Abstract
Foot-and-mouth disease virus (FMDV) causes a highly contagious viral disease of even-toed ungulates and is one of the most important economic diseases of livestock. Most studies of FMDV are done in countries where control measures are being implemented. In contrast, in areas such as sub-Saharan Africa, where FMDV is endemic and new strains are likely to emerge, there are only sporadic submissions to the World Reference Laboratory, Pirbright, United Kingdom. This paper describes the molecular epidemiology of FMDV in the Adamawa province of Cameroon based on a population sample of cattle herds. Serotypes SAT2 and A were isolated in the cross-sectional study. SAT2 isolates were all similar, with phylogenetic distances of <6%, and were most closely related to published sequences of isolates from Eritrea and Saudi Arabia. Serotype A isolates were more variable, with phylogenetic distances of 0 to 11%, and were most closely related to historic isolates from Cameroon. Use of a population-based sample gives a representative sample of virus diversity and will improve our understanding of the evolution of FMDV and its epidemiology. A supplementary study of pigs passing through the railhead collection yard at Ngaoundere detected a serotype O virus. A third pilot longitudinal study monitored viral persistence in three cattle herds over 12 months, and serotype O and A viruses were recovered from a herd 12 months after it was first recorded as being infected with SAT2 virus. The pig type O isolate was not closely related to that recovered from the cattle, suggesting that the pigs had not introduced the O virus into the cattle herds.
Foot-and-mouth disease virus (FMDV) (Picornaviridae, genus Aphthovirus) causes a highly contagious viral disease of even-toed ungulates (Artiodactyla) and is one of the most important economic diseases of livestock. Though it is generally referred to as a single disease, seven distinct viral serotypes are distributed globally. These have different geographical distributions and epidemiologies, though they are clinically indistinguishable. Asia1 (1) is restricted to the Middle East and Asia and has not spread into Africa or Europe, serotype SAT3 rarely occurs in species other than the Cape buffalo in southern Africa (38), and serotype O is widespread across the world (21).
Most studies of FMDV are done in countries where control measures are being implemented. In contrast, in areas such as sub-Saharan Africa, where FMDV is endemic, there are only sporadic submissions to the World Reference Laboratory (WRL), Pirbright, United Kingdom (13). These are usually from very limited geographical areas and time scales and as a result may give a very biased impression of the disease situation and epidemiology. However, regions where the disease is endemic are where new strains are most likely to evolve.
Typing of FMDVs was originally done by serology cross-protection, and on this basis, Brooksby (6) suggested that serotypes O, A, and C had probably not penetrated south of the equator before 1955. The SAT viruses were not described until the late 1940s in southern Africa (38) and had not yet moved north of the equator by the late 1950s (23). More recent reviews of the literature on submissions to the WRL for the whole of Africa between 1958 and 1992 (13) and from 1948 to 2002 (41) suggest that at least four of the six serotypes found in Africa have occurred in western and central Africa at some stage. These were serotypes SAT1, SAT2, A, and O. Reviews of submissions to WRL suggested that serotypes O and A have been circulating in Cameroon for the last 70 years (11, 13).
In recent years, molecular techniques have been used to define strains, to identify transmission events, and to characterize biodiversity (21, 29, 42). Most studies of FMDV have concentrated on the highly variable region of the capsid gene VP1, known to be an important antigenic site (14, 15, 25). A number of studies of SAT-type viruses have been done in cattle (42) in southern Africa and West Africa (34, 35) and in buffalo and impala in South Africa (2) and of type O viruses from northern Africa (29) and western and southern Africa (36). There do not appear to have been many studies on serotype A viruses in Africa, though they have been studied in other regions, particularly India (39).
This paper describes the molecular epidemiology of FMDV in the Adamawa province of Cameroon. At the time of the study, Cameroon had no FMD control program and no license had been issued for vaccine importation, presenting an opportunity to study the natural ecology and epidemiology of FMDV.
MATERIALS AND METHODS
Study design.
The Adamawa province lies between latitude 6° and 8° north latitude and covers an area of about 64,000 km2 with Ngaoundere as its provincial capital. Adamawa is divided into five administrative divisions serviced by 88 government veterinary centers. Samples were collected during three concurrent studies: a population-based cross-sectional study of cattle herds, a longitudinal study of pigs passing through the railhead collecting yard, and a pilot longitudinal study monitoring three cattle herds.
Cross-sectional study.
A stratified, two-stage random sample of cattle herds from Adamawa province were selected. The method is described in full elsewhere (5). Briefly, a sample frame of 13,006 herds was constructed with rinderpest vaccination records from the veterinary centers. A random sample of 162 herds was selected based on three herds from each of 54 veterinary centers. A standardized questionnaire was used to collect information from the herdsmen about herd management, movements, and contacts with other herds and wildlife (5). Random number tables were used to select a sample of five adult (>2 years old) and five juvenile (8 to 24 months old) cattle from each herd and five sheep or goats, if present. The within-herd sample size gave a 95% probability of detecting at least one positive animal, assuming a within-herd prevalence of 50% and a perfect test (7).
Each animal was cast in lateral recumbency, and the feet, mouth, and udder were examined for any sign of active or old FMDV lesions. Epithelial samples were taken from mouth or foot lesions of clinically affected animals with sterile scissors and forceps. Oropharyngeal samples, also known as probang samples, were taken with probang cups, which came in small-ruminant, calf, and adult cow sizes. Probang cups were washed in citric acid (0.2%, wt/vol) and rinsed three times in water between animals and flame sterilized between herds (19). Herds were visited between April and October 2000, and clinical epithelial samples and oropharyngeal samples were collected from 118 of the 162 herds.
Longitudinal study of the pigs at the railhead.
Pigs passing through the railhead collection yard at Ngaoundere Station were examined. Pigs are not common in Adamawa province, and these animals originated from the north of Cameroon. They were gathered and penned by dealers at the railhead until sufficient animals had been collected to fill a train car, at which point they were transported to the major cities of Douala and Yaounde in the south. Six visits, at roughly monthly intervals, were made to the railhead between April and October 2000. By use of random number tables, one pig was selected from each pen and examined for signs of disease. Serum samples were collected from all animals, and a whole-blood sample was collected for virus isolation from any animals that were febrile and showing signs of clinical disease. The sample size was limited by a strong reluctance of the dealers to allow blood samples to be obtained from their animals, which were about to be shipped for slaughter.
Pilot longitudinal study of cattle.
Three cattle herds were monitored over a 12-month period between 2000 and 2001. The herds were a convenience sample recruited on the basis of owner compliance and proximity to Ngaoundere and the presence of clinical disease at the time of the first examination. For each herd, 20 animals were randomly selected at the time of clinical disease (0 months, except herd 3) and at 3, 6, and 12 months postinfection. Each animal was examined clinically, and serum and oropharyngeal samples were collected. Epithelial samples were also collected from animals with active FMD lesions.
Sample collection and processing.
All samples were collected and processed following WRL and Office International des Epizooties guidelines (18, 19). After processing, the epithelial and serum samples were stored at 4°C in a portable gas fridge for up to 2 weeks and then at −20°C on returning to the laboratory in Ngaoundere. An aliquot of each oropharyngeal and whole-blood sample was stored in liquid nitrogen at the end of the working day. On completion of the fieldwork, samples were transported to the WRL either on dry ice (serum and epithelium) or in a liquid nitrogen dry shipper (oropharyngeal and whole blood). At the WRL, samples were stored at −20°C (serum and epithelium) or at −70°C (oropharyngeal and whole blood).
