Abstract
Laboratory diagnosis of typhoid fever requires isolation and identification of Salmonella enterica serotype Typhi. In many areas where this disease is endemic, laboratory capability is limited. Recent advances in molecular immunology have led to the identification of sensitive and specific markers for typhoid fever and technology to manufacture practical and inexpensive kits for their rapid detection. We evaluated three commercial kits for serologic diagnosis of typhoid fever. Patients presenting with ≥ 4 days of fever were enrolled at two hospitals in Southern Vietnam. Cases were patients with serotype Typhi isolated from blood samples, and controls were patients with other laboratory-confirmed illnesses. Serotype Typhi isolates were confirmed and tested for antimicrobial susceptibility at the Pasteur Institute in Ho Chi Minh City. The Widal test was run at the hospitals and the Pasteur Institute. Sera were shipped frozen to the Centers for Disease Control and Prevention and tested by using Multi-Test Dip-S-Ticks, TyphiDot, and TUBEX to detect immunoglobulin G (IgG), IgG and IgM, and IgM, respectively. Package insert protocol instructions were followed. We enrolled 59 patients and 21 controls. The sensitivity and specificity findings were as follows: 89 and 53% for Multi-Test Dip-S-Ticks, 79 and 89% for TyphiDot, 78 and 89% for TUBEX, and 64 and 76% for Widal testing in hospitals and 61% and 100% for Widal testing at the Pasteur Institute. For all assays, the sensitivity was highest in the second week of illness. The Widal test was insensitive and displayed interoperator variability. Two rapid kits, TyphiDot and TUBEX, demonstrated promising results.
Typhoid fever, caused by Salmonella enterica serotype Typhi, is a major cause of morbidity and mortality worldwide, causing an estimated 16.6 million new infections and 600,000 deaths each year (14). In Vietnam, typhoid fever is highly endemic, with the southern provinces most heavily affected. In a study conducted in Dong Thap Province in 1995 and 1996, the incidence of confirmed serotype Typhi infection was 198 per 100,000 for all ages (11).
Isolation of serotype Typhi from blood, urine, or stool is the most reliable means of confirming an infection. However, this requires laboratory equipment and technical training that are beyond the means of most primary health care facilities in the developing world. Most serotype Typhi infections are diagnosed purely on clinical grounds and treated presumptively. As a result, the diagnosis may be delayed or missed while other febrile illnesses are considered, and patients without typhoid fever may receive unnecessary and inappropriate antimicrobial therapy. Emerging drug resistance among circulating serotype Typhi strains in Vietnam (6, 15) and elsewhere (16) has complicated the treatment of typhoid fever and heightened the need for rapid accurate diagnosis and the appropriate and selective use of antimicrobial agents to which the organism has thus far remained susceptible.
Serodiagnosis of typhoid fever has been attempted since the late 19th century when Widal and Sicard showed that the serum of patients with typhoid fever agglutinated typhoid bacilli (20). Unfortunately, neither the Widal test, which remains in widespread use in the developing world, nor any of the serodiagnostic tests that have since been developed has proven sufficiently sensitive, specific, and practical to be of value in areas where this disease is endemic (9). Recent advances in molecular immunology have led to the identification of potentially more sensitive and specific markers in the blood and urine of patients with typhoid fever and have enabled the manufacture of practical and inexpensive kits for their detection. Here we report the results of an evaluation of three commercial serodiagnostic assays for diagnosis of acute serotype Typhi infection with specimens collected in southern Vietnam.
MATERIALS AND METHODS
Specimen collection.
Specimens were collected from patients at two hospitals in Southern Vietnam: Cai Lay District Hospital (180 beds) in Tien Giang Province and the Hospital for Tropical Diseases (Cho Quan Hospital) (500 beds) in Ho Chi Minh City. Patients ≥ 3 years old who presented with ≥ 4 days of fever between October 2000 and April 2002 were eligible for enrollment. Patients who met the criteria were asked to give informed consent and answer a brief questionnaire about clinical signs and symptoms, antimicrobial treatment, and history of typhoid fever and vaccination. Participants gave 5 ml of blood (3 ml from children 3 to 5 years old) upon routine venipuncture for blood culture. Only patients with a laboratory-confirmed etiology of their fever were included in the analysis.
