Abstract
Background: There is a correlation between temporal trends of obesity prevalence and papillary thyroid cancer (PTC) incidence in the United States. Obesity is a well-recognized risk factor for many cancers, but there are few studies on the association between obesity and PTC risk. We investigated the association between anthropometric measurements and PTC risk using pooled individual data from three case–control populations.
Methods: Height and weight information were obtained from three independent case–control studies, including 1917 patients with PTC (1360 women and 557 men) and 2127 cancer-free controls from the United States, Italy, and Germany. Body mass index (BMI), body fat percentage, and body surface area (BSA) were calculated. An unconditional logistic regression model was used to calculate odds ratios (ORs) and confidence intervals (CIs) with respect to risk of PTC, adjusted by age, sex, race/ethnicity, and study site.
Results: In the pooled population, for both men and women, an increased risk of PTC was found to be associated with greater weight, BMI, body fat percentage, and BSA, whereas a reduced risk of PTC was associated with greater height, in the pooled population for both men and women. Compared with normal-weight subjects (BMI 18.5–24.9 kg/m2), the ORs for overweight (BMI 25–29.9 kg/m2) and obese (BMI≥30 kg/m2) subjects were 1.72 [CI 1.48–2.00] and 4.17 [CI 3.41–5.10] respectively. Compared with the lowest quartile of body fat percentage, the ORs for the highest quartile were 3.83 [CI 2.85–5.15] in women and 4.05 [CI 2.67–6.15] in men.
Conclusion: Anthropometric factors, especially BMI and body fat percentage, were significantly associated with increased risk of PTC. Future studies of anthropometric factors and PTC that incorporate intermediate factors, including adiposity and hormone biomarkers, are essential to help clarify potential mechanisms of the relationship.
Introduction
An increase of thyroid cancer incidence has been reported in many countries, including the United States and some European countries (1). This temporal trend has been practically entirely attributed to trends in papillary thyroid cancer (PTC) incidence, which in the United States has increased by about 7% per year since 1992, making thyroid cancer the fastest-growing cancer in both men and women (2–4). While debate about the reason for the increased thyroid cancer incidence continues, this pronounced increase is unlikely to be entirely due to enhanced detection and may represent a true increase due to as yet undetermined environmental and/or lifestyle factors (5,6). Exposure to ionizing radiation during childhood and adolescence is the only confirmed environment risk factor for PTC but is not a major population-attributable risk factor because few patients with PTC have a known history of radiation exposure (7).
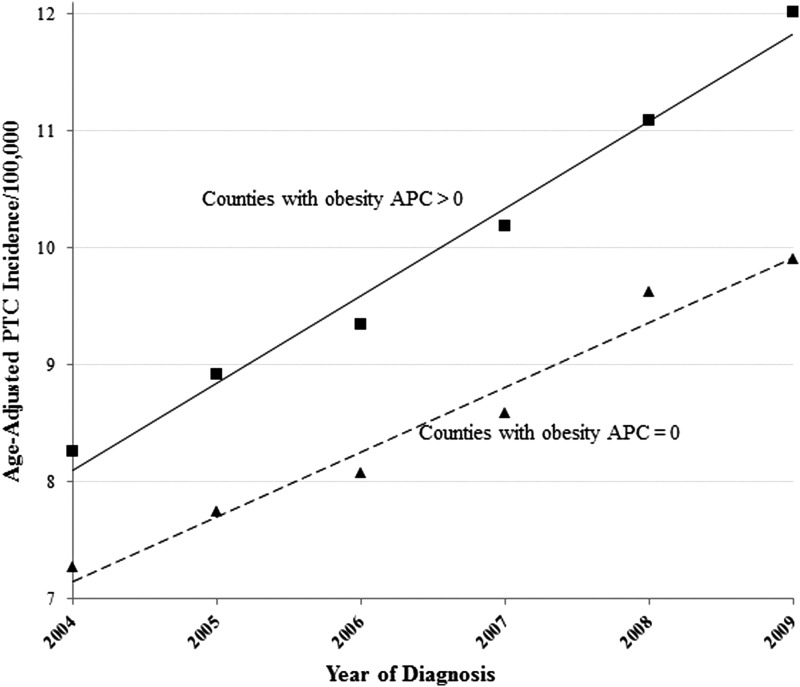
Coinciding with the increase of PTC incidence is an epidemic of obesity. Obesity is commonly defined as a body mass index (BMI)—expressed as weight in kilograms divided by height in meters squared—greater than 30 kg/m2. According to the National Health and Nutrition Examination Survey (NHANES), the prevalence of obesity increased from 13.4% to 35.7% among U.S. adults between 1960–1962 and 2009–2010; the increase was observed across subgroups defined by age, sex, race/ethnicity, and socioeconomic status (8,9). Similarly, PTC incidence increased in both sexes, in all racial/ethnic groups, and in subjects of both high and low socioeconomic status (3,6). To elucidate the correlation between temporal trends of obesity prevalence and PTC incidence at the county level, we linked the U.S. Surveillance, Epidemiology and End Results 18 registry data (2004–2009) with the NHANES obesity data (2004–2009) and grouped counties into two groups based on annual percentage change (APC) for obesity prevalence. As illustrated in Figure 1, for the counties with a significant positive APC for obesity prevalence, the age-adjusted PTC incidence rate increased from 8.3 (2004) to 12.0 (2009) per 100,000 population with a significant APC of 7.7% (p<0.05). For the counties with a nonsignificant APC for obesity prevalence, the increase in age-adjusted PTC incidence rate was lower: from 7.3 (2004) to 9.9 (2009) per 100,000 population with a significant APC of 6.7% (p<0.05).
