Abstract
The UT-A1 urea transporter plays an important role in the urinary concentration mechanism. However, the molecular mechanisms regarding UT-A1 trafficking, endocytosis, and degradation are still unclear. In this study, we identified the small GTPase Rab14 as a binding partner to the C terminus of UT-A1 in a yeast 2-hybrid assay. Interestingly, UT-A1 binding is preferential for the GDP-bound inactive form of Rab14. Coinjection of Rab14 in Xenopus oocytes results in a decrease of UT-A1 urea transport activity, suggesting that Rab14 acts as a negative regulator of UT-A1. We subsequently found that Rab14 reduces the cell membrane expression of UT-A1, as evidenced by cell surface biotinylation. This effect is blocked by chlorpromazine, an inhibitor of the clathrin-mediated endocytic pathway, but not by filipin, an inhibitor of the caveolin-mediated endocytic pathway. In kidney, Rab14 is mainly expressed in IMCD epithelial cells with a pattern identical to UT-A1 expression. Consistent with its role in participating in clathrin-mediated endocytosis, Rab14 localizes in nonlipid raft microdomains and codistributes with Rab5, a marker of the clathrin-mediated endocytic pathway. Taken together, our study suggests that Rab14, as a novel UT-A1 partner, may have an important regulatory function for UT-A1 urea transport activity in the kidney inner medulla.—Su, H., Liu, B., Fröhlich, O., Ma, H., Sands, J. M., Chen, G. Small GTPase Rab14 down-regulates UT-A1 urea transport activity through enhanced clathrin-dependent endocytosis.
Keywords: protein interaction, membrane protein, inner medulla
Urea plays a critical role in the urinary concentrating mechanism in the kidney inner medulla (IM). The major mechanism for delivering urea to the IM interstitium is facilitated by urea transport mediated by urea transporter A1 (UT-A1) of the terminal IM collecting duct (IMCD). The reabsorbed urea is then recycled within the IM and contributes to the development of the corticomedullary osmotic gradient (1, 2). Urea's important role in the urine-concentrating mechanism was appreciated by studies of a UT-A1/UT-A3-knockout mouse, which has seriously impaired urea reabsorption and urine-concentrating ability (3). Arginine vasopressin (AVP) is the primary hormone regulating UT-A1 transport activity in vivo (4, 5).
As a membrane protein, successful trafficking to and residing on the cell surface are the prerequisites for its proper functions. During the past decade, much attention has been paid to a group of important accessory proteins that determine the specificity of a membrane protein in sorting, membrane trafficking, and retrieval. A number of proteins, including SPA-1 (6), syntaxin-3 (7), syntaxin-4 (8), SNAP23 (9), Rho GTPase (10), dynein and dynactin (11), and actin (12–13), are involved in regulating water channel AQP2 trafficking and membrane expression. UT-A1 membrane trafficking, endocytosis, and degradation are regulated by the SNARE-associated proteins snapin (14), dynamin (15), caveolin (16), actin (17), and MDM2 (18).
Rab GTPase is the largest subfamily of the Ras-related GTPase superfamily and plays a key role in the regulation of intracellular membrane trafficking (19–21). Human cells contain ∼70 Rabs and Rab-like proteins (22). Most Rab proteins are ubiquitiously expressed, indicating a fundamental role for these proteins in membrane trafficking activity (22). Many isoforms of the Rab family localize to specific membrane compartments: Rab5 and 15 are on early endosomes (23–24); Rab6 is on the Golgi complex (25); and Rab7 and Rab9 are on the late endosomes (26–27). The C-terminal hypervariable domains are responsible for Rab protein localization (28). Rab proteins are anchored in the membrane through a geranylgeranyl group linked to cysteine residues in their carboxyl terminus (22).
Similar to all small GTPase proteins, the function of Rabs shifts between a GDP-bound inactive and a GTP-bound active form. The Rab proteins change their conformation on nucleotide binding. The existence of multiple Rab isoforms and their effector proteins allows Rab proteins to have multiple functions in regulating intracellular trafficking during endocytosis, exocytosis, and secretion (19, 22). Rab dysfunction has been linked to a variety of human diseases ranging from infectious diseases to cancer (29–30).
In this study, we employed a yeast 2-hybrid assay, screened a kidney cDNA library, and found that the small GTPase Rab14 could directly bind to the C terminus of UT-A1. Functionally, coexpression of Rab14 and UT-A1 in oocytes led to a reduction in urea transport. Furthermore, we found that Rab14, codistributed with Rab5 in cell membrane nonlipid raft domains and early endosomes, enhances UT-A1 clathrin-mediated endocytosis and protein degradation.
MATERIALS AND METHODS
Animals
The protocols used in this study were approved by the Institutional Animal Care and Use Committee of Emory University and complied with the U.S. National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Constructs
The pGEX-KG-Rab14 construct was generously provided by Dr. Richard Scheller (Genentech, South San Francisco, CA, USA; ref. 31). Rab14 S25N and Q70L mutants were generated by site-directed mutagenesis (Stratagene, La Jolla, CA, USA) and were verified by DNA sequencing. Hemagglutinin (HA)-tagged Rab14 was obtained by PCR by using pGEX-KG-Rab14 as a template and subcloned into pcDNA3 vector for transfection in HEK 293 cells. The bait construct (pGBKT7-C-UT-A1), which encoded the 48 C-terminal residues of UT-A1 for the yeast 2-hybrid assay, was described previously (17). UT-A1, engineered to contain an extracellular N-terminal Flag (pcDNA3-FLAG-Tac-UT-A1), was as described previously (32). pSuppressor-scramble and pSuppressor-μ2 were reported previously (15).
Yeast 2-hybrid analysis
The bait pGBKT7-C-UT-A1 construct was transformed into AH109 (BD Biosciences, San Jose, CA, USA) using the lithium acetate method and mated with Y187 pretransformed human kidney cDNAs (BD Biosciences) as described previously (17). The mating mixtures were plated on SD/Trp−Leu−Ade−His− medium. After 10–14 d, positive colonies were collected and reselected from the SD/Trp−Leu−Ade−His−/X-α-Gal plate. Positive colonies were collected and processed for plasmid DNA purification and sequencing.