Blood samples were centrifuged, and the serum was divided into aliquots and stored at −20°C. In order to minimize the degradation of the antibodies in the serum, the specimens were frozen immediately and remained frozen until the time of testing. At routine intervals, personnel from the Pasteur Institute retrieved the isolates and serum specimens from the hospitals; serum was stored at −70°C. All isolates were confirmed at the Pasteur Institute, and serum was reevaluated by using the Widal test. Serum specimens from all patients with a laboratory-confirmed illness were batched and shipped on ice every few months to the Centers for Disease Control and Prevention (CDC) in Atlanta, Ga., for further testing with the commercial assays. Patients with serotype Typhi isolated from blood were compared to patients with other laboratory-diagnosed pathogens by three commercial kits for rapid diagnosis of acute typhoid fever.
Laboratory analysis. (i) Blood culture.
At Cai Lay Hospital, 5 ml of patient blood was added to blood culture medium (biphasic tryptic soy agar and brain heart infusion broth with SPS [0.6 mg/ml]) supplied by the Pasteur Institute. The blood culture bottle was then incubated at 37°C for 24 h before being tilted so that the liquid flowed over the solid medium. The broth was subcultured on blood agar after 1, 2, 3, and 7 days, and the solid medium was subcultured any time there was a colony visible on the slant. Isolates were Gram stained and identified by standard biochemical methods. Serotyping was performed by using agglutination with Salmonella O, H, and Vi antisera. If there was no growth after 10 days, the culture was considered negative. The Hospital for Tropical Diseases used the BACTEC system and surveyed the results after 5 days. If there was any growth, colonies were subcultured to blood agar and identified as described above.
(ii) Confirmation and antimicrobial susceptibility testing of isolates at the Pasteur Institute.
The identification of suspect serotype Typhi isolates was confirmed at the Pasteur Institute by standard biochemical tests and Salmonella serotyping. Antimicrobial susceptibility testing was done by using the Kirby-Bauer disk diffusion method. The following antimicrobial agents (zone size for resistance) were used: ampicillin (≥ 17 mm), tetracycline (≥ 19 mm), chloramphenicol (≥ 18 mm), ceftriaxone (≥ 21 mm), ciprofloxacin (≥ 21 mm), ofloxacin (≥ 16 mm), norfloxacin (≥ 17 mm), nalidixic acid (≥ 19 mm), and gentamicin (≥ 15 mm).
(iii) Laboratory confirmation of other pathogens.
Confirmation of other pathogens was done as follows: blood smear for malaria, acid-fast bacilli (AFB) sputum smear for tuberculosis, blood or urine cultures for other bacterial pathogens, or serum immunoglobulin M (IgM) detection by antibody-capture enzyme immunosorbent assay (MAC EIA) for dengue. AFB smears and blood and urine cultures were done in the hospitals; sera were sent from Cai Lay Hospital to the Center for Preventive Medicine in Tien Giang province for dengue testing by using a MAC EIA kit produced by the Pasteur Institute (validated by comparison to an Omega, UK, commercial kit). The Hospital for Tropical Diseases did not test or refer specimens for dengue serology.
(iv) Widal test.
Widal testing was done by using the Sanofi qualitative agglutination test kits (Bio-Rad) by two different methods. In both methods, serum was serially diluted, starting at 1/10, in physiological saline and then further diluted 1/10 in suspensions containing serotype Typhi O and H antigens, separately. Cai Lay Hospital used the rapid centrifugation technique in which the tubes were centrifuged at 3,000 rpm for 5 min. The precipitate was resuspended by tapping the bottom of the tube; if agglutination was visible, the results were considered positive. The Hospital for Tropical Diseases and the Pasteur Institute used the classical technique with incubation in which the tubes were incubated in a 37°C water bath for 2 h for H suspensions and at room temperature overnight for O suspensions; if agglutination was visible, the results were considered positive.
(v) Rapid tests.
