Abstract
Combinations of anticancer therapies with high efficacy and low toxicities are highly sought after. Therefore, we studied the effect of polo-like kinase 1 (Plk1) inhibitors on prostate cancer cells as a single agent and in combination with histone deacetylase (HDAC) inhibitors valproic acid and vorinostat. IC50s of Plk1 inhibitors BI 2536 and BI 6727 were determined in prostate cancer cells by MTS assays. Morphological and molecular changes were assessed by immunoblotting, immunofluorescence, flow cytometry, real-time RT-PCR, and pulldown assays. Efficacy of combination therapy was assessed by MTS and clonogenic assays. IC50 values in DU145, LNCaP, and PC3 cells were 50, 75, and 175 nM, respectively, for BI 2536 and 2.5, 5, and 600 nM, respectively, for BI 6727. Human prostate fibroblasts and normal prostate epithelial cells were unaffected at these concentrations. While DU145 and LNCaP cells were solely arrested in mitosis on treatment, PC3 cells accumulated in G2 phase and mitosis, suggesting a weak spindle assembly checkpoint. Combining Plk1 inhibitors with HDAC inhibitors had synergistic antitumor effects in vitro. DMSO-treated prostate cancer cells were used as controls to study the effect of Plk1 and HDAC inhibition. Plk1 inhibitors decreased proliferation and clonogenic potential of prostate cancer cells. Hence, Plk1 may serve as an important molecular target for inhibiting prostate cancer. Combining HDAC inhibitors with BI 2536 or BI 6727 may be an effective treatment strategy against prostate cancer.—Wissing, M. D., Mendonca, J., Kortenhorst, M. S. Q., Kaelber, N. S., Gonzalez, M., Kim E., Hammers, H., van Diest, P. J., Carducci, M. A., Kachhap, S. K. Targeting prostate cancer cell lines with polo-like kinase 1 inhibitors as a single agent and in combination with histone deacetylase inhibitors.
Keywords: Plk1, HDAC, synergy, mitotic kinases, spindle assembly checkpoint
Of the five known polo-like kinases (Plks), Plk1 has been studied most extensively. Plk1 is a serine-threonine kinase characterized by a N-terminal protein kinase domain and two highly specific C-terminal polo-box domains (PBDs; ref. 1). Plk1 is crucial during cell division, playing an important role from G2/M transition through cytokinesis (2); it may have functions beyond mitosis maintaining genomic stability during DNA replication and as an important modulator of the DNA damage checkpoint (3, 4). Elevated levels of Plk1 have been found in multiple cancers, including prostate cancer (PCa), colorectal cancer, breast cancer and non-small cell lung cancer (5). Plk1 overexpression is associated with a poor prognosis in various cancer types, making Plk1 a potential marker for cancer progression (2, 5, 6). Because of its highly specific PBD domains and overexpression in multiple cancers, several Plk1 inhibitors have been developed and are being tested in phase I and/or II clinical trials for treating both solid and nonsolid tumors (2). Two dihydropteridinone derivatives, BI 2536 and BI 6727 (Volasertib), act as Plk1 inhibitors by inhibiting the enzyme activity of Plk1 in an ATP-competitive way, resulting in significant antitumor activity in vitro and in vivo in a wide variety of cancer cell lines (2, 7, 8). In in vitro kinase assays, BI 2536 inhibits Plk1, as well as the two closely related kinases, Plk2 and Plk3, at lower nanomolar concentrations [half maximal inhibitory concentration (IC50) values 0.83, 3.5, and 9 nM, respectively]; similarly, BI 6727 potently inhibits Plk1, Plk2, and Plk3 (IC50 values 0.87, 5, and 56 nM, respectively), but it is ineffective against a panel of 50 known kinases, even at 10 μM concentrations (7). Phase I and II studies conducted with BI 2536 as a single agent against various cancers, including metastatic castrate-resistant PCa, reported some antitumor effects in patients, while the compound was well tolerated (9–12). BI 6727 is expected to be more potent against tumors due to its favorable pharmacokinetic properties, demonstrating sustained tumor exposure, a high volume of distribution, a long terminal half-life, and good oral bioavailability (7). A phase I study with BI 6727 in patients with advanced solid tumors, including PCa, confirmed these preclinical observations, the compound having a favorable pharmacokinetic profile, promising antitumor activity and manageable toxicities (13). Combining Plk1 inhibitors, which arrest cells in mitosis, with agents that arrest cells in other phases of the cell cycle could potentially further enhance cancer cell death.
In this study, we tested BI 2536 and BI 6727 in PCa cell lines both as a single agent and in combination with histone deacetylase (HDAC) inhibitors valproic acid (VPA) and vorinostat [suberoylanilide hydroxamic acid (SAHA)]. HDACs deacetylate lysine residues in the N-terminal tails of histones, thereby blocking gene transcription; therefore, inhibition of HDACs changes the expression of a wide variety of genes in cancer cells, leading to growth arrest and/or apoptosis (14, 15). Although HDAC inhibitors were initially hypothesized to up-regulate silenced genes only, we and others have found a significant number of genes silenced on HDAC inhibition in PCa cell lines (16). Using analysis of functional annotation (AFA), we found multiple pathways down-regulated by HDAC inhibitors, several of these being involved in mitosis and the cell cycle, such as Plk1 (17). We speculated that combining Plk1 with HDAC inhibitors would have an additive and potentially synergistic effect in inhibiting PCa cells. Our rationale for combining the two inhibitors for treatment of prostate cancer was 2-fold. First, building on our AFA data, we hypothesized that combining HDAC inhibitors and Plk1 inhibitors might target Plk1 function through two different approaches. HDAC inhibition would lead to down-regulation of Plk1 transcript and, hence, less Plk1 protein molecule per cell, which could be effectively inhibited at enzymatic level with the Plk1 inhibitor. Second, HDAC inhibitors and PLK1 inhibitors inhibit cells in different stages of cell cycle. In an asynchronous culture, a HDAC inhibitor would effectively target cells in the G1/G2 phase of the cell cycle, while Plk1 inhibitor could target cells that are in the mitotic phase of the cell cycle. This could lead to an effective/enhanced inhibition in cell proliferation. Further, cells that are resistant to HDAC inhibition, and progress through the interphase could be halted at mitosis by Plk1 inhibition and vice versa. To the best of our knowledge, this is the first report that combines HDAC inhibitors with Plk1 inhibitors for prostate cancer treatment.
MATERIALS AND METHODS
Cell lines and treatment
PCa cell lines were obtained from the American Type Culture Collection (ATCC; Manassas, VA, USA). Human prostate fibroblasts, kindly provided by Dr. J. Isaacs (Johns Hopkins Medical Insitutions, Baltimore, MD, USA), were obtained from a prostate biopsy on a 62-yr-old patient with PCa having a Gleason score of 4. E/957 human telomerase reverse transcriptase (hTERT) cells (hTERT-immortalized normal prostate epithelial cells; ref. 18), also kindly provided by Dr. J. Isaacs, were grown in keratinocyte serum-free medium (K-SFM) supplemented with epithelial growth factor (EGF) and bovine pituitary extract (BPE; Invitrogen, Carlsbad, CA, USA). All other cells were grown in Roswell Park Memorial Institute (RPMI) 1640 (Invitrogen) medium supplemented with 10% FBS. Cells were grown in a humidified incubator at 37°C in a 5% CO2 atmosphere.
Stock solutions of 10 mM of SAHA (AtonPharma, Lawrenceville, NJ, USA), BI 2536 and BI 6727 (Boehringer Ingelheim, Ingelheim am Rhein, Germany), made in dimethyl sulfoxide (DMSO), and stock solutions of 1 M of sodium salt of VPA (Sigma-Aldrich, St. Louis, MO, USA), dissolved in complete RPMI medium, were used for all experiments. For experiments, compounds were further diluted in complete RPMI medium.
Proliferation assay
Cells were plated in 96-well plates containing 100 μl of complete RPMI medium and allowed to adhere overnight before treatment. At 48 h after treatment, cell viability was measured using the CellTiter 96 AQueous nonradioactive cell proliferation assay (Promega, Madison, WI, USA), according to the manufacturer's instructions. Absorption at 490 nm was determined using a microplate reader (Molecular Devices, Sunnyvale, CA, USA).
Clonogenic assay
PCa cells were plated in 60-mm dishes and drugged at 50–60% confluency. Dishes were incubated for 48 h, after which 1 × 103 cells (DU145/PC3) or 2 × 103 cells (LNCaP) from each treated or control dish were plated in triplicate in 60-mm dishes and incubated for 12 d. Colonies were stained with a crystal violet solution (Sigma-Aldrich) and counted manually.
Fluorescence microscopy
Cells were treated with BI 2536 or BI 6727 at 50–70% confluency. All cells, both floating and attached, were collected after 24 h of treatment, washed in phosphate-buffered saline (PBS), and fixed with 10% neutral buffered formalin (NBF). Plk1-inhibited (polo-arrested) cells exhibit condensed chromosomes that are randomly distributed through the cell and fail to congress at a single metaphase plate. For visualization and quantification of polo-arrested cells, fixed cells were washed in PBS, and their nuclei were counterstained with Hoechst 33258 (Sigma-Aldrich). Samples (25 μl) of the cell solution were mounted on slides. Cells were visualized with a Nikon Eclipse Ti inverted research microscope (Nikon Instruments, Linthicum, MD, USA). Multiple fields per slide were photographed, and the numbers of polo-arrested and non-polo-arrested cells were counted manually.
