Abstract
Heterotopic ossification (HO) and fatty infiltration (FI) often occur in diseased skeletal muscle and have been previously described in various animal models of Duchenne muscular dystrophy (DMD); however, the pathological mechanisms remain largely unknown. Dystrophin-deficient mdx mice and dystrophin/utrophin double-knockout (dKO) mice are mouse models of DMD; however, mdx mice display a strong muscle regeneration capacity, while dKO mice exhibit a much more severe phenotype, which is similar to patients with DMD. Our results revealed that more extensive HO, but not FI, occurred in the skeletal muscle of dKO mice versus mdx mice, and RhoA activation specifically occurred at the sites of HO. Moreover, the gene expression of RhoA, BMPs, and several inflammatory factors were significantly up-regulated in muscle stem cells isolated from dKO mice; while inactivation of RhoA in the cells with RhoA/ROCK inhibitor Y-27632 led to reduced osteogenic potential and improved myogenic potential. Finally, inactivation of RhoA signaling in the dKO mice with Y-27632 improved muscle regeneration and reduced the expression of BMPs, inflammation, HO, and intramyocellular lipid accumulation in both skeletal and cardiac muscle. Our results revealed that RhoA represents a major molecular switch in the regulation of HO and muscle regeneration in dystrophic skeletal muscle of mice.—Mu, X., Usas, A., Tang, Y., Lu, A., Wang, B., Weiss, K., Huard, J. RhoA mediates defective stem cell function and heterotopic ossification in dystrophic muscle of mice.
Keywords: ROCK, mdx, utrophin−/−, intramyocellular lipid accumulation
Heterotopic ossification (HO) and/or fatty infiltration (FI) are two distinct histological processes that often occur in diseased muscle tissues. HO refers to the formation of bone in the soft tissues of the body and can occur as a result of trauma, surgery, neurological injury, or genetic abnormalities (1). FI has been reported to be associated with aging, inactivity, obesity, and various diseases, such as diabetes, and results in the accumulation of fat cells outside the typical fat stores (2–3). FI located within skeletal muscle is often the result of disordered lipid metabolism (3); however, abnormal lipid metabolism can also cause another type of abnormal lipid deposition in skeletal muscle, known as intramyocellular lipid accumulation (IMCL; refs. 4–5). Notably, IMCL in cardiac muscle, (intramyocardial lipid accumulation) can be caused by lipid overload, which has the potential to lead to lipotoxicity and progressive cardiac dysfunction (6–9). However, the pathological mechanisms regulating these distinct processes in diseased muscles remain largely unknown.
Duchenne muscular dystrophy (DMD) features progressive muscle degeneration and has no cure yet. Obesity occurs in >50% of patients wtih DMD after 14 yr of age, and a reduction in myocardial fatty acid metabolism has been observed in ∼50% of patients wtih DMD (10, 11). FI is commonly observed in the skeletal muscles of patients wtih DMD, and it is one of the main factors responsible for patients' decline in muscular strength (12). Lipid mapping analysis of the hearts and skeletal muscles of patients wtih DMD revealed IMCL within the most damaged areas of the dystrophic muscles (11, 13); however, there are very few studies on the mechanisms and prevention of IMCL in patients wtih DMD. Although less documented in human patients wtih DMD, the presence of HO has been reported in the skeletal muscles of various animal models of human DMD, including mdx mice and golden retriever muscular dystrophy (GRMD) dogs (14–16). In vitro studies with muscle stem cells showed that bone morphogenetic protein (BMPs) or adipogenic media can promote the differentiation of muscle stem cells into osteogenic and adipogenic cells, respectively (17), suggesting that muscle stem cells may represent a cell source of HO and/or FI in skeletal muscle.
The experiments described in this article were conducted using two animal models of human DMD, dystrophin-deficient (mdx) mice and dystrophin/utrophin double-knockout (dKO) mice (14, 18–20). Compared with mdx mice, which actually feature potent muscle regeneration capacity, the phenotype of dKO mice is more severe and more closely resembles the phenotype seen in patients wtih DMD (19–20). For example, dKO mice feature a much shorter life span (∼8 wk compared with ∼2 yr), more necrosis and fibrosis in their skeletal muscles, scoliosis/kyphosis of the spine, and severe cardiac involvement and eventual cardiac failure (14, 19, 20). The occurrence of FI and HO in the skeletal muscles of mdx mice has been previously described (15), and more extensive HO in dKO mice has also been recently reported by our group (21). IMCL, on the other hand, has not been studied in either mdx or dKO mice or in any other DMD animal models. It is also clear that the knowledge about the molecular regulation of HO, fatty infiltration, and IMCL in dystrophic muscle remains limited.
Inflammation is directly involved in the dystrophic process and represents an important therapeutic target to treat DMD. For example, corticosteroids are capable of repressing systematic inflammation and are the only known effective drugs that can provide relief of the symptoms of DMD (22). Inflammation has been identified as a main contributor of HO (23); hence, the administrations of various anti-inflammatory medications have been used to prevent HO (24–25). For example, Cox-2 inhibitors were found to be effective at preventing HO after total hip arthroplasty (THA) and following spinal cord injury (26–27). Although inflammation and FI often occur simultaneously in diseased or injured skeletal muscles, inflammation has not been directly linked to FI (28–29). On the other hand, it has been well established, in studies of diabetes and obesity, that there is a close association between the occurrence of IMCL and chronic systematic inflammation during the progression of cardiac disease (30, 31). Similarly, lipid peroxidation has been shown to activate nuclear factor-κB (NF-κB), and consequently, has contributed to the histopathological cascade observed in mdx muscles (32). Finally, inflammatory cytokines have been shown to inhibit myogenic differentiation through the activation of NF-κB (33–34), and the activation of NF-κB signaling in skeletal muscle has been correlated with muscular dystrophies and inflammatory myopathies (34, 35).
In the current study, we examined the role that RhoA signaling pathway plays in regulating HO, FI, and IMCL in these models of DMD (dKO and mdx mice), due to the fact that RhoA signaling has been shown to play an important role in regulating osteogenesis, adipogenesis, myogenesis, and inflammation. RhoA is a small G protein in the Rho family that regulates cell morphology and migration by reorganizing the actin cytoskeleton in response to extracellular signaling (36). The RhoA signaling pathway is involved in the commitment of mesenchymal stem cells (MSCs) toward their osteogenic or adipogenic differentiation (37). RhoA signaling activation in MSCs in vitro induces osteogenesis potential and inhibits adipogenic potential of the cells; however, the application of Y-27632, a specific inhibitor of RhoA/Rho kinase (ROCK), reverses this process (37–39). RhoA also mediates BMP-induced signaling in MSCs and promotes osteoblastic cell survival (40, 41). Moreover, the inhibition of RhoA with Y-27632 was found to induce the adipogenic differentiation of muscle-derived cells in vitro, and resulted in the manifestation of FI in skeletal muscle (42). RhoA is also activated by Wnt5a, which results in the induction of osteogenic differentiation of human adipose stem cells (ASCs) and the repression of adipogenic differentiation (43). RhoA's role in the inflammatory process has been previously described, where TNF-α induces the activation of RhoA signaling in smooth muscle cells (44), RhoA regulates Cox-2 activity in fibroblasts (45), and RhoA induces the expression of inflammatory cytokines in adipocytes (46). Moreover, involvement of RhoA in mediating myocardial and pulmonary fibrosis has been described (47–48). In addition, previous studies have indicated that the sustained activation of the RhoA pathway can block the differentiation of muscle cells by inhibiting myoblast fusion (49–51).
Because of RhoA's potential involvement in the regulation of osteogenesis, adipogenesis, and myogenesis of stem cells and inflammation, we hypothesized that RhoA may act as a critical regulator of these processes in dystrophic muscle. In the current study, we investigated the status of HO, FI, IMCL, and muscle regeneration in the skeletal muscle of mdx and dKO mice, as well as the potential role that RhoA signaling plays in regulating these processes.
MATERIALS AND METHODS
Animals
Wild-type (C57BL/10J) mice were obtained from the Jackson Laboratory (Bar Harbor, ME, USA). The mdx and dKO (mdx; utrn−/−) mice were derived from our in-house colony. Mice were housed in groups of 4 on a 12:12-h light-dark cycle at 20–23°C. At least 6 mice were used in each experimental sample group. All procedures were approved by the Institutional Animal Care and Use Committee (IACUC) at the University of Pittsburgh.
Stem cell isolation from skeletal muscle
Muscle-derived stem cells (MDSCs) were isolated from the skeletal muscle of dKO and mdx mice (4 wk old) via a modified preplate technique (52). Mice were sacrificed in a carbon dioxide chamber, as described in the IACUC protocol. Cells were cultured in proliferation medium [DMEM supplemented with 20% fetal bovine serum (FBS), 1% penicillin-streptomycin antibiotics, and 0.5% chicken embryo extract (CEE)].
Micro-computed tomography (micro-CT)
To observe HO in the soft tissues of mdx and dKO mice, 8-wk-old mice were anesthetized with 3% isoflurane in O2 gas (1.5 L/min), and the lower extremities, including the pelvis, were scanned using the Viva CT 40 (Scanco, Wangen-Brüttisellen, Switzerland) with the following settings: energy, 70 kVp; intensity, 114 μA; integration time, 200 ms; isotropic voxel size, 35 μm; threshold, 163.
