Abstract
Pathological and physiological stimuli, including acute exercise, activate autophagy; however, it is unknown whether exercise training alters basal levels of autophagy and whether autophagy is required for skeletal muscle adaptation to training. We observed greater autophagy flux (i.e., a combination of increased LC3-II/LC3-I ratio and LC3-II levels and reduced p62 protein content indicating a higher rate of initiation and resolution of autophagic events), autophagy protein expression (i.e., Atg6/Beclin1, Atg7, and Atg8/LC3) and mitophagy protein Bnip3 expression in tonic, oxidative muscle compared to muscles of either mixed fiber types or of predominant glycolytic fibers in mice. Long-term voluntary running (4 wk) resulted in increased basal autophagy flux and expression of autophagy proteins and Bnip3 in parallel to mitochondrial biogenesis in plantaris muscle with mixed fiber types. Conversely, exercise training promoted autophagy protein expression with no significant increases of autophagy flux and mitochondrial biogenesis in the oxidative soleus muscle. We also observed increased basal autophagy flux and Bnip3 content without increases in autophagy protein expression in the plantaris muscle of sedentary muscle-specific Pgc-1α transgenic mice, a genetic model of augmented mitochondrial biogenesis. These findings reveal that endurance exercise training-induced increases in basal autophagy, including mitophagy, only take place if an enhanced oxidative phenotype is achieved. However, autophagy protein expression is mainly dictated by contractile activity independently of enhancements in oxidative phenotype. Exercise-trained mice heterozygous for the critical autophagy protein Atg6 showed attenuated increases of basal autophagy flux, mitochondrial content, and angiogenesis in skeletal muscle, along with impaired improvement of endurance capacity. These results demonstrate that increased basal autophagy is required for endurance exercise training-induced skeletal muscle adaptation and improvement of physical performance.—Lira, V. A., Okutsu, M., Zhang, M., Greene, N. P., Laker, R. C., Breen, D. S., Hoehn, K. L., Yan, Z. Autophagy is required for exercise training-induced skeletal muscle adaptation and improvement of physical performance.
Keywords: voluntary wheel running, mitophagy, Bnip3, mitochondrial biogenesis, angiogenesis
It is well known that regular exercise improves physical performance and brings about important health benefits that curb many pathological conditions, such as cancer, as well as cardiovascular, metabolic and neurodegenerative diseases (1–4). In fact, physical fitness is a predictor of all-cause mortality and a measure of quality of life (5). Human skeletal muscle represents 40–45% of body mass in a healthy, lean individual (6) and has an incredible capacity to adapt to use or disuse, which makes it a critical determinant of whole-body functional capacity and metabolic status (7–9). Exercise training induces phenotypic adaptations in skeletal muscle that include mitochondrial biogenesis, angiogenesis, fiber type transformation, and improved insulin sensitivity (10, 11). These phenotypic adaptations underscore the health benefits of regular exercise; however, the molecular and cellular mechanisms underlying exercise-induced skeletal muscle adaptation and improved physical performance are far from being completely understood.
Exercise training-induced skeletal muscle adaptation likely requires both addition and clearance of cellular components. Biosynthesis of contractile proteins and mitochondrial biogenesis during muscle adaptation has received considerable attention, whereas much less is known about the relevance of clearance in the adaptation process. Macroautophagy, hereafter referred to as autophagy, is an evolutionarily conserved catabolic process that is responsible for the degradation of cellular components, such as protein aggregates, long-lived proteins, excess or damaged organelles (e.g., mitochondria), and intracellular pathogens (12, 13). Autophagy involves the sequestration of proteins and/or organelles by double-membrane structures that form autophagosomes, which then fuse with lysosomes to degrade engulfed materials (14). Two recent studies have shown that an intense, single bout of forced treadmill exercise in mice resulted in activation of autophagy and increased autophagy flux in skeletal muscle (15, 16).
In contrast, basal autophagy, also referred to as quality control autophagy, is a continuous baseline process responsible for removal of dysfunctional cellular components and is required for normal cell function (12, 17). Despite the aforementioned evidences that an acute bout of exercise stimulates autophagy, investigations on the functional role and regulation of basal autophagy in skeletal muscle adaptation are currently few and inconclusive. It has been shown that basal autophagy is reduced in aging human skeletal muscle, which could be restored by calorie restriction alone or in combination with exercise training (18). It has also been demonstrated that deficiency in basal autophagy leads to muscle damage (19), but it is unknown whether basal autophagy rates in skeletal muscle are affected by exercise training. Most notably, it remains to be established whether an altered level of basal autophagy is required for exercise training-induced skeletal muscle adaptation and improved physical performance.
In the current study, we first compared basal autophagy among muscles of varying levels of locomotive activities and oxidative capacities. To gain further insights into the physiological regulatory steps in control of basal autophagy, we investigated glycolytic and oxidative muscles of sedentary and trained mice, as well as examined basal autophagy in sedentary muscle-specific Pgc-1α transgenic mice, which have enhanced mitochondrial biogenesis and oxidative phenotype independent of increases in contractile activity. Finally, we subjected haplodeficient mice for the autophagy related gene 6 (Atg6/Beclin1), a class III PI3K that is essential for autophagosome formation, to long-term voluntary wheel running and investigated phenotypic adaptations in skeletal muscle as well as physical performance. Our results reveal that increased basal autophagy is a feature of the enhanced oxidative muscle phenotype and is required for normal endurance exercise training-induced mitochondrial biogenesis and angiogenesis in skeletal muscle, as well as improvement in physical performance.
MATERIALS AND METHODS
Animals
C57BL/6J mice (male, 11 wk of age) were obtained from Jackson Laboratory (Bar Harbor, ME, USA). Mice with muscle-specific overexpression of peroxisome proliferator-activated receptor γ coactivator 1-α (Pgc-1α) under the promoter of muscle creatine kinase (MCK-Pgc-1α) were a kind gift of Dr. Bruce Spiegelman (Dana-Farber Cancer Institute, Boston, MA, USA; ref. 20). Atg6+/− mice were kindly provided by Dr. Beth Levine (University of Texas Southwestern Medical Center, Dallas, TX, USA; ref. 21). Mice were housed in temperature-controlled (22°C) quarters with a 12:12-h light-dark cycle with free access to water and normal chow (Harlan 7912; Harlan Bioproducts, Indianapolis, IN, USA). Muscle samples were harvested after the mice were anesthetized with isoflurane followed by euthanasia with cervical dislocation. All animal protocols were approved by the University of Virginia Animal Care and Use Committee.