Serum was evaluated by using the following three commercially available rapid diagnostic kits: Multi-Test Dip-S-Ticks (PANBIO INDX, Inc., Baltimore, Md.), TUBEX (IDL Biotech, Sollentuna, Sweden), and TyphiDot (Malaysian Biodiagnostic Research SDN BHD, Singapore, Malaysia). Briefly, the Multi-Test Dip-S-Ticks tests for five pathogens, including Salmonella serotype Typhi. The test is in a dipstick format that detects anti-O, anti-H, anti-Vi, IgM, or IgG antibodies in patient serum, plasma, or heparinized whole blood. We evaluated the IgG kit only. The TyphiDot is a DOT enzyme immunoassay that detects either IgM or IgG antibodies against a specific antigen on the outer membrane protein of serotype Typhi. For specimens that are indeterminate (IgM negative and IgG positive), a confirmatory test, TyphiDot-M, is recommended by the manufacturer. Due to manufacturing problems with the TyphiDot-M, only the TyphiDot was used in this evaluation. These first two tests, the Multi-Test Dip-S-Ticks and the TyphiDot, are qualitative. The third test was the TUBEX, a semiquantitative test that uses polystyrene particle agglutination to detect IgM antibodies to the O9 antigen. Specimens were run according to the protocol listed on the packet inserts.
Ethical review.
The study protocol was approved by the institutional review boards of the CDC and the National Institute of Hygiene and Epidemiology, Hanoi, Vietnam.
Statistical analysis.
Analyses were performed by using SPSS version 11.0.1 (SPSS, Inc., Chicago, Ill.). Medians were compared by using the median test for nonparametric data that calculates a chi-square statistic. For each assay, we calculated the sensitivity, specificity, and positive and negative predictive values. Fleiss quadratic 95% confidence intervals were calculated by using Epi Info 6 (CDC, Atlanta, Ga.). The patient age was calculated by using a mid-year birth date and date of interview.
RESULTS
We enrolled 59 serotype Typhi cases and 20 controls with other laboratory-confirmed febrile illnesses. The control diagnoses were as follows: 7 subjects with dengue fever, 4 subjects with Escherichia coli cultured from blood, 1 subject with E. coli cultured from urine, 2 subjects with malaria (Plasmodium falciparum), 2 subjects with tuberculosis, 2 subjects with Klebsiella pneumoniae cultured from blood, and 2 subjects with S. enterica serotype Paratyphi A cultured from blood. Serum was missing from one case and one control (serotype Paratyphi A).
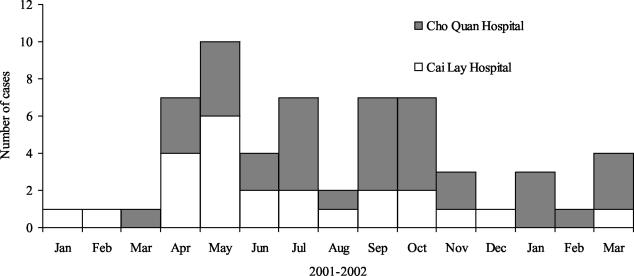
The demographic characteristics of the serotype Typhi patients and controls are listed in Table 1. In the Hospital for Tropical Diseases there was a slightly longer, but nonsignificant, time between fever onset and enrollment compared to Cai Lay Hospital (median number of days, 11 versus 8 [P = 0.07]). Twenty-five patients recalled taking antibiotics after fever onset (10 of 15 cases versus 6 of 10 controls), however, 74 of 75 (99%) reported taking any medicine in the same week. Most persons (54 of 79 [68%]) did not know if the medicine they took was an antibiotic or not. The dates of fever onset in patients with serotype Typhi ranged from January 2001 to March 2002, peaking in April through October (Fig. 1). None of the participants reported vaccination for typhoid fever; one patient and two controls reported having a history of typhoid fever.
TABLE 1.
Characteristics of patients enrolled in study by hospital and laboratory-confirmed diagnosis
| Parameter | Cai Lay Hospital
|
Hospital for Tropical Diseases
|
Total
|
|||
|---|---|---|---|---|---|---|
| Typhoid fever | Other | Typhoid fever | Other | Typhoid fever | Other | |
| Total no. of patients | 24 | 10 | 35 | 10 | 59 | 20 |
| Median age in yr (range) | 13 (2-72) | 15 (4-70) | 20 (3-67) | 36 (14-91) | 18 (2-72) | 26 (4-91) |
| No. (%) of female patients | 6 (25) | 6 (60) | 17 (49) | 5 (50) | 23 (39) | 11 (55) |
| Median days (range) between fever onset and enrollment | 9 (4-19) | 6 (4-15) | 11 (5-55) | 12 (5-129) | 11 (4-55) | 9 (4-129) |
| Median (range) duration of hospitalization | 10 (2-20) | 5 (2-9) | 10 (2-41) | 7 (4-18) | 10 (2-41) | 6 (2-18) |
| No. of patients (%) treated before presenting to the hospital/total no. | 24/24 (100) | 10/10 (100) | 31/32 (97) | 9/9 (100) | 55/56 (98) | 19/19 (100) |
| No. of patients (%) reportedly taking antibiotics at presentation/ total no. of patients who reported medicine type | 4/5 (80) | 2/4 (50) | 6/10 (60) | 4/6 (67) | 10/15 (67) | 6/10 (60) |
FIG. 1.
