Abstract
In humans, sterol 27-hydroxylase (CYP27A1) deficiency leads to cholesterol deposition in tendons and vasculature. Thus, in addition to its role in bile acid synthesis, where it converts cholesterol to 27-hydroxycholesterol (27-OHC), CYP27A1 may also be atheroprotective. Cyp27A1-deficient (Cyp27A1−/−) mice were crossed with apolipoprotein E (apoE)-deficient mice. Cyp27A1+/+/apoE−/− [ApoE-knockout (KO)], Cyp27A1+/−/apoE−/− heterozygous (het), and Cyp27A1−/−/apoE−/− [double-knockout (DKO)] mice were challenged with a Western diet (WD) for 3 and 6 mo. ApoE-KO mice fed a chow diet or a WD were used as the control. The severity of atherosclerosis in DKO mice was reduced 10-fold. Compared with the control, the DKO mice had no 27-OHC, total plasma cholesterol and low-density lipoprotein and very low density lipoprotein (LDL/VLDL) concentrations were reduced 2-fold, and HDL was elevated 2-fold. Expression of hepatic CYP7A1, CYP3A, and CYP8B1 were 5- to 10-fold higher. 3-Hydroxy-3-methyl-glutaryl-CoA reductase (HMGR) activity increased 4-fold. Fecal cholesterol was increased. In contrast, het mice fed a WD developed accelerated atherosclerosis and severe skin lesions, possibly because of reduced reverse cholesterol transport due to diminished 27-OHC production. CYP27A1 activity is involved in the control of cholesterol homeostasis and development of atherosclerosis with a distinct gene dose-dependent effect.—Zurkinden, L., Solcà, C., Vögeli, I. A., Vogt, B., Ackermann, D., Erickson, S. K., Frey, F. J., Sviridov, D., Escher, G. Effect of Cyp27A1 gene dosage on atherosclerosis development in ApoE-knockout mice.
Keywords: 27-hydroxycholesterol, LDL/VLDL fraction, CYP3A11
Sterol 27-hydroxylase (CYP27A1) is a ubiquitously expressed mitochondrial enzyme belonging to the cytochrome P450 family. CYP27A1 catalyzes the hydroxylation of cholesterol at C-27 to form 27-hydroxycholesterol (27-OHC) and cholestenoic acid (1, 2). The role of CYP27A1 in bile acid synthesis in the liver is well established; it catalyzes the initial, rate-limiting step in the “alternative” bile acid synthetic pathway and the intermediate step in the classic bile acid synthetic pathway (3, 4).
In addition to its role in bile acid synthesis, CYP27A1 has been implicated in several other pathways, including reverse cholesterol transport. Cholesterol efflux from macrophages in vitro and in vivo is enhanced when CYP27A1 is overexpressed (5–7). The importance of CYP27A1 beyond bile acid synthesis is illustrated by the pathology associated with cerebrotendinous xanthomatosis (CTX), an autosomal recessive genetic disorder caused by loss-of-function mutations in the Cyp27A1 gene (2). The clinical manifestations of CTX include juvenile cataract; a variety of neurologic symptoms; tendon xanthomas; increased incidence of chronic diarrhea in children; and, in 50% of patients with CTX, increased susceptibility to development of atherosclerosis (8–10). Some CTX manifestations have been attributed to compensatory overexpression of CYP7A1, leading to accumulation of cholestanol in a variety of tissues (11).
The Cyp27A1−/− mouse phenotype is similar in some aspects to that of human CTX (12, 13); however, to date, these mice have not been reported to develop the skin xanthomas or severe neurologic deficits observed in humans. The Cyp27A1−/− mouse phenotype includes reduced absorption of dietary cholesterol (12), increased cholesterol synthesis (12, 14), and accumulation of cholestanol in the brain (15).
Accelerated atherosclerosis is observed in ∼50% of patients with CTX despite normal or slightly decreased levels of total and low-density lipoprotein (LDL) cholesterol (16). The effect of CYP27A1 deficiency on development of atherosclerosis in animal models of this disease has not been reported.
In this study, we investigated the effects of CYP27A1 deficiency on the development of atherosclerosis in the apolipoprotein E (apoE)-deficient mouse model, which develops accelerated atherosclerosis. Cyp27A1+/− mice were crossed with apoE−/− mice to generate Cyp27A1+/+/apoE−/− [ApoE-knockout (KO)], Cyp27A1+/−/apoE−/− heterozygous (het), and Cyp27A1−/−/apoE−/− [double-knockout (DKO)] mice that were fed with a regular chow diet (CD) or a Western diet (WD) for 3 to 6 mo. We found that the DKO mice developed significantly less atherosclerosis on both the CD and the WD than did the ApoE-KO mice. In contrast, the het mice developed enhanced atherosclerosis on the WD.
MATERIALS AND METHODS
Materials
The chemicals sulfatase (S9626), choloylglycine hydroxylase (C4018), 1-methyl-3-nitro-1-nitrosoguanidine (A7231), hematoxylin solution Gill no. 3 (GHS316), and eosin Y solution aqueous (HT110216) were from Sigma-Aldrich (St. Louis, MO, USA) and Merck (Darmstadt, Germany); oligonucleotides were from Microsynth (Balgach, Switzerland); the hexanucleotide mix was from Roche Diagnostics (Mannheim, Germany); and TaqMan probes were from Life Technologies-Applied Biosystems (Rotkreuz, Switzerland).
Animals
Animal experimentation was approved by the Ethics Committee for Animal Experiments of the Veterinary Administration of the Canton of Berne, Switzerland, and conformed to the rules of the Swiss Federal Act on Animal Protection and the U.S. National Institutes of Health (NIH) Guide for the Care and Use of Laboratory Animals.
The Berne CYP27A1-deficient mouse-breeding colony was derived from Cyp27A1+/− mice provided by S.K.E. from her colony at the University of California (San Francisco, CA, USA). ApoE-KO mice were obtained from Charles River Laboratories (Sulzfeld, Germany). All mice were on the C57BL/6 background.
The breeding strategy was as follows: Cyp27A1+/− mice were crossed with apoE-deficient mice to generate Cyp27A1+/−/apoE+/− mice. These animals were interbred to obtain heterozygous mice that were then bred to produce ApoE-KO, het, and DKO mice. Mice were maintained under a standard 12:12-h light–dark cycle and had free access to chow and water. The pups were genotyped at postnatal d 18. (For primers and PCR conditions, see ref. 14.) Because of their low weight, they were weaned at the age of 28 d. The male mice used in all the experiments were fed a regular CD (3432; Provimi Kliba AG, Kaiseraugst, Switzerland) or a WD containing 21% fat and 0.15% cholesterol (D-12079B; Provimi Kliba AG) for 3 or 6 mo. For urine and feces collection, the mice were placed in metabolic cages, and 24-h samples were collected. The mice were denied access to food overnight before they were euthanized by pentobarbital injection (300 mg/kg, pentobarbital sodium, USP; Abbott Laboratories, North Chicago, IL, USA). The animals were weighed and blood was collected into a tube containing 20–50 U heparin, centrifuged at 4°C for 15 min at 13,000 rpm, and stored at −20°C. Organs were removed, washed with PBS, and fixed in formalin at 4°C or frozen in liquid nitrogen and stored at −70°C. The heart and aorta were dissected, fixed in 4% paraformaldehyde for 24 h, transferred to PBS, and stored at 4°C.
Characterization of atherosclerotic lesions
For en face analysis, the aorta was opened longitudinally, stained with 5% Sudan IV (Carl Roth, Karlsruhe, Germany), and pinned onto a white Styrofoam surface for imaging.
Lesions of the aortic root were analyzed in paraffin-embedded sections of 10 μm at 20 μm intervals for 700 μm and stained with hematoxylin and eosin (H&E).
Lesion areas were quantified in a blinded manner by image analysis software (ImageJ; NIH, Bethesda, MD, USA).
