Abstract
In a meta-analysis of 10 studies, the BACTEC 960/MGIT and BACTEC 460 systems showed a sensitivity and specificity in detecting mycobacteria (1,381 strains from 14,745 clinical specimens) of 81.5 and 99.6% and 85.8 and 99.9%, respectively. Combined with solid media, the sensitivity of the two systems increased to 87.7 and 89.7%, respectively.
Currently, a combination of conventional solid media with a broth-based method is the accepted reference standard for the diagnosis of mycobacterial infection (5, 26). Among the methods utilizing liquid media, the half-automated radiometric BACTEC 460 TB system (BACTEC 460) is widely accepted as the reference standard. This system, however, is also known for some well-established limitations, which include problems with the use of radioactive material, cumbersome manual loading and unloading, potential hazard of needle stick injury, risk of cross contamination, and lack of computerized data management.
Recently, the BACTEC Mycobacteria Growth Indicator Tube System (BACTEC 960/MGIT), a newly developed nonradiometric, fully automated, continuously monitoring system, was introduced as an alternative to the radiometric BACTEC 460 for growth and detection of mycobacteria. The results of several comparative studies show that BACTEC 960/MGIT is a suitable tool for the detection of Mycobacterium tuberculosis and other mycobacterial species, though rather wide variations in diagnostic performance have been reported (1-3, 6, 10-14, 16-18, 21, 23-25, 27, 28, 30-35). To provide an evaluation of the quality of the available reports and an overall summary of the diagnostic accuracy of BACTEC 960/MGIT versus that of BACTEC 460, we performed a systematic review and meta-analysis.
Literature search.
The literature was searched for the period from January 1990 to June 2003 with the Medline, Embase, and Cochrane libraries. The studies were included for analysis if they compared the results of BACTEC 960/MGIT and BACTEC 460TB with and without solid media, they reported data on false-positive (sample flagged as positive without any microscopic or cultural evidence of acid-fast bacilli by subculture at the end of the incubation period), false-negative (negative test samples found positive on subculture), true-positive, and true-negative results separately; the results of the tests were compared against the reference standard, which was defined as a positive culture with at least one of the three systems used, and the comparison between tests was performed prospectively with the same series of patients from a relevant clinical population, defined as a group of individuals requiring routine microbiologic investigations for suspected pulmonary or extrapulmonary mycobacterial infections.
Statistical analysis.
To evaluate if variations between primary studies in test threshold had an influence on the accuracy of diagnostic procedures, we used receiver operator characteristic (ROC) curves (9, 15, 20, 22).
The common value of noncomparative binary outcomes, as sensitivity and specificity, were obtained by transforming them into the corresponding logit. The related standard errors were calculated, and standard meta-analytical procedures were implemented (29). Heterogeneity and publication bias were assessed as previously described (8).
Studies included in the analysis.
Of the 498 articles identified in the literature search, 24 were reviewed in detail, and data from 14 of these articles (2, 3, 6, 10, 13, 14, 16, 21, 25, 30, 32-35) were excluded because they used a standard different from the accepted reference or they did not allow the construction of the 2 × 2 table of results. Table 1 summarizes the principal characteristics of the 10 studies included in the meta-analysis (1, 11, 12, 17, 18, 23, 24, 27, 28, 31).
TABLE 1.
Main characteristics of studies included in the meta-analysis
| Reference | Location(s) and yr | Total no. of specimens/no. positive (% positive) | No. of patients (mean no. of specimens per patient) | Sampling method | Mycobacterial species identifieda (no. of samples) |
|---|---|---|---|---|---|
| Hanna et al. (11) | USA, Germany, 1999 | 3,330/362 (10.8) | 2,346 (1.4) | Random | MBT (132), MOTT (230), MAC (172) |
| Tortoli et al. (31) | Italy, 1999 | 2,567/236 (9.1) | 1,631 (1.5) | Concecutive patients | MBT (169), MOTT (67), MAC (22) |
| Rohner et al. (24) | Switzerland, 2000 | 1,024/99 (9.6) | 503 (2.0) | Concecutive patients | MBT (83), MOTT (16), MAC (3) |
| Pinheiro and Ribeiro (23) | Portugal, 2000 | 330/33 (10.0) | 198 (1.6) | Random | MBT (31), MOTT (2), MAC (2) |
| Kanchana et al. (17) | Canada, 2000 | 1,742/104 (10.1) | Not specified | Not specified | MBT (59), MOTT (45), MAC (19) |
| Alcaide et al. (1) | Spain, 2000 | 1,068/120 (11.2) | Not specified | Consecutive patients | MBT (96), MOTT (24), MAC (2) |
| Somoskovi et al. (28) | Hungary, 2000 | 377/57 (15.1) | 243 (1.5) | Consecutive patients | MBT (55), MOTT (2), MAC (1) |
| Leitritz et al. (18) | Germany, 2001 | 2,624/127 (4.8) | 1,188 (2.2) | Consecutive patients | MBT (57), MOTT (70), MAC (38) |
| Huang et al. (12) | Taiwan, 2001 | 590/121 (20.5) | 297 (1.9) | Consecutive patients | MBT (81), MOTT (40) |
| Scarparo et al. (27) | Italy, 2002 | 1,093/122 (11.1) | 611 (1.7) | Consecutive patients | MBT (47), MOTT (75), MAC (6) |
MBT, Mycobacterium tuberculosis; MOTT, nontuberculous mycobacteria; MAC, Mycobacterium avium complex.
