SUMMARY
No single analgesic drug provides the perfect therapeutic/adverse effect profile for every pain condition. In addition to convenience and possibly improved compliance, a combination of analgesic drugs offers the potential, requiring verification, of providing greater pain relief and/or reduced adverse effects than the constituent drugs when used individually. We review here analgesic combinations containing oxycodone. We found surprisingly little preclinical information about the analgesic or adverse effect profiles of the combinations (with acetaminophen, paracetamol, nonsteroidal anti-inflammatory drugs, morphine, gabapentin or pregabalin). Clinical experience and studies suggest that the combinations are safe and effective and may offer certain advantages. As with all combinations, the profile of adverse effects must also be determined in order to provide the clinician with the overall benefit/risk assessment.
INTRODUCTION
The challenge in treating patients with acute or chronic pain has always been to find safe, effective and adequate analgesia. While numerous pharmacological advances have made more and better drugs available to manage pain, it often goes unrelieved (1–3). Inadequate analgesia can occur for many well-documented reasons: failure to prescribe according to guidelines (4, 5), poor understanding of analgesia on the part of prescribers (6), challenging patient populations such as the elderly (7), lack of availability of proper medications (8), excessive legal restrictions on opioids (9), patient misconceptions about drugs or resistance to them (10, 11), and changes in the patient’s condition or comorbidities requiring drug adjustments that may not be followed. Less frequently discussed is the fact that single agents, even when appropriately prescribed in an adherent patient, are not always sufficient to control pain or do so only at doses that produce excess adverse effects. To address the shortcomings of single-agent use, combination analgesics have long been prescribed for moderate to severe acute and chronic pain, and new drug formulations now offer the practicality of dual analgesic agents in a single tablet that may help address this unmet need.
Although oxycodone has been available as an analgesic agent for nearly a century, it is marketed in the United States, Canada and Australia primarily as part of a combination, such as oxycodone and aspirin or oxycodone and acetaminophen (U.S. name) or paracetamol (international name) (12). Oxycodone has been shown to be a safe and effective pain reliever with side effects comparable to those of other opioids. In 1995, the Food and Drug Administration (FDA) approved a sustainedrelease formulation of oxycodone (OxyContin®, Purdue Pharma); although the sustained-release formulation was designed to deter drug misuse, drug abusers could circumvent the design (13, 14). Oxycodone combination drugs can also be misused (15), and care should be taken on the part of healthcare providers to confirm and monitor appropriate use. Some oxycodone combination formulations are familiar to clinicians, and new combination products are being marketed. Herein, we review four oxycodone combinations for the treatment of chronic pain: oxycodone in combination with morphine, aspirin–nonsteroidal anti-inflammatory drugs (NSAIDs)–acetaminophen, pregabalin and gabapentin.
OXYCODONE AND MORPHINE (DUAL OPIOIDs)
Mechanisms of action
Receptors for the endogenous opioid peptides (namely, the endorphins, enkephalins, dynorphins and endomorphins) are located at multiple levels of nociceptive pathways (mainly in the spinal cord and brain, but also the periphery, particularly during injury) (16). Genes encoding three types of opioid receptors (named μ, δ, and κ) have been identified (17) and there is pharmacological evidence for subtypes of each type (μ1, μ2, δ1, δ2, and κ1 through κ3) (18), possibly arising from alternate splice variants of a common gene. All of the currently identified opioid receptors are members of the 7-transmembrane G protein-coupled receptor family (19).
Morphine (5α,6α-7,8-didehydro-4,5-epoxy-17-methylmorphinan-3,6-diol) is an agonist (determined by an increase in [35S]-GTPγS binding) at all three opioid receptors, having the highest affinity for the μ subtype (20). Agonist-induced activation of μ opioid receptors produces two well-established actions on neurons that express these receptors (21). On postsynaptic neurons, they enhance K+ efflux at inwardly rectifying K+ channels, and thereby hyperpolarize the postsynaptic neuron, rendering it less responsive to stimulation by presynaptic neurotransmitter release; on presynaptic neurons, they attenuate Ca2+ influx (at ligand-gated Ca2+ ion channels), and thereby reduce Ca2+-dependent neurotransmitter release from vesicular stores (22). Opioid-induced depression of neurotransmitter release appears to be quite general, since it has been demonstrated for neurotransmitters of diverse chemical classes, such as the excitatory amino acid glutamate, the catecholamine norepinephrine, the ester acetylcholine, the indoleamine 5-HT (5-hydroxytryptamine, also known as serotonin) and the undecapeptide substance P (23). Morphine is metabolized via UDP-glucuronosyltransferase-catalyzed conjugations to two active glucuronides: at the 6-hydroxyl position to morphine-6-glucuronide (M6G) and at the 3-hydroxyl position to morphine-3-glucuronide (M3G) (24). M6G is more potent (about 4–6-fold) as an analgesic than morphine, but whether M6G levels in the brain accumulate to levels that make a major contribution to morphine’s overall analgesic effect is not definitively known.
Oxycodone (5R,9R,13S,14S-4,5α-epoxy-14-hydroxy-3-methoxy-17-methylmorphinan-6-one) is an opioid receptor agonist (determined by an increase in [35S]-GTPγS binding) (25). A synergistic analgesic interaction with morphine would not be expected (untenable mechanistically), if it only interacts with the same opioid receptors or other analgesic pathways as does morphine. However, both in vitro and in vivo data suggest differences between the drugs (summarized in ref. 26). Oxycodone has relatively weak affinity for μ opioid receptors (Ki 300–400 nM), little or no affinity for δ opioid receptors, and affinity for only a subtype of κ opioid receptors (27–29), although the details of the receptor binding profile have not been unambiguously determined. Consistent with the binding data, intracerebroventricular (i.c.v.) oxycodone but not i.c.v. morphine, blocks antinociception by pretreatment with the κ-selective antagonist norbinaltorphimine (nor-BNI), whereas i.c.v. morphine blocks antinociception by pretreatment with the μ-selective antagonist naloxonazine (28). It appears that oxycodone does not affect the inwardly rectifying K+ channel in the same way as morphine (27). In animal models, the efficacy profile of oxycodone does not overlap that of morphine (30). A major metabolite of oxycodone is noroxycodone and a minor metabolite is oxymorphone, which is a powerful analgesic. Noroxycodone produces potent opioid receptor-mediated antinociception when it is administered spinally (31), but not systemically (due to poor penetration of the blood–brain barrier). An important role for a metabolite has been suggested (32, 33), but again the results are not definitive. In clinical practice, the observation of effective rotation from morphine to oxycodone suggests pharmacodynamic or pharmacokinetic differences.
Opioid efficacy is often discussed in terms of equianalgesia, a theory according to which a specific dose of one opioid at steady state will provide equivalent pain relief of a specific dose of another opioid at steady state. Parenteral morphine 10 mg was used in the initial equianalgesia studies and has since been taken as the “gold standard” in equianalgesia conversion (34). Oral oxycodone appears to be 1.5–2.0 times more potent than oral morphine (35–38). Much information in equianalgesic tables is derived from single-dose studies and expert opinion, and cannot accommodate variations in patients such as pain diagnosis, opioid experience and renal sufficiency, all of which can affect the efficacy (39). Some equianalgesic data are achieved by computational predictions rather than derived in the clinical context. For this reason, the literature often reports a range of equianalgesic dosing, that is often not clinically helpful.
Further confounding the issue of oxycodone and morphine efficacy is the fact that the oral bioavailability of morphine (15–64%) and oxycodone is variable (> 50%) (40). These differences alone could account for as much as a doubling of potency. Thus, it has been suggested that oral morphine should be considered to be about equipotent or half as potent as oral oxycodone. In a preclinical comparative study of six opioid analgesic agents, fentanyl was most potent, followed by burprenorphine, oxycodone, morphine, hydrocodone and codeine (41). In humans, intramuscular (i.m.) morphine is considered to be slightly more potent than i.m. oxycodone, whereas oral oxycodone is considered to be more potent than oral morphine (42). Oxycodone metabolizes in a more predictable way than morphine, and thus its dose titration is easier than with morphine. Collectively, it appears that oxycodone and morphine produce analgesia through mechanisms that do not completely overlap, and therefore their combination has the theoretical potential to produce synergistic action. Demonstration of actual synergy (or additive, or subadditive effect) requires testing of individual combinations.
Evaluation of mechanistic interaction
Basic principles
When drugs produce overtly similar effects, e.g., analgesia, it is common practice to use them in combination. In these situations, the combination effect may turn out to be less than, the same as or greater than the predicted magnitude (subadditive, additive or synergistic, respectively). Discerning which of these results actually apply requires measurements derived from a rigorous comparison of expected and actual effects, a procedure that is rooted in the concept of dose equivalence (doses of each drug that yield the same magnitude of effect when each used alone) (43–48). The analysis leading to metrics to distinguish between additive and nonadditive effects is also needed. No intimate knowledge of the drugs’ mechanism(s) is required; the entire procedure is built from the consequences of dose equivalency. Such a quantitative analysis for combinations involving two or more drugs is most often accomplished with ”isoboles”, a graph of dose combinations that produce an effect of specified magnitude. The initial procedure (49–52) has subsequently been applied to numerous combinations of drugs. The usual isobolographic procedure leading to linear isoboles of additive effects is applicable to drugs with a constant potency ratio (44–48), but has been extended to apply to cases in which the individual drugs have a variable potency ratio (53), as well as to application that is based on receptor occupation of the constituent compounds (54).
Oxycodone and morphine
Isobolographic analysis of oxycodone + morphine combination in rats demonstrated an antinociceptive synergy (55), whereas a study in mice found the interaction to be only additive (56). A clinical experimental study found an oxycodone + morphine combination to be additive (57), but the interpretation of the clinical study has been debated (58).
