Abstract
Human skin has the ability to synthesize glucocorticoids de novo from cholesterol or from steroid intermediates of systemic origin. By interacting with glucocorticoid receptors, they regulate skin immune functions as well as functions and phenotype of the epidermal, dermal and adnexal compartments. Most of the biochemical (enzyme and transporter activities) and regulatory (neuropeptides mediated activation of cAMP and protein kinase A dependent pathways) principles of steroidogenesis in the skin are similar to those operating in classical steroidogenic organs. However, there are also significant differences determined by the close proximity of synthesis and action (even within the same cells) allowing para-, auto- or intracrine modes of regulation. We also propose that ultraviolet light B (UVB) can regulate the availability of 7-dehydrocholesterol for transformation to cholesterol with its further metabolism to steroids, oxysterols or Δ7 steroids, because of its transformation to vitamin D3. In addition, UVB can rearrange locally produced Δ7 steroids to the corresponding secosteroids with a short- or no-side chain. Thus, different mechanisms of regulation occur in the skin that can be either stochastic or structuralized. We propose that local glucocorticosteroidogenic systems and their regulators, in concert with cognate receptors operate to stabilize skin homeostasis and prevent or attenuate skin pathology.
Keywords: corticosteroids, HPA axis, metabolism, receptors, skin, steroidogenesis, stress
Introduction
Skin functions involve barrier function, thermo- and electrolyte regulation, insulation, absorption of chemicals and production and inactivation of various factors (1–11). The maintenance of local homeostasis is achieved through close interaction of epidermal (10,12), adnexal (13–15), pigmentary (16,17), local immune (18,19), fibroblastic, vascular, adipose and neural systems (20,21), of which either stochastic or organized activities are determined by the cell type and differentiation level, and the local context determined by spatial location (11). As the involvement of the skin neuroendocrine system in the regulation of local homeostasis is appreciated (8,11,22), a similar role for the cutaneous glucocorticoisteroidogenis deserves special consideration (5,7).
General principles of glucocorticosteroidogenesis and mechanisms regulating it
Synthesis of all steroid hormones (corticosteroids, progesterone, androgens and oestrogens) starts with production of pregnenolone from cholesterol through its side chain cleavage catalysed by CYP 11A1, an obligatory enzyme of the pathway (Figs 1 and 2) (23–27). Pregnenolone is then modified by a tissue specific set of steroidogenic enzymes to produce corticosteroids, progesterone, androgens and oestrogens. Glucocorticoids (GC) are produced through a concerted and context-dependent action of CYP17A1, CYP21A2, CYP11B1 and 3β-HSD2, which are subject to central and local hormonal regulation (23,25,28–30). The major pathways of inactivation and activation of cortisol include the enzymes 11β-HSD2 and 11β-HSD1 (23). The detailed description of biochemical principles and regulatory mechanisms governing glucocorticosteroidogenesis is in the Data S1.
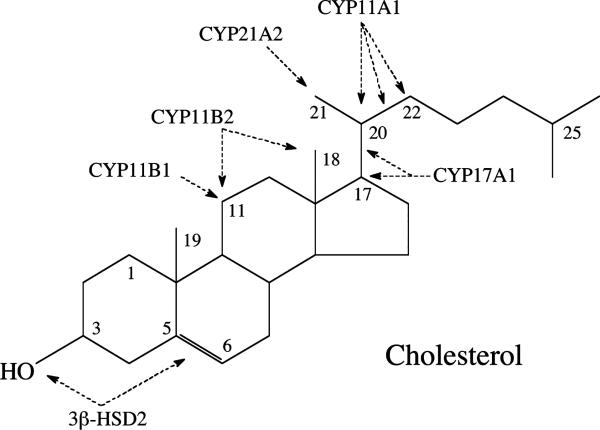
Figure 1.
The sites of modification of the steroid molecule by the steroidogenic enzymes present in the adrenal cortex. The sites of metabolism, using the cholesterol molecule as a template, are shown by arrows (for further details see Fig. 2). Note that the enzymes act in a specific order, dictated by the structure of their immediate substrate. Only CYP11A1 can act on cholesterol, cleaving six carbons from the side chain to produce pregnenolone (23,25). This reaction involves initial hydroxylation of the side chain at C22 to produce 22R-hydroxycholesterol, hydroxylation at C20 to produce 20R,22R-dihydroxycholesterol then oxidative cleavage of the C20-C22 bond producing pregnenolone and isocaproic aldehyde (25,27).
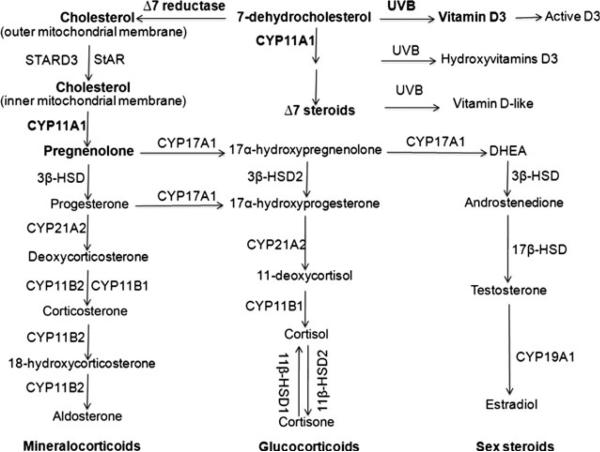
Figure 2.
Corticosteroid synthetic pathways in the skin. Initially the B ring of 7DHC is reduced by Δ7-reductase to produce cholesterol, which then has its side chain cleaved by CYP11A1 to produce pregnenolone. Pregnenolone is then metabolized in a context-dependent manner by steroidogenic enzymes to either mineralo- or glucocorticods or sex steroids. The side chain of 7DHC is also cleaved by CYP11A1 to produce 7DHP, which can be acted on by other steroidogenic enzymes producing Δ7 steroids (6,35,53). Furthermore, 7DHC (50), its hydroxyderivatives and Δ7 steroids (5,6) can be transformed by ultraviolet light B (UVB) to the corresponding secosteroids. Vitamin D3 can be activated through the classical pathway with production of 25(OH)D3 and 1,25(OH)2D3 (50,51) or the non-classical CYP11A1-dependent pathway, with products further modified by CYP27A1 and CYP27B1 (123,125). In addition, similar CYP11A1-dependent metabolism of vitamin D2 was reported recently in human epidermal keratinocytes (126).