Altogether, 1, 381 of 14,745 routine clinical specimens (primarily from the respiratory tract) grew mycobacteria in at least one of the three culture media. The overall isolation rate of acid-fast bacilli was 9.3%, ranging from 4.8% in a study from Germany to 20.5% in a study from Taiwan. The mycobacterial isolates ultimately recovered from these specimens included 810 strains of M. tuberculosis (58.6%) and 571 strains of mycobacteria other than those that cause tuberculosis (nontuberculous mycobacteria).
Comparison of systems.
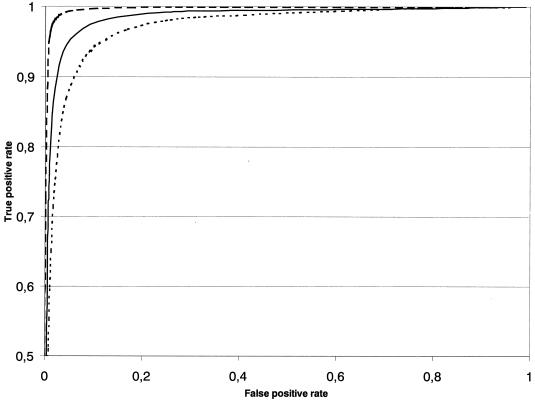
For all three diagnostic methods, the appearance of the summary ROC curve was symmetric, supporting the lack of relationship between the true- and false-positive rates (Fig. 1).
FIG. 1.
Graphs of summary ROC curves showing true-positive rate (sensitivity) versus false-positive rate (1 − specificity) for three mycobacterial culture systems: BACTEC 460 (broken line), BACTEC MGIT 960 (solid line), and solid media (dotted line).
The sensitivity of BACTEC 460 in detecting all mycobacteria, M. tuberculosis, all species of nontuberculous mycobacteria, and nontuberculous mycobacteria other than the M. avium complex was higher than that of BACTEC 960/MGIT, but in all cases these differences were not statistically significant (Table 2). By contrast, BACTEC 960/MGIT showed a higher, though not statistically significant, sensitivity in detecting M. avium complex than BACTEC 460. Compared to the individual systems, the mean overall sensitivity of BACTEC 960/MGIT and BACTEC 460 combined with solid media was increased by 6 and 4%, respectively.
TABLE 2.
Sensitivity of culture systems according to mycobacterial speciesa
| Mycobacteria (no. of isolates) | Sensitivity (95% confidence interval) |
||||
|---|---|---|---|---|---|
| BACTEC MGIT 960 | BACTEC 460 | Solid mediaa | BACTEC MGIT 960 + solid media | BACTEC 460 + solid media | |
| All species (1,381) | 0.81 (0.76-0.86) | 0.85 (0.81-0.89) | 0.67 (0.63-0.72) | 0.87 (0.82-0.91) | 0.89 (0.85-0.93) |
| M. tuberculosis (810) | 0.88 (0.84-0.91) | 0.90 (0.86-0.93) | 0.76 (0.73-0.79) | 0.92 (0.87-0.95) | 0.93 (0.89-0.96) |
| All nontuberculous mycobacteria (571) | 0.66 (0.55-0.76) | 0.75 (0.68-0.81) | 0.51 (0.40-0.60) | 0.76 (0.62-0.86) | 0.88 (0.80-0.93) |
| M. avium complex (265) | 0.80 (0.75-0.85) | 0.73 (0.67-0.78) | 0.57 (0.42-0.70) | 0.86 (0.75-0.93) | 0.83 (0.67-0.92) |
| Other nontuberculous mycobacteriab (306) | 0.53 (0.42-0.64) | 0.64 (0.57-0.75) | 0.47 (0.34-0.61) | 0.70 (0.55-0.81) | 0.88 (0.80-0.93) |
Lowenstein-Jensen in nine studies.