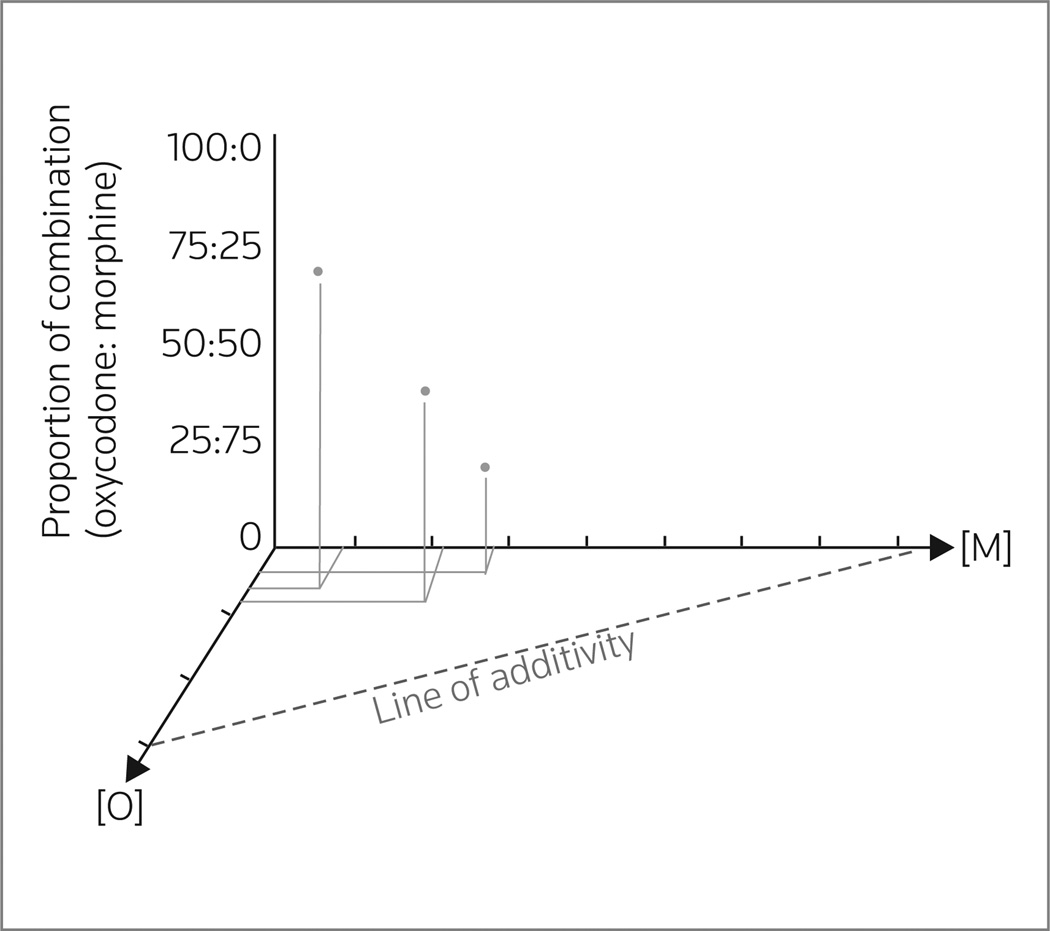
In the first study (55), oxycodone and morphine administered individually and in combination were tested for antinociceptive activity (in the radiant heat tail-flick test) in adult male Sprague-Dawley and Dark Agouti rats. Oxycodone and morphine were administered via i.c.v., intraperitoneal (i.p.) and subcutaneous (s.c.) routes. Dark Agouti rats were used for the systemic administrations because they generate only a small amount of oxycodone active metabolite (O-demethylate) due to a genetic deficiency in CYP2D1. Antinociceptive synergy was reported for the simultaneous administration of “subantinociceptive doses” of oxycodone and morphine by central or systemic administration. In the case of s.c. dosing, the antinociceptive ED50 values ± SD were 2.8 ± 0.2 and 8.5 ± 0.2 mg/kg respectively, for oxycodone and morphine. The rats were then dosed with a “subantinociceptive dosing combination” of ocycodone + morphine in three fixed-dose ratios of 3:1, 1:1 and 1:3 relative to the individual antinociceptive ED50 values. The isobologram generated using the results is shown in Figure 1. Since the experimentally determined ED50 values of each combination were statistically less than (P < 0.05) the theoretical additive ED50, a synergistic interaction was inferred.
Figure 1.
Three-dimensional representation of the isobologram generated for the antinociceptive interaction between oxycodone [O] (mg/kg) and morphine [M] (mg/kg). Antinociception was assessed for each drug alone (ED50 doses = the [O] and [M] axis intercepts of the line of additivity) and for three fixed-ratio combinations of oxycodone + morphine (75%:25%, 50%:50% and 25%:75%). The combination ED50 doses (filled circles) were between the origin and the line of additivity (projected onto the horizontal plane in this representation), which is indicative of a synergistic interaction (data obtained from 46).
In the second study (56), several opioids were tested alone and in fixed-ratio combinations for antinociceptive activity in adult male CD-1 mice (s.c. route, radiant heat tail-flick test). A synergistic antinociceptive interaction (identified when the composite line of additive effect for the combination was significantly different from the dose–response regression line of the experimentally determined combination) was reported (including the isobolograms) for combination of l-methadone with several opioids (morphine, M6G, codeine and 6-acetylmorphine [active metabolite of heroin]), but not others (fentanyl, alfentanyl, meperidine or oxymorphone). In contrast to the first study, the combination of morphine with oxycodone was found to be only additive, not synergistic. The authors did not comment on (or cite) the first study.
In the third study (57), an effort was made to investigate whether the synergy reported in the first study (55) would extend to humans. Healthy female volunteers (N = 47; predominantly from medical and nursing school) were enrolled in a double-blind, crossover trial. Nonstoic and placebo nonresponders were randomly assigned to receive oral morphine (0.5 mg/kg), the same dose of oral oxycodone (0.5 mg/kg), or a 1:1 combination of oral morphine and oral oxycodone each at half the dose given alone (i.e., 0.25 mg/kg). The reason stated in the study for giving equal doses of morphine and oxycodone was that it was commonly done in clinical practice. More recent literature states that the average relative potency ratio for oxycodone to morphine is 3:1 (58). Assessment measures included pain onset, magnitude and tolerance. The results failed to demonstrate a synergistic interaction between morphine and oxycodone on any of the three measures. The authors discuss possible reasons for the different findings from the rat study, in which synergy was found (55), and the authors of the rat study add more in an exchange of letters to the Editor (59). Neither discusses the agreement with lack of synergy reported in the mouse study (56).
Safety and efficacy
The oxycodone/morphine dual-opioid combination studies have been published (Table I). An animal study found that subanalgesic doses of both oxycodone and morphine produce antinociception without producing sedation (55). Grach and colleagues reported that oral oxycodone and morphine combined in a 1:1 ratio produced significantly higher latency to pain onset, i.e., impact on pain threshold, and provided greater analgesia, i.e., impact on pain tolerance, than morphine alone (P = 0.01 and P = 0.007, respectively) (57). While oxycodone alone provided improved analgesia compared to the combination (in terms of pain threshold and tolerance), the results were not significant. This study has been challenged in the literature with regards to dosages used (59). Ladd and colleagues (60) studied whether the combination of oxycodone and morphine might potentiate respiratory depression, and they found that although mean minute ventilation decreased during treatment when compared to pretreatment values, the results were deemed by investigators to be reasonable and not unexpected or disproportionate. Lauretti and colleagues reported that oxycodone and morphine in combination resulted in a 38% reduced use of rescue analgesics in cancer patients compared to morphine alone (61).
Table I.
Safety and efficacy of oxycodone/morphine.
| Study | Patients (n) | Design | Agents | Endpoint(s) | Results | Comments |
|---|---|---|---|---|---|---|
| Grach (57) 2004 | 30 healthy females | Enriched randomized design, double-blind crossover | Group 1, 0.5 mg/kg oral morphine; Group 2, 0.5 mg/kg oral oxycodone; Group 3, 0.25 mg/kg of both oxycodone and morphine, one week apart | Latency to pain onset (threshold), pain intensity (visual analog scale), pain tolerance measured 6 times in 3 h | Combination significantly reduced latency to pain onset and improved pain tolerance vs. morphine alone (but not oxycodone alone). No significant differences in pain magnitude | "Stoic" subjects and "placebo-responders" were excluded. This study was challenged in the literature regarding study design and doses (52) |
| Ladd (60) 2005 | 12 healthy males | Placebo-controlled randomized crossover | 1 h i.v. infusions of saline (placebo); 15 mg morphine; or 15 mg oxycodone; combinations of morphine/oxycodone in ratios of 1:2, 1:1, and 2:1 | Ventilatory effects | No systematic differences on the slopes or intercepts of the hypoxemic and hypercapnia ventilation responses. During treatment, mean minute ventilation at PETCO2 = 55 mmHg (VE55) decreased to 74% of pretreatment values | No disproportionate ventilator effects were observed in any of the combinations of oxycodone to morphine |
| Lauretti (61) 2003 | 22 patients with cancer pain | Open-label randomized titration phase (7 day) followed by double-blind crossover phases (2 periods of 14 days) | Patients received controlled-release morphine or oxycodone with immediate-release morphine in ratio of 1:1.8 (oxycodone: morphine) | Pain, satisfaction, AEs, rescue medication (10 mg immediate-release morphine) | Combination oxycodone and morphine (1:1.8 ratio) resulted in significantly less nausea and vomiting (P < 0.05). Morphine only patients consumed 38% more rescue analgesics (P < 0.05) than combination patients | No significant difference in AEs among patients except for significantly less nausea and vomiting with combination patients vs. morphine alone. No dyspnea reported in any patients |
AEs, adverse events; i.v., intravenous; PETCO2, end-tidal carbon dioxide tension.
OXYCODONE AND ASPIRIN/NSAIDs/ACETAMINOPHEN
Mechanisms of action
NSAIDs constitute a diverse family of analgesics that share a common mechanism of action and include derivatives of acetic acid (e.g., diclofenac, etodolac, indomethacin and sulindac), enolic acid (“oxicams”), fenamic acid (“fenamics”), proprionic acid (the ”profens” naproxen and oxaprozin) and the ”coxibs”. All produce their analgesic effect by reducing the production of pronociceptive and proinflammatory prostaglandins and other chemical mediators by inhibiting the biotransformation from arachidonic acid, a reaction catalyzed by cyclooxygenase (COX prostaglandin endoperoxidase H synthase) isozymes. NSAIDs reversibly inhibit the catalytic activity of cyclooxygenase (62, 63), whereas aspirin acts irreversibly. The two most relevant COX isoforms are COX-1 (prostaglandin G/H synthase 1) and COX-2 (prostaglandin G/H synthase 2). COX-1, generally a constitutive enzyme (its expression is induced throughout the cell life), shares about 60% sequence homology with COX-2, which is an inducible enzyme (transiently present during certain physiological states such as pain, inflammation and cancer) (65). The catalytic site of COX lies at the far end of a long and narrow hydrophobic tunnel defined by four alpha helices formed by residues 106–123, 325–353, 379–384 and 520–535. The NSAIDs block the tunnel in various ways, thereby preventing the migration of arachidonic acid to the active site (66). At least four molecular mechanisms of action have been proposed for NSAIDs: covalent modifications of an amino acid residue within the tunnel by aspirin (67); reversible competition for the substrate binding site within the tunnel by ibuprofen; formation of a salt bridge between carboxylate on the drug and Arg120 that lies within the tunnel, by fluribiprofen and indomethacin; and interaction between the selective COX-2 inhibitors with residues Arg513, His90 and Phe518 located within a side pocket (68). A central component of action of some NSAIDs adds to the possible site(s) of mechanistic interaction with oxycodone (69).
The mechanism of the analgesic action of acetaminophen (N-[4-hydroxyphenyl] acetamide), also known as paracetamol, remains elusive. To date, no single mechanism has been able to sufficiently describe all of its actions. It is reasonable to conclude that paracetamol likely has a pharmacological mechanism that interacts with a variety of physiological pathways, probably within the central nervous system (CNS). The major proposed mechanisms are the subject of a recent comprehensive review by Smith (70). These proposals among others involve: COX-1, COX-2, “COX-3”, peroxidase, nitric oxide synthase (NOS), endocannabinoids and 5-HT (71, 72).
