Abstract
Introduction
Treatment options and therapeutic guidelines have evolved substantially since highly active antiretroviral treatment (HAART) became the standard of HIV care in 1996. We conducted the present population-based analysis to characterize the determinants of direct costs of HAART over time in British Columbia (BC), Canada.
Methods
We considered individuals ever receiving HAART in BC from 1996–2011. Linear mixed effects regression models were constructed to determine the effects of demographic indicators, clinical stage and treatment characteristics on quarterly costs of HAART (in 2010$CDN) among individuals initiating in different temporal periods. Least square mean values were estimated by CD4 category and over time for each temporal cohort.
Results
Longitudinal data on N=9,601 HAART recipients (17.6% female, mean age at initiation=40.5) were analyzed. Multiple regression analyses identified demographics, treatment adherence and pharmacological class to be independently associated with quarterly HAART costs. Higher CD4 cell counts were associated with modestly lower costs among pre-HAART initiators (Least-Square Means (95% Confidence interval), CD4>500:4674(4632,4716); CD4:350–499:4765(4721,4809) CD4:200–349:4826(4780,4871); CD4<200:4809(4759,4859)); however these differences were not significant among post 2003-HAART initiators. Population-level mean costs increased through 2006 and stabilized Post-2003 HAART initiators incurred quarterly costs up to 23% lower than pre-2000 HAART initiators in 2010.
Conclusions
Our results highlight the magnitude of the temporal changes in HAART costs, and disparities between recent and pre-HAART initiators. This methodology can improve the precision of economic modeling efforts by using detailed cost functions for annual, population-level medication costs according to the distribution of clients by clinical stage and era of treatment initiation.
Keywords: Human Immunodeficiency Virus, Antiretroviral Treatment, Highly Active Antiretroviral Treatment
Introduction
HIV treatment has evolved substantially since the introduction of highly active antiretroviral therapy (HAART) in 1996 (1). HAART stops HIV replication on a sustained basis. As a result, plasma HIV-1 RNA concentrations (henceforth plasma viral load (pVL)) reach undetectable levels within a matter of weeks in the vast majority of adherent patients (2). This allows for immune reconstitution to take place, leading to long-term disease remission and deferral of the otherwise rapid fatal course (3, 4). The introduction of HAART has had a dramatic effect on the HIV/AIDS epidemic. By 2006, at least 3 million years of life had been saved in the USA as a direct result of HAART within a decade (5). In high-income countries, life expectancy for HIV-positive individuals aged 20 years and receiving HAART is now roughly two-thirds of that of the general population (6).
There has been a marked evolution in clinical treatment guidelines, as well as a substantial expansion in the number of antiretroviral agents available since 1996. The 1996 IAS-USA guidelines recommended HAART for those with CD4 cell counts below 500 cells/mm3 (1). By 2000, because of the emerging concerns regarding adverse effects, long term adherence and drug resistance the trend was to delay treatment initiation (7). By 2004, new drugs, and drug classes started to emerge, which both improved the safety and tolerability of the regimens, enhanced long term adherence, and remarkably facilitated the ability to treat drug resistant HIV. This coupled with a better understanding of the inflammatory consequences of untreated HIV infection led the emerging IAS-USA guidelines to recommend earlier initiation of HAART (8–11), a trend that has been confirmed in the most recent iteration of the guidelines (2). As a result, there is substantial variation in the costs of drug treatment between clients over time. The variance and distribution of combination antiretroviral therapy regimens used at the population level at any given time point is thus highly heterogeneous and may be influenced by the temporal phase of antiretroviral initiation and stage of disease progression, among other factors (12).
Evidence for the secondary preventive effect of HAART on HIV transmission (13–16) has spawned efforts to scale-up HIV treatment programs globally (17). A key challenge faced by these efforts is the substantial costs of scale-up, largely tied to the costs of antiretroviral medications. We draw upon detailed patient-level costing data for the population of HAART clients in BC throughout the HAART era in the interest of providing inputs for the development of more detailed cost functions to improve precision in economic modeling efforts (12), which are of vital importance to inform resource allocation decisions to maximize the public health impact of treatment and prevention programs across the spectrum of infectious and chronic diseases. Our objective was to characterize the determinants of the direct costs of HAART, and estimate fitted values of the quarterly costs of antiretroviral therapy for individuals initiating therapy at different stages of the evolution of HAART.
Methods
Study Population
We considered all individuals who had ever received antiretroviral treatment prior to September 30th, 2011, as observed in the BC Centre for Excellence in HIV/AIDS (BC-CfE) HIV Drug Treatment Program database. Individuals were included in the analysis if at least one antiretroviral drug pharmacy dispensation was recorded in the database. We excluded individuals initiating treatment under 19 years of age, and those initiating treatment in other provinces (thus missing information on antiretroviral treatment regimen at initiation). The study cohort is followed in a unique environment characterized by universal free medical care, including free in- and out-patient care, laboratory monitoring, and antiretroviral drugs. The antiretroviral drugs are centrally distributed by the BC-CfE according to the BC-CfE’s treatment guidelines, which have remained consistent with those put forward by the International AIDS Society since the summer of 1996 and until the guidelines released in 2010 (11).
