Abstract
Fibrosis, seen in the liver, lung, heart, kidney, and skin, is a significant global disease burden. Currently, therapeutic treatment is limited, and the number of cases continues to grow. Apoptosis has been identified as a potential initiator and propagator of fibrosis. This review specifically examines the correlation between the presence of apoptotic cells and their effect on fibroblast phenotype and collagen metabolism in several different experimental models of fibrosis. Fibrosis in these models is generally preceded by robust angiogenesis and vascular regression, suggesting that the vascular apoptotic burden may be important to fibrotic outcomes. This review considers the emerging evidence that angiogenesis or vascular regression contributes to fibrosis and identifies initial vascular outgrowth or vascular apoptotic cell presence as possible regulators of fibrosis. A further understanding of the cellular mechanisms of fibrosis may suggest novel methods for the reduction of the fibrotic response and promotion of regeneration.—Johnson, A., DiPietro, L. A. Apoptosis and angiogenesis: an evolving mechanism for fibrosis.
Keywords: wound healing, scar formation, liver, lung, allograft vasculopathy
Fibrosis can occur in many different types of tissue, such as lung, liver, heart, kidney, and skin. Several types of fibrosis are ranked in the top 20 Global Burdens of Disease in the World Health Organization's most recent publication (1). Burns, which often result in severe fibrotic scarring, ranked 15th, and liver fibrosis/cirrhosis from hepatitis viral infections ranked 18th in the global disease burden (1). Despite this international prevalence, a functional antifibrotic treatment has not been developed (2). The hallmarks of fibrosis, including increased presence, differentiation, and persistence of myofibroblasts (myoFBs); increased collagen deposition; decreased collagen degradation; and increased contraction, are conserved in most models of organ fibrosis. The normal progression of a healing wound is similarly characterized by an increase in collagen deposition and decrease in collagen degradation, and the presence and persistence of myoFBs, which results in a scar. In this review, we survey 4 types of fibrosis (wound healing/skin, liver, lung, and transplant vasculopathy) to illustrate commonalities in fibrosis and the mechanisms that drive this process. In particular, we consider the possible role of apoptotic cells as drivers of the fibrotic process.
APOPTOSIS
Apoptosis is nature's preprogrammed form of cell death. Apoptosis occurs throughout development, when damaged tissues are repaired, and as an ongoing event when tissues turn over in the human body. The classical understanding of apoptosis suggests that it provides a benign means for the necessary clearance of cells that are no longer needed or no longer functional. The attendant model holds that apoptotic cells do not elicit an immune response nor have any effect on surrounding cells. As described later, recent findings now challenge this paradigm of apoptosis as a process without consequence to neighboring tissue.
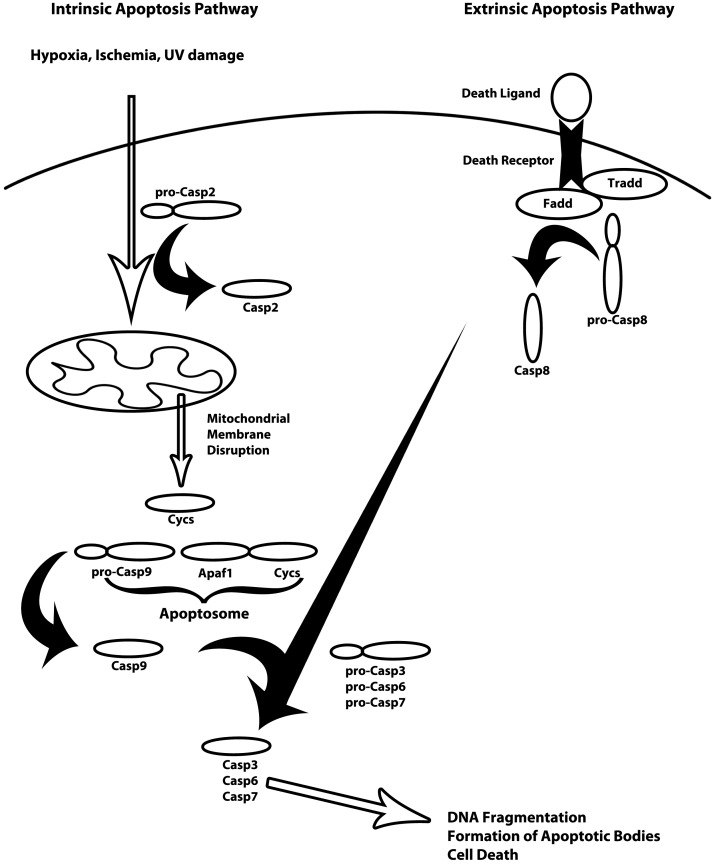
There are two main pathways to initiate apoptosis, termed intrinsic and extrinsic, that converge on a DNA fragmentation pathway (Fig. 1). Extrinsic signaling pathways involve the transmembrane death receptors of the tumor necrosis factor (TNF) receptor gene superfamily, FasR, TNFR1, and death receptors (DRs) 3, 4, and 5; all contain a death domain that transmits the death signal from the cell surface to intracellular signaling pathways. In the mitochondrial or intrinsic pathway, the balance of proapoptotic Bax and antiapoptotic Bcl-2 proteins, as well as caspase-2, determines cytochrome c release from mitochondria. There are numerous intracellular mediators for both pathways that are necessary for formation of death receptor complexes and caspase cleavage and activation. Caspases involved in apoptosis can be broken down into 3 broad categories: initiators (caspase-2, -8, -9, -10), executioners (caspase-3, -6, -7), and inflammatory caspases (caspase-1, -4, -5) (3, 4). Caspase-3 (Casp3) is the classical caspase common to all apoptosis pathways. The activation of the extrinsic pathway begins with recruitment of TNFR-associated death domain (TRADD), Fas-associated death domain (FADD), and procaspase-8; signaling is propagated by the cleavage and activation of procaspase-8 to caspase-8, which cleaves and activates Casp3. The intrinsic pathway is initiated by mitochondrial membrane disruption releasing cytochrome c. Cytochrome c binds to the cytosolic protein, apoptosis protease-activating factor 1 (APAF1), which recruits caspase-9 for cleavage and activation. Caspase-9, in turn, cleaves and activates Casp3 similar to the extrinsic pathway (refs. 3, 4 and Fig. 1).
Figure 1.