Genotyping
Mouse genomic DNA was isolated from the tail, and PCR-based genotyping for MCK-Pgc-1α mice was performed with the forward primer 5′-GCAGGATCACATAGGCAGGATGTGGCC-3′ and reverse primer 5′-GGAAGATCTGGGCAAAGAGGCTGGTCC-3′. The PCR reactions included an initial denaturation at 96°C for 4 min followed by 30 cycles of denaturation at 96°C for 30 s, annealing at 60°C for 30 s, and extension at 72°C for 45 s, and a final extension at 72°C for 7 min. Genotyping for Atg6+/− mice was also performed by using DNA isolated from the tail in PCR reaction with Atg6 wild-type, forward primer 5′-CTGGACACGAGTTTCAAGATCCTG-3′ and reverse primer 5′-GGGCATGGTAGCACACAGACCTC-3′, and Atg6-knockout, forward primer 5′-TGCGGGCCAGAGGCCACTTGTGTAGC-3′ and reverse primer 5′-GCTCCAGACTGCCTTGGGAAAAG-3′. The PCR reactions included an initial denaturation at 96°C for 4 min, followed by 35 cycles of denaturation at 96°C for 30 s, annealing at 60°C for 1 min, and extension at 72°C for 45 s, and a final extension at 72°C for 7 min.
Voluntary wheel-running exercise
Mice were individually housed in cages equipped with a running wheel. Animals were acclimatized to cages for 2 d with wheels locked. Wheels were unlocked on the third day, and running activity was monitored continually during the experiment (4 or 5 wk, as described in Results). Wheels were then locked for 28–30 h before muscle samples were harvested to avoid any acute effect of the last bout of exercise on autophagy.
Treadmill running test
Mice were acclimatized to treadmill running for 3 d (13.4 m/min, 10 min). On the fourth day, animals were tested on the treadmill starting with a speed of 13.4 m/min and 5% incline. The speed was increased by ∼2.7 m/min every 30 min until reaching the speed of 26.8 m/min. A brush located at the end of the treadmill was used to encourage the animals to run, and the test was terminated when a mouse stopped responding to tail brushing continuously for 20 s. Blood lactate level, used as a biochemical parameter to monitor exhaustion, was assessed before and at the termination of the test by tail bleeding using the Lactate Scout meter (SensLab, Leipzig, Germany).
Real-time PCR
Briefly, total RNA was extracted from skeletal muscle with TRIzol (Invitrogen, Madison, WI, USA) according to the manufacturer's instructions. Reverse transcriptase reaction was performed with 2 μg of total RNA using SuperScript II First-Strand Synthesis System for RT-PCR (Life Technologies, Carlsbad, CA, USA). The real-time PCR analysis was performed as described previously using the 2−ΔΔCT quantification method, where CT is the threshold cycle number (22). The following primers and probes were obtained from Applied Biosystems (Foster City, CA, USA): Pgc-1α (RefSeq NM_133249.2, assay no. Mm00504720_m1), cytochrome c (Cyc; RefSeq NM_025567.2, assay no. Mm00470540_m1), BCL2/adenovirus E1B 19-kDa protein-interacting protein 3 (Bnip3; RefSeq NM_009760.4, assay no. Mm01275600_g1), Atg6/Beclin1 (RefSeq NM_019584.3, assay no. Mm01265461_m1), and 18S rRNA (VIC/MGB probe, Primer Limited, cat. no. 4319413E). Equivalent PCR amplification efficiency for all primers was confirmed with serial dilutions of a standard sample. Results were then normalized to 18S and presented as fold change in relation to wild-type littermates.
Western blot analysis
For protein analysis, muscle samples were homogenized and analyzed as described previously (23). The following antibodies were used: Bnip3 (3769, 1:1000), Atg6/Beclin1 (3738, 1:1000), microtubule-associated protein light chain 3 (LC3) A/B (4108, 1:1000), Atg7 (2631, 1:1000), Cyc (4272, 1:1000), and glyceraldehyde 3-phosphate dehydrogenase (Gapdh; 2118, 1:2000) from Cell Signaling (Danvers, MA, USA); Pgc-1α (AB3234, 1:1000) from Millipore (Billerica, MA, USA); cytochrome c oxidase subunit 4 (Cox4; A21296, 1:1000) from Invitrogen (Grand Island, NY, USA); β-actin (81178,1:2000) from Santa Cruz Biotechnology (Santa Cruz, CA, USA); and myosin heavy chain IIa (MHC IIa; SC-71, 1:100) from theGerman Collection of Microorganisms and Cell Cultures (Braunschweig, Germany). Nitrocellulose membranes were used for all proteins listed above except for LC3, where PVDF membranes were used to improve LC3-II signal. We have observed 2 bands for p62/sequestosome 1 (Sqstm1) in skeletal muscle using the P0067 antibody (1:1000) from Sigma-Aldrich (St. Louis, MO, USA). The identity of the top band was confirmed as p62 through experiments in culture and in vivo with either chloroquine or colchicine, which blocks p62 degradation by inhibiting autophagasome-lysosome fusion (data not shown). Membranes were analyzed and quantified with an Odyssey Infrared Imaging System (LI-COR Biosciences, Lincoln, NE, USA). Protein expression was normalized to β-actin, except when β-actin levels were affected by training. In those cases, protein expression was normalized to Gapdh, as described in Results section.
Muscle capillary density
Plantaris muscle sections were analyzed by immunofluorescence for MHCIIa and CD31 as described previously (24). Briefly, frozen muscle sections were fixed with 4% paraformaldehyde, incubated with primary antibodies for MHCIIa (SC-71, 1:25) and cluster of differentiation 31 (CD31; MCA1364, 1:25; Serotec, Kidlington, UK) at 4°C overnight, followed by incubation with fluorescent-conjugated secondary antibodies (goat anti-mouse IgG-FITC and goat-anti-rat IgG-Cy5, respectively). Sections were then mounted with Vectashield (Vector Laboratories, Burlingame, CA, USA), and images were captured using a confocal microscope (Fluoview 1000; Olympus, Tokyo, Japan). Capillary density was calculated as previously reported (24).
Statistical analysis
The results are presented as means ± se. Student's t test was used to compare trained and sedentary samples as well as MCK-Pgc-1α mice and wild-type (WT) littermates. One-way ANOVA was used to assess differences across different muscles. Two-way ANOVA was used in the comparison of training adaptations in Atg6+/− mice and WT littermates. Both 1-way and 2-way ANOVA were followed by Newman-Keuls post hoc test when applicable. Values of P < 0.05 were considered statistically significant.