For immunofluorescence, cells fixed in formalin were permeabilized with 0.125% Triton X-100 for 5 min. The permeabilized cells were incubated with primary antibodies for Plk1 (antibody sc-5585; Santa Cruz Biotechnology, Santa Cruz, CA, USA) or phosphorylated aurora kinases (antibody 2914; Cell Signaling Technology, Danvers, MA, USA). Cells were washed in PBS and probed with Alexa Fluor-conjugated secondary antibodies (Molecular Probes, Invitrogen). Nuclei were counterstained with 4′,6-diamidino-2-phenylindole (DAPI; Sigma-Aldrich), and cells were mounted on slides. Confocal images were taken using the Zeiss LSM 510 metaconfocal microscope (Carl Zeiss, Thornwood, NY, USA).
Plk1 activity assay
To assess Plk1 activity, glutathione S-transferase (GST)-PBD pulldown assays were performed according to Park et al. (19), with some modifications. The assay is based on the principle that active Plk1 phosphorylates the centromeric protein polo box interacting domain 1 (PBIP1) at T78, which creates a docking site resulting in a strong interaction between PBIP1 and a PBD domain of Plk1. By using tandem-linked PBIP1 motifs (6 repeats in our experiments) harboring the T78 phosphorylation site, expressed in bacteria as a GST fusion protein, active Plk1 can be pulled out from cells and tissue lysates, which can then be analyzed by Western blotting.
In brief, GST-PBIPtides were expressed and purified from Escherichia coli BL21 by using glutathione (GSH)-Sepharose (GE Healthcare, Waukesha, WI, USA). Proteins bound to the beads were quantified by bicinchoninic acid (BCA) reagent (Pierce Biotechnology, Rockford, IL, USA). For GST-PBIPtide pulldown assays, PCa cells were lysed in lysis buffer [20 mM Tris-Cl, pH 8.0; 150 mM NaCl; 0.5% Nonidet P-40; 1.5 mM EDTA; 1× phosphoSTOP (Roche, Palo Alto, CA, USA), and 1× protease inhibitor (Roche)]. The resulting 500 μg of protein lysates was clarified by centrifugation at 15,000 g for 20 min at 4°C and incubated with bead-bound GST-PBIPtide (100 μg) to precipitate PBIPtide-bound Plk1. Bead-bound Plk1 was detected by Western blotting using a Plk1 antibody (Santa Cruz Biotechnology).
Flow cytometry
Cells were plated in 100-mm dishes and drugged at 50–70% confluency. After 24 h of treatment, both floating and attached cells were collected, washed in PBS, and fixed in a solution containing 10% NBF. Cells were permeabilized in 90% methanol and probed with a primary antibody against phosphorylated H3, followed by an Alexa Fluor 488-conjugated secondary antibody. Nuclei were stained with Hoechst 33258 in PBS containing 10% FBS. Flow cytometry was performed on the LSRII 4-laser, 18-color benchtop flow cytometer (BD Biosciences, San Jose, CA, USA). Data were analyzed using BD FACSDiva software and FlowJo (TreeStar Inc., Ashland, OR, USA).
Immunoblotting and densitometry
Immunoblotting was performed as described previously (17). Total protein (10 μg) was used for electrophoresis and blotted on polyvinylidene difluoride (PVDF) membrane. Primary antibodies were diluted in blocking buffer (5% milk) to a 1:1000 dilution, except for vinculin and actin, which were diluted 1:4000. Secondary antibodies were used at a 1:4000 dilution. Blots were developed using enhanced chemoluminescence (ECL; GE Healthcare) or Femto (Pierce Biotechnology), and density of bands was quantified by ImageJ software (U.S. National Institutes of Health, Bethesda, MD, USA).
Real-time quantitative reverse transcription polymerase chain reaction (RT-PCR) analysis
PCa cells were treated at 50–70% confluency with BI 2536 or BI 6727 for 6 or 24 h. Cells were lysed using TRIzol (Invitrogen) and total RNA was extracted. Total RNA (1 μg) was used for complementary DNA (cDNA) synthesis using the iScript cDNA synthesis kit (Bio-Rad, Hercules, CA, USA). One tenth of the first-strand cDNA reaction was used for RT-PCR amplification and analyzed further as described by Kachhap et al. (17).
Statistical methods
Studies to assess synergy in cell proliferation [i.e., in 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium (MTS) assays], and clonogenic survival assays were performed utilizing Calcusyn (Biosoft, Cambridge, UK), similar to previous studies (20, 21). This software compares the antitumor effect of combination therapy at the provided doses to the doses of single agents required to produce an equal antitumor effect. To quantify levels of synergy, Calcusyn calculates a combination index (CI) using the Chou-Talalay method (22). The calculated CI values were grouped in different levels of synergy (no synergy, CI>0.9; moderate synergy, 0.7<CI<0.9; synergy, 0.3<CI<0.7; strong synergy, 0.1<CI<0.3; very strong synergy, CI<0.1), according to the manufacturer's instructions.
RESULTS
BI 2536 and BI 6727 cause defects in mitotic progression and inhibit proliferation of PCa cells
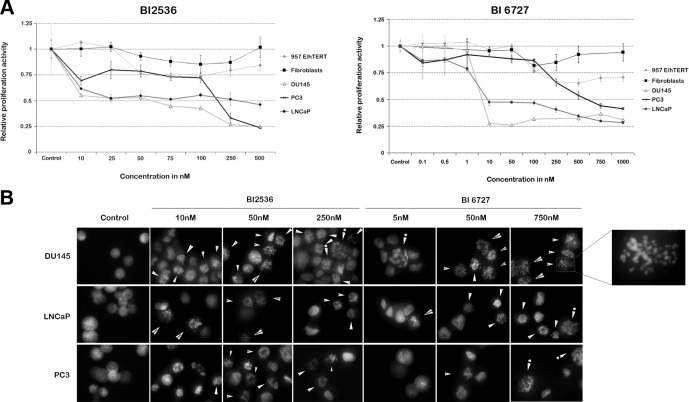
Proliferation of PCa cells, as judged by MTS assays, was considerably reduced by both BI 2536 and BI 6727 (Fig. 1A). For BI 2536, the IC50 in DU145 cells was ∼60 nM, in LNCaP cells ∼75 nM, and in PC3 cells ∼175 nM (Fig. 1A, left panel). These IC50 values were below serum plasma levels, as determined in patients treated with BI 2536, with Cmax (maximum measured concentration in plasma) of 921 ng/ml (1.765 μM) (23). It was noted that with increasing concentrations, proliferation of LNCaP cells was stably 50% lower compared to control cells, while in DU145 and PC3 cells, proliferation activity decreased to 25% relative to controls. We inferred that this was most likely due to the slower proliferative nature of untreated LNCaP cells (doubling time 48 h) compared to DU145 (doubling time 35 h) and PC3 (doubling time 30 h) cells. BI 6727 effectively inhibited DU145 and LNCaP cells: the IC50 in DU145 and LNCaP cells was in the low nanomolar range (<10 nM), but in PC3 cells, the IC50 was ∼600 nM. Despite the relatively high IC50 in PC3 cells, this concentration was still lower than serum plasma levels (Cmax=2.346 μM) established in patients treated with BI 6727 (13).
Figure 1.
Plk1 inhibitors BI 2536 and BI 6727 reduce cell viability in PCa cells by inducing polo arrest. A) Cells were treated with BI 2536 (left panel) or BI 6727 (right panel) for 48 h, after which MTS assays were performed. Control cells were treated with vehicle (0.1% DMSO) in complete RPMI medium. Proliferation of PCa cells was markedly decreased after BI treatment, while human prostate fibroblasts and 957E/hTERT cells were relatively unaffected by the BI compounds after similar treatment. DU145 and LNCaP cells had lower IC50 values than PC3 cells. Results represent average relative proliferative activity of 3 independent experiments. B) PCa cell lines were treated for 24 h with BI 2536 or BI 6727 and fixed, and their nuclei were stained with Hoechst 33258. At low nanomolar concentrations of BI compounds, DU145 and LNCaP cells exhibited a polo-arrest morphology, in which condensed chromosomes randomly organize through the cell (see enlarged image). At high nanomolar concentrations, part of the PC3 cells exhibited similar morphology. Arrows point to polo-arrested cells; arrows with asterisks point to enlarged polo-arrested cells. Image is representative of 3 independent experiments.
To explore whether the BI compounds have inhibitory effects on the proliferation of normal prostate cells, we treated E/957 hTERT (doubling time 60 h), an immortalized normal prostate cell line, and human prostate fibroblast cells (doubling time 55 h) with the Plk1 inhibitors. As depicted in Fig. 1A, both cell lines were relatively unaffected by the inhibitors at concentrations that severely reduced the viability of cancer cells, a feature that may be attributed to the rapid proliferative nature of cancer cells compared to their normal counterparts, suggesting that at concentrations at which Plk1 inhibitors exhibit their effect in prostate cancer cells, the inhibitors are not very toxic to healthy cells.
To investigate whether the reduction in cell proliferation was a result of Plk1 inhibition, we assessed the morphology of BI treated cells. Typical polo-arrested cells undergo mitotic dysregulation, exhibiting condensed chromosomes that fail to congress at the metaphase plate and appear randomly distributed through the cell, a feature attributed to monopolar spindles caused by Plk1 inhibition (2). We visualized Hoechst 33258-stained nuclei of cells treated with Plk1 inhibitors for 24 h under a fluorescent microscope. Dysregulated mitosis was evident in DU145 and LNCaP cells after treatment with either BI compound at low nanomolar concentrations (≤50 nM), indicating that these cells were polo arrested, while PC3 cells displayed mitotic dysregulation only at higher concentrations of the compounds (Fig. 1B, arrows). With an increase in concentration of the Plk1 inhibitors, an increased number of enlarged cells, suggestive of polyploid cells or cells in G2/M phase, were also visible (Fig. 1B, arrows with asterisk and enlarged image). We next quantified these polo-arrested cells. Our data indicate that irrespective of the compound, DU145 cells exhibit most polo-arrested cells as compared to other PCa cell lines (Supplemental Fig. S1). This response cannot be solely attributed to a shorter doubling time of DU145 cells, as PC3 cells, which have a comparable doubling time, exhibited only 40–50% of polo-arrested cells when treated with BI 2536 (Supplemental Fig. S1). This further suggested a differential response of PCa cells to both inhibitors.