In vitro translation and binding assay
The bait gene (C-UT-A1) in pGBKT7 was tagged with c-myc. The targeted gene Rab14 (clone 22) in pACT2 obtained from cDNA library screening was tagged with HA. [35S]Methionine-labeled proteins (C-UT-A1 and Rab14) were prepared by using the TNT T7-coupled rabbit reticulocyte lysate system (Promega, Madison, WI, USA) and then, either alone or mixed, immunoprecipitated by c-myc or HA antibody (Clontech, Mountain View, CA, USA). The proteins were resolved by SDS-PAGE. The gel was dried and analyzed by autoradiography (17).
GST fusion protein and pull-down assays
pGEX Rab14 constructs were transformed into Escherichia coli BL21 (Stratagene, La Jolla, CA, USA). Cells were grown at 30°C overnight and induced with 1 mM isopropyl thio-β-d-galactopyranoside (IPTG; Calbiochem, Billerica, MA, USA) for 4 h. The bacterial pellet was resuspended and sonicated in harvest buffer (10 mM HEPES, 50 mM NaCl, 1 mM benzamidine, 5 mM EDTA, and a cocktail of protease inhibitors). The lysates were centrifuged for 30 min at 15,000 rpm. The supernatants were collected, and the GST fusion proteins were purified by incubation with glutathione Sepharose 4B beads (Amersham Biosciences, Piscataway, NJ, USA) at 4°C overnight with gentle rotation. The beads were washed with harvest buffer. Equal amounts of kidney IM tissue lysates were incubated with GST alone or GST fusion Rab14 proteins at 4°C overnight. After washing, the proteins were eluted with Laemmli buffer and subjected to Western blot analysis.
Cell culture, transient transfection, and treatment
UT-A1 HEK 293 cells were grown in DMEM supplemented with 10% FCS, 25 mM HEPES and antibiotics in a 5% CO2 incubator at 37°C. HEK 293 cell transfection was performed using Lipofectamine (Invitrogen, Grand Island, NY, USA), according to the manufacturer's instructions. Prior to cell collection, some cells were subjected to chemical treatment for the indicated times. Cells were then processed for cell surface biotinylation, immunoprecipitation or immunofluorescence microscopy. Chlorpromazine and filipin were purchased from Sigma-Aldrich (St. Louis, MO, USA).
Cell surface biotinylation
Cell surface biotinylation assay was done as described previously (33).
Immunoprecipitation and Western blot
After transfection/treatment, cells were solubilized in RIPA buffer (33). The supernatant was recovered after 10,000 rpm centrifugation for 10 min. The supernatants were incubated with relevant antibodies overnight at 4°C, followed by incubation with protein-A or protein-G Sepharose beads (Santa Cruz Biotechnology, Dallas, TX, USA) for 2 h. After washing, beads were eluted in Laemmli sample buffer and run on SDS-PAGE for Western blot analysis. The antibodies used in this study include UT-A1 (33), Rab14 (a generous gift of Richard Scheller), Rab5 (Santa Cruz Biotechnology), c-myc (Clontech), HA (Clontech), Na+/K+ ATPase α subunit (Sigma-Aldrich), caveolin (BD Bioscience), transferrin receptor (Invitrogen), and actin (Sigma-Aldrich).
Lipid raft fractionation
Lipid raft isolation was performed with a 5–40% sucrose discontinuous gradient as described previously (34). Briefly, rat IMCD suspensions were prepared (36) and lysed in ice-cold 0.5% Brij96-TNEV buffer for 30 min on ice. Postnuclear supernatant (500 μl) was mixed with an equal volume of 80% sucrose in TNEV and transferred to 13- × 51-mm Beckman centrifuge tubes. Sucrose (35%, 3 ml) in TNEV was layered carefully on top of the mixture, followed by another 1-ml layer of 5% sucrose. The sucrose gradient was centrifuged in a SW 50.1 rotor (Beckman, Brea, CA, USA) at 34,000 rpm (110,000 g) for 18 h at 4°C. Equal sizes of fractions (∼400 μl) were collected from the top to bottom of the tube and analyzed by Western blotting with respective antibodies.
Plasma membrane (PM) and endosome preparation
PM from HEK 293 cells was prepared from a 5-step sucrose gradient (2.0, 1.6, 1.4, 1.2, and 0.8 M) ultracentrifugation performed as described previously (33). The fraction from the interface of 1.6/1.4 density was collected as PM.
Early endosome preparation followed the protocol reported by Butterworth et al. (36). Briefly, after the cells were lysed, the postnuclear supernatant was diluted 1:1 with 62% sucrose in HEPES buffer and placed at the bottom of a 5-ml ultracentrifuge tube. Sucrose (35%, 1.5 ml) was layered on top, followed by 1.5 ml of 25% sucrose and 0.5 ml of HEPES buffer. The gradients were centrifuged in a SW50.1 rotor at 37,000 rpm (∼167,000 g) for 75 min at 4°C. The interface between 25 and 35% sucrose was collected as the early endosomal fraction.
UT-A1 endocytosis and confocal microscopy
HEK 293 cells stably expressing an external FLAG-tagged UT-A1 were transfected with pcDNA3-HA-Rab14 or pSuppressor-μ2 (15). After 48 h, the cells were washed with ice-cold PBS and incubated with FLAG monoclonal antibody on ice for 30 min. Unbound antibody was removed with 3 washes of ice-cold PBS. Prewarmed complete culture medium was added, and the cells were switched to 37°C for 30 min to allow internalization. After fixation with 3% paraformaldehyde, the cells (without permeabilization treatment) were incubated with Texas Red-conjugated anti-mouse antibody. Cells were mounted with Vectashield (Vector Laboratories, Burlingame, CA, USA) and examined under a confocal microscope.
Immunohistochemistry
The kidneys used for immunohistochemistry were perfused with 4% paraformaldehyde and processed for paraffin section. After dewaxing and quenching endogenous peroxidase activity, the sections were incubated with primary antibody against Rab14 or UT-A1, followed by biotinylated anti-rabbit IgG and avidin-biotin peroxidase complex (Vector Laboratories). Colors were developed using aminoethyl carbozole (AEC, Zymed, South San Francisco, CA, USA) as substrate and examined under a microscope.
Oocyte isolation and urea flux experiment
A female Xenopus laevis was anesthetized with 0.2% Tricaine (Sigma-Aldrich), and oocytes were harvested. The oocytes were defolliculated twice with 2 mg/ml collagenase I–V (Sigma-Aldrich). Stage V–VI oocytes were used for cRNA injection (17). Capped cRNAs for UT-A1 and Rab14 were transcribed with T7 polymerase using the mMessage mMachine T7 Ultra Kit (Ambion, Austin, TX, USA. UT-A1 and Rab14 cRNA (5 ng) in 23 nl of water were injected into each oocyte. After 3 d, good and healthy oocytes were selected for protein expression and urea transport functional measurements, as described previously (17).