In vitro RhoA inactivation with Y-27632 and multipotent differentiation assays
dKO MDSCs cultured in vitro were treated with the RhoA/Rock inhibitor Y-27632 (10 μM; EMD Millipore, Billerica, MA, USA) in proliferation medium for 2 d, before being plated in 12-well flasks and set up for osteogenesis, adipogenesis, or myogenesis assays. The osteogenesis assay was conducted with osteogenic medium (DMEM supplemented with 110 μg/ml sodium pyruvate, 584 μg/ml l-glutamine, 10% FBS, 1% penicillin/streptomycin, 10−7 μM dexamethasone, 50 μg/ml ascorbic-acid-2-phosphate, and 10−2 μM β-glycerophosphate), supplemented with BMP2 (50 ng/ml for 7 d). Calcium deposition was assessed with alizarin red stain. Adipogenesis assay was conducted with adipogenic induction medium (Lonza, Basel, Switzerland) for 10 d and tested for lipid droplets with Oil red O stain (Sigma, St. Louis, MO, USA). The myogenesis assay was conducted by switching the proliferation medium into myogenic differentiation medium (DMEM containing 2% horse serum). Myotube formation was tracked during the following 4 d. 10 μM of Y-27632 was continuously present in the differentiation medium.
mRNA analysis with reverse transcriptase-PCR
Total RNA was obtained from MDSCs or the skeletal muscles of mice using a RNeasy Mini Kit (Qiagen, Valencia, CA, USA), according to the manufacturer's instructions. Reverse transcription was performed using the iScript cDNA synthesis kit (Bio-Rad, Hercules, CA, USA). PCR reactions were performed using an iCycler Thermal Cycler (Bio-Rad). The cycling parameters used for all primers were as follows: 95°C for 10 min; PCR, 40 cycles of 30 s at 95°C for denaturation, 1 min at 54°C for annealing, and 30 s at 72°C for extension. Products were separated by size, and they were visualized on 1.5% agarose gels stained with ethidium bromide. All data were normalized to the expression of GAPDH. Genes and primers used in the study included GAPDH: TCCATGACAACTTTGGCATTG (sense) and TCACGCCACAGCTTTCCA (antisense); RhoA: GTAGAGTTGGCTTTATGGGACAC (sense), and TGGAGTCCATTTTTCTGGGATG (antisense); BMP2: TCTTCCGGGAACAGATACAGG (sense), and TGGTGTCCAATAGTCTGGTCA (antisense); BMP4: ATTCCTGGTAACCGAATGCTG (sense), and CCGGTCTCAGGTATCAAACTAGC (antisense); TNF-α: GATTATGGCTCAGGGTCCAA (sense), and CTCCCTTTGCAGAACTCAGG (antisense); IL6: GGAAATCGTGGAAATGAG (sense), and GCTTAGGCATAACGCACT (antisense); Klotho: CCCAAACCATCTATGAAAC (sense), and CTACCGTATTCTATGCCTTC (antisense); and peroxisome proliferator-activated receptor γ (PPARγ): CCACCAACTTCGGAATCAGCT (sense) and TTTGTGGATCCGGCAGTTAAGA (antisense).
In vivo RhoA inactivation with Y-27632
Intramuscular injections into the gastrocnemius muscles (GMs) of dKO mice were conducted with Y-27632 (5 mM in 30 μl of PBS solution; left limb) or control (30 μl of PBS; right limb), starting from 4 wk of age. Intramuscular injections were conducted 3×/wk for 4 wk. Differential HO formation in the skeletal muscle with or without Y-27632 treatment was assessed by micro-CT scan or alizarin red stain. Systematic inhibition of RhoA signaling was conducted by intraperitoneal injection of Y-27632 (5 mM in PBS, 10 mg/kg/mouse) or control (PBS only) into dKO mice from 3 wk of age. Intraperitoneal injections into dKO mice were conducted 3×/wk for 4 wk.
Histology
Cryostat sections (10 μm) were prepared using standard techniques from GMs of mice. HO in muscle tissue was assessed by alizarin red stain: tissue sections of skeletal muscle were fixed with 4% formalin (10 min) and rinsed with ddH2O; slides were then incubated with alizarin red working solution for 10 min before being washed with ddH2O. FI was detected by Oil red O stain: fixed tissue sections were rinsed with ddH2O and 60% isopropanol; slides were then incubated with Oil red O working solution for 15 min before being rinsed with 60% isopropanol and ddH2O. The IMCL was detected by AdipoRed assay reagent (Lonza): fixed tissue sections were rinsed with PBS and incubated with AdipoRed assay reagent for 15 min before being washed with PBS. For immunofluorescent staining of tissue sections, the sections were blocked with horse serum (10%) for 1 h, and the primary antibodies RhoA (Santa Cruz Biotechnology, Santa Cruz, CA, USA), CD68 (Abcam, Cambridge, MA, USA), or MyoD (Santa Cruz Biotechnology) were applied at 1:100–1:200. Negative controls were performed concurrently with all immunohistochemical staining. Necrosis with damaged myofibers in muscle was assayed by incubating with biotinylated anti-mouse IgG (1:300; Vector Laboratory, Burlingame, CA, USA) for 1 h at room temperature, which was followed by a 15-min incubation with streptavidin Cy3 conjugate (1:500; Sigma-Aldrich). All incubations were performed at room temperature. All slides were analyzed using fluorescent microscopy (Leica Microsystemic, Buffalo Grove, IL, USA) and photographed at ×4–40 view.
Measurement of results and statistical analysis
The measurement of results from images was performed using commercially available software (Northern Eclipse 6.0; Empix Imaging, Mississauga, ON, Canada) and ImageJ 1.32j (U.S. National Institutes of Health, Bethesda, MD, USA). Data from ≥3 samples from each subject were pooled for statistical analysis. Results are given as means ± sd. Statistical significance of any difference was calculated using Student's t test. Values of P < 0.05 were considered statistically significant.
RESULTS
Extensive intramuscular HO and IMCL occurred in the skeletal muscle of dKO mice
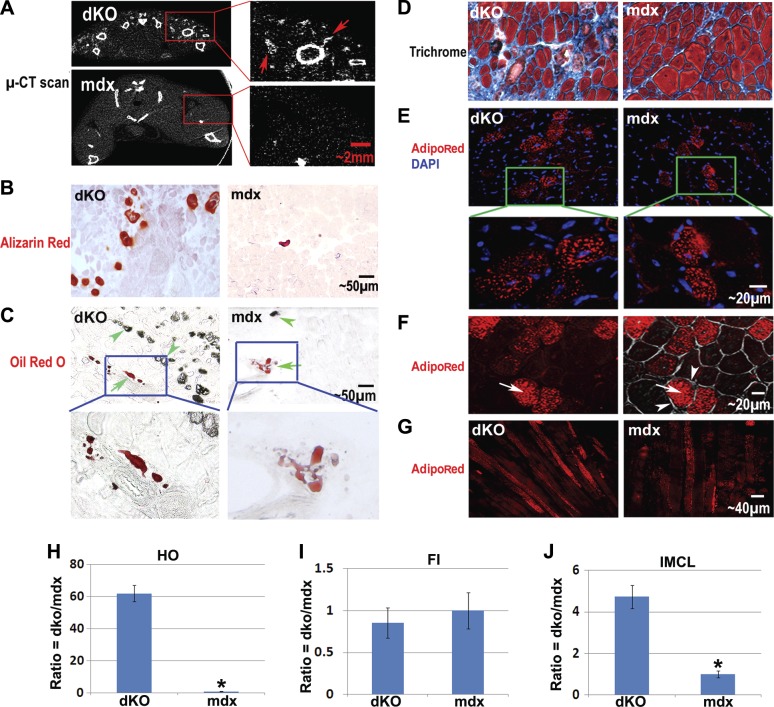
Micro-CT scan and alizarin red staining revealed extensive HO in the hind-limb muscles (i.e., GMs) of dKO mice at 8 wk of age (Fig. 1A, B), while in age-matched mdx mice, only mild HO was observed (Fig. 1A, B). Oil red O stain revealed mild intramuscular FI in the GMs of both 8-wk-old mdx and dKO mice (Fig. 1C). Notably, the FI/HO ratio was much lower in the dKO mice, and the sites of intramuscular HO and FI did not colocalize (Fig. 1C and Supplemental Fig. S1A). The fact that HO and FI never colocalized in the muscle suggests that these two processes are mutually exclusive and could implicate different cell types and/or niches involved in the two processes. In addition, trichrome staining of the skeletal muscle tissue further revealed more fibrosis in the skeletal muscle of dKO mice when compared with skeletal muscle of the mdx mice (Fig. 1D). These observations suggest that the microenvironment differs between dKO and mdx skeletal muscle, and the micromilieu in the dKO skeletal muscle is more conductive to osteogenesis or fibrogenesis processes. Much like our observation in the dKO mice, other important animal models of human DMD, including the GRMD dog and the canine X-linked muscular dystrophy (CXMD) dog, feature extensive HO and mild FI in their skeletal muscle, especially before the age of 4–6 mo (16, 53–54).