RESULTS
Tonic, oxidative muscle has greater basal autophagy, autophagy, and mitophagy protein expression than phasic, glycolytic muscle
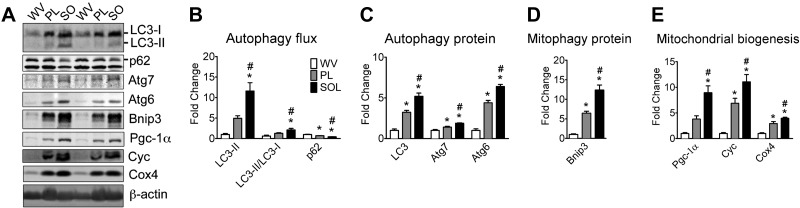
Skeletal muscles exhibit different phenotypic features due to different levels of locomotive activities. Tonic muscles, such as postural muscles, are mostly composed of oxidative fibers, with a robust mitochondrial network and predominant expression of MHCIIa and MHCI proteins. Conversely, phasic muscles are mainly composed of glycolytic fibers with low mitochondrial content and predominant expression of MHCIIb or MHCIIx proteins (25). It is currently unknown whether basal autophagy levels are regulated in parallel to muscle contractile and metabolic properties. We addressed this question by comparing basal autophagy among tonic, oxidative soleus muscle with phasic, glycolytic white vastus lateralis muscle and plantaris muscle, which is composed of both glycolytic and oxidative fibers. Basal autophagy was assessed as nonstimulated autophagy flux. Increased levels of LC3-II, the phosphatidylethanolamine (PE)-conjugated form of LC3-I that is found attached to both internal and external autophagosome membranes (26), coupled with increased LC3-I conversion into LC3-II, assessed via LC3-II/LC3-I ratio, and reduced p62 protein content, a cargo receptor for ubiquitinated substrates that is itself degraded during autophagy, collectively denote higher rates of initiation and resolution of autophagic events, a.k.a. autophagy flux (15, 16, 27). Here, we observed that LC3-II protein and LC3-II/LC3-I ratio were the lowest in white vastus lateralis muscle, the highest in soleus muscle and at an intermediate level in plantaris muscle. Accordingly, p62 protein content showed the opposite pattern among these muscles (Fig. 1A, B). These changes were accompanied by graded increases from white vastus lateralis to plantaris and then to soleus muscles of total LC3, Atg6, and Atg7 proteins; the latter is involved in the process of LC3-I conjugation to PE during formation of the autophagosome (26), as well as of Bnip3 expression, a mitochondrial protein that interacts with LC3 and plays a critical role in mitophagy (refs. 28–31 and Fig. 1A, C, D). A very similar pattern was observed with Pgc-1α, a master regulator of mitochondrial gene expression (20), and the mitochondrial proteins Cyc and Cox4, which jointly were used to evaluate mitochondrial biogenesis (Fig. 1A, E). These results demonstrate that basal autophagy, autophagy, and mitophagy proteins are positively correlated with locomotive activity and oxidative phenotype in skeletal muscle.
Figure 1.
Tonic, oxidative muscles have higher autophagy flux, autophagy, and mitophagy protein expression than phasic, glycolytic muscles. Immunoblot analyses were performed for homogenates of tonic, oxidative soleus (SO), plantaris (PL) with mixed fiber types, and phasic, glycolytic white vastus lateralis (WV) muscles. A) Representative immunoblot images of LC3, p62, Atg7, Atg6, Bnip3, Pgc-1α, Cyc, Cox4, and β-actin. B) Quantification of LC3-II, LC3-II/LC3-I ratio, and p62 protein collectively as an index of autophagy flux. C) Quantification of LC3 (LC3-I+LC3-II), Atg7, and Atg6 proteins. D) Quantification of the mitophagy protein Bnip3. E) Quantification of Pgc-1α, Cyc, and Cox4 proteins as indexes of mitochondrial biogenesis. Protein expression comparisons were performed after normalization to β-actin. Results are represented as means ± se (n=4/muscle). *P < 0.05 vs. WV; #P < 0.05 vs. PL.
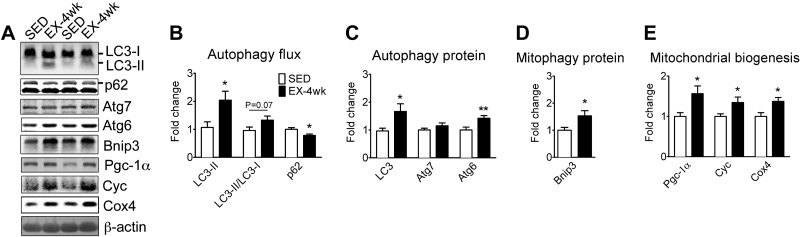
Exercise training promotes basal autophagy, autophagy, and mitophagy protein expression in plantaris muscle of mixed fiber types
To determine whether exercise training promotes basal autophagy, we took advantage of a mouse model of physiological exercise that causes adaptation in plantaris muscle with mixed fiber types (23, 24). Long-term (4-wk) voluntary wheel running led to significantly increased basal autophagy in the plantaris muscle compared to sedentary control mice. This was evidenced by elevated LC3-II protein expression, a trend of increased LC3-II/LC3-I ratio (P = 0.07) and reduced p62 protein content (Fig. 2A, B). Exercise training also resulted in increased protein expression of Atg6 and LC3 (Fig. 2A, C), but not Atg7. In addition, long-term voluntary wheel running led to increased Bnip3 protein levels (Fig. 2A, D). As expected, endurance exercise training resulted in increased protein expression of Pgc-1α, Cyc, and Cox4, indicating increased mitochondrial content (Fig. 2A, E). Altogether, these findings demonstrate that endurance exercise training promotes basal autophagy along with enhanced expression of autophagy and mitophagy proteins in a skeletal muscle that becomes more oxidative with training.
Figure 2.
Exercise training promotes basal autophagy, autophagy, and mitophagy protein expression in the plantaris muscle with mixed fiber types. Immunoblot analyses were performed for homogenates of plantaris muscles from mice that underwent 4 wk of voluntary wheel-running exercise (EX-4 wk) compared to the sedentary control (SED). A) Representative immunoblot images of LC3, p62, Atg7, Atg6, Bnip3, Pgc-1α, Cyc, Cox4, and β-actin in sedentary and trained mice. B) Quantification of LC3-II, LC3-II/LC3-I ratio, and p62 protein collectively as an index of autophagy flux. C) Quantification of LC3 (LC3-I+LC3-II), Atg7, and Atg6 proteins. D) Quantification of the mitophagy protein Bnip3. E) Quantification of Pgc-1α, Cyc, and Cox4 proteins as indexes of mitochondrial biogenesis. Protein expression comparisons were performed after normalization to β-actin. Results are represented as means ± se (n=10/group). *P < 0.05, **P < 0.01.