Statistics
Urea flux data are expressed as means ± sd. One-way ANOVA followed by Tukey's HSD test was used to assess statistically significant differences.
RESULTS
Identification of Rab14 as a UT-A1 interaction partner
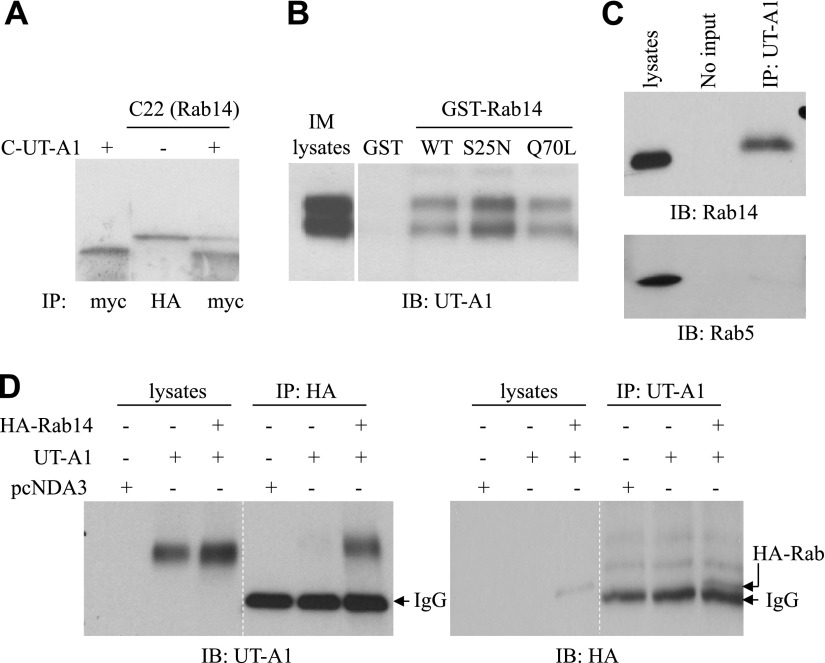
To identify proteins that associate with, and potentially regulate, UT-A1, we used a 48-aa C-terminal fragment of UT-A1 as bait in a yeast 2-hybrid assay. A clone (C22) was obtained after a screening 5 × 107 CFU kidney cDNA library. DNA sequencing revealed that it is Rab14, a member of the Rab family of small GTPases. The interaction between Rab14 and UT-A1 was verified by an in vitro binding assay. As shown in Fig. 1A, the UT-A1 C terminus could be immunoprecipitated by Rab14 (C22).
Figure 1.
Direct interaction between Rab14 and UT-A1. A) In vitro binding assay. The UT-A1 binding protein C22 (Rab14) and C-UT-A1 bait were in vitro translated in rabbit reticulocyte lysates and labeled with 35S methionine. Rab14 tagged by HA and C-UT-A1 tagged by c-myc, alone or combined, were then immunoprecipitated with HA or C-myc antibody for 1 h. The samples were resolved by 10–20% gradient gel and analyzed by autoradiography. B) GST pull-down assay. GST-Rab14 fusion proteins (WT, S25N, and Q70L) were prepared from E. coli BL21, then incubated with kidney IM tissue lysates overnight at 4°C. After washing, the precipitated products were processed for immunoblotting with UT-A1 antibody. WT, wild type. C) Coimmunoprecipitation of Rab14 and UT-A1 from kidney IM. Tissue lysates from normal rat kidney IM was immunoprecipitated by UT-A1, then immunoblotted with Rab14 or Rab5 antibody. D) Coimmunoprecipitation of Rab14 and UT-A1 in mammalian HEK 293 cells. HEK 293 cells were transiently transfected with pcDNA3 vector, pcDNA3-UT-A1, or pcDNA3-UT-A1 plus HA-Rab14 for 2 d. Cell lysates were incubated with HA or UT-A1 antibody overnight at 4°C, followed by a 1-h incubation of protein-A beads. The precipitated samples were processed for immunoblotting with antibodies to UT-A1 (left panel) or HA (right panel).
GDP-bound Rab14 preferentially binds to UT-A1
To further confirm the interaction of Rab14 and UT-A1, we performed GST pull-down experiments using rat kidney IM tissue lysates. UT-A1 expressed in IM exhibits two different glycosylation forms of 97 and 117 kDa (34, 35). Figure 1B shows that Rab14 binds to both 97- and 117-kDa forms of UT-A1. However, the binding was stronger for Rab14 S25N, a GDP-bound Rab14, than for wild-type (WT) Rab14 or Rab14 Q70L (GTP-bound Rab14), indicating that the nucleotide state of Rab14 affects the ability to bind UT-A1.
To clarify the specificity of Rab14 interaction with UT-A1 in vivo, kidney IM tissue lysates were used for immunoprecipitation by UT-A1 antibody and followed by immunoblotting for Rab14 and Rab5. As shown in Fig. 1C, Rab14, but not Rab5, was immunoprecipitated by UT-A1.
The specific protein-protein interaction between Rab14 and UT-A1 was also examined in a mammalian cell line, HEK 293 cells. The UT-A1 and Rab14 complex could be immunoprecipited by either HA antibody or UT-A1 antibody (Fig. 1D).
Rab14 reduces UT-A1 activity in Xenopus oocyte
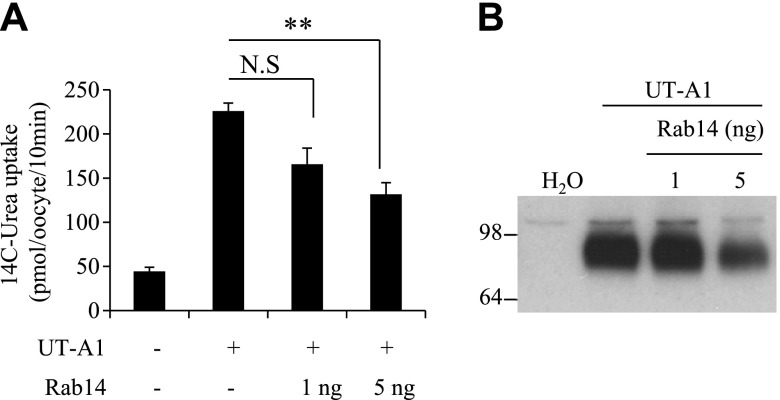
Next, we assessed the functional role of Rab14 on UT-A1 urea transport activity in the Xenopus oocyte system. UT-A1 and Rab14 cRNAs were coinjected into oocytes. UT-A1 urea transport activity, measured by 14C-urea flux, was significantly reduced when the oocytes were coinjected with Rab14 cRNAs (Fig. 2A). Western blot analysis revealed that Rab14 reduced UT-A1 protein expression (Fig. 2B).