Diagram of the intrinsic and extrinsic apoptosis signaling pathways (adapted from ref. 80). Intrinsic apoptosis (left side) is usually the result of hypoxia, ischemia, or UV damage, but may also be induced via stress from low levels of growth factors. These damage signals are propagated through Casp2- and Trp53-mediated disruption of the mitochondrial membrane. Cytochrome c (Cycs) is released following mitochondrial membrane disruption and recruited to form the apoptosome with Apaf1 and pro-Casp9. Pro-Casp9 is cleaved and activates the caspase cascade resulting in cleavage and activation of Casp3, Casp6, and Casp7. The downstream effect of activation of the caspase cascade is DNA fragmentation, formation of apoptotic bodies, and cell death. The extrinsic apoptosis signaling pathway (right side) requires binding of the death ligand (FasL or TNF-α) to its respective receptor (FasR or Tnfrsf1b). Binding of the death ligand to the death signals the recruitment of Tradd, Fadd, and pro-Casp8. Pro-Casp8 is cleaved by the complex and begins the caspase cleavage cascade resulting in the cleavage and activation of Casp3, Casp6, and Casp7. Similar to the intrinsic pathway, the result of the caspase cleavage cascade is DNA fragmentation, formation of apoptotic bodies, and cell death.
APOPTOSIS AND FIBROSIS
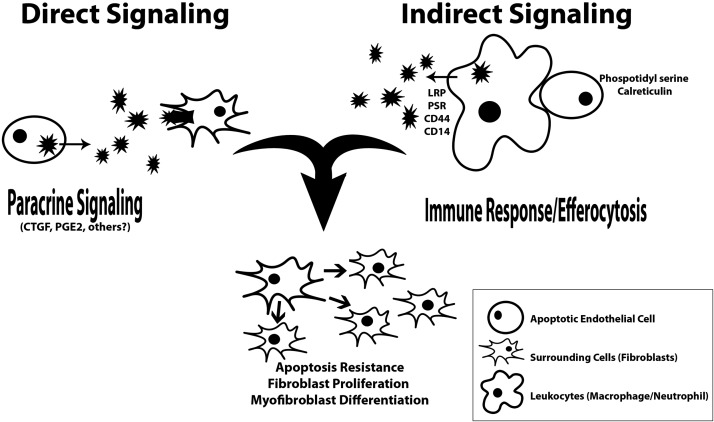
For decades, apoptotic cells were thought to be inconsequential to the final outcome of processes such as wound healing, and viewed simply as a necessary occurrence in the function of the human body. This model has recently been challenged, as apoptosis has been identified as a key player in the initiation, propagation, and resolution of organ fibrosis. High levels of apoptosis have been identified in nearly all types of fibrosis, and many mechanisms by which the apoptotic cells might dictate fibrotic outcomes have been suggested (5). Characteristics of fibrosis, such as increased levels of myoFB-like cells, increased collagen synthesis, and decreased collagen degradation, can be seen in wound healing, liver and lung fibrosis, and chronic transplant vasculopathy (CTV). In addition, high levels of apoptosis are seen in all models of fibrosis, either as initiators or perpetuators of the fibrotic response (Table 1). The three most notable pathways that dictate the severity of the fibrotic outcomes include apoptosis resistance of fibroblasts (FBs) and myoFBs, increased FB and myoFB proliferation, and increased myoFB differentiation (6). Apoptotic cells can stimulate these fibrotic outcomes either indirectly or directly. Macrophages, neutrophils, and other leukocytes may be stimulated to secrete factors that mediate fibrotic effects following the engulfment of apoptotic cells. Direct paracrine signaling from early and late apoptotic cells themselves can also cause fibrotic outcomes (Fig. 2).
Table 1.
Characteristics of fibrosis, apoptosis, and vascularity in different tissues
| Parameter | Fibrosis type |
|||
|---|---|---|---|---|
| Wound healing | Liver fibrosis | Lung fibrosis | CTV, CAV, RAV | |
| Collagen synthesis | Increase | Increase | Increase | Increase |
| Presence and persistence of fibrotic cells | Increase | Increase | Increase | Increase |
| Fibrotic cell type | MyoFBs | Hepatic stellate cells | Pneumocytes | MyoFBs |
| Fibrotic outcome | Scarring | Cirrhosis | Idiopathic pulmonary fibrosis | Intimal hyperplasia |
| Collagen degradation | Decrease | Decrease | Decrease | Increase |
| Source of apoptotic cells | Neutrophils/macrophages (early); endothelial cells (late) | Hepatocytes | Endothelial cells; alveolar epithelial cells | Cardiomyocytes; donor organ endothelial cells |
| Angiogenesis | Higher than necessary | Increase | Increase | Increase |
CAV, cardiac allograft vasculopathy; CTV, chronic transplant vasculopathy; RAV, renal allograft vasculopathy.
Figure 2.
Roles of apoptosis in fibrosis: immune response modulation and paracrine signaling. Apoptotic cells, such as apoptotic ECs, can affect surrounding cells through paracrine signaling directly from the apoptotic ECs or through immune modulation of leukocytes that efferocytose apoptotic ECs. The downstream effects on the surrounding cells include apoptosis resistance, increased proliferation, and differentiation.
In further considering the indirect effects of apoptotic cells, modulation of the immune response by apoptotic cells is a concept that has recently gained support. The phagocytosis of apoptotic cells by macrophages, a process termed efferocytosis, can define the inflammatory state of macrophages and the resolution of inflammation in general (7). Most of the research on this topic has attempted to identify the initiating event in the macrophage switch from a “proinflammatory” to “prohealing” phenotype (8). Following this switch, macrophages begin secreting cytokines and growth factors (8) that stimulate tissue growth. These factors may contribute to a profibrotic outcome via multiple mechanisms (9, 10). Macrophages with the prohealing phenotype are known to secrete lower levels of TNF α and interleukin (IL)-6 and increased levels of IL-10, transforming growth factor β1 (TGF-β1) and insulin-like growth factor 1 (7, 11, 12). Notably, IL-10, TGF-β1, and insulin-like growth factor 1 have also been shown to promote cell survival in the surrounding cells. In doing so, these signals could also initiate a profibrotic response by conferring apoptosis resistance and promoting proliferation and differentiation in FBs and myoFBs (8, 9).
Direct effects of apoptotic cells also play a part in modulating fibrotic outcomes; apoptotic cells may act directly on FBs and other cell types, enhancing cellular proliferation and profibrotic phenotypes. In the skin, subcutaneous injection of conditioned medium (CM) from apoptotic endothelial cells (ECs) was sufficient to increase the presence and persistence of myoFBs (13). In the liver, lung, cardiac allografts, and renal allografts, administration of apoptotic cells relevant to the corresponding organ induced a limited immune response while increasing the fibrotic response (13–21). These studies suggest that paracrine signaling mechanisms from apoptotic cells may influence the fibrotic response.
SOURCES OF APOPTOTIC CELLS IN FIBROTIC RESPONSES
Since the presence and persistence of apoptotic cells may contribute to the fibrotic outcome via immune modulation and paracrine signaling, the identification of the source of the apoptotic cells is important. Several studies have identified apoptotic inflammatory cells as contributing to fibrotic lesions (21, 22). In a mouse model of lung fibrosis, introduction of apoptotic macrophages via intratracheal administration resulted in increased macrophage recruitment and increased collagen deposition, and contributed to the overall initiation and propagation of pulmonary fibrosis (21, 22).