Evaluation of mechanistic interaction
Based on his testing experience, Dr. William Beaver made the early clinical observation that: “There is substantial evidence that combining an optimal dose of acetaminophen or aspirin with an oral opioid such as codeine, hydrocodone, or oxycodone produces an analgesic effect greater than that obtained by doubling the dose of either constituent administered alone” (73, 74). A more systematic examination of combinations of opioids and NSAIDs was subsequently made using an animal model (radiant heat tail-flick test), which the investigators considered to be a model of moderate to severe pain, that correlates with the clinical situations in which combinations are most commonly used (75). Dose–response curves were first obtained for the opioids (fentanyl, hydrocodone, levorphanol, methadone and morphine) when administered alone orally to mice, and were then reobtained when each opioid was administered in combination with a constant oral dose of each NSAID (aspirin, ibuprofen, ketorolac or naproxen). The NSAIDs were inactive in this test when administered alone. Ibuprofen ”potentiated” the analgesic action of oxycodone (defined as a significant reduction in oxycodone’s antinociceptive ED50 value when tested in combination). Ibuprofen did not potentiate the antinociceptive activity of any opioids tested. In addition, not all of the NSAIDs potentiated the antinociceptive activity of oxycodone. As far as we can determine, this is the only rigorous testing for analgesic synergy between oxycodone and NSAIDs.
At the time of writing, no studies were found that tested for a nonadditive analgesic interaction between oxycodone and acetaminophen (paracetamol).
Safety and efficacy
Agents combining low doses of oxycodone with aspirin, acetaminophen and NSAIDs have considerable history and are the subject of many studies in the literature, dating back to 1974 (76). Some of the more recent trials are summarized in Table II. Many of these studies use the dental pain model, which provides short-term results consistent with oxycodone combination drug labeling (77). Potential issues involving opioid dependence or potential misuse must be assessed when using these drugs off label in longer-term analgesia. One study reported stable, modest-dose, long-term oxycodone/acetaminophen treatment of pain, suggesting that there are pain populations which might be managed effectively on oxycodone combination therapy over the long term (78).
Table II.
Safety and efficacy of oxycodone and aspirin/NSAIDs/acetaminophen.
| Study | Patients (n) | Design | Agents | Endpoint(s) | Results | Comments |
|---|---|---|---|---|---|---|
| Corsinovi (79) 2009 | 154 geriatric females with moderate to severe osteoarthritis pain | Randomized, single-blind, controlled study (6 weeks) | Oxycodone + acetaminophen; codeine + acetaminophen; control group took acetaminophen, COX-2-inh, NSAIDs, or Cox-2-inh plus NSAIDs. Average doses at end of study were: Oxycodone 16 mg/acetamino-phen 900 mg, codeine 115 mg/acetaminophen 1916 mg | Pain relief, pain at rest, pain in movement, depressive symptoms, AEs | Combination opioid therapy (oxycodone + acetaminophen and codeine + acetaminophen) significantly reduced depressive symptoms and pain vs. conventional therapy although all 3 groups showed significant improvement in pain endpoints | 78% of oxycodone combo, 63% of codeine combo, and 45% of control group achieved clinically meaningful pain reduction (≥30% reduction in pain). 19.2% of oxycodone combo, 30.8% of codeine combo, and 34.0% of control group discontinued treatment because of AEs. AEs were mild to moderate |
| Daniels (80) 2002 | 205 and 201 patients with moderate to severe dental pain following removal of 2 or more molars | Two studies combined: double-blind, double-dummy, placebo-controlled trials | Single dose of valdecoxib (20 or 40 mg) or oxycodone 10 mg/acetamino-phen 1000 mg or placebo | Pain relief over 24 h, AEs | Pain relief was equivalent for both active agents but valdecoxib had more rapid onset of analgesia at 40 mg and both valdecoxib doses provided longer duration of analgesia than oxycodone combo | AE rates were 36% valdecoxib (20 mg), 27% valdecoxib (40 mg), 70% (oxycodone combo), 53% placebo. When AE data were pooled, valdecoxib AEs were significantly lower than oxycodone combo AEs |
| Desjardins (81) 2007 | 270 patients with moderate to severe dental pain following removal of 2 or more molars | Randomized, double-blind, double-dummy, parallel group, placebo- and active-comparator-controlled study | Single oral dose of rofecoxib 50 mg or oxycodone 10 mg/acetaminophen 650 mg, followed by oxycodone 5 mg/acetaminophen 325 mg every 6 hours as needed (both groups) or placebo. Rescue medication was oxycodone 5 mg/acetaminophen 325 mg | Pain relief, AEs | Both active agents provided pain relief at 24 h, significantly better than placebo (P < 0.001) and rofecoxib was as effective as oxycodone/acetaminophen for analgesia. Significantly fewer rofecoxib patients took rescue medication (20.3% vs. 37.7%, P = 0.003) and rofecoxib patients had significantly fewer AEs than oxycodone combo patients (P < 0.001) or placebo (P = 0.007) | No serious AEs reported. GI AEs occurred in 22.9% (rofecoxib) vs. 46.7% oxycodone combo) vs. 33.3% placebo). Overall AE rates were significantly lower in rofecoxib group vs. other groups |
| Dionne (82) 1999 | 118 patients with moderate to severe dental pain following removal of 2 or more molars | Comparison of various agents, no control | 400 mg ibuprofen; 400 mg ibuprofen + oxycodone (2.5, 5 or 10 mg) | Pain (visual analog scale) 15 min post dosing to 6 h | 2.5 and 5 mg oxycodone + ibuprofen provided no additive analgesia over ibuprofen alone, but 10 mg oxycodone + ibuprofen was more effective at relieving pain | AEs were not significantly increased over 400 mg ibuprofen alone with 2.5 or 5 mg oxycodone, but increased significantly with 10 mg oxycodone: drowsiness (P < 0.001) and vomiting (P < 0.05) |
| Gammaitoni (83) 2003 | 141 patients with moderate to severe dental pain following removal of 2 or more molars | Randomized, controlled trial | Oxycodone 10 mg/acetaminophen 325 mg; controlled-release oxycodone 20 mg; placebo | Pain intensity and pain relief over 6 h | Combo agent provided significantly greater analgesia in 4 out of 5 outcome measures (both agents provided significantly better analgesia than placebo). Combo offered faster onset of action and 24% reduction in patients reporting treatment-emergent AEs | Treatment-emergent AE rates were 44.1% for combo patients, 55.7% for controlled-release oxycodone patients, and 16.7% for placebo. No subjects discontinued because of AEs. No serious AEs. |
| Korn (84) 2004 | 212 patients with moderate to severe dental pain following removal of 2 or more molars | Randomized, double-blind, placebo- and active-comparator-controlled study | Single dose of oral rofecoxib 50 mg; oxycodone 5 mg/acetaminophen 325 mg; placebo | Pain relief at 6 h, onset of analgesia, AEs | Rofecoxib had greater analgesic effect, significant at many points, and fewer AEs than oxycodone/acetaminophen | AE rates were 51.1% rofecoxib, 64.8% oxycodone combo, and 48.4% placebo. There was significantly less nausea (18.9% vs. 39.6%, P < 0.001) and vomiting (6.7% vs. 23.1%, P < 0.001) with rofecoxib vs. oxycodone/acetaminophen |
| Litkowski (85) 2005 | 249 patients with moderate to severe dental pain following removal of 2 or more molars | Randomized, double-blind, placebo-controlled study | Oxycodone 5 mg/ibuprofen 400 mg; oxycodone 5 mg/acetaminophen 325 mg; hydrocodone 7.5 mg/acetaminophen 500 mg; placebo | Total pain relief through 6 h after dosing, pain intensity differences, AEs | Oxycodone 5 mg/ibuprofen 400 mg provided significantly better analgesia (P < 0.001) and was associated with lowest rate of nausea and vomiting of all active agents (6.5%), placebo 3.2% | Oxycodone 5 mg/ibuprofen 400 mg was associated with significantly lower rates of nausea and vomiting than oxycodone 5 mg/acetaminophen 325 mg (P = 0.011) but difference not significant with hydrocodone 7.5 mg/acetaminphen 500 mg |
| Lovell (86) 2004 | 51 patients in an emergency department with musculoskeletal pain (extremities, neck, back) | Randomized, double-blind, controlled study | Oral valdecoxib 40 mg; oral oxycodone 10 mg/acetaminophen 650 mg. Pain monitored at 30 and 60 min with phone follow-up at 24 h | Pain relief, AEs | Both agents provided similar analgesia but valdecoxib patients had fewer AEs and required significantly less rescue medication (44% vs. 74%, P = 0.04) | Significantly fewer valdecoxib patients had sedation or dizziness (15% vs. 44% P = 0.03) but rates of nausea and dyspepsia were similar (12% each, P = 0.96) |
| Palangio (87) 2002 | 147 patients with moderate to severe chronic low back pain | Randomized, double-blind, parallel-group study (8 days), intention-to-treat analysis | Hydrocodone 7.5 mg + ibuprofen 200 mg versus oxycodone 5 mg + acetaminophen 325 mg; patients took one tablet every 4–6 h not to exceed 5 per day. Rescue medication was 200 mg ibuprofen or 325 mg acetaminophen | Daily pain relief, number of tablets, rescue medication, global evaluation, questionnaire (Short-Form Health Survey SF-36) | Both groups had significant pain relief at 8 days vs. baseline but there was no significant difference in any endpoint between groups and no significant difference in AEs | AEs occurred in 62.7% of hydrocodone combo and 62.5% of oxycodone combo patients. Most common AEs were somnolence, dizziness, nausea, headache, constipation, sweating, vomiting, pruritus |
| Palangio (88) 2000 | 180 patients with moderate to severe pain following obstetric or gynecological surgery | Randomized, double-blind, parallel-group, single-dose, active-comparator, placebo-controlled study 8 h) | 2-tablet dose of either hydrocodone 7.5 mg + i buprofen 200 mg; 2-tablet dose of oxycodone 5 mg + acetaminophen 325 mg; placebo. Rescue medication was available but removed subject from study. | Pain relief, pain intensity differences, median time to remedication, AEs | Pain relief endpoints were similar, with both active agents significantly better than placebo and hydrocodone combo significantly better than oxycodone combo at certain time points. AEs were similar for both active agents | Rate of AEs was 18.0% for hydrocodone combo, 11.9% for oxycodone combo, and 10.0% for placebo, not significant. Most common AE was nausea. 14.8% of hydrocodone combo patients, 6.8% of oxycodone combo, and 100% of placebo patients were removed from study because they took rescue medication |
| Singla (89) 2005 | 456 female patients undergoing abdominal or pelvic surgery | Randomized, double-blind, placebo and active-controlled, parallel-group study | Oxycodone 5 mg/ibuprofen 400 mg; ibuprofen alone; oxycodone alone; placebo in 3:3:1:1 ratio, administered 14 to 48 h post-op | Total pain relief, pain intensity, time to first rescue medication, AEs | Oxycodone 5 mg/ibuprofen 400 mg was associated with significantly greater pain relief (P < 0.02 vs. ibuprofen, 0.015 vs. oxycodone, P < 0.001 vs. placebo), significantly longer time to rescue medication (P < 0.05), and fewest treatment-emergent AEs | Oxycodone 5 mg/ibuprofen was associated with fewest AEs 40.8%) even compared to placebo (55.0%) |
| Van Dyke (90) 2004 | 498 patients with moderate to severe dental pain following removal of 2 or more molars | Double-blind, double-dummy, parallel-group placebo-controlled and active-controlled | Oxycodone 5 mg/ibuprofen 400 mg; ibuprofen 400 mg alone; oxycodone 5 mg alone; placebo | Pain relief, and sum of pain intensity differences over 6 h | Combination therapy provided significatly better analgesia (total relief and sum of pain intensity differences) than monotherapy or placebo | No serious AEs; highest rate of AEs occurred in oxycodone 5 mg group (27.0%) with 15.5% for oxyycodone/ibuprofen, 11.3% placebo, and 10.8% ibuprofen alone. Most common AE was nausea in all three active groups |
AEs, adverse events; GI, gastrointestinal; NSAIDs, nonsteroidal anti-inflammatory drugs.