FIG. 1.
Papillary thyroid cancer (PTC) incidence trends in the United States by annual percentage change (APC) in obesity prevalence, 2004 through 2009, for counties with nonsignificant APC in obesity prevalence (APC=0) and significantly positive APC in obesity prevalence (APC>0). APCs for PTC incidence trends were 7.7% among counties with obesity-prevalence APC>0, and 6.7% among counties with obesity-prevalence APC=0. Incidence source: U.S. Surveillance, Epidemiology and End Results 18 registry data (2004–2009); obesity percentage source: National Health and Nutrition Examination Survey (2004–2009).
The association between obesity and thyroid cancer risk has been reported epidemiologically (10–15), but there is considerable interstudy heterogeneity, which may arise from the relatively small number of cases due to the relative rarity of thyroid cancer. In the present study, we investigated the association between anthropometric measurements and PTC risk using individual data from three case–control populations. We included only PTC cases because they are epidemiologically and etiologically distinct from other histological subtypes, such as follicular and medullary thyroid cancers. Pooled data analysis with a large number of subjects enhanced the study power and enabled precise risk estimates.
Materials and Methods
Study population
Data for the pooled analysis were retrieved from three case–control studies conducted in the United States, Italy, and Germany. The U.S. study included 417 patients with newly diagnosed PTC and 489 controls prospectively recruited at The University of Texas MD Anderson Cancer Center between 1999 and February 2013. The controls were unrelated visitors to the institution using the same exclusion criteria as cases (no prior cancer history except nonmelanoma skin cancer, no current use of steroids or immunosuppressive medication, no blood transfusion in the previous six months). The Italy study included 1040 patients with PTC who presented to the University Hospital of Cisanello and 851 workers (mainly physicians, nurses, and paramedical staff) at the same hospital with no prior history of cancer or thyroid disease recruited between 2009 and 2012. The Germany study included 460 patients diagnosed with PTC and 787 cancer-free controls enrolled through the German University Hospitals of Hannover Medical School, University Clinic Würzburg, and the Central Hospital of the German Federal Armed Forces Koblenz between February 2008 and March 2010. The Germany controls were patients without tumor who presented to a surgery department of the Central Hospital of the German Federal Armed Forces Koblenz (n=105) or healthy volunteer blood donors who presented to Hannover Medical School (n=682). All cases from three study sites had histologically or cytologically confirmed diagnosis of papillary histology thyroid carcinoma (ICD-O-3 codes 8050, 8260, 8340-8341, 8343-8344, and 8350). Subjects were excluded from the analyses if they were younger than 18 years old at recruitment or if they had missing data for both weight and height. The studies were approved by the institutional review boards or local ethics committees of the participating institutions. Written informed consent was obtained from all subjects recruited to the studies.
Anthropometric measurements
Height and weight were measured at recruitment. BMI was determined by dividing weight in kilograms by height in meters squared (kg/m2). The body fat percentage was estimated from BMI by using the formula of Deurenberg et al. (16): body fat percentage=(1.20×BMI)+(0.23×Age) − (10.8×Sex) − 5.4, where age is in years and sex is set to 0 for women and 1 for men (16). Body surface area (BSA), an indicator of metabolic mass that is less affected than BMI by abnormal adipose mass, was calculated from height and weight by using the formula of Du Bois and Du Bois: BSA=0.007184×Weight0.425×Height0.725 (17).