Time of fever onset in patients with Salmonella Typhi by hospital.
A comparison of the three assays in presented in Table 2. A refrigerator is needed for storage of all of the kits but very little additional equipment is needed. The Multi-Test Dip-S-Ticks method requires a water bath, and the TyphiDot requires a calibrated pipette. At approximately $10 per test, the Multi-Test Dip-S-Ticks is the most expensive, followed by TUBEX at approximately $4 per test and the TyphiDot at approximately $1 per test.
TABLE 2.
Characteristics of assays
| Characteristic | Multi-Test Dip-S-Ticks | TyphiDot | TUBEX | Widal |
|---|---|---|---|---|
| Approximate cost (U.S. dollars)/specimena | 10 | 2.14 | 4.00 | 0.50 |
| No. of tests/kit | 50 | 56 | 30 | 55 |
| Antibody | IgM and IgG | IgM or IgG | IgM | IgM and IgG |
| Antigen | O, H, and Vi | OMPb | O9 | O, H, and Vi |
| Amt of serum needed (μl) | 10 | 2.5 | 35-40 ± (one drop) | 300 ± (two dilutions) |
| Reaction time (min) | 90 | 60 | 3 | 5c |
| Temp for storage (°C) | 2-8 | 2-8 | 2-8 | 2-8 |
Personal communication (Prue Griffin, PANBIO INDX, Inc.; Kok-hai Ong, Malaysian Bio-Diagnostic Research Sdn. Bhd.; Helena Goike, IDL Biotech). TyphiDot price includes running each sample twice; once for IgM and once for IgG (an additional $2.93 should be added for each TyphiDot-M run on IgM-negative, IgG-positive specimens). All costs assume samples are batched to maximize kit use; use of the kits for single use increases the price per specimen. Costs do not include shipping.
OMP, outer membrane protein.
As determined by the rapid centrifugation technique (the classical technique is 2 h to overnight).
The sensitivity, specificity, and predictive values are shown in Table 3. Although the sensitivity of the Multi-Test Dip-S-Ticks was quite high (89%), it had low specificity (50%). The TyphiDot and the TUBEX both had high sensitivities (79 and 78%, respectively) and specificities (89 and 94%, respectively). The Widal test was the least sensitive of the assays, and the results varied by place performed (64% sensitive and 76% specific in the hospitals and 61% sensitive and 100% specific at the Pasteur Institute).
TABLE 3.
Senstivity, specificity, positive predictive value, negative predictive value, and 95% confidence intervals of rapid diagnostic assays for typhoid fever
| Assaya | No. of samples/total no. of samples, % (95% confidence interval)
|
|||
|---|---|---|---|---|
| Sensitivity | Specificity | Positive predictive value | Negative predictive value | |
| Multi-Test Dip-S-Ticks for Serotype Typhi | 51/57, 89 (78-96) | 9/18, 50 (27-73) | 51/60, 85 (73-93) | 9/15, 60 (33-83) |
| TyphiDot | 46/58, 79 (66-88) | 17/19, 89 (66-98) | 46/48, 96 (85-99) | 17/29, 59 (39-76) |
| TUBEX | 43/55, 78 (65-88) | 17/18, 94 (71-100) | 43/44, 98 (87-100) | 17/29, 59 (39-76) |
| Widal testing in the hospitalb | 30/47, 64 (49-77) | 13/17, 76 (50-92) | 30/34, 88 (72-96) | 13/30, 43 (26-62) |
| Widal testing at the Pasteur Institute | 33/54, 61 (47-74) | 19/19, 100 (79-100) | 33/33, 100 (87-100) | 19/40, 48 (32-64) |
Widal, TO or TH agglutinin ≥100.
At any time during hospitalization.