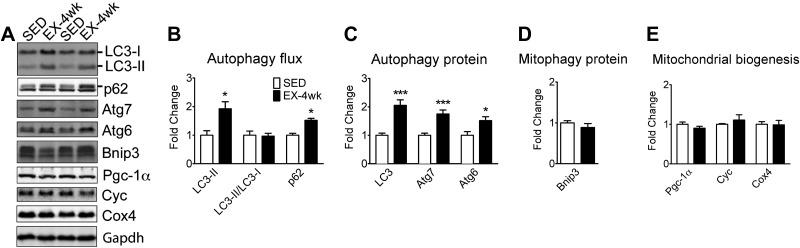
Endurance exercise training promotes autophagy protein expression without increases in basal autophagy and mitophagy protein content in oxidative soleus muscle
Endurance exercise training involves chronically increased contractile activity and results in an enhanced oxidative phenotype, either of which may promote basal autophagy and expression of autophagy genes. To gain further insight into the necessity for an enhanced oxidative phenotype for the elevation of basal autophagy with exercise training, we first measured autophagy flux and autophagy protein expression in oxidative soleus muscle. Because of its high postural and locomotive activities soleus muscle has already a highly oxidative phenotype that is minimally enhanced by the long-term increases in contractile activity during voluntary running. In fact, we did not observe any increases in Pgc-1α, Cyc, and Cox4 protein expression with voluntary wheel running (Fig. 3A, E). Interestingly, voluntary wheel running resulted in increased LC3-II expression, but without increases in the LC3-II/LC3-I ratio and with elevated, rather than reduced p62 protein content (Fig. 3A, B). These findings collectively show that endurance exercise training does not result in increased basal autophagy flux in oxidative soleus muscle when there is no significant enhancement of oxidative phenotype. Increased LC3-II levels seemed to be a result of increased total LC3 (LC3-I+LC3-II) protein expression as shown in Fig. 3C. On the other hand, exercise training promoted total LC3, Atg7, and Atg6 protein expression (Fig. 3A, C), but not Bnip3 (Fig. 3A, D). Therefore, endurance exercise training promotes autophagy protein expression, but does not increase basal autophagy and the expression of the mitophagy protein Bnip3 in skeletal muscle that does not become more oxidative with training.
Figure 3.
Exercise training promotes autophagy expression, but not basal autophagy and mitophagy protein expression in tonic, oxidative muscle. Immunoblot analyses were performed for homogenates of soleus muscle from mice that underwent 4 wk of voluntary wheel-running exercise (EX-4 wk) compared to the sedentary control (SED). A) Representative immunoblot images of LC3, p62, Atg7, Atg6, Bnip3, Pgc-1α, Cyc, Cox4, and Gapdh in sedentary and trained mice. B) Quantification of LC3-II, LC3-II/LC3-I ratio, and p62 protein collectively as an index of autophagy flux. C) Quantification of LC3 (LC3-I+LC3-II), Atg7, and Atg6 proteins. D) Quantification of the mitophagy protein Bnip3. E) Quantification of Pgc-1α, Cyc, and Cox4 proteins as indexes of mitochondrial biogenesis. Protein expression comparisons were performed after normalization to Gapdh. Results are represented as means ± se (n=10/group). *P < 0.05, ***P < 0.001.
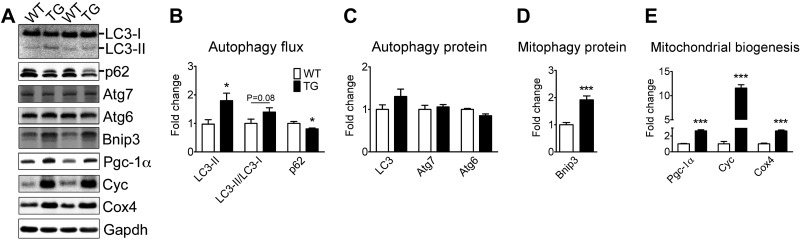
Muscle-specific Pgc-1α transgenic mice have elevated basal autophagy and mitophagy protein expression in plantaris muscle with mixed fiber types
To further address the question of whether increased basal autophagy observed following endurance exercise training in the plantaris muscle is due to chronically increased contractile activity and/or enhanced oxidative phenotype, we investigated the plantaris muscle of sedentary MCK-Pgc-1α mice. MCK-Pgc-1α mice are known to have enhanced oxidative phenotype and mitochondrial biogenesis in plantaris muscle (20). Accordingly, we observed increased LC3-II, a trend of increased LC3-II/LC3-I ratio (P = 0.08) and reduced p62 levels in MCK-Pgc-1α mice compared to WT littermates, denoting increased basal autophagy flux (Fig. 4A, B). Elevated levels of Bnip3, without significant changes in Atg7, Atg6 and LC3, followed this increased autophagic flux in MCK-Pgc-1α mice (Fig. 4A, C, D). The Bnip3 gene did not seem to be transcriptionally regulated by Pgc-1α because Bnip3 mRNA levels were the same between MCK-Pgc-1α mice and WT littermates (Supplemental Fig. S1). As expected, MCK-Pgc-1α mice showed significant increases in Pgc-1α, Cox4, and Cyc protein expression in plantaris muscle (Fig. 4A, E). Taken together, these findings demonstrate that an enhanced oxidative phenotype is sufficient to promote enhanced basal autophagy flux and Bnip3 protein expression, but not the expression of autophagy proteins, in the absence of chronically increased contractile activity in skeletal muscle.
Figure 4.
Muscle-specific Pgc-1α overexpression promotes basal autophagy and the mitophagy protein Bnip3 expression, but not autophagy expression in skeletal muscle. Immunoblot analyses were performed for homogenates of plantaris muscles from MCK-Pgc-1α transgenic mice (TG) and WT littermates (WT). A) Representative immunoblot images of LC3, p62, Atg7, Atg6, Bnip3, Pgc-1α, Cyc, Cox4, and Gapdh. B) Quantification of LC3-II, LC3-II/LC3-I ratio, and p62 protein collectively as an index of autophagy flux. C) Quantification of LC3 (LC3-I+LC3-II), Atg7, and Atg6 proteins. D) Quantification of the mitophagy protein Bnip3. E) Quantification of Pgc-1α, Cyc, and Cox4 proteins as indexes of mitochondrial biogenesis. Protein expression comparisons were performed after normalization to Gapdh. Results are represented as mean ± se (n=9–12/group). *P < 0.05, ***P < 0.001.