Glucocorticosteroidogenesis in the skin
Description of the pathway
Besides the very active and well-characterized steroidogenic organs comprising the adrenal cortex, ovary, testis and placenta, a number of other cells and tissues are capable of de novo steroid synthesis from cholesterol, defining them as steroidogenic (5,7,23,28,31,32). These tissues include the brain, gut, heart, prostate, thymus, immune system and skin. Corticosteroidogenesis in these tissues is relatively low and may also include metabolic transformations of steroid intermediates (progesterone, 17α-hydroxyprogesterone) from other tissues, or activation of biologically inactive precursors. The steroid products likely play paracrine, autocrine and perhaps even intracrine roles, rather than an endocrine role (5,7). The pathways for corticosteroid synthesis in the skin are almost identical to the adrenal cortex as detailed in Fig. 2 (5,23). Specifically, human skin expresses CYP11A11, 3β-HSD, CPY17A1, CYP21A2 and CYP11B1, and produces corticosterone and cortisol (33–43). It expresses steroidogenic acute regulatory protein (StAR), and StAR-related lipid transfer protein (STARD3; also called metastatic lymph node 64, MLN64) that are required for cholesterol transport into mitochondria necessary for de novo steroidogenesis from cholesterol (5,28,35,41). The glucocorticosteroidogenic activity has been detected in epidermal and follicular keratinocytes, melanocytes and dermal fibroblasts (36–42), as well as in melanoma cells (7,34) cultured in vitro. Local levels of cholesterol and steroids can also be regulated by steroid sulphatase, which catalyses transformation of sulphated cholesterol or steroids to non-sulphated biologically active compounds or their precursors (44,45). Skin cells express 11β-HSD1 and 11β-HSD2 that transform cortisol to cortisone and vice versa, respectively (42,46), permitting rapid activation or inactivation of GC in the skin. Rodent skin expresses CYP11A1 (35) and has the ability to produce corticosterone (47,48). It should be noted that production of corticosteroids is context and cell type dependent. For example, under standard conditions of culture of HaCaT keratinocytes, evaluation of cortisol and corticosterone production requires sensitive methods because of the abundance of several other steroids that are easily detectable by less sensitive methods (49). On the other hand, high production of corticosterone was observed in melanoma cells, with the rate of synthesis from progesterone or deoxycorticosterone being even higher than in an immortalized adrenocortical cell line (34).
There are also differences between human skin and the major or even secondary steroidogenic tissues (Fig. 2). Specifically, the highest body concentration of 7-dehydrocholesterol (7DHC), the precursor to cholesterol, is in the epidermis. After exposure to ultraviolet light B (UVB) solar radiation, 7DHC undergoes transformation to vitamin D3 or lumi- and tachysterol, depending on the energy of the UVB (50,51). Vitamin D3 can be activated locally in the epidermis through the sequential action of 25-hydroxylase (CYP27A1 and CYP2R1) and 1α-hydroxylase (CYP27B1), to the biologically active 1,25-diydroxyvitamin D3 (1,25(OH)2D3 (51). Thus, UVB can determine the cholesterol availability for local production of either steroids or oxysterols, which are modulators of cholesterol synthesis and metabolism (7). Furthermore, CYP11A1 has a higher catalytic efficiency with 7DHC as substrate than with cholesterol, indicating that 7DHC can be used preferentially (35). In fact, we and others have elucidated the following pathway for the metabolism of 7DHC by CYP11A1: 7DHC→22-hydroxy-7DHC → 20,22-dihydroxy-7DHC → 7-dehydropregnenolone (7DHP) (35,52), which can operate in vivo in epidermal keratinocytes (53,54). 7DHP can be further hydroxylated by steroidogenic enzymes expressed in the skin to produce Δ7-steroids (35,53,54), which are characteristic of the Smith-Lemli-Optiz (SLOS) syndrome (55). These are biologically active on skin cells and can be phototransformed to the corresponding secosteroids that are also biologically active (6,54,56–58). Thus, in the skin, the activity of 7-dehydrocholesterol reductase (Δ7-reductase) determines whether cholesterol-derived or 7DHC-derived Δ7-steroids are produced, and thus the amount of unsaturated B-ring steroids available for photochemical transformation on exposure to the UVB. For the corresponding schemes outlining these novel pathways, we refer to the following papers (6,7,53).
The skin expresses the genes encoding 7-hydroxylases that can hydroxylate steroids and cholesterol at either the 7α- or 7β-positions (7). Because non-enzymatic oxidation of the B ring in sterols and steroids appears to be conserved in evolution (59,60), it is likely that such process can occur in the skin under oxidative conditions, perhaps induced by UVR. Although 7α-hydroxysteroids are converted to 7β-hydroxysteroids through enzymatic reactions (61–67), oxidative stress-induced non-enzymatic conversions between 7α-hydroxy- and 7-oxo-intermediates, and 7β-hydroxy metabolites (59,60), are also possible in the skin following oxidative stress (59,60). Interestingly, 7DHC may also be a substrate for oxidation by CYP7A1, which would lead to production of 7-ketocholesterol, which is toxic in the liver and is a major oxysterol in human atherosclerotic plaques and photodamaged rat retina (68). In relation to other oxysterols, local production of 25-, 26- or 27-hydroxycholesterol or 25-, 26- or 27-hydroxy-7DHC should be considered because of cutaneous expression of CYP27A1, which can produce in the liver 25-hydroxy and 26/27-hydroxy7DHC (69). This is in addition to production of 22-hydroxy- or 20,22-dihydroxy-cholesterol or 22-hydroxy- or 20,22-dihydroxy-7DHC, because of the expression of CYP11A1 in skin cells (53,54). Production of 20-hydroxycholesterol is also possible, as it was clearly detected in the brain and placenta (70).
Skin also is a recognized site of androgen and oestrogens synthesis and metabolism (5,14,71,72) being the main, if not sole, body producent of oestrogen in postmenopausal women (73). This topic is covered by several extensive reviews and therefore not further discussed in this viewpoint (5,14,71,72).