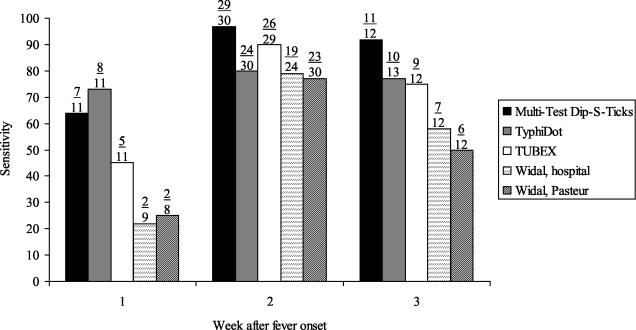
We examined the sensitivity of the assays by week after fever onset (Fig. 2). These were not tests performed on specimens from the same patient at weeks 1, 2, and 3 but rather the merged results of single samples collected at a single time point from each patient. The sensitivity of all tests was highest for serum specimens obtained during the second week of illness. The numbers were too small to do a meaningful evaluation of the specificity of the assays by the week after fever onset (data not shown).
FIG. 2.
Sensitivity by week after fever onset.
There were no discernible differences between the 10 controls with a false-positive result on one of the three commercial assays and the 9 other controls, although the numbers were small. The controls with a false-positive result were slightly younger (mean age, 31 versus 37 years [P = 0.6]) and less likely to be women (50% versus 55%, P = 1.0); these differences are not statistically significant. Nine of the ten controls were positive on the Multi-Test Dip-S-Ticks, and four of these had dengue fever. One control with a false-positive result reported having a history of typhoid fever.
Antibiotic susceptibility.
A total of 58 of the 59 serotype Typhi isolates were available for testing. Of the 58 isolates tested, 14 (24%) were pansensitive. All of the remaining 44 isolates were resistant to nalidixic acid; 33 were also resistant to chloramphenicol and tetracycline, and 29 of these were also resistant to ampicillin. Only two isolates were also resistant to cefotaxime, one of which was also resistant to norfloxacin. Among the 57 cases with serologic results, there was no statistically significant difference in the typhoid assay results by sensitivity as defined by pansensitivity or resistance to at least one antimicrobial agent.
DISCUSSION
We evaluated three commercial rapid diagnostic kits for serotype Typhi with sera collected from patients with acute febrile illness of ≥ 4 days' duration at two hospitals in Vietnam. Overall, the TyphiDot and TUBEX, both of which detect IgM antibodies, demonstrated the most promising results. However, the performance of the TyphiDot assay may not have been optimized since we were unable to run our 15 indeterminate specimens (seven cases and eight controls) on the TyphiDot-M assay for confirmation. The Multi-Test Dip-S-Ticks, which only detects IgG antibodies, had poor specificity. The Multi-Test Dip-S-Ticks to detect IgM was not evaluated. The Widal test had low sensitivity and was highly operator dependent. Since the other three assays were performed at only one laboratory, we could not assess their operator variability.
The hospitals participating in this evaluation were quite different and had the potential to enroll patients at different stages of illness. Cai Lay is a small, rural hospital with minimal laboratory capability, whereas the Hospital for Tropical Diseases is a large, urban referral hospital with good laboratory capability. Despite this, there were few differences in the patients enrolled. Patients at the Hospital for Tropical Diseases were slightly older, more likely to be women, and were seen a median of 2 days later in their illness.
In our evaluation, the sensitivity of the TyphiDot was high beginning in the first week of illness onset. Presumably, this is because the TyphiDot relies more heavily on IgM results that occur earlier in the course of the illness, whereas IgG rises later; however, we did not see this effect with the TUBEX, which also detects IgM antibodies. In the Widal test, the O and H agglutinins usually appear around day 8 and days 10 to 12, respectively.
The Multi-Test Dip-S-Ticks was the most costly assay, presumably because the dipstick measures antibodies to five different pathogens. Although all three assays were relatively easy to use, the TUBEX was the simplest. A limitation of the TUBEX test, which uses a colorimetric reaction, is the potential for difficulty in interpreting the results of hemolyzed samples. Another concern is that the TUBEX may produce a false positive in persons with recent S. enterica serotype Enteritidis infection and result in inappropriate antibiotic treatment (13).
Ideally, the Widal test should be run on both acute- and convalescent-phase sera to detect an increase in the agglutination titer. However, to inform treatment decisions before convalescent samples can be obtained, it is common for a single acute-phase serum sample to be run. The results from a single sample are difficult to interpret because high background rates of circulating antibodies to serotype Typhi or other Salmonella serotypes may produce a false-positive result. In Vietnam, an area of high endemicity, a single Widal test can lead to many false-positive and false-negative results (17). Operator variability also contributes to unreliable results, as evidenced in the present study.