Virus isolation.
Virus isolation was attempted from all samples in either primary bovine thyroid cell cultures for all epithelial and oropharyngeal samples or renal swine cell cultures (RS) for epithelial and pig whole-blood samples by the WRL standard protocol (18). All cultures showing FMDV cytopathic effect were harvested and serotyped with the WRL indirect sandwich enzyme-linked immunosorbent assay (12, 18, 27).
Identification codes for isolated viruses.
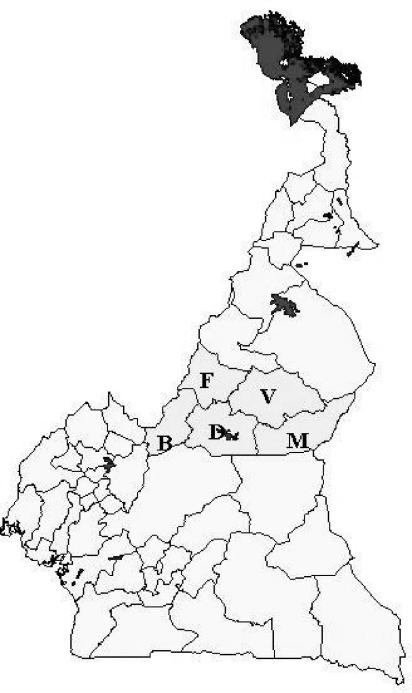
The viruses isolated during this study were named in one of two ways. Viruses isolated from epithelial or whole-blood samples were attributed to clinical disease and given WRL codes. These consist of a three-letter uppercase country code, a sample number, and a two- or four-digit year code, e.g., CAR/12/2000 was the 12th sample submitted for virus isolation from a clinical outbreak from Cameroon in 2000. Isolates obtained from probang samples kept their original project collection code, consisting of a lowercase three-letter veterinary center code, a three-digit herd code, and a two-digit animal code, e.g., vdi/044/05. The first letter of the veterinary center code, v, m, d, b, or f, indicates that the isolate came from the Vina, Mbere, Djerem, Mayo Banyo, or Faro et Deo division, respectively. Therefore, vdi/044/05 was a probang sample from the Vina Division, Dibi Veterinary Center, herd 044, and the fifth animal. The Adamawa province and the locations of the five administrative divisions are shown in Fig. 1.
FIG. 1.
Map of Cameroon showing Adamawa province (grey) and the five divisions (V, Vina; M, Mbere; D, Djerem; B, Mayo Banyo; and F, Faro et Deo).
Sequencing.
All viruses that were sequenced underwent a maximum of two passages in cell culture. The viral RNA was extracted from 460 μl of either the original viral suspension or the tissue culture supernatant, following the manufacturer's instructions (RNeasy Protect minikit; Qiagen Ltd.), eluted into 50 μl of diethyl pyrocarbonate-treated H2O, and stored at −70°C until used for reverse transcription.
A one-stage reverse transcription-PCR was used to amplify the 3′ end of the VP1 gene from 5 μl of purified RNA according to the manufacturer's instructions (Ready-To-Go reverse transcription-PCR tube; Amersham Pharmacia Biotech United Kingdom Ltd.). The primers were used at a concentration of 0.2 to 0.5 pmol/μl and depended on the FMDV serotype (Tables 1 and 2). For the reverse transcription phase, the samples were incubated at 42°C for 30 min, followed by 5 min at 90°C to inactivate the reverse transcriptase. After further incubation at 94°C for 3 min, the samples were subjected to 30 cycles of PCR, with 1 cycle consisting of template denaturation at 94°C for 1 min, primer annealing at 55°C for 1 min, and primer extension at 72°C for 1.5 min. There was a final incubation at 72°C for 5 min.
TABLE 1.
PCR primers used in this study
| FMDV serotype | Reverse transcription primer | PCR forward primer | PCR reverse primer | Amplicon length (bp) | Region |
|---|---|---|---|---|---|
| O | NK61 | ARS4 | NK61 | 1,301 | VP1 + 2A |
| A | NK61 | A-1C562 | NK61 | 863-866 | VP1 + 2A |
| SAT2 | 2B208R | 1D209F | 2B208R | 450 | Part of VP1 + 2A |
TABLE 2.
Sequences of PCR and sequencing primers
| Primer | Serotype | Direction for sequencinga | Sequence (5′ to 3′) |
|---|---|---|---|
| ARS4 | O | PCR/seq/forward | ACCAACCTCCTTGATGTGGCT |
| A-1C562 | A | PCR/seq/forward | TACCAAATTACACACGGGAA |
| 1D209F | SAT2 | PCR/seq/forward | CCACATACTACTTTTGTGACCTGGA |
| NK61 | O/A | PCR/reverse | GACATGTCCTCCTGCATCTG |
| 2B208R | SAT2 | PCR/reverse | ACAGCGGCCATGCACGACAG |
| NK72 | O/A/SAT2 | seq/reverse | GAAGGGCCCAGGGTTGGACTC |
seq, sequencing primer; PCR, PCR primer.
Amplicons were purified to remove unincorporated primers and nucleotides (Wizard Prep DNA purification kit; Promega) and extracted with phenol-chloroform (28) to remove any viral protein or infectivity. The purified amplicons were sequenced by MWG-Biotech as recommended by the manufacturer (ABI Prism BigDye Terminators version 3.0 cycle sequencing kits; Applied Biosystems). All amplicons were forward sequenced with the PCR primer for that serotype and reverse sequenced with internal primer NK72 (Table 2).
Archive samples and GenBank data.
In addition to the samples collected in this study, five historical isolates from Cameroon, including four type A viruses and one type O virus submitted to WRL in the 1970s and 1980s, were also sequenced (type A, CAR/14/75, CAR/1/76, CAR/5/85, and CAR/4/86; type O, CAR/12/88).
Previously published sequences of the same section of the VP1 region were also included in the analysis; these included all published sequences of serotypes SAT2 and A from Africa. However, not many such sequences were available. In addition, some representative sequences from other parts of the world were included for comparison. Isolates from the Middle East and India as well as examples from South America were included. For serotype A, these were BRA/55 (GenBank accession no. FD1251476), MOR/83 (M16090); IRQ/24/64 (AJ251474) (4); ARG/68 (AJ308694) (22); ARG/87 (FDU62261); and IND/17/77 (AF204108), IND/302/88 (AF390641), IND/395/88 (AF390645), IND/299/94 (AF390639), IND/76/96 (AF390662), IND/490/97 (AF390652), IND/456/98 (AF390651), IND/84/2000 (AF390668), and IND/126/2000 (AF390599) (39). The SAT2 sequences previously published by Sangare et al. (34) (AF426068-71, AF426074, AF426076, AF426078-89, AF426091-98, AF367139-40, and AF367100) and Bastos et al. (2) (AF136987 to AF137019) were included, as well as KEN/3/57 (AJ251473) and MOS/4/83 (AF023519) (3). Sequences for SAU/2/00 and ERI/1/98 were kindly provided by N. Knowles (WRL).