OXYCODONE, GABAPENTIN AND PREGABALIN
Mechanisms of action
The mechanism of analgesic action of gabapentin 2-(1-[aminomethyl]cyclohexyl)acetic acid is the subject of several recent comprehensive reviews (91–94). Initially designed with the intent of creating a γ-aminobutyric acid (GABA) analogue having greater lipophilicity (CNS penetration), gabapentin does not bind to GABA receptors (types A, B, or C), nor is it metabolized to GABA (95, 96). Gabapentin-induced antinociceptive or antihyperalgesic effects are not blocked by GABAB antagonists in animal models (97–99), discounting a direct action on these receptors or an indirect action involving elevation of GABA levels via inhibition of GABA transaminase (100), activation of glutamic acid decarboxylase (101) or other mechanism (102).
Autoradiographic studies demonstrated heterogeneous distribution of gabapentin binding in the brain, with highest levels in the cortex, hippocampus and cerebellum (103). The current view is that gabapentin produces its effects by binding to the α2-δ subunit (Cavα2-δ) of the voltage-gated Ca2+ channels (VGCCs) located in these regions (104). The evidence to support this view includes data from Maneuf and colleagues (93) in models of neuropathic pain; Cavα2-δ expression is upregulated in sensory neurons and the dorsal horn of the spinal cord (105–108); blocking Cavα2-δ upregulation (rhizotomy or antisense oligonucleotides) reverses neuropathic pain (108); gabapentin’s antiallodynic effects are only observed when Cavα2-δ is upregulated in the dorsal root ganglion or the spinal cord (109); and transgenic mice overexpressing Cavα2-δ in sensory and dorsal horn neurons exhibit mechanical and thermal hypersensitivities compared to wild-type littermates (107).
Pregabalin ([S]-3-[aminomethyl]-5-methylhexanoic acid), like gabapentin, was designed as a more lipophilic GABA analogue. Like gabapentin, pregabalin does not bind to GABA receptors, metabolize to GABA or alter GABA uptake or degradation (110). Pregabalin, like gabapentin, is believed to produce its analgesic effect by binding to the α2-δ subunit (Cavα2-δ) of presynaptic VGCCs (111). By their actions on Cavα2-δ subunits, both gabapentin and pregabalin attenuate the neuronal activity in pain transmission pathways by reducing presynaptic Ca2+ channel influx, and consequently reducing the release of neurotransmitters, including glutamate (112) and substance P (113) from presynaptic neurons.
Evaluation of mechanistic interaction
At the time of writing, no studies were found that tested for a nonadditive analgesic interaction between oxycodone and gabapentin, or oxycodone and pregabalin.
Safety and efficacy
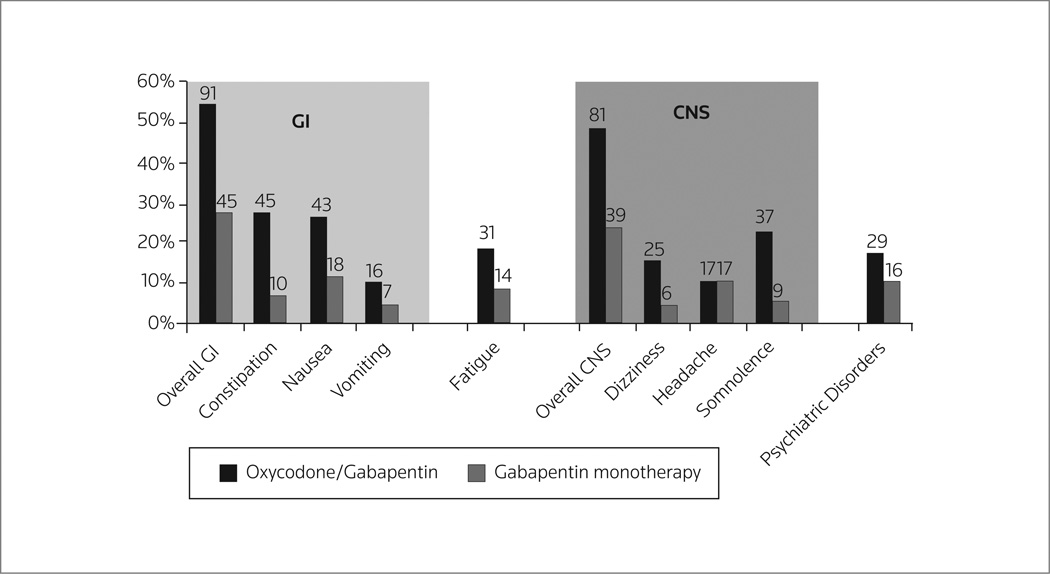
There are very little empirical data on oxycodone combinations with anticonvulsants such as pregabalin or gabapentin. Pregabalin has been reported to be safe and effective in patients with trigeminal neuralgia (114), postherpetic neuralgia, painful diabetic neuropathy (115) and fibromyalgia (116). Although safe, pregabalin was no more effective than placebo in treating pain associated with HIV neuropathy (117, 118). Not all neuropathic pain patients respond to pregabalin analgesia in monotherapy (119) and pregabalin is sometimes combined with other analgesic agents (120), including, but not limited to, oxycodone. Analgesia is sometimes dose–limited with combination therapy because of increased side effects (Fig. 2) (121).
Figure 2.
Increased side effects with gabapentin/oxycodone versus gabapentin monotherapy in 338 patients with painful diabetic neuropathy (121). GI, gastrointestinal; CNS, central nervous system.
Preoperative gabapentin for the management of post-surgical pain has produced mixed results. Gabapentin did not reduce pain or morphine consumption in patients (N = 126) undergoing total hip arthroplasty (neither acute postoperative pain nor pain in the first 6 months postsurgery) (122) or patients given interscalene brachial plexus blocks for arthroscopic shoulder procedures (N = 60) (123). However, it did significantly reduce pain and reduce opioid consumption in a study of 40 knee arthroscopy patients (124), in a study of 70 patients undergoing orthopedic surgery to a lower extremity (125), coronary artery bypass graft patients (N = 60) (126), nasal surgery patients (N = 60) (127), inguinal herniorrhaphy patients (N = 60) (128) and patients requiring surgery for brachial plexus injury (N = 20) (129). In treating severe cancer pain, an open-label study in Japan determined that addition of gabapentin provided an additive analgesic benefit that was statistically significant (visual analog scale) but clinically insignificant (130).
Rajpal and colleagues used 100 historical spinal surgery patients who received patient-controlled analagesia (PCA) of either morphine (1–2 mg) or hydromorphone (0.2–0.4 mg) with a 6–10-min lockout interval (131). These controls were compared to a prospective group of similar surgical patients (n = 100) who received preoperatively controlled-release oxycodone 20 mg, gabapentin 600 mg and acetaminophen 1 g. A prophylactic dose of i.v. dolasetron was administered perioperatively.
Following surgery, these patients received controlled-release oxycodone 10–20 mg b.i.d., gabapentin 300 to 600 mg t.i.d., and acetaminophen 1 g t.i.d. started upon return to the nursing unit and continuing for 3 days after surgery. Breakthrough pain was managed by immediate-release oxycodone (5–20 mg every 3 h, as needed). The combination patients consumed less opioids, suffered less pain and had improved patient satisfaction scores compared to the control group. While this study does not test a specific new oxycodone/gabapentin formulation, it indicates the potential value of these agents in a multimodal therapeutic regimen.
No randomized, controlled trials directly comparing gabapentin to pregabalin appear in the literature, but a recent open-label study of neuropathic pain patients (N = 146) suggests that pregabalin may provide greater analgesia than gabapentin (132). Both gabapentin (118) and pregabalin (133) have been shown to have opioidsparing effects when paired with a concomitant narcotic analgesic. Studies combining gabapentin or pregabalin with oxycodone (although not necessarily in a combination formulation, but rather as two discreet agents) appear in Table III.
Table III.
Safety and efficacy of oxycodone, pregabalin and gabapentin combinations.
| Study | Patients (n) | Design | Agents | Endpoint(s) | Results |
|---|---|---|---|---|---|
| Gatti (133) 2009 | 409 patients with moderate to severe neuropathic pain | Randomized, controlled study, 90 days | Patients received oral controlled-release oxycodone as monotherapy, oral pregabalin as monotherapy, or both for 90 days. Drugs were dosed per labeling with mean daily dose of oxycodone 24.1 (mono) and 19.4 (combo); mean daily dose of pregabalin was 85.6 (mono) and 108.1 (combo) | Safety, efficacy, quality of life | Combination therapy was significantly more effective for pain relief (P ≤ 0.003) and significantly improved quality of life compared to monotherapies (P = 0.0009). 91.2% of combo patients were effective or very effective |
| Hanna (121) 2008 | 338 patients with moderate to severe diabetic neuropathic pain | Randomized, double-blind, placebo-controlled, 12 weeks | All patients were on pregabalin at baseline. Randomization to receive controlled-release oxycodone 5 mg every 12 h vs. placebo. Rescue analgesia was 1 g paracetamol | Pain relief, use of rescue medication, sleep quality | Combination therapy improved pain relief vs. gabapentin alone (P = 0.003), was associated with less rescue medication (P = 0.03), and fewer nights of disturbed sleep (P < 0.05). AEs were more common in patients taking oxycodone and were typical opioid side effects |
| Rajpal (131) 2010 | 200 patients undergoing spinal surgery | Historical control using IV patient-controlled analgesia vs. a prospective group taking the active agent. Patient surveys and chart audits to measure pain and side effects pre-op and for 24 h post-op | Test group received extended -release oxycodone, gabapentin, and acetaminophen, intraoperative dolasetron and as needed immediate-release oral oxycodone | Pain intensity, functional interference, opioid consumtion, side effects | Combo patients had significantly lower opioid consumption (P < 0.001), lower pain ratings (P < 0.01), and less nausea (P < 0.001), drowsiness (P < 0.05), and lower interference with walking (P = 0.05) and less coughing and breathing problems (P < 0.05) |
| Zin (115) 2009 | 62 patients with postherpetic neuralgia or painful diabetic neuropathy | 7 day washout with dosing for 1 week, then open-label according to forced-titration dosing regimen of pregabalin with stable oxycodone or placebo over 4 weeks | Pregabalin: 75, 150, 300 or 600 mg/day. Patients randomized to receive stable dose of oxycodone 10 mg per day or placebo | Pain relief, sleep interference, neuropathic pain scale, AEs | Pregabalin was effective at relieving pain in these patients, but oxycodone did not enhance analgesia. AEs were comparable in oxycodone group (89%) vs. pregabalin alone group (80%); 77–88% of AEs were mild |
AEs, adverse events.