Another potential source of apoptotic cells is the angiogenic response. A vigorous angiogenic response is frequently associated with fibrotic outcomes (23), and antiangiogenic therapies have been proposed to modulate fibrosis (16, 24–26). The role of dynamic angiogenesis in fibrosis and scar formation has been generally considered to involve the need for oxygen and nutrient delivery to support fibrotic processes (27). Recent studies in wound healing, however, suggest that the angiogenic response may influence fibrosis through the generation of apoptotic ECs (28–31). As the angiogenic process resolves, the vascular bed is pruned back via apoptosis of ECs. The resolution of robust angiogenesis thus creates a significant apoptotic burden (28, 29). Upon vessel regression, pericytes may be released and develop myoFB features via paracrine signals from apoptotic ECs, further encouraging the development of fibrosis (32). The increased levels of apoptotic vascular cells may contribute via immune modulation and paracrine signaling to the resulting fibrotic outcomes. Fibrocytes, a circulatory cell type that enters the wound and secretes extracellular matrix (ECM) proteins, have been linked to angiogenesis and scar formation (33). Interestingly, recent studies have suggested that fibrocytes that enter the wound promote angiogenesis through secretion of proangiogenic factors and factors that encourage EC migration, such as bFGF, vascular endothelial growth factor (VEGF), G-CSF, and MMP-9. Fibrocytes may therefore contribute to the fibrotic environment of the wound through the promotion of angiogenesis, leading to increased levels of apoptotic ECs from the subsequent vessel regression, and through secreted profibrotic factors (33).
WOUND HEALING
In wound healing, fibrotic outcomes can vary from minor scars to hypertrophic scars and severe fibrotic lesions. In all cases, the collagen content and architecture of the scar are significantly different from normal skin (34, 35). Fibrosis in skin is characterized by increased collagen synthesis; specifically, increased collagen type I (Col1), decreased collagen type III (Col3), and decreased collagen degradation attributed to an imbalance of matrix metalloproteinases and their inhibitors, tissue inhibitor metalloproteinases (34–36). Hypertrophic scarring, in particular, is characterized as raised, red, fibrous lesions that typically remain within the area of the original wound and undergo at least partial spontaneous resolution over a long period of time. Hypertrophic scars can occur following minimal skin trauma, and result in physical pain and limited mobility due to contractures, in addition to psychological stress due to the physical appearance of the scar (36). Because the collagen architecture of hypertrophic scars is abnormal, the scar tissue has less tensile strength than normal skin, which contributes to high levels of wound dehiscence (37). Due to limited therapeutic treatments, hypertrophic scars continue to be a significant clinical problem.
During wound healing, apoptosis maintains the homeostasis of the wound environment by balancing cell elimination with cell proliferation (38). Early apoptosis in wound healing primarily involves neutrophils and macrophages, cells that are eliminated as inflammation resolves. In the later phases of repair, both FBs and ECs undergo apoptosis while the wound is remodeling (38, 39). Notably, in vitro studies by Laplante et al. (13) have shown that CM from Casp3- mediated apoptotic ECs can increase focal adhesion formation and gene expression of α-smooth muscle actin (α-SMA), a myoFB marker, in FB culture. The fibrotic effect of this CM was also demonstrated in vivo with subcutaneous injection of CM from apoptotic ECs. Following proteomic analysis, the fibrotic effect was shown to derive from the presence of connective tissue growth factor (CTGF), a member of the CCN family of proteins that has been previously implicated in fibrosis (5, 13). These studies suggest that apoptotic ECs (and perhaps CTGF) might mediate critical paracrine signals that influence fibrosis, and contribute to the accumulation of apoptotic cells via synergistic effects of CTGF and the extrinsic apoptosis pathway (5).
Vessel regression in the remodeling phase of wound healing is a significant source of apoptotic ECs (29). The modulation of blood vessel growth is an important component of wound repair; angiogenesis peaks during the proliferative phase, and vessel density decreases gradually in the remodeling phase. As the wound progresses through the remodeling phase, there is a shift from a predominance of proangiogenic mediators to antiangiogenic mediators (28, 29, 40). When the antiangiogenic mediators outweigh the proangiogenic mediators, the result is increased levels of apoptotic ECs and vascular regression (29–31). The balance between pro- and antiangiogenic mediators determines the levels of apoptotic ECs in wound healing (30, 31). Since persistent and high apoptotic loads are related to the etiology of several other types of fibrosis (19, 41, 42), we hypothesize that the increase in the vascular cell apoptotic load during vessel regression in normal wound healing may influence FB function, promoting formation of fibrotic scars and preventing regeneration.
Robust angiogenesis has now been associated with hypertrophic scarring (34, 35). A recent study compared the capillary content of human normotrophic and hypertrophic scars and demonstrated that hypertrophic scars contain higher levels of angiogenesis and vascular remodeling (20). Given these observations, antiangiogenic therapy has been suggested to reduce scar formation (16, 25, 43, 44). Recent studies by us and others now suggest that a reduction in angiogenesis may be beneficial to healing outcomes and does not impair wound closure (16, 45, 46). For example, neutralization of vascular VEGF via antibody treatment has been used to reduce the angiogenic response in adult skin wounds by ∼50% (ref. 46 and unpublished results). The anti-VEGF-treated wounds close normally and have reduced scarring, an outcome more closely resembling regeneration. The results from this study support the hypothesis that reducing angiogenesis also reduces the potential apoptotic load that occurs during vascular regression; the reduction in the apoptotic load may be connected to the improved scarring outcome.
LIVER FIBROSIS
Liver fibrosis has been studied extensively because the liver is the only mammalian organ known to regenerate after injury, and because of the clinical importance of fibrosis in this organ. Liver fibrosis is characterized by chronic activation of the wound healing response and an increase in the differentiation of hepatic stellate cells (HSCs) to myoFB-like cells (47, 48). In addition to HSCs, other cell types contribute to liver fibrosis, specifically resident portal FBs, bone marrow-derived or circulating fibrocytes, and epithelial cells that have undergone epithelial-to-mesenchymal-transition (EMT) (47, 48). Perturbations in the expression of collagen, matrix metalloproteinases, and tissue inhibitor metalloproteinases have also been described in the fibrotic response of the liver (49). In addition, changes in the vasculature are a hallmark of liver fibrosis, as the microvascular fenestrations that are critical to healthy liver function are occluded by deposition of ECM (47). A well-characterized model of liver fibrosis in mice results from chronic CCl4 administration; cessation of the CCl4 administration is accompanied by spontaneous resolution of liver fibrosis over a period of a year (24, 50). Most researchers agree that the spontaneous resolution of liver fibrosis is related to apoptosis of hepatic myoFBs (48), or through the transition of HSCs to quiescent HSCs (47). Similar to fibrosis of the skin, the increased presence and persistence of myoFB-like cells contributes to liver fibrosis; however, unlike skin, the liver may regenerate after the initial injury.