The type O sequences included in the analysis were ANG/10/74 (AF300810), NGR/1/88 (AF300801), BKF/1/92 (AF300804), GHA/6/93 (AF300807) GHA/5/93 (AJ303488), SA/11/00 (AF306646), SAR/12/00 (AY009087) (36); SAU/100/94 (AJ004660), SAU/2/95 (AJ004662) (33); ETH/8/94 (AJ303487), KEN/2/95 (AJ303514), UGA/5/96 (AJ296327), TAN/7/98 (AJ290320), ALG/1/99 (AJ303481), CIV/8/99 (AJ303485), IRQ/30/2000 (AJ303499) (31), and IRN/16/2000 (AJ318840).
Phylogenetic analysis.
The forward and reverse sequences obtained from each isolate were aligned, and a consensus sequence was derived with MatchTools Navigator (ABI Perkin Elmer). For 14 of 69 sequences, the forward sequence was of relatively poor quality. In these cases, only the reverse sequence was used in further analysis. The use of single-stranded sequences in these cases was justified on the basis that when double-stranded sequence was available, ambiguities occurred in less than 0.1% of nucleotides. All sequences were aligned and trimmed to a standard length in Bioedit (16). This resulted in a final useable sequence of 537 bp for serotype A, 357 bp for serotype SAT2, and 490 bp for serotype O. Uncorrected neighbor-joining trees were constructed with Clustal-X version 1.82′ (37) and viewed with Treeview (24). Bootstrap values of >70 out of 100 replications are shown at the relevant major nodes. The geographic positions of the herds were plotted with ArcGIS version 8.0 (Environmental Systems Research Institute, Inc.).
Distance distribution analysis.
Previous studies on FMDV have used various lengths and regions of the VP1 sequence to calculate distance values between isolates. In order to determine the effect of the length and region of nucleotide sequence used on the resulting phylogenetic trees, a sliding window distance analysis was conducted (Plotsimilarity in the GCG package [10]). This analysis was performed both on the full set of sequences (to get an average) and for a pair of unrelated sequences (to get an estimate for the maximum variation) for serotype SAT2 and A viruses only.
RESULTS
Based on the results of the cross-sectional study, a description of the farming practices of the Fulani tribe of the Adamawa can be found in Bronsvoort et al. (5). The herdsmen reported an estimated prevalence of FMD outbreaks for the previous 12 months of 57.9% of herds (50.4 to 65.4% [90% confidence interval]). The prevalence varied across the divisions, with the highest prevalences in the Vina (77%) and Faro et Deo (73%) divisions, compared to Mbere (57%), Djerem (37%), and Mayo Banyo (44%).
Virus isolation.(i) Cross-sectional study.
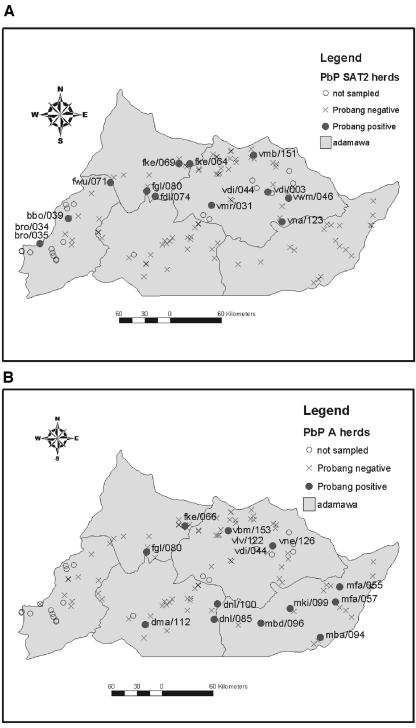
A total of 1,097 bovine and 55 ovine or caprine oropharyngeal samples and one bovine epithelial sample were collected from 118 herds. Thirty-eight (3.5%) of the bovine samples were FMDV positive, of which 18 were serotype SAT2 and 20 were serotype A. The epithelial sample was also SAT2 positive. No virus was isolated from any of the small-ruminant samples. This was the first record of a SAT2 virus from Cameroon. The geographic positions of the herds are given in Fig. 2; the positive herds have been highlighted and labeled.
FIG. 2.
(A) Distribution of serotype SAT2 probang-positive herds in Adamawa province in 2000. (B) Distribution of serotype A probang-positive herds in Adamawa in 2000.
(ii) Longitudinal study of pigs.
A total of 58 pigs were examined at the Ngaoundere lairage over six visits. Only three animals showed clinical disease, all at the fifth visit, and serotype O virus was isolated from two of these (3.4%).
(iii) Longitudinal study in cattle.
A total of 172 oropharyngeal samples and six epithelial samples were collected from the three herds. Twenty-seven oropharyngeal and four epithelial samples were virus positive. The oropharyngeal samples from herds vdi/003 and vdi/044 were positive for SAT2 FMDV at 3 months but negative at 6 months. Herd vdi/044 was again positive at 12 months, but serotypes O and A were isolated on this occasion. Herd vlv/122 had serotype A-positive oropharyngeal and epithelial samples at the first sampling, when animals were clinically affected, but all subsequent samples up to 12 months later were culture negative for virus. One additional epithelial convenience sample from a clinically diseased herd (bbo/042) was also positive for serotype SAT2.
High-quality sequence was obtained for 67 of the 73 isolates, and these are listed along with the five historic isolates from Cameroon in Table 3 (serotype SAT2), Table 4 (serotype A), and Table 5 (serotype O).
TABLE 3.
Serotype SAT2 isolates from Cameroon in 2000 used in this analysis
| Isolate | Samplea | Date (day/mo/yr) | Division and center | Time since last outbreakc (mo) | Accession no. |
|---|---|---|---|---|---|
| CAR/5/00 (bbo/42/10)b | Epi/nrs | 23/05/00 | Mayo Banyo, Banyo | NA | AY254444 |
| CAR/10/00 (vdi/44/10) | Epi/nrs | 31/05/00 | Vina, Dibi | NA | AY254467 |
| CAR/11/00 (fwu/71/09) | Epi/rs | 05/07/00 | Faro et Deo, Wogomdou | NA | AY254446 |
| vdi/003/30 | Pro/rs/ls | 18/04/00 | Vina, Dibi | 3 | AY254452 |
| vmr/031/07 | Pro/rs | 15/05/00 | Vina, Makor | 4 | AY254453 |
| vmr/031/08 | Pro/rs | AY254455 | |||
| vmr/031/09 | Pro/rs | AY254454 | |||
| bro/034/08 | Pro/rs | 18/05/00 | Mayo Banyo, Ribao | 7 | AY254459 |
| bro/035/10 | Pro/rs | 18/05/00 | Mayo Banyo, Ribao | 3 | AY254460 |
| bbo/039/06 | Pro/rs | 22/05/00 | Mayo Banyo, Banyo | 5 | AY254448 |
| vdi/044/01 | Pro/ls | 31/05/00 | Vina, Dibi | 0 | AY254463 |
| vdi/044/04 | Pro/ls | AY254464 | |||
| vdi/044/07 | Pro/ls | AY254465 | |||
| vdi/044/08 | Pro/ls | AY254466 | |||
| vdi/044/09 | Pro/ls | AY254461 | |||
| vdi/044/10 | Pro/ls | AY254462 | |||
| vdi/044/27 | Pro/ls | 27/09/00 | Vina, Dibi | 3 | AY254468 |
| vdi/044/30 | Pro/ls | AY254469 | |||
| vwm/046/06 | Pro/ls | 01/06/00 | Vina, Mangom | 7 | AY254458 |
| fke/064/02 | Pro/ls | 28/06/00 | Faro et Deo, Karedje | 6 | AY254450 |
| fke/069/08 | Pro/rs | 29/06/00 | Faro et Deo, Karedje | 24 | AY254449 |
| fwu/071/07 | Pro/rs | 05/07/00 | Faro et Deo, Wogomdou | 0 | AY254445 |
| fdl/074/04 | Pro/rs | 28/07/00 | Faro et Deo, Doualayel | 4 | AY254451 |
| fdl/074/10 | Pro/rs | AY254471 | |||
| fgl/080/09 | Pro/rs | 29/07/00 | Faro et Deo, Gassanguel | 0 | AY254447 |
| vna/123/10 | Pro/rs | 22/09/00 | Vina, Nyambaka | 10 | AY254470 |
| vmb/151/08 | Pro/rs | 22/10/00 | Vina, Mbang-Bouhari | 1 | AY254456 |
| vmb/151/09 | Pro/rs | AY254457 |
Epi, epithelial sample; Pro, probang sample; WB, whole-blood sample; ls, cattle longitudinal study; rs, cattle cross-sectional study; nrs, cattle convenience sample; pig, pig longitudinal study.