Biochemical analysis of plasma, liver, bile, and feces
Glucose, alanine aminotransferase (ALT) and aspartate aminotransferase (AST) were measured in plasma, by using an electrochemiluminescence immunoassay from Roche Diagnostics (Zug, Switzerland). Plasma and biliary cholesterol, plasma triglycerides (TGs), LDL/very low density lipoprotein (VLDL), and high-density lipoprotein (HDL) were quantified with a kit from Wako Chemicals GmbH (Neuss, Germany). In liver homogenates, TGs were measured using a TG quantification kit (BioVision, Mountain View, CA, USA), and cholesterol and cholesteryl esters (CEs) were assayed with a CE quantification kit (Calbiochem-Merck Millipore, Zug, Switzerland). In feces, the cholesterol and TGs were quantified as in the liver homogenates.
27-OHC was quantified by GC-MS, as described by Burkard et al. (17), except that 100 ng 5α-cholestan-3β,6α-diol was used as the extraction standard and 100 ng stigmasterol as the derivation standard.
Quantification of bile acids in plasma, bile, liver, urine, and feces
Bile acids were quantified in 200 μl plasma, 1 μl bile, 100 mg liver tissue, 200 μl urine, and 250 mg feces in the presence of 400 ng 23-nordeoxycholic acid as internal standard. Bile acids from urine and bile were extracted with Sep-Pack (Waters Corp., Milford, MA, USA) as already described (18). Bile acids from feces were extracted with 10 ml butanol:water (1:1 vol:vol) overnight and centrifuged for 10 min at 3000 rpm, whereas those from powdered liver and plasma were extracted successively with 2 ml ethanol at 100%, 2 ml ethanol at 80%, and 2 ml methanol at 100°C, as described by Locket and Gallaher (19). For plasma, liver, and feces, after extraction, the supernatant was transferred into a fresh tube, evaporated, resuspended in 5 ml water, and extracted on a Sep-Pak C18 cartridge. For all the samples, the subsequent solvolysis, hydrolysis, and derivatization were performed according to the same procedure as the one used for the urine (18).
RNA extraction and real-time PCR in liver and intestine
RNA extraction and real-time PCR in liver, ileal, and jejunal homogenates were conducted according to standard procedures. Reverse transcription was performed with 2 μg RNA in a reaction containing 100 U SuperScript Reverse Transcriptase type II (Roche, Basel, Switzerland). Real-time PCR was performed in triplicate with 100 ng cDNA/reaction. The following primers and probes were used: CYP3A11 (Mm00731567_m1), 3-hydroxy-3-methyl-glutaryl-CoA reductase (HMGR; hmgr; Mm01282499_m1), pregnane X receptor (PXR; Nr1i; Mm01344139_m1), fatty acid synthase (FAS; Fasn; Mm00662319_m1), sterol regulatory element binding protein 2 (SREBP2; Srebf2; Mm01306292_m1), diacylglycerol acyltransferase 1 (DGAT1; Dgat1; Mm00515643_m1), CYP7A1 (Mm00484152_m1), CYP27A1 (Mm00470430_m1), CYP8B1(Mm00501637_s1), liver X receptor α (LXRα; Mm00443451_m1), farnesoid X receptor (FXR; Nr1h4; (Mm00436419_m1), SR-B1 (Mm00450234_m1), LDL receptor (LDLR; Mm01177349_m1), ATP-binding cassette transporter A1 (ABCA1; Mm00442646_m1), ABCG5 (Mm00446241_m1), ABCG8 (Mm00445970_m1), SREBP-1c (Srebf-1; Mm00550338_m1), SREBP2 (Mm01306292_m1), small heterodimer partner (SHP; Nr0b2; Mm00442278_m1), Bsep (Abcb11; Mm00445168_m1), fibroblast growth factor receptor 4 (FGFR4; Mm01341852_m1), and fibroblast growth factor 15 (FGF15; Mm00433278_m1). β-Actin (AM1720) was used as the internal standard.
Quantification was performed by the relative quantification method with ApoE-KO mice fed the CD as the calibrator.
Immunohistochemistry
Macrophages were stained with purified rat anti-mouse MAC 3-antibodies (BD Biosciences, Franklin Lakes, NJ, USA) in 5–7 paraffin-embedded sections of aorta per animal and quantified with Image J.
Enzymatic assays
Hepatic microsomes were prepared from individual frozen livers, CYP7A1 activity was measured using the conversion of cholesterol to 7α-hydroxycholesterol, and HMGR activity was assessed by quantifying the formation of mevalonate from HMGCoA, as described elsewhere (20). All assays were performed in triplicate.
Statistical analysis
To determine statistically significant differences, 1- or 2-way ANOVA was used, followed by Bonferroni or Dunn's post hoc test for multiple comparisons.
RESULTS
Effect of Cyp27A1 gene dosage and WD feeding on body and organ weights
To investigate the importance of CYP27A1 expression in proatherosclerotic conditions, Cyp27A1−/− mice were crossed with apoE−/− mice, and the resulting ApoE-KO, het and DKO offspring were challenged with WD. As expected, body and liver weights increased in response to WD in the ApoE-KO mice (Table 1). DKO mice were smaller than their ApoE-KO littermates and failed to gain weight with WD. The hepatomegaly observed in the Cyp27A1−/− mice (12) was also observed in DKO mice, but did not deteriorate when they consumed the WD (Table 1). On the ApoE-KO background, the amount of steatosis was of the same magnitude (S1). Het mice underwent changes similar to those of the ApoE-KO after 3 mo on the WD; however, in those mice, longer periods on WD led to the development of severe skin lesions, necessitating discontinuation of the experiment. The weights of spleen, lungs, kidney, adrenal glands, testis, and brain were similar for all genotypes and were unaffected by diet (data not shown).
Table 1.
Effect of CYP27A1 expression on body and liver weight in ApoE-KO, het, and DKO mice fed a CD or WD for 3 or 6 mo
| Parameter | ApoE-KO |
Het |
DKO |
P | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CD |
WD |
CD |
WD |
CD |
WD |
||||||||
| 3 mo | 6 mo | 3 mo | 6 mo | 3 mo | 6 mo | 3 mo | 6 mo | 3 mo | 6 mo | 3 mo | 6 mo | ||
| Body weight (g) | 30 ± 2 | 32 ± 1 | 32 ± 3 | 37 ± 1*,# | 29 ± 2 | 32 ± 5 | 32 ± 3* | N.D. | 28 ± 2@ | 29 ± 1 | 31 ± 2** | 27 ± 2@@@,**,## | <0.0001 |
| Liver weight (g) | 1.4 ± 0.2 | 1.4 ± 0.2 | 1.5 ± 0.3 | 2.5 ± 0.4***,### | 1.5 ± 0.2 | 1.5 ± 0.3 | 1.8 ± 0.3* | N.D. | 2.3 ± 0.2@@@ | 2.3 ± 0.4@ | 2.3 ± 0.5@@@ | 2.0 ± 0.2 | <0.0001 |
| Liver/body weight | 4.6 ± 0.7 | 4.4 ± 0.5 | 4.6 ± 0.8 | 6.9 ± 1.1**,### | 5.1 ± 0.6 | 4.7 ± 0.2 | 5.6 ± 0.7 | N.D. | 8.1 ± 0.7@@@ | 7.9 ± 1.3@@ | 7.5 ± 1.6@@@ | 7.6 ± 0.4 | <0.0001 |
Results are presented as means ± sd (n=4–14).
P < 0.01,
P < 0.001,
P < 0.0001 vs. CD;
P < 0.01,
P < 0.001,
P < 0.0001 vs. 3 mo;
P < 0.01,
P < 0.001,
P < 0.0001 vs. ApoE-KO.