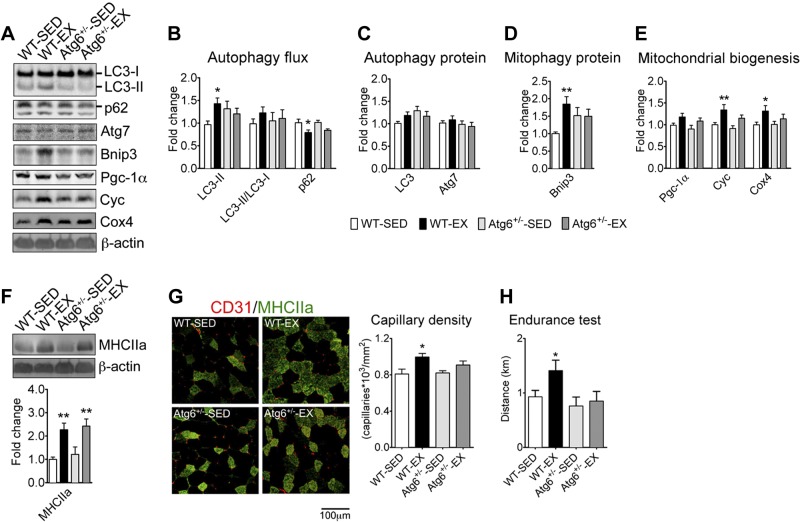
Atg6+/− mice have attenuated increases of autophagy flux, mitochondrial content, and angiogenesis in skeletal muscle, and no improvement in performance following endurance exercise training
In light of our observations of increased basal autophagy in oxidative or trained muscles, we decided to investigate the role of endurance exercise training-induced increase in basal autophagy on skeletal muscle adaptation and improvement of physical performance. We hypothesized that Atg6+/− mice, which have reduced expression of the critical Atg6 protein in skeletal muscle and several other tissues, but display normal basal autophagy (16, 21), would have a limited capacity to increase basal autophagy in response to exercise training and, as such, would serve as an ideal model to test the functional importance of increased basal autophagy flux in exercise-induced adaptations. Atg6+/− mice and WT littermates had similar voluntary running activity (Supplemental Fig. S2A) and ran a total of ∼400 km in 5 wk of training (Supplemental Fig. S2B). These mice also showed a similar degree of increased heart and soleus muscle weight with training (Supplemental Fig. S2C, E). Exercise training resulted in increased basal autophagy flux (i.e., increased LC3-II, and reduced p62 protein levels with a small, but not significant, increase in LC3-II/LC3-I ratio) in plantaris muscle with mixed fiber types in WT mice (Fig. 5A, B) despite a small, nonsignificant increase in total LC3 (Fig. 5A, C). This was accompanied by significant increases in the expression of the mitophagy protein Bnip3 (Fig. D) and Cyc and Cox4 proteins (Fig. 5A, E) that were paralleled by a nonsignificant increase in Pgc-1α. Overall these results are consistent with the findings in the plantaris muscle of WT mice presented in Fig. 2. However, none of these changes were observed in Atg6+/− mice. Taken together, these observations suggest that exercise training-induced increases in autophagy flux, Atg and mitophagy protein Bnip3 expression, and mitochondrial biogenesis were blunted in Atg6+/− mice. Consistent with our previous observations that contractile and metabolic adaptations (mitochondrial biogenesis and angiogenesis) in skeletal muscle are separable events (32, 33), Atg6+/− mice also showed normal increase of MHCIIa protein (Fig. 5F), but attenuated increase of capillary density in plantaris muscle (Fig. 5G). In addition, Atg6+/− mice failed to improve endurance capacity assessed by maximal treadmill running distance (Fig. 5H), where equivalent levels of exertion were confirmed by 2-fold or greater increases in blood lactate levels at the end of the test (Supplemental Fig. S3).
Figure 5.
Atg6+/− mice present deficient metabolic adaptations to exercise training in skeletal muscle and no improvement in endurance capacity. Immunoblot and immunofluorescence analyses were performed for plantaris muscles from Atg6+/− mice and WT littermates following 5 wk of voluntary wheel-running exercise (EX) or sedentary cage activity (SED). A) Representative immunoblot images of LC3, p62, Atg7, Bnip3, Pgc-1α, Cyc, Cox4, and β-actin. B) Quantification of LC3-II, LC3-II/LC3-I ratio, and p62 protein collectively as an index of autophagy flux. C) Quantification of LC3 (LC3-I+LC3-II) and Atg7 proteins. D) Quantification of the mitophagy protein Bnip3. E) Quantification of Pgc-1α, Cyc, and Cox4 proteins as indexes of mitochondrial biogenesis. F) Representative immunoblot images of MHCIIa and β-actin (top panel) and quantification of MHCIIa protein (bottom panel). G) Left panel: representative immunofluorescence images of plantaris cross-sections stained with antibodies for MHCIIa (green) and endothelial cell marker CD31 (red). Right panel: quantification of capillary density (right, n=6/group). H) Quantification of treadmill running distance during endurance test. Protein expression comparisons were performed after normalization to β-actin. Results are represented as means ± se (n=9–14/group, unless specified otherwise). *P < 0.05, **P < 0.01 vs. SED condition within the same genotype.
DISCUSSION
The discovery of autophagy as a major catabolic cellular process has triggered special interest in muscle research in recent years. Most of these studies investigate the induction of autophagy in catabolic muscle models associated with increased protein turnover, such as starvation, hypoxia and cachexia (28, 29, 34, 35). However, the regulation and functional role of basal autophagy in skeletal muscle adaptation remains largely uncharted. Here we document for the first time that endurance exercise training promotes basal autophagy, autophagy, and mitophagy protein expression in recruited skeletal muscle. Elevated levels of basal autophagy are also readily observed in a tonic, oxidative muscle when compared to a muscle of mixed fiber types and a phasic, glycolytic muscle. We have also taken advantage of a model of increased contractile activity that does not result in an enhanced oxidative phenotype (i.e., trained vs. sedentary soleus muscle), and a genetic model of enhanced oxidative phenotype that is independent of increased contractile activity in skeletal muscle (i.e., MCK-Pgc-1α mice) to dissect the relationship of contractile activity and oxidative phenotype to basal autophagy. Our findings support a link between mitochondrial biogenesis, or oxidative phenotype, and basal autophagy flux and expression of the mitophagy protein Bnip3. The results also demonstrate a link between chronic contractile activity and autophagy protein expression in skeletal muscle that is not sufficient to affect basal autophagy flux. Most notably, we have obtained evidence that endurance exercise training-induced increases in basal autophagy are required for metabolic adaptations (mitochondrial biogenesis and angiogenesis) and improved physical performance, but not for contractile adaptation in skeletal muscle.
Our first observation was that high levels of basal autophagy flux, autophagy, and mitophagy protein expression are inherent features of tonic, oxidative muscles. These results demonstrated that basal autophagy is regulated in parallel to muscle contractile and metabolic properties and raised the question of whether exercise training could promote basal autophagy levels in skeletal muscle. Here we report that endurance exercise training (voluntary running for 4 wk) in mice resulted in elevated autophagy flux and increased expression of autophagy and mitophagy proteins in recruited plantaris muscle. Of note, we harvested the muscle samples at 28–30 h after the last bout of exercise to avoid any acute exercise-mediated induction of autophagy as previously documented (15, 16). Consequently, our results clearly demonstrate that endurance exercise training promotes basal autophagy in skeletal muscle. Since autophagy is the main mechanism of mitochondrial turnover (36, 37), it seems logical that muscles with high mitochondrial content would require high basal autophagy flux. However, several models of increased use (e.g., exercise training) and disuse (e.g., casting and denervation) have demonstrated that muscle mitochondrial volume is fine tuned to contractile demands (23, 33, 38, 39). These observations raise the question of whether mitochondrial volume per se, or contractile activity, is the major determinant of basal autophagy in skeletal muscle. To address this question we first investigated the oxidative soleus muscle that does not increase mitochondrial content in response to the voluntary wheel running model of exercise training.