Regulation of glucocorticosteroidogenesis in the skin
The general principles regulating local glucocorticosteroidogenesis are similar to those operating in classical steroidogenic tissues (see Data S1). Briefly, in skin cells, corticosteroid synthesis is stimulated by corticotropin releasing hormone (CRH), ACTH, cAMP and cytokines (36–40,42). Furthermore, human skin expresses CRH, CRH receptors and proopiomelanocortin (POMC) with the entire machinery necessary to produce the final regulatory peptides including ACTH, α-MSH and β-endorphin, and also expresses the corresponding receptors including MC2R [reviewed in (11,22,74)]. There is evidence that stimulation of cutaneous corticosteroidogenesis can occur via this skin homologue of the HPA axis which is dependent on the functional activity of CRH receptor type 1 (CRH-R1) and expression and processing of POMC (36–38). The stimulation of cortisol synthesis by IL-1 (42) is intriguing because IL-1 serves as a local signal of skin injury. It is possible that IL-1 may stimulate corticosteroidogenesis indirectly, through upregulation of CRH or POMC peptides or by itself, which has been described in the adrenals [reviewed in (22)]. For CRH, in addition to indirectly stimulating local corticosteroidogenesis, it can also directly stimulate it because it increases cAMP (75), and a similar direct action of CRH on adrenocortical cells has been reported [reviewed in (22)]. The same tissue location of all elements of the HPA axis and of cytokine production provide a background for multiple levels of regulation of local steroidogenesis through direct or indirect mechanisms, which would be impossible at a systemic level because of anatomical separation of the immune system, hypothalamus, pituitary and adrenals (76). An additional mechanism of regulation involves the activation or inactivation of glucocorticosteroids by locally expressed 11b-hydroxysteroid dehydrogenase type1 (11β-HSD1) and 11β-HSD2 (40,42,46,77). Thus, different regulatory circuits (direct or indirect involving HPA organization) could operate in the skin, some of which could even bypass MC2R (via stimulation of cAMP in skin cells), all acting in concert to regulate local steroidogenesis [reviewed in (5,7,11,22,38,40,42,78,79)].
There is a shortage of information concerning inhibitory feedback of GC on the CRH and POMC signalling system. However, inhibition of POMC and CRH expression by dexamethasone was documented in mouse skin and cultured human skin cells, respectively (80,81). Nevertheless, detailed studies have to be performed to dissect which circuits are inhibited by GC and which are resistant to it, as described for some peripheral organs [reviewed in (22)]. It must be noted that GC are potent inhibitors of proinflammatory cytokines production in the skin, including IL-1.
An additional important factor that directly affects steroidogenesis in the skin but not in internal organs is UVR. Specifically, short wavelengths of UVR (UVB and UVC) but not UVA, stimulate cortisol and corticosterone synthesis in mammalian skin (46,48,82), which is associated with upregulation of CRH, POMC, ACTH, MC1R, MC2R, CYP11A1, CYP11B1 (human but not mouse skin) and 11β-HSD1, and downregulation of 11β-HSD2 (46,82). Thus, highly energetic short wavelengths of UVR are necessary to stimulate corticosteroidogenesis. How this UVB-induced corticosteroidogenesis is regulated is still unclear. However, it is likely that one mechanism would involve activation of local elements of the HPA axis (CRH, urocortin or POMC peptides) to stimulate cortisol production (78). Also, UVB-induced POMC expression has been shown to be dependent on activation of CRH-R1 (83). An additional mechanism could involve changes in the NADPH/NADP+ ratio determined by the activity of hexose-6-phosphate dehydrogenase and the pentose phosphate pathway, which would dictate whether 11β-HSD1 operates as a reductase, converting cortisone to cortisol.
Oxysterols act as ligands for liver X receptors (LXRα and LXRβ), members of the nuclear receptor superfamily of transcription factors. Potent ligands for LXR include the intermediates in the conversion of cholesterol to pregnenolone by CYP11A1, particularly 22R-hydroxycholesterol (84). LXR ligands such as 20-, 25-, 26- and 27-hydroxycholesterol have been shown to be involved in the stimulation of StAR expression and steroid biosyn-thesis in different steroidogenic cell models (85–88). Induction of LXR-regulated steroidogenesis is mediated by an LXR responsive element in the StAR promoter (87,88). Oxysterols and LXRs are expressed in skin keratinocytes, where they play important roles in epidermal development, differentiation and function (89–91). LXR heterodimerizes with retinoid A receptors (RARs) and retinoid X receptors (RXRs), and has been shown to regulate the transcription of a number of genes involved in cholesterol utilization and metabolism, including sterol regulatory element-binding protein 1c (SREBP-1c), ATP-binding cassette transporter 1 (ABCA1) and StAR (87,92,93). In support of this, an increase in hormone-sensitive lipase (HSL) has recently been shown to be correlated with the expression of LXR-regulated genes, SREBP-1c and ABCA1 in gonadal and adrenal cells (88). Noteworthy, HSL is a multifunctional lipase that catalyses the hydrolysis of cholesteryl- as well as retinyl-esters; thus, it facilitates cholesterol accessibility for steroidogenesis. Both RARs and RXRs mediate the biological action of retinoids that exert a wide range of effects on development, differentiation and epidermal homeostasis (94–96). Importantly, the therapeutic and preventive effects of retinoids in numerous skin disorders, including epithelial tumors, premature skin ageing, skin cancer prevention and cutaneous squamous cell carcinoma (SCC) have long been recognized (94,97,98). Recently, it has been reported that regulation of steroid biosynthesis involves cooperation/interaction between LXR and RAR/RXR signalling (88). Furthermore, activation of LXR has been shown to induce lipid accumulation and inflammation in HaCaT skin keratinocytes, suggesting that agents that regulate the LXR-RAR/RXR heterodimer could be used as therapeutic targets for the treatment of skin ageing and inflammatory diseases (99–101). LXR activators also play important roles in keratinocyte differentiation and anti-inflamma-tory activity (102,103). It has been shown that expression of SREBP-1c mRNA increases during differentiation of HaCaT cells, demonstrating that LXR influences epidermal barrier function and keratinocyte differentiation (104). This suggests that oxysterols are capable of influencing steroidogensis in skin tissues; however, a role of HSL in the regulation of cutaneous steroidogenesis remains unknown. We recently observed that overexpression of HSL increased the efficacies of LXR ligands in StAR expression and steroid synthesis in gonadal and HaCaT cells, suggesting HSL-mediated steroidogenesis entails enhanced oxysterol production [(88); P. R. Manna unpublished observations]. Identification of LXR ligand(s) and its correlation with genomic and non-genomic effects of GC may have significant biological and clinical impact in the context of pertinent dermatological treatments.