Effect of Cyp27A1 genotype on the development of atherosclerosis
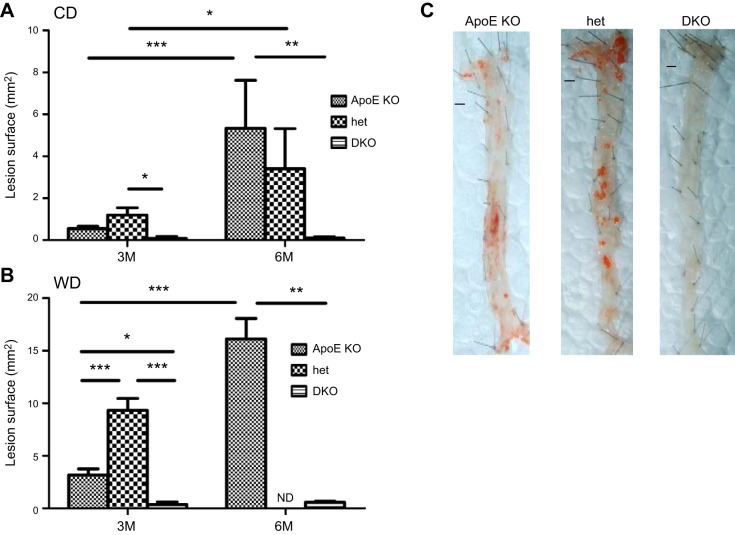
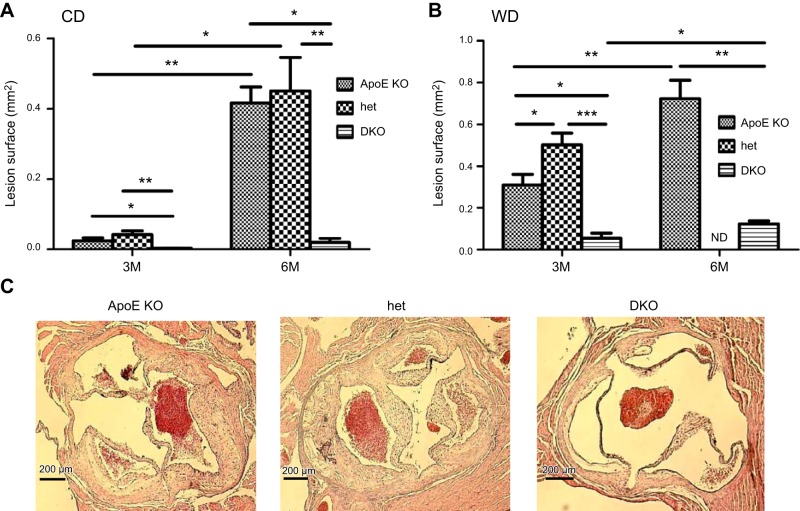
The analysis of atherosclerotic plaque development was assessed by both en face analysis (Fig. 1) and analysis of atherosclerotic lesions in sections of aortic root (Fig. 2). Cyp27A1−/− mice on the apoE+/+ background did not develop atherosclerosis, when on either the CD or the WD (Supplemental Fig. S2). After 3 mo on the CD, the ApoE-KO and het mice developed atherosclerotic lesions, as observed both in en face analysis (Fig. 1A) and on sections of aortic root (Fig. 2A). In contrast, the DKO mice had very little atherosclerosis. The amount of atherosclerotic plaque was up to 6- and 15-fold lower than that in their ApoE-KO littermates when assessed en face and in aortic root sections (Figs. 1A and 2A). Extension of the length of the dietary regimen to 6 mo in these animals did not increase atherosclerosis formation, whereas extended plaque formation was observed in the ApoE-KO and het mice (Figs. 1A and 2A).
Figure 1.
Effect of CYP27A1 deficiency on the development of atherosclerosis: en face analysis. ApoE-KO, het, and DKO mice were fed for 3 or 6 mo with a CD or WD before they were euthanized, and aorta was analyzed en face. A, B) Quantification of atherosclerotic lesions after 3 or 6 mo of CD (A) or WD (B). Data are means ± sem (n=4–6). *P < 0.05, **P < 0.01, ***P < 0.001. C) Representative en face Sudan IV staining of sections of aortas of mice fed the WD for 3 mo. Scale bars = 1 mm. ND, not done.
Figure 2.
Effect of CYP27A1 deficiency on the development of atherosclerosis: analysis of aortic root sections. ApoE-KO, het, and DKO mice were fed for 3 or 6 mo with a CD or WD before they were euthanized. Atherosclerotic lesions in sections of aortic root were analyzed as described in Materials and Methods. A, B) Quantification of atherosclerotic lesions after 3 or 6 mo of CD (A) or WD (B).*P < 0.05, **P < 0.01, ***P < 0.001. Data are means ± sem (n=5–7). C) Representative photomicrographs of aortic root in paraffin-embedded sections stained with H&E in mice fed the WD for 3 mo. Scale bars = 200 μm. ND, not done.
As expected, feeding the ApoE-KO mice WD for 3 and 6 mo significantly accelerated the development of atherosclerosis (Figs. 1B and 2B). Atherosclerotic plaque in these mice increased on average 3-fold after 3 mo on the WD (Figs. 1A, B and 2A, B) and 2–3-fold when compared to animals fed the CD (Figs. 1B and 2B). However, feeding DKO mice the WD did not result in significant changes in development of atherosclerosis, even after 6 mo (compare Figs. 1A, B and 2A, B). A massive increase in atherosclerosis was observed in the het mice when compared to the ApoE-KO mice, both in en face analysis (P<0.001; Fig. 1B) and in sections of the aortic root (P<0.05; Fig. 2B). These findings are illustrated by micrographs showing aortas of all 3 genotypes after 3 mo of WD stained with Sudan IV (Fig. 1C) and aortic root sections stained with H&E (Fig. 2C).
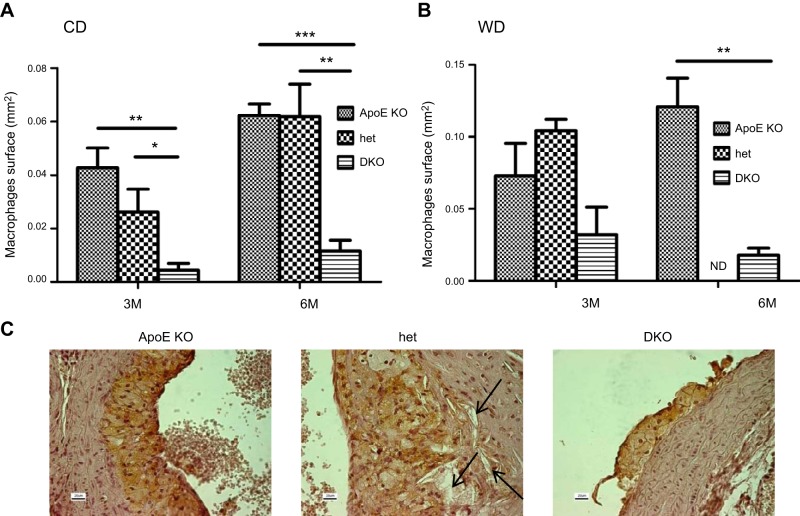
The histologic analysis of plaque composition revealed a pronounced accumulation of macrophages in the aortic root of the ApoE-KO and het mice fed CD for 3 or 6 mo when compared to those in the DKO mice (Fig. 3A). The effect was enhanced by feeding mice the WD (Fig. 3A, B). The presence of cholesterol clefts and fibrosis was also more pronounced in the het than in the ApoE-KO mice, whereas little evidence of these 2 components of atherosclerotic lesions was seen in the DKO aortas (Fig. 3C).
Figure 3.
Effect of CYP27A1 deficiency on macrophage accumulation in atherosclerotic lesions. ApoE-KO, het, and DKO mice were fed for 3 to 6 mo with a CD or WD. Macrophages were stained with anti- MAC-1 antibody. A, B) Quantification of abundance of macrophages in atherosclerotic lesions after 3 or 6 mo of CD (A) or WD (B). *P < 0.05, **P < 0.01, ***P < 0.001. Data are means ± sem (n=4). C) Representative photomicrographs of aortic root in paraffin-embedded sections stained with anti-MAC-1 antibody in mice fed the WD for 3 mo. Arrows indicate the cholesterol cleft. Scale bars = 20 μm. ND, not done.