Measures
The primary outcome of this study was the quarterly cost of dispensed antiretroviral treatment. Individual-level costs were estimated by summing the total amount of medications dispensed for consumption during each 3-month period from the point of HAART initiation in which medication was dispensed. The BC-CfE procures and distributes all antiretroviral medications for the treatment of HIV in the province of BC; unit costs of antiretroviral medications were drawn from financial records of purchase costs for the province. Total quarterly costs included the costs of pharmacy dispensation, and summed across all antiretroviral medications dispensed within the person-quarter. These quarterly costs excluded the cost of physician visits for prescription refills and were presented in 2010$CDN, adjusted using the Canadian consumer price index.
As determinants of the costs of HAART, we considered the effects of demographic indicators, clinical stage and characteristics of treatment. Patient demographics included age and gender, ethnicity (Aboriginal status) and mode of transmission (history of injection drug use (IDU) or ‘other’); demographic factors are commonly associated with disparities in levels of health resource utilization (18–21). Indicators of clinical stage included CD4 at treatment initiation, current CD4 cell count (including CD4 measurements within 6 months of the earliest date in a given quarter), and the area under the pVL curve in the year of the quarterly observation. The latter specification was chosen as a measure of cumulative viral load, found to be a more sensitive indicator of disease severity (22). As pVL and CD4 measures are known to have independent effects on AIDS-related illness and mortality (23,24), time-varying effects for each measure were included. The last observation of CD4 or pVL was carried forward in quarters with missing pVL or CD4 measures. Finally, treatment characteristics included past-year adherence and eight mutually-exclusive stratifications of current therapy type: (i) combination regimens featuring all nucleoside/nucleotide reverse transcriptase inhibitors (NRTI); (ii) those featuring non-nucleoside reverse transcriptase inhibitors (NNRTI); (ii) Those featuring a single protease inhibitor (PI); (iv) those featuring a boosted PI (bPI); (v) those featuring a PI and NNRTI (PI+NNRTI); (vi) those featuring an integrase inhibitor and/or fusion inhibitor (INT/FI); (vii) multiple drug rescue therapy regimens (MDRT) with combinations of 5 or more antiretrovirals; and (viii) other antiretroviral combinations.
Statistical analysis
Given substantial changes in clinical guidelines and subsequent prescribing practices, we stratified our cohort based on four distinct temporal periods of HAART initiation: pre-1996; 1996–1999; 2000–2003; and post-2003. These categories distinguished pre-HAART era antiretroviral medication initiators, early-HAART era initiators (pre-2000), when IAS-USA guidelines for treatment initiation were less stringent (CD4<500) (1,2), and 2000–2003, when emerging data on drug resistance shifted HAART initiation to CD4<200 (7–11). These changes in clinical guidelines and practice resulted in observed differences in costs and their determinants within the dataset.
Statistical analysis then proceeded in two steps. First, multiple generalized linear mixed effects regression models were considered to produce mean marginal costs of HAART on a quarterly basis for a range of client characteristics within the study time horizon. We used a modified Park test to assess the extent of heteroscedasticity and identify the appropriate distributional family (25,26). Generalized linear models with gamma distribution and log link are commonly used in econometric analyses of healthcare costs due to their asymptotic properties for non-negative outcomes (27). However non-zero quarterly medication costs may not feature the same distributional properties as, for instance, costs derived from counts of physician visits and days of inpatient care, thereby necessitating diagnostic testing to indicate the best modeling strategy given the outcome measure’s distribution and variance. Mixed-effects regression models allow for the inter-individual correlation in balanced or unbalanced repeated measures data.(28). Regression coefficients were presented in their natural units, as marginal effects of a one-unit change in the covariate on the quarterly costs of HAART. In addition, results were presented as least-squares means by CD4 category and over time, for each temporal stage, based on within-sample mean covariate values. All analyses were executed using SAS version 9.3.
Results
The distributions of initial antiretroviral regimens in the post-HAART era throughout the study period are presented in Figure S1 (Supplemental Digital Content). These histograms display the movement towards standardization of initial treatment regimens over time. Whereas in the 1996–1999 period, there was wide variation in starting regimens, with the three most common regimens (AZT/3TC/NVP, 3TC/D4T/RTV/LPV, and 3TC/D4T) comprising roughly 30% of all cases. By 2008, the two most common regimens (EFV/TEN/FTC and RIT/TEN/ATV/FTC) accounted for nearly 75% of all cases. On aggregate, whereas PI-based regimens and NRTI regimens were predominantly prescribed from 1996–2000, NNRTI-based regimens and boosted PI-based regimens have since become the most commonly prescribed (Figure S2).
Summary statistics of ART initiators stratified by the selected temporal groups are presented in Table 1. Most notably, a higher proportion of females have initiated HAART from 1996 onward, from 9.1% prior to 1996, to 17.2% (1996–1999) and 22.3% in 2000–2003. Initial CD4 counts also varied substantially between the study cohorts; specifically, a higher proportion of individuals had an initial CD4 count <200 cells/mm3 in later temporal periods (2004–2011: 41.8% vs. pre-1996: 24,4%). As a result of mortality and increasing HAART uptake over time, the total population of clients on HAART has evolved to feature a progressively smaller proportion of pre-HAART and early-HAART initiators (Figure 1). However, it is important to note that in 2011 nearly 40% of the population of clients receiving HAART initiated pre-1999, with 15% initiating treatment in the pre-HAART era.