Other nontuberculous mycobacteria more commonly isolated were M. xenopi (n = 53), M. gordonae (n = 40), M. fortuitum (n = 20), M. kansasii (n = 18), and M. chelonae (n = 14).
Six studies reported data on sensitivity according to smear results. Both BACTEC 460 and BACTEC 960/MGIT had a higher sensitivity for smear-positive specimens (0.92 and 0.89, respectively) than for the smear-negative specimens (0.80 and 0.73, respectively). The fixed-effects model was used for analysis of BACTEC 460 and solid medium data, as the homogeneity hypothesis was tenable (P = 0.91 and 0.93, respectively). The random-effects model was more appropriate for the data on the BACTEC 960/MGIT, as the homogeneity hypothesis was rejected (P < 0.001).
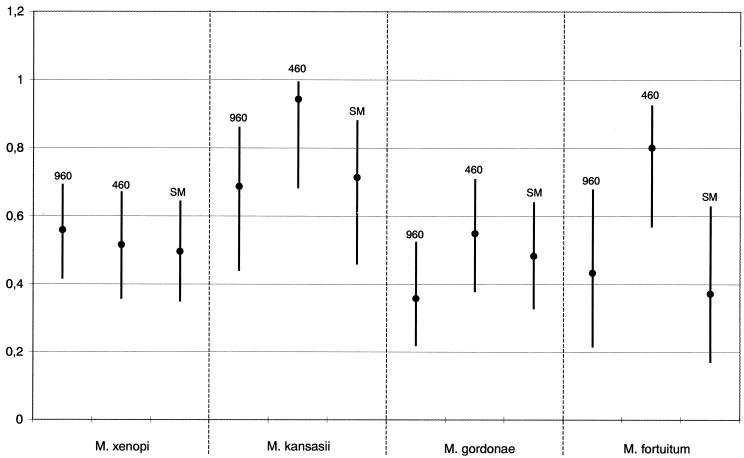
Data for other less commonly encountered nontuberculous mycobacteria showed variable results in term of recovery rates (Fig. 2). With M. xenopi, the BACTEC 960/MGIT system was comparable to the BACTEC 460; the latter system, however, exhibited a slightly better performance than the BACTEC 960/MGIT with M. kansasii, M. fortuitum, and M. gordonae.
FIG. 2.
Sensitivity of the BACTEC 960/MGIT (960), BACTEC 460 (460), and solid media (SM) for nontuberculous mycobacteria other than M. avium complex. Circles show weighted rates, and vertical bars show 95% confidence intervals.
The specificity of the BACTEC 960/MGIT was high (99.6%), though significantly lower than the 99.9% sensitivity of the BACTEC 460 (P = 0.01) and solid media (P = 0.03).
Rates of breakthrough contamination (presumptively positive medium subsequently found positive for bacteria other than acid-fast bacilli) were 12.8% (95% confidence interval, 12.3 to 13.4) with solid media, 8.6% (95% confidence interval, 8.2 to 9.9) with the BACTEC 960/MGIT and 4.4% (95% confidence interval, 4.1 to 4.7) with the BACTEC 460. These differences were statistically significant (P < 0.001) in each possible comparison.
The time to detection was significantly shorter for the BACTEC 960/MGIT than for BACTEC 460 and solid media for overall mycobacteria as well as for each of the species evaluated (Table 3).
TABLE 3.
Time to detection by mycobacterial speciesa
| Species (no. of studies reporting these data) | Time to detection (days) |
||
|---|---|---|---|
| BACTEC MGIT 960 | BACTEC 460 | Lowenstein-Jensen medium | |
| All mycobacteria (10) | 12.9 | 15.0 | 27.0 |
| M. tuberculosis (10) | 13.2 | 15.2 | 25.8 |
| Smear-positive specimens (6) | 11.7 | 11.6 | 22.3 |
| Smear-negative specimens (6) | 16.5 | 18.0 | 33.7 |
| All nontuberculous mycobacteria (6) | 16.3 | 20.2 | 24.3 |
| M. avium complex (5) | 7.6 | 10.7 | 23.3 |
Time to detection for solid media was available for five studies for overall mycobacteria and M. tuberculosis (three reported rates according to smear results), for three studies for nontuberculous mycobacteria, and for one study only for M. avium complex.