Figure 1.
Differential formation of HO, FI, fibrosis, and IMCL in the skeletal muscle of dKO mice and mdx mice. A) Micro-CT scan revealed extensive HO formation (arrows) in the hind-limb skeletal muscles of dKO mice (8 wk of age), especially in the thigh and GM. Much less HO was observed in mdx mice (8 wk of age). B) Alizarin red stain of muscle tissue sections also showed more extensive HO formation in the GMs of the dKO mice. C) Oil red O stain revealed comparable amounts of intramuscular FI in dKO and mdx mice (but a much lower ratio of fat/HO), and the distinct localization of fat and HO (HO is visible with brightfield light). Arrows denote FI; arrowheads denote HO. D) Trichrome stain showed more fibrotic tissue (blue) and less normal myofibers in the skeletal muscle of dKO mice. E) AdipoRed stain indicated that IMCL occurred in the skeletal muscle of both mdx and dKO mice but was more extensive in the dKO mice. F) Representative images showing the localization of lipids (arrows) inside the membrane (arrowheads) of myofibers. Left panel: fluorescent image showing AdipoRed signal. Right panel: overlay of fluorescent and brightfield images. G) AdipoRed stain of longitudinal sections of GMs, verifying that the identity of the cells with positive signals were myofibers and not fat cells. H) Statistics of HO in GMs of dKO mice compared to mdx mice. I) Statistics of FI in GMs of dKO mice compared to mdx mice. J) Statistics of IMCL in GMs of dKO mice compared to mdx mice. *P < 0.05.
Interestingly, contrary to the mild FI observed in the skeletal muscles of dKO mice, extensive IMCL was observed; while in the skeletal muscle of mdx mice, less extensive amounts of IMCL were noted (Fig. 1E–G). This severe lipid accumulation in the mature muscle cells (myofibers) of dKO muscle is indicative of disordered lipid metabolism in their skeletal muscle (55).
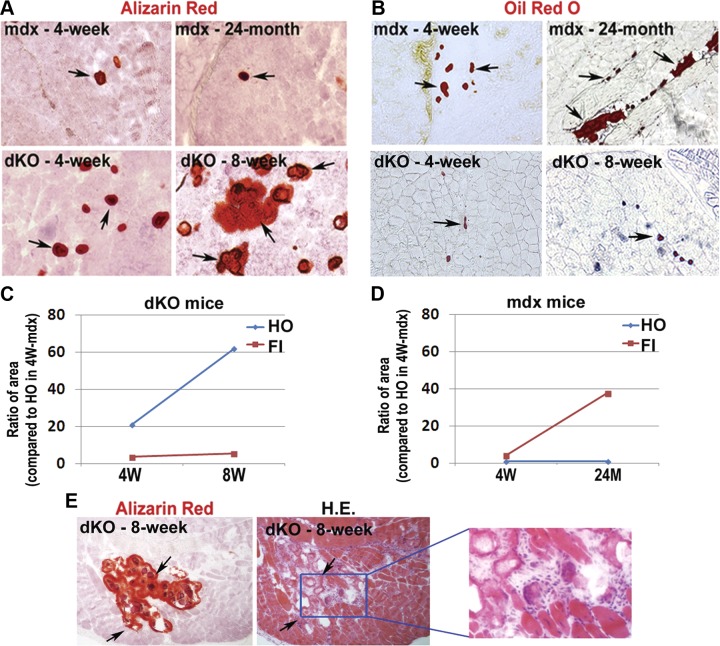
In addition, intramuscular HO and FI development was compared between the dKO and mdx mice at different ages (dKO mice at 4 or 8 wk and mdx mice at 4 wk or 24 mo). Eight weeks and 24 mo represent ∼100% of the life span of the dKO and mdx mice, respectively. Results showed that HO increased with age in the dKO mice within their very short life span, but it remained mild in the mdx mice across their entire life span (Fig. 2A, C, D). Meanwhile, FI increased with age in both the mdx and dKO mice and became quite excessive in aged mdx mice but not in the 8-wk-old dKO mice (Fig. 2B–D). Notably, in both the 4- and 8-wk-old dKO mice, the presence of FI was much less extensive than HO (Fig. 2C), while in mdx mice, FI became more extensive than HO with aging (Fig. 2D).
Figure 2.
Development of HO and FI in the skeletal muscle of “younger” and “older” dKO mice (4 and 8 wk) and mdx mice (4 wk and 24 mo). A) HO (arrows) increased significantly with age in dKO mice but not in mdx mice. B) FI (arrows) increased with age in both mdx mice and dKO mice, but to a lesser extent in the dKO mice. C) Statistics on the change of HO and FI with aging in dKO mice. D) Statistics on the change of HO and FI with aging in mdx mice. E) Serial sections of the skeletal muscle of 8-wk-old dKO mice were stained with either alizarin red or hematoxylin and eosin (H.E.); it shows that HO was generally localized at the sites enriched with damaged myofibers and fibrosis (arrows) but not at the sites possessing normal myofibers.
HO localizes at the sites of necrosis and fibrosis in the skeletal muscle of dKO mice
Alizarin red or hematoxylin and eosin staining was performed on serial sections of the skeletal muscle of 8-wk-old dKO mice, and HO generally localized at the sites enriched with damaged myofibers, but not in areas where normal myofibers existed (Fig. 2E). Also, Trichrome stain and IgG stain further indicated that localization of HO is generally surrounded by fibrotic tissues (Supplemental Fig. S1B) or necrotic tissues (Supplemental Fig. S1C). Therefore, it appears that HO formation in the skeletal muscle of dKO mice is concurrent with necrosis and fibrosis.
RhoA signaling was activated in the skeletal muscle of dKO mice and RhoA+ cells specifically localized at the sites of necrosis and HO
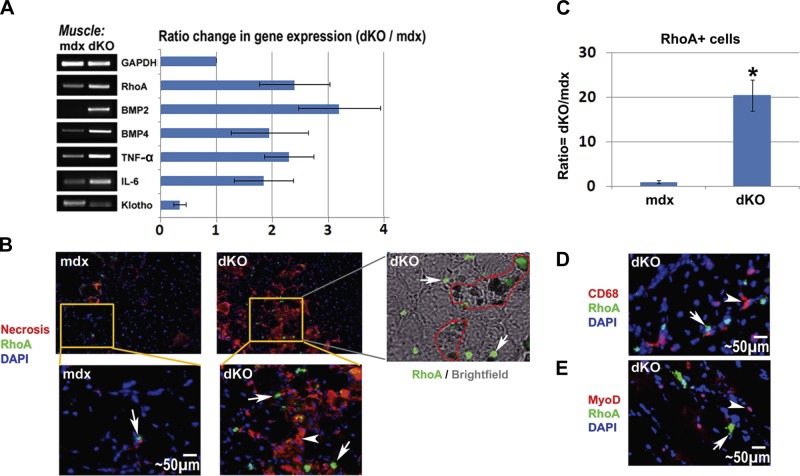
Semiquantitative RT-PCR analyses revealed that the expression of the inflammatory factor TNF-α, and osteogenesis-related factors BMP2, BMP4, and RhoA were all up-regulated in the dKO skeletal muscle, when compared to the age-matched mdx skeletal muscle (Fig. 3A). BMPs are known to induce HO in damaged skeletal muscle, and elevated BMP signaling has been observed in satellite cells of patients wtih DMD (56). We also found that the expression of Klotho, an antiinflammatory and antiaging factor (57), was down-regulated in the dKO skeletal muscle when compared to the mdx skeletal muscle (Fig. 3A). Because inflammation has been implicated as an important contributor to HO (1, 27, 58), it is possible that highly activated inflammation signaling in the dKO skeletal muscle is involved with the extensive HO observed in this animal model. A similar differential expression of these genes was found in MDSCs isolated from dKO and mdx mice.
Figure 3.
RhoA+ cells in the skeletal muscle of dKO and mdx mice. A) RT-PCR of mRNA isolated from the skeletal muscle of dKO and mdx mice (8 wk of age) showed that the expression of RhoA, BMP2/4, TNF-α, IL-6 was up-regulated in dKO mice, and the expression of the anti-inflammation factor Klotho was down-regulated. B) Immunofluorescent staining of RhoA demonstrated a greater number of RhoA+ cells (arrows) in the skeletal muscle of dKO mice. Necrotic areas (arrowheads) were localized by staining with fluorescent anti-mouse IgG, and RhoA+ cells were found to localize at or around the necrotic areas; overlay of images of brightfield and immunofluorescent staining of RhoA further revealed that the same areas enriched with RhoA+ cells (arrows) are the area of HO (circled) too. C) Statistics of RhoA+ cells in mdx and dKO skeletal muscle. D) Representative image of immunofluorescent staining of CD68 and RhoA in the dKO muscle demonstrated that CD68+ cells (arrowheads) and RhoA+ cells (arrows) were two different cell populations. E) Representative image of immunofluorescent staining of MyoD and RhoA in the dKO muscle demonstrated that MyoD+ cells (arrowheads) and RhoA+ cells (arrows) were two different cell populations. *P < 0.05.