Mechanism of action of GC in the skin
Glucocorticoids exert their physiological and pharmacological actions through the GC receptor (GR), a ligand-dependent transcription factor that mediates most of the known biological effects of GCs (11,32,105,106). A large variety of synthetic GC derivatives are widely used therapeutic agents for treating numerous skin immune and inflammatory diseases. GCs bind and activate the GR which is expressed as two major and well-characterized iso-forms, GRα and GRβ, produced by alternative splicing. In humans and mice, each of the GRα and GRβ splice isoforms has at least eight translational variants that display diverse cytoplasm-to-nucleus trafficking patterns and discrete transcriptional activities (105,107,108). The abundance and functional relevance of these translational isoforms uniquely differ in tissue- and cell-specific manners and show differential sensitivity to GCc. Both GRα and GRβ are ubiquitously expressed in all organs and tissues, including skin. Whereas the former is the predominant isoform that binds GCs, the latter does not bind endogenous or synthetic ligands.
The mechanisms by which GCs modulate gene expression are conventionally referred to as transactivation (direct interaction between the GR and the GRE) and transrepression (independent of DNA binding) (Fig. 3, and see Data S1). Whereas the former action of GR is known to be linked to certain adverse side effects (most notably skin atrophy), the later function has been ascribed to its anti-inflammatory action (11,32,109,110). It is noteworthy, however, that genomic effects of GR in skin are also mediated by monomers and trimers. Given its pleiotropic effects in many vital organs, GR is essential for survival and plays an important role in skin homeostasis and epidermal barrier competence. GR is also required for proper skin development, as demonstrated by the analysis of genetically modified mice with gain and loss of function (111–113). Mice lacking GR display incomplete epidermal stratification and abnormal keratinocyte differentiation, resulting in a defective skin barrier (110,114). Multiple signalling pathways such as PKA, PKC, MAPK and PI3K mediate the action of GR in a variety of tissues (115,116).
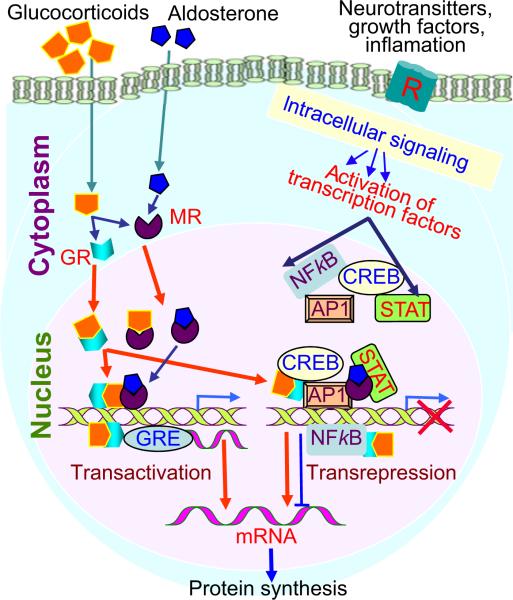
Figure 3.
A model illustrating the main mode of action of glucocorticoids (GC). Transactivation involves binding of GC receptor (GR) dimers to GREs in promoter regions or introns of responsive genes, which results in either activation or repression (for negative GREs). Transrepression involves interaction of GR or MR monomers with activated transcription factors via other signalling pathways.
Notably, non-genomic effects of GR and other steroid hormones are well known, and it is conceivable that GR action in skin is influenced, either individually or in combination, with MR, androgen, oestrogen and progesterone receptors, all present in skin tissues. Most recently, evidence for non-genomic signalling by GC in skin cells has been provided (116).
There is a shortage of information on the regulation of GR expression in the skin; however, recent data have shown that both UVB and UVC, but not UVA, inhibit the expression of GR in the human skin (46,82). Hence, it is conceivable that an epidermal mechanism is involved in attenuating the long-term immunosuppression of cortisol through downregulation of its receptor. In many autoimmune skin disorders, GC resistance has been shown to be associated with a qualitative or quantitative deficiency in GR activity. Additional studies are necessary to elucidate the regulatory mechanisms of GRα and GRβ expressions and activity in the context of clinical significance.
The local glucocorticosteroidogenic system secures skin functions and its integrity
Evidence is accumulating that deregulation of local glucocorticosteroidogeneis contributes significantly to skin pathology including inflammatory and autoimmune disorders, and carcinogenesis. First, expression of StAR mRNA is decreased in the skin of psoriatic and atopic dermatitis patients (40,41,117). Furthermore, the coiled-coil α-helical rod protein 1 (CCHCR1), which is involved in skin steroidogenesis, localizes with StAR, and CCHCR1 is downregulated in psoriatic keratinocytes (118). The expression of StAR mRNA is aberrant in several additional skin pathologies, including eczema, intertrigo and seborrheic keratosis, indicating that acute steroid synthesis is disrupted in these conditions (5,7). In addition, StAR expression decreases in skin tissues of ageing populations (47–83 years) of both men and women when compared with young (14–29 years) individuals (P. R. Manna et al., unpublished observations). Also, reduced production or improper inactivation/activation of GC can lead to hyperproliferative benign and malignant skin disorders including basal and SCC (7,119). It is known that elderly individuals are more prone to numerous skin conditions, including thinning, dryness, hair loss and propensity to blister. These data indicate that deterioration of cutaneous steroidogenesis is responsible, at least in part, for these skin abnormalities. Therefore, it is plausible that restoration and/or modulation of the skin steroidogenic activity may potentially rescue and/or delay the ageing process. This is supported by studies with genetically modified mice with gain and loss of function of GR which demonstrate its requirement for proper skin development, normal keratinocyte differentiation and formation of a proper skin barrier (110–114). Also, synthetic GC are widely used for treating numerous skin diseases that require anti-inflammatory activity and immunosuppression (11,110,116,120,121). Furthermore, in wound healing, the initial proinflammatory response is controlled by GC, with acceleration of wound closure occurring when their levels are reduced (42,122).
Finally, UVB and UVC exposure stimulate de novo cortisol/corticosterone synthesis and/or its activation by 11β-HSD1 (46,48,82), which is accompanied by significant downregulation of GR in the epidermis (46). This paradoxical disconnection between signalling molecules and the receptor target should help in preserving skin integrity. Thus, the local accumulation of cortisol/corticosterone would generate a protective field against incoming immune cells that would mount an autoimmune response against epidermal antigens released through the action of UVR, providing protection against autorejection of the epidermis. Downregulation of GR in the epidermal keratinocytes would protect the epidermis from glucocorticoid-induced atrophy and allow its regeneration. Thus, in addition to attenuating or preventing cutaneous pathology, the local corticosteroidogenic system would preserve the skin integrity through sophisticated regulatory mechanisms.