Figure 2.
Rab14 reduces UT-A1 activity in Xenopus oocytes. UT-A1 cRNA (5 ng/cell) alone or together with Rab14 cRNA (1–5 ng/cell) was microinjected into oocytes and incubated at 18°C for 3 d. A) Urea transport activity was measured by [14C]urea flux at 10 min (n=5–6, means ± sd). **P < 0.01. B) Cell lysates (10 oocytes/group) were prepared in RIPA lysis buffer and immunoblotted with UT-A1 antibody.
Rab14 reduces UT-A1 membrane expression
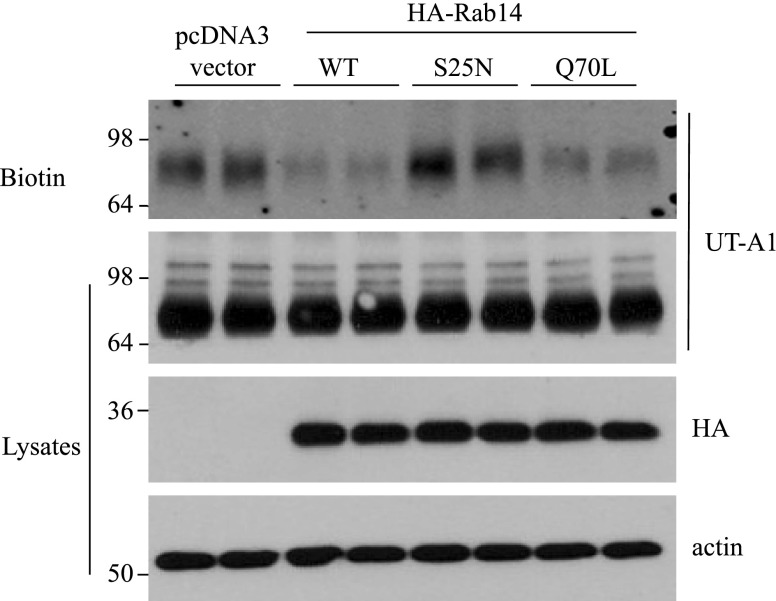
We then specifically examined the effect of Rab14 on UT-A1 cell membrane expression. UT-A1-HEK 293 cells were transfected with pcDNA3 vector or 3 types of Rab14: WT, the GDP-bound inactive form (S25N), and the GTP-bound active form (Q70L). Cell surface UT-A1 abundance was examined by biotinylation. Overexpression of Rab14 significantly reduced UT-A1 cell membrane expression (Fig. 3).
Figure 3.
Rab14 reduces UT-A1 membrane expression. UT-A1-HEK 293 cells grown in 6-well plates were transfected with pcDNA3 vector or HA-Rab14 (WT, S25N, Q70L). After 48 h, the cells were processed for cell surface biotinylation. The biotinylated samples and the total proteins were analyzed for UT-A1 expression by Western blot. The same samples were also probed with HA and actin antibodies to evaluate Rab14 protein expression and an equal amount of protein loading.
Chlorpromazine treatment or μ2 siRNA transfection prevents Rab14-induced cell membrane UT-A1 reduction
We previously reported that UT-A1 internalization is through both caveolin- and clathrin-mediated endocytic pathways (15). To explore whether Rab14 causing UT-A1 membrane reduction could influence UT-A1 endocytosis through one or both pathways, UT-A1-HEK 293 cells were transfected with or without Rab14, and then were treated for 1 h prior to cell collection with 10 μg/ml chlorpromazine, which inhibits clathrin-coated pit-mediated endocytosis, or 1 μM filipin, which inhibits caveolae-mediated endocytosis. UT-A1 accumulation in the cell PM was measured by biotinylation. Overexpression of Rab14 reduced cell surface UT-A1 expression; however this effect was blocked when the clathrin endocytic pathway was inhibited by chlorpromazine. Inhibition of caveolin endocytic pathway with filipin did not affect Rab14 induced UT-A1 membrane expression (Fig. 4A).
Figure 4.
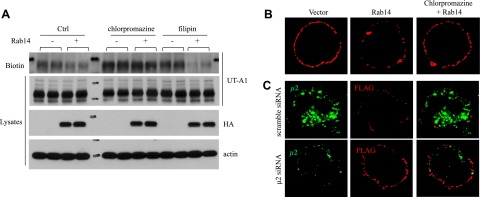
Inhibition of clathrin endocytic pathway blocks Rab14-induced UT-A1 membrane reduction. A) Cell surface biotinylation assay. UT-A1-HEK 293 cells were transfected with or without Rab14. After 48 h, cells were treated with 10 μg/ml chlorpromazine or 1 μM filipin for 1 h, and then processed for biotinylation. The cells were lysed, and the biotinylated samples and total proteins were analyzed by Western blot with antibodies to UT-A1, Rab14 (HA) or actin. B) Chlorpromazine treatment. External FLAG-tagged UT-A1 HEK 293 cells were transfected with pcDNA3-HARab14 for 48 h. Cells were treated with or without 10 μg/ml chlorpromazine for 1 h. Cell surface UT-A1 was labeled with anti-FLAG antibody on ice for 1 h. After washing, the cells were rewarmed at 37°C for 30 min. Cells were then fixed, and the UT-A1 remaining on the cell surface was detected by Texas Red-conjugated anti-mouse antibody without membrane permeabilization. C) Knocking down μ2 by siRNA. External FLAG-tagged UT-A1 HEK 293 cells were cotransfected with Rab14 together with pSuppressor-scramble or pSuppressor-μ2 for 48 h. UT-A1 internalization was induced, and cell surface UT-A1 was detected as described above.