The effects of apoptotic hepatocytes in liver fibrosis range from immune modulation to direct interaction of apoptotic hepatocytes with surrounding cells. HSCs are induced to develop into myoFB-like cells in proximity to the apoptotic hepatocytes (47–49). Fas and CD40, extrinsic apoptosis signaling pathway ligands, are up-regulated in the development and persistence of liver fibrosis (51). However, activated HSCs (myoFB-like cells) are resistant to apoptosis, especially after sustained activation (52). Apoptosis resistance aids in maintaining the profibrotic environment and sustains the presence of activated HSCs (47, 48). Inducing apoptosis of HSCs has been a major therapeutic target to promote the resolution of liver fibrosis (47, 48). Interestingly, in nonalcoholic fatty liver disease (NAFLD) and nonalcoholic steatohepatitis (NASH), hepatocyte apoptosis is increased, as is the inflammatory response. After the initiation of inflammation and activation of HSCs, cirrhosis and fibrosis develop (41, 53, 54). In this situation, there is no toxin to remove in order to reverse the fibrotic response. Therefore, in some cases of liver fibrosis, specifically NAFLD and NASH, the initiation of fibrosis may be related to the apoptotic load in the liver, a load which may be above and beyond the phagocytic clearance levels. This apoptotic burden could therefore directly contribute to fibrosis via paracrine signaling to the surrounding HSCs, or indirectly via immune modulation of the macrophage phenotype.
Several different apoptotic cell types have been identified in the liver. The largest contribution during the initiation of liver fibrosis is made by hepatocytes. Most studies suggest that as liver fibrosis progresses, angiogenesis occurs (24, 50). Increasing levels of VEGF from activated HSCs and other myoFB-like hepatocytes encourage new vessel growth (48). However, very little is known about how the vasculature then returns to the original sinusoidal architecture nor about the regulation of vessel regression during the resolution of liver fibrosis. During regression of fibrosis, the fate of the activated HSCs is also important. One group identified collagen- and α-SMA-expressing HSCs undergoing apoptosis; however, not all do. Some HSCs revert to an inactive phenotype and, in doing so, lower the apoptotic load during the resolution of fibrosis (55). The mechanism with which the liver promotes and controls vessel regression and remodeling may be similar to the mechanism for the removal of HSCs, a key component in liver regeneration.
LUNG FIBROSIS
Lung fibrosis is characterized by heavy collagen deposition centered around fibrotic foci that contain ECs (56–59). Idiopathic pulmonary fibrosis (IPF) is initiated by an injury to the alveolar epithelium, which is followed by a mild to moderate inflammatory response (58, 59). Neighboring alveolar epithelial cells then migrate to the site and are activated (58, 59). Resident FBs are recruited from the surrounding tissue, as are fibrocytes from the bone marrow (56). In addition, epithelial cells may contribute to the FB and myoFB population via EMT (60). EMT in this case occurs as the protein microenvironment changes from being prohealing to profibrotic (56). These cellular and biochemical changes signal the initiation of normal tissue repair, similar to the normal wound healing response; however, in IPF, the wound healing process never concludes (58, 59). Chronic activation of the healing response results in fibrosis, similar to the chronic activation of the wound healing response in the liver.
The presence of apoptotic alveolar epithelium is thought to initiate the wound healing response and ultimately the fibrotic response (58). However, there are several other hypotheses that relate apoptosis to the progression of lung fibrosis: paracrine signaling confers apoptosis resistance to FBs and myoFBs that perpetuate the fibrotic response; or impaired apoptotic cell clearance by macrophages results in the release of the potentially toxic or profibrotic cellular contents of apoptotic pulmonary cells and profibrotic activation of FBs (19, 44, 61, 62). In experimental models of lung fibrosis, such as bleomycin-induced fibrosis, the fibrotic response can be bolstered by concurrent administration of apoptotic lung cells, and fibrosis can be induced by exposure to apoptotic pneumocytes alone (21). Interestingly, in bleomycin-induced lung fibrosis, there is increased expression of Fas on alveolar epithelial cells (60, 63), suggesting that the extrinsic pathway is activated in chemically induced lung fibrosis. To support this observation, an antibody was used to constitutively activate the Fas receptor-ligand binding pathway; this also resulted in a fibrotic response (63). Taken together, this research supports the hypothesis that the presence of apoptotic pneumocytes in the injured lung is sufficient to induce a fibrotic response.
As of yet, only two studies have examined the necessity of apoptosis in the induction of lung fibrosis. The first study used aerosol administration of z-VAD-fmk, a pan-caspase inhibitor, in a model of bleomycin-induced fibrosis (64). The results were consistent with another study that examined the fibrotic response in bleomycin-induced lung fibrosis after blocking the extrinsic apoptosis pathway with ANG-converting enzyme inhibitor or the pan-caspase inhibitor z-VAD-fmk (21). Both studies showed significant reduction in the subsequent fibrotic response under conditions in which apoptosis was inhibited.
Studies to identify of the cell types responsible for apoptosis in lung fibrosis suggest several potential sources. One study utilized dexamethasone to induce apoptosis of macrophages, neutrophils, and other leukocytes in the bleomycin model of lung fibrosis. The results indicated that the fibrotic response was significantly reduced (65); however repeated studies have not been able to duplicate this response (58). Histologically, apoptotic alveolar epithelial cells have been found in proximity to the heaviest myoFB activity and collagen accumulation in both clinical cases of IPF and in experimental models of bleomycin-induced pulmonary fibrosis (65). The effect of alveolar epithelial cell apoptosis has been studied to determine the role it plays in lung fibrosis (62). Several signaling pathways that experimentally control alveolar epithelial cell apoptosis have been identified, including ANG-converting enzyme inhibitor and TNF-α (59, 62).
Another potential source of apoptotic cells in lung fibrosis is EC apoptosis during vascular remodeling (19, 66). Experimental lung fibrosis is characterized by an increase in blood vessel density (56, 62). Clinical observation has shown increased vascular remodeling in the normal parenchyma, while the fibrotic regions have fewer vessels, similar to wound healing and liver fibrosis (56). The fibrotic regions contain both apoptotic and proliferating ECs, resulting in aberrant vascular architecture and anastomoses (67). These changes in vascular architecture can be attributed to an imbalance of proangiogenic and antiangiogenic mediators (66, 68, 69). The pro- and antiangiogenic mediators found in the fibrotic lung are similar to those found in healing wounds: increased angiotensin 1 and 2, angiostatin, pigment epithelium-derived growth factor, TGF-β, and endostatin, and decreased chemokine ligand 8 (CXCL8), VEGF, and fibroblast growth factor-2 (66). In addition to promoting vascular remodeling, these factors may lead to vascular injury. Vascular injury could lead to aberrant EC proliferation, which results in intimal proliferation, plexiform lesions, media hypertrophy, and adventitia fibrosis, all leading to pulmonary hypertension and exacerbation of the pulmonary fibrotic response (66).