Effect of CYP27A1 expression on plasma, liver, bile, feces, and urine composition
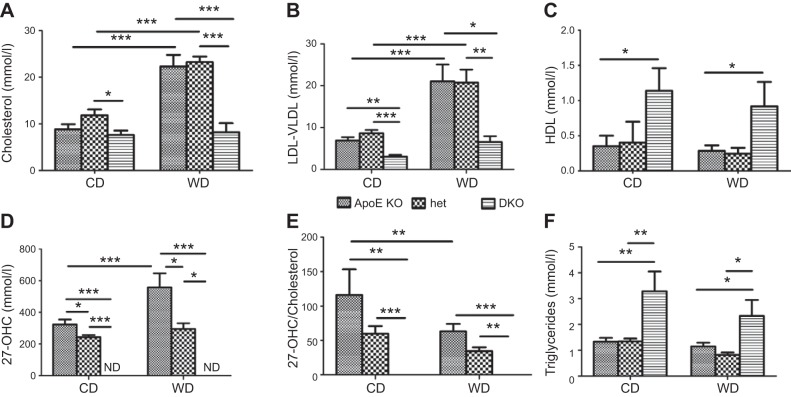
To explain the differences in the atherosclerotic phenotype observed in the different groups of mice, plasma composition was analyzed at 3 mo. In mice fed the CD, plasma total cholesterol (TC) concentrations were similar in the ApoE-KO and het mice and slightly lower in the DKO mice (Fig. 4A). TC more than doubled when the ApoE-KO and het mice were fed the WD. In contrast, the WD had no effect on TC in the DKO mice, in which it was almost 3-fold lower compared to that in the ApoE-KO and het mice (Fig. 4A). Regardless of diet, LDL/VLDL in the DKO mice was 2- to 3-fold lower than that in both the het and ApoE-KO mice (Fig. 4B). HDL was 2- to 3-fold higher in the DKO mice on both diets (Fig. 4C).
Figure 4.
Effect of CYP27A1 expression and diet on plasma lipids and CYP27A1 activity. ApoE-KO, het, and DKO mice were fed for 3 mo with a CD or WD. Cholesterol (A), LDL/VLDL (B), HDL (C), 27-OHC (D), CYP27A1 activity measured as 27-OHC/cholesterol ratio (E), and TGs (F) were quantified in plasma of mice after overnight food withdrawal. Data are means ± sem (n=5–10). ND, not detectable (<1 ng/ml). *P < 0.05, **P < 0.01, ***P < 0.001.
As expected, plasma 27-OHC was not detectable in the DKO mice. It was reduced by 50% in het when compared to that in the ApoE-KO mice (Fig. 4D). Feeding WD increased 27-OHC plasma concentration significantly in the ApoE-KO, but not in the het mice (Fig. 4D).
To assess apparent CYP27A1 activity, we calculated the 27-OHC/TC ratio. This ratio was reduced by approximately half in the het mice on both diets when compared to that in the ApoE-KO mice, indicating that CYP27A1 activity was reduced. WD led to a further 50% decrease in the ratio (Fig. 4E). As expected, no 27-OHC was detectable in the plasma of the DKO mice; the calculated 27-OHC/TC ratio was 0, indicating suppression of CYP27A1 activity.
Plasma TG concentration was higher in the DKO mice on both diets when compared to that in the het and ApoE-KO mice (Fig. 4F). WD did not have a significant effect on TG concentrations in any mouse genotype (Fig. 4D).
Levels of liver transaminase ALT were not significantly affected either by genotype or by diet (Table 2). AST was higher in the DKO mice and decreased in all 3 genotypes after mice consumed the WD (Table 2). The ALT/AST ratio, an indicator of liver damage, remained unchanged (data not shown). The Cyp27A1 genotype had little influence on plasma glucose levels (Table 2). WD markedly increased plasma levels of bilirubin in the ApoE-KO and het mice, but not in the DKO mice (Table 2).
Table 2.
Effect of CYP27A1 expression on plasma, liver, and bile biochemistry and on bile acid concentration in ApoE-KO, het, and DKO mice fed a CD or WD for 3 mo
| Parameter | ApoE-KO |
Het |
DKO |
P | |||
|---|---|---|---|---|---|---|---|
| CD | WD | CD | WD | CD | WD | ||
| Plasma | |||||||
| ALT (U/L) | 57 ± 50 | 32 ± 12 | 59 ± 53 | 37 ± 25 | 88 ± 57 | 85 ± 40 | NS |
| AST (U/L) | 208 ± 103 | 66 ± 75* | 257 ± 171 | 123 ± 84* | 506 ± 374 | 325 ± 235## | <0.0002 |
| Glucose (mM) | 8.2 ± 3.6 | 8.4 ± 1.8 | 8.6 ± 1.9 | 9.1 ± 3.1 | 10.5 ± 4.4 | 8.9 ± 2.2 | NS |
| Bilirubin (μM) | 1.0 ± 1.6 | 24.8 ± 20** | 2.9 ± 1.6# | 21.8 ± 1.8** | <0.1 | 0.3 ± 1.0## | <0.0001 |
| Bile acids (μg/ml) | 9.8 ± 12.4 | 2.5 ± 1.0 | 6.3 ± 8.7 | 2.5 ± 1.8 | 4.4 ± 1.7 | 4.7 ± 2.4 | NS |
| Liver | |||||||
| TG (nmol/mg) | 39 ± 11 | 55 ± 23 | 57 ± 25 | 56 ± 23 | 78 ± 20# | 65 ± 21 | <0.02 |
| TC (mmol/mg) | 4.4 ± 0.7 | 5.6 ± 1.9 | 4.4 ± 0.6 | 5.9 ± 1.6 | 3.8 ± 0.5 | 4.2 ± 0.6 | <0.0189 |
| CE (μg/mg) | 0.9 ± 0.5 | 0.4 ± 0.2 | 0.5 ± 0.4 | 0.4 ± 0.2 | 0.3 ± 0.3 | 0.3 ± 0.2 | <0.0483 |
| Bile acids (μg/g) | 7.4 ± 3.7 | 45.9 ± 45.6 | 17.8 ± 10.9 | 26.7 ± 16.6 | 5.9 ± 3.6 | 6.2 ± 2.4* | <0.0045 |
| Bile | |||||||
| TC (M) | 2.8 ± 1.2 | 4.0 ± 2.5 | 3.2 ± 0.7 | 4.0 ± 1.9 | 2.0 ± 0.7 | 1.8 ± 0.9 | NS |
| Bile acids (μg/μl) | 1.6 ± 1.3 | 1.3 ± 1.4 | 1.8 ± 2.3 | 0.6 ± 0.4 | 0.3 ± 0.0.5## | 0.3 ± 0.02## | <0.0008 |
| Urine bile acids (μg/μl) | 18.7 ± 11.2 | 11.0 ± 18.0 | 10.4 ± 10.3 | 14.2 ± 7.2 | 8.2 ± 7.7 | 1.0 ± 0.5## | <0.0001 |
Results are presented as means ± sd (n=4–14).
P < 0.01,
P < 0.001 vs. CD;
P < 0.01,
P < 0.001 vs. ApoE-KO.
In the liver, TGs were higher in DKO mice fed the CD, compared with their ApoE-KO and het littermates, but differences decreased when the mice were fed the WD (Table 2). Liver and biliary cholesterol had a tendency to increase with the WD in the ApoE-KO and het mice, but not in the DKO mice (Table 2). Liver CEs were lower in the DKO mice, irrespective of diet (Table 2).
To rule out cholestasis and gain more understanding in lipid reabsorption, we quantified bile acids in plasma, liver, bile, feces, and urine. There were no significant changes in plasma bile acid concentrations (Table 2). WD led to an increase in hepatic bile acid concentrations in the ApoE-KO and het mice, but not in the DKO mice, which produced significantly less bile acids (Table 2). Bile acid concentration was significantly reduced in bile, urine, and feces of the DKO mice fed the WD and in bile and feces in the mice fed the CD (Table 2 and Fig. 5).
Figure 5.
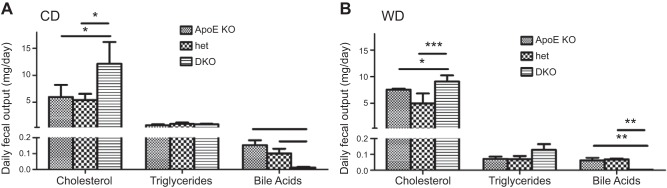
Effect of CYP27A1 expression on fecal lipid content. Daily excretion of cholesterol, TGs, and bile acids was measured in feces collected from ApoE-KO, het, and DKO mice placed for 24 h in metabolic cages after 3 mo of CD (A) or WD (B). Results are presented as means ± sem (n=5). *P < 0.01, **P < 0.001, ***P < 0.0001.