To directly visualize the effect of Rab14 on UT-A1 endocytosis, we used FLAG-Tac-fused UT-A1. The recombinant FLAG NH2-terminal end is present in the extracellular space (32). The HEK 293 cells transfected with FLAG UT-A1 were incubated with FLAG antibody on ice. When the cells warm up at 37°C, UT-A1 internalization occurs, and the cell surface FLAG antibody-conjugated UT-A1 is decreased. After fixation, the cells were incubated with secondary fluorescent antibody without any membrane permeabilization treatment, so that only the UT-A1 remaining on the cell membrane was labeled. As seen in Fig. 4B, comparing with the control cells without Rab14 transfection, overexpression of Rab14 reduced cell surface UT-A1. However, chlorpromazine treatment prevented UT-A1 loss from the cell membrane induced by Rab14. Alternatively, we employed siRNA technology to knock down AP-2 clathrin adaptor complex component μ2 and observed whether Rab14-induced UT-A1 endocytosis is mediated through the clathrin endocytic pathway. Figure 4C shows that knocking down μ2 blocked Rab14-induced UT-A1 endocytosis. However, knocking down caveolin, which affects the caveolae-mediated endocytic pathway, did not change UT-A1 endocytosis induced by Rab14 (data not shown).
Rab14 colocalizes with UT-A1 in PM and early endosomes
To verify Rab14 expression and function at the cell membrane, cell PM from UT-A1-HEK 293 cells was prepared by a sucrose gradient ultracentrifugation (37). Western blot showed that Rab14, as well as UT-A1, is present in cell PM (Fig. 5A). Na+/K+ ATPase was used as the PM marker. We also isolated early endosomes (Fig. 5B) and found that Rab14 was highly abundant in early endosomes. Rab5 served as a marker for the early endosomal fractions. Actin was used as a negative control. Rab14 and UT-A1 are codistributed in the endosomes.
Figure 5.

Rab14 expression in PM and endosomes. A) Isolation of PM by sucrose gradient ultracentrifugation. UT-A1-HEK 293 cells, transfected with or without Rab14, were lysed, and the supernatants were loaded on a 5-step discontinuous sucrose gradient as described in Materials and Methods. After ultracentrifugation, the fraction between 1.6/1.4 M sucrose was collected as PM. B) Early endosome preparation. UT-A1-HEK 293 cell lysates were loaded on a sucrose gradient as described in Materials and Methods. The fractions between 25 and 35% sucrose were collected as the early endosome. The UT-A1 and Rab14 in the PM (A) and endosome (B) were analyzed by Western blot. Rab5, Na+/K+ ATPase, and actin were used as controls. C) UT-A1-HEK 293 cells, transfected with or without Rab14, were fixed in with 3% paraformaldehyde, permeabilized with 0.2% Triton X-100, and immunostained with UT-A1, Rab14 (HA), or Rab5. The cells were examined under a confocal microscope.
We then employed an immunocytochemical study and evaluated whether UT-A1 and Rab14 are colocalized in the clathrin-mediated endocytic pathway with Rab5 in HEK 293 cells. Figure 5C shows that the Rab14 and Rab5 distribution are exactly identical. There is partial colocalization of Rab14 and UT-A1 in HEK cells (Fig. 5C, bottom panel).
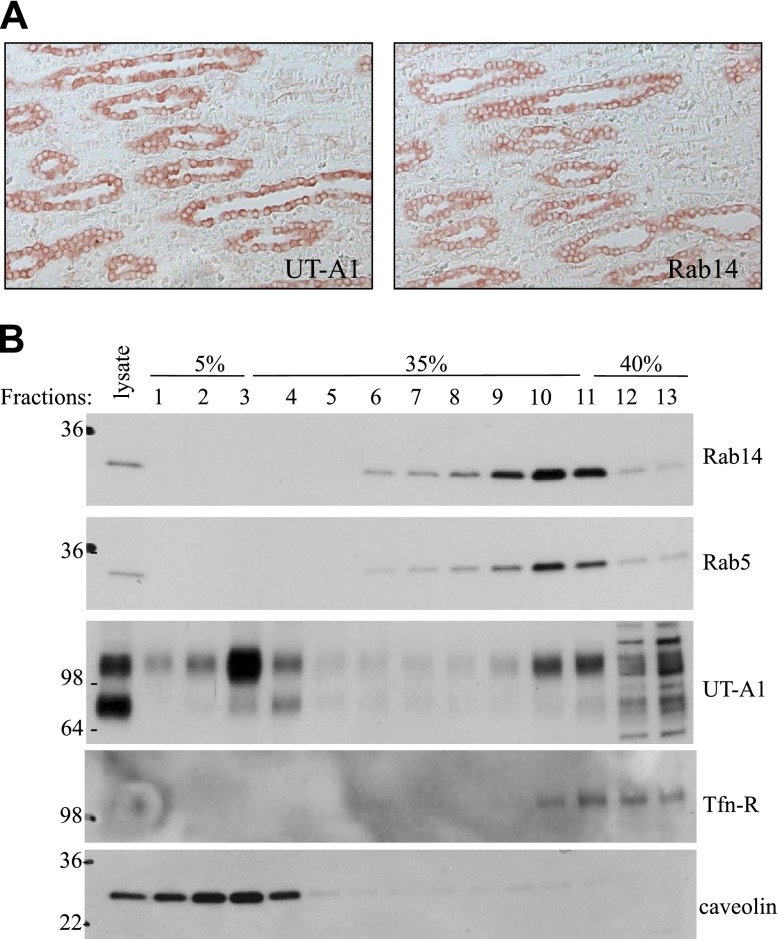
Rab14 expression in kidney IM
Finally, we explored Rab14 expression in kidney. Rab14 is mainly expressed in IM, less in OM, and very low in cortex. In IM, Rab14 is localized to IMCD epithelial cells, the same distribution as UT-A1 in IM (Fig. 6A). Some positive staining for Rab14 can also be observed in cortical and outer medullary collecting duct epithelial cells but not in proximal and distal tubular cells (data not shown). We then prepared an IMCD tubule suspension and fractionated cell PM by sucrose gradient ultracentrifugation. As we reported before, fractions 1–4 are the lipid raft domains, as indicated by caveolin, and fractions 8–11 are the nonlipid raft domains. The majority of UT-A1 is in lipid raft microdomains (16, 34). Consistent with its possible role in promoting UT-A1 internalization through the clathrin-mediated endocytic pathway, Rab14 is localized in nonlipid raft microdomains, the same distribution as Rab5 and transferrin receptor (TfR) in PM fractions (Fig. 6B).
