test performance. The CHEKIT ELISA Se dropped to 15% while the Sp did not change, and for the C-ELISA the Se dropped to 57% although the Sp increased slightly, to 94%. The changes in test parameters are reflected in the ROC curves with the AUC decreasing for both tests, although the C-ELISA still performed better than the CHEKIT ELISA (Fig. 2).
TABLE 2.
Estimates of Se and Sp for the CHEKIT ELISA and the C-ELISA based on subset of juvenile animals and compared to the cVNT as the gold standarda
| cVNT result | CHEKIT result | No. of animals with C-ELISA result that was:
|
|
|---|---|---|---|
| + | − | ||
| Seropositiveb | + | 46 | 2 |
| − | 136 | 134 | |
| Seronegativec | + | 2 | 4 |
| − | 17 | 310 | |
Total number of animals tested, 651. The recommended cutoffs of 30% for the CHEKIT ELISA and 50% for the C-ELISA were used.
Se of CHEKIT ELISA = 0.151; Se of C-ELISA = 0.572; kappa value of 0.212 between CHEKIT and C-ELISA results.
Sp of CHEKIT ELISA = 0.982; Sp of C-ELISA = 0.942; kappa value of 0.136 between CHEKIT and C-ELISA results.
FIG. 2.
ROC curves for CHEKIT ELISA (AUC = 0.707) and C-ELISA (AUC = 0.829) based on juveniles only (n = 651). Two-tailed test of AUC Z statistic = 4.64; P < 0.01.
Comparison of test results for probang-positive animals.
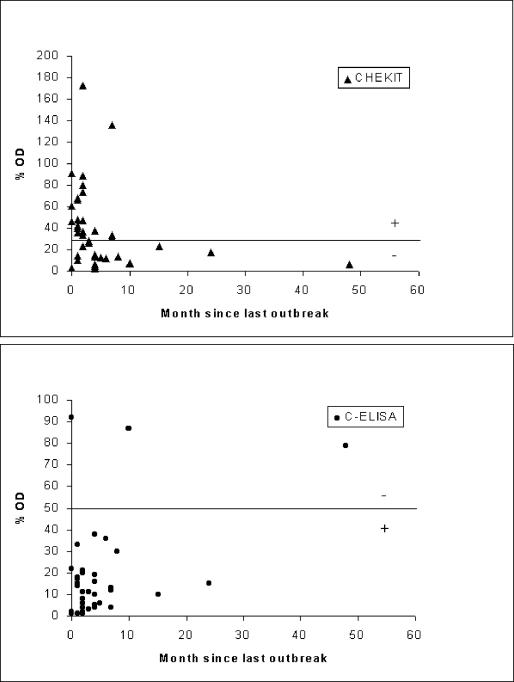
In order to investigate the Se of the tests for carrier animals, the ELISA results for the 38 animals that had a positive probang result (after FMDV was cultured and typed) were examined. The ELISA test results were plotted against the number of months since the last outbreak of FMD in the herd reported by the herdsman (Fig. 3). In this study most carrier animals came from herds with FMD reported in the previous 12 months. The range of OD percentage values recorded for each of the two tests varied, with a more clustered pattern well below the test cutoff value for the C-ELISA and a more dispersed range of readings straddling the cutoff for the CHEKIT ELISA. The C-ELISA detected 35 of 38 (92%) positive animals, while the CHEKIT ELISA detected only 18 of 38 (47%).
FIG. 3.
Scatter plots of the OD readings for the CHEKIT ELISA (+, >30%) and C-ELISA (+, ≤50%) plotted against the number of months since the herdsman reported the last outbreak of FMD for animals that were probang culture positive (n = 38). Cutoffs are marked with horizontal lines.
Implications of using the CHEKIT ELISA and C-ELISA at a herd level.