DISCUSSION
The motivation to combine therapeutic agents must be based on producing benefits for the patient. On a practical level, oxycodone combinations reduce the pill burden and provide for dosing convenience, and it can be hoped that these will foster better compliance. While those alone may be worthwhile benefits, the most important clinical measures for oxycodone combinations reside in enhancing analgesic benefits to the patient or reducing adverse effects. The analgesic benefit from a combination formulation may be additive, subadditive or synergistic. This analgesic benefit goes beyond “better pain relief” in that adding an agent to oxycodone may in some cases allow for analgesia to be provided with less amount of either agent administered alone. Since each analgesic is associated with dose-dependent adverse effects, lowering the dose of each drug may mitigate side effects associated with higher-dose monotherapy. By the same token, combinations may give rise to new or magnified (synergistic) adverse effects, which is something that needs to be determined.
Oxycodone combinations with aspirin, acetaminophen and NSAIDs have a long history and many studies demonstrate their safety and effectiveness when carefully monitored. It is unclear if they produce an opioid-sparing effect, but they do provide reliable analgesia and are particularly well-suited to acute pain syndromes, since long-term use of acetaminophen, NSAIDs and aspirin has become increasingly questionable (134, 135). Patients taking such combination drugs should be made aware of the risks of long-term use of acetaminophen, NSAIDs and aspirin. Many popular over-the-counter medications contain acetaminophen, aspirin or NSAIDs but may not be prominently labeled to that effect; patients must be counseled to avoid these drugs when taking oxycodone combinations with acetaminophen, aspirin or NSAIDs to prevent accidental overdose. This is crucial; a study of patients’ knowledge of common medications found that just 7% knew the safe daily dose of acetaminophen and up to 90% could not correctly state whether certain familiar medications contained acetaminophen (136).
The few studies of oxycodone/morphine combination agents and oxycodone combinations with pregabalin and gabapentin suggest that they may provide improved analgesic benefits with reduced side effects. Combination drugs are important additions to the armamentarium of pain relievers. They can offer far more than convenient “packaging”. Oxycodone combinations merit consideration and further study.
CONCLUSIONS
Oxycodone combination drugs include dual-opioid formulations (oxycodone/morphine), oxycodone with aspirin or NSAIDs or acetaminophen, and oxycodone paired with gabapentin or pregabalin. These formulations each offer potential benefits in that the combination of drugs appears to exert a safe and effective (and possibly enhanced) analgesic effect, with possibly reduced side effects. These drugs may also offer other benefits, such as the opioid-sparing properties of combinations of pregabalin and gabapentin (and possibly NSAIDs and acetaminophen) with oxycodone. There is a paucity of information about the nature of the interaction. However, the combination of oxycodone with acetaminophen, NSAIDs and aspirin has been thoroughly studied in the literature and reported to be safe and effective, although newly raised concerns about acetaminophen, NSAIDs and aspirin require physicians to prescribe these agents prudently, ideally in the acute pain setting and with counseling to patients. New combinations of oxycodone/pregabalin and oxycodone/gabapentin, as well as dual-opioid drugs, merit greater investigation to better assess their appropriate role in the clinical setting as analgesic agents in acute and chronic pain syndromes.
Footnotes
DISCLOSURES
Drs. Raffa and Tallarida are speakers, consultants and/or basic science investigators for several pharmaceutical companies involved in analgesics research, but receive no royalties (cash or otherwise) from the sale of any product. Dr. Pergolizzi is a consultant for Grünenthal, Baxter, Hospira and Endo Pharmaceuticals. Dr. Segarnick states no conflicts of interest. This article was prepared with editorial support from LeQ Medical in Angleton, Texas.
REFERENCES
- 1.Davies E, Higginson IJ. Better palliative care for older people. [Last accessed 8 Mar 2010];World Health Organization Regional Office for Europe. 2004 Available at: http://www.euro.who.int/document/e82933.pdf.
- 2.Koshy RC, Rhodes D, Devi S, Grossman SA. Cancer pain management in developing countries: A mosaic of complex issues resulting in inadequate analgesia. Support Care Cancer. 1998;6(5):430–437. doi: 10.1007/s005200050190. [DOI] [PubMed] [Google Scholar]
- 3.Oldenmenger WH, Sillevis Smitt PA, van Dooren S, van der Rijt CC. A systematic review on barriers hindering adequate cancer pain management and interventions to reduce them: A critical appraisal. Eur J Cancer. 2009;45(8):1370–1380. doi: 10.1016/j.ejca.2009.01.007. [DOI] [PubMed] [Google Scholar]
- 4.Al-Rowalli A, Al-aqeel SA, Al-Naim LS, Al-Diab AI. Appropriateness of cancer pain management in Saudi teaching hospital. Gulf J Oncolog. 2009;(5):37–43. [PubMed] [Google Scholar]
- 5.Coluzzi F, Savoia G, Paoletti F, Costantini A, Mattia C. Postoperative pain survey in Italy (POPSI): A snapshot of current national practices. Minerva Anestesiol. 2009;75(11):622–631. [PubMed] [Google Scholar]
- 6.Murnion BP, Gnjidic D, Hilmer SN. Prescription and administration of opioids to hospital in-patients and barriers to effective use. Pain Med. 2010;11(1):58–66. doi: 10.1111/j.1526-4637.2009.00747.x. [DOI] [PubMed] [Google Scholar]
- 7.Pergolizzi J, Böger RH, Budd K, et al. Opioids and the management of chronic severe pain in the elderly: Consensus statement of an international expert panel with focus on the six clinically most often used World Health Organization step III opioids (buprenorphine, fentanyl, hydromorphone, methadone, morphine, oxycodone) Pain Practice. 2008;8(4):287–313. doi: 10.1111/j.1533-2500.2008.00204.x. [DOI] [PubMed] [Google Scholar]
- 8.Osifo OD, Oriafo IA. Circumcision mishaps in Nigerian children. Ann Afr Med. 2009;8(4):266–270. doi: 10.4103/1596-3519.59583. [DOI] [PubMed] [Google Scholar]
- 9.Joransen DE. Improving patient access to opioids through consensus building with government. [Last accessed 8 Mar 2010];World Health Organization Collaborating Center. Available at: http://paincenter.stanford.edu/iasp/Speaker%20Slide%20Handouts/Actual%20Joransen.pdf.
- 10.Potter VT, Wiseman CE, Dunn SM, Boyle FM. Patient barriers to optimal cancer pain control. Psychooncology. 2003;12(2):153–160. doi: 10.1002/pon.627. [DOI] [PubMed] [Google Scholar]
- 11.Fishman SM. Pain management consultation-information for patients: Opioid side effects, addiction and anti-inflammatory medications. J Pain Palliative Care Pharmacother. 2005;19(1):51–55. [PubMed] [Google Scholar]
- 12.Kalso E. Oxycodone: Proceedings of the symposium “Updates of the clinical pharmacology of opioids with special attention to long-acting drugs”. J Pain Symptom Manage. 2005;29(5) Suppl.:S47–S56. doi: 10.1016/j.jpainsymman.2005.01.010. [DOI] [PubMed] [Google Scholar]
- 13.Cicero TJ, Inciardi JA, Munoz A. Trends in abuse of OxyContin® and other opioid analgesics in the United States: 2002–2004. J Pain. 2005;6(10):662–672. doi: 10.1016/j.jpain.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 14.McCormick CG, Henningfield JE, Haddox JD, et al. Case histories in pharmaceutical risk management. Drug Alcohol Depend. 2009;105(Suppl. 1):S42–S55. doi: 10.1016/j.drugalcdep.2009.08.003. [DOI] [PubMed] [Google Scholar]
- 15.Wu LT, Pilowsky DJ, Patkar AA. Non-prescribed use of pain relievers among adolescents in the United States. Drug Alcohol Depend. 2008;94(103):1–11. doi: 10.1016/j.drugalcdep.2007.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marvizon JC, Ma Y-Y, Charles AC, Walwyn W, Evans CJ. Pharmacology of the opioid system. In: Beaulieu P, Lussier D, Porreca F, Dickenson AH, editors. Pharmacology of Pain. Seattle: IASP Press; 2010. pp. 87–110. [Google Scholar]
- 17.Wei LN, Law PY, Loh HH. Post-transcriptional regulation of opioid receptors in the nervous system. Front Biosci. 2004;9:1665–1679. doi: 10.2741/1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pasternak GW. Multiple opiate receptors: Déjà vu all over again. Neuropharmacology. 2004;47(Suppl. 1):312–323. doi: 10.1016/j.neuropharm.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 19.Kieffer BL, Evans CJ. Opioid receptors: From binding sites to visible molecules in vivo. Neuropharmacology. 2009;56(Suppl. 1):205–212. doi: 10.1016/j.neuropharm.2008.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Crooks PA, Kottayil SG, Al-Ghananeem AM, Byrn SR, Butterfield DA. Opiate receptor binding properties of morphine-, dihydromorphine-, and codeine 6-O-sulfate ester congeners. Bioorg Med Chem Lett. 2006;16(16):4291–4295. doi: 10.1016/j.bmcl.2006.05.060. [DOI] [PubMed] [Google Scholar]
- 21.Inturrisi CE. Clinical pharmacology of opioids for pain. Clin J Pain. 2004;18(4) Suppl.:S3–S13. doi: 10.1097/00002508-200207001-00002. [DOI] [PubMed] [Google Scholar]
- 22.Crowder JM, Norris DK, Bradford HF. Morphine inhibition of calcium fluxes, neurotransmitter release and protein and lipid phosphorylation in brain slices and synaptosomes. Biochem Pharmacol. 1986;35(15):2501–2507. doi: 10.1016/0006-2952(86)90046-8. [DOI] [PubMed] [Google Scholar]