Figure 6.
Rab14 expression in normal rat kidney. A) Immunohistochemistry. Normal rat kidney paraffin sections were dewaxed, hydrated, and then incubated with primary antibodies Rab14 or UT-A1, followed by secondary antibody and avidin-biotin peroxidase complex. Colors were developed by AEC and examined under a microscope. B) Lipid raft experiment. Rat IMCD suspensions were prepared and lysed in TENV buffer. The lysate supernatants were loaded on a 5–40% sucrose gradient as described in Materials and Methods. After 16 h ultracentrifugation, equal amounts of fractions were collected and used for immunoblotting with relevant antibodies.
DISCUSSION
Rab GTPase proteins function in multiple steps in the intracellular movement of various vesicles and cargo proteins during biosynthetic/secretory and endocytotic pathways (19, 20, 30, 38). Specific Rab proteins are associated with the trafficking of different cargo molecules to discrete membranes. Accumulating studies report that Rab proteins could directly bind to the cargo proteins, such as Rab3 and Rab27a association with the epithelial sodium channel (ENaC; ref. 39), Rab4 with cystic fibrosis transmembrane conductance regulator (CFTR; ref. 40), and Rab11a with the epithelial calcium channels TRPV5 and TRPV6 (41). In the current study, we identified one Rab protein, Rab14, which directly binds to a cargo protein, UT-A1. Their physical interaction has been confirmed by both in vitro and in vivo coimmunoprecipitation studies. Similar to Rab11a, which recognizes a conserved stretch in the carboxyl terminus of TRPV5 (41), Rab14 also binds UT-A1 at the carboxyl terminus.
Although many Rab proteins in vesicular transport have been investigated, and their functions are well recognized, the studies of Rab14 are far less explored. Larance et al. (42) reported that Rab14, together with Rab10 and Rab11, is expressed in GLUT4 vesicles, suggesting that Rab14 is probably implicated in insulin-stimulated GLUT4 vesicle translocation. A new study by Linford et al. (43) shows that Rab14 is required for cell migration. Interestingly, Rab14 interacts specifically with some apical targeting proteins (44). Here, for the first time, we provide evidence that the small GTPase Rab14 directly interacts with UT-A1 and demonstrate that Rab14 may play an important role in kidney urea transport regulation. By switching between the GDP-bound inactive and the GTP-bound active state, the Rab proteins function as perfect cargo carriers to recruit or release the molecule onto membranes in a specific compartment by changing nucleotide forms. In our study, we found that both forms have the capability to bind UT-A1; however, GDP-bound Rab14 more strongly binds to UT-A1. Saxena et al. (39) similarly found that the epithelial calcium channels TRPV5 and TRPV6 preferentially interact with Rab11a in its inactive configuration.
The discovery of Rab14 association with UT-A1 raised the possibility that Rab14 may participate in the regulation of urea reabsorption in vivo. Indeed, the kidney is one of the organs in which Rab14 protein is highly expressed (45). Immunostaining (Fig. 6A) revealed that Rab14 is mainly expressed in the IMCD epithelial cells, similar to UT-A1 in IM, supporting a possible regulatory role of Rab14 for UT-A1. Our functional study (Fig. 2) demonstrated that Rab14 reduces UT-A1 protein expression and functional activity, indicating that Rab14 acts as a negative regulator for UT-A1.
Several studies have reported that Rab proteins negatively regulate transporter activity, but the mechanisms are quite different for each case. Overexpression of Rab4 in HT-29 cells inhibits both basal and cAMP-stimulated CFTR activity (40). Rab3 and Rab27a inhibit ENaC activity, and their inhibitory effect is due to reduced cell membrane expression of ENaC (39). GDP-locked Rab11a decreased TRPV5 and TRPV6 channel cell surface expression and Ca2+ uptake (41). We explored the possible mechanism of Rab14 down-regulation of UT-A1 activity and found that Rab14 reduces UT-A1 cell membrane expression by promoting UT-A1 clathrin-mediated endocytosis.
Excellent work by Junutula et al. (31) has revealed Rab14's subcellular distribution mainly in Golgi and endosomes. In this study, we confirmed the high abundance of Rab14 in endosomes. However, using immunoelectron microscopy, the researchers also observed a number of Rab14 molecules expressed in the cell PM. To support that Rab14 protein is present in PM and is capable of involvement in membrane UT-A1 regulation, we isolated PMs by a sucrose gradient ultracentrifugation and clearly showed that Rab14 is present in the cell membrane. These Rab14 molecules may contribute to the regulation of UT-A1 at the cell surface by bringing UT-A1 from the cell surface to the endosome. Interestingly, we fractionated cell PM from IM tissue and discovered that Rab14 is present in nonlipid raft domains, cofractionated with Rab5, the clathrin-mediated endocytosis pathway marker. Our data are consistent with an early immunostaining report (38) showing that Rab14 is colocalized with the AP-1 adaptor subunit γ-adaptin. This provides strong evidence for Rab14's possible role at the cell membrane by mediating UT-A1 endocytosis through a clathrin-mediated pathway. Indeed, when the clathrin-dependent endocytic pathway is inhibited by chlorpromazine or siRNA knocking down μ2, the loss of UT-A1 from the membrane induced by Rab14 is obviously blocked (Fig. 4).
Junutula et al. (31) further reported that Rab14 Q70L (the GTP-bound active form of Rab14) is mainly distributed in the early endosome-associated vesicles, while the inactive Rab14 S25N is in the Golgi region. Overexpression of Rab14Q70L significantly enhanced Rab14 in early endosomes but decreased it in the Golgi stack. TfR internalization is typically through the clathrin endocytic pathway (37). The researchers observed that TfR distribution was shifted from the Golgi region in Rab14 S25N transfected cells to small tubulo-vesicular structures in Rab14 Q70L transfected cells (31). In our study, we found that the Rab14-inactive form (Rab14 S25N) strongly binds to UT-A1. Presumably, the GDP-bound form of Rab14 may bind UT-A1 at the cell membrane, and/or UT-A1 in the Golgi, and bring UT-A1 to endosome, and thus decrease cell surface UT-A1 expression and down-regulate UT-A1 urea transport activity.