Codes in parentheses are the animals from which epithelial samples were collected.
As reported by herdsmen. NA, not applicable.
TABLE 4.
Serotype A FMDV isolates from Cameroon used in this analysisa
| Isolate | Sample | Date (yr or day/mo/yr) | Division and center | Time since last outbreak (mo) | Accession no. |
|---|---|---|---|---|---|
| CAR/14/75 | Epi | 1975 | NA | AY254440 | |
| CAR/1/76 | Epi | 1976 | NA | AY254443 | |
| CAR/5/85 | Epi | 1985 | NA | AY254442 | |
| CAR/4/86 | Epi | 1986 | NA | AY254441 | |
| CAR/12/00 (vlv/122/11) | Epi/ls | 19/09/00 | Vina, Lahore Vina | NA | AY254410 |
| CAR/13/00 | Epi/ls | 19/09/00 | Vina, Lahore Vina | NA | AY254412 |
| CAR/14/00 | AY254411 | ||||
| CAR/15/00 (vlv/122/20) | AY254413 | ||||
| vdi/044/66 | Pro/ls | 26/07/01 | Vina, Dibi | 12 | AY254439 |
| mfa/055/03 | Pro/rs | 17/06/00 | Mbere, Fada | 8 | AY254408 |
| mfa/057/01 | Pro/rs | 19/06/00 | Mbere, Fada | 48 | AY254419 |
| fke/066/08 | Pro/rs | 28/06/00 | Faro et Deo, Karedje | 15 | AY254437 |
| fgl/080/10 | Pro/rs | 29/07/00 | Faro et Deo, Gassanguel | 0 | AY254438 |
| dnl/085/01 | Pro/rs | 06/08/00 | Djerem, Ngaoundal | 2 | AY254423 |
| dnl/085/08 | Pro/rs | AY254424 | |||
| dnl/085/09 | Pro/rs | AY254425 | |||
| dnl/085/10 | Pro/rs | AY254432 | |||
| mba/094/09 | Pro/rs | 15/08/00 | Mbere, Beka | 4 | AY254435 |
| mbd/096/03 | Pro/rs | 17/08/00 | Mbere, Bindiba | 2 | AY254434 |
| mki/099/01 | Pro/rs | 18/08/00 | Mbere, Kalaidi | 7 | AY254409 |
| dnl/100/05 | Pro/rs | 28/08/00 | Djerem, Ngaoundal | 1 | AY254418 |
| dnl/100/06 | Pro/rs | AY254416 | |||
| dnl/100/08 | Pro/rs | AY254431 | |||
| dma/112/01 | Pro/rs | 09/09/00 | Djerem, Meijamba | 1 | AY254405 |
| dma/112/03 | Pro/rs | AY254406 | |||
| dma/112/06 | Pro/rs | AY254407 | |||
| vlv/122/02 | Pro/ls | 19/09/00 | Vina, Lahore Vina | 0 | AY254426 |
| vlv/122/03 | Pro/ls | 19/09/00 | AY254429 | ||
| vlv/122/04 | Pro/ls | 19/09/00 | AY254420 | ||
| vlv/122/06 | Pro/ls | 19/09/00 | AY254421 | ||
| vlv/122/08 | Pro/ls | 19/09/00 | AY254430 | ||
| vlv/122/11 | Pro/ls | 19/09/00 | AY254427 | ||
| vlv/122/13 | Pro/ls | 19/09/00 | AY254428 | ||
| vlv/122/16 | Pro/ls | 19/09/00 | AY254417 | ||
| vlv/122/19 | Pro/ls | 19/09/00 | AY254414 | ||
| vlv/122/20 | Pro/ls | 19/09/00 | AY254422 | ||
| vne/126/01 | Pro/rs | 25/06/00 | Vina, Ngaoundere | 2 | AY254436 |
| vne/126/09 | Pro/rs | AY254415 | |||
| vbm/153/09 | Pro/rs | 26/10/00 | Vina, Beka Mangare | 2 | AY254433 |
See Table 3, footnotes a, b, and c.
TABLE 5.
Serotype O isolates used in this analysisa
| Isolate | Sample | Date (yr or day/mo/yr) | Division and center | Time since last outbreak (mo) | Accession no. |
|---|---|---|---|---|---|
| CAR/12/88 | WRL | 1988 | Vina | NA | AY254400 |
| CAR/16/2000 | WB/pig | 20/09/00 | Vina, Ngaoundere | NA | AY254404 |
| CAR/17/2000 | WB/pig | 20/09/00 | Vina, Ngaoundere | NA | AY254403 |
| vdi/044/62 | Pro/ls | 26/07/01 | Vina, Dibi | 12 | AY254401 |
| vdi/044/70 | Pro/ls | 26/07/01 | Vina, Dibi | 12 | AY254402 |
See Table 3, footnotes a, b, and c.
Phylogenetic analysis.
The neighbor-joining trees based on the VP1 gene for each serotype are given in Fig. 3 (serotype SAT2), Fig. 4 (serotype A), and Fig. 5 (serotype O).
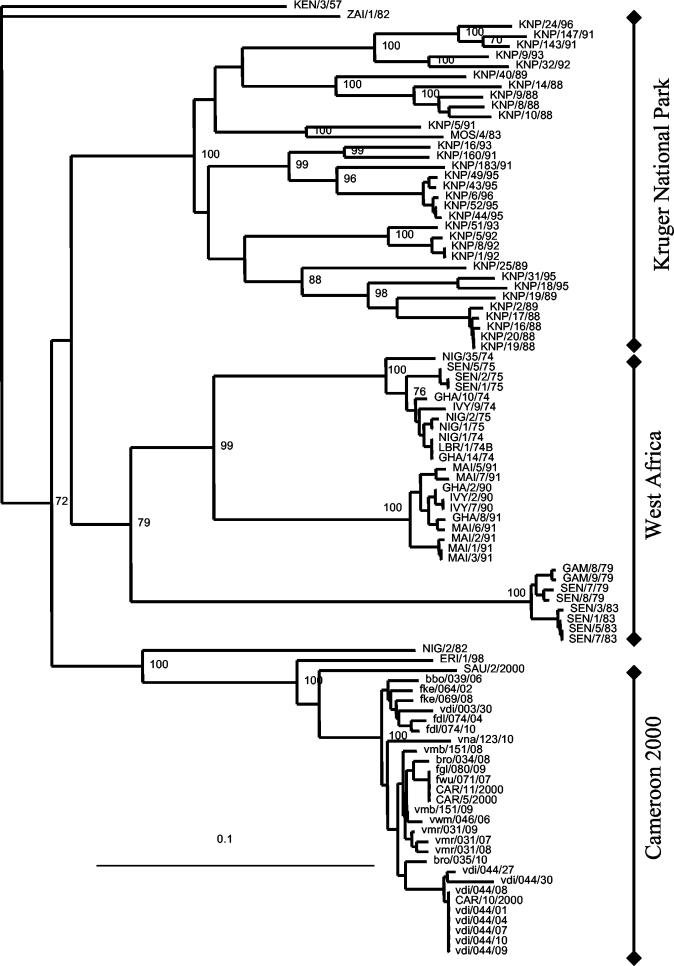
FIG. 3.