Figure 1.
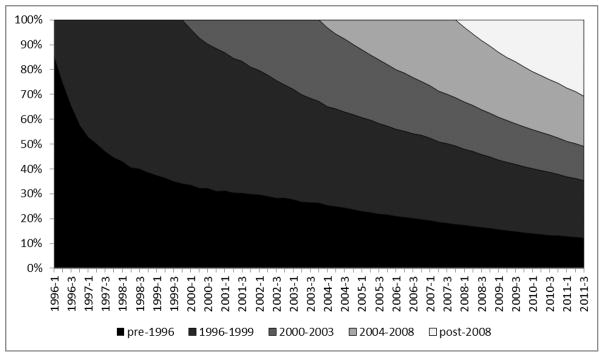
Changes in HIV client mix, by period of antiretroviral treatment initiation in British Columbia, Canada: 1996–2011
The pre-1996 ART initiator cohort had a median 41 (Inter-quartile range (IQR): 14, 59) observations per individual, while the 1996–1999 ART initiator cohort had 32 (11, 49), the 2000–2003 ART initiator cohort had 32 (12, 37), and the post-2003 ART initiator cohort had 11 (5, 18) observations per individual.
Separate multivariate regression models were executed for each of the four cohorts defined by temporal period of HAART initiation. Modified Park Tests were executed for each temporal cohort; in each case, results indicated a normal distribution provided the greatest model fit; we therefore proceeded with multiple linear mixed effects regression models (equivalent to a generalized linear mixed effects regression model with normal distribution and identity link function specified). Controlling for fixed and time-varying prognostic variables as well as indicators of treatment regimen type, patient demographics had an influence on quarterly drug costs. Among post-2003 ART initiators, known history of IDU and known aboriginal ethnicity were each associated with higher quarterly costs. Older age was negatively associated with quarterly costs among pre-1996 and 1996–1999 initiators, however not among post-2000 initiator cohorts (Table 2).
Within each cohort, quarterly ART costs increased over time but at a diminishing rate – indicated by positive coefficients on the linear, and negative coefficients on the quadratic time trend variables.
Intermediate indicators of HIV disease progression were independently associated with quarterly drug costs. Controlling for other factors, higher CD4 at ART initiation was associated with lower costs among recent (post-2004) initiators; however this effect was diminished within cohorts with longer periods of follow-up. Conversely, higher current CD4 levels were consistently associated with lower quarterly drug costs, however the effect diminished in cohorts with shorter durations of follow-up. On the other hand, those with consistently low plasma viral load over the previous 12 month period (indicated by AUC of pVL) had higher antiretroviral drug costs, independent of regimen type, treatment adherence and CD4 cell count – a result that was consistent across all temporal cohorts. Finally, drug treatment regimen indicators had the largest independent effect magnitudes on quarterly drug costs. NRTI-based regimens were the least costly, while INT/FI and MDRT-based regimens were the most costly, at an increased cost of $1920 (2000–2003) to $3572 (post-2003) per quarter in comparison to NRTI-based regimens, respectively.
Least-squares means of quarterly drug costs, evaluated in the 2010 calendar year, were presented by CD4 category in Table 3. Based on observed distributions of the additional covariates estimated in the model, drug costs were uniformly lower among later HAART initiators - $4674 (95% CI: $4632, $4716) per person-quarter among pre-1996 initiators with CD4 > 500 versus $3740 ($3716, $3764) per person-quarter for post-2003 initiators.
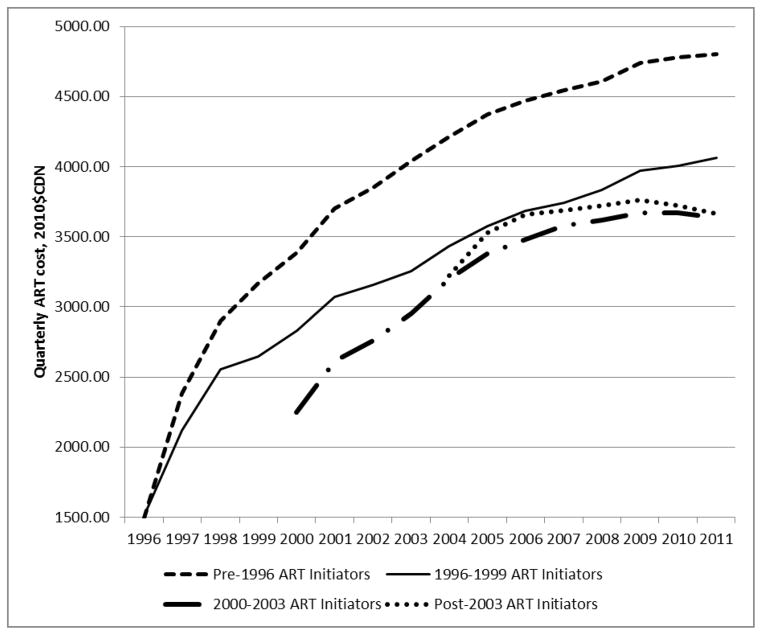
The fitted values of these models, based on population-level covariate values for each temporal cohort throughout the study period, were presented in Figure 2. It is important to note that those initiating treatment in the pre-HAART era incurred the highest quarterly treatment costs of any of the initiation cohorts, at $4403 per person-quarter in treatment in 2010. In each cohort, the direct costs of treatment increased over time. This increase was steepest and most pronounced for pre-HAART initiators, however it is also observed in the 1996–1999 ($1513 in 1996 to $4066 per person-quarter in 2010) and 2000–2003 ($2251 in 2000 to $3636 per person-quarter in 2010) cohorts. The increase is slightest in the post-2003 initiators (from $3222 in 2004 to $3664 per person-quarter in 2010).