Furthermore, immunofluorescent staining for the RhoA protein revealed an increased number of RhoA+ cells in the skeletal muscle of dKO mice when compared to mdx mice (Fig. 3B, C), further confirming elevated RhoA signaling in the dKO skeletal muscle. Notably, it was noted that RhoA+ cells were usually localized at the sites of excessive necrosis and HO (Fig. 3B and Supplemental Fig. S2), but not in area of FI, indicating potential involvement of RhoA+ cells in the progression of HO.
In addition, through colocalization analyses, it was demonstrated that RhoA+ cells do not colocalize with CD68+ inflammatory cells (Fig. 3D) and MyoD+ myogenic cells (Fig. 3E), indicating that the RhoA+ cells did not represent inflammatory or myogenic cells.
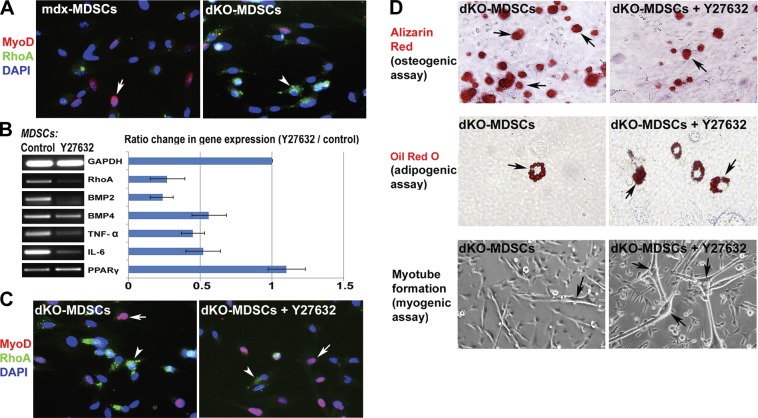
RhoA inactivation of dKO MDSCs decreased their osteogenic potential, while it increased their adipogenic and myogenic potentials
Similar to what was observed with skeletal muscle tissues, immunofluorescent staining of MDSCs isolated from dKO and mdx mice for MyoD and RhoA also demonstrated a greater number of RhoA+ cells in the MDSCs isolated from the dKO mice than the mdx mice (Fig. 4A). Therefore, we hypothesized that the inactivation of RhoA signaling in the MDSCs may change the osteogenic, adipogenic, and myogenic potentials of the cells because RhoA is a proosteogenic and antiadipogenic/myogenic signaling pathway. To test the hypothesis, dKO MDSCs were cultured in proliferation medium and pretreated with the RhoA/Rock inhibitor, Y-27632, for 2 d before undergoing osteogenic, adipogenic, and myogenic assays. Y-27632 pretreatment of dKO MDSCs significantly down-regulated the expression of the inflammation-related genes (TNF-α and IL-6), BMPs, and RhoA (Fig. 4B). Also, Y-27632 pretreatment up-regulated the expression of the key adipogenic factor, PPARγ, which is known to have anti-inflammatory activities (59). Immunostaining of RhoA and MyoD showed that RhoA+ cells and MyoD+ cells were 2 distinct cell populations, and the number of RhoA+ cells was decreased, while the number of MyoD+ cells was increased with Y-27632 treatment (Fig. 4C), indicating decreased osteogenic but increased myogenic potentials of the treated cell population. Osteogenesis and adipogenesis assays revealed a decreased osteogenic potential and increased adipogenic potential of the MDSCs treated with Y-27632 (Fig. 4D). Meanwhile, the dKO MDSCs treated with Y-27632 showed an increase in their myogenic potential (Fig. 4D).
Figure 4.
RhoA in MDSCs and in vitro inactivation of RhoA in cultured MDSCs from dKO mice. A) Immunofluorescent staining of MyoD and RhoA in MDSCs isolated from mdx and dKO mice demonstrated that RhoA+ cells (arrowhead) and MyoD+ cells (arrow) were two distinct cell populations, and there were more RhoA+ cells in the MDSCs isolated from dKO mice than mdx mice. B) Pretreatment of dKO-MDSCs with the RhoA/Rock inhibitor Y-27632 in proliferation medium for 2 d down-regulated the expression of RhoA, BMP2/4, TNF-α, and IL-6, and up-regulated the expression of PPARγ. C) Y-27632 treatment decreased the number of RhoA+ cells (arrowheads) and increased the number of MyoD+ cells (arrows). D) In vitro osteogenesis, adipogenesis, and myogenesis assays indicated that, Y-27632 pretreated dKO-MDSCs demonstrated decreased osteogenic potential (less cells with positive alizarin red signal) in osteogenic medium and increased adipogenesis potential (more cells with positive Oil red O signal) in adipogenic medium, as well as increased myogenic potential (more myotube formation) in the myogenic medium.
RhoA inactivation in dKO skeletal muscle decreased HO and IMCL, while it increased muscle regeneration
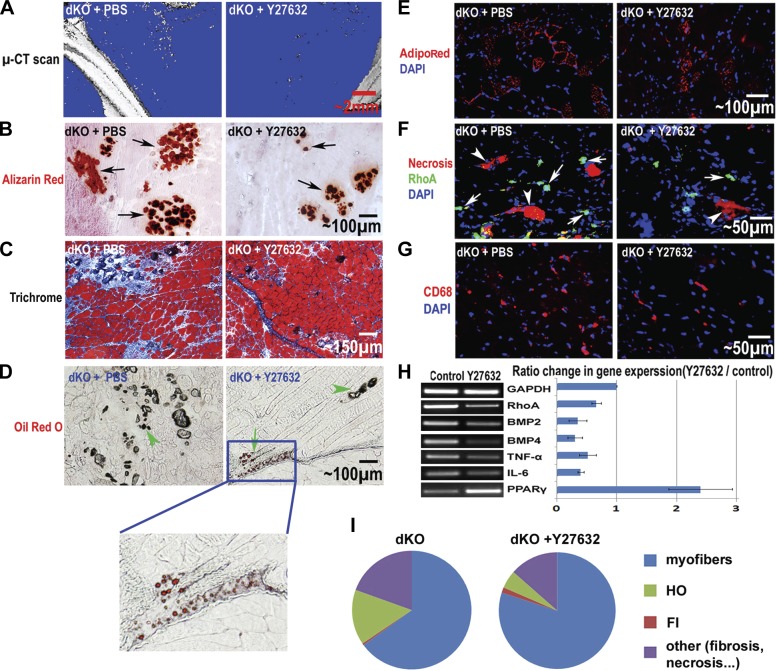
The RhoA/Rock inhibitor Y-27632 was injected intramuscularly into the GM muscles of 4-wk-old dKO mice to determine the effect of RhoA inactivation on the development of HO, FI, IMCL, and muscle regeneration. Four weeks after the administration of Y-27632, we observed slower development of HO in the dKO skeletal muscle, as determined by micro-CT scanning and histological staining (Fig. 5A, B). At the same time, skeletal muscle regeneration was enhanced in the treated dKO skeletal muscle (Fig. 5C) despite slightly increased FI (Fig. 5D). Notably, the increase of FI in the dKO muscle with Y-27632 administration was mild compared to the increase of muscle regeneration, and the overall phenotype of the dystrophic muscle was greatly improved (Fig. 5). Finally, IMCL was decreased (Fig. 5E), indicating improved fatty acid metabolism in the skeletal muscle with RhoA inactivation. Immunofluorescent staining of RhoA revealed that the number of RhoA+ cells decreased with the administration of Y-27632 (Fig. 5F), which may correlate with the reduced number of osteogenic cells in the skeletal muscle. Furthermore, the number of CD68+ inflammatory cells (mainly macrophages; ref. 60) was also reduced after the administration of Y-27632 (Fig. 5G). In addition, semiquantitative RT-PCR showed that the expression of RhoA, BMPs, and inflammation-related factors were down-regulated in skeletal muscle treated with Y-27632, while the expression of PPARγ was up-regulated (Fig. 5H). PPARγ activation may have improved fatty acid metabolism and reduced the accumulation of lipid within the muscle cells (61–62). The overall phenotypic change in the dKO skeletal muscle treated with Y-27632 is summarized in Fig. 5I.
Figure 5.
In vivo inactivation of RhoA in the skeletal muscle of dKO mice. A) Micro-CT scan of the hind-limb skeletal muscles, including the GM, 4 wk after beginning the administration of the RhoA/Rock inhibitor Y-27632 in dKO mice (from 4 to 8 wk of age) showed a reduction in HO. B) Alizarin red stain of muscle tissues also revealed greatly reduced HO (arrows) in the GM muscles with Y-27632 treatment. C) Hematoxylin and eosin stain showed improved muscle regeneration with Y-27632 treatment. D) Oil red O stain showed slightly increased FI (arrows) with Y-27632 treatment. HO is also visible with brightfield microscopy (arrowheads). E) AdipoRed stain showed reduced IMCL in dKO mice with Y-27632 treatment. F) Immunofluorescent stain showed reduced numbers of RhoA+ cells (arrows) and necrotic myofibers (arrowheads) with Y-27632 treatment. G) Immunofluorescent stain showed reduced numbers of CD68+ inflammatory cells with Y-27632 treatment. H) RT-PCR of the muscle tissues revealed down-regulated expression of RhoA, BMP2/4, TNF-α, and IL-6, and up-regulated expression of PPARγ with Y-27632 treatment. I) The overall phenotypic change of the muscle treated with Y-27632 is summarized in the pie graph, including myofibers, HO, FI, and other (fibrosis and necrosis). It is clear that HO, fibrosis, and necrosis were reduced with Y-27632 treatment, while muscle regeneration was improved; FI was also increased, but only very slightly.
dKO cardiac muscle featured increased IMCL, fibrosis, and HO when compared to mdx mice
Cardiac involvement is the leading cause of early death in patients wtih DMD (63), and intramyocardial lipid accumulation in cardiac muscle has been observed in patients wtih DMD, especially in the most damaged areas of the hearts (11, 13). We hypothesized that IMCL observed in the mdx and dKO mice was not limited to the skeletal muscle but would also be found in cardiac muscle and could be related to the formation of fibrosis observed in the dystrophic hearts (cardiomyopathy) of the mice.
