Abstract
To investigate the prevalence of spotted fever group rickettsioses in Korea, a serosurvey of Japanese spotted fever rickettsiosis in patients with acute febrile illness was conducted with an indirect immunofluorescence assay. Overall, 19.88% of the patients were found to have polyvalent antibody against Rickettsia japonica. This study is the first documentation of spotted fever group rickettsiosis in Korea.
Japanese spotted fever, a member of the spotted fever group rickettsioses, is an emerging tick-transmitted infectious disease (1). Japanese spotted fever is characterized by fever, headache, shaking chills, skin eruptions, tick bite eschars, erythemas, and malaise (9). The causative organism has been isolated from the blood of a patient with febrile exanthematous illness (14). It was characterized as a new spotted fever group rickettsia and named Rickettsia japonica (13). The agent is known to be associated with Dermacentor taiwanensis, Haemaphysalis flava, H. longicornis, H. formosensis, Ixodes ovatus, and I. persulcatus, which have been reported as being positive for R. japonica in areas where Japanese spotted fever is endemic (4, 6, 9, 15).
Japanese spotted fever was first described in 1984 in Japan (10). Now the disease is known to be endemic in the warm climate on the coast of southwestern and central Japan, where 144 cases were described between 1984 and 1995 (9). In most other parts of the world, including Korea, the epidemiology of Japanese spotted fever rickettsioses has not been well studied. In southern China and Taiwan, serologic surveys of patients have confirmed the prevalence of spotted fever group rickettsioses; however, there are few reports of Japanese spotted fever (3, 11, 12). Until now, no rickettsioses of the spotted fever group, including Japanese spotted fever, are considered to exist in Korea.
In this study, a serosurvey of Japanese spotted fever rickettsioses in patients with acute febrile illness was conducted in Korea through an indirect immunofluorescence assay (IFA). The findings were compared with those of scrub typhus, a disease caused by Orientia tsutsugamushi. Scrub typhus has been known as an important acute febrile illness and is considered the major rickettsiosis in Korea.
A total of 3,401 serum samples were included in the study. The serum samples were obtained from patients in Korea with acute febrile illness from December 1992 to November 1993. One serum sample was collected per patient. The age and sex of the patient, province of sample collection, and collection date were recorded. The sera were submitted to the Institute of Endemic Disease at Seoul National University Medical Research Center for laboratory diagnosis of scrub typhus, leptospirosis, and hemorrhagic fever with renal syndrome caused by hantavirus. Serologic tests were performed by IFA and a microagglutination test. Four individuals were seropositive for Leptospira species (0.12%), and 79 (2.32%) were seropositive for hantaan virus. The others were seronegative for these infectious agents.
The sera were tested retrospectively for immunoglobulin (Ig) G and IgM antibodies to R. japonica by IFA as described previously (7). Teflon-coated slides with L929 cells heavily infected with the YH strain of R. japonica (ATCC VR-1363) were acetone fixed before use. Serum samples diluted in phosphate-buffered saline with 3% nonfat powdered milk at the cutoff point of 1:40 were added to the antigen-coated spot of the slide and incubated for 60 min in a moist chamber at room temperature. The fluorescein isothiocyanate-conjugated goat anti-human IgG (heavy plus light chain) (109-095-003; Jackson ImmunoResearch Labs) antibody diluted 1:100 in phosphate-buffered saline was used as a secondary antibody. The stained slides were examined with a fluorescent microscope (BX51; Olympus). Positive sera were subsequently assayed at twofold dilutions with monovalent fluorescein isothiocyanate-conjugated goat anti-human IgG (Fcγ fragment specific) (109-095-008; Jackson ImmunoResearch Labs) and IgM (Fc5μ fragment specific) (109-095-043; Jackson ImmunoResearch Labs) antibodies as secondary antibodies.
To validate the IFA system, we used five human sera from patients with Rocky Mountain spotted fever (obtained from David Walker at the University of Texas Medical Branch, Galveston, Tex.) and hyperimmune sera immunized with O. tsutsugamushi or R. japonica. The test showed that all of the patient sera were seropositive for R. japonica antigen, but normal human sera and hyperimmune sera from mice immunized with O. tsutsugamushi were seronegative. The five human sera from patients with Rocky Mountain spotted fever and hyperimmune sera from mice immunized with R. japonica were seronegative for O. tsutsugamushi.
SAS Windows version 8.2 (SAS Institute Inc.) was used to analyze data. Chi-square or Fisher's exact test was used to determine the significance of differences in proportions between groups. A P value of less than 0.05 was considered to indicate statistical significance.