Figure 2.
Fitted values of quarterly costs of HAART (2010$CDN) by year of initiation in British Columbia, Canada: 1996–2011
* Least-square mean values based on multivariate regression model parameter estimates and observed distributions of specified covariates, by calendar year over time.
Discussion
Our results demonstrate substantial heterogeneity in antiretroviral drug costs across individuals and over time. Recent HAART initiators incurred the lowest quarterly costs, which increased through 2006 and stabilized thereafter for those initiating treatment after 2003. Individuals initiating treatment in the pre-HAART era and continuing to receive treatment more recently incurred quarterly costs roughly 23% higher than recent HAART initiators. Within each of the HAART initiation-era cohorts, quarterly costs increased over time, reflecting the shift towards progressively more costly medication switches. The observed differences in mean quarterly costs in a given calendar year are thus influenced by individual case-mix, according to clinical stage, as well as time since HAART initiation.
As the paradigm for HIV treatment shifts to account for both the individual and public health benefits of HAART, the reliance on economic forecasting has, and will continue to increase (31–35). A recent review noted most analyses forecasting the effect of ‘treatment as prevention’ programs assume a uniform cost per patient, citing differences in factor prices and the scale and scope of operations as potentially influential factors (12). While these factors are fixed in BC due to centralized drug purchasing and standardized physician and pharmacy billing rates, our analysis suggests detailed cost functions for annual, population-level HAART costs should be developed according to the distribution of clients by clinical stage and era of HAART initiation. Our study thus demonstrates the importance of using a flexible cost function for current cost estimation, and subsequently for future projection in efforts to estimate the economic impact of HIV ‘treatment as prevention’ initiatives, and is directly applicable to other chronic and infectious diseases.
The relationships between CD4, pVL and drug costs have not always been clearly understood and articulated in past analyses on the costs of health resource use among individuals with HIV, in part because these are commonly combined with the costs of inpatient and outpatient care (18–20). Primary care and hospital based care costs are unequivocally positively related to disease progression (measured as baseline, pre-ART CD4 cell count level), as later disease stage necessitates closer physician monitoring and carries a higher probability of inpatient care due to the occurrence of opportunistic infections, adverse drug reactions and related medical complications (20,21, -29).
Our results demonstrate modest differences in HAART costs by CD4 stratum among early initiators, and primarily not statistically significant differences in quarterly costs among recent initiators. In a recent review of the published literature on direct costs of HIV, articles disaggregating the costs of HAART from inpatient and outpatient care (published between 1999 and 2007) showed an inverse relationship between costs and CD4 cell counts similar to those found amongst early initiators in our analysis (30). In contrast, a more recent empirical study found little difference in annual HAART costs across CD4 strata among those accessing treatment (21), a result reinforced by a study modeling the lifetime costs of early HAART initiators, which estimated stable treatment costs across CD4 cell count strata among recent initiators (31). These results are consistent with those of the recent initiators in our study, and demonstrate a process pertaining to antiretroviral drug costs that is distinct from other health resource utilization patterns in HIV/AIDS.
Furthermore, we found that the effect of disease progression on quarterly drug costs is somewhat more nuanced given the variable course of the intermediate outcomes used to measure progression. In this study, individuals with sustained virologic suppression tended to have higher treatment costs, controlling for other factors, including CD4 count. High CD4 counts may be indicative of early stages of disease progression, whereas sustained pVL suppression (and thus low pVL AUC values) may be obtained at any stage of disease progression, with aggressive treatment (often requiring switches to more costly regimens) to achieve and sustain suppression.
The increases in the costs of HAART over time have been driven by the introduction of more effective, safer and better tolerated medications, medication switches as a result of drug resistance or intolerance in the course of disease progression (most evident in pre-HAART initiators with longer duration of follow-up), and new and more effective therapeutic options for multi-drug resistance. Given the availability of new, more effective treatment regimens (resulting in longer delays, or entirely averted therapeutic switches resulting from developed resistance), increasing numbers of generic antiretroviral formulations (32) and the natural evolution of the HIV-positive population in treatment, it may be plausible to expect annual per-patient costs of drug treatment to plateau and even decline in future years in British Columbia. The rate of change, however, is likely to differ substantially across jurisdictions, and will rely heavily on local factor costs, prescribing practices and the stage of the HIV epidemic itself. These considerations must be taken into account when projecting localized costs of antiretroviral treatment scale-up.
Figures S1 and S2 indicate variation from evidence-based standards of care according to IAS-USA guidelines in the early-HAART era, with considerable subsequent movement towards standardization. Specifically, triple therapy with 2 NRTIs and 1 PI was recommended practice through 2000 (1,33,34), yet by 2000 only 40% of prescribed regimens were NRTI- or PI/boosted PI-based. The proliferation of pharmacological regimens used in 2000 was in accordance to changes in the contemporary guidelines (35,36). Since 2004, NNRTI- and boosted-PI-based regimens became the preferred options (8–11). Boosted-PI and NNRTI-based regimens comprised nearly 80% of all prescribed regimens in 2011; along with improving rates of early detection (CD4>500) HAART uptake, and viral suppression (37,38), this, further demonstrates increasing uptake of evidence-based standards of HIV care in British Columbia.