CTV
CTV is characterized by fibrosis of the vasculature of the transplanted organ (70–72). CTV in cardiac as well as renal allografts leads to failure of the transplanted organ and may lead to death (70–72). Cardiac allograft vasculopathy (CAV) leads to cardiac vessel fibrosis and ultimately silent myocardial infarction, heart failure, or sudden death (71, 72). One of the characteristics of CAV is mononuclear cell infiltration and accumulation of vascular smooth muscle cells (VSMCs) in the neointima of the mid and dorsal coronary vessels (73, 75). In addition, there is accumulation of fibrillar collagens, Col1, Col3, and Col4, in the neointima (73–75). These vascular changes are all diagnostic of intimal thickening and hyperplasia (73–75) As rejection progresses, so does cardiac vessel stiffness, and the stiffness is thought to be related to cellular infiltration and differentiation of FBs to myoFBs (74). Apoptosis has been identified as an initiator and perpetuator of the fibrogenic reaction in CTV (15). In both CAV and renal allograft vasculopathy (RAV), EC apoptosis is the initiator of hyperplasia of the neointima and the downstream fibrotic response (15, 73). α-SMA positive cells accumulate in close proximity to apoptotic ECs in the neointima and acquire apoptotic resistance, very similar to what has been observed in other models of fibrosis (15). In CTV, EC apoptosis plays a central role in the progression of vasculopathy and fibrogenesis by inducing a hyperadhesive and thrombogenic state in the interior of the vessels of the donor organ (15). In addition, many of the apoptotic ECs are phagocytosed by macrophages, increasing the production of TGF-β in proximity to the vessel wall and likely promoting the initiation of neointima formation (15, 76). Lastly, EC apoptosis can trigger proteolysis and production of fibrogenic mediators in the surrounding tunica intima and tunica media that act as recruitment signals for FBs and encouragee FB to myoFB differentiation (15).
In any transplant, the ECs lining the blood vessels of the donor organ are the first cells that directly contact the host's immune system (15). Fas signaling has been implicated in graft vs. host immune-mediated vascular injury (15). The circulating immune cells of the host present the ECs lining the vasculature in the donor organ with Fas ligand (15). Recent studies have shown that blocking Fas signaling prevents vascular fibrogenesis (77). Also, when apoptosis of ECs is blocked, immune cell infiltration can still occur, but there is significantly less fibrogenesis (78). The maintenance of sustained levels of apoptotic ECs is also correlated with the development of CAV in heart transplant in pigs (79).
CONCLUSIONS
In summary, apoptosis is a prominent feature of several different types of fibrosis during initiation, progression, and resolution. In liver fibrosis, apoptosis plays a critical role in the initiation and propagation from liver injury to liver fibrosis. In lung fibrosis, apoptosis has been identified as the initiator of the fibrotic response and is both necessary and sufficient to induce a fibrotic response. In CTV, EC apoptosis as a result of graft vs. host interactions is the initiator of the fibrotic response that will ultimately lead to transplant rejection. In wound healing, the role of apoptosis in the development of fibrosis is not well understood; however, fibrosis as a result of dermal wound healing can and should be compared to other models of fibrosis. Similar to CTV and liver and lung fibrosis, skin fibrosis, such as hypertrophic scarring, is characterized by excessive production and deposition of ECM, in particular, Col1 and Col3; FB hyperproliferation and apoptosis resistance; differentiation of FBs into myoFBs; and a decrease in collagen degradation.
One mode of modulating the apoptotic load in wound healing is regulation of vessel regression. Aberrant vessel regression and angiogenesis has already been identified in exaggerated fibrotic outcomes of wound healing, such as hypertrophic or keloid scarring. The balance of pro- and antiangiogenic mediators controls angiogenesis and vessel regression and has been identified as pathogenic in liver fibrosis, lung fibrosis, and CTV. The chronic activation of the wound healing response and the imbalance of pro- and antiangiogenic mediators together may contribute to the increased apoptotic load and ultimately to fibrosis. The increased apoptotic load seen in vascular regression during normal wound healing may be necessary and sufficient to induce and promote the normotrophic scarring response, and aberrant angiogenic control may promote the formation of hypertrophic scars. In the future, by controlling vessel regression and the apoptotic load, researchers may be able to halt the chronic activation of the wound healing response and promote tissue regeneration instead of tissue repair. We hypothesize that in order to achieve regeneration, similar to the resolution of liver fibrosis, circumstances that result in fibrosis, such as a healing wound, would benefit from a controlled induction of vascular regression resulting in a low apoptotic load. By controlling the induction of vascular regression, we may be able to control the apoptotic EC load present during resolution, and thus the effect of the apoptotic load on the surrounding tissue.
Acknowledgments
This work was supported by U.S. National Institutes of Health grants R01-GM50875 and T32 DE018381.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
- α-SMA
- α-smooth muscle actin
- APAF1
- apoptosis protease-activating factor-1
- Casp3
- caspase-3
- CAV
- cardiac allograft vasculopathy
- CM
- conditioned medium
- Col1
- collagen type I
- Col3
- collagen type III
- CTGF
- connective tissue growth factor
- CTV
- chronic transplant vasculopathy
- DR
- death receptor
- EC
- endothelial cell
- ECM
- extracellular matrix
- EMT
- epithelial to mesenchymal transition
- FADD
- Fas-associated death domain
- FB
- fibroblast
- HSC
- hepatic stellate cell