Next, to explain the absence of the WD-induced hypercholesterolemia observed in the DKO mice, we determined fecal cholesterol, TGs, and bile acids, to assess lipid absorption. The daily fecal output of cholesterol was markedly increased in the DKO mice on both diets (Fig. 5). The amount of cholesterol excreted, however, was of the same magnitude for both diets in all 3 genotypes. Triglyceride excretion was reduced in animals fed the WD (P<0.0001) but of the same magnitude in the 3 groups of mice. Bile acid excretion increased considerably when mice of all genotypes were moved from the CD to the WD (P<0.0001). As expected for the genotype, bile acid excretion was significantly lower in the DKO mice that in their ApoE-KO and het littermates on both diets. The effects on urinary excretion of bile acids paralleled those for fecal excretion (Table 2).
Effect of the Cyp27A1 genotype on expression of liver and intestinal genes
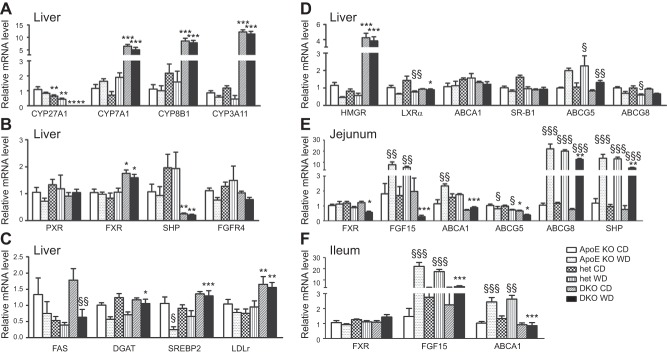
First, we assessed the effect of the Cyp27A1 genotype on the expression of genes involved in bile acid metabolism and detoxification. As expected, CYP27A1 mRNA in the liver was undetectable in the DKO mice and was reduced by ∼50% in the het mice when compared to that in the ApoE-KO mice (Fig. 6A). Liver levels of mRNA of CYP7A1, encoding the enzyme catalyzing the first step in the classic bile acid biosynthesis pathway, increased 6-fold in the DKO mice and was similar in the het mice when compared to the control group (Fig. 6A; ref. 14). CYP7A1 activity increased by 14-fold on the CD and 24-fold in the DKO mice on the WD. It did not change in the het mice when compared to the ApoE-KO mice (Fig. 7A). Effects on mRNA levels for CYP8B1 mRNA, a key determinant of cholic acid production, showed that CYP8B1 increased 8-fold in the DKO mice on both diets when compared to the ApoE-KO mice (Fig. 6A).
Figure 6.
Genotype- and diet-induced effects on expression of genes involved in lipid metabolism in liver and intestine. A–D) Using β-actin as internal standard, expression of cytochromes (A), nuclear receptors (B), regulators of cholesterol and TG biosynthesis (C), and reverse cholesterol transport (D) was assessed in livers of ApoE-KO, het, and DKO mice fed for 3 mo with CD or WD. E, F) Similarly, gene expression of enzymes involved in bile acid and lipid metabolism in the jejunum (E) and ileum (F) was investigated. Results are presented as means ± sem (n=5–14). *P < 0.01, **P < 0.001, ***P < 0.0001 vs. ApoE-KO; §P < 0.01, §§P < 0.001, §§§P < 0.0001 vs. CD.
Figure 7.
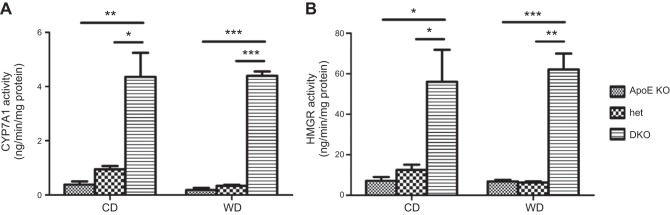
Effect of diet and CYP27A1 genotype on CYP7A1 and HMGR activities in the liver. Conversion of cholesterol to 7α-cholesterol (A) and HMGCo-A to mevalonic acid (B) was measured in liver microsomes of ApoE-KO, het, and DKO mice fed for 3 mo with CD or WD as described in Materials and Methods. Results are presented as means ± sd of triplicate determinations (n=3 animals/condition). *P < 0.05, **P < 0.01, ***P < 0.001.
Next, we examined the effect of the Cyp27A1 genotype on the expression of CYP3A11. CYP3A11 mRNA levels were increased 14- and 20-fold in the liver of the DKO mice fed the CD or WD, respectively, compared with levels in the ApoE-KO mice. CYP3A11 expression in the het mice was similar to that in the ApoE-KO mice (Fig. 6A). The mRNA level of PXR, a regulator of CYP3A11 expression, was affected by neither genotype nor diet (Fig. 6B). Given the increased CYP7A1 and CYP8B1 expression found in liver tissues, we analyzed the expression of FXR. FXR reduces the conversion of cholesterol to bile acids by down-regulating the expression of CYP7A1 and CYP8B1. FXR expression was increased in the livers of the DKO mouse fed both diets but not in the het livers (Fig. 6B). Expression of Bsep, an FXR target gene, was unaffected by genotype and diet (data not shown). Expression of Shp, another FXR target gene, was lower in the DKO mice than in the ApoE-KO and het mice (Fig. 6B). Finally, we investigated the enterohepatic pathway involving FGF15 signaling via FGFR4. No change in mRNA expression of FGFR4 was observed in the liver (Fig. 6B), but FGF15 mRNA was significantly increased in the jejunum and ileum of the ApoE-KO and het mice on the WD (Fig. 6E, F).
Next, we examined the effect of the Cyp27A1 genotype on the expression of genes involved in cholesterol efflux. In the liver, levels of LXRα mRNA were decreased in the het mice on the WD diet, compared with those in the ApoE-KO mice, but were similar in mice on the CD (Fig. 6D). Expression of ABCA1 and SR-B1 was unaffected by Cyp27A1 genotype or diet (Fig. 6D). ABCG5 mRNA expression was induced by the WD, but remained significantly lower in the DKO mice. In contrast, the mRNA expression levels of ABCG8 were reduced in mice fed the WD, but no difference was seen among the genotypes. In the jejunum, ABCG5 and ABCG8 expression was diminished in the DKO mice on both diets when compared to expression in the ApoE-KO mice (Fig. 6E), suggesting decreased cholesterol absorption. Similarly, unlike in the ApoE-KO and het mice fed the WD, FGF15 mRNA expression decreased, in both the jejunum and the ileum (Fig. 6E, F).
Further, we investigated the effect of the Cyp27A1 genotype on the expression of genes involved in cholesterol biosynthesis. Expression of SREPB2 was higher in the DKO mice than in the ApoE-KO and het mice; the differences were accentuated in animals on the WD (Fig. 6C). The increased SREBP2 expression in DKO correlated with the increased levels of LDLR mRNA (Fig. 6C), suggesting subsequent increased LDL cholesterol uptake by the liver for catabolism by increased CYP7A1. Not only was there evidence of increased cholesterol uptake in the DKO mice, but, as suggested by the increased LDLR expression, cholesterol biosynthesis was also increased, as shown by the increased HMGR mRNA levels, which were 4-fold higher in the livers of the DKO mice fed the CD and 8-fold higher in those fed the WD, than in livers of their ApoE-KO littermates (Fig. 6D). In contrast, in the het mice, the expression level of HMGR was similar to that in the control group. HMGR activity in the DKO mice was 8- and 10-fold higher than that in the ApoE-KO mice on the CD or the WD (Fig. 7B). No difference in HMGR activity was found between the het and ApoE-KO mice on either diet.
Finally, we assessed the effect of the Cyp27A1 genotype on the expression of genes involved in fatty acid and TGs synthesis. WD reduced FAS mRNA levels in all genotypes (P<0.01; Fig. 6C). In mice fed the CD, FAS mRNA levels were significantly reduced in the het mice compared with those in the ApoE-KO or DKO mice (P<0.05; Fig. 6C). DGAT-1 mRNA was increased in the DKO mice on the WD (Fig. 6C).
DISCUSSION
In addition to its well-established role in bile acid synthesis in the liver, CYP27A1 participates in sterol metabolism in other tissues. Deficiency of CYP27A1 has been implicated in the pathogenesis of Alzheimer's disease (21), retinal abnormalities (22), neurologic disorders (23), and hypertension (24). Some, but not all, patients with CTX, a disease caused by a congenital defect in CYP27A1, have higher risk of atherosclerosis (16). CYP27A1 has a high degree of polymorphism (25), but association of this polymorphism with cardiovascular disease has not been reported.