Supplementary Material
Acknowledgments
The authors greatly acknowledge Richard Scheller (Genentech, South San Francisco, CA, USA) for the Rab14 antibody and pGEX-KG-Rab14 construct.
This work was supported by U.S. National Institutes of Health grants R01-DK087838 (to G.C.) and R01-DK041707 (to J M.S.).
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
- AEC
- aminoethyl carbozole
- AVP
- arginine vasopressin
- CFTR
- cystic fibrosis transmembrane conductance regulator
- ENaC
- epithelial sodium channel
- HA
- hemagglutinin
- IM
- inner medulla
- IMCD
- inner medulla collecting duct
- PM
- plasma membrane
- TfR
- transferrin receptor
- UT
- urea transporter
- WT
- wild type
REFERENCES
- 1. Sands J. M. (2004) Renal urea transporters. Curr. Opin. Nephrol. Hypertens. 13, 525–532 [DOI] [PubMed] [Google Scholar]
- 2. Sands J. M. (2003) Molecular mechanisms of urea transport. J. Membr. Biol. 191, 149–163 [DOI] [PubMed] [Google Scholar]
- 3. Fenton R. A., Chou C. L., Stewart G. S., Smith C. P., Knepper M. A. (2004) Urinary concentrating defect in mice with selective deletion of phloretin-sensitive urea transporters in the renal collecting duct. Proc. Natl. Acad. Sci. U. S. A. 101, 7469–7474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Knepper M. A., Nielsen S. (1993) Kinetic model of water and urea permeability regulation by vasopressin in collecting duct. Am. J. Physiol. Renal Physiol. 265, F214–F224 [DOI] [PubMed] [Google Scholar]
- 5. Nielsen S., Knepper M. A. (1993) Vasopressin activates collecting duct urea transporters and water channels by distinct physical processes. Am. J. Physiol. Renal Physiol. 265, F204–F213 [DOI] [PubMed] [Google Scholar]
- 6. Kinashi T., Hattori M., Minato N., Sasaki S., Noda Y., Horikawa S., Furukawa T., Hirai K., Katayama Y., Asai T., Kuwahara M., Katagiri K. (2004) Aquaporin-2 trafficking is regulated by PDZ-domain containing protein SPA-1. FEBS Lett. 568, 139–145 [DOI] [PubMed] [Google Scholar]
- 7. Procino G., Barbieri C., Tamma G., De Benedictis L., Pessin J. E., Svelto M., Valenti G. (2008) AQP2 exocytosis in the renal collecting duct—involvement of SNARE isoforms and the regulatory role of Munc18b. J. Cell Sci. 121, 2097–2106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mistry A. C., Mallick R., Klein J. D., Weimbs T., Sands J. M., Fröhlich O. (2009) Syntaxin specificity of aquaporins in the inner medullary collecting duct. Am. J. Physiol. Renal Physiol. 297, F292–F300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Valenti G., Procino G., Tamma G., Carmosino M., Svelto M. (2005) Minireview: aquaporin 2 trafficking. Endocrinology 146, 5063–5070 [DOI] [PubMed] [Google Scholar]
- 10. Tamma G., Klussmann E., Maric K., Aktories K., Svelto M., Rosenthal W., Valenti G. (2001) Rho inhibits cAMP-induced translocation of aquaporin-2 into the apical membrane of renal cells. Am. J. Physiol. Renal Physiol. 281, F1092–F1101 [DOI] [PubMed] [Google Scholar]
- 11. Marples D., Schroer T. A., Ahrens N., Taylor A., Knepper M. A., Nielsen S. (1998) Dynein and dynactin colocalize with AQP2 water channels in intracellular vesicles from kidney collecting duct. Am. J. Physiol. Renal Physiol. 274, F384–F394 [DOI] [PubMed] [Google Scholar]
- 12. Noda Y., Horikawa S., Katayama Y., Sasaki S. (2004) Water channel aquaporin-2 directly binds to actin. Biochem. Biophys. Res. Commun. 322, 740–745 [DOI] [PubMed] [Google Scholar]
- 13. Noda Y., Horikawa S., Kanda E., Yamashita M., Meng H., Eto K., Li Y., Kuwahara M., Hirai K., Pack C., Kinjo M., Okabe S., Sasaki S. (2008) Reciprocal interaction with G-actin and tropomyosin is essential for aquaporin-2 trafficking. J. Cell Biol. 182, 587–601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mistry A. C., Mallick R., Fröhlich O., Klein J. D., Rehm A., Chen G., Sands J. M. (2007) The UT-A1 urea transporter interacts with snapin, a SNARE-associated protein. J. Biol. Chem. 282, 30097–30106 [DOI] [PubMed] [Google Scholar]
- 15. Huang H., Feng X., Zhuang J., Fröhlich O., Klein J. D., Cai H., Sands J. M., Chen G. (2010) Internalization of UT-A1 urea transporter is dynamin dependent and mediated by both caveolae- and clathrin-coated pit pathways. Am. J. Physiol. Renal Physiol. 299, F1389–F1395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Feng X., Huang H., Yang Y., Fröhlich O., Klein J. D., Sands J. M., Chen G. (2009) Caveolin-1 directly interacts with UT-A1 urea transporter: the role of caveolae/lipid rafts in UT-A1 regulation at the cell membrane. Am. J. Physiol. Renal Physiol. 296, F1514–F1520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Xu G., Su H., Carter B. C., Fröhlich O., Chen G. (2012) Depolymerization of cortical actin inhibits UT-A1 urea transporter endocytosis but promotes forskolin stimulated membrane trafficking. Am. J. Physiol. Cell Physiol. 302, C1012–C1018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chen G., Huang H., Fröhlich O., Yang Y., Klein J. D., Price S. R., Sands J. M. (2008) MDM2 E3 ubiquitin ligase mediates UT-A1 urea transporter ubiquitination and degradation. Am. J. Physiol. Renal Physiol. 295, F1528–F1534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Takai Y., Sasaki T., Matozaki T. (2001) Small GTP-binding proteins. Physiol. Rev. 81, 153–208 [DOI] [PubMed] [Google Scholar]
- 20. Somsel Rodman J., Wandinger-Ness A. (2000) Rab GTPases coordinate endocytosis. J. Cell Sci. 113, 183–192 [DOI] [PubMed] [Google Scholar]
- 21. Stenmark H. (2009) Rab GTPases as coordinators of vesicle traffic. Nat. Rev. Mol. Cell Biol. 10, 513–525 [DOI] [PubMed] [Google Scholar]