In order to demonstrate the effect of using these tests at the herd level, the two tests were applied to a theoretical herd of 100 cattle with a range of prevalences of 0 to 30%. The probability of correctly identifying a herd as infected, i.e., the HSe, increases with true prevalence and sample size (Tables 3 and 4). For the CHEKIT ELISA (Table 3), the only commercially available test, the HSe is 9.8% at a prevalence of 1% with a sample size of 5 cattle, while the HSe is 99.9% with a sample size of 55 cattle from a herd with a prevalence of 30%. This higher probability would be achieved with a sample size of about 15 cattle with the C-ELISA (Table 4). In contrast, the probability of correctly classifying a negative herd (HSp) declines with an increasing sample size. For the CHEKIT ELISA, this probability declines from 90.2% with a sample size of 5 to 20% with a sample size of 55. Because of the poorer test Sp, this decline in HSp is a greater problem with the C-ELISA.
TABLE 3.
HSe and HSp of the CHEKIT ELISAa
| No. of animals tested | HSp (0, 2)b | HSe for herd with indicated characteristics
|
||||
|---|---|---|---|---|---|---|
| 1, 2b | 5, 3 | 10, 4 | 20, 6 | 30, 8 | ||
| 5 | 0.902 | 0.098 | 0.144 | 0.188 | 0.271 | 0.347 |
| 15 | 0.721 | 0.279 | 0.389 | 0.484 | 0.633 | 0.741 |
| 25 | 0.561 | 0.439 | 0.582 | 0.690 | 0.831 | 0.909 |
| 35 | 0.420 | 0.580 | 0.730 | 0.827 | 0.931 | 0.973 |
| 45 | 0.300 | 0.700 | 0.838 | 0.913 | 0.976 | 0.993 |
| 55 | 0.200 | 0.800 | 0.912 | 0.962 | 0.993 | 0.999 |
Assumptions: cutoff point, 1; Se, 0.23; Sp, 0.98. Sampling without replacement. Herd size, 100 animals. Hypergeometric distribution used.
Number of infected animals in herd, number of test positives in herd.
TABLE 4.
HSe and HSp of the C-ELISAa
| No. of animals tested | HSp (0, 10)b | HSe for herd with indicated characteristics
|
||||
|---|---|---|---|---|---|---|
| 1, 11b | 5, 13 | 10, 16 | 20, 22 | 30, 28 | ||
| 5 | 0.584 | 0.449 | 0.509 | 0.590 | 0.720 | 0.814 |
| 15 | 0.181 | 0.849 | 0.896 | 0.942 | 0.983 | 0.995 |
| 25 | 0.048 | 0.965 | 0.982 | 0.994 | 0.999 | 1.000 |
| 35 | 0.010 | 0.994 | 0.998 | 1.000 | 1.000 | 1.000 |
| 45 | 0.002 | 0.999 | 1.000 | 1.000 | 1.000 | 1.000 |
| 55 | 0.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 |
Assumptions: cutoff point, 1; Se, 0.71; Sp, 0.9. Sampling without replacement. Herd size, 100 animals. Hypergeometric distribution used.
Number of infected animals in herd, number of test positives in herd.
DISCUSSION
Serological tests which differentiate FMDV-vaccinated animals from unvaccinated, exposed animals that are either recovering or that are persistently infected could be used (i) for herd surveillance following emergency vaccination in a disease-free country after an outbreak or as part of an eradication program in a country where the disease is endemic or (ii) to screen individuals or groups of animals at import-export stations. An added advantage of the NSP tests is that a single test can be used to detect antibodies to any of the seven serotypes of FMDV. Our original intention was to use the CHEKIT ELISA to classify the herds in this unvaccinated cattle population as having been exposed or not exposed to FMDV. This would have avoided the need to perform multiple, time-consuming VNTs.