BI 2536 and BI 6727 affect Plk1 enzyme activity in PCa cells differently
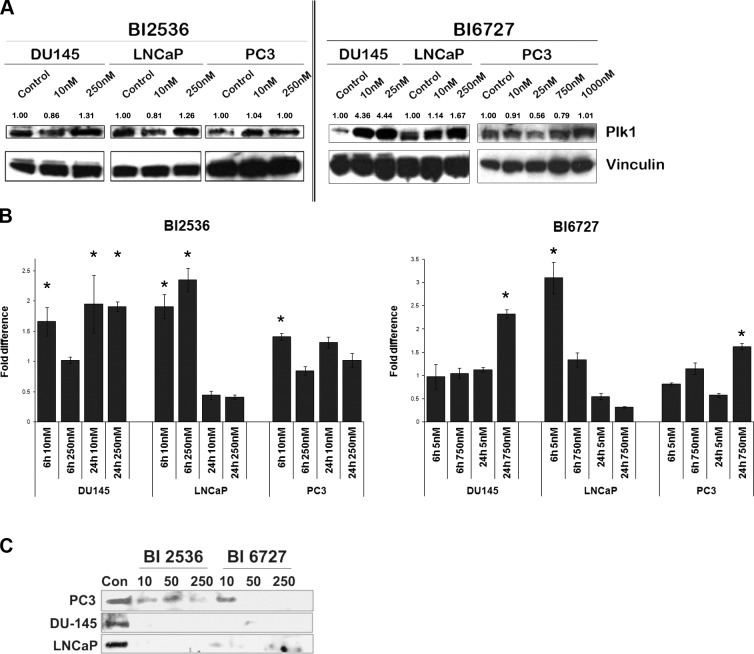
Cancer cells are known to overexpress Plk1, which may provide a selective growth advantage (2, 5). As expected, both normal prostate cell lines had lower level of total Plk1 than their cancer counterpart (Supplemental Fig. S2). The differential response of PCa cells to Plk1 inhibitors could result from differences in dependence on Plk1 among the cell lines. Therefore, we first studied protein expression levels of total Plk1 after Plk1 inhibition by Western blot analysis (Fig. 2A). In general, cells treated with low nanomolar concentrations of the BI compounds did not exhibit a significant change in total Plk1 protein levels compared to untreated cells. However, with increasing concentrations of the BI compounds, an increase in total Plk1 was evident (Fig. 2A). BI 6727-treated DU145 cells were an exception: low nanomolar concentrations of the inhibitor increased the amount of Plk1 to its saturation level, as increasing concentrations did not demonstrate any further increase in Plk1 expression. This result may reflect the difference in response to BI 6727 as the compound is highly effective against DU145 cells, even at lower nanomolar concentrations (as evident in Fig. 1A). To investigate whether the increase in Plk1 protein after treatment with the inhibitors was a direct effect of increased transcription, we quantified Plk1 transcripts by real-time RT-PCR. Messenger RNA (mRNA) levels of total Plk1 was measured after 6 h (early response) and 24 h (delayed response) of BI treatment. Plk1 expression was found to be induced in DU145 and LNCaP cells at different time points. Although LNCaP cells demonstrated an earlier response with both of the inhibitors, DU-145 cells exhibited a delayed response when treated with BI6727. Further, induction of Plk1 transcript in DU-145 cells was restricted to a higher concentration of BI 6727. On the other hand, Plk1 expression in PC3 cells was modestly induced only when treated with a high concentration (750 nM) of BI 6727 (>1.5-fold increase; Fig. 2B). These results led us to infer that induction of Plk1 in DU145 and LNCaP cells could be a consequence of the cells being arrested in mitosis, wherein Plk1 protein levels peak. We speculated that the increase in Plk1 protein after Plk1 inhibition seen in our Western blots may not reflect an increase in Plk1 activity. To ascertain this, Plk1 activity was determined by pulling down active Plk1 using the T78 PBIP1-GST peptide (see Materials and Methods). GST fusion PBIPtide proteins were expressed in BL 21 cells and purified for the assay (Supplemental Fig. S3 and ref. 19). Lysates of Plk1 inhibitor-treated and control PCa cells were used to pull down active Plk1. Active Plk1 levels were assessed by Western blot analysis. As depicted in Fig. 2C, a marked reduction in Plk1 activity was evident in both DU145 and LNCaP cells after treatment with Plk1 inhibitors. Although Plk1 activity decreased after BI treatment compared to untreated cells in all PCa cells, Plk1 activity was markedly higher in BI-treated PC3 cells compared to BI-treated DU145 and LNCaP cells (Fig. 2C).
Figure 2.
Plk1 inhibition leads to differential responses in Plk1 expression and activity among PCa cell lines. A) Exponentially growing PCa cells were treated with Plk1 inhibitors for 24 h. Proteins were extracted, and Western blot analyses for total Plk1 were performed. Numbers indicate densitometric quantification of protein expression levels relative to untreated controls, normalized to the housekeeper vinculin. BI treatment led to an increase of total Plk1 levels in cells. Blots depict a representative image of 3 independent experiments. B) Real-time RT-PCRs performed after 6 and 24 h of BI treatment revealed increases in mRNA levels of Plk1 in DU145 and LNCaP cells (>1.5-fold), while in PC3 cells Plk1 mRNA levels were induced below 1.5-fold with BI 2536 and 1.62-fold with BI6727. Error bars represent sd of averaged fold values of 3 independent experiments. Fold changes were compared to controls using Student's t test. *P < 0.01. C) GST-PBD pulldown assays were performed with BI-treated PCa cell lysates to detect levels of active Plk1. Western blots display the levels of PBIPtide precipitated active Plk1. Residual Plk1 activity was seen in PC3 cells both after BI 2536 and BI 6727 treatment, while Plk1 activity was completely inhibited in DU145 and LNCaP cells. Concentrations are nanomolar. Image is a representative blot of 2 independent experiments.
BI 2536 and BI 6727 affect the subcellular localization of Plk1 in PCa cells
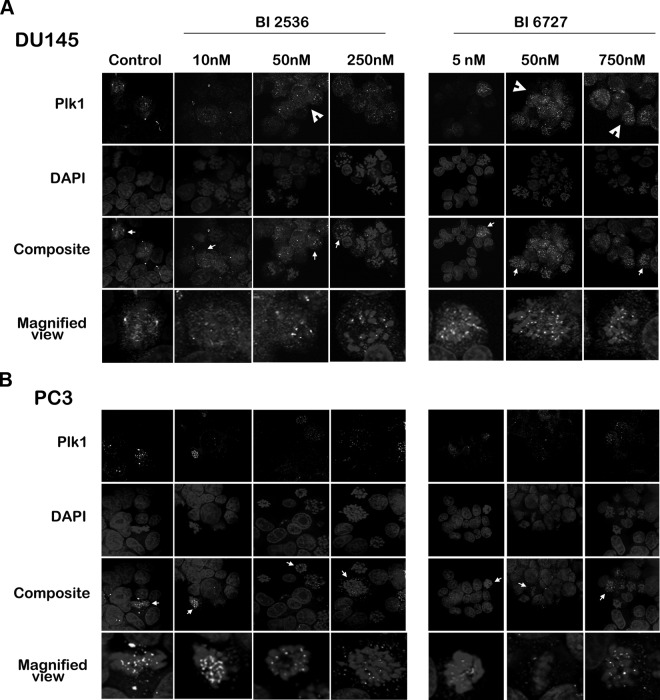
Because the subcellular localization of Plk1 changes relative to its function in different stages of mitosis and cytokinesis, we visualized Plk1 distribution by immunofluorescence after treating the cells with different concentrations of the BI compounds (Fig. 3). In untreated cells, Plk1 staining was most evident in mitosis where it seemed to localize to kinetochores as punctate dots, in the central spindle, in the centrosomal regions, and in the midbody during cytokinesis. This corroborates other reports in which Plk1 localized to the mitotic spindle poles and kinetochores during mitosis (24, 25). Consistent with earlier reports, we also observed centrosomal and faintly diffused cytoplasmic staining for Plk1 during interphase in untreated cells (24). When BI 2536 and BI 6727 were administered, Plk1 localized to condensed chromosomes. Furthermore, an increase in cytoplasmic Plk1 staining was observed in DU145 cells, which seemed to be dose-dependent. However, PC3 cells exhibited this effect to a much lesser extent after similar treatment. Overall, PC3 cells demonstrated a distinct reduction in Plk1 staining and mitotic figures after BI treatment compared to DU145 cells. This observation is in accordance with our Western blot data, which indicated a larger increase in total Plk1-expression in DU145 cells compared to PC3 cells (Fig. 2A). Although there is an increase in Plk1 expression after treatment with the inhibitors, Plk1 activity is markedly reduced in DU145 cells, as demonstrated by the pulldown activity assay (Fig. 2C). It is therefore unlikely that the increased cytoplasmic localization of Plk1 seen through immunofluorescence is enzymatically active. The differences in subcellular localization of Plk1 further indicate a differential response of PCa cells to the inhibitors.
Figure 3.
Plk1 inhibition results in a differential response in the cellular distribution of Plk1 among PCa cell lines. DU145 (A) and PC3 (B) cells were treated for 24 h with BI 2536 (left) or BI 6727 (right), fixed, and stained with DAPI and Plk1 antibody. Cells were visualized under a confocal microscope. BI treatment led to an increase in cytoplasmic staining of Plk1 in DU145 cells (arrowheads), which seemed to be dose dependent. A representative cell in the composite image (arrow) is magnified below. Plk1 is visible on spindles, kinetochores, and poles of control cells. BI-treated cells retain kinetochore Plk1 staining while exhibiting increased cytoplasmic staining in DU-145 cells. PC3 cells exhibit a relative absence of cytoplasmic Plk1 staining. Image is a representative image of ≥3 independent experiments.