Table 1 lists the prevalence of IF antibodies to R. japonica antigen in 3,401 serum samples. Of the 3,401 sera tested, 676 (19.88%) were found to have polyvalent antibody at a 1:40 serum dilution against the antigen. Of these, 377 sera were found to have IgM antibody (11.08%), and 167 sera had IgG antibody (4.91%). IgM titers to R. japonica varied from 1:40 to 1:2,560, with 109 sera at 1:40, 133 at 1:80, 50 at 1:160, 38 at 1:320, 33 at 1:640, 10 at 1:1,280, and 4 at 1:2,560. IgG titers varied from 1:40 to 1:5,120, with 82 sera at 1:40, 55 at 1:80, 11 at 1:160, 9 at 1:320, 3 at 1:640, 6 at 1:1,280, and 1 at 1:5,120. Of the 3,401 sera tested, 1,196 (35.17%) sera were found to have polyvalent antibody (titer, ≥1:40) against O. tsutsugamushi (strain Boryong).
TABLE 1.
Results of IFA of 3,401 serum samples from patients with acute febrile illness
| Antibody titer by IFA | No. (%) of sera with antibody to R. japonicaa
|
||
|---|---|---|---|
| Polyvalent antibody | IgG antibody | IgM antibody | |
| 1:40 | 676 (19.88) | 82 (2.41) | 109 (3.20) |
| 1:80 | ND | 55 (1.62) | 133 (3.91) |
| 1:160 | ND | 11 (0.32) | 50 (1.47) |
| 1:320 | ND | 9 (0.26) | 38 (1.12) |
| 1:640 | ND | 3 (0.09) | 33 (0.97) |
| 1:1,280 | ND | 6 (0.18) | 10 (0.29) |
| 1:2,560 | ND | 0 | 4 (0.12) |
| 1:5,120 | ND | 1 (0.03) | 0 |
| Total | 676 (19.88) | 167 (4.91) | 377 (11.08) |
ND, not done.
Some differences in the distribution of the seroprevalence of Japanese spotted fever and scrub typhus by age and sex were noted (Table 2). Of the 3,401 serum samples, 58.89% (n = 2,003) were from men and 40.02% (n = 1,361) were from women. For 1.09% (n = 37) of the samples, there was no information on the sex of the patient. The difference in results between the sexes was significant for Japanese spotted fever (positive samples: female, 21.01%; male, 18.47%) (P < 0.001). For scrub typhus, seroprevalence was significantly higher among females (43.94%) than males (28.81%) (P < 0.001). For Japanese spotted fever, seroprevalence among subjects 1 to 10 years of age was 21.95%, which decreased among subjects 11 to 20 years of age. It reached the lowest level (15.84%) among subjects 31 to 40 years of age and increased again among subjects 41 to 50 years of age. For scrub typhus, seroprevalence among subjects 1 to 10 years of age was 29.97%. It decreased among subjects 11 to 20 years of age, reached 18.93% among subjects 21 to 30 years of age, and increased again among subjects 31 to 40 years of age.
TABLE 2.
Seropositivity of IgG and IgM antibodies against R. japonica and O. tsutsugamushi between 1992 and 1993 in Korea
| Sample category | No. of sera tested (% of total) | No. (%) showing polyvalent seropositivity against:
|
|
|---|---|---|---|
| R. japonica | O. tsutsugamushi | ||
| Total | 3,401 (100) | 676 (19.88) | 1,196 (35.17) |
| Sex | |||
| Male | 2,003 (40.02) | 370 (18.47) | 577 (28.81) |
| Female | 1,361 (58.89) | 286 (21.01) | 598 (43.94) |
| Unknown | 37 (1.09) | 20 (54.05) | 21 (56.76) |
| Age (yr) | |||
| ≤10 | 41 (1.21) | 9 (21.95) | 12 (29.27) |
| 11-20 | 180 (5.29) | 32 (17.78) | 52 (28.89) |
| 21-30 | 412 (12.11) | 71 (17.23) | 78 (18.93) |
| 31-40 | 562 (16.52) | 89 (15.84) | 183 (32.56) |
| 41-50 | 918 (26.99) | 186 (20.26) | 324 (35.29) |
| 51-60 | 588 (17.29) | 118 (20.07) | 246 (41.84) |
| ≥61 | 794 (22.35) | 137 (17.25) | 261 (67.10) |
| Unknown | 106 (3.12) | 34 (32.08) | 40 (37.74) |
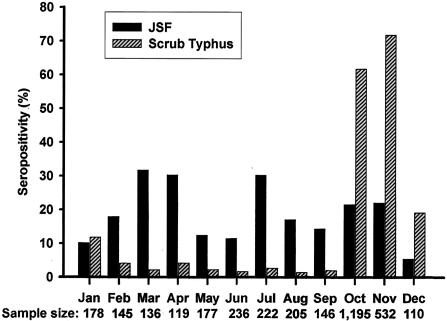
Japanese spotted fever infection occurred from spring to winter. The monthly incidence reached a peak between March (31.62%) and April (30.25%). It reached a second peak in July (30.18%). Major scrub typhus infection occurred from autumn to winter. The monthly incidence of the infection reached a peak between October (61.76%) and November (71.80%). Figure 1 summarizes the seasonal seroprevalence of Japanese spotted fever and scrub typhus rickettsioses.