The comparison of the two NSP ELISAs with the cVNT as the gold standard indicates that the CHEKIT ELISA has very high Sp (98%) but very low Se (23% for adults and juveniles or 15% for juveniles only). No previous publications mention this kit, although several studies used a similar format with an E. coli-expressed protein. The parameter estimates were high, with an Se of 100% and an Sp of 99% in cattle 20 days postinfection (31) and an Se of 92% and an Sp of 90% in sheep (7). However, these studies were based on small, experimentally infected groups rather than naturally infected populations. The problem with this test population is that the test is then not being implemented against the range of disease stages likely to be encountered in the field. In comparison, the results of the present study were based on a population sample from naturally infected cattle.
The Danish 3ABC C-ELISA had much higher Se but lower Sp. This test has been used more widely in both a 3AB and a 3ABC format. In Chinese cattle in Taiwan, the parameters for the 3AB format were estimated to be an Se of 64% and an Sp of 99% (22). In pigs, also in Taiwan, the estimates were an Se of 73% and an Sp of 90% (13), although in a later communication they were revised to be an Se of 96% and an Sp of 99% (12). The 3ABC format was used in a small sample of experimentally infected cattle (n = 58), and the estimates were an Se of 88% and an Sp of 99.8% (41). These estimates compare with an Sp of 90 to 94% and an Se of 57 to 71% in the present study. There may be several reasons for this difference. There may be nonspecific cross-reactions with other pathogens or antigens to which animals are exposed in the tropics or differences between breeds and management systems (21). It has also been suggested that tests based on these long recombinant proteins may cross-react with antibodies to the system in which they were produced, such as insect proteins. Using peptides for specific epitopes may help overcome this problem (37).
Se and Sp are not fixed values and will differ between subpopulations and between populations depending on the distribution of influential covariates (e.g., age and stage of disease). It has been suggested that the 3ABC test be used with young animals during surveillance (3); however, our results suggest that in fact there is a lower relative Se in the juvenile population for both of these tests. The CHEKIT ELISA had a particularly low Se of 15% in this age group. Therefore an even greater risk of failing to identify seropositive animals would exist if only juveniles were selected.
VNTs were used as the gold standard in this comparison, using three (O, A, and SAT 2) of the six serotypes likely to occur in Africa. These three were selected because they were the only serotypes isolated from the population. SAT 1 has never been identified in Cameroon, although its presence has been recorded in neighboring Nigeria, and serotypes SAT 3 and C were unlikely to occur in this region (43). Previous studies have suggested that the antibody response to the 3ABC proteins occurs slightly later than the VN response to the structural proteins (41) and that the 3ABC antibodies may not persist for as long as the VN antibodies. The estimates range from only a few months (8) up to 3.5 years (13) for the 3ABC antibodies, compared to 1 to 3 years (1) up to 4.5 years (14) for the VN antibodies. Therefore, comparing the 3ABC ELISAs with the cVNT is likely to produce a low Se estimate if the durations of the antibody response are different. Another approach would be to compare the NSP tests using a model without a gold standard (23). Both maximum likelihood and Bayesian approaches are being investigated, and the results of this investigation will be presented in a technical statistical paper.
The problems of these tests at the level of the individual animal change when the tests are used to classify herds as positive or negative. Poor individual test Se can be improved by increasing the sample size, although the very poor Se of the CHEKIT ELISA will never be overcome unless the true herd prevalence is very high. However, one of the suggested roles for these tests is to differentiate vaccinated from exposed or carrier animals following an outbreak and emergency vaccination program. If vaccination has been successful and transmission has been reduced, it is likely that the prevalence of exposed and carrier animals within a herd will be very low. Even with perfect test Sp, the CHEKIT ELISA would not be able to detect herds with low prevalences, although the C-ELISA would perform much better. However, because the Sp is less than perfect for both tests, there is in fact a counterintuitive increase in HSe due to the false positives. The C-ELISA has much better HSe for a given true prevalence, but the cost will be many more false-positive herds that will need to be retested with another, more specific test. While this requirement may protect an importing country, it would be a major problem for an exporting country. In particular, the increasing problem of false positives can be combated by altering the cutoff number of positives or by using a second, more specific test. The current recommendation by the OIE is a combination of a 3ABC ELISA and a subsequent enzyme-linked immunoelectrotransfer blot assay to confirm all positives (6). Such an approach has been used successfully in South America, where vaccination has been used for eradication (5).