- 23.Kondo I, Marvizon JCG, Song B, Salgado F, Codeluppi S, Hua XY, Yaksh TL. Inhibition by spinal mu- and deltaopioid agonists of afferent-evoked substance P release. J Neurosc. 2005;25(14):3651–3660. doi: 10.1523/JNEUROSCI.0252-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Andersen G, Christrup L, Sjogren P. Relationship among morphine metabolism, pain, and side effects during longterm treatment: An update. J Pain Symptom Management. 2003;25(1):74–91. doi: 10.1016/s0885-3924(02)00531-6. [DOI] [PubMed] [Google Scholar]
- 25.Narita M, Nakamura A, Ozaki M, Imai S, Miyoshi K, Suzuki M, Suzuki T. Comparative pharmacological profiles of morphine and oxycodone under a neuropathic pain-like state in mice: Evidence for less sensitivity to morphine. Neuropsychopharmacology. 2008;33(5):1097–1112. doi: 10.1038/sj.npp.1301471. [DOI] [PubMed] [Google Scholar]
- 26.Smith MT. Differences between and combinations of opioids re-visited. Curr Opin Anaesthesiol. 2008;21(5):596–601. doi: 10.1097/ACO.0b013e32830a4c4a. [DOI] [PubMed] [Google Scholar]
- 27.Virk MS, Williams JT. Agonist-specific regulation of mu-opioid receptor desensitization and recovery from desensitization. Mol Pharmacol. 2008;73(4):1301–1308. doi: 10.1124/mol.107.042952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nielsen CK, Ross FB, Lotfipour S, Saini KS, Edwards SR, Smith MT. Oxycodone and morphine have distinctly different pharmacological profiles: Radioligand binding and behavioral studies in two rat models of neuropathic pain. Pain. 2007;132(3):289–300. doi: 10.1016/j.pain.2007.03.022. [DOI] [PubMed] [Google Scholar]
- 29.Lalovic B, Kharasch E, Hoffer C, Risler L, Liu-Chen LY, Shen DD. Pharmacokinetics and pharmacodynamics of oral oxycodone in healthy human subjects: Role of circulating active metabolites. Clin Pharmacol Ther. 2006;79(5):461–479. doi: 10.1016/j.clpt.2006.01.009. [DOI] [PubMed] [Google Scholar]
- 30.Minami K, Hasegawa M, Ito H, et al. Morphine, oxycodone, and fentanyl exhibit different analgesic profiles in mouse pain models. J Pharmacol Sci. 2009;111(1):60–72. doi: 10.1254/jphs.09139fp. [DOI] [PubMed] [Google Scholar]
- 31.Lemberg KK, Sjiskonen AO, Kontinen VK, Yli-Kauhaluoma JT, Kalso EA. Pharmacological characterization of noroxymorphone as a new opioid for spinal analgesia. Anesth Analg. 2008;106(2):463–470. doi: 10.1213/ane.0b013e3181605a15. [DOI] [PubMed] [Google Scholar]
- 32.Lemberg KK, Kontinen V, Siiskonen AO, Viljakka KM, Yli-Kauhaluoma JT, Korpi ER, Kalso EA. Antinociception by spinal and systemic oxycodone: Why does the route make the difference?: In vitro and in vivo studies in rats. Anesthesiology. 2006;105(4):801–812. doi: 10.1097/00000542-200610000-00027. [DOI] [PubMed] [Google Scholar]
- 33.Nielsen CK, Ross FB, Smith MT. Incomplete, asymmetric, and route-dependent cross-tolerance between oxycodone and morphine in the dark agouti rat. J Pharmacol Exp Ther. 2000;295(1):91–99. [PubMed] [Google Scholar]
- 34.Berdine HJ. Equianalgesic dosing of opioids. J Pain Palliative Care Pharmacother. 2006;20:79–84. [PubMed] [Google Scholar]
- 35.Heiskanen T, Kalso E. Controlled release oxycodone and morphine in cancer related pain. Pain. 1997;73:37–45. doi: 10.1016/s0304-3959(97)00072-9. [DOI] [PubMed] [Google Scholar]
- 36.Bruera E, Belzile M, Pituksin E, et al. Randomized double blind crossover trial comparing safety and efficacy of oral controlled release oxydone with controlled release morphine in patients with cancer pain. J Clin Oncol. 1998;16(10):3222–3229. doi: 10.1200/JCO.1998.16.10.3222. [DOI] [PubMed] [Google Scholar]
- 37.Silvasti M, Rosenberg P, Seppala T, Svartling N, Pitkanen M. Comparison of analgesic efficacy of oxycodone and morphine in postoperative intranvenous patient controlled analgesia. Acta Anaesth Scand. 1998;42(5):576–580. doi: 10.1111/j.1399-6576.1998.tb05169.x. [DOI] [PubMed] [Google Scholar]
- 38.Ordonez Gallego A, Gonzalez Baron M, Espinosa Arranz E. Oxycodone: A pharmacological and clinical review. Clin Transl Oncol. 2007;9(5):298–307. doi: 10.1007/s12094-007-0057-9. [DOI] [PubMed] [Google Scholar]
- 39.Shaheen PE, Walsh D, Lasheen W, Davis MP, Lagman RL. Opioid equianalgesic tables: Are they all equally dangerous? J Pain Symptom Managem. 2009;38(3):409–417. doi: 10.1016/j.jpainsymman.2009.06.004. [DOI] [PubMed] [Google Scholar]
- 40.Knotkova H, Fine PG, Portenoy RK. Opioid rotation: The science and the limitations of the equianalgesic dose table. J Pain Symptom Managem. 2009;38(3):426–439. doi: 10.1016/j.jpainsymman.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 41.Meert TF, Vermeirsch HA. A preclinical comparison between different opioids: Antinociceptive versus adverse effects. Pharmacol Biochem Behavior. 2005;80(2):309–326. doi: 10.1016/j.pbb.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 42.Reisine T, Pasternak G. Opioid analgesics and antagonists. In: Hardman JG, Limbird LE, editors. Goodman and Gilman’s the Pharmacological Basis of Therapeutics. New York: McGraw-Hill; 1996. pp. 521–556. [Google Scholar]
- 43.Tallarida RJ, Stone DJ, Jr, Raffa RB. Efficient designs for studying synergistic drug combinations. Life Sci. 1997;61(26):417–425. doi: 10.1016/s0024-3205(97)01030-8. [DOI] [PubMed] [Google Scholar]
- 44.Tallarida RJ. Drug synergism and dose-effect data analysis (monograph) Boca Raton: CRC/Chapman-Hall; 2000. [Google Scholar]
- 45.Tallarida RJ. Drug synergism: Its detection and applications. Perspectives in pharmacology. J Pharmacol Exp Ther. 2001;298(3):865–872. [PubMed] [Google Scholar]
- 46.Tallarida RJ, Cowan A, Raffa RB. Antinociceptive synergy, additivity, and subaddivitiy with combinations of oral glucosamine plus nonopioid analgesics in mice. J Pharmacol Exp Ther. 2003;307(2):699–704. doi: 10.1124/jpet.103.054320. [DOI] [PubMed] [Google Scholar]
- 47.Tallarida RJ. An overview of drug combination analysis with isobolograms. Perspectives in pharmacology. J Pharmacol Exp Ther. 2006;319(1):1–7. doi: 10.1124/jpet.106.104117. [DOI] [PubMed] [Google Scholar]
- 48.Tallarida RJ. Interactions between drugs and occupied receptors. Pharmacol Ther. 2007;113(1):197–209. doi: 10.1016/j.pharmthera.2006.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Loewe S. Die Mischiarnei. Klin Wochenschr. 1927;6:1077–1085. [Google Scholar]
- 50.Loewe S. Die quantitativen Probleme der Pharmakologie. Ergebn Physiol. 1928;27:47–187. [Google Scholar]
- 51.Loewe S. The problem of synergism and antagonism of combined drugs. Arzneimittelforschung. 1953;3(6):285–290. [PubMed] [Google Scholar]
- 52.Loewe S. Antagonisms and antagonists. Pharmacol Rev. 1957;9(2):237–242. [PubMed] [Google Scholar]
- 53.Grabovsky Y, Tallarida RJ. Isobolographic analysis for combinations of full and partial agonist: Curved isoboles. J Pharmacol Exper Ther. 2004;310(3):981–986. doi: 10.1124/jpet.104.067264. [DOI] [PubMed] [Google Scholar]
- 54.Braverman AS, Tallarida RJ, Ruggieri MR., Sr The use of occupation isoboles for analysis of a response mediated by two receptors: M2 and M3 muscarinic receptor subtype-induced mouse stomach contraction. J Pharmacol Exp Ther. 2008;325(3):954–960. doi: 10.1124/jpet.108.137018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ross FB, Wallis SC, Smith MT. Co-administration of sub-antinociceptive doses of oxycodone and morphine produces marked antinociceptive synergy with reduced CNS side-effects in rats. Pain. 2000;84(2–3):421–428. doi: 10.1016/s0304-3959(99)00230-4. [DOI] [PubMed] [Google Scholar]
- 56.Bolan EA, Tallarida RJ, Pasternak GW. Synergy between mu opioid ligands: Evidence for functional interactions among mu opioid receptor subtypes. J Pharmacol Exp Ther. 2002;303(2):557–562. doi: 10.1124/jpet.102.035881. [DOI] [PubMed] [Google Scholar]
- 57.Grach M, Massalha W, Pud D, Adler R, Eisenberg E. Can coadministration of oxycodone and morphine produce analgesic synergy in humans? An experimental cold pain study. Br J Clin Pharmacol. 2004;58(3):235–242. doi: 10.1111/j.1365-2125.2004.02141.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zacny JP, Lichtor SA. Within-subject comparison of the psychopharmacological profiles of oral oxycodone and oral morphine in non-drug-abusing volunteers. Psychopharmacology. 2008;196:105–116. doi: 10.1007/s00213-007-0937-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Smith MT, de la Iglesia FA. Co-administration of oxycodone and morphine and analgesic synergy re-examined. Br J Clin Pharmacol. 59(4):486–487. Letter to the editors. Author’s response: Grach M., Massalha W., Pud D., Adler R., Eisenberg E.: Response to Smith M.T. and de la Iglesia F.A.: ‘Co-administration of oxycodone and analgesic synergy reamined.’ Br J Pharmacol 2005, 59(4): 487–8. [Google Scholar]
- 60.Ladd LA, Kam PC, Williams DB, Wright AW, Smith MT, Mather LE. Ventilatory responses of healthy subjects to intravenous combinations of morphine and oxycodone under imposed hypercapnic and hypoxaemic conditions. Br J Clin Pharmacol. 2005;59(5):524–535. doi: 10.1111/j.1365-2125.2005.02368.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lauretti GR, Oliveira GM, Pereira NL. Comparison of sustained-release morphine with sustained-release oxycodone in advanced cancer patients. Br J Cancer. 2003;89(11):2027–2030. doi: 10.1038/sj.bjc.6601365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Smith MT, de la Iglesia FA. Co-administration of oxycodone and morphine and analgesic synergy re-examined. Br J Clin Pharmacol. 2005;59(4):486–488. doi: 10.1111/j.1365-2125.2005.02345_1.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Vane JR, Botting RM. The mechanism of action of aspirin. Thromb Res. 2003;110(5–6):255–258. doi: 10.1016/s0049-3848(03)00379-7. [DOI] [PubMed] [Google Scholar]