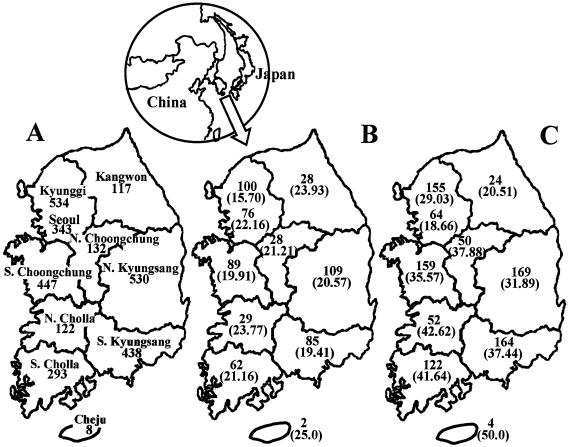
FIG. 1.
Map of South Korea showing the geographic distribution of Japanese spotted fever and scrub typhus rickettsioses from 1992 to 1993. (A) Number of serum samples obtained by province. (B) Seroprevalence of Japanese spotted fever disease as number (percent) of patients with acute febrile illness. (C) Seroprevalence of scrub typhus disease as number (percent) of patients with acute febrile illness.
The map of the incidence rate of Japanese spotted fever shows that the geographic distribution of patients was homogenous; fewer samples were taken from patients in Cheju province, which is an island (Fig. 2). For scrub typhus, the southern part of South Korea had a higher incidence rate than the northern part.
FIG. 2.
Seasonal seroprevalence of Japanese spotted fever (JSF) and scrub typhus rickettsioses in patients with acute febrile illness from 1992 to 1993 in Korea.
This report gives the first details of the seroepidemiology of spotted fever group rickettsioses in Korea. The results indicated that Japanese spotted fever occurred throughout the country. The presence of R. japonica and R. rickettsii nucleic acid in Haemaphysalis longicornis was recently reported for the first time (8). The detection of pathogenic bacteria in vector arthropods suggested the possibility of the presence of Japanese spotted fever and/or spotted fever group rickettsioses due to ticks in Korea. These findings inspired further investigation of Japanese spotted fever in patients with acute febrile illness.
Because the serum samples tested in this study were obtained from patients experiencing the early stage of febrile illness, a screening IFA cutoff value of ≥1:40 was selected. The IFA results demonstrated that 676 of the 3,401 sera tested contained polyvalent antibodies against R. japonica. Of the positive sera, 377 contained IgM antibody and 167 had IgG antibody. These findings indicate that most of the specimens were obtained from patients with acute disease or in early convalescence and that Japanese spotted fever was prevalent in Korea from 1992 to 1993.
One report described that 11 of 28 sera seropositive for spotted fever group rickettsiae were also positive for scrub typhus group rickettsiae and 4 were positive for scrub typhus group and typhus group rickettsiae (5). In this study, 256 of 676 R. japonica polyvalent antibody-positive sera were also seropositive for O. tsutsugamushi. This suggested that one acute infection was occurring in a patient with circulating antibodies from a previous infection with the other agent. In Korea, it has been reported that 34.3% of febrile hospitalized patients were positive for O. tsutsugamushi infection (2). Since the clinical symptoms (high fever, skin eruption, and tick bite eschar) and signs of Japanese spotted fever are similar to those of scrub typhus, Japanese spotted fever may be misdiagnosed as scrub typhus.
The first serologic evidence that the agents of spotted fever group rickettsioses are present in Korea has been determined. Spotted fever group rickettsioses, especially Japanese spotted fever, should therefore be included in the differential diagnoses for persons in Korea who have been exposed to ticks and have febrile episodes.
Acknowledgments
This study was supported by a grant from the Korea Health 21 R&D Project, Ministry of Health & Welfare, Republic of Korea (01-PJ10-PG6-01GM01-0004).