This analysis featured several limitations. First, while the study was based on a population-level registry of antiretroviral treatment dispensation, initiated in 1992 (in the pre-HAART era), given the characteristics of clients, the nature of the HIV epidemic in BC, healthcare delivery policies and current and historical factor prices, caution must be exercised in applying these estimates to other settings. If sufficient data are not available, extrapolation using the regression models, and based on localized population and policy conditions, with some adjustment for differential factor prices, may reduce bias. Second, current CD4 and pVL measurements were not always available in all time periods where treatment was delivered. CD4 markers have been noted to exhibit considerable variability as a result of intra-person temporal fluctuation, for example diurnal variation, as well as from measurement error introduced by the process of blood collection or the method of collection (flow cytometry and PCR) itself (39). While lagged measurements were used to maximize the use of available data in the multivariate models, missing, or non-representative measures likely attenuated the effect of disease course on quarterly drug costs. Baseline pVL measures were not available for early-HAART initiators, as the pVL test only became available in BC in June of 1996 (15); therefore this covariate was excluded from all regression models, though its inclusion in the post-1996 models had no meaningful effect on covariate or least-square means estimates (results not presented). Further, self-reported indicators of history of IDU and aboriginal ethnicity had high levels of missing data, likely to be non-differential, resulting in coefficient estimated attenuated towards the null hypothesis. Efforts to improve data quality on these critical indicators via triangulation with provincial registries and administrative databases are currently underway. Finally, like all non-experimental studies, our coefficient estimates may have been subject to some degree of bias as a result of unmeasured confounding factors (40).
To conclude, this analysis has highlighted the magnitude and dimensions of variations in the direct costs of antiretroviral therapy over time and by disease stage. Population-level mean HAART costs increased through 2006 and stabilized thereafter. Post-2003 treatment initiators incurred HAART costs up to 23% lower than pre-2000 initiators in 2010. These factors are cause for careful consideration of estimates of medication costs in economic models to project the costs of HAART scale-up in HIV ‘treatment as prevention’ programs.
Supplementary Material
Acknowledgments
We acknowledge the assistance of Guillaume Colley, David Milan and Suzanne Humphreys in early efforts towards this manuscript, as well as all BCMoH and Vancouver Coastal Health Decision Support Staff involved in data access and procurement, including Monika Lindegger, Clinical Prevention Services, BC Centre for Disease Control; Elsie Wong, Public Health Agency of Canada; Al Cassidy, BC Ministry of Health Registries and Joleen Wright and Karen Luers, Vancouver Coastal Health decision support. This study was funded by the BC Ministry of Health-funded ‘Seek and treat for optimal prevention of HIV & AIDS’ pilot project. Bohdan Nosyk is a Michael Smith Foundation for Health Research Scholar.
Funding: This study was funded by the BC Ministry of Health, as well as through an Avant-Garde Award (No. 1DP1DA026182) from the National Institute of Drug Abuse, at the US National Institutes of Health.
The STOP HIV/AIDS Study Group is comprised of the following
Rolando Barrios, MD, FRCPC, Senior Medical Director, VCH; Adjunct Professor, School of Population and Public Health, UBC.
Patty Daly, MD, Vancouver Coastal Health Authority
Mark Gilbert, Clinical Prevention Services, BC Centre for Disease Control; School of Population & Public Health, University of British Columbia.
Reka Gustafson, MD, Vancouver Coastal Health Authority
Perry RW Kendall, OBC, MBBS, MSc, FRCPC. Provincial Health Officer, British Columbia Ministry of Health; Clinical Professor, Faculty of Medicine UBC
Ciro Panessa, British Columbia Ministry of Health
Nancy South, British Columbia Ministry of Health
Kate Heath, BC Centre for Excellence in HIV/AIDS
Julio SG Montaner, BC Centre for Excellence in HIV/AIDS
Robert S Hogg, BC Centre for Excellence in HIV/AIDS
Bohdan Nosyk, BC Centre for Excellence in HIV/AIDS
Footnotes
Conflicts of Interest: Dr. Julio Montaner has received grants from Abbott, Biolytical, Boehringer Ingelheim, Bristol-Myers Squibb, Gilead Sciences, Janssen, Merck and ViiV Healthcare. He is also is supported by the Ministry of Health, from the Province of British Columbia; through a Knowledge Translation Award from the Canadian Institutes of Health Research (CIHR); and through an Avant-Garde Award (No. 1DP1DA026182) from the National Institute of Drug Abuse, at the US National Institutes of Health. He has also received support from the International AIDS Society, United Nations AIDS Program, World Health Organization, National Institute on Drug Abuse, National Institutes of Health Research-Office of AIDS Research, National Institute of Allergy & Infectious Diseases, The United States President’s Emergency Plan for AIDS Relief (PEPfAR), Bill & Melinda Gates Foundation, French National Agency for Research on AIDS & Viral Hepatitis (ANRS), Public Health Agency of Canada. All other authors have no conflicts of interest to declare.