Expression and localization of active aurora kinases differ in PCa cells after BI 2536 and BI 6727 treatment
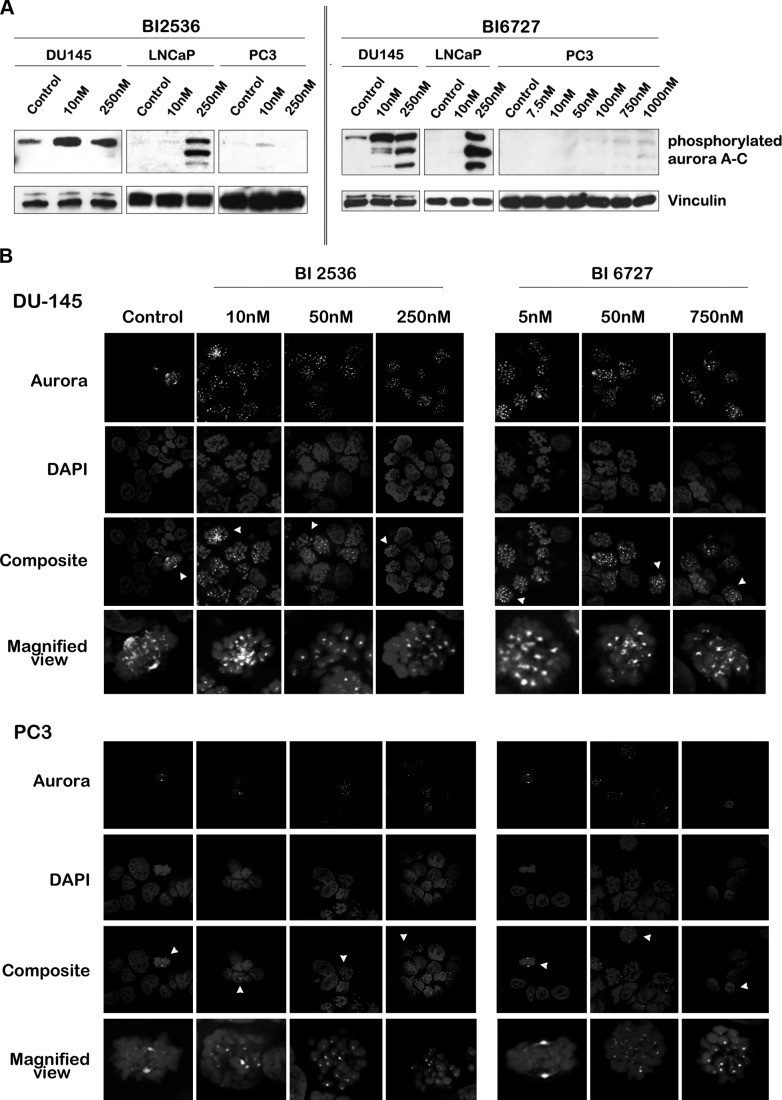
During mitosis, there is extensive crosstalk between Plk1 and aurora A and B kinases, two other key regulators of mitosis (26). Aurora A phosphorylates Plk1 during G2/M transition, while Plk1 controls aurora A localization to the spindle poles through hBora (27, 28). Previous reports have indicated that Plk1 inhibition leads to mislocalization of aurora A (27). Therefore, we assessed changes in aurora expression and localization after Plk1 inhibition. Because phosphorylation of aurora is necessary for the enzyme to exert its kinase activity (29), we determined active aurora A–C using an antibody that recognizes phosphorylated aurora A–C. As indicated by Western blots, protein expression levels of phosphorylated aurora A–C in LNCaP cells were increased after treatment with high nanomolar concentrations of the BI compounds (Fig. 4A). Phosphorylated aurora A protein levels were elevated in DU145 cells when treated with BI 2536; in BI 6727-treated cells an increase in phosphorylated aurora A–C levels was observed with increasing dosage. Intriguingly, PC3 cells did not exhibit an increase in phosphorylated aurora proteins. To investigate whether this was not due to an aurora-null phenotype in PC3 cells, we performed a Western blot analysis in PC3 cells for total aurora kinase A and B (Supplemental Fig. S4A). Aurora A was the predominant isoform present in PC3 cells, which showed a slight increase on treatment with BI compounds. These data indicated that PC3 cells were not null for aurora protein kinase. To test whether PC3 cells lacked the ability to phosphorylate aurora kinase, protein expression levels of phosphorylated aurora A–C proteins were tested after nocodazole treatment. Phosphorylation of aurora kinases increased after nocodazole treatment in DU145, LNCaP, and PC3 cells, suggesting that the lack of phosphorylated aurora kinases in PC3 cells after treatment with the BI compounds was not due to their inability to phosphorylate aurora kinases (Supplemental Fig. S4B). Thus, expression of phosphorylated aurora in response to treatment with the BI compounds differed among PCa cells.
Figure 4.
Expression and localization of active aurora kinases differ between PCa cells after treatment with BI 2536 and BI 6727. A) Western blot analyses for phosphorylated aurora A–C (from high to low molecular weight: aurora A, B, C) were performed after 24 h of Plk1 inhibition with BI 2536 and BI 6727 in PCa cell lines. Protein expression levels of phosphorylated aurora were increased in DU145 and LNCaP cells after BI treatment, but not in PC3 cells. B) DU145 (top panels) and PC3 (bottom panels) cells were treated for 24 h with BI 2536 (left panels) or BI 6727 (right panels), fixed and stained with DAPI, and aurora antibody. Cells were visualized under a confocal microscope. A representative cell (arrowhead) is magnified below. Phosphorylated aurora localized to microtubules and mitotic poles of control cells undergoing mitosis. Cells treated with BI 2536 or BI 6727 lacked polar aurora staining but exhibited aurora at the kinetochores. Image is representative of ≥3 independent experiments.
As with Plk1, the localization of aurora kinases changes during progression through the cell cycle (29). We visualized phosphorylated aurora A–C in untreated and BI-treated PCa cells (Fig. 4B). Similar to previous reports, phosphorylated aurora A–C seemed to localize to kinetochores, poles, and spindles of mitotic cells in untreated controls (29, 30). PC3 cells arrested in mitosis after treatment with the compounds exhibited aurora localization similar to mitotically arrested BI treated DU145 cells. However, the total number of mitotic figures in PC3 cells was lower compared to DU145 cells, which may explain the lack of signal in the Western blot. Although we did not observe any marked increase in cytoplasmic aurora staining as observed in Plk1 after treatment, we did find a total lack of polar and spindle staining for aurora, while kinetochore staining was retained. In this respect, our results differ from a previous report which found an increase in cytoplasmic aurora A after Plk1 knockdown (27). One explanation could be the differences in antibodies used: while our observations are solely based on active/phosphorylated forms of aurora kinases, previous localization experiments were based on antibodies that recognized total aurora A. However, our results are in agreement with other reports that have observed impaired recruitment of aurora A to the centrosome and mitotic spindle (28, 30).
PCa cell lines exhibit variable degrees of mitotic arrest on treatment with BI 2536 and BI 6727
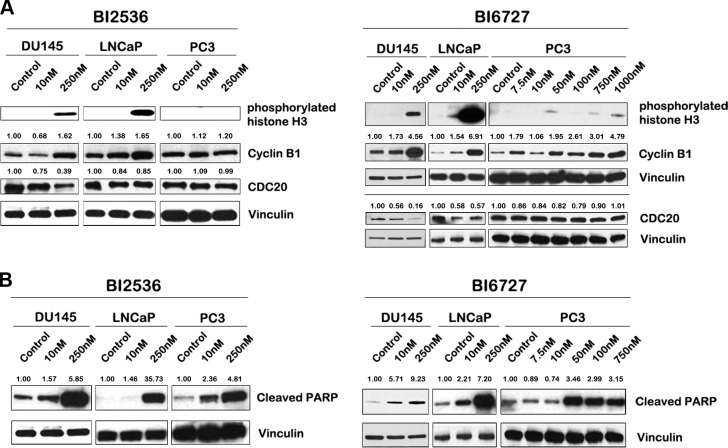
Next, we determined the effect of BI 2536 and BI 6727 on known markers of mitosis. Cells were treated for 24 h with the BI compounds at varying concentrations, and protein expression levels were assessed by Western blots (Fig. 5A). At a final treatment concentration of 250 nM with either compound, DU145 and LNCaP cells had a marked increase in phosphorylated histone H3, suggesting an increase in mitotic population. However, PC3 cells demonstrated only a slight increase in phosphorylated histone H3 when treated with higher nanomolar concentrations of BI 6727, while there was an absence of any detectable protein when treated with BI 2536. Cyclin B1, another mitotic marker, increases late in G2 phase, and is degraded by cell-division cycle protein 20 (cdc20) when cells progress through the spindle assembly checkpoint (SAC), marking the progression from metaphase to anaphase (25, 31). DU145 and LNCaP cells had increased levels of cyclin B1 after BI treatment, suggesting cells are arrested early in mitosis. On the other hand, cyclin B1 protein levels remained unchanged compared to untreated controls in PC3 cells after treatment with BI 2536. Treatment of PC3 cells with high nanomolar concentrations of BI 6727 yielded an increase in cyclin B1 expression. This suggested that on treatment with BI compounds, PC3 cells do undergo mitotic arrest, albeit at higher doses of BI compounds than DU145 and LNCaP cells. We next assessed the levels of cdc20, a protein that plays a key role in the anaphase-promoting complex (31). Activation of the SAC leads to proteolysis of cdc20, causing a (pro)metaphase arrest. Treatment of DU145 and LNCaP cells with BI compounds led to a decrease in cdc20 protein levels, demonstrating activation of the SAC. However, PC3 cells did not show an appreciable change in cdc20 protein levels. This further adds to the notion that PC3 cells lack a strong SAC, which results in cells progressing through mitosis despite Plk1 inhibition, as was evident in the MTS assay results (Fig. 1A).