Uncorrected neighbor-joining tree of SAT2 isolates based on a 357-bp segment on the VP1 gene and rooted to KEN/3/57. Bootstrap values greater than 70 out of 100 repetitions are shown at the relevant major nodes. WRL codes: ALG, Algeria; BRA, Brazil; CAR, Cameroon; IVY/CIV, Côte d'Ivoire; ERI, Eritrea; ETH, Ethiopia; GAM, Gambia; GHA, Ghana; IND, India; IRQ, Iraq; IRN, Iran; KEN, Kenya; KNP, Kruger National Park, South Africa; LBR, Liberia; MAI, Mali; MOR, Morocco; MOS, Mozambique; NGR, Niger; NIG, Nigeria; SAU, Saudi Arabia; SAR, South Africa; SEN, Senegal; TAN, Tanzania; ZAI, Zaire.
FIG. 4.
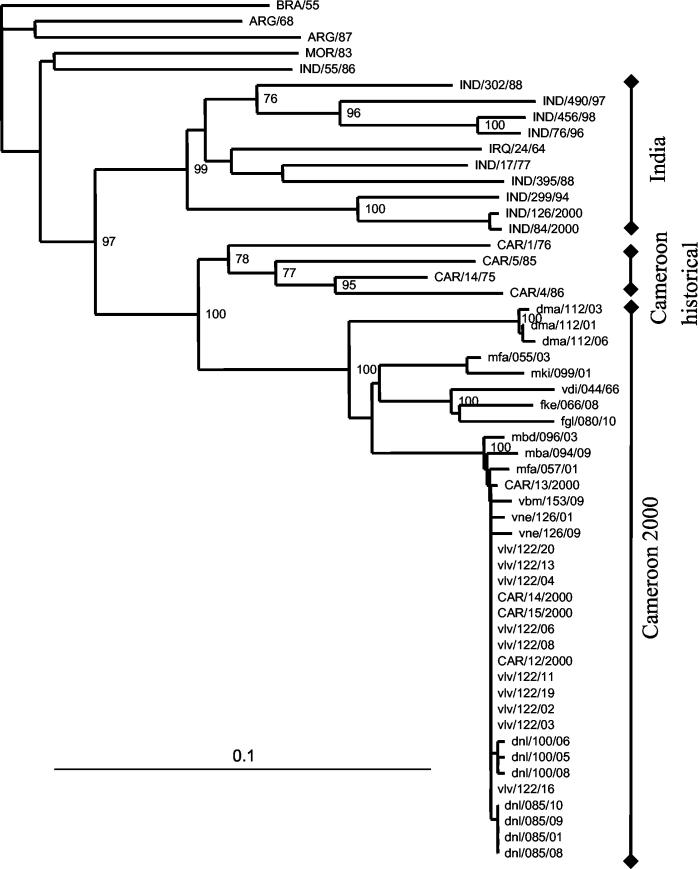
Uncorrected neighbor-joining tree of type A isolates based on a 537-bp region of the VP1 gene and rooted to A/BRA/55. For WRL codes, see the legend to Fig. 3.
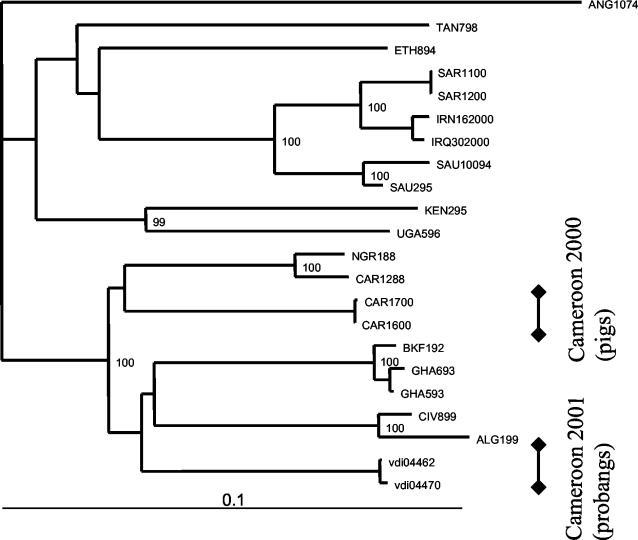
FIG. 5.
Uncorrected neighbor-joining tree of type O isolates based on a 489-bp segment of the VP1 gene and rooted to ANG/10/74. For WRL codes, see the legend to Fig. 3.
Serotype SAT2.
The phylogenetic distances for the cross-sectional study of herds from Cameroon ranged from 0 to 5.9%. There were several herds with multiple samples, and the within-herd distances were from 1.7 to 2.8% for herd vmr/031, 1.2% for herd vmb/151, and 0.8% for herd fdl/074. The distance between herds sampled at the same center within a few days of each other was 2% for fke/064/02 and fke/069/08 and 1.9% for isolates bro/034/08 and bro/035/10. Sequences from herds bbo/042 (epithelial isolate CAR/5/00) and vdi/044 (including epithelial isolate CAR/10/2000 and eight probang isolates) which were not part of the cross-sectional study were closely related to the other SAT2 isolates. Isolates from herd vdi/044, which was monitored over 12 months, formed a separate cluster, with identical sequences from all the probang and epithelial isolates taken at the time of clinical disease (vdi/044/01 to vdi/044/10 and CAR/10/2000, respectively). Two animals from this herd were probang positive after 3 months, and isolates vdi/044/27 and vdi/044/30 were 2% different from each other and between 2.2 and 6.2% different from the rest of the Cameroon isolates.
Distance comparisons with the next closest sequences ranged from 8.1 to 11.5% for a Saudi Arabian isolate (SAU/2/2000), from 10.1 to 12.3% for an Eritrean isolate (ERI/1/98), and from 19.9 to 21.0% for a Nigerian isolate (NIG/2/82). The distances then increased to 22 to 32% for the three clusters of western African isolates collected between the 1970s and 1990s and to 26 to 32.5% for those from buffalo and impala in Kruger National Park. The maximum distance value between all SAT2 sequences was 34%.
Serotype A.