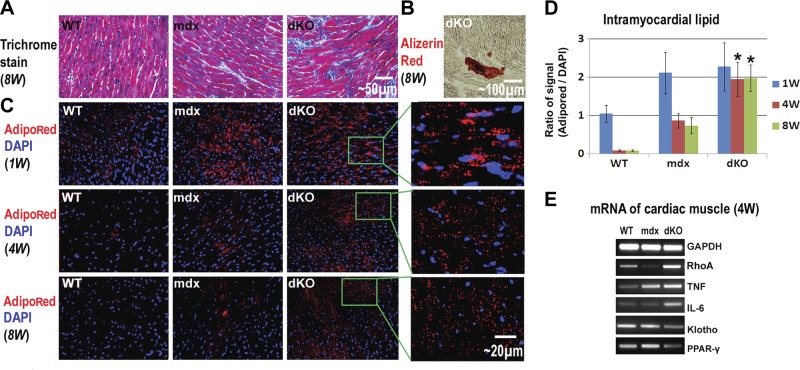
Trichrome staining of cardiac muscles from 8-wk-old WT, mdx, and dKO mice was conducted to characterize ECM collagen deposition, and it revealed that fibrosis formation is generally severe in dKO mice, mild in mdx mice, and absent in WT mice (Fig. 6A). Alizarin red staining of the cardiac muscle revealed occurrences of HO in the dKO mice (Fig. 6B), but not in the WT or mdx mice (data not shown). Meanwhile, we did not observe any FI in the cardiac muscles in these three mouse models (data not shown), but we did observe intramyocardial lipid accumulation in both the mdx and dKO mice (Fig. 6C, D).
Figure 6.
Fibrosis formation and intramyocardial lipid accumulation in the cardiac muscles of WT, mdx, and dKO mice. A) Trichrome stain showed the progression of fibrosis formation (collagen deposition, blue) in the cardiac muscles as dKO > mdx > WT. B) Alizarin red stain revealed HO (although mild) in the cardiac muscle of dKO mice. C) High levels of intramyocardial lipids were observed in all three mouse models at 1 wk after birth. At 4 and 8 wk after birth, intramyocardial lipid accumulation decreased to an undetectable level in WT mice and decreased to a moderate level in mdx mice but still maintained a very high level in the dKO mice. D) Statistical analysis of the percentage of area positive for lipid accumulation in WT, mdx, and dKO mice of different ages. E) RT-PCR results showed that the expression of inflammatory factors (TNF-α and IL-6) were up-regulated in the cardiac muscles of mdx and dKO mice, compared with WT mice. The strongest up-regulation of TNF-α and IL-6 occurred to the dKO mice. The expression of the anti-inflammatory factor Klotho was down-regulated in the cardiac muscle of mdx and dKO mice, compared to WT mice. *P < 0.05.
Extensive intramyocardial lipid accumulation at 1 wk of age was observed in all 3 mouse models (Fig. 6C). Intramyocardial lipid accumulation in fetal WT mice is a common occurrence because, unlike adult hearts, the fetal hearts use glucose instead of fatty acids as a source of energy (64). Intramyocardial lipid accumulation was found to decrease rapidly in WT mice 1 wk after birth and became nearly undetectable at 4 wk of age (Fig. 6C); however, in both mdx and dKO mice, intramyocardial lipid accumulation was still observed at 4 wk of age, and the dKO mice exhibited more intramyocardial lipid accumulation than the mdx mice (Fig. 6C, D). Compared to the WT and mdx mice, the expression of RhoA and inflammation-related factors (TNF-α and IL-6) were also found to be up-regulated in the dKO cardiac muscle, while the expression of the Klotho gene was down-regulated (Fig. 6E). We suggest that the activation of RhoA and inflammation signaling in dKO cardiac muscle may account for higher levels of HO, intramyocardial lipid accumulation, and fibrosis.
Systemic RhoA inactivation via intraperitoneal injection (IP) of Y-27632 reduced IMCL, fibrosis, and HO in dKO cardiac muscle
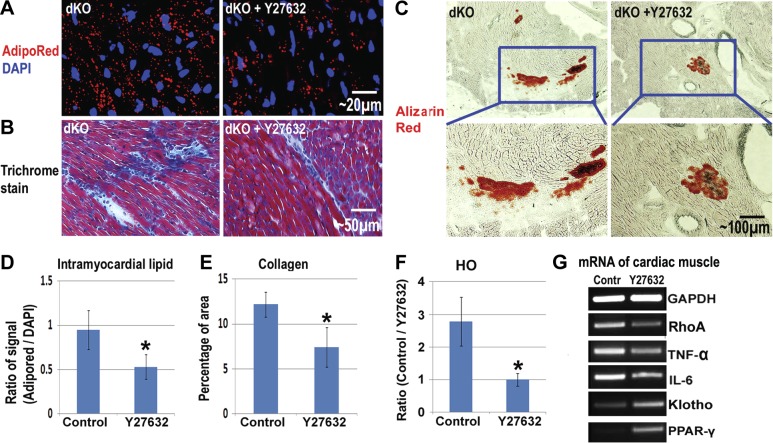
We hypothesized that RhoA inactivation could reduce HO, IMCL, and fibrosis in the dKO cardiac muscle. To confirm this hypothesis, Y-27632 was injected intraperitoneally (IP) to achieve the systemic inhibition of RhoA signaling in 3-wk-old dKO mice. As expected, after 4 wk of continuous injection. IMCL, fibrosis, and HO in the cardiac muscle decreased compared to nontreated mice (Fig. 7A–F). Actually, involvement of RhoA/ROCK in mediating myocardial fibrosis in type 2 diabetes has been previously demonstrated (48). Semiquantitative PCR further revealed that the expression of RhoA and inflammatory factors were down-regulated with Y-27632 administration, while the expression of Klotho and PPARγ was up-regulated (Fig. 7G).
Figure 7.
Reduction of intramyocardial lipid accumulation and fibrosis in the cardiac muscles of dKO mice with inactivated RhoA. A) AdipoRed stain showed that intramyocardial lipid accumulation in dKO mice was reduced 4 wk after the initiation of Y-27632 administration. B) Trichrome stain showed that fibrosis formation was reduced with Y-27632 administration. C) Alizarin red stain showed that HO formation in the cardiac muscles of dKO mice was reduced with Y-27632 administration. D) Statistical analysis of the percentage of areas positive for IMCL in dKO mice, with and without Y-27632 administration. E) Statistical analysis of the percentage of dKO mice positive for HO in their cardiac muscles, with and without Y-27632 administration. F) RT-PCR of mRNA from the cardiac muscles showed that the expression of RhoA and inflammatory factors (TNF-α and IL-6) were down-regulated with Y-27632 administration, while the expressions of Klotho and PPAR-γ were up-regulated. *Significant difference, P < 0.05.
DISCUSSION
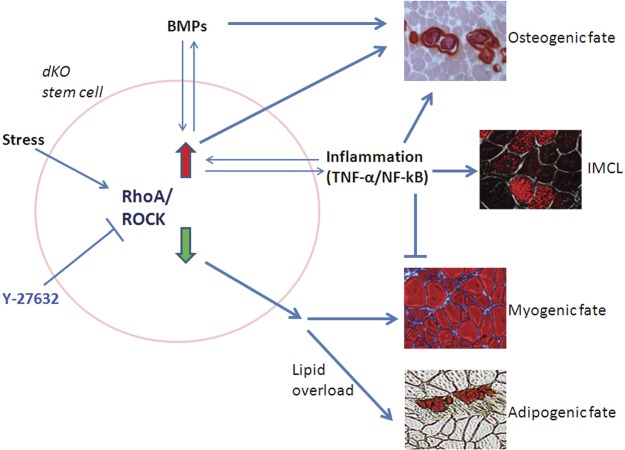
Heterotopic ossification (HO) and ectopic fatty infiltration (FI) have been reported in the dystrophic muscle of patients wtih DMD and related animal models (12, 14–16, 65). Our results revealed that mouse models of DMD featuring differing severities of muscular dystrophy have varied potentials for developing HO and FI, and that RhoA signaling could represent a critical mediator in the process, including progression toward HO, FI, and normal muscle regeneration. We noted that the inactivation of RhoA repressed the development of HO and IMCL and improved muscle regeneration in dKO mice. RhoA signaling was previously demonstrated to promote the osteogenic potential of MSCs, while preventing their adipogenic and myogenic potentials (37, 40). Here, we further identified a similar role of RhoA signaling in muscle stem cells. We suggest that HO in skeletal muscle could be a cell-mediated process involving the transdifferentiation of myogenic cells into osteogenic cells in a stressful microenvironment. The potential mechanisms underlying our current results are proposed in Fig. 8.