Figure 5.
Mitotic and apoptotic markers are differentially expressed in PCa cell lines after treatment with BI 2536 and BI 6727. A) Lysates from PCa cells treated with BI compounds were probed for various mitotic markers. DU145 and LNCaP cells demonstrated elevated levels of phosphorylated histone H3 and cyclin B1, and a decrease in cdc20 levels, indicating an increased cell population in early mitosis. A lack of phosphorylated histone H3 induction and cdc20 down-regulation was evident in PC3 lysates. While cyclin B1 levels did not change after BI 2536 treatment in PC3 cells, an increase was evident at high nanomolar concentration of BI 6727. B) Western blot analyses for cleaved PARP in PCa cells after 24 h of treatment with BI compounds. PARP cleavage was increased in all PCa cell lines after treatment. The numbers above Western blots indicate densitometric quantification of protein expression levels relative to untreated controls, normalized to the housekeeper vinculin. All Western blots are representative of ≥3 independent experiments.
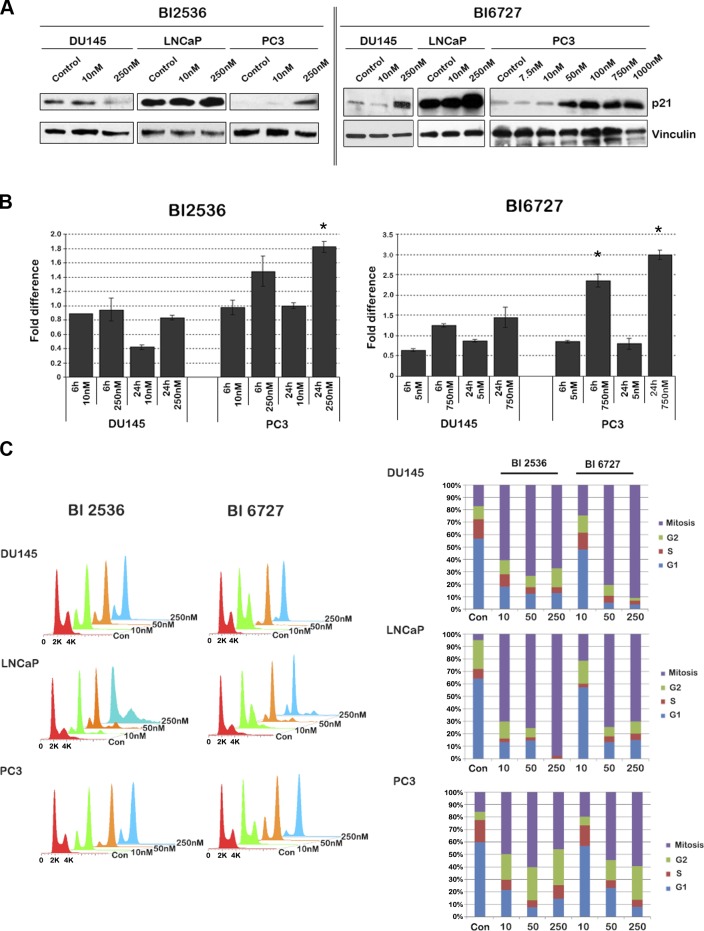
Prolonged mitotic arrest may trigger apoptosis (32, 33). To assess whether BI treatment leads to induction of apoptosis, we assessed, poly (ADP-ribose) polymerase (PARP) cleavage as a surrogate marker for apoptosis (Fig. 5B). Cleaved PARP was increased in all three PCa cell lines with increasing dosage of both BI compounds. After treatment with BI 6727, DU145, and LNCaP cells demonstrated an increase in cleaved PARP at 10 nM, while cleaved PARP was increased in PC3 cells at concentrations of 50 nM and higher only. Thus, both BI 2536 and BI 6727 have the ability to trigger apoptosis in all PCa cell lines, albeit to variable degrees. Since PC3 cells do undergo apoptosis, we wondered whether these cells are arrested in a cell cycle phase other than mitosis. We used p21 as a marker for G1 arrest and were surprised to find a significant dose-dependent induction of p21 in PC3 cells on treatment with both compounds (Fig. 6A). Analysis of mRNA levels of p21 revealed that PC3 cells demonstrated a marked increase in p21 transcripts after treatment with both compounds, while p21 was induced in DU145 cells only at higher nanomolar concentrations of BI 6727 (Fig. 6B). Since both PC3 and DU145 cells are deficient in p53, induction of p21 on Plk1 inhibition is through a p53-independent mechanism. Induction of p21 in PC3 cells suggests a G1 arrest; however, p21 is also known to cause G2 arrest in p53-deficient cells (34, 35). To distinguish between these possibilities, we conducted a fluorescence-activated cell sorting (FACS) analysis of PCa cells after treatment with BI 2536 and BI 6727 (Fig. 6C). Cells were stained for phosphorylated histone H3 to differentiate between cells in G2 phase and in mitosis. Our data indicated that all PCa cell lines exhibited mitotic arrest after BI treatment as indicated by the phosphorylated H3-positive fraction. However, the degree of arrest differed among cell lines. DU145 cells were most sensitive to BI 6727, followed by LNCaP and PC3 cells. LNCaP cells were slightly more sensitive to BI 2536, with 98% of the cells accumulated in mitosis at the highest concentration, while in DU145 and PC3 cells, only 67 and 45% of the cells were in mitosis, respectively. Although the baseline percentage of PC3 cells in G2 phase was low compared to other PCa cell lines, an increase in cells in G2 phase was observed on treatment with either BI compound. Thus, at higher concentrations, the BI compounds caused DU145 and LNCaP cells to accumulate in mitosis and PC3 cells to accumulate both in the G2 and mitotic phase of the cell cycle arrest, eventually triggering apoptosis. Moreover, LNCaP cells exhibited an increase in polyploid cells after Plk1 inhibition.
Figure 6.
DU145 and LNCaP cells are arrested exclusively in mitosis after BI 2536 and BI 6727 treatment, while PC3 cells accumulate both in G2 and M phase. A) Western blot analysis of p21 after treatment of PCa cells with BI 2536 or BI 6727 for 24 h revealed a marked increase in p21 expression in PC3 cells, while DU145 and LNCaP cells only had a marginal increase in p21 expression. B) Real-time RT-PCRs performed after 6 and 24 h of BI treatment of DU145 and PC3 cells revealed increased mRNA levels of p21 in PC3 cells, but not in DU145 cells. Error bars represent sd of averaged fold values of 3 independent experiments. Fold changes are compared to untreated control levels using Student t test. *P < 0.01. C) Analysis of flow cytometry data acquired from PCa cell lines after treatment with BI 2536 and BI 6727 for 24 h revealed the percentage of cells at a respective cell cycle stage. With increasing concentration of the BI compounds, higher percentages of DU145 and LNCaP cells were in mitosis. While the percentage of PC3 cells in mitosis increased with increasing concentrations of the BI compounds, there was a concomitant increase in the percentage of PC3 cells in G2 phase. Concentrations are nanomolar. Flow cytometry data represent 3 independent experiments.