- IL
- interleukin
- IPF
- idiopathic pulmonary fibrosis
- myoFB
- myofibroblast
- NAFLD
- nonalcoholic fatty liver disease
- NASH
- nonalcoholic steatohepatitis
- TGF
- transforming growth factor
- TNF
- tumor necrosis factor
- TRADD
- tumor necrosis factor receptor-associated death domain
- VEGF
- vascular endothelial growth factor
REFERENCES
- 1. World Health Organization (2008) The Global Burden of Disease: 2004 Update. World Health Organization, Geneva, Switzerland [Google Scholar]
- 2. Chen C., Raghunath M. (2009) Focus on collagen: in vitro systems to study fibrogenesis and antifibrosis state of the art. Fibrogenesis Tissue Repair 2, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cohen G. (1997) Caspases the executioner of apoptosis. Biochem. J. 326, 1–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rai N. K. (2005) Apoptosis: a basic physiologic process in wound healing. Int. J. Low. Extrem. Wounds 4, 138–144 [DOI] [PubMed] [Google Scholar]
- 5. Chen C. C., Lau L. F. (2010) Deadly liaisons: fatal attraction between CCN matricellular proteins and the tumor necrosis factor family of cytokines. J. Cell Commun. Signal. 4, 63–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kisseleva T., Brenner D. A. (2008) Mechanisms of fibrogenesis. Exp. Biol. Med. 233, 109–122 [DOI] [PubMed] [Google Scholar]
- 7. Khanna S., Biswas S., Shang Y., Collard E., Azad A., Kauh C., Bhasker V., Gordillo G. M., Sen C. K., Roy S. (2010) Macrophage dysfunction impairs resolution of inflammation in the wounds of diabetic mice. PLoS One 5, e9539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Stout R., Jiang C., Matta B., Tietzel I., Watkins S. K., Suttles J. (2005) Macrophages sequentially change their functional phenotype in response to changes in microenvironmental influences. J. Immunol. 175, 342–349 [DOI] [PubMed] [Google Scholar]
- 9. Mathai S. K., Gulati M., Peng X., Russell T. R., Shaw A. C., Rubinowitz A. N., Murray L. A., Siner J. M., Antin-Ozerkis D. E., Montgomery R. R., Reilkoff R. A., Bucala R. J., Herzog E. L. (2010) Circulating monocytes from systemic sclerosis patients with interstitial lung disease show an enhanced profibrotic phenotype. Lab. Invest. 90, 812–823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Murray L. A., Chen Q., Kramer M. S., Hesson D. P., Argentieri R. L., Peng X., Gulati M., Homer R. J., Russell T., van Rooijen N., Elias J. A., Hogaboam C. M., Herzog E. L. (2011) TGF-beta driven lung fibrosis is macrophage dependent and blocked by serum amyloid P. Int. J. Biochem. Cell Biol. 43, 154–162 [DOI] [PubMed] [Google Scholar]
- 11. Horowitz J., Cui Z., Moore T. A, Meier T. R., Reddy R. C., Toews G. B., Standiford T. J., Thannickal V. J. (2006) Constitutive activation of prosurvival signaling in alveolar mesenchymal cells isolated from patients with nonresolving acute respiratory distress syndrome. Am. J. Physiol. Lung Cell. Mol. Physiol. 290, L415–L425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wynes M. W., Frankel S. K., Riches D. W. (2004) IL-4-induced macrophage-derived IGF-I protects myofibroblasts from apoptosis following growth factor withdrawal. J. Leukoc. Biol. 76, 1019–1027 [DOI] [PubMed] [Google Scholar]
- 13. Laplante P., Sirois I., Raymond M. A., Kokta V., Béliveau A., Prat A., Pshezhetsky A. V., Hébert M. J. (2010) Caspase-3-mediated secretion of connective tissue growth factor by apoptotic endothelial cells promotes fibrosis. Cell Death Differ. 17, 291–303 [DOI] [PubMed] [Google Scholar]
- 14. Berger A. C., Feldman A. L., Gnant M. F., Kruger E. A., Sim B. K., Hewitt S., Figg W. D., Alexander H. R., Libutti S. K. (2000) The angiogenesis inhibitor, endostatin, does not affect murine cutaneous wound healing. J. Surg. Res. 91, 26–31 [DOI] [PubMed] [Google Scholar]
- 15. Cailhier J. F., Laplante P., Hebert M. J. (2006) Endothelial apoptosis and chronic transplant vasculopathy: recent results, novel mechanisms. Am. J. Transplant. 6, 247–253 [DOI] [PubMed] [Google Scholar]
- 16. Diao J. S., Xia W. S., Guo S. Z. (2010) Bevacizumab: a potential agent for prevention and treatment of hypertrophic scar. Burns 36, 1136–1137 [DOI] [PubMed] [Google Scholar]
- 17. Havasi A., Borkan S. C. (2011) Apoptosis and acute kidney injury. Kidney Int. 80, 29–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Szpaderska A. M., Walsh C. G., Steinberg M. J., DiPietro L. A. (2005) Distinct patterns of angiogenesis in oral and skin wounds. J. Dent. Res. 84, 309–314 [DOI] [PubMed] [Google Scholar]
- 19. Uhal B. D. (2008) The role of apoptosis in pulmonary fibrosis. Eur. Respir. Rev. 17, 138–144 [Google Scholar]
- 20. Van der Veer W., Niessen F. B., Ferreira J. A., Zwiers P. J., de Jong E. H., Middelkoop E., Molema G. (2011) Time course of the angiogenic response during normotrophic and hypertrophic scar formation in humans. Wound Repair Regen. 19, 292–301 [DOI] [PubMed] [Google Scholar]
- 21. Wang L., Scabilloni J. F., Antonini J. M., Rojanasakul Y., Castranova V., Mercer R. (2006) Induction of secondary apoptosis, inflammation, and lung fibrosis after intratracheal instillation of apoptotic cells in rats. Am. J. Physiol. Lung Cell. Mol. Physiol. 290, L696–L702 [DOI] [PubMed] [Google Scholar]
- 22. Wang R., Alam G., Zagariya A., Gidea C., Pinllos H., Lalude O., Choudhary G., Oezatalay D., Uhal B. D. (2000) Apoptosis of lung epithelial cells in response to TNF-alpha requires angiotensin II generation de novo. J. Cell. Physiol. 185, 253–259 [DOI] [PubMed] [Google Scholar]
- 23. Wynn T. A. (2007) Common and unique mechanisms regulate fibrosis in various fibroproliferative diseases. J. Clin. Invest. 117, 524–529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lai W. K., Adams D. H. (2005) Angiogenesis and chronic inflammation: the potential for novel therapeutic approaches in chronic liver disease. J. Hepatol. 42, 7–11 [DOI] [PubMed] [Google Scholar]
- 25. Song B., Zhang W., Guo S., Han Y., Zhang Y., Ma F., Zhang L., Lu K. (2009) Adenovirus-mediated METH1 gene expression inhibits hypertrophic scarring in a rabbit ear model. Wound Repair Regen. 17, 559–568 [DOI] [PubMed] [Google Scholar]