- 64.Rao P, Knaus EE. Evolution of nonsteroidal anti-inflammatory drugs (NSAIDs): Cyclooxygenase (COX) inhibition and beyond. J Pharm Pharm Sci. 2008;11(2):81s–110s. doi: 10.18433/j3t886. [DOI] [PubMed] [Google Scholar]
- 65.Vergne-Salle P, Beneytout J-L. Targeting the cyclooxygenase pathway. In: Beaulieu P, Lussier D, Porreca F, Dickenson AH, editors. Pharmacology of Pain. Seattle: IASP Press; 2010. pp. 43–64. [Google Scholar]
- 66.Cyclooxygenase structure and mechanism: how aspirin and NSAIDs work. [Last accessed 5 Mar 2010]; Available at: http://cti.itc.virginia.edu/~cmg/Demo/pdb/cycox/cycox.html. [Google Scholar]
- 67.Fitzpatrick FA. Cyclooxygenase enzymes: Regulation and function. Curr Pharm Des. 2004;10(6):577–588. doi: 10.2174/1381612043453144. [DOI] [PubMed] [Google Scholar]
- 68.Kurumbail RG, Stevens AM, Gierse JK, et al. Structural basis for selective inhibition of cyclooxygenase-2 by antiinflammatory agents. Nature. 1996;384(6610):644–648. doi: 10.1038/384644a0. [DOI] [PubMed] [Google Scholar]
- 69.Yaksh TL, Dirig DM, Malmberg AB. Mechanism of action of nonsteroidal anti-inflammatory drugs. Cancer Invest. 1998;16(7):509–527. doi: 10.3109/07357909809011705. [DOI] [PubMed] [Google Scholar]
- 70.Smith HS. Potential analgesic mechanisms of acetaminophen. Pain Physician. 2009;12(1):269–280. [PubMed] [Google Scholar]
- 71.Toussaint K, Yang XC, Zielinski MA, et al. What do we (not) know about how paracetamol (acetaminophen) works? J Clin Pharm Ther. doi: 10.1111/j.1365-2710.2009.01143.x. In press. [DOI] [PubMed] [Google Scholar]
- 72.Mallet C, Eschalier A. Pharmacology and mechanism of action of acetaminophen. In: Beaulieu P, Lussier D, Porreca F, Dickenson AH, editors. Pharmacology of Pain. Seattle: IASP Press; 2010. pp. 65–85. [Google Scholar]
- 73.Beaver WT. Aspirin and acetaminophen as constituents of analgesic combinations. Arch Intern Med. 1981;141(3, Spec No.):293–300. doi: 10.1001/archinte.141.3.293. [DOI] [PubMed] [Google Scholar]
- 74.Beaver WT. Combination analgesics. Am J Med. 1984;10(3A):38–53. doi: 10.1016/s0002-9343(84)80101-1. [DOI] [PubMed] [Google Scholar]
- 75.Zelcer S, Kloesnikov Y, Kovalyshyn I, Pasternak DA, Pasternak GW. Selective potentiation of opioid analgesia by nonsteroidal anti-inflammatory drugs. Brain Res. 2005;1040(1–2):151–156. doi: 10.1016/j.brainres.2005.01.070. [DOI] [PubMed] [Google Scholar]
- 76.Moertel CG, Ahmann DL, Taylor WF, Schwartau N. Relief of pain by oral medications. A controlled evaluation of analgesic combinations. JAMA. 1974;229(1):55–59. [PubMed] [Google Scholar]
- 77. [Last accessed 2 Mar 2010];Combunox package insert. Label Data Plus. 2010 Available at: http://www.labeldataplus.com/detail.php?c=2497. [Google Scholar]
- 78.Hermos JA, Young MM, Gagnos DR, Fiore LD. Characterizations of long-term oxycodone/acetaminophen prescriptions in veteran patients. Arch Intern Med. 2004;164(21):2361–2366. doi: 10.1001/archinte.164.21.2361. [DOI] [PubMed] [Google Scholar]
- 79.Corsinovi L, Marintelli E, Fonte G, et al. Efficacy of oxycodone/acetaminophen and codeine/acetaminophen vs. conventional therapy in elderly women with persistent, moderate to severe osteoarthritis-related pain. Arch Gerontol Geriatr. 2009;49(3):378–382. doi: 10.1016/j.archger.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 80.Daniels SE, Desjardins PJ, Talwalker S, Recker DP, Verburg KM. The analgesic efficacy of valdecoxib vs. oxycodone/acetaminophen after oral surgery. J Am Dent Assoc. 2002;133(5):611–621. doi: 10.14219/jada.archive.2002.0237. [DOI] [PubMed] [Google Scholar]
- 81.Desjardins PJ, Black PM, Daniels SE, et al. A double-blind randomized controlled trial of rofecoxib and multidose oxycodone/acetaminophen in dental impaction pain. J Oral Maxillofac Surg. 2007;65(8):1624–1632. doi: 10.1016/j.joms.2006.06.268. [DOI] [PubMed] [Google Scholar]
- 82.Dionne RA. Additive analgesic effects of oxycodone and ibuprofen in the oral surgery model. J Oral Maxillofac Surg. 1999;57(6):673–678. doi: 10.1016/s0278-2391(99)90429-9. [DOI] [PubMed] [Google Scholar]
- 83.Gammaitoni AR, Galer BS, Bulloch S, Lacouture P, Caruso F, Ma T, Schlagheck T. Randomized, double-blind, placebo-controlled comparison of analgesic efficacy of oxycodone 10 mg/acetaminophen 325 mg versus controlled-release oxycodone 20 mg in postsurgical pain. J Clin Pharmacol. 2003;43(3):296–304. doi: 10.1177/0091270003251147. [DOI] [PubMed] [Google Scholar]
- 84.Korn S, Vassil TC, Kotey PN, Fricke JR., Jr Comparison of rofecoxib and oxycodone plus acetaminophen in the treatment of acute pain: A randomized, double-blind, placebo-controlled study in patients with moderate to severe postoperative pain in the third molar extraction model. Clin Ther. 2004;26(5):769–778. doi: 10.1016/s0149-2918(04)90076-8. [DOI] [PubMed] [Google Scholar]
- 85.Litkowski LJ, Christensen SE, Adamson DN, Van Dyke T, Han SH, Neman KB. Analgesic efficacy and tolerability of oxycodone 5 mg/ibuprofen 400 mg compared with those of oxycodone 5 mg/acetaminophen 325 mg and hydrocodone 7.5 mg/acetaminophen 500 mg in patients with moderate to severe postoperative pain: A randomized, double-blind, placebo-controlled, single-dose, parallel-group study in a dental pain model. Clin Ther. 2005;27(4):418–429. doi: 10.1016/j.clinthera.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 86.Lovell SJ, Taira T, Rodriguez E, Wackett A, Gulla J, Singer AJ. Comparison of valdecoxib and an oxycodone-racetaminophen combination for acute musculoskeletal pain in the emergency department: A randomized controlled trial. Acad Emerg Med. 2004;11(12):1278–1282. doi: 10.1197/j.aem.2004.06.011. [DOI] [PubMed] [Google Scholar]
- 87.Palangio M, Morris E, Doyle RT, Jr, Dornseif BE, Valente TJ. Combination hydrocone and ibuprofen versus combination oxycodone and acetaminophen in the treatment of moderate or severe acute low back pain. Clin Ther. 2002;24(1):87–99. doi: 10.1016/s0149-2918(02)85007-x. [DOI] [PubMed] [Google Scholar]
- 88.Palangio M, Wideman GL, Keffer M, et al. Combination hydrocodone and ibuprofen versus combination oxycodone and acetaminophen in the treatment of postoperative obstetric or gynecological pain. Clin Ther. 2000;22(5):600–612. doi: 10.1016/s0149-2918(00)80047-8. [DOI] [PubMed] [Google Scholar]
- 89.Singla N, Pong A, Newman K. Combination oxycodone 5 mg/ibuprofen 400 mg for the treatment of pain after abdominal or pelvic surgery in women: A randomized, double-blind, placebo- and active-controlled parallel-group study. Clin Ther. 2005;27(1):45–57. doi: 10.1016/j.clinthera.2005.01.010. [DOI] [PubMed] [Google Scholar]
- 90.Van Dyke T, Litkowski LJ, Kiersch TA, Zarringhalam NM, Zheng H, Newman K. Combination oxycodone 5 mg/ibuprofen 400 mg for the treatment of postoperative pain: A double-blind, placebo- and active-controlled parallel-group study. Clin Ther. 2004;26(12):2003–2014. doi: 10.1016/j.clinthera.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 91.Baillie JK, Power I. The mechanism of action of gabapentin in neuropathic pain. Curr Opin Investig Drugs. 2006;7(1):33–39. [PubMed] [Google Scholar]
- 92.Cheng JK, Chiou LC. Mechanisms of the antinociceptive action of gabapentin. J Pharmacol Sci. 2006;100(5):471–486. doi: 10.1254/jphs.cr0050020. [DOI] [PubMed] [Google Scholar]
- 93.Maneuf YP, Luo ZD, Lee K. Alpha2delta and the mechanism of action of gabapentin in the treatment of pain. Semin Cell Dev Biol. 2006;17(5):565–570. doi: 10.1016/j.semcdb.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 94.McGivern JG, McDonough SI. Voltage-gated calcium channels as targets for the treatment of chronic pain. Curr Drug Targets CNS Neurol Disord. 2004;3(6):457–478. doi: 10.2174/1568007043336743. [DOI] [PubMed] [Google Scholar]
- 95.Taylor CP, Angelotti T, Fauman E. Pharmacology and mechanism of action of pregabalin: The calcium channel alpha2-delta (alpha2-delta) subunit as a target for antiepileptic drug discovery. Epilepsy Res. 2007;73(2):137–150. doi: 10.1016/j.eplepsyres.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 96.Suman-Chauhan N, Webdale L, Hill DR, Woodruff GN. Characterisation of [3H]gabapentin binding to a novel site in rat brain: Homogenate binding studies. Eur J Pharmacol. 1993;244(3):293–301. doi: 10.1016/0922-4106(93)90155-3. [DOI] [PubMed] [Google Scholar]
- 97.Patel S, Naeem S, Kesingland A, Froestl W, Capogna M, Urban L, Fox A. The effects of GABA(B) agonists and gabapentin on mechanical hyperalgesia in models of neuropathic and inflammatory pain in the rat. Pain. 2001;90(3):217–226. doi: 10.1016/S0304-3959(00)00404-8. [DOI] [PubMed] [Google Scholar]
- 98.Cheng JK, Lee SZ, Yang JR, Wang CH, Liao YY, Chen CC, Chiou LC. Does gabapentin act as an agonist at native GABA(B) receptors? J Biomed Sci. 2004;11(3):346–355. doi: 10.1007/BF02254439. [DOI] [PubMed] [Google Scholar]
- 99.Shimizu S, Honda M, Tanabe M, Ono H. GABAB receptors do not mediate the inhibitory actions of gabapentin on the spinal reflex in rats. J Pharmacol Sci. 2004;96(4):444–449. doi: 10.1254/jphs.fp0040537. [DOI] [PubMed] [Google Scholar]
- 100.Goldlust A, Su TZ, Welty DF, Taylor CP, Oxender DL. Effects of anticonvulsant drug gabapentin on the enzymes in metabolic pathways of glutamate and GABA. Epilepsy Res. 1995;22(1):1–11. doi: 10.1016/0920-1211(95)00028-9. [DOI] [PubMed] [Google Scholar]