The present analysis was aimed to provide an overall summary of diagnostic accuracy from the multitude of sometimes conflicting results from reports on the comparative diagnostic yield of the BACTEC 960/MGIT and BACTEC 460 systems. Using meta-analysis, we provide data from the available literature, which include a larger number of specimens than can be encompassed by individual studies. However, as the individual reports collected their data independently, heterogeneity and biases may be introduced in assembling patient groups and associating the data.
While in the present analysis, publication bias assessments gave inconsistent results with the different methods used, there was evidence of some degree of heterogeneity. Neither the study design, as supported by subgroup analyses (data not shown), nor the interpretation of the test results or changes in the diagnostic threshold can explain the statistical heterogeneity observed with the BACTEC 960/MGIT. We hypothesize that the heterogeneity may be to some extent attributed to the variable contamination rate observed in individual studies (from 3.8 to 16.6%). Another source of heterogeneity may be attributed to the variety of samples examined. Since blood interferes with the detection of fluorescence, the BACTEC 960/MGIT system is not suitable for the evaluation of blood and bone marrow specimens (4, 7). Though most of the specimens evaluated in the meta-analysis were from the respiratory tract, different rates of specimens from other sources or body fluids, which may have been contaminated by blood, were included in individual studies. For instance, one of the studies explicitly included a certain number of bone marrow specimens.
In conclusion, the present data provide additional evidence of the value of the BACTEC 960/MGIT system for recovery of mycobacteria from clinical specimens. The radiometric BACTEC 460 system, in combination with solid media, remains the gold standard for diagnosis of acid-fast bacilli. On the other hand, the BACTEC 960/MGIT shows new and interesting features, such as a shorter time to detection of acid-fast bacilli and more convenient technology. These favorable features, coupled with an elevated diagnostic accuracy on almost all the clinically most important mycobacterial species, make the BACTEC 960/MGIT system in combination with conventional solid media a valuable alternative to the radiometric system.
Acknowledgments
We thank all the authors who replied to our letters and supplied data (F. Alcaide, M. Cormican, A. Somoskovi, T. S. Huang, M. D. Pinheiro, and L. Leitritz). We also thank AnnaLisa Rossi for helpful suggestions in preparing the manuscript.
REFERENCES
- 1.Alcaide, F., M. A. Benitez, J. M. Escriba, and R. Martin. 2000. Evaluation of the BACTEC MGIT 960 and the MB/BacT systems for recovery of mycobacteria from clinical specimens and for species identification by DNA Accur Probe. J. Clin. Microbiol. 38:398-401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Augustinowic-Kopec, E., A. Jaworsky, A., and Z. Zwolska. 2002. Evaluation of the Bactec MGIT 960 fluorescent method in diagnosis of tuberculosis. Pneumonol. Alergol. Pol. 70:450-457. [PubMed] [Google Scholar]
- 3.Banaiee, N., M. Bobadilla-Del-Valle, S. Bardarov, Jr., P. F. Riska, P. M. Small, A. Ponce-De-Leon, W. R. Jacobs, Jr., G. F. Hatfull, and J. Sifuentes-Osornio. 2001. Luciferase report mycobacteriophages for detection, identification, and antibiotic susceptibility testing of Mycobacterium tuberculosis in Mexico. J. Clin. Microbiol. 39:3883-3888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Becton Dickinson and Company. 1994. BBL MGIT products for the detection of mycobacteria. Becton Dickinson Microbiology Systems, Cockeysville, Md.