Figure 8.
Schematic diagram of the potential mechanism involving RhoA/ROCK signaling in regulating HO, FI, and muscle regeneration in the dystrophic muscle of dKO mice. RhoA/ROCK is responsive to a variety of stresses. Multipotent stem cells, either local stem cells in the dystrophic muscle, bone-marrow-derived stem cells mobilized to the dystrophic muscle of dKO mice, or stem cells of other origins, may be stimulated to differentiate into an osteogenic lineage and may participate in HO formation by highly activated RhoA/Rock signaling, whereas normal myogenic differentiation or potential adipogenic differentiation in dKO mice were repressed by RhoA/Rock signaling. Critical HO-inducing factors, such as BMPs and inflammatory signaling, are interactive with RhoA/Rock signaling, and contribute to HO formation by stem cells or other progenitor cells. RhoA/Rock inactivation with Y-27632 has the potential to reverse the progression of differentiation of the stem cells toward HO, and improve myogenic or adipogenic differentiation. Inflammation-induced IMCL may be directly responsible for HO formation in the muscle (72).
Muscle stem cells from normal mice are known to possess multipotent differentiation potentials and can differentiate into osteogenic, chrondrogenic, adipogenic, and myogenic lineages with appropriate induction stimuli (66). In the current study, we observed that the osteogenic potential of muscle stem cells appeared to be promoted, while adipogenic and myogenic potentials appeared to be repressed in the severely dystrophic muscle of dKO mice, a process likely mediated, at least in part, by RhoA activation. RhoA activation was shown to occur in response to stresses, including mechanical stress and oxidative stress (67, 68), suggesting that RhoA activation in the dystrophic muscle of dKO mice could be related to the multiple stresses to which the skeletal muscle of dKO mice is exposed. Compared to mdx mice, stresses in the dystrophic muscle of dKO mice may include more myofiber damage and abnormal neuromuscular junctions created by the absence of utrophin; stronger production of profibrotic factors, such as TGF-β1; and severe inflammation caused by extensive muscle degeneration and an abnormally high fat-to-skeletal muscle ratio.
Inactivation of RhoA signaling could be beneficial for improving the severe myopathological phenotype present in dKO mice. Interestingly, RhoA signaling was found to be increased in the spinal cord of an intermediate spinal muscular atrophy (SMA) mouse model, and the inactivation of RhoA signaling with Y-27632 improved the survival of these SMA mice (69). Our results showed that RhoA inactivation in dKO mice led to a less severe dystrophic muscle phenotype that more closely resembled the phenotype observed in mdx mice. RhoA signaling has been found to interact with inflammatory signaling and acts as a proinflammatory factor (46, 70). In our current study, we found the level of inflammation to be higher in dKO mice compared to mdx mice. By inactivating RhoA with Y-27632, we observed that TNF-α and IL-6 were down-regulated, while Klotho and PPAR-γ were up-regulated in both dKO muscle stem cells and skeletal muscle tissues, indicating a repression of inflammation (59). Therefore, we suggest that the improved muscle phenotype of dKO mice with RhoA inactivation is at least partially due to a reduction in inflammation. Moreover, the up-regulation of PPARγ expression via RhoA inactivation could also improve the metabolism of fatty acids in dystrophic muscle since PPARγ was previously reported to increase free fatty acid (FFA) disposal in skeletal muscle through oxidation augmentation, resulting in the reduction of IMCL (71). PPARγ activation in muscle has also been reported to prevent IMCL normally observed in both fat-fed wild-type mice, as well as in the muscles of obese diabetic patients (61, 62). Thus, the up-regulation of PPARγ via RhoA inactivation in dKO muscle improved fat metabolism by promoting the uptake of lipids by fat cells and not by muscle cells. Since obesity, inflammation, and IMCL have also been commonly observed in human DMD, we suggest that DMD should also be investigated for the prevention of IMCL by reducing obesity, inflammation, and metabolic abnormalities.
Previous researchers have demonstrated that intramyocardial lipid accumulation is potentially involved with cardiac dysfunction in the dystrophic heart. Cardiac failure is the leading cause for early death of patients wtih DMD (63), and it has been reported that intramyocardial lipid accumulation occurs in the damaged areas of cardiac muscle in patients wtih DMD (11); however, research is sparse regarding the mechanisms and prevention of intramyocardial lipid accumulation. Compared with mdx mice, the dKO mouse model exhibits a more severe abnormal cardiac phenotype (i.e., fibrosis formation) and is considered to be an important model for studying DMD-associated cardiomyopathy (14, 19, 20). Our results showed that, when comparing the cardiac muscles of WT, mdx, and dKO mice, the severity of intramyocardial lipid accumulation appeared to be closely related to progressive cardiac muscle degeneration. More importantly, our results indicate that intramyocardial lipid accumulation can be reduced by inactivating RhoA, an effect potentially associated with repressed systematic inflammation.
In summary, our results indicated that RhoA signaling could play a major role in regulating the processes of HO, FI, IMCL, and muscle regeneration in the dystrophic skeletal muscle of mice, and consequently, RhoA inactivation may represent a therapeutic target to improve the muscle histopathology associated with DMD. Moreover, RhoA signaling may also serve as a potential target for repressing the development of HO in cases of trauma, neurological injury, and genetic abnormalities. The status of RhoA activation in human patients wtih DMD and the potential effect of RhoA inactivation are, therefore, very promising but require further investigation.
Supplementary Material
Acknowledgments
This research was supported, in part, by the Henry J. Mankin endowed chair at the University of Pittsburgh, and the William F. and Jean W. Donaldson endowed chair at the Children's Hospital of Pittsburgh.
The authors also thank Ms. Bria King and Mr. James Cummins for their editorial assistance in completing this manuscript.
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
- BMP
- bone morphogenetic protein
- dKO
- dystrophin/utrophin double knockout
- DMD
- Duchenne muscular dystrophy
- FBS
- fetal bovine serum
- FI
- fatty infiltration
- GM
- gastrocnemius muscle
- GRMD
- golden retriever muscular dystrophy
- HO
- heterotopic ossification
- IMCL
- intramyocellular lipid accumulation
- MDSC
- muscle-derived stem cell
- mdx
- dystrophin-deficient
- micro-CT
- micro-computed tomography
- MSC
- mesenchymal stem cell
- NF-κB
- nuclear factor-κB
- PPARγ
- peroxisome proliferator-activated receptor γ
REFERENCES
- 1. Cipriano C. A., Pill S. G., Keenan M. A. (2009) Heterotopic ossification following traumatic brain injury and spinal cord injury. J. Am. Acad. Orthop. Surg. 17, 689–697 [DOI] [PubMed] [Google Scholar]
- 2. Marcus R. L., Addison O., Kidde J. P., Dibble L. E., Lastayo P. C. (2010) Skeletal muscle fat infiltration: impact of age, inactivity, and exercise. J. Nutr. Health Aging 14, 362–366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Miljkovic-Gacic I., Wang X., Kammerer C. M., Gordon C. L., Bunker C. H., Kuller L. H., Patrick A. L., Wheeler V. W., Evans R. W., Zmuda J. M. (2008) Fat infiltration in muscle: new evidence for familial clustering and associations with diabetes. Obesity 16, 1854–1860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Savage D. B., Petersen K. F., Shulman G. I. (2007) Disordered lipid metabolism and the pathogenesis of insulin resistance. Physiol. Rev. 87, 507–520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hulver M. W., Dohm G. L. (2004) The molecular mechanism linking muscle fat accumulation to insulin resistance. Proc. Nutr. Soc. 63, 375–380 [DOI] [PubMed] [Google Scholar]