The phylogenetic distances between the random samples of Cameroon herds ranged from 0 to 11.2%. Again there were several herds with multiple samples, and the within-herd distances ranged from 0% for herd dnl/085, 0.4 to 0.6% for herds dnl/100 and dma/112, and 1.3% for herd vne/126. There were two isolates from separate herds from the same center in Mbere (mfa/055/03 and mfa/057/01) which showed a 7.1% difference. There were four more distantly related isolates, but pairs that were geographically closer were more closely related (mfa/055/03 and mki/099/01 isolates and fke/066/08 and fgl/080/10 isolates), with distances of 9.1 to 11.2% from the largest cluster. The probang and epithelial samples from clinically infected herd vlv/122 accounted for most of the isolates in the largest cluster and had between 0 and 0.4% difference, though most of the sequences were identical. Isolate vdi/044/66, collected in the longitudinal study of cattle 12 months after the herd was infected with a SAT2 virus, was between 5 and 11.2% different from the other Cameroon isolates.
The isolates from this study were most closely related to four historic isolates from Cameroon. The phylogenetic distances between these historic sequences and the contemporary isolates ranged from 13.0 to 17.7%. The contemporary Cameroon isolates were not closely related to any of the other published sequences, though very few suitable sequences were available for Africa. The maximum variation between the serotype A sequences was 24.6%.
Serotype O.
None of the serotype O isolates came from the random sample of herds. Only four isolates were recovered and were grouped phylogenetically into two clusters. One cluster contained isolates from clinically infected pigs collected in 2000 (CAR/16/2000 and CAR/17/2000), with only 0.4% phylogenetic distance between them. The other contained two isolates, vdi/044/62 and vdi/044/70, which were 0.8% different. These came from probang samples collected from herd vdi/044 concurrently with a type A virus, 12 months after originally being infected with a SAT2 virus. The phylogenetic distances between these two small clusters ranged from 11.9 to 12.1%.
There was only one historic serotype O sequence available for Cameroon (CAR/12/88), which was most closely related to sequence NGR/1/88 from neighboring Niger. The phylogenetic distances between the contemporary Cameroon isolates and this historic isolate were between 9.8 and 11%. These contemporary Cameroon sequences were not closely related to any of the other suitable published sequences for Africa or the Middle East. The pan-Asian O strain, responsible for several major outbreaks around the world, is represented here by the two South African isolates SAR/11/2000 and SAR/12/2000.
Effect of sequence length on observed variation between sequences.
The results of the sliding-window analysis of data for serotypes SAT2 and A are given in Table 6. For both serotypes, as the window size was reduced, the range in variation increased. When all the sequences were included, there was more variation in the SAT2 than in the A sequences, but the window size appeared to have a greater effect on the range of variation for type A than for type SAT2 sequences.
TABLE 6.
Estimation of the effect of length of sequence and region of VP1 used on distance calculations for serotypes SAT2 and Aa
| Window size | Distance (%)
|
|||||
|---|---|---|---|---|---|---|
| SAT2 (357 bp)
|
A (537 bp)
|
O (490 bp)
|
||||
| All | Unrelated | All | Unrelated | All | Unrelated | |
| Full alignment | 24 | 26 | 13 | 20 | 15 | 21 |
| 250 | 23-25 | 27-29 | 11-14 | 16-22 | 14-17 | 19-24 |
| 200 | 23-27 | 25-31 | 11-15 | 15-24 | 13-18 | 17-26 |
| 150 | 22-27 | 23-32 | 10-16 | 14-24 | 13-19 | 15-26 |
| 100 | 20-30 | 19-38 | 7-20 | 9-28 | 11-22 | 11-29 |
For each window size, the range of distance values obtained for all possible window positions is shown. All, full alignment of sequences as in Fig. 2 and 3. In order to estimate the maximum variation in distance values, two unrelated sequences were used. For SAT2, these were KEN/57 and NIG/2/82; for A, they were VLV/122/11 and ARG/68; and for O, they were ANG/10/74 and CAR/16/00.
DISCUSSION
This study represents the first population-based study of FMDVs from a region of Africa where they are endemic but there is no current control strategy. Serotypes SAT2 and A were recovered from the random samples. This was the first record of a SAT2 virus from Cameroon. Though not recovered from the random samples, serotype O was recovered from a probang sample from the longitudinal cattle study and from two pigs in the longitudinal pig study, though these viruses were very different, suggesting that the virus had not been transmitted to the cattle from the pigs. The lack of type O isolates from the random sampling was surprising because type O virus has been recorded regularly in sub-Saharan Africa (41).
The origin of the SAT2 viruses is not certain. The SAT2 viruses were more closely related to isolates from Eritrea (1998) and Saudi Arabia (2000) than to any of the isolates from western Africa. However, these were all that were available from the WRL and GenBank and probably do not give a complete picture of the viruses in the region. Unfortunately, there are no isolates from the Sudan and the Central African Republic to fill the gaps. However, it is well-known that animals are traded from Sudan across to Cameroon, particularly through the Ngoui market on the border with the Central African Republic, and it is quite plausible that the virus could have spread from eastern Africa with the movement of cattle.
The relationship between these Cameroon isolates and those from the rest of western Africa is more difficult to interpret. In other parts of western Africa, there appears to be a clustering of isolates temporally but not geographically, which was interpreted as evidence of unrestricted movement of livestock across the region, transmitting the virus over a wide geographical area (34). However, from the phylogenetic tree and the sequences supplied by Sangare et al. (34), it is clear that at least three unrelated viruses have been circulating at different times over the last three decades in western Africa. The variation within each of these clusters is very small but the variation between clusters is large. This raises several questions. Have there been repeated introductions of unrelated viruses from outside the region, or is this the same evolving virus that is only being sampled when it has changed sufficiently to cause a new outbreak? One very intriguing cluster is that from Gambia and Senegal from 1979, with sequences that are very similar to those of viruses from an outbreak in 1983.
In southern Africa, the cape buffalo is believed to be an important reservoir of FMD for livestock (38), in particular the SAT viruses, although experimental attempts to reproduce transmission have proved difficult (8, 9). It was not possible to sample buffalo or other wildlife species within the limitations of this project. However, the forest buffalo found in Cameroon have never been studied for their role in FMD, and this is an area that needs attention. It was possible, however, to identify herds that came close to buffalo herds with the questionnaire, and 62% of herdsmen reported seeing buffalo near their herds during the last transhumance (seasonal migration), while only 9% of herdsmen reported seeing buffalo at their home pastures (5). A preliminary univariate analysis showed no association between being probang positive for either SAT2 or A and contact with buffalo (M. Bronsvoort, unpublished data).
The origins of the type A virus are even less clear. On the basis of the available sequences, it seems likely that the type A virus has been endemic in Cameroon for many decades and has been evolving continuously, causing outbreaks as new strains that can escape the protective immune response in the population evolve. The contemporary Cameroon isolates are quite distinct from the historic isolates from the 1970s and 1980s, with between 13 and 18% difference, which is sufficient to class them as unrelated. However, given that these viruses have been evolving for 20 to 30 years, it may be presumptive to do so.
The origins of the serotype O isolates were also difficult to determine due to the lack of available sequences from the region. They do not appear to be closely related to any of the other sequences from western Africa.
The isolates collected in this study show a range of variation within herds, between herds, and between veterinary centers. Generally, isolates from the same herd appear very similar, while isolates from different herds had slightly more variation. A major problem in discussing the relationships between isolates and their sequence data is deciding what the observed phylogenetic distances actually mean. As a rule of thumb, distances of <5% are considered to indicate the same “strain” (30, 31). At the other end of the spectrum, distances of more than 15% have been considered sufficient to classify isolates as unrelated (26, 32), although because of the greater variation in the SATs, a higher threshold of 20% has been suggested (20). However, this leaves a range of 5 to 15% phylogenetic distances that are undefined. Until this occurs, the epidemiological relevance of related and unrelated strains will remain unclear. It is only through well-structured population-based samples that this missing information can be obtained.