Combinations of Plk1 inhibitors BI 6727 or BI 2536 with HDAC inhibitors VPA or SAHAhave synergistic effects in vitro
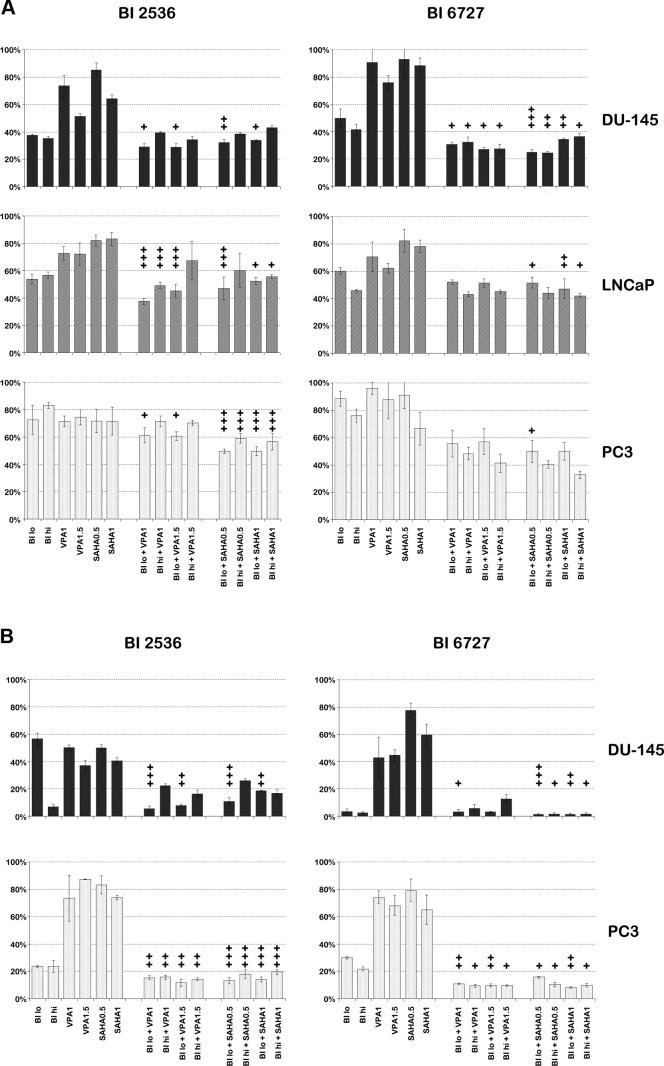
On the basis of previous findings that HDAC inhibitors down-regulate Plk1 expression, we argued that combining HDAC inhibitors with Plk1 inhibitors would increase cell death, as the combination would maximize inhibition of the Plk1 pathway. Besides, we and others have shown that HDAC inhibitors can inhibit growth and proliferation of cancer cells through multiple pathways, such as cell cycle arrest, apoptosis, angiogenesis inhibition, and senescence (15, 36, 37). To ascertain whether down-regulation of Plk1 by HDAC inhibitors at the transcript level was translated to protein expression levels, we determined protein expression levels of Plk1 by Western blots after treatment of DU145 and PC3 cells with HDAC inhibitors VPA and SAHA. As expected, Plk1 expression was decreased after treatment with the HDAC inhibitors at concentrations that correspond with serum plasma levels established in cancer patients treated with VPA (3 mM) or SAHA (6.17 μM) at the maximum tolerated dose (MTD) or below in phase I clinical trials (Supplemental Fig. S5 and refs. 38, 39). Next, we treated PCa cells with combinations of Plk1 inhibitors BI 2536 or BI 6727 and HDAC inhibitors VPA or SAHA. Proliferation and survival were assessed by MTS and clonogenic assays, respectively (Fig. 7). For the MTS assays, two concentrations were chosen per inhibitor based on the IC50 values for the respective cell line. Concentrations used in clonogenic assays were derived from results of the MTS assays. All concentrations used were at or below the plasma serum levels at the MTD in patients as determined in clinical studies (13, 23, 38, 39). Clonogenic results suggested that combining the BI compounds with HDAC inhibitors had a synergistic effect in DU-145 and PC3 cells (Fig. 7 and Table 1). There were three important features evident from these studies. First, combinations of HDAC inhibitors with BI 2536 had a greater synergistic effect on all PCa cell lines than combinations with BI 6727. The lower synergistic effect in combinations with BI 6727 was most likely due to this compound being highly effective as a single agent at very low concentrations: i.e., BI 6727 at a concentration of 10 nM resulted in <5% clonogenic survival in DU145 cells compared to untreated DU145 cells (Fig. 7G, H). Second, combining BI compounds with SAHA led to more synergy than combinations with VPA. This is well illustrated in MTS assays performed after treatment of PCa cells with BI 6727: while combinations of BI 6727 with SAHA did exhibit a synergistic effect, in general, combinations of BI 6727 with VPA failed to exhibit clear synergy, as synergism was limited to DU145 cells (Fig. 7C, D). Third, and most important, increasing treatment concentrations overall diminished synergistic effects; at times, it even seemed to result in antagonistic effects. A clear example is evident in clonogenic assays performed with DU145 cells treated with BI 2536 and/or VPA: at low concentrations of the single agents, survival of DU145 cells was inhibited by 40–50%, while the combination of the two compounds inhibited survival of DU145 cells highly effectively (>90%); on the other hand, at higher nanomolar concentrations, cells were effectively inhibited by BI 2536 alone (<10% clonogenic survival), while the combination of BI 2536 with VPA led to 15–30% clonogenic survival (Fig. 7E). To further validate that combination of low-dose HDAC inhibitors and Plk1 inhibitors does lead to an enhanced decrease in clonogenic potential, we performed the clonogenic experiments with a range of Plk1 inhibitor concentrations with low doses of HDAC inhibitors (Supplemental Fig. S6). We did find a significant increase in cell death in both DU145 and PC3 cells at lower concentrations of Plk1 inhibitor when combined with HDAC inhibitors.
Figure 7.
Combination treatment of Plk1 inhibitors BI 2536 and BI 6727 with HDAC inhibitors VPA and SAHA in PCa cell lines has a synergistic effect in both MTS (A) and clonogenic (B) assays after 48 h of treatment. The antitumor effect was most apparent at low concentrations of the agents, in BI 2536 compared to BI 6727, and in SAHA compared to VPA. +, synergy; ++, strong synergy; +++, very strong synergy, as determined by Calcusyn. For DU145 cells: BI 2536 lo, 10 nM; BI 2536 hi, 100 nM (MTS), or 250 nM (clonogenics); BI 6727 lo, 10 nM; BI 6727 hi, 25 nM. For LNCaP cells: BI lo, 50 nM; BI hi, 250 nM. For PC3 cells: BI 2536 lo, 50 nM; BI 2536 hi, 250 nM; BI 6727 lo, 300 nM; BI 6727 hi, 500 nM. Clonogenic and MTS data represent ≥3 independent experiments.
Table 1.
Combination indices of different combinations of Plk1 inhibitors with HDAC inhibitors in DU145, LNCaP, and PC3 PCa cell lines
| Inhibitor combination | Combination index |
||||
|---|---|---|---|---|---|
| MTS assays |
Clonogenic assays |
||||
| DU145 | LNCaP | PC3 | DU145 | PC3 | |
| BI 2536 | |||||
| BI lo + VPA1 | 0.447 | 0.000 | 0.554 | 0.152 | 0.294 |
| BI hi + VPA1 | 53.814 | 0.019 | 1.164 | 4.963 | 0.299 |
| BI lo + VPA1.5 | 0.670 | 0.012 | 0.426 | 0.290 | 0.170 |
| BI hi + VPA1.5 | 1.103 | 318.620 | 0.714 | 3.381 | 0.189 |
| BI lo + SAHA0.5 | 0.232 | 0.031 | 0.079 | 0.090 | 0.013 |
| BI hi + SAHA0.5 | 25.581 | 6.777 | 0.042 | 5.939 | 0.021 |
| BI lo + SAHA1 | 0.488 | 0.451 | 0.078 | 0.279 | 0.029 |
| BI hi + SAHA1 | 1855.938 | 0.494 | 0.032 | 3.115 | 0.048 |
| BI 6727 | |||||
| BI lo + VPA1 | 0.444 | 0.813 | 0.737 | 0.690 | 0.246 |
| BI hi + VPA1 | 0.686 | 1.034 | 0.847 | 10.378 | 0.327 |
| BI lo + VPA1.5 | 0.538 | 0.981 | 0.951 | 0.835 | 0.215 |
| BI hi + VPA1.5 | 0.656 | 1.399 | 0.901 | 96.972 | 0.346 |
| BI lo + SAHA0.5 | 0.062 | 0.395 | 0.684 | 0.079 | 0.432 |
| BI hi + SAHA0.5 | 0.132 | 0.835 | 0.731 | 0.451 | 0.398 |
| BI lo + SAHA1 | 0.216 | 0.253 | 1.055 | 0.129 | 0.218 |
| BI hi + SAHA1 | 0.616 | 0.681 | 0.894 | 0.398 | 0.400 |
In summary, both BI 2536 and BI 6727 were successful in inhibiting PCa growth and survival in vitro. Combining these Plk1 inhibitors with HDAC inhibitors VPA or SAHA resulted in a synergistic effect depending on the type of inhibitor, the concentration of the inhibitor, and the PCa cell line.
DISCUSSION
Insights gained from various studies indicate that cancer is fueled by multiple pathways, which are either mutated or amplified, to gain growth and invasive potential. The oncogene addiction hypothesis, put forward by Weinstein, states that cancer cells are addicted to such molecular pathways, which, if targeted, could reverse or inhibit the cancer phenotype (40). This has been the rationale for several successful targeted therapies, such as tamoxifen and trastuzumab for breast cancer, and imatinib for gastrointestinal cancers and leukemia (41–43). A protein up-regulated in several cancers that could serve as a target in PCa is Plk1. Multiple small molecules have been generated to inhibit Plk1 and are currently in clinical trials (2). Our study investigates two such compounds, BI 2536 and BI 6727. Multiple-phase II clinical trials have been conducted with BI 2536 (9–12). All trials concluded that BI 2536 was well tolerated (9). A newer, potentially improved compound is BI 6727 (2, 7, 13). Phase I dose escalation studies in patients with advanced solid tumors reported partial responses in patients with sarcoma, head and neck cancer, urothelial cancer, ovarian cancer, and melanoma, the limiting side effect being of hematological origin, similar to BI 2536 (13). Currently, a phase II clinical trial for this compound is being performed in patients with metastatic urothelial cancer (44). Preliminary results of this study are promising, the drug being well tolerated in patients, 14% of the patients having a partial response and 24% of the patients having stable disease.
In the current study, we assessed the effect of BI 2536 and BI 6727 in PCa cells at the molecular level. We further assessed the in vitro potency of both compounds as chemotherapeutics in the treatment of PCa, both as a single agent and in combination with HDAC inhibitors. Our results reveal that both BI 2536 and BI 6727 are potent inhibitors of PCa cell growth, BI 6727 being the more effective of the two. However, we did observe a difference in response to the BI compounds across PCa cell lines. We argued that this differential response could be on account of the inherent molecular differences between the cell lines. Our data indicate that changes in Plk1 activity as a result of treatment with the BI compounds differ among the cell lines: while Plk1 activity was effectively decreased in DU145 and LNCaP cells on treatment, the activity in PC3 cells was relatively refractory to BI treatment. As our study does not include mutation analysis of Plk1 in PCa cell lines, the possibility of PC3 cells harboring a Plk1 mutant, which render the cells less sensitive to the BI compounds, cannot be ruled out. Alternatively, differences in response may arise from differential reliance on Plk1 among PCa cells, which may reflect the heterogeneity in molecular pathways that drive PCa. Another difference may be the presence of a strong SAC in DU145 and LNCaP cells compared to PC3 cells, which may account why PC3 cells are not effectively arrested in mitosis after drug treatment. Nonetheless, we found that the BI compounds can arrest PC3 cells in G2 and M phase, decrease their clonogenic potential, and induce apoptosis, albeit at higher nanomolar concentrations. Arrest of PC3 cells in G2 phase by the Plk1 inhibitors may suggest that Plk1 has functions beyond mitosis (4).