References
- 1.Carpenter CC, Fischl MA, Hammer SM, Hirsch MS, Jacobsen DM, Katzenstein DA, Montaner JS, Richman DD, Sáag MS, Schooley RT, Thompson MA, Vella S, Yeni PG, Volberding PA. Antiretroviral therapy for HIV infection in 1996. Recommendations of an international panel. International AIDS Society-USA. JAMA. 1996;276(2):146–54. [PubMed] [Google Scholar]
- 2.Thompson MA, Aberg JA, Hoy JF, Telenti A, Benson C, Cahn P, Eron JJ, Günthard HF, Hammer SM, Reiss P, Richman DD, Rizzardini G, Thomas DL, Jacobsen DM, Volberding PA. Antiretroviral treatment of adult HIV infection: 2012 recommendations of the International Antiviral Society-USA panel. JAMA. 2012 Jul 25;308(4):387–402. doi: 10.1001/jama.2012.7961. [DOI] [PubMed] [Google Scholar]
- 3.Hogg RS, O’Shaughnessy MV, Gataric N, et al. Decline in deaths from AIDS due to new antiretrovirals. Lancet. 1997;349:1294. doi: 10.1016/S0140-6736(05)62505-6. [DOI] [PubMed] [Google Scholar]
- 4.The Antiretroviral Therapy in Lower Income Countries (ART-LINC) Collaboration and ART Cohort Collaboration (ART-CC) groups. Mortality of HIV-1-infected patients in the first year of antiretroviral therapy: comparison between low-income and high-income countries. Lancet. 2006;367:817–24. doi: 10.1016/S0140-6736(06)68337-2. [DOI] [PubMed] [Google Scholar]
- 5.Walensky RP, Paltiel AD, Losina E, et al. The survival benefits of AIDS treatment in the United States. J Infect Dis. 2006;194:11–19. doi: 10.1086/505147. [DOI] [PubMed] [Google Scholar]
- 6.Antiretroviral Cohort Collaboration. Life expectancy of individuals on combination antiretroviral therapy in high-income countries: a collaborative analysis of 14 cohort studies. Lancet. 2008;372(9635):293–9. doi: 10.1016/S0140-6736(08)61113-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carpenter CC, Cooper DA, Fischl MA, Gatell JM, Gazzard BG, Hammer SM, Hirsch MS, Jacobsen DM, Katzenstein DA, Montaner JS, Richman DD, Saag MS, Schechter M, Schooley RT, Thompson MA, Vella S, Yeni PG, Volberding PA. Antiretroviral therapy in adults: updated recommendations of the International AIDS Society-USA Panel. JAMA. 2000;283(3):381–90. doi: 10.1001/jama.283.3.381. [DOI] [PubMed] [Google Scholar]
- 8.Yeni PG, Hammer SM, Hirsch MS, Saag MS, Schechter M, Carpenter CC, Fischl MA, Gatell JM, Gazzard BG, Jacobsen DM, Katzenstein DA, Montaner JS, Richman DD, Schooley RT, Thompson MA, Vella S, Volberding PA. Treatment for adult HIV infection: 2004 recommendations of the International AIDS Society-USA Panel. JAMA. 2004;292(2):251–65. doi: 10.1001/jama.292.2.251. [DOI] [PubMed] [Google Scholar]
- 9.Hammer SM, Saag MS, Schechter M, Montaner JS, Schooley RT, Jacobsen DM, Thompson MA, Carpenter CC, Fischl MA, Gazzard BG, Gatell JM, Hirsch MS, Katzenstein DA, Richman DD, Vella S, Yeni PG, Volberding PA International AIDS Society-USA panel. Treatment for adult HIV infection: 2006 recommendations of the International AIDS Society-USA panel. JAMA. 2006;296(7):827–43. doi: 10.1001/jama.296.7.827. [DOI] [PubMed] [Google Scholar]
- 10.Hammer SM, Eron JJ, Jr, Reiss P, Schooley RT, Thompson MA, Walmsley S, Cahn P, Fischl MA, Gatell JM, Hirsch MS, Jacobsen DM, Montaner JS, Richman DD, Yeni PG, Volberding PA International AIDS Society-USA. Antiretroviral treatment of adult HIV infection: 2008 recommendations of the International AIDS Society-USA panel. JAMA. 2008;300(5):555–70. doi: 10.1001/jama.300.5.555. [DOI] [PubMed] [Google Scholar]
- 11.Thompson MA, Aberg JA, Cahn P, Montaner JS, Rizzardini G, Telenti A, Gatell JM, Günthard HF, Hammer SM, Hirsch MS, Jacobsen DM, Reiss P, Richman DD, Volberding PA, Yeni P, Schooley RT International AIDS Society-USA. Antiretroviral treatment of adult HIV infection: 2010 recommendations of the International AIDS Society-USA panel. JAMA. 2010 Jul 21;304(3):321–33. doi: 10.1001/jama.2010.1004. [DOI] [PubMed] [Google Scholar]
- 12.Meyer-Rath G, Over M. HIV Treatment as Prevention: Modeling the cost of antiretroviral treatment – state of the art and future directions. PLoS Medicine. 2012;9(7):e1001247. doi: 10.1371/journal.pmed.1001247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Montaner JSG, Hogg R, Wood E, et al. The case for expanding access to highly active antiretroviral therapy to curb the growth of the HIV epidemic. Lancet. 2006;368:531–36. doi: 10.1016/S0140-6736(06)69162-9. [DOI] [PubMed] [Google Scholar]