REFERENCES
- 1.Brouqui, P., and D. Raoult. 1996. Clinical aspects of human SFG rickettsiae infections in the era of molecular biology: an update, p. 195-210. In J. Kazar and R. Toman (ed.), Proceedings of the 5th International Symposium on Rickettsial Diseases. Slovak Academy of Sciences, Bratislava, Slovakia.
- 2.Choi, M. S., S. K. Park, W. J. Chang, M. S. Huh, H. R. Kim, T. H. Han, I. S. Kim, and W. H. Chang. 1994. A seroepidemiological survey on the scrub typhus in Korea, 1994. J. Korean Soc. Microbiol. 30:593-602. [Google Scholar]
- 3.Feng, H. M., T. S. Chen, B. H. Lin, P. E. Wang, Q. H. Su, H. B. Xia, K. Kumano, and T. Uchida. 1991. Serologic survey of spotted fever group rickettsiosis on Hainan Island of China. Microbiol. Immunol. 35:687-694. [DOI] [PubMed] [Google Scholar]
- 4.Fournier, P. E., H. Fujita, N. Takada, and D. Raoult. 2002. Genetic identification of rickettsiae isolated from ticks in Japan. J. Clin. Microbiol. 40:2176-2181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Graves, S., L. Wang, Z. Nack, and S. Jones. 1999. Rickettsia serosurvey in Kimberley, Western Australia. Am. J. Trop. Med. Hyg. 60:786-789. [DOI] [PubMed] [Google Scholar]
- 6.Ishikura, M., H. Fujita, S. Ando, K. Matsuura, and M. Watanabe. 2002. Phylogenetic analysis of spotted fever group rickettsiae isolated from ticks in Japan. Microbiol. Immunol. 46:241-247. [DOI] [PubMed] [Google Scholar]
- 7.Jang, W. J., M. S. Huh, K. H. Park, M. S. Choi, and I. S. Kim. 2003. Evaluation of an immunoglobulin M capture enzyme-linked immunosorbent assay for diagnosis of Orientia tsutsugamushi infection. Clin. Diagn. Lab. Immunol. 10:394-398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee, J. H., H. S. Park, K. D. Jung, W. J. Jang, S. E. Koh, S. S. Kang, I. Y. Lee, W. J. Lee, B. J. Kim, Y. H. Kook, K. H. Park, and S. H. Lee. 2003. Identification of the spotted fever group rickettsiae detected from Haemaphysalis longicornis in Korea. Microbiol. Immunol. 47:301-304. [DOI] [PubMed] [Google Scholar]
- 9.Mahara, F. 1997. Japanese spotted fever: report of 31 cases and review of the literature. Emerg. Infect. Dis. 3:105-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mahara, F., K. Koga, S. Sawada, T. Taniguchi, F. Shigemi, T. Suto, Y. Tsuboi, A. Ooya, H. Koyama, T. Uchiyama, and T. Uchida. 1985. The first report of the rickettsial infections of spotted fever group in Japan: three clinical cases. J. Jpn. Assoc. Infect. Dis. 59:1165-1172. [DOI] [PubMed] [Google Scholar]
- 11.Okabayashi, T., K. Tsuchiya, Y. Muramatsu, H. Ueno, and C. Morita. 1996. Serologic survey of spotted fever group rickettsia in wild rats in Thailand in the 1970s. Microbiol. Immunol. 40:895-898. [DOI] [PubMed] [Google Scholar]
- 12.Takada, N., H. Fujita, Y. Yano, W. H. Huang, and C. Khamboonruang. 1993. Serosurveys of spotted fever and murine typhus in local residents of Taiwan and Thailand compared with Japan. Southeast Asian J. Trop. Med. Public Health 24:354-356. [PubMed] [Google Scholar]
- 13.Uchida, T., T. Uchiyama, K. Kumano, and D. H. Walker. 1992. Rickettsia japonica sp. nov., the etiological agent of spotted fever group rickettsiosis in Japan. Int. J. Syst. Bacteriol. 42:303-305. [DOI] [PubMed] [Google Scholar]
- 14.Uchida, T., F. Tashiro, T. Funato, and Y. Kitamura. 1986. Isolation of a spotted fever group rickettsia from a patient with febrile exanthematous illness in Shikoku, Japan. Microbiol. Immunol. 30:1323-1326. [DOI] [PubMed] [Google Scholar]
- 15.Uchida, T., Y. Yan, and S. Kitaoka. 1995. Detection of Rickettsia japonica in Haemaphyslis lingicornis ticks by restriction fragment length polymorphism of PCR product. J. Clin. Microbiol. 33:824-828. [DOI] [PMC free article] [PubMed] [Google Scholar]