- 26. Wang X., Zhu H., Yang X., Bi Y., Cui S. (2010) Vasohibin attenuates bleomycin induced pulmonary fibrosis via inhibition of angiogenesis in mice. Pathology. 42, 457–462 [DOI] [PubMed] [Google Scholar]
- 27. Fraisl P., Mazzone M., Schmidt T., Carmeliet P. (2009) Regulation of angiogenesis by oxygen and metabolism. Dev. Cell. 16, 167–179 [DOI] [PubMed] [Google Scholar]
- 28. Cheresh D. A., Stupack D. G. (2008) Regulation of angiogenesis: apoptotic cues from the ECM. Oncogene 27, 6285–6298 [DOI] [PubMed] [Google Scholar]
- 29. Dimmeler S., Zeiher A. M. (2000) Endothelial cell apoptosis in angiogenesis and vessel regression. Circ. Res. 87, 434–439 [DOI] [PubMed] [Google Scholar]
- 30. Nyberg P., Xie L., Kalluri R. (2005) Endogenous inhibitors of angiogenesis. Cancer Res. 65, 3967–3979 [DOI] [PubMed] [Google Scholar]
- 31. Stoneman V., Bennett M. R. (2009) Role of Fas/Fas-L in vascular cell apoptosis. J. Cardiovasc. Pharmacol. 53, 100–108 [DOI] [PubMed] [Google Scholar]
- 32. Dulauroy S., Di Carlo S. E., Langa F., Eberl G., Peduto L. (2012) Lineage tracing and genetic ablation of ADAM12(+) perivascular cells identify a major source of profibrotic cells during acute tissue injury. Nat. Med. 18, 1262–1270 [DOI] [PubMed] [Google Scholar]
- 33. Hartlapp I., Abe R., Saeed RW., Peng T., Voelter W., Bucala R., Metz C. (2001) Fibrocytes induce an angiogenic phenotype in cultured endothelial cells and promote angiogenesis in vivo. FASEB J. 15, 2215–2224 [DOI] [PubMed] [Google Scholar]
- 34. Sarrazy V., Billet F., Micallef L., Coulomb B., Desmouliere A. (2011) Mechanisms of pathological scarring: role of myofibroblasts and current developments. Wound Repair Regen. 19(Suppl 1), s10–s15 [DOI] [PubMed] [Google Scholar]
- 35. Wolfram D., Tzankov A., Pulzl P., Piza-Katzer H. (2009) Hypertrophic scars and keloids–a review of their pathophysiology, risk factors, and therapeutic management. Dermatol. Surg. 35, 171–181 [DOI] [PubMed] [Google Scholar]
- 36. Van der Veer W., Bloemen M. C., Ulrich M. M., Molema G., van Zuijlen P. P., Middelkoop E., Niessen F. B. (2009) Potential cellular and molecular causes of hypertrophic scar formation. Burns 35, 15–29 [DOI] [PubMed] [Google Scholar]
- 37. Riou J., Cohen J. R., Johnson H., Jr. (1992) Factors influencing wound dehiscence. Am. J. Surg. 163, 324–330 [DOI] [PubMed] [Google Scholar]
- 38. Greenhalgh D. (1998) The role of apoptosis in wound healing. Int. J. Biochem. Cell Biol. 30, 1019–1030 [DOI] [PubMed] [Google Scholar]
- 39. Desmoulire A., Redard M., Darby I., Gabbiani G. (1995) Apoptosis mediates the decrease in cellularity during the transition between granulation tissue and scar. Am. J. Pathol. 146, 56–66 [PMC free article] [PubMed] [Google Scholar]
- 40. Benjamin L. (2000) The controls of microvascular survival. Cancer Metastasis Rev. 19, 75–81 [DOI] [PubMed] [Google Scholar]
- 41. Canbay A., Kip S. N., Kahraman A., Gieseler R. K., Nayci A., Gerken G. (2005) Apoptosis and fibrosis in non-alcoholic fatty liver disease. Turk. J. Gastroenterol. 16, 1–6 [PubMed] [Google Scholar]
- 42. Gurtl B., Kratky D., Guelly C., Zhang L., Gorkiewicz G., Das S. K., Tamilarasan K. P., Hoefler G. (2009) Apoptosis and fibrosis are early features of heart failure in an animal model of metabolic cardiomyopathy. Int. J. Exp. Pathol. 90, 338–346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Eltanani M., Campbell F., Kurisetty V., Jin D., McCann M., Rudland P. (2006) The regulation and role of osteopontin in malignant transformation and cancer. Cytokine Growth Factor Rev. 17, 463–474 [DOI] [PubMed] [Google Scholar]
- 44. Tzortzaki E., Antoniou K. M., Zervou M. I., Lambiri I., Koutsopoulos A., Tzanakis N., Plataki M., Maltezakis G., Bouros D., Siafakas N. M., (2007) Effects of antifibrotic agents on TGF-β1, CTGF and IFN-γ expression in patients with idiopathic pulmonary fibrosis. Respir. Med. 101, 1821–1829 [DOI] [PubMed] [Google Scholar]
- 45. Jang Y., Arumugam S., Gibran N. S., Isik F. F. (1999) Role of αv integrins and angiogenesis during wound repair. Wound Repair Regen. 7, 375–380 [DOI] [PubMed] [Google Scholar]
- 46. Wilgus T. A., Ferreira A. M., Oberyszyn T. M., Bergdall V. K., Dipietro L. A. (2008) Regulation of scar formation by vascular endothelial growth factor. Lab. Invest. 88, 579–590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Hernandez-Gea V., Friedman S. L. (2011) Pathogenesis of liver fibrosis. Ann. Rev. Pathol. Mech. Dis. 6, 425–456 [DOI] [PubMed] [Google Scholar]
- 48. Povero D., Busletta C., Novo E., di Bonzo L. V., Cannito S., Paternostro C., Parola M. (2010) Liver fibrosis: a dynamic and potentially reversible process. Histol. Histopathol. 25, 1075–1091 [DOI] [PubMed] [Google Scholar]
- 49. Murphy F. R., Issa R., Zhou X., Ratnarajah S., Nagase H., Arthur M. J., Benyon C., Iredale J. P. (2002) Inhibition of apoptosis of activated hepatic stellate cells by tissue inhibitor of metalloproteinase-1 is mediated via effects on matrix metalloproteinase inhibition: implications for reversibility of liver fibrosis. J. Biol. Chem. 277, 11069–11076 [DOI] [PubMed] [Google Scholar]
- 50. Lemos Q., Andrade Z. A. (2010) Angiogenesis and experimental hepatic fibrosis. Mem. Inst. Oswaldo Cruz 105, 611–614 [DOI] [PubMed] [Google Scholar]
- 51. Hannivoort R. A., Hernandez-Gea V., Friedman S. L. (2012) Genomics and proteomics in liver fibrosis and cirrhosis. Fibrogenesis Tissue Repair 5, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Novo E. (2005) Overexpression of Bcl-2 by activated human hepatic stellate cells: resistance to apoptosis as a mechanism of progressive hepatic fibrogenesis in humans. Gut 55, 1174–1182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Lee J., Lee K. S., Paik Y., Nyun Park Y., Han K. H., Chon C. Y., Moon Y. M. (2003) Apoptosis of hepatic stellate cells in carbon tetrachloride induced acute liver injury of the rat: analysis of isolated hepatic stellate cells. J. Hepatol. 39, 960–966 [DOI] [PubMed] [Google Scholar]