Conclusions and perspectives
In the skin, all of the elements for corticosteroid synthesis and its regulation, as well as corticosteroid action, are in close proximity or even within the same cells, permitting para-, auto- or intracrine modes of regulation. This provides a level of fluidity enabling different mechanisms of regulation that can be either stochastic or structuralized. This is in contrast to the systemic level where such elements are separated anatomically and structurally and follow organized regulatory structures (HPA or HP-gonadal axes) with precise assignment of functions to different histological layers (zona glomerulosa, fasciculata and reticularis in adrenal cortex). An additional difference relates to the relatively high concentration of 7DHC in the epidermis and access to the UVB, which in concert with the action of CYP11A1 or other hydroxylating enzymes, leads to alternative steroidogenic or secosteroidogenic pathways (Fig. 2).
Thus, production and metabolism of different steroids and secosteroids in the skin that mediate their actions via distinct nuclear hormone receptors, which themselves are the subject to regulation by sterols, steroids and secosteroid, make future work at deciphering the complex interactions between different signalling pathways extremely exciting, with therapeutic implications for glucocorticoid-based therapies. In the simplest model, the regulation of local levels of corticosteroids and the activity and availability of their cognate receptors, is central to homeostatic prevention or attenuation of skin pathology. The complexity of regulatory mechanisms indicates that there is no one simple pathway for therapy, with signalling targets identifiable in a context-dependent manner (5,7,11,22,123,124). However, the proof of the concept would require demonstrating that deficiency of the human steroidogenic enzymes in the skin or mouse knockouts leads to detectable changes in the skin phenotype. Such changes in phenotype are seen in defects in steroid sulphatase (45) or 11β-HSD2−/− mice (77). A review of the literature does not reveal any direct link between genetic deficiencies in the enzymes of glucocorticoid synthesis and skin disorders. The major issue here is that any genetic deficiency resulting in reduced cortisol results in increased production of POMC and ACTH in the pituitary, and one cannot dissect the indirect effects of this (and the low cortisol) from the direct effects of the mutation on skin steroidogenesis. Such information will require conditional skin-specific gene knockouts to be performed. The most instructive would be the conditional CYP11A1 knockout in the skin, as this mutation at the systemic level is incompatible with survival.
Supplementary Material
Acknowledgements
This work was supported by NIH Grants 2R01AR052190-A6 and 1R01AR056666-01A2 and NSF Grant # IOS-0918934 to AS. ATS, PRM and RCT contributed equally in design, research analysis and writing of the paper.
Footnotes
Conflict of interest
There are no conflict of interest to declare for all the authors.
Supporting Information
Additional Supporting Information may be found in the online version of this article:
Data S1. Cutaneous glucocorticosteroidogenesis: securing local homeostasis and the skin integrity.
References
- 1.Grando SA, Pittelkow MR, Schallreuter KU. J Invest Dermatol. 2006;126:1948–1965. doi: 10.1038/sj.jid.5700151. [DOI] [PubMed] [Google Scholar]
- 2.Botchkarev VA, Yaar M, Peters EM, et al. J Invest Dermatol. 2006;126:1719–1727. doi: 10.1038/sj.jid.5700270. [DOI] [PubMed] [Google Scholar]
- 3.Feingold KR, Schmuth M, Elias PM. J Invest Dermatol. 2007;127:1574–1576. doi: 10.1038/sj.jid.5700774. [DOI] [PubMed] [Google Scholar]
- 4.Elias PM, Menon G, Wetzel BK, et al. Am J Hum Biol. 2010;22:526–537. doi: 10.1002/ajhb.21043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Slominski A, Zbytek B, Nikolakis G, et al. J Steroid Biochem Mol Biol. 2013;137:107–123. doi: 10.1016/j.jsbmb.2013.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Slominski A, Kim T-K, Zmijewski MA, et al. Dermatoendocrinol. 2013;5:7–19. [Google Scholar]
- 7.Slominski AT, Zmijewski MA, Semak I, et al. Anti-Cancer Agents Med Chem. 2014;14:77–96. doi: 10.2174/18715206113139990308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Slominski A, Wortsman J. Endocr Rev. 2000;21:457–487. doi: 10.1210/edrv.21.5.0410. [DOI] [PubMed] [Google Scholar]
- 9.Botchkarev VA, Gdula MR, Mardaryev AN, et al. J Invest Dermatol. 2012;132:2505–2521. doi: 10.1038/jid.2012.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eckert RL, Crish JF, Robinson NA. Physiol Rev. 1997;77:397–424. doi: 10.1152/physrev.1997.77.2.397. [DOI] [PubMed] [Google Scholar]
- 11.Slominski AT, Zmijewski MA, Skobowiat C, et al. Adv Anat Embryol Cell Biol. 2012;212:v, vii, 1–115. doi: 10.1007/978-3-642-19683-6_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Elias PM, Williams ML, Feingold KR. Clin Dermatol. 2012;30:311–322. doi: 10.1016/j.clindermatol.2011.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stenn KS, Paus R. Physiol Rev. 2001;81:449–494. doi: 10.1152/physrev.2001.81.1.449. [DOI] [PubMed] [Google Scholar]