Each of these three commercial kits has been previously evaluated but, to our knowledge, this is the first time they have all been included together in the same evaluation. In a recent evaluation of the Multi-Test Dip-S-Ticks in Singapore, the sensitivity varied greatly depending upon the case definition (5). Among clinical and blood culture-positive cases it was 51%, and among blood culture-positive patients only it was 78%. The specificity among control patients who had a clinical diagnosis of typhoid but were culture negative, had other laboratory diagnoses, or had pyrexia of unknown origin was 81%. The TyphiDot was evaluated in Pakistan and Singapore by using a variety of case definitions (2, 5). The sensitivity ranged from 59 to 93% for the TyphiDot and 73 to 84% with the addition of the TyphiDot-M. Specificity was consistently higher when TyphiDot-M was used; 89% compared to 77% or lower with only the Typhi-Dot. An evaluation of the TyphiDot in India was 100% sensitive and 80% specific compared to a blood culture “gold standard” (8). In an early evaluation, the TUBEX demonstrated 100% sensitivity and specificity (10). However, this was before the kit was commercially available. In Vietnam, it was 87% sensitive among blood culture positive patients and 76% sensitive among hospitalized patients with fever (7).
One limitation in the previous and current evaluations is that blood culture-confirmed cases were used as the gold standard. Since blood culture is less sensitive than bone marrow culture for diagnosing typhoid fever (4), the results should be interpreted with caution. It is possible that the rapid diagnostic tests are more sensitive than blood culture. If so, a result that appears to be a false-positive test compared to a blood culture may in fact be a true positive. This hypothesis requires further evaluation. Alternatively, a false-positive may be the result of past infection with serotype Typhi or another nontyphoidal Salmonella serotype that shares common antigens.
Researchers continue to search for the ideal rapid test to diagnose acute typhoid fever. Several urine assays have been developed (1, 3, 12, 18), although none has proved optimal. In the field, there is a definite advantage to collecting urine instead of blood; urine collection is simple, less invasive than venipuncture, and requires less training and equipment. In addition, some antigens may be excreted in higher concentration in the urine. With the recent sequencing of the entire serotype Typhi genome, it may now be possible to identify other antigens, such as fimbrial antigens, that may produce an antibody response specific to serotype Typhi (19). More sophisticated molecular techniques for diagnosis, such as PCR, are also being explored. However, their use in developing countries will most likely be limited.
Acknowledgments
We thank Tran Thi My Trinh and Bui Thu Hien of the Pasteur Institute in Ho Chi Minh for their assistance with laboratory testing.
REFERENCES
- 1.Barrett, T. J., J. D. Snyder, P. A. Blake, and J. C. Feeley. 1982. Enzyme-linked immunosorbent assay for detection of Salmonella typhi Vi antigen in urine from typhoid patients. J. Clin. Microbiol. 15:235-237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bhutta, Z. A., and N. Mansurali. 1999. Rapid serologic diagnosis of pediatric typhoid fever in an endemic area: a prospective comparative evaluation of two dot-enzyme immunoassays and the Widal test. Am. J. Trop. Med. Hyg. 61:654-657. [DOI] [PubMed] [Google Scholar]
- 3.Fadeel, M. A., J. A. Crump, F. J. Mahoney, I. A. Nakhla, A. M. Mansour, B. Reyad, D. E. Melegi, Y. Sultan, E. D. Mintz, and W. F. Bibb. 2004. Rapid diagnosis of typhoid fever by enzyme-linked immunosorbent assay detection of Salmonella serotype Typhi antigens in urine. Am. J. Trop. Med. Hyg. 70:323-328. [PubMed]
- 4.Gilman, R. H., M. Terminel, M. M. Levine, P. Hernandez-Mendoza, and R. B. Hornick. 1975. Relative efficacy of blood, urine, rectal swab, bone-marrow, and rose-spot cultures for recovery of Salmonella typhi in typhoid fever. Lancet i:1211-1213. [DOI] [PubMed] [Google Scholar]
- 5.Gopalakrishnan, V., W. Y. Sekhar, E. H. Soo, and S. Devi. 2002. Typhoid fever in Kuala Lumpur and a comparative evaluation of two commercial diagnostic kits for detection of antibodies to Salmonella typhi. Singapore Med. J. 43:354-358. [PubMed] [Google Scholar]
- 6.Hoa, N. T., T. S. Diep, J. Wain, C. M. Parry, T. T. Hien, M. D. Smith, A. L. Walsh, and N. J. White. 1998. Community-acquired septicaemia in southern Viet Nam: the importance of multidrug-resistant Salmonella typhi. Trans. R. Soc. Trop. Med. Hyg. 92:503-508. [DOI] [PubMed] [Google Scholar]
- 7.House, D., J. Wain, V. A. Ho, T. S. Diep, N. T. Chinh, P. V. Bay, H. Vinh, M. Duc, C. M. Parry, G. Dougan, N. J. White, T. T. Hien, and J. J. Farrar. 2001. Serology of typhoid fever in an area of endemicity and its relevance to diagnosis. J. Clin. Microbiol. 39:1002-1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jesudason, M., E. Esther, and E. Mathai. 2002. Typhidot test to detect IgG and IgM antibodies in typhoid fever. Indian J. Med. Res. 116:70-72. [PubMed] [Google Scholar]
- 9.Levine, M. M., and W. A. Orenstein. 1999. Typhoid fever vaccines, p. 781-814. In S. A. Plotkin (ed.), Vaccines, 3rd ed. W. B. Saunders Co., Philadelphia, Pa.