To gain insight into the possible roles of CYP27A1 in atherosclerosis, we studied the effect of Cyp27A1 gene dosage in the apoE-deficient mouse model of atherosclerosis. The two main findings were that complete deficiency of CYP27A1 in the apoE-deficient background (DKO) mice was atheroprotective, whereas partial deficiency of CYP27A1 on this background in het mice accelerated the development of atherosclerosis.
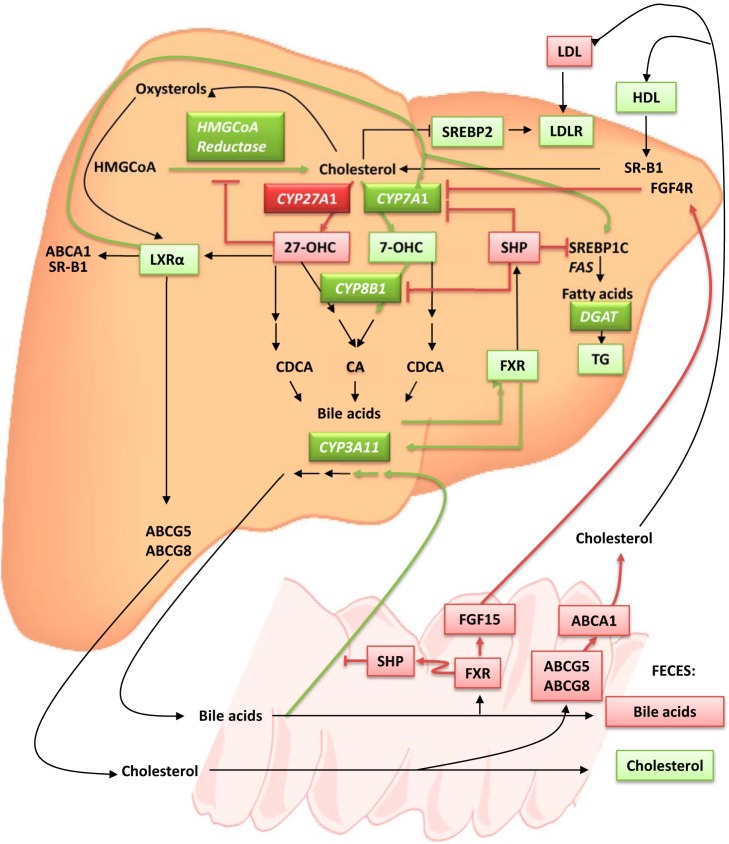
Possible explanations of the antiatherosclerotic effect in the DKO mice are shown in Fig. 8. DKO mice have markedly reduced levels of cholesterol and LDL/VLDL and increased HDL. Atherosclerosis in apoE-deficient mice, as in humans, is thought to be driven mainly by elevation of TC and VLDL/LDL; thus, reducing these levels would be expected to decrease atherosclerosis. The low plasma TC and VLDL/LDL in the DKO mice relative to the controls may be explained, at least in part, by the increased SREBP2 and LDLR mRNA levels, as also found in Cyp27A1−/− mice on an apoE+/+ background, suggesting that LDL uptake by the liver was enhanced. Second, fecal output of cholesterol was modestly increased, suggesting increased reverse cholesterol transport to the liver and decreased intestinal cholesterol reabsorption; this observation could also explain the absence of weight gain in animals fed the WD. Third, hepatic bile acid synthesis was relatively maintained in the DKO mice because of the induction of CYP7A1, CYP8B1, and CYP3A11. Finally, the reduced bile acid pool in the DKO mice may also contribute to decreased plasma LDL/VLDL, secondary to decreased cholesterol and fat absorption.
Figure 8.
Possible atheroprotective mechanisms in DKO mice fed a WD. Atherosclerosis is mainly driven by high circulating cholesterol concentrations, low HDL, and high LDL. In plasma of DKO mice fed the WD, cholesterol values were low, HDL increased, and LDL decreased when compared to levels in ApoE-KO mice. In the absence of 27-OHC in DKO mice, the negative feedback regulation of the rate-limiting enzyme of cholesterol biosynthesis HMGCoAR is suppressed, and cholesterol production is increased, but because of CYP7A1 and CYP8B1 up-regulation, cholesterol is metabolized. The low level of LDL can be attributed to the increase of SREBP2 in the liver, which increases LDLR expression and LDL uptake. 27-OHC is also a ligand for LXRα, which, on activation, stimulates ABCA1, ABCG5, ABCG8, SR-B1, CYP7A1, and SREBP1c. The LXRα activation induces TG biosynthesis and CYP7A1 activity, which increases bile acid biosynthesis. Bile acids are natural ligands for FXR, a mediator of SHP, which interferes with the activity of LXRα to induce SREBP1c and CYP7A1. FXR reduces bile acid toxicity in the liver by increasing CYP3A11. In DKO mice, SHP is down-regulated so that the inhibitory effect on SREBP1c and CYP7A1 is suppressed, and TGs and bile acid synthesis are increased. In the intestine, FXR promotes the release of FGF15 from the intestine to the liver. On binding to the hepatic receptor FGF4R, FGF15 inhibits CYP7A1 expression. In DKO mice fed the WD, FXR is down-regulated so that the release of FGF15 to the circulation followed by activation of FGF4R is reduced. As a consequence, CYP7A1 transcription is enhanced in the liver. The decreased expression of FXR leads also to a reduction in SHP inhibition of bile acid absorption, bile acid reabsorption is increased, and excretion is reduced. The reduced intestinal absorption attributed to reduced expression of ABCA1, ABCG5, and ABCG8 leads to an increase in cholesterol excretion in feces. Genes and molecules overexpressed are in green; those down-regulated are in red.
The impairment of the first step of the alternative bile acid biosynthesis pathway led, in our model, to a compensatory enhancement of the first step of the classic pathway, the conversion of cholesterol to 7α-hydroxycholesterol by CYP7A1; the same phenomenon has been observed in patients with CTX (26–28) and in CYP27A1-deficient mice (12, 14). In the present investigation, we have shown that CYP8B1 which catalyzes an intermediate step in the classic bile acid biosynthesis pathway is overexpressed, as well. Because the alternative bile acid biosynthesis pathway usually contributes to ∼25% of total bile acid output, the magnitude of activation of the classic bile acid synthesis pathway is consistent with overcompensation, with the overall rate of bile acid synthesis being enhanced, thus increasing the overall rate of cholesterol degradation and elimination.
Regulation of bile acid synthesis and transport involves, in part, the nuclear receptor FXR, which up-regulates the expression of SHP, which in turn inhibits CYP7A1, the key enzyme of the classic bile acid biosynthesis pathway, and CYP8B1, which catalyzes the formation of cholic acid from 7α-hydroxycholesterol (12–14). In Cyp27A1−/− mice SHP expression was reduced and CYP7A1 expression and activity were greatly increased (12, 27). In our experiments SHP was also reduced in DKO mice compared to ApoE-KO mice, despite increased expression of FXR, suggesting a reduced FXR activation, potentially secondary to the decreased bile acid pool in the liver and its altered composition. CYP7A1 up-regulation could also be explained as follows. In the intestine of DKO mice fed the WD, FXR and FGF15 expression was reduced. FGF15 is a ligand for the FGF4 receptor located in the liver, which, on activation, inhibits CYP7A1. With reduced FGF15 availability, the repression of CYP7A1 via FGFR4 activation is decreased. With the decreased negative feedback regulation of HMGCoR via 27-OHC, which is not synthesized in DKO, increased de novo cholesterol synthesis also contributed to up-regulation of CYP7A1 and CYP8B; de novo synthesized cholesterol is a preferred substrate for CYP7A1 (29). Finally, hepatic capacity, per se, may play a role in the observed atheroprotective effect in the DKO genotype. The percentage of liver/body weight doubled in the DKO vs. the ApoE-KO mice. Thus, our results indicate that the apoE background did not modify the CYP27A1 phenotype significantly but the CYP27A1 had a profound effect on ApoE-KO susceptibility to development of atherosclerosis.