- 6. Schulze P. C. (2009) Myocardial lipid accumulation and lipotoxicity in heart failure. J. Lipid Res. 50, 2137–2138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Axelsen L. N., Lademann J. B., Petersen J. S., Holstein-Rathlou N. H., Ploug T., Prats C., Pedersen H. D., Kjolbye A. L. (2010) Cardiac and metabolic changes in long-term high fructose-fat fed rats with severe obesity and extensive intramyocardial lipid accumulation. Am. J. Physiol. Regul. Integr. Comp. Physiol. 298, R1560–R1570 [DOI] [PubMed] [Google Scholar]
- 8. Ruberg F. L. (2007) Myocardial lipid accumulation in the diabetic heart. Circulation 116, 1110–1112 [DOI] [PubMed] [Google Scholar]
- 9. Sharma S., Adrogue J. V., Golfman L., Uray I., Lemm J., Youker K., Noon G. P., Frazier O. H., Taegtmeyer H. (2004) Intramyocardial lipid accumulation in the failing human heart resembles the lipotoxic rat heart. FASEB J. 18, 1692–1700 [DOI] [PubMed] [Google Scholar]
- 10. Momose M., Iguchi N., Imamura K., Usui H., Ueda T., Miyamoto K., Inaba S. (2001) Depressed myocardial fatty acid metabolism in patients with muscular dystrophy. Neuromusc. Disord. 11, 464–469 [DOI] [PubMed] [Google Scholar]
- 11. Saini-Chohan H. K., Mitchell R. W., Vaz F. M., Zelinski T., Hatch G. M. (2012) Delineating the role of alterations in lipid metabolism to the pathogenesis of inherited skeletal and cardiac muscle disorders: Thematic Review Series: Genetics of Human Lipid Diseases. J. Lipid Res. 53, 4–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ionasescu V., Monaco L., Sandra A., Ionasescu R., Burmeister L., Deprosse C., Stern L. Z. (1981) Alterations in lipid incorporation in Duchenne muscular dystrophy. Studies of fresh and cultured muscle. J. Neurol. Sci. 50, 249–251 [DOI] [PubMed] [Google Scholar]
- 13. Tahallah N., Brunelle A., De La Porte S., Laprevote O. (2008) Lipid mapping in human dystrophic muscle by cluster-time-of-flight secondary ion mass spectrometry imaging. J. Lipid Res. 49, 438–454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Duan D. (2006) Challenges and opportunities in dystrophin-deficient cardiomyopathy gene therapy. Hum. Mol. Genet. 15(Spec. 2), R253–R261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kikkawa N., Ohno T., Nagata Y., Shiozuka M., Kogure T., Matsuda R. (2009) Ectopic calcification is caused by elevated levels of serum inorganic phosphate in mdx mice. Cell Struct. Funct. 34, 77–88 [DOI] [PubMed] [Google Scholar]
- 16. Nguyen F., Cherel Y., Guigand L., Goubault-Leroux I., Wyers M. (2002) Muscle lesions associated with dystrophin deficiency in neonatal golden retriever puppies. J. Comp. Pathol. 126, 100–108 [DOI] [PubMed] [Google Scholar]
- 17. Starkey J. D., Yamamoto M., Yamamoto S., Goldhamer D. J. (2011) Skeletal muscle satellite cells are committed to myogenesis and do not spontaneously adopt nonmyogenic fates. J. Histochem. Cytochem. 59, 33–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sicinski P., Geng Y., Ryder-Cook A. S., Barnard E. A., Darlison M. G., Barnard P. J. (1989) The molecular basis of muscular dystrophy in the mdx mouse: a point mutation. Science 244, 1578–1580 [DOI] [PubMed] [Google Scholar]
- 19. Grady R. M., Teng H., Nichol M. C., Cunningham J. C., Wilkinson R. S., Sanes J. R. (1997) Skeletal and cardiac myopathies in mice lacking utrophin and dystrophin: a model for Duchenne muscular dystrophy. Cell 90, 729–738 [DOI] [PubMed] [Google Scholar]
- 20. Deconinck A. E., Rafael J. A., Skinner J. A., Brown S. C., Potter A. C., Metzinger L., Watt D. J., Dickson J. G., Tinsley J. M., Davies K. E. (1997) Utrophin-dystrophin-deficient mice as a model for Duchenne muscular dystrophy. Cell 90, 717–727 [DOI] [PubMed] [Google Scholar]
- 21. Isaac C., Wright A., Usas A., Li H., Tang Y., Mu X., Greco N., Dong Q., Vo N., Kang J., Wang B., Huard J. (2013) Dystrophin and utrophin “double knockout” dystrophic mice exhibit a spectrum of degenerative musculoskeletal abnormalities. J. Orthop. Res. 31, 343–349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Muntoni F., Fisher I., Morgan J. E., Abraham D. (2002) Steroids in Duchenne muscular dystrophy: from clinical trials to genomic research. Neuromusc. Disord. 12(Suppl. 1), S162–S165 [DOI] [PubMed] [Google Scholar]
- 23. Mavrogenis A. F., Soucacos P. N., Papagelopoulos P. J. (2011) Heterotopic ossification revisited. Orthopedics 34, 177. [DOI] [PubMed] [Google Scholar]
- 24. Neal B., Rodgers A., Dunn L., Fransen M. (2000) Non-steroidal anti-inflammatory drugs for preventing heterotopic bone formation after hip arthroplasty. Cochrane Database Syst. Rev., CD001160. [DOI] [PubMed] [Google Scholar]
- 25. Dahners L. E., Mullis B. H. (2004) Effects of nonsteroidal anti-inflammatory drugs on bone formation and soft-tissue healing. J. Am. Acad. Orthop. Surg. 12, 139–143 [DOI] [PubMed] [Google Scholar]
- 26. Vasileiadis G. I., Sioutis I. C., Mavrogenis A. F., Vlasis K., Babis G. C., Papagelopoulos P. J. (2011) COX-2 inhibitors for the prevention of heterotopic ossification after THA. Orthopedics 34, 467. [DOI] [PubMed] [Google Scholar]
- 27. Banovac K., Williams J. M., Patrick L. D., Levi A. (2004) Prevention of heterotopic ossification after spinal cord injury with COX-2 selective inhibitor (rofecoxib). Spinal Cord 42, 707–710 [DOI] [PubMed] [Google Scholar]
- 28. Joe A. W., Yi L., Natarajan A., Le Grand F., So L., Wang J., Rudnicki M. A., Rossi F. M. (2010) Muscle injury activates resident fibro/adipogenic progenitors that facilitate myogenesis. Nat. Cell Biol. 12, 153–163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Cox F. M., Reijnierse M., van Rijswijk C. S., Wintzen A. R., Verschuuren J. J., Badrising U. A. (2011) Magnetic resonance imaging of skeletal muscles in sporadic inclusion body myositis. Rheumatology 50, 1153–1161 [DOI] [PubMed] [Google Scholar]
- 30. Mei M., Zhao L., Li Q., Chen Y., Huang A., Varghese Z., Moorhead J. F., Zhang S., Powis S. H., Ruan X. Z. (2011) Inflammatory stress exacerbates ectopic lipid deposition in C57BL/6J mice. Lipids Health Dis. 10, 110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hotamisligil G. S., Shargill N. S., Spiegelman B. M. (1993) Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science 259, 87–91 [DOI] [PubMed] [Google Scholar]
- 32. Messina S., Altavilla D., Aguennouz M., Seminara P., Minutoli L., Monici M. C., Bitto A., Mazzeo A., Marini H., Squadrito F., Vita G. (2006) Lipid peroxidation inhibition blunts nuclear factor-kappaB activation, reduces skeletal muscle degeneration, and enhances muscle function in mdx mice. Am. J. Pathol. 168, 918–926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Langen R. C., Schols A. M., Kelders M. C., Wouters E. F., Janssen-Heininger Y. M. (2001) Inflammatory cytokines inhibit myogenic differentiation through activation of nuclear factor-κB. FASEB J. 15, 1169–1180 [DOI] [PubMed] [Google Scholar]
- 34. Lu A., Proto J. D., Guo L., Tang Y., Lavasani M., Tilstra J. S., Niedernhofer L. J., Wang B., Guttridge D. C., Robbins P. D., Huard J. (2012) NF-κB negatively impacts the myogenic potential of muscle-derived stem cells. Mol. Ther. 20, 661–668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Monici M. C., Aguennouz M., Mazzeo A., Messina C., Vita G. (2003) Activation of nuclear factor-κB in inflammatory myopathies and Duchenne muscular dystrophy. Neurology 60, 993–997 [DOI] [PubMed] [Google Scholar]
- 36. Ridley A. J. (2001) Rho GTPases and cell migration. J. Cell Sci. 114, 2713–2722 [DOI] [PubMed] [Google Scholar]
- 37. McBeath R., Pirone D. M., Nelson C. M., Bhadriraju K., Chen C. S. (2004) Cell shape, cytoskeletal tension, and RhoA regulate stem cell lineage commitment. Dev. Cell. 6, 483–495 [DOI] [PubMed] [Google Scholar]
- 38. Khatiwala C. B., Kim P. D., Peyton S. R., Putnam A. J. (2009) ECM compliance regulates osteogenesis by influencing MAPK signaling downstream of RhoA and ROCK. J. Bone. Miner. Res. 24, 886–898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Meyers V. E., Zayzafoon M., Douglas J. T., McDonald J. M. (2005) RhoA and cytoskeletal disruption mediate reduced osteoblastogenesis and enhanced adipogenesis of human mesenchymal stem cells in modeled microgravity. J. Bone Miner. Res. 20, 1858–1866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wang Y. K., Yu X., Cohen D. M., Wozniak M. A., Yang M. T., Gao L., Eyckmans J., Chen C. S. (2012) Bone morphogenetic protein-2-induced signaling and osteogenesis is regulated by cell shape, RhoA/ROCK, and cytoskeletal tension. Stem Cells Dev. 21, 1176–1186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Fromigue O., Hay E., Modrowski D., Bouvet S., Jacquel A., Auberger P., Marie P. J. (2006) RhoA GTPase inactivation by statins induces osteosarcoma cell apoptosis by inhibiting p42/p44-MAPKs-Bcl-2 signaling independently of BMP-2 and cell differentiation. Cell Death Differ. 13, 1845–1856 [DOI] [PubMed] [Google Scholar]