- 10.Lim, P. L., F. C. Tam, Y. M. Cheong, and M. Jegathesan. 1998. One-step 2-minute test to detect typhoid-specific antibodies based on particle separation in tubes. J. Clin. Microbiol. 36:2271-2278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lin, F. Y., A. H. Vo, V. B. Phan, T. T. Nguyen, D. Bryla, C. T. Tran, B. K. Ha, D. T. Dang, and J. B. Robbins. 2000. The epidemiology of typhoid fever in the Dong Thap Province, Mekong Delta region of Vietnam. Am. J. Trop. Med. Hyg. 62:644-648. [DOI] [PubMed] [Google Scholar]
- 12.Nguyen, N. Q., P. Tapchaisri, M. Chongsa-nguan, V. V. Cao, T. T. Doan, Y. Sakolvaree, P. Srimanote, and W. Chaicumpa. 1997. Diagnosis of enteric fever caused by Salmonella spp. in Vietnam by a monoclonal antibody-based dot blot ELISA. Asian Pac. J. Allergy Immunol. 15:205-212. [PubMed] [Google Scholar]
- 13.Oracz, G., W. Feleszko, D. Golicka, J. Maksymiuk, A. Klonowska, and H. Szajewska. 2003. Rapid diagnosis of acute Salmonella gastrointestinal infection. Clin. Infect. Dis. 36:112-115. [DOI] [PubMed] [Google Scholar]
- 14.Pang, T., M. M. Levine, B. Ivanoff, J. Wain, and B. B. Finlay. 1998. Typhoid fever-important issues still remain. Trends in Microbiology 6:131-133. [DOI] [PubMed] [Google Scholar]
- 15.Parry, C., J. Wain, N. T. Chinh, H. Vinh, and J. J. Farrar. 1998. Quinolone-resistant Salmonella typhi in Vietnam. Lancet 351:1289. [DOI] [PubMed] [Google Scholar]
- 16.Parry, C. M., T. T. Hien, G. Dougan, N. J. White, and J. J. Farrar. 2002. Typhoid fever. N. Engl. J. Med. 347:1770-1782. [DOI] [PubMed] [Google Scholar]
- 17.Parry, C. M., N. T. Hoa, T. S. Diep, J. Wain, N. T. Chinh, H. Vinh, T. T. Hien, N. J. White, and J. J. Farrar. 1999. Value of a single-tube Widal test in diagnosis of typhoid fever in Vietnam. J. Clin. Microbiol. 37:2882-2886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rockhill, R. C., L. W. Rumans, M. Lesmana, and D. T. Dennis. 1980. Detection of Salmonella typhi D, Vi, and d antigens, by slide coagglutination, in urine from patients with typhoid fever. J. Clin. Microbiol. 11:213-216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wain, J., D. House, J. Parkhill, C. Parry, and G. Dougan. 2002. Unlocking the genome of the human typhoid bacillus. Lancet Infect. Dis. 2:163-170. [DOI] [PubMed] [Google Scholar]
- 20.Widal, F. 1896. Serodiagnostique de la fievre typhoide. Semaine Med. 16:259. [Google Scholar]