Despite increased contractile activity and adaptive responses induced by voluntary wheel running, including increased muscle weight (40), the soleus muscle did not show significant increases in autophagy flux and expression of the mitophagy protein Bnip3. Accordingly, there were no significant increases of proteins involved in mitochondrial biogenesis in the soleus muscle after 4 wk of voluntary wheel running. This is consistent with the notion that increases in basal autophagy require enhancements of the oxidative phenotype and occurs in parallel to mitochondrial biogenesis. Our observations in the trained oxidative soleus muscle also reveal that increased contractile activity is sufficient to cause an induction of autophagy protein expression despite not affecting basal autophagy.
Pgc-1α is a transcription cofactor that is not only sufficient to promote mitochondrial biogenesis and induce an oxidative phenotype (20), but is also required for exercise-induced metabolic adaptations in skeletal muscle (32). MCK-Pgc-1α mice display increased mitochondrial content with normal organelle function in both adults and aged rodents, without increases in contractile activity (20, 41). Therefore, these animals serve as an ideal genetic model to address whether an enhanced oxidative phenotype per se can drive increased levels of basal autophagy. Our results unveil that indeed the increased mitochondrial biogenesis in MCK-Pgc-1α mice is associated with elevated basal autophagy in skeletal muscle. It is important to notice that these findings were observed in association with enhanced Bnip3 expression but in the absence of increased autophagy protein expression, suggesting that increased oxidative phenotype or mitochondrial content promotes basal autophagy, likely via increased mitophagy rates, but not the expression of autophagy proteins. These observations are physiologically sound because a mismatch between mitochondrial biogenesis and mitochondrial turnover (i.e., mitophagy) would result in accumulation of damaged/dysfunctional mitochondria. In addition, we have ruled out the possibility that Pgc-1α overexpression transcriptionally up-regulates Bnip3 as no differences in Bnip3 mRNA expression were observed between MCK-Pgc-1α mice and WT littermates. Altogether the findings observed in sedentary MCK-Pgc-1α mice and in soleus muscle following exercise training support that basal autophagy and the mitophagy protein Bnip3 expression are dictated by the oxidative phenotype or mitochondrial content of the muscle, whereas autophagy protein expression is dictated primarily by chronic contractile activity. To our knowledge this is the first evidence for a dichotomous regulation of basal autophagy and autophagy protein expression in skeletal muscle. It is also important to note that Pgc-1α overexpression mediates up-regulation of Bnip3 protein expression and enhanced autophagy flux in skeletal muscle through a yet unexplored transcription-independent mechanism. Of note, our observations that basal autophagy flux increases only in conditions that enhance mitochondrial content and Bnip3 expression (i.e., trained plantaris muscle and overexpression of Pgc-1α) point to mitophagy as the major determinant of basal autophagy in skeletal muscle.
Previous studies have documented the importance of basal autophagy in skeletal muscle function. Deletion of the Atg7 gene compromises muscle mass, force generation and mitochondrial function (42). A similar muscle phenotype is present in mice lacking Atg5 expression (43), another protein involved in the early phases of autophagosome formation (14). Because of our findings that increased basal autophagy was an adaptation to endurance exercise and a feature of the oxidative phenotype in skeletal muscle, we wanted to ascertain its functional role in exercise training-induced adaptations. Previous reports have documented normal basal autophagy levels in sedentary Atg6+/− mice (16, 21), a finding that we have replicated here; however, these animals were unable to elevate basal autophagy and Bnip3 protein expression in response to long-term voluntary wheel running. Normal basal autophagy under sedentary conditions, but with blunted increases in response to exercise training made the Atg6+/− mice an ideal model to test the functional importance of basal autophagy changes for the adaptations in muscle. In fact, we observed blunted mitochondrial biogenesis and angiogenesis in skeletal muscle with normal induction of MHCIIa expression in these animals. Therefore, increased basal autophagy is required for exercise training-induced metabolic adaptations (mitochondrial biogenesis and angiogenesis), but not for contractile adaptation (fiber type transformation). Interestingly, these findings in trained Atg6+/− mice phenocopy our previous observations in trained p38γ and Pgc-1α muscle-specific knockout mice (32, 33), where signaling transduction toward metabolic adaptations were disrupted. Further studies are required to precisely address the mechanisms responsible for the crosstalk between basal autophagy and metabolic adaptation in skeletal muscle.
Another interesting observation in Atg6+/− mice was the lack of improvement in endurance capacity despite having similar voluntary running activity compared with WT littermates. One possible explanation is that blunted mitochondrial biogenesis and angiogenesis in the skeletal muscles of Atg6+/− mice were more functionally limiting for continuous and maximal performance than for the intermittent submaximal exercise, which is characteristic of the voluntary running model. Of note, we cannot exclude the possibility that reduced Atg6 expression in other tissues, such as the heart, brain and liver, may also have contributed to the loss of improvement in endurance performance in Atg6+/− mice. Nevertheless, this is a very important observation because exercise capacity is a major predictor of all-cause mortality (5). It also demonstrates that a failure to increase basal autophagy in skeletal muscle, and possibly other tissues, could be associated with the low ability of certain individuals to enhance endurance capacity in response to exercise training (44).
In summary, the present study demonstrates that exercise training promotes basal autophagy and expression of autophagy proteins and the mitophagy protein Bnip3 in skeletal muscle. In addition, we provide evidence that increased basal autophagy is a feature of the enhanced oxidative phenotype in skeletal muscle, while increased autophagy protein expression is mainly dictated by contractile activity. Our results also reveal that deficient increases in basal autophagy in skeletal muscle lead to impaired metabolic adaptations induced by exercise training, such as mitochondrial biogenesis and angiogenesis, without compromising contractile adaptation. In addition, deficient increases in skeletal muscle basal autophagy were associated with important limitations in endurance exercise training-induced improvements in performance.
Supplementary Material
Acknowledgments
The authors thank Dr. Beth Levine (University of Texas Southwestern Medical Center, Dallas, TX, USA) for her generosity in providing Atg6-deficient mice and Dr. Ira G. Shulman for insightful comments on the manuscript.