In situations in areas of endemicity at the start of an eradication program, the C-ELISA will clearly be the better test to use since the sample sizes will be smaller and the problem of false positives will be reduced. However, as the prevalence declines, the same problems will arise as previously discussed, and the strategy may need to be adjusted accordingly. In terms of screening import-export animals, the CHEKIT ELISA is likely to be of use only to exporters needing to declare animals seronegative since the test has such a low Se. The C-ELISA, in contrast, will have problems with many false positives. The tests should not be used or interpreted on a herd basis unless the animals constitute a complete herd or epidemiological group, which in many import-export situations is not the case. It would, however, be useful to model the different situations and costs of the different tests in order to identify the most efficient strategies for each situation.
The main hope for these 3ABC tests is that they will help to identify carrier animals and subclinical infections in a vaccinated population during the surveillance phase after an outbreak or that they can be used during an eradication based on vaccination to monitor areas with continuing transmission. However, in the present study only 47% of carrier animals (those that showed no clinical signs at the time of sampling but yielded probang samples that were FMDV positive) were detected by the commercial test. The C-ELISA performed better but still failed to detect 8% (3 of 38) of probang-positive animals. One of these animals may have been at a very early stage of infection, while it had been several years since the last reported infection for another. In both cases the titers might be expected to be low. Although the present study was carried out with an unvaccinated population, the tests need to be able to detect seropositive animals that are carriers or subclinically infected regardless of whether the animals had been vaccinated; if the tests fail to detect these exposed seropositive populations, the issue of differentiating them from vaccinated animals becomes meaningless. Clearly, however, it would be useful to have a vaccinated exposed population with which to compare these results.
The recent changes in OIE regulations will allow countries to resume trading after 6 instead of 12 months if the animals are vaccinated and can then be screened and demonstrated to be free from disease. While this change will facilitate trade in line with the current World Trade Organization agreements, it requires a test that will distinguish vaccinated from subclinically infected or convalescing animals. This study does not address the difficulties of differentiating vaccinated animals but highlights the problems of detecting infected animals and herds. It shows that both the commercially available CHEKIT ELISA and the C-ELISA have problems at the level of the individual animal. Some of these problems can be overcome by using the tests at the herd level. This study demonstrates the importance of evaluating these tests at the population level in an environment in which they will be used. This requirement is essential in ensuring that commercial pressures do not compromise international animal health.
Acknowledgments
Mark Bronsvoort was funded by a Wellcome Trust Research Training Fellowship in Clinical Tropical Epidemiology (grant no. 053840).
We thank the Provincial Delegate for MINEPA, Adamou Abba, and all his staff, without whom the study would have not been possible. Finally, we thank the cattle owners and herdsmen for their hospitality and for their time and effort in allowing their herds to be examined.