Plk1 inhibitors, such as BI 6727, have a relatively low toxicity profile in humans, but more than half of the patients with metastatic urothelial cancer treated with BI 6727 had progressive disease after 6 wk of treatment (44). Better treatment outcomes for patients with PCa could be accomplished by combining BI compounds with other chemotherapeutics, to accomplish an additive or synergistic effect, while keeping toxicities manageable. To our knowledge, only one phase II clinical trial assessing combinational therapy with a BI compound is currently being performed, in which BI 6727 is combined with cisplatin and carboplatin in patients with advanced solid tumors (45). In this study, preliminary data show a partial response in 8% and stable disease in 50% of the patients. In this study, we combined BI 2536 and BI 6727 with HDAC inhibitors. Taking lead from our AFA study and those of others that indicated that HDAC inhibitors down-regulate mitotic checkpoint genes, Plk1 being one of them (17, 46), we reasoned that Plk1 would be inhibited from a transcriptional angle by the HDAC inhibitors and by direct enzyme inhibition by the Plk1 inhibitors, leading to enhanced cell death. As hypothesized, combining BI compounds with HDAC inhibitors had synergistic effects both in MTS and clonogenic assays. Synergism was more pronounced in the combination of BI 2536 with HDAC inhibitors, and when both types of inhibitors were added in lower concentrations. Intriguingly, higher concentrations of BI compounds and HDAC inhibitors had the same effect as a single agent or were even found to be seemingly antagonistic. Our data, therefore, underscores the importance of correct dosing of both compounds to achieve the desired effect.
In summary, this study tested the Plk1 inhibitors BI 2536 and BI 6727 in PCa cells, and elucidated the molecular mechanism by which the BI compounds effectively inhibit PCa cells. Furthermore, this study clearly indicates that the combination of two cell-cycle-arresting chemotherapeutical agents, Plk1 inhibitors with HDAC inhibitors, has a synergistic effect in inhibiting PCa cells. However, to fully validate this claim, we feel in vivo studies are warranted before further evaluation in clinical trials for PCa.
Supplementary Material
Acknowledgments
The authors thank Boehringer Ingelheim (Ingelheim am Rhein, Germany) for the Plk1 inhibitors, Dr. Kyung S. Lee [U.S. National Cancer Institute (NCI), Bethesda, MD, USA] for providing the GST-PBIPtide-A6 vector, Eugene Kim (Johns Hopkins Medical Institutions, Baltimore, MD, USA) for his help with the Western blots, and Prof. J. W. R. Nortier (Leiden University Medical Centre, Leiden, The Netherlands) and Prof. E. Van der Wall (University Medical Centre Utrecht, Utrecht, The Netherlands) for their support and discussion.
This study was supported by the Flight Attendant Medical Research Institute (FAMRI), an NCI Specialized Programs of Research Excellence (SPORE) grant (P50CA58236), Aegon, and the Prostate Cancer Foundation (Koch Award). M.W. was financially supported by fellowships from the Dr. Saal van Zwanenbergstichting, the Huygens Scholarship Programme (HSP) Talentenprogramma, and Leiden University.
The authors declare no conflicts of interest. The funding sources had no role in the study design, data collection, analysis and interpretation, decision to publish, or preparation of the manuscript.
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
- AFA
- analysis of functional annotation
- BI
- Boehringer Ingelheim
- BPE
- bovine pituitary extract
- cdc20
- cell-division cycle protein 20
- cDNA
- complementary DNA
- CI
- combination index
- DAPI
- 4′,6-diamidino-2-phenylindole
- DMSO
- dimethyl sulfoxide
- EGF
- epithelial growth factor
- GST
- glutathione S-transferase
- hTERT
- human telomerase reverse transcriptase
- Plk1
- polo-like kinase 1
- HDAC
- histone deacetylase
- IC50
- half maximal inhibitory concentration
- mRNA
- messenger RNA
- MTD
- maximum tolerated dose
- MTS
- 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium
- NBF
- neutral buffered formalin
- PARP
- poly (ADP-ribose) polymerase
- PBD
- polo-box domain
- PBID
- polo box interacting domain
- PBS
- phosphate-buffered saline
- PCa
- prostate cancer
- RPMI
- Roswell Park Memorial Institute
- RT-PCR
- reverse transcription polymerase chain reaction
- SAC
- spindle assembly checkpoint
- SAHA
- suberoylanilide hydroxamic acid (vorinostat)
- VPA
- valproic acid
REFERENCES
- 1. De Carcer G., Manning G., Malumbres M. (2011) From Plk1 to Plk5: functional evolution of polo-like kinases. Cell Cycle 10, 2255–2262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chopra P., Sethi G., Dastidar S. G., Ray A. (2010) Polo-like kinase inhibitors: an emerging opportunity for cancer therapeutics. Expert Opin. Invest. Drugs 19, 27–43 [DOI] [PubMed] [Google Scholar]
- 3. El Bahassi M. (2011) Polo-like kinases and DNA damage checkpoint: beyond the traditional mitotic functions. Exp. Biol. Med. 236, 648–657 [DOI] [PubMed] [Google Scholar]
- 4. Takaki T., Trenz K., Costanzo V., Petronczki M. (2008) Polo-like kinase 1 reaches beyond mitosis–cytokinesis, DNA damage response, and development. Curr. Opin. Cell Biol. 20, 650–660 [DOI] [PubMed] [Google Scholar]
- 5. Schmit T. L., Ledesma M. C., Ahmad N. (2009) Modulating polo-like kinase 1 as a means for cancer chemoprevention. Pharmaceut. Res. 27, 989–998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Deeraksa A., Pan J., Sha Y., Liu X. D., Eissa N. T., Lin S. H., Yu-Lee L. Y. (2013) Plk1 is upregulated in androgen-insensitive prostate cancer cells and its inhibition leads to necroptosis. Oncogene 32, 2973–2983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rudolph D., Steegmaier M., Hoffmann M., Grauert M., Baum A., Quant J., Haslinger C., Garin-Chesa P., Adolf G. R. (2009) BI 6727, a Polo-like kinase inhibitor with improved pharmacokinetic profile and broad antitumor activity. Clin. Cancer Res. 15, 3094–3102 [DOI] [PubMed] [Google Scholar]
- 8. Schoffski P. (2009) Polo-like kinase (PLK) inhibitors in preclinical and early clinical development in oncology. Oncologist 14, 559–570 [DOI] [PubMed] [Google Scholar]
- 9. Pandha H. S., Protheroe A., Wylie J., Parker C., Chambers J., Bell S., Munzert G. (2008) An open label phase II trial of BI 2536, a novel Plk1 inhibitor, in patients with metastatic hormone refractory prostate cancer (HRPC). J. Clin. Oncol. 26, 14547 [Google Scholar]
- 10. Gandhi L., Chu Q. S., Stephenson J., Johnson B. E., Govindan R., Bonomi P., Eaton K., Fritsch H., Munzert G., Socinski M. (2009) An open label phase II trial of the Plk1 inhibitor BI 2536, in patients with sensitive relapse small cell lung cancer (SCLC). J. Clin. Oncol. 27, 8108 [Google Scholar]
- 11. Sebastian M., Reck M., Waller C. F., Kortsik C., Frickhofen N., Schuler M., Fritsch H., Gaschler-Markefski B., Hanft G., Munzert G., von Pawel J. (2010) The efficacy and safety of BI 2536, a novel Plk-1 inhibitor, in patients with stage IIIB/IV non-small cell lung cancer who had relapsed after, or failed, chemotherapy: results from an open-label, randomized phase II clinical trial. J. Thorac. Oncol. 5, 1060–1067 [DOI] [PubMed] [Google Scholar]
- 12. Schoffski P., Blay J. Y., De Greve J., Brain E., Machiels J. P., Soria J. C., Sleijfer S., Wolter P., Ray-Coquard I., Fontaine C., Munzert G., Fritsch H., Hanft G., Aerts C., Rapion J., Allgeier A., Bogaerts J., Lacombe D. (2010) Multicentric parallel phase II trial of the polo-like kinase 1 inhibitor BI 2536 in patients with advanced head and neck cancer, breast cancer, ovarian cancer, soft tissue sarcoma and melanoma. The first protocol of the European Organization for Research and Treatment of Cancer (EORTC) Network of Core Institutes (NOCI). Eur. J. Cancer 46, 2206–2215 [DOI] [PubMed] [Google Scholar]
- 13. Schoffski P., Awada A., Dumez H., Gil T., Bartholomeus S., Wolter P., Taton M., Fritsch H., Glomb P., Munzert G. (2012) A phase I, dose-escalation study of the novel Polo-like kinase inhibitor volasertib (BI 6727) in patients with advanced solid tumours. Eur. J. Cancer 48, 179–186 [DOI] [PubMed] [Google Scholar]
- 14. Rokhlin O. W., Glover R. B., Guseva N. V., Taghiyev A. F., Kohlgraf K. G., Cohen M. B. (2006) Mechanisms of cell death induced by histone deacetylase inhibitors in androgen receptor-positive prostate cancer cells. Mol. Cancer Res. 4, 113–123 [DOI] [PubMed] [Google Scholar]
- 15. Xu W. S., Parmigiani R. B., Marks P. A. (2007) Histone deacetylase inhibitors: molecular mechanisms of action. Oncogene 26, 5541–5552 [DOI] [PubMed] [Google Scholar]