The increased atherosclerosis in the het mice was unexpected. As for the Cyp27A1+/− mice (14), no effect was observed on cyp7A1 expression or activity in the Cyp27A1+/− on the ApoE-deficient background, resulting in no compensatory effect on hepatic cholesterol and biliary homeostasis. Cyp3A11 and Cyp8B1 were also unchanged. It is conceivable that under these circumstances, degradation and elimination of cholesterol are impaired; however, lack of changes in HMGR expression and activity argues against the expected elevation of plasma cholesterol content. Daily fecal output was of the same magnitude in the het mice as in the ApoE-KO. Based on the data, partial CYP27A1 deficiency either cannot override the apoE ko susceptibility to atherosclerosis, or it may actually enhance it. Of interest, decreased 27-OHC plasma concentration was the only parameter that changed significantly between the het and ApoE-KO mice. This may modulate vascular events such that susceptibility to atherosclerosis in the apoE ko background is intact or increased. CYP27A1 has been implicated in cholesterol efflux from macrophages via 2 different mechanisms: generation of 27-OHC, an nLXRα ligand, followed by stimulation of ABCA1 expression (5); and by changing plasma membrane configuration, allowing efflux of both cholesterol and the more hydrophilic cholesterol derivative 27-OHC (30). Reduced 27-OHC production may impair macrophage cholesterol efflux and contribute to atherosclerotic plaque formation.
The question of why the DKO mice were protected from the development of atherosclerosis, whereas some patients with CTX, despite nondetectable or very low CYP27A1 activity and generally normal to low levels of plasma cholesterol, show an acceleration of its development, can be partly explained by the apoE background. The CYP27A1 gene dose and/or differences in pathogenesis of atherosclerosis in mice and humans are some obvious possibilities. Honda et al. (31) have shown that patients with CTX and Cyp27A1−/− mice also differ in their ability to compensate for the increased production of cholestanol and bile alcohols induced by CYP27A1 deficiency. In Cyp27A1−/− mice, CYP27A1 deficiency was compensated for by induction of CYP3A11, an observation confirmed in our study, whereas induction of CYP3A4/A5 did not occur in the liver of the patients with CTX investigated (26).
The findings of this study may have important clinical implications. They suggest that the interplay of CYP27A1 deficiency with apoE deficiency may play a role in the development of atherosclerosis. Thus, it may be useful to screen patients with CTX for apoE to assess their susceptibility to development of atherosclerosis and other apoE genotype-related pathologies. The data also suggest that the presence of heterozygote CYP27A1 mutations may increase relative susceptibility to atherosclerosis. The current therapy for CTX consists of chenodeoxycholic acid, which inhibits CYP7A1 activity by preventing accumulation of bile alcohols and stanols and normalizes endogenous cholesterol biosynthesis (11). The outcome of symptoms and atherosclerosis development of a combined treatment of chenodeoxycholic acid with statin to reduce LDL levels is unknown (32). We now may anticipate that the effects of CYP27A1 deficiency on atherosclerosis depend on whether the deficiency is complete or partial and whether apoE is or is not deficient, providing an explanation for the finding that the risk of cardiovascular disease is enhanced in only some patients with CTX. Therefore, assessment of the degree of CYP27A1 activity left in patients with CTX and apoE should be taken into account in the therapeutic design.
The observation that the DKO mice on the WD were protected against weight gain could also have some potential clinical implications toward the development of new strategies in public health. Targeting bile acids could be beneficial for the treatment of obesity, provided that a dosage below cytotoxicity is used.
In summary, we demonstrated that homozygous CYP27A1 deficiency is atheroprotective in the apoE gene knockout model because of low levels of plasma TC and VLDL/LDL cholesterol, and elevated HDL—evidence of increased hepatic uptake of LDL via LDLR and a compensatory increases in cholesterol catabolism and cholesterol excretion. In contrast, heterozygous CYP27A1 deficiency in this model is proatherogenic, as it fails to activate the compensatory mechanisms and thus may decrease, not reverse, cholesterol transport, including efflux from lesional macrophages.
Supplementary Material
Acknowledgments
The authors thank Prof. Jürg Reichen for critical discussion of the manuscript and Ms. Beatrice Blanchard for technical help.
This work was supported by grants from the Foundation Banca del Ceresio (to C.S.) and Swiss National Foundation grant 310030-122133 (to G.E.). D.S. is a fellow of the National Health and Medical Research Council of Australia.
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
- 27-OHC
- 27-hydroxycholesterol
- ABC
- ATP-binding cassette transporter
- ALT
- alanine aminotransferase
- ApoE
- apolipoprotein E
- AST
- aspartate aminotransferase
- CD
- chow diet
- CE
- cholesteryl ester
- CTX
- cerebrotendinous xanthomatosis
- CYP27A1
- sterol 27-hydroxylase
- DGAT
- diacylglycerol acyltransferase
- DKO
- double-knockout
- FAS
- fatty acid synthase
- FGF
- fibroblast growth factor
- FGF
- fibroblast growth factor receptor
- FXR
- farnesoid X receptor
- H&E
- hematoxylin and eosin
- HDL
- high-density lipoprotein
- het
- cyp27A1+/−/apoE−/− heterozygous
- HMGR
- 3-hydroxy-3-methyl-glutaryl-CoA reductase
- KO
- knockout
- LDL
- low-density lipoprotein
- LDLR
- low-density lipoprotein receptor
- LXR
- liver X receptor
- PXR
- pregnane X receptor
- SHP
- small heterodimer partner
- SREBP
- sterol regulatory element binding protein
- TC
- total cholesterol
- TG
- triglyceride
- VLDL
- very low density lipoprotein
- WD
- Western diet
REFERENCES
- 1. Norlin M., von Bahr S., Bjorkhem I., Wikvall K. (2003) On the substrate specificity of human CYP27A1: implications for bile acid and cholestanol formation. J. Lipid Res. 44, 1515–1522 [DOI] [PubMed] [Google Scholar]
- 2. Cali J. J., Hsieh C. L., Francke U., Russell D. W. (1991) Mutations in the bile acid biosynthetic enzyme sterol 27-hydroxylase underlie cerebrotendinous xanthomatosis. J. Biol. Chem. 266, 7779–7783 [PMC free article] [PubMed] [Google Scholar]
- 3. Bjorkhem I. (1992) Mechanism of degradation of the steroid side chain in the formation of bile acids. J. Lipid Res. 33, 455–471 [PubMed] [Google Scholar]
- 4. Russell D. W., Setchell K. D. (1992) Bile acid biosynthesis. Biochemistry 31, 4737–4749 [DOI] [PubMed] [Google Scholar]
- 5. Escher G., Krozowski Z., Croft K. D., Sviridov D. (2003) Expression of sterol 27-hydroxylase (CYP27A1) enhances cholesterol efflux. J. Biol. Chem. 278, 11015–11019 [DOI] [PubMed] [Google Scholar]
- 6. Mukhamedova N., Escher G., D'Souza W., Tchoua U., Grant A., Krozowski Z., Bukrinsky M., Sviridov D. (2008) Enhancing apolipoprotein A-I-dependent cholesterol efflux elevates cholesterol export from macrophages in vivo. J. Lipid Res. 49, 2312–2322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Babiker A., Andersson O., Lund E., Xiu R. J., Deeb S., Reshef A., Leitersdorf E., Diczfalusy U., Bjorkhem I. (1997) Elimination of cholesterol in macrophages and endothelial cells by the sterol 27-hydroxylase mechanism: comparison with high density lipoprotein-mediated reverse cholesterol transport. J. Biol. Chem. 272, 26253–26261 [DOI] [PubMed] [Google Scholar]