The phylogenetic analysis of the Cameroon SAT2 serotype sequences demonstrated that the overall range of phylogenetic distances was 0 to <6%, which the authors interpreted as suggesting that all the isolates had a common recent ancestor or were possibly part of the same single quasi-species. This suggests that there may have been an outbreak of SAT2 in which the virus spread through the population with only small genetic changes. This would be consistent with the suggestion that this was the first record of SAT2 in Cameroon. In contrast, the serotype A viruses from the random sample were much more diverse overall, with phylogenetic distances of up to 11%, suggesting that they have been in the region for some time and evolved from a distant common ancestor. Alternatively, their diversity could be interpreted as the result of several separate introductions. Superimposed on the endemic appearance of serotype A was a cluster of very closely related, if not the same, quasi-species of virus causing what appeared to be an outbreak with this virus strain widely dispersed across the province. This strain was only distantly related to the other type A viruses and may represent a genetic divergence that has produced an antigenic shift in the virus, allowing a new wave of type A disease to spread across the province.
Some impression of the rate of evolution may be obtained from the two samples from herd vdi/044 taken 3 months apart (vdi/044/27 and vdi/044/30). Isolate vdi/044/27 had hardly evolved from the virus recovered 3 months earlier from the herd, but the other isolate, vdi/044/30, had already accumulated 2% differences in sequence. This may not be reflected in the amino acid sequences. This much variation appears greater than published estimates for SAT2 in buffalo of 1.64% nucleotide substitutions per year (40). Here there are several genotypes, with many intermediate strains, which begins to give an impression of the evolutionary changes of these persistent viruses and the immunological pressure they are under (17).
The analysis of the relationship between the length of fragment used and the effect this has on the estimate of the similarity (diversity) of the sequences suggests that longer fragments should be used. In the published literature for FMDV molecular epidemiology, little attempt appears to have been made to standardize the region of the genome used for analysis beyond use of some part of the VP1 gene. The importance of sequence length on the relationships illustrated in dendrograms has been described for polioviruses (26). Those authors concluded that as long as a minimum of 60% of the fragment used came from the VP1 coding region and was the same for all the isolates, sequence length had no effect on the final tree. The issue of using the same region for all the pairwise comparisons is important if the comparisons are to be meaningful. This was one of the problems encountered in this study when looking for published sequences. Many of the published isolates only partially overlapped the region sequenced here, and the fragments were very short. With improved technology, it is increasingly feasible to sequence the whole genome. This may be at the other extreme, however, and possibly the aim should be to compare complete capsid gene sequences, as this would include all the antigenic sites.
Most molecular epidemiological studies on FMDV attempt to relate isolates from outbreaks or persistently infected animals over extended time periods and geographical areas with sequences derived from a convenience sample of viruses available from GenBank or at the WRL. However, such an approach may give a biased picture of the viral diversity in any one geographic region and may not reflect the true epidemiology of the disease. In contrast, structured sampling of endemically infected populations has rarely been performed. Using such a structured sampling approach, we have highlighted the very complex epidemiological situation that exists in Cameroon, with multiple serotypes of FMDV and various degrees of diversity within serotypes circulating simultaneously in a region or herd.
Acknowledgments
Mark Bronsvoort is funded by a Wellcome Trust Research Training Fellowship in Clinical Tropical Epidemiology (grant 053480).
We thank the Provincial Delegate for MINEPA, Adamou Abba, and all his staff, without whom the study would have not been possible. In addition, we thank O. Sangare for generously supplying the SAT2 sequences for western Africa and Nick Knowles for sequences from Eritrea and Sandi Arabia. Finally, we thank the cattle owners and herdsmen for their hospitality and their time and effort in allowing their herds to be examined.
REFERENCES
- 1.Ansell, D. M., A. R. Samuel, W. C. Carpenter, and N. J. Knowles. 1994. Genetic relationships between foot-and-mouth disease type Asia 1 viruses. Epidemiol. Infect. 112:213-224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bastos, A., C. Boshoff, D. Keet, R. Bengis, and G. Thomson. 2000. Natural transmission of foot-and-mouth disease virus between African buffalo (Syncerus caffer) and impala (Aepyceros melampus) in the Kruger National Park, South Africa. Epidemiol. Infect. 124:591-598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bastos, A. D. S. 1998. Detection and characterization of foot-and-mouth disease virus in sub-Saharan Africa. Onderstepoort J. Vet. Res. 65:37-47. [PubMed] [Google Scholar]
- 4.Bolwell, C., A. L. Brown, P. V. Barnett, R. O. Campbell, B. E. Clarke, N. R. Parry, E. J. Ouldridge, F. Brown, and D. J. Rowlands. 1989. Host-cell selection of antigenic variants of foot-and-mouth-disease virus. J. Gen. Virol. 70:45-57. [DOI] [PubMed] [Google Scholar]
- 5.Bronsvoort, B. M. D., V. N. Tanya, R. P. Kitching, C. Nfon, S. M. Hamman, and K. L. Morgan. 2003. Foot-and-mouth disease and livestock husbandry practices in the Adamawa Province of Cameroon. Trop. Anim. Health Prod. 35:491-507. [DOI] [PubMed] [Google Scholar]
- 6.Brooksby, J. B. 1982. Portraits of viruses: foot-and-mouth disease virus. Intervirology 18:1-23. [DOI] [PubMed] [Google Scholar]
- 7.Cannon, R. M., and R. T. Roe. 1982. Livestock disease surveys: a field manual for veterinarians. Australian Government Publishing Service, Canberra, Australia.