- 14.Wood E, Kerr T, Marshall BDL, Li K, Zhang R, Hogg RS, Harrigan PR, Montaner JSG. Longitudinal community plasma HIV-1-RNA concentrations and incidence of HIV-1 among injecting drug users: a prospective cohort study. BMJ. 2009;338:b1649, 1191–94. doi: 10.1136/bmj.b1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Montaner JSG, Lima VD, Barrios R, Yip B, Wood E, Kerr T, Shannon K, Harrigan RP, Hogg RS, Daly P, Kendall P. Association of highly active antiretroviral therapy coverage, population viral load, and yearly new HIV diagnoses in British Columbia, Canada: A population-based study. Lancet. 2010;376:532–9. doi: 10.1016/S0140-6736(10)60936-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cohen MS, Chen YQ, McCauley M, gamble T, Hosseinipour MC, Kumarasamy N, Hakim JG, Kumwenda J, Grinsztejn B, Pilotto JHS, Godbole SV, Mehendale S, Chariyalertsak S, Santos BR, Mayer KH, Hoffman IF, Eshleman SH, Piwowar-Manning E, Wang L, Makhema J, Mills LA, de Bruyn G, Sanne I, Eron J, Gallant J, Havlir D, Swindells S, Ribaudo H, Elharrar V, Burns D, Taha TE, Nielsen-Saines K, Celentano D, Essex M, Fleming TR for the HPTN 052 study team. Prevention of HIV-1 infection with early antiretroviral therapy. New England Journal of Medicine. 2011 doi: 10.1056/NEJMoa1105243. [DOI] [Google Scholar]
- 17.Granich R, Gupta S, Suthar A, Smyth C, Hoos D, Vitoria M, Simao M, Hankins C, Schwartlander B, Ridzon R, Bazin B, Williams B, Lo Y-R, McClure C, Montaner J, Hirnschall G on behalf of the ART in Prevention of HIV and TB Research Writing Group. ART in prevention of HIV and TB: Update on current research efforts. Current HIV Research. 2011;9:446–69. doi: 10.2174/157016211798038597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barnett PG, Chow A, Joyce VR, Bayoumi AR, Griffin SC, Nosyk B, Holodniy M, Brown ST, Sculpher M, Anis AH, Owens DK for the OPTIMA team. Determinants of the costs of health services used by veterans with HIV. Med Care. 2011;49(9):848–56. doi: 10.1097/MLR.0b013e31821b34c0. [DOI] [PubMed] [Google Scholar]
- 19.Bozzette SA, Joce G, McCaffrey DF, et al. Expenditures for the care of HIV-infected patients in the era of highly active antiretroviral therapy. N Engl J Med. 2001;344:817–23. doi: 10.1056/NEJM200103153441107. [DOI] [PubMed] [Google Scholar]
- 20.Chen RY, Accort NA, Westfall AO, et al. Distribution of health care expenditures for HIV-infected patients. Clinc Infect Dis. 2006;42:1003–10. doi: 10.1086/500453. [DOI] [PubMed] [Google Scholar]
- 21.Gebo KA, Fleishman JA, Conviser R, Hellinger FJ, Josephs JS, Keiser P, Gaist P, Moore RD HIV Research Network. Contemporary Costs of HIV Healthcare in the HAART Era. AIDS. 2010;24(17):2705–2715. doi: 10.1097/QAD.0b013e32833f3c14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lima VD, Zhang W, Yip B, Chan K, Nosyk B, Kanters S, Kozai T, Montaner JSG. Assessing the effectiveness of antiretroviral regimens in cohort studies involving HIV-positive injection drug users. AIDS. 2012;26(12):1491–500. doi: 10.1097/QAD.0b013e3283550b68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mellors JW, Munoz A, Giorgi JV, Margolick JB, Tassoni CJ, Gupta P, Kingsley LA, Todd JA, Saah AJ, Detels R, Phair JP, Rinaldo CR., Jr Plasma viral load and CD4+ lymphocytes as prognostic markers of HIV-1 infection. Ann Intern Med. 1997;126(12):946–54. doi: 10.7326/0003-4819-126-12-199706150-00003. [DOI] [PubMed] [Google Scholar]
- 24.O’Brien WA, Hartigan PM, Martin D, Esinhart J, Hill A, Benoit S, Rubin M, Simberkoff MS, Hamilton JD. Changes in plasma HIV-1 RNA and CD4+ lymphocyte counts and the risk of progression to AIDS. Veterans Affairs Cooperative Study Group on AIDS. N Engl J Med. 1996;334(7):426–31. doi: 10.1056/NEJM199602153340703. [DOI] [PubMed] [Google Scholar]
- 25.Park R. Estimation with heteroskedastic error terms. Econometrica. 1966;34:888. [Google Scholar]
- 26.Manning WG, Mullahy J. Estimating log models: to transform or not ot transform? Journal of Health Economics. 2001;20:461–94. doi: 10.1016/s0167-6296(01)00086-8. [DOI] [PubMed] [Google Scholar]