REFERENCES
- 1.Bachrach, H. L. 1968. Foot-and-mouth disease. Annu. Rev. Microbiol. 22:201-244. [DOI] [PubMed] [Google Scholar]
- 2.Berger, H. G., O. C. Straub, R. Ahl, M. Tesar, and O. Marquardt. 1990. Identification of foot-and-mouth disease virus replication in vaccinated cattle by antibodies to non-structural virus proteins. Vaccine 8:213-216. [DOI] [PubMed] [Google Scholar]
- 3.Bergmann, I. E., V. Astudillo, V. Malirat, and E. Neitzert. 1998. Serodiagnostic strategy for estimation of foot-and-mouth disease viral activity through highly sensitive immunoassays using bioengineered nonstructural proteins. Vet. Q. 20(Suppl. 2):S6-S9. [DOI] [PubMed] [Google Scholar]
- 4.Bergmann, I. E., P. A. de Mello, E. Neitzert, E. Beck, and I. Gomes. 1993. Diagnosis of persistent aphthovirus infection and its differentiation from vaccination response in cattle by use of enzyme-linked immunoelectrotransfer blot analysis with bioengineered nonstructural viral antigens. Am. J. Vet. Res. 54:825-831. [PubMed] [Google Scholar]
- 5.Bergmann, I. E., V. Malirat, L. E. Dias, and R. Dilandro. 1996. Identification of foot-and-mouth disease virus-free regions by use of a standardized enzyme-linked immunoelectrotransfer blot assay. Am. J. Vet. Res. 57:972-974. [PubMed] [Google Scholar]
- 6.Bergmann, I. E., V. Malirat, E. Neitzert, E. Beck, N. Panizzutti, C. Sanchez, and A. Falczuk. 2000. Improvement of a serodiagnostic strategy for foot-and-mouth disease virus surveillance in cattle under systematic vaccination: a combined system of an indirect ELISA-3ABC with an enzyme-linked immunoelectrotransfer blot assay. Arch. Virol. 145:473-489. [DOI] [PubMed] [Google Scholar]
- 7.Blanco, E., L. J. Romero, M. El Harrach, and J. M. Sanchez-Vizcaino. 2002. Serological evidence of FMD subclinical infection in the sheep population during the 1999 epidemic in Morocco. Vet. Microbiol. 85:13-21. [DOI] [PubMed] [Google Scholar]
- 8.Brocchi, E., M. I. De Diego, A. Berlinzani, D. Gamba, and F. De Simone. 1998. Diagnostic potential of Mab-based ELISAs for antibodies to non-structural proteins of foot-and-mouth disease virus to differentiate infection from vaccination. Vet. Q. 20(Suppl. 2):S20-S24. [PubMed] [Google Scholar]
- 9.Bronsvoort, B. M. D., V. N. Tanya, R. P. Kitching, C. Nfon, S. M. Hamman, and K. L. Morgan. 2003. Foot-and-mouth disease and livestock husbandry practices in the Adamawa Province of Cameroon. Trop. Anim. Health Prod. 35:491-507. [DOI] [PubMed] [Google Scholar]
- 10.Cannon, R. M., and R. T. Roe. 1982. Livestock disease surveys: a field manual for veterinarians. Australian Government Publishing Service, Canberra.
- 11.Carroll, A. R., D. J. Rowlands, and B. E. Clarke. 1984. The complete nucleotide sequence of the RNA coding for the primary translation product of foot-and-mouth disease virus. Nucleic Acids Res. 12:2461-2472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chung, W. B., P. C. Liao, P. C. Yang, S. P. Chen, M. H. Jong, and T. W. Sheu. 2003. Surveillance of FMD virus non-structural protein antibodies in pig populations involved in an eradication programme. Vet. Rec. 152:395-397. [DOI] [PubMed] [Google Scholar]
- 13.Chung, W.-B., K. J. Sorensen, P.-C. Liao, P.-C. Yang, and M.-H. Jong. 2002. Differentiation of foot-and-mouth disease virus-infected from vaccinated pigs by enzyme-linked immunosorbent assay using nonstructural protein 3AB as the antigen and application to an eradication program. J. Clin. Microbiol. 40:2843-2848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cuncliffe, H. R. 1962. Observations on the duration of immunity in cattle after experimental infection with foot-and-mouth disease. Cornell Vet. 54:501-510. [PubMed] [Google Scholar]