- 16. Kortenhorst M. S., Zahurak M., Shabbeer S., Kachhap S., Galloway N., Parmigiani G., Verheul H. M., Carducci M. A. (2008) A multiple-loop, double-cube microarray design applied to prostate cancer cell lines with variable sensitivity to histone deacetylase inhibitors. Clin. Cancer Res. 14, 6886–6894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kachhap S. K., Rosmus N., Collis S. J., Kortenhorst M. S., Wissing M. D., Hedayati M., Shabbeer S., Mendonca J., Deangelis J., Marchionni L., Lin J., Hoti N., Nortier J. W., DeWeese T. L., Hammers H., Carducci M. A. (2010) Downregulation of homologous recombination DNA repair genes by HDAC inhibition in prostate cancer is mediated through the E2F1 transcription factor. PloS One 5, e11208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yasunaga Y., Nakamura K., Ewing C. M., Isaacs W. B., Hukku B., Rhim J. S. (2001) A novel human cell culture model for the study of familial prostate cancer. Cancer Res. 61, 5969–5973 [PubMed] [Google Scholar]
- 19. Park J. E., Li L., Park J., Knecht R., Strebhardt K., Yuspa S. H., Lee K. S. (2009) Direct quantification of polo-like kinase 1 activity in cells and tissues using a highly sensitive and specific ELISA assay. Proc. Natl. Acad. Sci. U. S. A. 106, 1725–1730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cordoba E. V., Arnaiz E., De La Mata F. J., Gomez R., Leal M., Pion M., Munoz-Fernandez M. A. (2013) Synergistic activity of carbosilane dendrimers in combination with maraviroc against HIV in vitro. [E-pub ahead of print] AIDS 10.1097/QAD.0b013e328361fa4a [DOI] [PubMed] [Google Scholar]
- 21. Yun S. M., Jung K. H., Lee H., Son M. K., Seo J. H., Yan H. H., Park B. H., Hong S., Hong S. S. (2013) Synergistic anticancer activity of HS-173, a novel PI3K inhibitor in combination with Sorafenib against pancreatic cancer cells. Cancer Lett. 331, 250–261 [DOI] [PubMed] [Google Scholar]
- 22. Chou T. C., Talalay P. (1984) Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Adv. Enzyme Regul. 22, 27–55 [DOI] [PubMed] [Google Scholar]
- 23. Mross K., Frost A., Steinbild S., Hedbom S., Rentschler J., Kaiser R., Rouyrre N., Trommeshauser D., Hoesl C. E., Munzert G. (2008) Phase I dose escalation and pharmacokinetic study of BI 2536, a novel Polo-like kinase 1 inhibitor, in patients with advanced solid tumors. J. Clin. Oncol. 26, 5511–5517 [DOI] [PubMed] [Google Scholar]
- 24. Lee K. S., Oh D. Y., Kang Y. H., Park J. E. (2008) Self-regulated mechanism of Plk1 localization to kinetochores: lessons from the Plk1-PBIP1 interaction. Cell Div. 3, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Strebhardt K. (2010) Multifaceted polo-like kinases: drug targets and antitargets for cancer therapy. Nat. Rev. 9, 643–660 [DOI] [PubMed] [Google Scholar]
- 26. Lens S. M., Voest E. E., Medema R. H. (2010) Shared and separate functions of polo-like kinases and aurora kinases in cancer. Nat. Rev. Cancer 10, 825–841 [DOI] [PubMed] [Google Scholar]
- 27. Chan E. H., Santamaria A., Sillje H. H., Nigg E. A. (2008) Plk1 regulates mitotic Aurora A function through betaTrCP-dependent degradation of hBora. Chromosoma 117, 457–469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hanisch A., Wehner A., Nigg E. A., Sillje H. H. (2006) Different Plk1 functions show distinct dependencies on Polo-Box domain-mediated targeting. Mol. Biol. Cell 17, 448–459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Crane R., Gadea B., Littlepage L., Wu H., Ruderman J. V. (2004) Aurora A, meiosis and mitosis. Biol. Cell 96, 215–229 [DOI] [PubMed] [Google Scholar]
- 30. De Luca M., Lavia P., Guarguaglini G. (2006) A functional interplay between Aurora-A, Plk1 and TPX2 at spindle poles: Plk1 controls centrosomal localization of Aurora-A and TPX2 spindle association. Cell Cycle 5, 296–303 [DOI] [PubMed] [Google Scholar]
- 31. Bolanos-Garcia V. M. (2009) Assessment of the mitotic spindle assembly checkpoint (SAC) as the target of anticancer therapies. Curr. Cancer Drug Targets 9, 131–141 [DOI] [PubMed] [Google Scholar]
- 32. Weaver B. A., Cleveland D. W. (2005) Decoding the links between mitosis, cancer, and chemotherapy: The mitotic checkpoint, adaptation, and cell death. Cancer Cell 8, 7–12 [DOI] [PubMed] [Google Scholar]
- 33. Valsasina B., Beria I., Alli C., Alzani R., Avanzi N., Ballinari D., Cappella P., Caruso M., Casolaro A., Ciavolella A., Cucchi U., De Ponti A., Felder E., Fiorentini F., Galvani A., Gianellini L. M., Giorgini M. L., Isacchi A., Lansen J., Pesenti E., Rizzi S., Rocchetti M., Sola F., Moll J. (2012) NMS-P937, an orally available, specific small-molecule polo-like kinase 1 inhibitor with antitumor activity in solid and hematologic malignancies. Mol. Cancer Therapeut. 11, 1006–1016 [DOI] [PubMed] [Google Scholar]
- 34. Cazzalini O., Scovassi A. I., Savio M., Stivala L. A., Prosperi E. (2010) Multiple roles of the cell cycle inhibitor p21(CDKN1A) in the DNA damage response. Mutat. Res. 704, 12–20 [DOI] [PubMed] [Google Scholar]
- 35. Cayrol C., Knibiehler M., Ducommun B. (1998) p21 binding to PCNA causes G1 and G2 cell cycle arrest in p53-deficient cells. Oncogene 16, 311–320 [DOI] [PubMed] [Google Scholar]
- 36. Shabbeer S., Kortenhorst M. S., Kachhap S., Galloway N., Rodriguez R., Carducci M. A. (2007) Multiple molecular pathways explain the anti-proliferative effect of valproic acid on prostate cancer cells in vitro and in vivo. Prostate 67, 1099–1110 [DOI] [PubMed] [Google Scholar]
- 37. Richon V. M., Sandhoff T. W., Rifkind R. A., Marks P. A. (2000) Histone deacetylase inhibitor selectively induces p21WAF1 expression and gene-associated histone acetylation. Proc. Natl. Acad. Sci. U. S. A. 97, 10014–10019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Atmaca A., Al-Batran S. E., Maurer A., Neumann A., Heinzel T., Hentsch B., Schwarz S. E., Hovelmann S., Gottlicher M., Knuth A., Jager E. (2007) Valproic acid (VPA) in patients with refractory advanced cancer: a dose escalating phase I clinical trial. Brit. J. Cancer 97, 177–182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Rubin E. H., Agrawal N. G., Friedman E. J., Scott P., Mazina K. E., Sun L., Du L., Ricker J. L., Frankel S. R., Gottesdiener K. M., Wagner J. A., Iwamoto M. (2006) A study to determine the effects of food and multiple dosing on the pharmacokinetics of vorinostat given orally to patients with advanced cancer. Clin. Cancer Res. 12, 7039–7045 [DOI] [PubMed] [Google Scholar]
- 40. Weinstein I. B., Joe A. K. (2006) Mechanisms of disease: Oncogene addiction—a rationale for molecular targeting in cancer therapy. Nat. Clin. Pract. 3, 448–457 [DOI] [PubMed] [Google Scholar]
- 41. Baselga J., Tripathy D., Mendelsohn J., Baughman S., Benz C. C., Dantis L., Sklarin N. T., Seidman A. D., Hudis C. A., Moore J., Rosen P. P., Twaddell T., Henderson I. C., Norton L. (1996) Phase II study of weekly intravenous recombinant humanized anti-p185HER2 monoclonal antibody in patients with HER2/neu-overexpressing metastatic breast cancer. J. Clin. Oncol. 14, 737–744 [DOI] [PubMed] [Google Scholar]
- 42. Deininger M. W., Goldman J. M., Lydon N., Melo J. V. (1997) The tyrosine kinase inhibitor CGP57148B selectively inhibits the growth of BCR-ABL-positive cells. Blood 90, 3691–3698 [PubMed] [Google Scholar]
- 43. Riggs B. L., Hartmann L. C. (2003) Selective estrogen-receptor modulators—mechanisms of action and application to clinical practice. N. Engl. J. Med. 348, 618–629 [DOI] [PubMed] [Google Scholar]
- 44. Stadler W. M., Vaughn D. J., Sonpavde G., Vogelzang N. J., Tagawa S. T., Petrylak D. P., Rosen P. J., Lin C., Mahoney J. F., Modi S. S., Lee P., Ernstoff M. S., Su W., Spira A. I., Ould K. M., Taube T., Vinisko R., Schloss C., Zhao C., Carducci M. A. (2011) Clinical outcome of single agent volasertib (BI 6727) as second-line treatment of patients (pts) with advanced or metastatic urothelial cancer (UC). J. Clin.Oncol. 29, 4567 [Google Scholar]
- 45. Deleporte A., Dumez H., Awada A., Costermans J., Meeus M., Berghmans T., Ould K. M., Juhel N., Berge A., Taube T., Schoffski P. (2011) Phase I trial of volasertib (BI 6727), a polo-like kinase 1 (Plk1) inhibitor, in combination with cisplatin or carboplatin in patients with advanced solid tumors. J. Clin. Oncol. 29, 3031 [Google Scholar]
- 46. Prystowsky M. B., Adomako A., Smith R. V., Kawachi N., McKimpson W., Atadja P., Chen Q., Schlecht N. F., Parish J. L., Childs G., Belbin T. J. (2009) The histone deacetylase inhibitor LBH589 inhibits expression of mitotic genes causing G2/M arrest and cell death in head and neck squamous cell carcinoma cell lines. J. Pathol. 218, 467–477 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.