- 14.Chen WC, Zouboulis CC. Dermatoendocrinol. 2009;1:81–86. doi: 10.4161/derm.1.2.8354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gilhar A, Etzioni A, Paus R. N Engl J Med. 2012;366:1515–1525. doi: 10.1056/NEJMra1103442. [DOI] [PubMed] [Google Scholar]
- 16.Slominski A, Tobin DJ, Shibahara S, et al. Physiol Rev. 2004;84:1155–1228. doi: 10.1152/physrev.00044.2003. [DOI] [PubMed] [Google Scholar]
- 17.Slominski A, Wortsman J, Plonka PM, et al. J Invest Dermatol. 2005;124:13–21. doi: 10.1111/j.0022-202X.2004.23528.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Di Meglio P, Perera GK, Nestle FO. Immunity. 2011;35:857–869. doi: 10.1016/j.immuni.2011.12.003. [DOI] [PubMed] [Google Scholar]
- 19.Gallo RL, Hooper LV. Nat Rev Immunol. 2012;12:503–516. doi: 10.1038/nri3228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roosterman D, Goerge T, Schneider SW, et al. Physiol Rev. 2006;86:1309–1379. doi: 10.1152/physrev.00026.2005. [DOI] [PubMed] [Google Scholar]
- 21.Klein J, Permana PA, Owecki M, et al. Exp Dermatol. 2007;16:45–70. doi: 10.1111/j.1600-0625.2006.00519_1.x. [DOI] [PubMed] [Google Scholar]
- 22.Slominski AT, Zmijewski MA, Zbytek B, et al. Endocr Rev. 2013;34:827–884. doi: 10.1210/er.2012-1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miller WL, Auchus RJ. Endocr Rev. 2011;32:81–151. doi: 10.1210/er.2010-0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miller WL, Bose HS. J Lipid Res. 2011;52:2111–2135. doi: 10.1194/jlr.R016675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tuckey RC. Placenta. 2005;26:273–281. doi: 10.1016/j.placenta.2004.06.012. [DOI] [PubMed] [Google Scholar]
- 26.Hu J, Zhang Z, Shen WJ, et al. Nutr Metab (Lond) 2010;7:47. doi: 10.1186/1743-7075-7-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tuckey RC, Cameron KJ. Biochim Biophys Acta. 1993;1163:185–194. doi: 10.1016/0167-4838(93)90180-y. [DOI] [PubMed] [Google Scholar]
- 28.Anuka E, Gal M, Stocco DM, et al. Mol Cell Endocrinol. 2013;371:47–61. doi: 10.1016/j.mce.2013.02.003. [DOI] [PubMed] [Google Scholar]
- 29.Rainey WE, Carr BR, Sasano H, et al. Trends Endocrinol Metab. 2002;13:234–239. doi: 10.1016/s1043-2760(02)00609-4. [DOI] [PubMed] [Google Scholar]
- 30.Dorr HG, Heller A, Versmold HT, et al. J Clin Endocrinol Metab. 1989;68:863–868. doi: 10.1210/jcem-68-5-863. [DOI] [PubMed] [Google Scholar]
- 31.Talaber G, Jondal M, Okret S. Mol Cell Endocrinol. 2013;380:89–98. doi: 10.1016/j.mce.2013.05.007. [DOI] [PubMed] [Google Scholar]
- 32.Taves MD, Gomez-Sanchez CE, Soma KK. Am J Physiol Endocrinol Metab. 2011;301:E11–E24. doi: 10.1152/ajpendo.00100.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Slominski A, Ermak G, Mihm M. J Clin Endocrinol Metab. 1996;81:2746–2749. doi: 10.1210/jcem.81.7.8675607. [DOI] [PubMed] [Google Scholar]
- 34.Slominski A, Gomez-Sanchez CE, Foecking MF, et al. FEBS Lett. 1999;455:364–366. doi: 10.1016/s0014-5793(99)00889-3. [DOI] [PubMed] [Google Scholar]
- 35.Slominski A, Zjawiony J, Wortsman J, et al. Eur J Biochem. 2004;271:4178–4188. doi: 10.1111/j.1432-1033.2004.04356.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Slominski A, Zbytek B, Szczesniewski A, et al. Am J Physiol Endocrinol Metab. 2005;288:E701–E706. doi: 10.1152/ajpendo.00519.2004. [DOI] [PubMed] [Google Scholar]
- 37.Slominski A, Zbytek B, Semak I, et al. J Neuroimmunol. 2005;162:97–102. doi: 10.1016/j.jneuroim.2005.01.014. [DOI] [PubMed] [Google Scholar]
- 38.Ito N, Ito T, Kromminga A, et al. FASEB J. 2005;19:1332–1334. doi: 10.1096/fj.04-1968fje. [DOI] [PubMed] [Google Scholar]
- 39.Slominski A, Zbytek B, Szczesniewski A, et al. J Invest Dermatol. 2006;126:1177–1178. doi: 10.1038/sj.jid.5700204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cirillo N, Prime SS. J Cell Biochem. 2011;112:1499–1505. doi: 10.1002/jcb.23081. [DOI] [PubMed] [Google Scholar]
- 41.Hannen RF, Michael AE, Jaulim A, et al. Biochem Biophys Res Commun. 2011;404:62–67. doi: 10.1016/j.bbrc.2010.11.059. [DOI] [PubMed] [Google Scholar]
- 42.Vukelic S, Stojadinovic O, Pastar I, et al. J Biol Chem. 2011;286:10265–10275. doi: 10.1074/jbc.M110.188268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Labrie F, Luu-The V, Labrie C, et al. Horm Res. 2000;54:218–229. doi: 10.1159/000053264. [DOI] [PubMed] [Google Scholar]
- 44.Reed MJ, Purohit A, Woo LW, et al. Endocr Rev. 2005;26:171–202. doi: 10.1210/er.2004-0003. [DOI] [PubMed] [Google Scholar]
- 45.Elias PM, Williams ML, Choi EH, et al. Biochim Biophys Acta. 2014;1841:353–361. doi: 10.1016/j.bbalip.2013.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Skobowiat C, Sayre RM, Dowdy JC, et al. Br J Dermatol. 2013;168:595–601. doi: 10.1111/bjd.12096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Slominski A, Gomez-Sanchez C, Foecking MF, et al. Biochim Biophys Acta. 2000;1474:1–4. doi: 10.1016/s0304-4165(99)00215-9. [DOI] [PubMed] [Google Scholar]
- 48.Skobowiat C, Nejati R, Lu L, et al. Gene. 2013;530:1–7. doi: 10.1016/j.gene.2013.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Slominski A, Wortsman J, Foecking MF, et al. J Invest Dermatol. 2002;118:310–315. doi: 10.1046/j.0022-202x.2001.01648.x. [DOI] [PubMed] [Google Scholar]
- 50.Holick MF, Vitamin D. J Cell Biochem. 2003;88:296–307. doi: 10.1002/jcb.10338. [DOI] [PubMed] [Google Scholar]
- 51.Bikle DD. Exp Dermatol. 2011;20:7–13. doi: 10.1111/j.1600-0625.2010.01202.x. [DOI] [PubMed] [Google Scholar]