- 101.Taylor CP, Vartanian MG, Andruszkiewicz R, Silverman RB. 3-alkyl GABA and 3-alkylglutamic acid analogues: Two new classes of anticonvulsant agents. Epilepsy Res. 1992;11(2):103–110. doi: 10.1016/0920-1211(92)90044-t. [DOI] [PubMed] [Google Scholar]
- 102.Sweatt AJ, Garcia-Espinosa MA, Wallin R, Hutson SM. Branched-chain amino acids and neurotransmitter metabolism: Expression of cytosolic branched-chain amino-transferase (BCATc) in the cerebellum and hippocampus. J Comp Neurol. 2004;477:360–370. doi: 10.1002/cne.20200. [DOI] [PubMed] [Google Scholar]
- 103.Hill DR, Suman-Chauhan N, Woodruff GN. Localization of [3H]gabapentin to a novel site in rat brain: Autoradiogrpahic studies. Eur J Pharmacol. 1993;244(3):303–309. doi: 10.1016/0922-4106(93)90156-4. [DOI] [PubMed] [Google Scholar]
- 104.Gee NS, Brown JP, Dissanayake VU, Offord J, Thurlow R, Woodruff GN. The novel anticonvulsant drug, gabapentin (Neurontin), binds to the alpha2delta subunit of a calcium channel. J Biol Chem. 1996;271(10):5768–5776. doi: 10.1074/jbc.271.10.5768. [DOI] [PubMed] [Google Scholar]
- 105.Luo ZD, Chaplan SR, Hiquera ES, Sorkin LS, Stauderman KA, Williams ME, Yaksh TL. Upregulation of dorsal root ganglion (alpha)2(delta) calcium channel subunit and its correlation with allodynia in spinal nerve-injured rats. J Neurosci. 2001;21(6):1868–1875. doi: 10.1523/JNEUROSCI.21-06-01868.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Newton RA, Bingham S, Case PC, Sanger GJ, Lawson SN. Dorsal root ganglion neurons show increased expression of the calcium channel alpha2delta-1 subunit following partial sciatic nerve injury. Brain Res Mol Brain Res. 2001;95(1–2):1–8. doi: 10.1016/s0169-328x(01)00188-7. [DOI] [PubMed] [Google Scholar]
- 107.Li CY, Li KW, Kurwa A, et al. Calcium channel a2d-1 subunit is a molecular determinant of pain behaviors: evidence from injury-free transgenic mice. Soc for Neurosci. 2004 Abst 64.9. [Google Scholar]
- 108.Li CY, Song YH, Higuera ES, Luo ZD. Spinal dorsal horn calcium channel alpha2delta-1 subunit upregulation contributes to peripheral nerve injury-induced tactile allodynia. J Neurosci. 2004;24(39):8494–8499. doi: 10.1523/JNEUROSCI.2982-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Luo ZD, Calcutt NA, Higuera ES, Valder CR, Song YH, Svensson CI, Myers RR. Injury type-specific calcium channel alpha 2 delta-1 subunit up-regulation in rat neuropathic pain models correlates with antiallodynic effects of gabapentin. J Pharmacol Exp Ther. 2002;303(3):1199–1205. doi: 10.1124/jpet.102.041574. [DOI] [PubMed] [Google Scholar]
- 110.Sills GJ. The mechanisms of action of gabapentin and pregabalin. Curr Opin Pharmacol. 2006;6(1):108–113. doi: 10.1016/j.coph.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 111.Gajraj NM. Pregabalin: Its pharmacology and use in pain management. Anesth Analg. 2007;105(6):1805–1815. doi: 10.1213/01.ane.0000287643.13410.5e. [DOI] [PubMed] [Google Scholar]
- 112.Maneuf YP, Huges J, McKnight AT. Gabapentin inhibits the substance P-facilitated K(+)-evoked release of [(3)H]glutamate from rate caudial trigeminal nucleus slices. Pain. 2001;93(2):191–196. doi: 10.1016/S0304-3959(01)00316-5. [DOI] [PubMed] [Google Scholar]
- 113.Fehrenbacher JC, Taylor CP, Vasko MR. Pregabalin and gabapentin reduce release of substance P and CGRP from rat spinal tissues only after inflammation or activation of protein kinase C. Pain. 2003;105(1–2):133–141. doi: 10.1016/s0304-3959(03)00173-8. [DOI] [PubMed] [Google Scholar]
- 114.Perez C, Saldana MT, Navarro A, Martinez S, Rejas J. Trigeminal neuralgia treated with pregabalin in family medicine settings: Its effect on pain alleviation and cost reduction. J Clin Pharmacol. 2009;49(5):582–590. doi: 10.1177/0091270009333017. [DOI] [PubMed] [Google Scholar]
- 115.Zin CS, Nissen LM, O’Callaghan P, et al. A randomized controlled trial of oxycodone vs. placebo in patients with postherpetic neuralgia and painful diabetic neuropathy treated with pregabalin. J Pain. 2009 doi: 10.1016/j.jpain.2009.09.003. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 116.Straube S, Derry S, Moore RA, McQuay HJ. Pregabalin in fibromyalgia: Meta-analysis of efficacy and safety from company clinical trial reports. Rheumatology. 2010 doi: 10.1093/rheumatology/kep432. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 117.Simpson DM, Schifitto G, Clifford DB, et al. Pregabalin for painful HIV neuropathy. Neurology. 2010;74(5):413–420. doi: 10.1212/WNL.0b013e3181ccc6ef. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ho KY, Gan TJ, Habib AS. Gapapentin and postoperative pain—A systematic review of randomized controlled trials. Pain. 2006;126(1–3):91–101. doi: 10.1016/j.pain.2006.06.018. [DOI] [PubMed] [Google Scholar]
- 119.Namaka M, Gramlich CR, Ruhlen D, Melanson M, Sutton I, Major J. A treatment algorithm for neuropathic pain. Clin Ther. 2004;26(7):951–979. doi: 10.1016/s0149-2918(04)90171-3. [DOI] [PubMed] [Google Scholar]
- 120.Mizoguchi H, Watanabe C, Yonezawa A, Sakurada S. New therapy for neuropathic pain. Int Rev Neurobiol. 2009;85:249–260. doi: 10.1016/S0074-7742(09)85019-8. [DOI] [PubMed] [Google Scholar]
- 121.Hanna M, O’Brien C, Wilson MC. Prolonged-release oxycodone enhances the effects of existing gabapentin therapy in painful diabetic neuropathy patients. Eur J Pain. 2008;12(6):804–813. doi: 10.1016/j.ejpain.2007.12.010. [DOI] [PubMed] [Google Scholar]
- 122.Clarke H, Pereira S, Kennedy D, et al. Adding gabapentin to a multimodal regiment does not reduce acute pain, opioid consumption or chronic pain after total hip arthroplasty. Acta Anaesthesiol Scand. 2009;53(8):1073–1083. doi: 10.1111/j.1399-6576.2009.02039.x. [DOI] [PubMed] [Google Scholar]
- 123.Adam F, Menigaux C, Sessler DI, Chauvin M. A single preoperative dose of gabapentin (800 milligrams) does not augment postoperative analgesia in patients given interscalene brachial plexus blocks for arthroscopic shoulder surgery. Anesth Analg. 2006;103(5):1278–1282. doi: 10.1213/01.ane.0000237300.78508.f1. [DOI] [PubMed] [Google Scholar]
- 124.Clarke H, Pereira S, Kennedy D, Gilron I, Katz J, Gollish J, Kay J. Gabapentin decreases morphine consumption and improves functional recovery following total knee arthroplasty. Pain Res Manag. 2009;14(3):217–222. doi: 10.1155/2009/930609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Montazeri K, Kashefi P, Honamand A. Pre-emptive gabapentin significantly reduces postoperative pain and morphine demand following lower extremity orthopaedic surgery. Singapore Med J. 2007;48(8):748–751. [PubMed] [Google Scholar]
- 126.Menda F, Köner O, Sayin M, Ergenoglu M, Kücükaksu S, Aykac B. Effects of single-dose gabapentin on postoperative pain and morphine consumption after cardiac surgery. J Cardiothorac Vasc Anesth. 2010 doi: 10.1053/j.jvca.2009.10.023. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 127.Kazak Z, Meltern Mortimer N, Sekerci S. Single dose of preoperative analgesia with gabapentin (600 mg) is safe and effective in monitored anesthesia care for nasal surgery. Eur Arch Otorhinolaryngol. 2009 doi: 10.1007/s00405-009-1175-5. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 128.Sen H, Sizlan A, Yanarates O, et al. The effects of gabapentin on acute and chronic pain after inguinal herniorrhaphy. Eur J Anaesthesiol. 2009;26(9):772–776. doi: 10.1097/EJA.0b013e32832ad2fa. [DOI] [PubMed] [Google Scholar]
- 129.Prabhakar H, Arora R, Bithal PK, Rath GP, Dash HH. The analgesic effect of preemptive gabapentin in patients undergoing surgery for brachial plexus injury—A preliminary study. J Neurosurg Anesthesiol. 2007;19(4):235–238. doi: 10.1097/ANA.0b013e3181271863. [DOI] [PubMed] [Google Scholar]
- 130.Takahashi H, Shimoyama N. A prospective open-label trial of gabapentin as an adjuvant analgesic with opioids for Japanese patients with neuropathic cancer pain. Int J Clin Oncol. 2010 doi: 10.1007/s10147-009-0009-1. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 131.Rajpal S, Gordon DB, Pellino TA, et al. Comparison of perioperative oral multimodal analgesia versus IV PCA for spine surgery. J Spinal Disor Tech. 2010;23(2):139–145. doi: 10.1097/BSD.0b013e3181cf07ee. [DOI] [PubMed] [Google Scholar]
- 132.Toth C. Substitution of gabapentin therapy with pregabalin therapy in neuropathic pain due to peripheral neuropathy. Pain Med. 2010 doi: 10.1111/j.1526-4637.2009.00796.x. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 133.Gatti A, Sabato AF, Occhioni R, Colini Baldeschi G, Reale C. Controlled-release oxycodone and pregabalin in the treatment of neuropathic pain: Results of a multicenter Italian study. Eur Neurol. 2009;61(3):129–137. doi: 10.1159/000186502. [DOI] [PubMed] [Google Scholar]
- 134.Krenzelok EP. The FDA Acetaminophen Advisory Committee Meeting—What is the future of acetaminophen in the United States? The perspective of a committee member. Clin Toxicol. 2009;47(8):784–789. doi: 10.1080/15563650903232345. [DOI] [PubMed] [Google Scholar]
- 135.Brune K, Hinz B, Otterness I. Aspirin and acetaminophen: should they be available over the counter? Curr Rheumatol Rep. 2009;11(1):36–40. doi: 10.1007/s11926-009-0006-4. [DOI] [PubMed] [Google Scholar]
- 136.Fosnocht D, Taylor JR, Caravati EM. Emergency department patient knowledge concerning acetaminophen (paracetamol) in over-the-counter and prescription analgesics. Emerg Med J. 2008;25(4):213–216. doi: 10.1136/emj.2007.053850. [DOI] [PubMed] [Google Scholar]