- 8. Monson D. M., DeBarber A. E., Bock C. J., Anadiotis G., Merkens L. S., Steiner R. D., Stout A. U. (2011) Cerebrotendinous xanthomatosis: a treatable disease with juvenile cataracts as a presenting sign. Arch. Ophthalmol. 129, 1087–1088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rafiq M., Sharrack N., Shaw P. J., Hadjivassiliou M. (2011) A neurological rarity not to be missed: cerebrotendinous xanthomatosis. Pract. Neurol. 11, 296–300 [DOI] [PubMed] [Google Scholar]
- 10. Reichwaldt I., Zustin J., Wenke K., Ridderbusch I. (2010) Differential diagnosis of tendon tumors: xanthomas caused by hyperlipidemia in children. J. Pediatr. Surg. 45, e9–e12 [DOI] [PubMed] [Google Scholar]
- 11. Bjorkhem I., Hansson M. (2010) Cerebrotendinous xanthomatosis: an inborn error in bile acid synthesis with defined mutations but still a challenge. Biochem. Biophys. Res. Commun. 396, 46–49 [DOI] [PubMed] [Google Scholar]
- 12. Repa J. J., Lund E. G., Horton J. D., Leitersdorf E., Russell D. W., Dietschy J. M., Turley S. D. (2000) Disruption of the sterol 27-hydroxylase gene in mice results in hepatomegaly and hypertriglyceridemia: reversal by cholic acid feeding. J. Biol. Chem. 275, 39685–39692 [DOI] [PubMed] [Google Scholar]
- 13. Rosen H., Reshef A., Maeda N., Lippoldt A., Shpizen S., Triger L., Eggertsen G., Bjorkhem I., Leitersdorf E. (1998) Markedly reduced bile acid synthesis but maintained levels of cholesterol and vitamin D metabolites in mice with disrupted sterol 27-hydroxylase gene. J. Biol. Chem. 273, 14805–14812 [DOI] [PubMed] [Google Scholar]
- 14. Dubrac S., Lear S. R., Ananthanarayanan M., Balasubramaniyan N., Bollineni J., Shefer S., Hyogo H., Cohen D. E., Blanche P. J., Krauss R. M., Batta A. K., Salen G., Suchy F. J., Maeda N., Erickson S. K. (2005) Role of CYP27A in cholesterol and bile acid metabolism. J. Lipid Res. 46, 76–85 [DOI] [PubMed] [Google Scholar]
- 15. Bavner A., Shafaati M., Hansson M., Olin M., Shpitzen S., Meiner V., Leitersdorf E., Bjorkhem I. (2010) On the mechanism of accumulation of cholestanol in the brain of mice with a disruption of sterol 27-hydroxylase. J. Lipid Res. 51, 2722–2730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bjorkhem I. (1994) Inborn errors of metabolism with consequences for bile acid biosynthesis: a minireview. Scand. J. Gastroenterol. Suppl. 204, 68–72 [DOI] [PubMed] [Google Scholar]
- 17. Burkard I., Rentsch K. M., von Eckardstein A. (2004) Determination of 24S- and 27-hydroxycholesterol in plasma by high-performance liquid chromatography-mass spectrometry. J. Lipid Res. 45, 776–781 [DOI] [PubMed] [Google Scholar]
- 18. Quattropani C., Vogt B., Odermatt A., Dick B., Frey B. M., Frey F. J. (2001) Reduced activity of 11 beta-hydroxysteroid dehydrogenase in patients with cholestasis. J. Clin. Invest. 108, 1299–1305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Locket P. L., Gallaher D. D. (1989) An improved procedure for bile acid extraction and purification and tissue distribution in the rat. Lipids 24, 221–223 [DOI] [PubMed] [Google Scholar]
- 20. Erickson S. K., Lear S. R., Deane S., Dubrac S., Huling S. L., Nguyen L., Bollineni J. S., Shefer S., Hyogo H., Cohen D. E., Shneider B., Sehayek E., Ananthanarayanan M., Balasubramaniyan N., Suchy F. J., Batta A. K., Salen G. (2003) Hypercholesterolemia and changes in lipid and bile acid metabolism in male and female cyp7A1-deficient mice. J. Lipid Res. 44, 1001–1009 [DOI] [PubMed] [Google Scholar]
- 21. Brown J., III, Theisler C., Silberman S., Magnuson D., Gottardi-Littell N., Lee J. M., Yager D., Crowley J., Sambamurti K., Rahman M. M., Reiss A. B., Eckman C. B., Wolozin B. (2004) Differential expression of cholesterol hydroxylases in Alzheimer's disease. J. Biol. Chem. 279, 34674–34681 [DOI] [PubMed] [Google Scholar]
- 22. Omarova S., Charvet C. D., Reem R. E., Mast N., Zheng W., Huang S., Peachey N. S., Pikuleva I. A. (2012) Abnormal vascularization in mouse retina with dysregulated retinal cholesterol homeostasis. J. Clin. Invest. 122, 3012–3023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Diekstra F. P., Saris C. G., van Rheenen W., Franke L., Jansen R. C., van Es M. A., van Vught P. W., Blauw H. M., Groen E. J., Horvath S., Estrada K., Rivadeneira F., Hofman A., Uitterlinden A. G., Robberecht W., Andersen P. M., Melki J., Meininger V., Hardiman O., Landers J. E., Brown R. H., Jr., Shatunov A., Shaw C. E., Leigh P. N., Al-Chalabi A., Ophoff R. A., van den Berg L. H., Veldink J. H. (2012) Mapping of gene expression reveals CYP27A1 as a susceptibility gene for sporadic ALS. PLoS ONE 7, e35333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mateos L., Ismail M. A., Gil-Bea F. J., Schule R., Schols L., Heverin M., Folkesson R., Bjorkhem I., Cedazo-Minguez A. (2011) Side chain-oxidized oxysterols regulate the brain renin-angiotensin system through a liver X receptor-dependent mechanism. J. Biol. Chem. 286, 25574–25585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Dias V., Ribeiro V. (2011) Ethnic differences in the prevalence of polymorphisms in CYP7A1, CYP7B1 AND CYP27A1 enzymes involved in cholesterol metabolism. J. Pharm. Bioallied Sci. 3, 453–459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Goodwin B., Gauthier K. C., Umetani M., Watson M. A., Lochansky M. I., Collins J. L., Leitersdorf E., Mangelsdorf D. J., Kliewer S. A., Repa J. J. (2003) Identification of bile acid precursors as endogenous ligands for the nuclear xenobiotic pregnane X receptor. Proc. Natl. Acad. Sci. U. S. A. 100, 223–228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Draper N., Walker E. A., Bujalska I. J., Tomlinson J. W., Chalder S. M., Arlt W., Lavery G. G., Bedendo O., Ray D. W., Laing I., Malunowicz E., White P. C., Hewison M., Mason P. J., Connell J. M., Shackleton C. H., Stewart P. M. (2003) Mutations in the genes encoding 11beta-hydroxysteroid dehydrogenase type 1 and hexose-6-phosphate dehydrogenase interact to cause cortisone reductase deficiency. Nat. Genet. 34, 434–439 [DOI] [PubMed] [Google Scholar]
- 28. Honda A., Salen G., Matsuzaki Y., Batta A. K., Xu G., Hirayama T., Tint G. S., Doy M., Shefer S. (2005) Disrupted coordinate regulation of farnesoid X receptor target genes in a patient with cerebrotendinous xanthomatosis. J. Lipid Res. 46, 287–296 [DOI] [PubMed] [Google Scholar]
- 29. Stone B. G., Erickson S. K., Craig W. Y., Cooper A. D. (1985) Regulation of rat biliary cholesterol secretion by agents that alter intrahepatic cholesterol metabolism: evidence for a distinct biliary precursor pool. J. Clin. Invest. 76, 1773–1781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bjorkhem I., Andersson O., Diczfalusy U., Sevastik B., Xiu R. J., Duan C., Lund E. (1994) Atherosclerosis and sterol 27-hydroxylase: evidence for a role of this enzyme in elimination of cholesterol from human macrophages. Proc. Natl. Acad. Sci. U. S. A. 91, 8592–8596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Honda A., Salen G., Matsuzaki Y., Batta A. K., Xu G., Leitersdorf E., Tint G. S., Erickson S. K., Tanaka N., Shefer S. (2001) Side chain hydroxylations in bile acid biosynthesis catalyzed by CYP3A are markedly up-regulated in Cyp27−/− mice but not in cerebrotendinous xanthomatosis. J. Biol. Chem. 276, 34579–34585 [DOI] [PubMed] [Google Scholar]
- 32. Verrips A., Wevers R. A., Van Engelen B. G., Keyser A., Wolthers B. G., Barkhof F., Stalenhoef A., De Graaf R., Janssen-Zijlstra F., Van Spreeken A., Gabreels F. J. (1999) Effect of simvastatin in addition to chenodeoxycholic acid in patients with cerebrotendinous xanthomatosis. Metabolism 48, 233–238 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.