- 54. Maher J. (2001) Interactions between hepatic stellate cells and the immune system. Semin. Liver. Dis. 21, 417–426 [DOI] [PubMed] [Google Scholar]
- 55. Kisseleva T., Cong M., Paik Y., Scholten D., Jiang C., Benner C., Iwaisako K., Moore-Morris T., Scott B., Tsukamoto H., Evans S. M., Dillmann W., Glass C. K., Brenner D. A. (2012) Myofibroblasts revert to an inactive phenotype during regression of liver fibrosis. Proc. Natl. Acad. Sci. U. S. A. 109, 9448–9453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. King T., Jr., Pardo A., Selman M. (2011) Idiopathic pulmonary fibrosis. Lancet. 378, 1949–1961 [DOI] [PubMed] [Google Scholar]
- 57. Meltzer E., Noble P. W. (2008) Idiopathic pulmonary fibrosis. Orphanet J. Rare Dis. 3, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Pardo A. (2006) Matrix metalloproteases in aberrant fibrotic tissue remodeling. Proc. Am. Thorac. Soc. 3, 383–388 [DOI] [PubMed] [Google Scholar]
- 59. Razzaque M., Taguchi T. (2003) Pulmonary fibrosis: cellular and molecular events. Pathol. Int. 53, 133–145 [DOI] [PubMed] [Google Scholar]
- 60. Hagimoto N., Kuwano K., Nomoto Y., Kunitake R., Hara N. (1997) Apoptosis and expression of Fas Flas ligand mRNA in bleomycin induced pulmonary fibrosis in mice. Am. J. Respir. Cell Mol. Biol. 16, 91–101 [DOI] [PubMed] [Google Scholar]
- 61. Arenberg D., Kunkelfl S. A., Polverini P. J., Morris S. B., Burdick M. D., Glass M. C., Taub D. T., Iannettoni M. D., Whyte R. I., Strieter R. M. (1996) Interferon-γ-inducible protein 10 (IP-10) is an angiostatic factor that inhibits human non-small cell lung cancer (NSCLC) tumorigenesis and spontaneous metastases. J. Exp. Med. 184, 981–992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Noble P. W., Homer R. J. (2004) Idiopathic pulmonary fibrosis: new insights into pathogenesis. Clin. Chest Med. 25, 749–758, vii [DOI] [PubMed] [Google Scholar]
- 63. Hagimoto N., Kuwano K., Miyazaki H., Kunitake R., Fujita M., Kawasaki M., Kaneko Y., Hara N. (1997) Induction of apoptosis and pulmonary fibrosis in mice in response to ligation of Fas antigen. Am. J. Respir. Cell Mol. Biol. 17, 272–278 [DOI] [PubMed] [Google Scholar]
- 64. Kuwano K., Kunitake R., Maeyama T., Hagimoto N., Kawasaki M., Matsuba T., Yoshimi M., Inoshima I., Yoshida K., Hara N. (2001) Attenuation of bleomycin induced pneumopathy in mice by a caspase inhibitor. Am. J. Physiol. Lung Cell. Mol. Physiol. 280, L316–L325 [DOI] [PubMed] [Google Scholar]
- 65. Chen F., Gong L., Zhang L., Wang H., Qi X., Wu X., Xiao Y., Cai Y., Liu L., Li X., Ren J. (2006) Short courses of low dose dexamethasone delay bleomycin-induced lung fibrosis in rats. Eur. J. Pharmacol. 536, 287–295 [DOI] [PubMed] [Google Scholar]
- 66. Farkas L., Gauldie J., Voelkel N. F., Kolb M. (2010) Pulmonary hypertension and idiopathic pulmonary fibrosis: a tale of angiogenesis, apoptosis, and growth factors. Am. J. Respir. Cell Mol. Biol. 45, 1–15 [DOI] [PubMed] [Google Scholar]
- 67. Renzoni E. A. (2002) Interstitial vascularity in fibrosing alveolitis. Am. J. Respir. Crit. Care Med. 167, 438–443 [DOI] [PubMed] [Google Scholar]
- 68. Cosgrove G. P. (2004) Pigment epithelium-derived factor in idiopathic pulmonary fibrosis: a role in aberrant angiogenesis. Am. J. Respir. Crit. Care Med. 170, 242–251 [DOI] [PubMed] [Google Scholar]
- 69. Ebina M. (2004) Heterogeneous increase in CD34-positive alveolar capillaries in idiopathic pulmonary fibrosis. Am. J. Respir. Crit. Care Med. 169, 1203–1208 [DOI] [PubMed] [Google Scholar]
- 70. De Fijter J. (2010) Rejection and function and chronic allograft dysfunction. Kidney Int. 78, S38–S41 [DOI] [PubMed] [Google Scholar]
- 71. Schmauss D., Weis M. (2008) Cardiac allograft vasculopathy: recent developments. Circulation. 117, 2131–2141 [DOI] [PubMed] [Google Scholar]
- 72. Taylor D., Edwards L. B., Boucek M. M., Trulock E. P., Waltz D. A., Keck B. M., Hertz M. I. (2006) Registry of the International Society for Heart and Lung Transplantation: Twenty-third Official Adult Heart Transplantation Report—2006. J. Heart Lung Transplant. 25, 869–879 [DOI] [PubMed] [Google Scholar]
- 73. Denton M., Davis S. F., Baum M. A., Melter M., Reinders M. E. J., Exeni A., Samsonov D. V., Fang J., Ganz P., Briscoe D. M. (2000) The role of the graft endothelium in transplant rejection: Evidence that endothelial activation may serve as a clinical marker for the development of chronic rejection. Pediatr. Transplant. 4, 252–260 [DOI] [PubMed] [Google Scholar]
- 74. Huibers M., De Jonge N., Van Kuik J., Koning E., Van Wichen D., Dullens H., Schipper M., De Weger R. (2011) Intimal fibrosis in human cardiac allograft vasculopathy. Transpl. Immunol. 25, 124–132 [DOI] [PubMed] [Google Scholar]
- 75. Reinders M. E. J. (2006) Angiogenesis and endothelial cell repair in renal disease and allograft rejection. (2006) J. Am. Soc. Nephrol. 17, 932–942 [DOI] [PubMed] [Google Scholar]
- 76. Reinders M., Fang J. C., Wong W., Ganz P., Briscoe D. M. (2003) Expression patterns of vascular endothelial growth factor in human cardiac allografts. Transplantation 76, 224–230 [DOI] [PubMed] [Google Scholar]
- 77. Wang W. (2002) Notch3 signaling in vascular smooth muscle cells induces c-FLIP expression via ERK/MAPK activation. Resistance to Fas ligand-induced apoptosis. J. Biol. Chem. 277, 21723–21729 [DOI] [PubMed] [Google Scholar]
- 78. Choy J., Kerjner A., Wong B. W., McManus B. M., Granville D.J. (2004) Perforin mediates endothelial cell death and resultant transplant vascular disease in cardiac allografts. Am. J. Pathol. 165, 127–133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Shimizu A., Yamada K., Sachs D. H., Colvin R. B. (2002) Persistent rejection of peritubular capillaries and tubules is associated with progressive interstitial fibrosis. Kidney Int. 61, 1867–1879 [DOI] [PubMed] [Google Scholar]
- 80. Johnson A., Francis M., DiPietro L. (2013) Differential apoptosis in mucosal and dermal wound healing. Adv. Wound Care In press [DOI] [PMC free article] [PubMed] [Google Scholar]