The study was supported by U.S. National Institutes of Health (NIH) grant AR050429 and American Diabetes Association research award 7-06-RA-165 (Z.Y.), an American Physiological Society postdoctoral fellowship in physiological genomics (V.A.L.), and NIH training grant T32HL07284 (N.P.G.).
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
- Atg6/Beclin1
- autophagy-related gene 6
- Atg7
- autophagy-related gene 7
- Bnip3
- BCL2/adenovirus E1B 19-kDa protein-interacting protein 3
- CD31
- cluster of differentiation 31
- Cox4
- cytochrome c oxidase subunit 4
- Cyc
- cytochrome c
- Gapdh
- glyceraldehyde 3-phosphate dehydrogenase
- LC3
- microtubule-associated protein light chain 3
- MCK
- muscle creatine kinase
- MHC
- myosin heavy chain
- p62
- sequestosome 1
- PE
- phosphatidylethanolamine
- Pgc-1α
- peroxisome proliferator-activated receptor γ co-activator 1-α
- WT
- wild type
REFERENCES
- 1. Manson J. E., Hu F. B., Rich-Edwards J. W., Colditz G. A., Stampfer M. J., Willett W. C., Speizer F. E., Hennekens C. H. (1999) A prospective study of walking as compared with vigorous exercise in the prevention of coronary heart disease in women. N. Engl. J. Med. 341, 650–658 [DOI] [PubMed] [Google Scholar]
- 2. Hu F. B., Manson J. E., Stampfer M. J., Colditz G., Liu S., Solomon C. G., Willett W. C. (2001) Diet, lifestyle, and the risk of type 2 diabetes mellitus in women. N. Engl. J. Med. 345, 790–797 [DOI] [PubMed] [Google Scholar]
- 3. Buchman A. S., Boyle P. A., Yu L., Shah R. C., Wilson R. S., Bennett D. A. (2012) Total daily physical activity and the risk of AD and cognitive decline in older adults. Neurology 78, 1323–1329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Colditz G. A., Cannuscio C. C., Frazier A. L. (1997) Physical activity and reduced risk of colon cancer: implications for prevention. Cancer Causes Control 8, 649–667 [DOI] [PubMed] [Google Scholar]
- 5. Kodama S., Saito K., Tanaka S., Maki M., Yachi Y., Asumi M., Sugawara A., Totsuka K., Shimano H., Ohashi Y., Yamada N., Sone H. (2009) Cardiorespiratory fitness as a quantitative predictor of all-cause mortality and cardiovascular events in healthy men and women: a meta-analysis. JAMA 301, 2024–2035 [DOI] [PubMed] [Google Scholar]
- 6. Janssen I., Heymsfield S. B., Baumgartner R. N., Ross R. (2000) Estimation of skeletal muscle mass by bioelectrical impedance analysis. J. Appl. Physiol. 89, 465–471 [DOI] [PubMed] [Google Scholar]
- 7. DeFronzo R. A., Ferrannini E., Sato Y., Felig P., Wahren J. (1981) Synergistic interaction between exercise and insulin on peripheral glucose uptake. J. Clin. Inv. 68, 1468–1474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. DeFronzo R. A., Jacot E., Jequier E., Maeder E., Wahren J., Felber J. P. (1981) The effect of insulin on the disposal of intravenous glucose. Results from indirect calorimetry and hepatic and femoral venous catheterization. Diabetes 30, 1000–1007 [DOI] [PubMed] [Google Scholar]
- 9. Katz L. D., Glickman M. G., Rapoport S., Ferrannini E., DeFronzo R. A. (1983) Splanchnic and peripheral disposal of oral glucose in man. Diabetes 32, 675–679 [DOI] [PubMed] [Google Scholar]
- 10. Yan Z., Okutsu M., Akhtar Y. N., Lira V. A. (2011) Regulation of exercise-induced fiber type transformation, mitochondrial biogenesis, and angiogenesis in skeletal muscle. J. Appl. Physiol. 110, 264–274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lira V. A., Benton C. R., Yan Z., Bonen A. (2010) PGC-1alpha regulation by exercise training and its influences on muscle function and insulin sensitivity. Am. J. Physiol. Endocrinol. Metab. 299, E145–161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Levine B., Klionsky D. J. (2004) Development by self-digestion: molecular mechanisms and biological functions of autophagy. Dev. Cell. 6, 463–477 [DOI] [PubMed] [Google Scholar]
- 13. Cecconi F., Levine B. (2008) The role of autophagy in mammalian development: cell makeover rather than cell death. Dev. Cell 15, 344–357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ravikumar B., Sarkar S., Davies J. E., Futter M., Garcia-Arencibia M., Green-Thompson Z. W., Jimenez-Sanchez M., Korolchuk V. I., Lichtenberg M., Luo S., Massey D. C., Menzies F. M., Moreau K., Narayanan U., Renna M., Siddiqi F. H., Underwood B. R., Winslow A. R., Rubinsztein D. C. (2010) Regulation of mammalian autophagy in physiology and pathophysiology. Physiolog. Rev. 90, 1383–1435 [DOI] [PubMed] [Google Scholar]
- 15. Grumati P., Coletto L., Schiavinato A., Castagnaro S., Bertaggia E., Sandri M., Bonaldo P. (2011) Physical exercise stimulates autophagy in normal skeletal muscles but is detrimental for collagen VI-deficient muscles. Autophagy 7, 1415–1423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. He C., Bassik M. C., Moresi V., Sun K., Wei Y., Zou Z., An Z., Loh J., Fisher J., Sun Q., Korsmeyer S., Packer M., May H. I., Hill J. A., Virgin H. W., Gilpin C., Xiao G., Bassel-Duby R., Scherer P. E., Levine B. (2012) Exercise-induced BCL2-regulated autophagy is required for muscle glucose homeostasis. Nature 481, 511–515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lee J. Y., Koga H., Kawaguchi Y., Tang W., Wong E., Gao Y. S., Pandey U. B., Kaushik S., Tresse E., Lu J., Taylor J. P., Cuervo A. M., Yao T. P. HDAC6 controls autophagosome maturation essential for ubiquitin-selective quality-control autophagy. EMBO J. 29, 969–980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wohlgemuth S. E., Seo A. Y., Marzetti E., Lees H. A., Leeuwenburgh C. (2010) Skeletal muscle autophagy and apoptosis during aging: effects of calorie restriction and life-long exercise. Exp. Gerontol. 45, 138–148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Masiero E., Sandri M. (2010) Autophagy inhibition induces atrophy and myopathy in adult skeletal muscles. Autophagy 6, 307–309 [DOI] [PubMed] [Google Scholar]
- 20. Lin J., Wu H., Tarr P. T., Zhang C. Y., Wu Z., Boss O., Michael L. F., Puigserver P., Isotani E., Olson E. N., Lowell B. B., Bassel-Duby R., Spiegelman B. M. (2002) Transcriptional co-activator PGC-1 alpha drives the formation of slow-twitch muscle fibres. Nature 418, 797–801 [DOI] [PubMed] [Google Scholar]