- 15.De Diego, M., E. Brocchi, D. Mackay, and F. De Simone. 1997. The non-structural polyprotein 3ABC of foot-and-mouth disease virus as a diagnostic antigen in ELISA to differentiate infected from vaccinated cattle. Arch. Virol. 142:2021-2033. [DOI] [PubMed] [Google Scholar]
- 16.DeLong, E. R., D. M. DeLong, and D. L. Clarke-Pearson. 1988. Comparing the areas under two or more correlated receiver operating characteristic curves: a non-parametric approach. Biometrics 44:837-845. [PubMed] [Google Scholar]
- 17.Ferris, N. P., and M. Dawson. 1988. Routine application of enzyme-linked immunosorbent assay in comparison with complement fixation for the diagnosis of foot-and-mouth and swine vesicular diseases. Vet. Microbiol. 16:201-209. [DOI] [PubMed] [Google Scholar]
- 18.Forss, S., K. Strebel, E. Beck, and H. Schaller. 1984. Nucleotide sequence and genome organisation of foot-and-mouth disease virus. Nucleic Acids Res. 12:6587-6601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gardner, I. A., H. Stryhn, P. Lind, and M. T. Collins. 2000. Conditional dependence between tests affects the diagnosis and surveillance of animal diseases. Prev. Vet. Med. 45:107-122. [DOI] [PubMed] [Google Scholar]
- 20.Golding, S. M., R. S. Hedger, and P. Talbot. 1976. Radial immuno-diffusion and serum neutralisation techniques for the assay of antibodies to swine vesicular disease. Res. Vet. Sci. 20:142-147. [PubMed] [Google Scholar]
- 21.Greiner, M., and I. A. Gardner. 2000. Epidemiologic issues in the validation of veterinary diagnostic tests. Prev. Vet. Med. 45:3-22. [DOI] [PubMed] [Google Scholar]
- 22.Huang, C. C., F. Lee, W. J. Tu, S. H. Lee, T. S. Huang, Y. L. Lin, M. H. Jong, and S. Y. Lin. 2002. Anti-3AB antibodies in the Chinese yellow cattle infected by the O/Taiwan/99 foot-and-mouth disease virus. Vet. Microbiol. 84:317-326. [DOI] [PubMed] [Google Scholar]
- 23.Hui, S. L., and S. D. Walter. 1980. Estimating the error rates of diagnostic tests. Biometrics 36:167-171. [PubMed] [Google Scholar]
- 24.Karber, G. 1931. Beitrag zur kollectiven Behandlung pharmakologischer Reikenversuche. Arch. Exp. Pathol. Pharmakol. 162:480-483. [Google Scholar]
- 25.King, A. M. Q. 2000. Picornaviridae, p. 657-673. In M. H. V. Van Regenmortel, C. M. Fauquet, and D. H. L. Bishop (ed.), Virus taxonomy: classification and nomenclature of viruses. Academic Press, San Diego, Calif.
- 26.Kitching, R. P. 2002. Clinical variation in foot and mouth disease: cattle. Rev. Sci. Tech. Off. Int. Epizoot. 21:499-504. [DOI] [PubMed] [Google Scholar]
- 27.Kitching, R. P., and S. Alexandersen. 2002. Clinical variation in foot and mouth disease: pigs. Rev. Sci. Tech. Off. Int. Epizoot. 21:513-518. [DOI] [PubMed] [Google Scholar]
- 28.Kitching, R. P., P. V. Barnett, A. I. Donaldson, and D. Mackay. 2000. Foot-and-mouth disease, p. 77-92. In S. Linnane (ed.), Manual of standards for diagnostic tests and vaccines, 4th ed., vol. 1. Office International des Epizooties, Paris, France. [Google Scholar]
- 29.Kitching, R. P., and G. J. Hughes. 2002. Clinical variation in foot and mouth disease: sheep and goats. Rev. Sci. Tech. Off. Int. Epizoot. 21:505-512. [DOI] [PubMed] [Google Scholar]
- 30.Mackay, D. K. J., M. A. Forsyth, P. R. Davies, A. Berlinzani, G. J. Belsham, M. Flint, and M. D. Ryan. 1998. Differentiating infection from vaccination in foot-and-mouth disease using a panel of recombinant, non-structural proteins in ELISA. Vaccine 16:446-459. [DOI] [PubMed] [Google Scholar]