- 52.Guryev O, Carvalho RA, Usanov S, et al. Proc Natl Acad Sci USA. 2003;100:14754–14759. doi: 10.1073/pnas.2336107100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Slominski AT, Kim TK, Chen J, et al. Int J Biochem Cell Biol. 2012;44:2003–2018. doi: 10.1016/j.biocel.2012.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Slominski AT, Zmijewski MA, Semak I, et al. PLoS ONE. 2009;4:e4309. doi: 10.1371/journal.pone.0004309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shackleton CH. Lipids. 2012;47:1–12. doi: 10.1007/s11745-011-3605-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zmijewski MA, Li W, Zjawiony JK, et al. Steroids. 2009;74:218–228. doi: 10.1016/j.steroids.2008.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zmijewski MA, Li W, Chen J, et al. Steroids. 2011;76:193–203. doi: 10.1016/j.steroids.2010.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Slominski AT, Li W, Bhattacharya SK, et al. J Invest Dermatol. 2011;131:1167–1169. doi: 10.1038/jid.2010.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lathe R. Steroids. 2002;67:967–977. doi: 10.1016/s0039-128x(02)00044-2. [DOI] [PubMed] [Google Scholar]
- 60.Larsson H, Bottiger Y, Iuliano L, et al. Free Radic Biol Med. 2007;43:695–701. doi: 10.1016/j.freeradbiomed.2007.04.033. [DOI] [PubMed] [Google Scholar]
- 61.Hennebert O, Montes M, Favre-Reguillon A, et al. J Steroid Biochem Mol Biol. 2009;114:57–63. doi: 10.1016/j.jsbmb.2008.12.015. [DOI] [PubMed] [Google Scholar]
- 62.Muller C, Pompon D, Urban P, et al. J Steroid Biochem Mol Biol. 2006;99:215–222. doi: 10.1016/j.jsbmb.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 63.Schweizer RA, Zurcher M, Balazs Z, et al. J Biol Chem. 2004;279:18415–18424. doi: 10.1074/jbc.M313615200. [DOI] [PubMed] [Google Scholar]
- 64.Nashev LG, Chandsawangbhuwana C, Balazs Z, et al. PLoS ONE. 2007;2:e561. doi: 10.1371/journal.pone.0000561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jellinck PH, Croft G, McEwen BS, et al. J Steroid Biochem Mol Biol. 2005;93:81–86. doi: 10.1016/j.jsbmb.2004.11.008. [DOI] [PubMed] [Google Scholar]
- 66.Chalbot S, Morfin R. Steroids. 2005;70:319–326. doi: 10.1016/j.steroids.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 67.Li A, Bigelow JC. Steroids. 2010;75:404–410. doi: 10.1016/j.steroids.2010.02.003. [DOI] [PubMed] [Google Scholar]
- 68.Shinkyo R, Xu L, Tallman KA, et al. J Biol Chem. 2011;286:33021–33028. doi: 10.1074/jbc.M111.282434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Endo-Umeda K, Yasuda K, Sugita K, et al. J Steroid Biochem Mol Biol. 2014;140:7–16. doi: 10.1016/j.jsbmb.2013.11.010. [DOI] [PubMed] [Google Scholar]
- 70.Lin YY, Welch M, Lieberman S. J Steroid Biochem Mol Biol. 2003;85:57–61. doi: 10.1016/s0960-0760(03)00137-7. [DOI] [PubMed] [Google Scholar]
- 71.Zouboulis CC, Chen WC, Thornton MJ, et al. Horm Metab Res. 2007;39:85–95. doi: 10.1055/s-2007-961807. [DOI] [PubMed] [Google Scholar]
- 72.Chen W, Thiboutot D, Zouboulis CC. J Invest Dermatol. 2002;119:992–1007. doi: 10.1046/j.1523-1747.2002.00613.x. [DOI] [PubMed] [Google Scholar]
- 73.Zouboulis CC. Dermatoendocrinol. 2009;1:250–252. [Google Scholar]
- 74.Slominski A, Wortsman J, Luger T, et al. Physiol Rev. 2000;80:979–1020. doi: 10.1152/physrev.2000.80.3.979. [DOI] [PubMed] [Google Scholar]
- 75.Slominski A, Zbytek B, Pisarchik A, et al. J Cell Physiol. 2006;206:780–791. doi: 10.1002/jcp.20530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Slominski A. J Clin Invest. 2007;117:3166–3169. doi: 10.1172/JCI33508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tiganescu A, Tahrani AA, Morgan SA, et al. J Clin Invest. 2013;123:3051–3060. doi: 10.1172/JCI64162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Slominski A, Wortsman J, Tuckey RC, et al. Mol Cell Endocrinol. 2007;265–266:143–149. doi: 10.1016/j.mce.2006.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zhu G, Janjetovic Z, Slominski A. Exp Dermatol. 2014;23:15–17. doi: 10.1111/exd.12275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ermak G, Slominski A. J Invest Dermatol. 1997;108:160–167. doi: 10.1111/1523-1747.ep12332925. [DOI] [PubMed] [Google Scholar]
- 81.Slominski A, Ermak G, Mazurkiewicz JE, et al. J Clin Endocrinol Metab. 1998;83:1020–1024. doi: 10.1210/jcem.83.3.4650. [DOI] [PubMed] [Google Scholar]
- 82.Skobowiat C, Dowdy JC, Sayre RM, et al. Am J Physiol Endocrinol Metab. 2011;301:E484–E493. doi: 10.1152/ajpendo.00217.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zbytek B, Wortsman J, Slominski A. Mol Endocrinol. 2006;20:2539–2547. doi: 10.1210/me.2006-0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Edwards PA, Kennedy MA, Mak PA. Vascul Pharmacol. 2002;38:249–256. doi: 10.1016/s1537-1891(02)00175-1. [DOI] [PubMed] [Google Scholar]
- 85.Lukyanenko YO, Chen JJ, Hutson JC. Biol Re-prod. 2001;64:790–796. doi: 10.1095/biolreprod64.3.790. [DOI] [PubMed] [Google Scholar]
- 86.King SR, Matassa AA, White EK, et al. J Mol Endocrinol. 2004;32:507–517. doi: 10.1677/jme.0.0320507. [DOI] [PubMed] [Google Scholar]
- 87.Cummins CL, Volle DH, Zhang Y, et al. J Clin Invest. 2006;116:1902–1912. doi: 10.1172/JCI28400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Manna PR, Cohen-Tannoudji J, Counis R, et al. J Biol Chem. 2013;288:8505–8518. doi: 10.1074/jbc.M112.417873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hanley K, Ng DC, He SS, et al. J Invest Dermatol. 2000;114:545–553. doi: 10.1046/j.1523-1747.2000.00895.x. [DOI] [PubMed] [Google Scholar]