- 42. Hosoyama T., Ishiguro N., Yamanouchi K., Nishihara M. (2009) Degenerative muscle fiber accelerates adipogenesis of intramuscular cells via RhoA signaling pathway. Differentiation 77, 350–359 [DOI] [PubMed] [Google Scholar]
- 43. Santos A., Bakker A. D., de Blieck-Hogervorst J. M., Klein-Nulend J. (2010) WNT5A induces osteogenic differentiation of human adipose stem cells via rho-associated kinase ROCK. Cytotherapy 12, 924–932 [DOI] [PubMed] [Google Scholar]
- 44. Goto K., Chiba Y., Sakai H., Misawa M. (2009) Tumor necrosis factor-alpha (TNF-alpha) induces upregulation of RhoA via NF-κB activation in cultured human bronchial smooth muscle cells. J. Pharmacol. Sci. 110, 437–444 [DOI] [PubMed] [Google Scholar]
- 45. Slice L. W., Bui L., Mak C., Walsh J. H. (2000) Differential regulation of COX-2 transcription by Ras- and Rho-family of GTPases. Biochem. Biophys. Res. Commun. 276, 406–410 [DOI] [PubMed] [Google Scholar]
- 46. Nakayama Y., Komuro R., Yamamoto A., Miyata Y., Tanaka M., Matsuda M., Fukuhara A., Shimomura I. (2009) RhoA induces expression of inflammatory cytokine in adipocytes. Biochem. Biophys. Res. Commun. 379, 288–292 [DOI] [PubMed] [Google Scholar]
- 47. Zhou Y., Huang X., Hecker L., Kurundkar D., Kurundkar A., Liu H., Jin T. H., Desai L., Bernard K., Thannickal V. J. (2013) Inhibition of mechanosensitive signaling in myofibroblasts ameliorates experimental pulmonary fibrosis. J. Clin. Invest. 123, 1096–1108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Zhou H., Li Y. J., Wang M., Zhang L. H., Guo B. Y., Zhao Z. S., Meng F. L., Deng Y. G., Wang R. Y. (2011) Involvement of RhoA/ROCK in myocardial fibrosis in a rat model of type 2 diabetes. Acta Pharmacol. Sin. 32, 999–1008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Charrasse S., Comunale F., Grumbach Y., Poulat F., Blangy A., Gauthier-Rouviere C. (2006) RhoA GTPase regulates M-cadherin activity and myoblast fusion. Mol. Biol. Cell 17, 749–759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Castellani L., Salvati E., Alema S., Falcone G. (2006) Fine regulation of RhoA and Rock is required for skeletal muscle differentiation. J. Biol. Chem. 281, 15249–15257 [DOI] [PubMed] [Google Scholar]
- 51. Beqaj S., Jakkaraju S., Mattingly R. R., Pan D., Schuger L. (2002) High RhoA activity maintains the undifferentiated mesenchymal cell phenotype, whereas RhoA down-regulation by laminin-2 induces smooth muscle myogenesis. J. Cell Biol. 156, 893–903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Gharaibeh B., Lu A., Tebbets J., Zheng B., Feduska J., Crisan M., Peault B., Cummins J., Huard J. (2008) Isolation of a slowly adhering cell fraction containing stem cells from murine skeletal muscle by the preplate technique. Nat. Protoc. 3, 1501–1509 [DOI] [PubMed] [Google Scholar]
- 53. Thibaud J. L., Monnet A., Bertoldi D., Barthelemy I., Blot S., Carlier P. G. (2007) Characterization of dystrophic muscle in golden retriever muscular dystrophy dogs by nuclear magnetic resonance imaging. Neuromusc. Disord. 17, 575–584 [DOI] [PubMed] [Google Scholar]
- 54. Valentine B. A., Cooper B. J., Cummings J. F., de Lahunta A. (1990) Canine X-linked muscular dystrophy: morphologic lesions. J. Neurol. Sci. 97, 1–23 [DOI] [PubMed] [Google Scholar]
- 55. Greenberg A. S., Coleman R. A., Kraemer F. B., McManaman J. L., Obin M. S., Puri V., Yan Q. W., Miyoshi H., Mashek D. G. (2011) The role of lipid droplets in metabolic disease in rodents and humans. J. Clin. Invest. 121, 2102–2110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Lounev V. Y., Ramachandran R., Wosczyna M. N., Yamamoto M., Maidment A. D., Shore E. M., Glaser D. L., Goldhamer D. J., Kaplan F. S. (2009) Identification of progenitor cells that contribute to heterotopic skeletogenesis. J. Bone Joint Surg. Am. 91, 652–663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Arking D. E., Krebsova A., Macek M., Sr., Macek M., Jr., Arking A., Mian I. S., Fried L., Hamosh A., Dey S., McIntosh I., Dietz H. C. (2002) Association of human aging with a functional variant of klotho. Proc. Natl. Acad. Sci. U. S. A. 99, 856–861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Salisbury E., Rodenberg E., Sonnet C., Hipp J., Gannon F. H., Vadakkan T. J., Dickinson M. E., Olmsted-Davis E. A., Davis A. R. (2011) Sensory nerve induced inflammation contributes to heterotopic ossification. J. Cell. Biochem. 112, 2748–2758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Straus D. S., Glass C. K. (2007) Anti-inflammatory actions of PPAR ligands: new insights on cellular and molecular mechanisms. Trends Immunol. 28, 551–558 [DOI] [PubMed] [Google Scholar]
- 60. Holness C. L., Simmons D. L. (1993) Molecular cloning of CD68, a human macrophage marker related to lysosomal glycoproteins. Blood 81, 1607–1613 [PubMed] [Google Scholar]
- 61. Amin R. H., Mathews S. T., Camp H. S., Ding L., Leff T. (2010) Selective activation of PPARgamma in skeletal muscle induces endogenous production of adiponectin and protects mice from diet-induced insulin resistance. Am. J. Physiol. Endocrinol. Metab. 298, E28–E37 [DOI] [PubMed] [Google Scholar]
- 62. Ye J. M., Doyle P. J., Iglesias M. A., Watson D. G., Cooney G. J., Kraegen E. W. (2001) Peroxisome proliferator-activated receptor (PPAR)-α activation lowers muscle lipids and improves insulin sensitivity in high fat-fed rats: comparison with PPAR-γ activation. Diabetes 50, 411–417 [DOI] [PubMed] [Google Scholar]
- 63. Finsterer J., Stollberger C. (2003) The heart in human dystrophinopathies. Cardiology 99, 1–19 [DOI] [PubMed] [Google Scholar]
- 64. Lehman J. J., Kelly D. P. (2002) Transcriptional activation of energy metabolic switches in the developing and hypertrophied heart. Clin. Exp. Pharmacol. Physiol. 29, 339–345 [DOI] [PubMed] [Google Scholar]
- 65. Ahmad N., Bygrave M., De Zordo T., Fenster A., Lee T. Y. (2010) Detecting degenerative changes in myotonic murine models of Duchenne muscular dystrophy using high-frequency ultrasound. J. Ultrasound. Med. 29, 367–375 [DOI] [PubMed] [Google Scholar]
- 66. Qu-Petersen Z., Deasy B., Jankowski R., Ikezawa M., Cummins J., Pruchnic R., Mytinger J., Cao B., Gates C., Wernig A., Huard J. (2002) Identification of a novel population of muscle stem cells in mice: potential for muscle regeneration. J. Cell Biol. 157, 851–864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Smith P. G., Roy C., Zhang Y. N., Chauduri S. (2003) Mechanical stress increases RhoA activation in airway smooth muscle cells. Am. J. Respir. Cell Mol. Biol. 28, 436–442 [DOI] [PubMed] [Google Scholar]
- 68. Aghajanian A., Wittchen E. S., Campbell S. L., Burridge K. (2009) Direct activation of RhoA by reactive oxygen species requires a redox-sensitive motif. PLoS One 4, e8045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Bowerman M., Beauvais A., Anderson C. L., Kothary R. (2010) Rho-kinase inactivation prolongs survival of an intermediate SMA mouse model. Hum. Mol. Genet. 19, 1468–1478 [DOI] [PubMed] [Google Scholar]
- 70. Segain J. P., Raingeard de la Bletiere D., Sauzeau V., Bourreille A., Hilaret G., Cario-Toumaniantz C., Pacaud P., Galmiche J. P., Loirand G. (2003) Rho kinase blockade prevents inflammation via nuclear factor κB inhibition: evidence in Crohn's disease and experimental colitis. Gastroenterology 124, 1180–1187 [DOI] [PubMed] [Google Scholar]
- 71. Ciaraldi T. P., Cha B. S., Park K. S., Carter L., Mudaliar S. R., Henry R. R. (2002) Free fatty acid metabolism in human skeletal muscle is regulated by PPARγ and RXR agonists. Ann. N. Y. Acad. Sci. 967, 66–70 [DOI] [PubMed] [Google Scholar]
- 72. Demer L. L. (2002) Vascular calcification and osteoporosis: inflammatory responses to oxidized lipids. Int. J. Epidemiol. 31, 737–741 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.