- 31.Malirat, V., E. Neitzert, I. E. Bergmann, E. Maradei, and E. Beck. 1998. Detection of cattle exposed to foot-and-mouth disease virus by means of an indirect ELISA test using bioengineered nonstructural polyprotein 3ABC. Vet. Q. 20(Suppl. 2):S24-S26. [DOI] [PubMed] [Google Scholar]
- 32.Martin, S. W., M. Shoukri, and M. A. Thorburn. 1992. Evaluating the health status of herds based on tests applied to individuals. Prev. Vet. Med. 14:33-43. [Google Scholar]
- 33.McVicar, J. W., and P. Sutmoller. 1970. Foot-and-mouth disease: the agar gel diffusion precipitin test for antibody to virus-infection-associated (VIA) antigen as a tool for epizootiologic surveys. Am. J. Epidemiol. 92:273-279. [DOI] [PubMed] [Google Scholar]
- 34.Meyer, R. F., G. D. Babcock, J. F. Newman, T. G. Burrage, K. Toohey, J. Lubroth, and F. Brown. 1997. Baculovirus expressed 2C of foot-and-mouth disease virus has the potential for differentiating convalescent from vaccinated animals. J. Virol. Methods 65:33-43. [DOI] [PubMed] [Google Scholar]
- 35.Pinto, A. A., and J. M. Garland. 1979. Immune response to virus-infection-associated (VIA) antigen in cattle repeatedly vaccinated with foot-and-mouth disease virus inactivated by formalin or acetylethyleneimine. J. Hyg. Camb. 82:41-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Roeder, P. L., and P. M. Le Blanc Smith. 1987. Detection and typing of foot-and-mouth disease virus by enzyme-linked immunosorbent assay: a sensitive, rapid and reliable technique for primary diagnosis. Res. Vet. Sci. 43:225-232. [PubMed] [Google Scholar]
- 37.Shen, F., P. D. Chen, A. M. Walfield, J. Ye, J. House, F. Brown, and C. Y. Wang. 1999. Differentiation of convalescent animals from those vaccinated against foot-and-mouth disease by a peptide ELISA. Vaccine 17:3039-3049. [DOI] [PubMed] [Google Scholar]
- 38.Snowdon, W. A. 1966. Growth of foot-and-mouth disease virus in monolayer cultures of calf thyroid cells. Nature 210:1079-1080. [DOI] [PubMed] [Google Scholar]
- 39.Sørensen, K. J., C. M. Hansen, E. S. Madsen, and K. G. Madsen. 1998. Blocking ELISAs using the FMDV nonstructural proteins 3D, 3AB, and 3ABC produced in the baculovirus expression system. Vet. Q. 20:S17-S20. [PubMed] [Google Scholar]
- 40.Sørensen, K. J., R. L. Madekurozwa, and P. Dawe. 1992. Foot-and-mouth disease: detection of antibodies in cattle sera by blocking ELISA. Vet. Microbiol. 32:253-265. [DOI] [PubMed] [Google Scholar]
- 41.Sørensen, K. J., K. G. Madsen, E. S. Madsen, J. S. Salt, J. Nqindi, and D. K. J. Mackay. 1998. Differentiation of infection from vaccination in foot-and-mouth disease by the detection of antibodies to the non-structural proteins 3D, 3AB and 3ABC in ELISA using antigens expressed in baculovirus. Arch. Virol. 143:1461-1476. [DOI] [PubMed] [Google Scholar]
- 42.Sugimura, T., T. Suzuki, A. Chatchawanchonteera, P. Sinuwonkwat, T. Tsuda, and Y. Murakami. 2000. Application of latex beads agglutination test for the detection of the antibody against virus-infection-associated (VIA) antigen of foot-and-mouth disease (FMD) virus. J. Vet. Med. Sci. 62:805-807. [DOI] [PubMed] [Google Scholar]
- 43.Vosloo, W., A. D. S. Bastos, O. Sangare, S. K. Hargreaves, and G. R. Thomson. 2002. Review of the status and control of foot and mouth disease in sub-Saharan Africa. Rev. Sci. Tech. Off. Int. Epizoot. 21:437-449. [DOI] [PubMed] [Google Scholar]