- 5.Centers for Diseases Control. 1995. Essential components of a tuberculosis prevention and control program. Recommendations of the Advisory Council for the Elimination of Tuberculosis. Morb. Mortal. Wkly. Rep. 44:1-16. [PubMed] [Google Scholar]
- 6.Chien, H. P., M. C. Yu, M. H. Wu, T. P. Lin, and K. T. Luh. 2000. Comparison of the BACTEC MGIT 960 with Lowenstein-Jensen medium for recovery of mycobacteria from clinical specimens. Int. J. Tuberc. Lung Dis. 9:866-870. [PubMed] [Google Scholar]
- 7.Cornfield, D. B., K. Gleason Beavis, J. A. Greene, M. Bojak, and J. Bondi. 1997. Mycobacterial growth and bacterial contamination in the mycobacteria growth indicator tube and BACTEC 460 culture system. J. Clin. Microbiol. 35:2068-2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cruciani, M., M. Malena, O. Bosco, S. Nardi, G. Serpelloni, and C. Mengoli. 2003. Reappraisal with meta-analysis of the addition of gram-positive prophylaxis to fluoroquinolone in neutropenic patients J. Clin. Oncol. 21:4127-4137. [DOI] [PubMed] [Google Scholar]
- 9.Deeks, J. J. 2001. Systematic reviews of evaluations of diagnostic and screening tests. Br. Med. J. 323:157-162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Flanagan, P. G., R. Williams, and A. Paull. 1999. Comparison of the automated systems for the isolation of mycobacteria from clinical specimens Eur. J. Clin. Microbiol. Infect. Dis. 18:912-914. [DOI] [PubMed] [Google Scholar]
- 11.Hanna, B. A., A. Ebrahimzadeh, L. B. Elliott, M. A. Morgan, S. M. Novak, S. Rusch-Gerdes, M. Acio, D. F. Dunbar, T. M. Holmes, C. H. Rexer, C. Savthyakumar, and A. M. Vannier. 1999. Multicenter evaluation of the BACTEC MGIT 960 system for recovery of mycobacteria J. Clin. Microbiol. 37:748-752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang, T. S., C. S. Chen, S. S. Lee, W. K. Huang, and Y. C. Liu. 2001. Comparison of the BACTEC MGIT 960 and BACTEC 460TB systems for detection of mycobacteria in clinical specimens. Ann. Clin. Lab. Sci. 31:279-283. [PubMed] [Google Scholar]
- 13.Idigoras, P., X. Beristain, A. Iturzaeta, D. Vicente, and E. Perez-Trallero. 2000. Comparison of the automated nonradiometric Bactec MGIT 960 system with Lowenstein-Jensen, Coletsos, and Middlebrook 7H11 solid media for recovery of mycobacteria. Eur. J. Clin. Microbiol. Infect. Dis. 19:350-354. [DOI] [PubMed] [Google Scholar]
- 14.Irtuganova, O. A., N. S. Smirnova, L. V. Slogotskaia, A. M. Moroz, and V. I. Litvinov. 2001. Automated methods of culture determination of M. tuberculosis in liquid media. Probl. Tuberk. 3:53-55. [PubMed] [Google Scholar]
- 15.Irwig, L., A. N. Tosteson, C. Gatsonis, J. Lau, T. C. Chalmers, and F. Mosteller. 1994. Guidelines for meta-analyses evaluating diagnostic test. Ann. Intern. Med. 120:667-676. [DOI] [PubMed] [Google Scholar]
- 16.Jayakumar, K. V., T. Forster, and M. S. Kyi. 2001. Improved detection of Mycobacterium spp. using the Bactec MGIT 960 system. Br. J. Biomed. Sci. 58:154-158. [PubMed] [Google Scholar]
- 17.Kanchana, M. V., D. Cheke, I. Natyshak, B. Condor, A. Warner, and T. Martin. 2000. Evaluation of BACTEC MGIT 960 system for recovery of mycobacteria. Diagn. Microbiol. Infect. Dis. 37:31-36. [DOI] [PubMed] [Google Scholar]
- 18.Leitritz, L. L., S. Schubert, B. Bucherl, A. Masch, J. Heesemann, and A. Roggenkamp. 2001. Evaluation of BACTEC MGIT 960 and BACTEC 460TB systems for recovery of mycobacteria from clinical specimens of a university hospital with low incidence of tuberculosis. J. Clin. Microbiol. 39:3764-3767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lijmer, J. G., B. W. Mol, S. Heisterkamp, G. J. Bonsel, M. H. Prins, and J. H. van der Meulen. 1999. Empirical evidence of design-related bias in studies of diagnostic tests. JAMA 282:1061-1066. [DOI] [PubMed] [Google Scholar]