- 21. Qu X., Yu J., Bhagat G., Furuya N., Hibshoosh H., Troxel A., Rosen J., Eskelinen E. L., Mizushima N., Ohsumi Y., Cattoretti G., Levine B. (2003) Promotion of tumorigenesis by heterozygous disruption of the beclin 1 autophagy gene. J. Clin. Inv. 112, 1809–1820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lira V. A., Brown D. L., Lira A. K., Kavazis A. N., Soltow Q. A., Zeanah E. H., Criswell D. S. (2010) Nitric oxide and AMPK cooperatively regulate PGC-1 in skeletal muscle cells. J. Physiol. 588, 3551–3566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Akimoto T., Ribar T. J., Williams R. S., Yan Z. (2004) Skeletal muscle adaptation in response to voluntary running in Ca2+/calmodulin-dependent protein kinase IV-deficient mice. Am. J. Physiol. Cell Physiol. 287, C1311–C1319 [DOI] [PubMed] [Google Scholar]
- 24. Waters R. E., Rotevatn S., Li P., Annex B. H., Yan Z. (2004) Voluntary running induces fiber type-specific angiogenesis in mouse skeletal muscle. Am. J. Physiol. Cell Physiol. 287, C1342–C1348 [DOI] [PubMed] [Google Scholar]
- 25. Schiaffino S., Reggiani C. (2011) Fiber types in mammalian skeletal muscles. Physiol. Rev. 91, 1447–1531 [DOI] [PubMed] [Google Scholar]
- 26. Kabeya Y., Mizushima N., Ueno T., Yamamoto A., Kirisako T., Noda T., Kominami E., Ohsumi Y., Yoshimori T. (2000) LC3, a mammalian homologue of yeast Apg8p, is localized in autophagosome membranes after processing. EMBO J. 19, 5720–5728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mizushima N., Levine B., Cuervo A. M., Klionsky D. J. (2008) Autophagy fights disease through cellular self-digestion. Nature 451, 1069–1075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Romanello V., Guadagnin E., Gomes L., Roder I., Sandri C., Petersen Y., Milan G., Masiero E., Del Piccolo P., Foretz M., Scorrano L., Rudolf R., Sandri M. (2010) Mitochondrial fission and remodelling contributes to muscle atrophy. EMBO J. 29, 1774–1785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Band M., Joel A., Hernandez A., Avivi A. (2009) Hypoxia-induced BNIP3 expression and mitophagy: in vivo comparison of the rat and the hypoxia-tolerant mole rat, Spalax ehrenbergi. FASEB J. 23, 2327–2335 [DOI] [PubMed] [Google Scholar]
- 30. Zhang J., Ney P. A. (2009) Role of BNIP3 and NIX in cell death, autophagy, and mitophagy. Cell Death Differ. 16, 939–946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Quinsay M. N., Thomas R. L., Lee Y., Gustafsson A. B. Bnip3-mediated mitochondrial autophagy is independent of the mitochondrial permeability transition pore. Autophagy 6, 855–862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Geng T., Li P., Okutsu M., Yin X., Kwek J., Zhang M., Yan Z. (2010) PGC-1α plays a functional role in exercise-induced mitochondrial biogenesis and angiogenesis but not fiber-type transformation in mouse skeletal muscle. Am. J. Physiol. Cell Physiol. 298, C572–C579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Pogozelski A. R., Geng T., Li P., Yin X., Lira V. A., Zhang M., Chi J. T., Yan Z. (2009) p38gamma mitogen-activated protein kinase is a key regulator in skeletal muscle metabolic adaptation in mice. PLoS One 4, e7934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Yamada E., Bastie C. C., Koga H., Wang Y., Cuervo A. M., Pessin J. E. (2012) Mouse skeletal muscle fiber-type-specific macroautophagy and muscle wasting are regulated by a Fyn/STAT3/Vps34 signaling pathway. Cell Rep. 1, 557–569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Smuder A. J., Kavazis A. N., Min K., Powers S. K. Exercise protects against doxorubicin-induced markers of autophagy signaling in skeletal muscle. J. Appl. Physiol. 111, 1190–1198 [DOI] [PubMed] [Google Scholar]
- 36. Lemasters J. J. (2005) Selective mitochondrial autophagy, or mitophagy, as a targeted defense against oxidative stress, mitochondrial dysfunction, and aging. Rejuvenation Res. 8, 3–5 [DOI] [PubMed] [Google Scholar]
- 37. Gottlieb R. A., Carreira R. S. (2010) Autophagy in health and disease. 5. Mitophagy as a way of life. Am. J. Physiol. Cell Physiol. 299, C203–C210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Nielsen J., Suetta C., Hvid L. G., Schroder H. D., Aagaard P., Ortenblad N. (2010) Subcellular localization-dependent decrements in skeletal muscle glycogen and mitochondria content following short-term disuse in young and old men. Am. J. Physiol. Endocrinol. Metab. 299, E1053–1060 [DOI] [PubMed] [Google Scholar]
- 39. Eisenberg H. A., Hood D. A. (1994) Blood flow, mitochondria, and performance in skeletal muscle after denervation and reinnervation. J. Appl. Physiol. 76, 859–866 [DOI] [PubMed] [Google Scholar]
- 40. Li P., Akimoto T., Zhang M., Williams R. S., Yan Z. (2006) Resident stem cells are not required for exercise-induced fiber-type switching and angiogenesis but are necessary for activity-dependent muscle growth. Am. J. Physiol. Cell Physiol. 290, C1461–C1468 [DOI] [PubMed] [Google Scholar]
- 41. Wenz T., Rossi S. G., Rotundo R. L., Spiegelman B. M., Moraes C. T. (2009) Increased muscle PGC-1alpha expression protects from sarcopenia and metabolic disease during aging. Proc. Natl. Acad. Sci. U. S. A. 106, 20405–20410 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 42. Masiero E., Agatea L., Mammucari C., Blaauw B., Loro E., Komatsu M., Metzger D., Reggiani C., Schiaffino S., Sandri M. (2009) Autophagy is required to maintain muscle mass. Cell Metab. 10, 507–515 [DOI] [PubMed] [Google Scholar]
- 43. Raben N., Hill V., Shea L., Takikita S., Baum R., Mizushima N., Ralston E., Plotz P. (2008) Suppression of autophagy in skeletal muscle uncovers the accumulation of ubiquitinated proteins and their potential role in muscle damage in Pompe disease. Hum. Mol. Gen. 17, 3897–3908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Bouchard C., Rankinen T. (2001) Individual differences in response to regular physical activity. Med. Sci. Sports Exer. 33, S446–S451; discussion S452–443 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.