- 22. Pfeffer S. R. (2005) Structural clues to Rab GTPase functional diversity. J. Biol. Chem. 280, 15485–15488 [DOI] [PubMed] [Google Scholar]
- 23. Nielsen E., Severin F., Backer J. M., Hyman A. A., Zerial M. (1999) Rab5 regulates motility of early endosomes on microtubules. Nat Cell Biol. 1, 376–382 [DOI] [PubMed] [Google Scholar]
- 24. Zuk P. A., Elferink L. A. (2000) Rab15 differentially regulates early endocytic trafficking. J. Biol. Chem. 275, 26754–26764 [DOI] [PubMed] [Google Scholar]
- 25. Martinez O., Schmidt A., Salaméro J., Hoflack B., Roa M., Goud B. (1994) The small GTP-binding protein rab6 functions in intra-Golgi transport. J. Cell Biol. 127, 1575–1588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chavrier P., Parton R. G., Hauri H. P., Simons K., Zerial M. (1990) Localization of low molecular weight GTP binding proteins to exocytic and endocytic compartments. Cell 62, 317–329 [DOI] [PubMed] [Google Scholar]
- 27. Pfeffer S. (2003) Membrane domains in the secretory and endocytic pathways. Cell 112, 507–517 [DOI] [PubMed] [Google Scholar]
- 28. Chavrier P., Gorvel J. P., Stelzer E., Simons K., Gruenberg J., Zerial M. (1991) Hypervariable C-terminal domain of rab proteins acts as a targeting signal. Nature 353, 769–772 [DOI] [PubMed] [Google Scholar]
- 29. Seabra M. C., Mules E. H., Hume A. N. (2002) Rab GTPases, intracellular traffic and disease. Trends Mol. Med. 8, 23–30 [DOI] [PubMed] [Google Scholar]
- 30. Hutagalung A. H., Novick P. J. (2011) Role of Rab GTPases in membrane traffic and cell physiology. Physiol. Rev. 91, 119–149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Junutula J. R., De Maziere A. M., Peden A. A., Ervin K. E., Advani R. J., van Dijk S. M., Klumperman J., Scheller R. H. (2004) Rab14 is involved in membrane trafficking between the Golgi complex and endosomes. Mol. Biol. Cell 15, 2218–2229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Su H., Carter B. C., Laur O., Sands J. M., Chen G. (2012) Forskolin stimulation promotes urea transporter UT-A1 ubiquitination, endocytosis and degradation. Am. J. Physiol. Renal Physiol. 303, F1325–F1332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Chen G., Froehlich O., Yang Y., Klein J. D., Sands J. M. (2006) Loss of N-linked glycosylation reduces urea transporter UT-A1 response to vasopressin. J. Biol. Chem. 281, 27436–27442 [DOI] [PubMed] [Google Scholar]
- 34. Chen G., Howe A. G., Xu G., Fröhlich O., Klein J. D., Sands J. M. (2011) Mature N-linked glycans facilitate UT-A1 urea transporter lipid raft compartmentalization. FASEB J. 25, 4531–4539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bradford A. D., Terris J. M., Ecelbarger C. A., Klein J. D., Sands J. M., Chou C. L., Knepper M. A. (2001) 97- and 117-kDa forms of collecting duct urea transporter UT-A1 are due to different states of glycosylation. Am. J. Physiol. Renal Physiol. 281, F133–F143 [DOI] [PubMed] [Google Scholar]
- 36. Butterworth M. B., Edinger R. S., Ovaa H., Burg D., Johnson J. P., Frizzell R. A. (2007) The deubiquitinating enzyme UCH-L3 regulates the apical membrane recycling of the epithelial sodium channel. J. Biol. Chem. 282, 37885–37893 [DOI] [PubMed] [Google Scholar]
- 37. Abdel Shakor A. B., Atia M. M., Kwiatkowska K., Sobota A. (2012) Cell surface ceramide controls translocation of transferrin receptor to clathrin-coated pits. Cell. Signal. 24, 677–684 [DOI] [PubMed] [Google Scholar]
- 38. Proikas-Cezanne T., Gaugel A., Frickey T., Nordheim A. (2006) Rab14 is part of the early endosomal clathrin-coated TGN microdomain. FEBS Lett. 580, 5241–5246 [DOI] [PubMed] [Google Scholar]
- 39. Saxena S., Singh M., Engisch K., Fukuda M., Kaur S. (2005) Rab proteins regulate epithelial sodium channel activity in colonic epithelial HT-29 cells. Biochem. Biophys. Res. Commun. 337, 1219–1223 [DOI] [PubMed] [Google Scholar]
- 40. Saxena S. K., Kaur S., George C. (2006) Rab4GTPase modulates CFTR function by impairing channel expression at plasma membrane. Biochem. Biophys. Res. Commun. 341, 184–191 [DOI] [PubMed] [Google Scholar]
- 41. Van de Graaf S. F., Chang Q., Mensenkamp A. R., Hoenderop J. G., Bindels R. J. (2006) Direct interaction with Rab11a targets the epithelial Ca2+ channels TRPV5 and TRPV6 to the plasma membrane. Mol. Cell. Biol. 26, 303–312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Larance M., Ramm G., Stöckli J., van Dam E. M., Winata S., Wasinger V., Simpson F., Graham M., Junutula J. R., Guilhaus M., James D. E. (2005) Characterization of the role of the Rab GTPase-activating protein AS160 in insulin-regulated GLUT4 trafficking. J. Biol. Chem. 280, 37803–37813 [DOI] [PubMed] [Google Scholar]
- 43. Linford A., Yoshimura S., Nunes Bastos R., Langemeyer L., Gerondopoulos A., Rigden D. J., Barr F. A. (2012) Rab14 and its exchange factor FAM116 link endocytic recycling and adherens junction stability in migrating cells. Dev. Cell 22, 952–966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kitt K. N., Hernández-Deviez D., Ballantyne S. D., Spiliotis E. T., Casanova J. E., Wilson J. M. (2008) Rab14 regulates apical targeting in polarized epithelial cells. Traffic 9, 1218–1231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Liu G. Y., Ge C. R., Zhang X., Gao S. Z. (2008) Isolation, sequence identification and tissue expression distribution of three novel porcine genes: RAB14, S35A3 and ITM2A. Mol. Biol. Rep. 35, 201–206 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.