- 20.Littenberg, B., and L. E. Moses. 1993. Estimating diagnostic accuracy from multiple conflicting reports: a new meta-analytic method. Med. Decision Making 13:313-321. [DOI] [PubMed] [Google Scholar]
- 21.Lu, D., B. Heeren, and W. M. Dunne. 2002. Comparison of automated Mycobacteria Growth Indicator Tube System (BACTEC 960/MGIT) with Lowenstein-Jensen medium for recovery of mycobacteria from clinical specimens. Am. J. Clin. Pathol. 118:542-545. [DOI] [PubMed] [Google Scholar]
- 22.Moses, L. E., D. Shapiro, and B. Littenberg. 1993. Combining independent studies of a diagnostic test into a summary ROC curve: data analytic approaches and some additional considerations. Stat. Med. 12:1293-1316. [DOI] [PubMed] [Google Scholar]
- 23.Pinheiro, M. D., and M. M. Ribeiro. 2000. Comparison of the BACTEC 460TB system and the BACTEC MGIT 960 system in recovery of mycobacteria from clinical specimens. Clin. Microbiol. Infect. 6:171-173. [DOI] [PubMed] [Google Scholar]
- 24.Rohner, P., B. Ninet, A. M. Benri, and R. Auckentaler. 2000. Evaluation of the BACTEC 960 automated nonradiometric system for isolation of mycobacteria from clinical specimens. Eur. J. Clin. Microbiol. Infect. Dis. 19:715-717. [DOI] [PubMed] [Google Scholar]
- 25.Saitoh, H., and N. Hamane. 2000. Comparative evaluation of BACTEC MGIT 960 system with MB/BacT and egg-based media for recovery of mycobacteria. Rinsho Biseibutshu Jinsoku Shindan Kenkyukai Shi 11:19-26. [PubMed] [Google Scholar]
- 26.Salfinger, M., Y. M. Hale, and J. R Driscoll. 1998. Diagnostic tool in tuberculosis. Respiration 65:163-170. [DOI] [PubMed] [Google Scholar]
- 27.Scarparo, C., P. Piccoli, A. Rigon, G. Ruggiero, P. Ricordi, and C. Piersimoni. 2002. Evaluation of BACTEC MGIT 960 in comparison with BACTEC 460TB for detection and recovery of mycobacteria from clinical specimens. Diagn. Microbiol. Infect. Dis. 44:157-161. [DOI] [PubMed] [Google Scholar]
- 28.Somoskovi, A., C. Kodmon, A. Lantos,. Z. Bartfai, L. Tamasi, J. Fuzy, and P. Magyar. 2000. Comparison of recoveries of Mycobacterium tuberculosis using the automated BACTEC MGIT 960 system, the BACTEC 460TB system, and Lowenstein-Jensen medium. J. Clin. Microbiol. 38:2395-2397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sutton, A. J., K. R. Abrams, D. R. Jones, T. A. Sheldon, and F. Song. 2000. Methods for meta-analysis in medical research, p. 17-19. Wiley & Sons Ltd., New York, N.Y.
- 30.Tazawa, Y., S. Ishikawa, Y. Furuhata, Y. Kukuchi, and J. Okada. 1998. Experience and clinical usefulness of BACTEC MGIT 960. Rinsho Biseibutshu Jinsoku Shindan Kenkyukai Shi 9:59-65. [PubMed] [Google Scholar]
- 31.Tortoli, E., P. Cichero, Piersimoni, M. T. Simonetti, G. Gesu, and D. Nista. 1999. Use of BACTEC MGIT 960 for recovery of mycobacteria from clinical specimens: multicenter study. J. Clin. Microbiol. 37:3578-3582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Whyte, T., M. Cormican, B. Hanahoe, G. Doran, T. Collins, and G. Corbett-Feney. 2000. Comparison of BACTEC MGIT 960 and BACTEC 460TB for culture of mycobacteria. Diagn. Microbiol. Infect. Dis. 38:123-126. [DOI] [PubMed] [Google Scholar]
- 33.Williams-Bouyer, N., R. Yorke, H. I. Lee, and G. L. Woods. 2000. Comparison of the BACTEC MGIT 960 and ESP Culture System II for growth and detection of mycobacteria. J. Clin. Microbiol. 38:4167-4170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yan, J. J., A. H. Huang, S. H. Tsai, W. C. Ko, Y. T. Jin, and J. J. Wu. 2000. Comparison of the MB/BacT and BACTEC MGIT 960 system for recovery of mycobacteria from clinical specimens. Diagn. Microbiol. Infect. Dis. 37:25-30. [DOI] [PubMed] [Google Scholar]
- 35.Zaruba, R., and M. Kralova. 2002. Evaluation of the effectiveness of the BACTEC MGIT automatic system for culture of mycobacteria in comparison with classical methods of culture. Experience after one year of use. Epidemiol. Mikrobiol. Imunol. 51:66-70. [PubMed] [Google Scholar]