- 90.Kielar D, Kaminski WE, Liebisch G, et al. J Invest Dermatol. 2003;121:465–474. doi: 10.1046/j.1523-1747.2003.12404.x. [DOI] [PubMed] [Google Scholar]
- 91.Schmuth M, Jiang YJ, Dubrac S, et al. J Lipid Res. 2008;49:499–509. doi: 10.1194/jlr.R800001-JLR200. [DOI] [PubMed] [Google Scholar]
- 92.Repa JJ, Berge KE, Pomajzl C, et al. J Biol Chem. 2002;277:18793–18800. doi: 10.1074/jbc.M109927200. [DOI] [PubMed] [Google Scholar]
- 93.Volle DH, Lobaccaro JM. Mol Cell Endocrinol. 2007;265–266:183–189. doi: 10.1016/j.mce.2006.12.018. [DOI] [PubMed] [Google Scholar]
- 94.Bushue N, Wan YJ. Adv Drug Deliv Rev. 2010;62:1285–1298. doi: 10.1016/j.addr.2010.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Torma H. Dermatoendocrinol. 2011;3:136–140. doi: 10.4161/derm.3.3.15026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Chapman MS. Semin Cutan Med Surg. 2012;31:11–16. doi: 10.1016/j.sder.2011.11.009. [DOI] [PubMed] [Google Scholar]
- 97.Memezawa A, Takada I, Takeyama K, et al. Oncogene. 2007;26:5038–5045. doi: 10.1038/sj.onc.1210320. [DOI] [PubMed] [Google Scholar]
- 98.Esteban-Pretel G, Marin MP, Cabezuelo F, et al. J Nutr. 2010;140:792–798. doi: 10.3945/jn.109.119388. [DOI] [PubMed] [Google Scholar]
- 99.Chang KC, Shen Q, Oh IG, et al. Mol Endocrinol. 2008;22:2407–2419. doi: 10.1210/me.2008-0232. [DOI] [PubMed] [Google Scholar]
- 100.Hong I, Rho HS, Kim DH, et al. Arch Pharm Res. 2010;33:1443–1449. doi: 10.1007/s12272-010-0919-5. [DOI] [PubMed] [Google Scholar]
- 101.Shen Q, Bai Y, Chang KC, et al. J Biol Chem. 2011;286:14554–14563. doi: 10.1074/jbc.M110.165704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Komuves LG, Schmuth M, Fowler AJ, et al. J Invest Dermatol. 2002;118:25–34. doi: 10.1046/j.0022-202x.2001.01628.x. [DOI] [PubMed] [Google Scholar]
- 103.Fowler AJ, Sheu MY, Schmuth M, et al. J Invest Dermatol. 2003;120:246–255. doi: 10.1046/j.1523-1747.2003.12033.x. [DOI] [PubMed] [Google Scholar]
- 104.Yokoyama A, Makishima M, Choi M, et al. J Invest Dermatol. 2009;129:1395–1401. doi: 10.1038/jid.2009.15. [DOI] [PubMed] [Google Scholar]
- 105.Revollo JR, Cidlowski JA. Ann N Y Acad Sci. 2009;1179:167–178. doi: 10.1111/j.1749-6632.2009.04986.x. [DOI] [PubMed] [Google Scholar]
- 106.Sarabdjitsingh RA, Joels M, de Kloet ER. Physiol Behav. 2012;106:73–80. doi: 10.1016/j.physbeh.2011.09.017. [DOI] [PubMed] [Google Scholar]
- 107.Lu NZ, Cidlowski JA. Mol Cell. 2005;18:331–342. doi: 10.1016/j.molcel.2005.03.025. [DOI] [PubMed] [Google Scholar]
- 108.Duma D, Jewell CM, Cidlowski JA. J Steroid Biochem Mol Biol. 2006;102:11–21. doi: 10.1016/j.jsbmb.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 109.Schacke H, Rehwinkel H, Asadullah K, et al. Exp Dermatol. 2006;15:565–573. doi: 10.1111/j.1600-0625.2006.00453.x. [DOI] [PubMed] [Google Scholar]
- 110.Perez P. Dermatoendocrinol. 2011;3:166–174. [Google Scholar]
- 111.Perez P, Page A, Bravo A, et al. FASEB J. 2001;15:2030–2032. doi: 10.1096/fj.00-0772fje. [DOI] [PubMed] [Google Scholar]
- 112.Fuchs E. Nature. 2007;445:834–842. doi: 10.1038/nature05659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Sevilla LM, Bayo P, Latorre V, et al. Mol Endocrinol. 2010;24:2166–2178. doi: 10.1210/me.2010-0183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Bayo P, Sanchis A, Bravo A, et al. Endocrinology. 2008;149:1377–1388. doi: 10.1210/en.2007-0814. [DOI] [PubMed] [Google Scholar]
- 115.Yu XJ, Ren XH, Xu YH, et al. Neuropeptides. 2010;44:407–411. doi: 10.1016/j.npep.2010.05.002. [DOI] [PubMed] [Google Scholar]
- 116.Stojadinovic O, Sawaya A, Pastar I, et al. PLoS ONE. 2013;8:e63453. doi: 10.1371/journal.pone.0063453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Tiala I, Suomela S, Huuhtanen J, et al. J Mol Med (Berl) 2007;85:589–601. doi: 10.1007/s00109-006-0155-0. [DOI] [PubMed] [Google Scholar]
- 118.Suomela S, Elomaa O, Skoog T, et al. PLoS ONE. 2009;4:e6030. doi: 10.1371/journal.pone.0006030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Terao M, Itoi S, Murota H, et al. Exp Dermatol. 2013;22:98–101. doi: 10.1111/exd.12075. [DOI] [PubMed] [Google Scholar]
- 120.Jackson S, Gilchrist H, Nesbitt LT., Jr Dermatol Ther. 2007;20:187–205. doi: 10.1111/j.1529-8019.2007.00133.x. [DOI] [PubMed] [Google Scholar]
- 121.Stahn C, Lowenberg M, Hommes DW, et al. Mol Cell Endocrinol. 2007;275:71–78. doi: 10.1016/j.mce.2007.05.019. [DOI] [PubMed] [Google Scholar]
- 122.Terao M, Murota H, Kimura A, et al. PLoS ONE. 2011;6:e25039. doi: 10.1371/journal.pone.0025039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Slominski AT, Kim TK, Li W, et al. J Steroid Biochem Mol Biol. 2013 doi: 10.1016/j.jsbmb. 2013.10.012. [Epub a head of print] [Google Scholar]
- 124.Slominski AT, Carlson AJ. Mayo Clin Proc. 2014;89:429–433. doi: 10.1016/j.mayocp.2014.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Slominski AT, Kim T-K, Shehabi HZ, et al. FASEB J. 2012;26:3901–3915. doi: 10.1096/fj.12-208975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Slominski AT, Kim TK, Shehabi HZ, et al. Mol Cell Endocrinol. 2014;383:181–192. doi: 10.1016/j.mce.2013.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.