- 27.Liu L, Strawderman RL, Cowen ME, Shih YC. A flexible two-part random effects model for correlated medical costs. J Health Econ. 2010;29(1):110–23. doi: 10.1016/j.jhealeco.2009.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Raudenbush SW, Bryk AS. Hierarchical linear models: Applications and data analysis methods. 2. Newbury Park, CA: Sage; 2002. [Google Scholar]
- 29.Levy AR, James D, Johnston KM, Hogg RS, Harrigan PR, Harrigan BP, Sobolev B, Montaner JS. The direct costs of HIV/AIDS care. Lancet Infect Dis. 2006 Mar;6(3):171–7. doi: 10.1016/S1473-3099(06)70413-3. [DOI] [PubMed] [Google Scholar]
- 30.Levy A, Johnston K, Annemans L, Tramarin A, Montaner J. The impact of disease stage on direct medical costs of HIV management: a review of the international literature. Pharmacoeconomics. 2010;28 (Suppl 1):35–47. doi: 10.2165/11587430-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 31.Sloan CE, Champenois K, Choisy P, Losina E, Walensky RP, Schackman BR, Ajana F, Melliez H, Paltiel AD, Freedberg KA, Yazdanpanah Y Cost-Effectiveness of Preventing AIDS Complications (CEPAC) investigators. Newer drugs and earlier treatment: impact on lifetime cost of care for HIV-infected adults. AIDS. 2012;26(1):45–56. doi: 10.1097/QAD.0b013e32834dce6e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Walensky RP, Sax PE, Nakamura YM, Weinstein MC, Pei PP, Freedberg KA, Paltiel AD, Schackman BR. Economic savings versus health losses: the cost-effectiveness of generic antiretroviral therapy in the United States. Ann Intern Med. 2013;158(2):84–92. doi: 10.7326/0003-4819-158-2-201301150-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Carpenter CC, Fischl MA, Hammer SM, Hirsch MS, Jacobsen DM, Katzenstein DA, Montaner JS, Richman DD, Saag MS, Schooley RT, Thompson MA, Vella S, Yeni PG, Volberding PA. Antiretroviral therapy for HIV infection in 1997. Updated recommendations of the International AIDS Society-USA panel. JAMA. 1997;277(24):1962–9. [PubMed] [Google Scholar]
- 34.Carpenter CC, Fischl MA, Hammer SM, Hirsch MS, Jacobsen DM, Katzenstein DA, Montaner JS, Richman DD, Saag MS, Schooley RT, Thompson MA, Vella S, Yeni PG, Volberding PA. Antiretroviral therapy for HIV infection in 1998: updated recommendations of the International AIDS Society-USA Panel. JAMA. 1998;280(1):78–86. doi: 10.1001/jama.280.1.78. [DOI] [PubMed] [Google Scholar]
- 35.Carpenter CC, Cooper DA, Fischl MA, Gatell JM, Gazzard BG, Hammer SM, Hirsch MS, Jacobsen DM, Katzenstein DA, Montaner JS, Richman DD, Saag MS, Schechter M, Schooley RT, Thompson MA, Vella S, Yeni PG, Volberding PA. Antiretroviral therapy in adults: updated recommendations of the International AIDS Society-USA Panel. JAMA. 2000 Jan 19;283(3):381–90. doi: 10.1001/jama.283.3.381. [DOI] [PubMed] [Google Scholar]
- 36.Yeni PG, Hammer SM, Carpenter CC, Cooper DA, Fischl MA, Gatell JM, Gazzard BG, Hirsch MS, Jacobsen DM, Katzenstein DA, Montaner JS, Richman DD, Saag MS, Schechter M, Schooley RT, Thompson MA, Vella S, Volberding PA. Antiretroviral treatment for adult HIV infection in 2002: updated recommendations of the International AIDS Society-USA Panel. JAMA. 2002 Jul 10;288(2):222–35. doi: 10.1001/jama.288.2.222. Review. Erratum in: JAMA. 2003 Jan-Feb;11(1):32. [DOI] [PubMed] [Google Scholar]
- 37.Nosyk B, Montaner JSG, Colley G, Lima VD, Chan K, Yip B, Heath K, Gilbert M, Gustafson R, Hogg RS on behalf of the STOP HIV/AIDS Study Group. Population-level retrospective cohort study: The Cascade of HIV Care in British Columbia, Canada: 1996–2011. Lancet Infectious Diseases. 2013 Sep 26; doi: 10.1016/S1473-3099(13)70254-8. pii: S1473–3099(13)70254–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lima VD, Le A, Nosyk B, Barrios R, Yip B, Hogg RS, Harrigan PR, Montaner JSG. Development and Assessment of a Composite Programme Assessment Score for Initial HIV Therapy. PLoS One. 2012;7(11):e47859. doi: 10.1371/journal.pone.0047859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hoover DR, Graham NM, Chen B, Taylor JM, Phair J, Zhou SY, Munoz A. Effect of CD4+ cell count measurement variability on staging HIV-1 infection. Journal of Acquired Immune Deficiency Syndrome. 1992;5(8):794–802. [PubMed] [Google Scholar]
- 40.Grimes DA, Schulz KF. Bias and causal associations in observational research. Lancet. 2002;359:248–52. doi: 10.1016/S0140-6736(02)07451-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.