- 8.Condy, J. B., and R. S. Hedger. 1974. The survival of foot-and-mouth disease in African buffalo with non-transference of infection to domestic cattle. Res. Vet. Sci. 16:182-185. [PubMed] [Google Scholar]
- 9.Dawe, P. S., K. J. Sørensen, N. P. Ferris, I. T. Barnett, R. M. Armstrong, and N. J. Knowles. 1994. Experimental transmission of foot-and-mouth disease virus from carrier African buffalo Syncerus caffer to cattle in Zimbabwe. Vet. Rec. 134:211-215. [DOI] [PubMed] [Google Scholar]
- 10.Deveraux, J., P. Haeberli, and O. Smithies. 1984. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 12:387-395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ekue, N. F., V. N. Tanya, and C. Ndi. 1990. Foot-and-mouth disease in Cameroon. Trop. Anim. Health Prod. 22:34-36. [DOI] [PubMed] [Google Scholar]
- 12.Ferris, N. P., and M. Dawson. 1988. Routine application of enzyme-linked immunosorbent-assay in comparison with complement-fixation for the diagnosis of foot-and-mouth and swine vesicular diseases. Vet. Microbiol. 16:201-209. [DOI] [PubMed] [Google Scholar]
- 13.Ferris, N. P., and A. I. Donaldson. 1992. The World Reference Laboratory for Foot and Mouth Disease: a review of thirty-three years of activity (1958-1991). Rev. Sci. Tech. Off. Int. Epizoot. 11:657-684. [DOI] [PubMed] [Google Scholar]
- 14.González, M., M. G. Mateu, M. A. Martínez, C. Carrillo, and F. Sobrino. 1992. Comparison of capsid protein VP1 of the viruses used for the production and challenge of foot-and-mouth disease vaccines in Spain. Vaccine 10:731-734. [DOI] [PubMed] [Google Scholar]
- 15.Gurumurthy, C. B., A. Sanyal, R. Venkataramanan, C. Tosh, M. George, and D. Hemadri. 2002. Genetic diversity in the VP1 gene of foot-and-mouth disease virus serotype Asia 1. Arch. Virol. 147:85-102. [DOI] [PubMed] [Google Scholar]
- 16.Hall, T. A. 1999. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 41:95-98. [Google Scholar]
- 17.Haydon, D. T., A. D. Bastos, N. J. Knowles, and A. R. Samuel. 2001. Evidence for positive selection in foot-and-mouth disease virus capsid genes from field isolates. Genetics 157:7-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kitching, R. P., P. V. Barnett, A. I. Donaldson, and D. Mackay. 2000. Foot-and-mouth disease, p. 77-92. In S. Linnane (ed.), Manual of standards for diagnostic tests and vaccines, 4th ed., vol. 1. Office International des Epizooties, Paris, France. [Google Scholar]
- 19.Kitching, R. P., and A. I. Donaldson. 1987. Collection and transportation of specimens for vesicular virus investigation. Rev. Sci. Tech. Off. Int. Epizoot. 6:263-272. [Google Scholar]
- 20.Knowles, N. J., and A. R. Samuel. 2003. Molecular epidemiology of foot-and-mouth disease virus. Virus Res. 91:65-80. [DOI] [PubMed] [Google Scholar]
- 21.Knowles, N. J., A. R. Samuel, P. R. Davies, R. P. Kitching, and A. I. Donaldson. 2001. Outbreak of foot-and-mouth disease virus serotype O in the United Kingdom caused by a pandemic strain. Vet. Rec. 148:258-259. [PubMed] [Google Scholar]
- 22.Konig, G., C. Blanco, N. J. Knowles, E. L. Palma, E. Maradei, and M. E. Piccone. 2001. Phylogenetic analysis of foot-and-mouth disease viruses isolated in Argentina. Virus Genes 23:175-181. [DOI] [PubMed] [Google Scholar]
- 23.Libeau, J. 1960. Foot-and-mouth disease in Africa south of the Sahara: the present position. Bull. Off. Int. Epizoot. 54:90-104. [Google Scholar]
- 24.Page, R. D. 1996. Treeview: an application to display phylogenetic trees on personal computers. Comput. Appl. Biosci. 12:357-358. [DOI] [PubMed] [Google Scholar]
- 25.Pattnaik, B., R. Venkataramanan, C. Tosh, A. Sanyal, D. Hemadri, A. R. Samuel, N. J. Knowles, and R. P. Kitching. 1998. Genetic heterogeneity of Indian field isolates of foot-and-mouth disease virus serotype O as revealed by partial sequencing of 1D gene. Virus Res. 55:115-127. [DOI] [PubMed] [Google Scholar]
- 26.Rico-Hesse, R., M. Pallansch, B. Nottay, and O. Kew. 1987. Geographic distribution of wild poliovirus type 1 genotypes. Virology 160:311-322. [DOI] [PubMed] [Google Scholar]
- 27.Roeder, P. L., and P. M. Le Blanc Smith. 1987. Detection and typing of foot-and-mouth disease virus by enzyme-linked immunosorbent assay: a sensitive, rapid and reliable technique for primary diagnosis. Res. Vet. Sci. 43:225-232. [PubMed] [Google Scholar]
- 28.Sambrook, J., and D. W. Russell. 2001. Molecular cloning, 3rd ed. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 29.Samuel, A. R., N. J. Knowles, and D. K. Mackay. 1999. Genetic analysis of type O viruses responsible for epidemics of foot-and-mouth disease in North Africa. Epidemiol. Infect. 122:529-538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Samuel, A. R. 1997. Genetic and antigenic studies on foot-and-mouth disease virus type O. University of Hertfordshire, Woking, England.
- 31.Samuel, A. R., and N. J. Knowles. 2001. Foot-and-mouth disease type O viruses exhibit genetically and geographically distinct evolutionary lineages (topotypes). J. Gen. Virol. 82:609-621. [DOI] [PubMed] [Google Scholar]
- 32.Samuel, A. R., N. J. Knowles, and R. P. Kitching. 1988. Serological and biochemical analysis of some recent type A foot-and-mouth disease virus isolates from the Middle East. Epidemiol. Infect. 101:577-590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Samuel, A. R., N. J. Knowles, R. P. Kitching, and S. M. Hafez. 1997. Molecular epidemiology of foot-and-mouth disease type O viruses isolated in Saudi Arabia between 1983 and 1995. Epidemiol. Infect. 119:381-389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sangare, O., A. Bastos, D. S., E. H. Venter, and W. Vosloo. A first molecular epidemiological study of SAT-2 type foot-and-mouth disease viruses in West Africa. Epidemiol. Infect., in press. [DOI] [PMC free article] [PubMed]
- 35.Sangare, O., A. Bastos, D. S., E. H. Venter, and W. Vosloo. 2003. Retrospective genetic analysis of SAT-1 type foot-and-mouth disease outbreaks in West Africa. Vet. Microbiol. 93:279-289. [DOI] [PubMed] [Google Scholar]
- 36.Sangare, O., A. D. S. Bastos, O. Marquardt, E. H. Venter, W. Vosloo, and G. R. Thomson. 2001. Molecular epidemiology of serotype O foot-and-mouth disease virus with emphasis on West and South Africa. Virus Genes 22:345-351. [DOI] [PubMed] [Google Scholar]
- 37.Thompson, J. D., F. Plewniak, and O. Poch. 1999. A comprehensive comparison of multiple sequence alignment programs. Nucleic Acids Res. 27:2682-2690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Thomson, G. R. 1994. Foot-and-mouth disease, p. 823-852. In J. A. W. Coetzer, G. R. Thomson, and R. C. Tustin (ed.), Infectious diseases of livestock with special reference to southern Africa, 1st ed., vol. 2. Oxford University Press, Oxford, England. [Google Scholar]
- 39.Tosh, C., A. Sanyal, D. Hemadri, and R. Venkataramanan. 2002. Phylogenetic analysis of serotype A foot-and-mouth disease virus isolated in India between 1977 and 2000. Arch. Virol. 147:493-513. [DOI] [PubMed] [Google Scholar]
- 40.Vosloo, W., A. D. Bastos, E. Kirkbride, J. J. Esterhuysen, D. J. van Rensburg, R. G. Bengis, D. W. Keet, and G. R. Thomson. 1996. Persistent infection of African buffalo (Syncerus caffer) with SAT-type foot-and-mouth disease viruses: rate of fixation of mutations, antigenic change and interspecies transmission. J. Gen. Virol. 77:1457-1467. [DOI] [PubMed] [Google Scholar]
- 41.Vosloo, W., A. D. S. Bastos, O. Sangare, S. K. Hargreaves, and G. R. Thomson. 2002. Review of the status and control of foot and mouth disease in sub-Saharan Africa. Rev. Sci. Tech. Off. Int. Epizoot. 21:437-449. [DOI] [PubMed] [Google Scholar]
- 42.Vosloo, W., N. J. Knowles, and G. R. Thomson. 1992. Genetic relationships between southern African SAT-2 isolates of foot-and-mouth disease virus. Epidemiol. Infect. 109:547-558. [DOI] [PMC free article] [PubMed] [Google Scholar]