Statistical analysis
The t-test or Wilcoxon rank-sum test was used to compare continuous variables between cases and controls as appropriate. The chi-square test was used to compare proportions between cases and controls. An unconditional logistic regression model was used to estimate crude and age-, sex-, race/ethnicity-, and study site-adjusted odds ratios (ORs) and confidence intervals (CIs) for each of the anthropometric measurements with respect to risk of PTC, including height, weight, body fat percentage, BSA, and BMI. In these analyses, anthropometric measurements were examined as continuous variables and as categorical variables. For the analysis as categorical variables, measurements were categorized into quartiles on the basis of their distribution among controls for each sex separately. BMI was also categorized as underweight (<18.5 kg/m2), normal weight (18.5–24.9 kg/m2), overweight (25.0–29.9 kg/m2), or obese (≥30 kg/m2) according to the World Health Organization definition. Tests for trend were performed by treating ordinal category scales of the anthropometric measurements as continuous variables. Tests for interaction were determined by Wald chi-square test of the interaction term. Associations with risk of PTC were also evaluated using logistic regression models stratified by study sites, age (<45, ≥45 years old), and sex (men, women). A two-sided significance level of 0.05 was adopted for all tests. Statistical analyses were performed using SAS software, v9.2 (SAS Institute, Inc., Cary, NC).
Results
A total of 1917 PTC cases and 2127 controls were included in the pooled analysis. Among the PTC cases, 1360 (70.9%) were women and 557 (29.1%) were men. Table 1 shows characteristics of the subjects and anthropometric measurement distributions for men and women by study site. In these studies, cases were not matched with controls for these demographic characteristics.
Table 1.
Characteristics of Cases and Controls in Three Case–Control Studies Included in the Pooled Analysis
| Women | Men | |||
|---|---|---|---|---|
| Characteristic | Cases | Controls | Cases | Controls |
| U.S. study | n=292 | n=335 | n=125 | n=154 |
| Age, years | 42 (18–86) | 52 (21–82) | 44 (19–77) | 51 (22–84) |
| Height, cm | 164 (137–189) | 162 (147–208) | 177 (158–195) | 175 (155–196) |
| Weight, kg | 72 (42–157) | 68 (38–155) | 91 (59–186) | 79 (60–159) |
| Body fat, % | 37.8 (18.5–69.1) | 36.6 (20.3–67.1) | 29.1 (13.7–64.6) | 26.7 (14.4–54.1) |
| BSA, m2 | 1.79 (1.39–2.61) | 1.73 (1.37–2.54) | 2.11 (1.64–2.86) | 1.97 (1.62–2.70) |
| BMI, kg/m2 | 27.0 (15.0–51.9) | 24.9 (13.9–53.5) | 29.1 (18.1–57.4) | 25.8 (20.7–47.5) |
| Race/ethnicity, % of Caucasians | 67.8 | 70.9 | 75.6 | 73.8 |
| Italy study | n=732 | n=513 | n=308 | n=338 |
| Age, years | 45 (18–83) | 43 (25–65) | 44 (18–84) | 46 (26–91) |
| Height, cm | 162 (140–180) | 164 (140–188) | 175 (155–198) | 177 (158–193) |
| Weight, kg | 66 (42–155) | 60 (36–118) | 84 (50–130) | 79 (52–125) |
| Body fat, % | 35.1 (18.8–74.2) | 31.5 (21.8–54.3) | 27.0 (10.2–46.1) | 24.5 (13.5–44.0) |
| BSA, m2 | 1.71 (1.36–2.50) | 1.66 (1.19–2.43) | 2.00 (1.50–2.49) | 1.95 (1.58–2.43) |
| BMI, kg/m2 | 24.9 (16.5–55.6) | 22.3 (16.0–39.4) | 26.8 (16.9–42.4) | 24.9 (18.1–37.7) |
| Race/ethnicity, % of Caucasians | 100 | 100 | 100 | 100 |
| Germany study | n=336 | n=264 | n=124 | n=523 |
| Age, years | 53 (19–83) | 30 (18–93) | 55 (22–81) | 36 (18–84) |
| Height, cm | 168 (142–192) | 168 (158–193) | 177 (160–198) | 179 (165–201) |
| Weight, kg | 74 (44–160) | 69 (51–136) | 85 (53–145) | 82 (58–136) |
| Body fat, % | 39.5 (22.5–74.7) | 31.1 (21.3–58.5) | 29.0 (13.3–49.5) | 23.0 (11.5–39.9) |
| BSA, m2 | 1.84 (1.37–2.54) | 1.79 (1.53–2.49) | 2.02 (1.55–2.62) | 2.03 (1.64–2.71) |
| BMI, kg/m2 | 26.8 (17.5–58.8) | 24.1 (18.3–46.5) | 27.0 (18.4–44.8) | 25.1 (18.4–37.9) |
| Race/ethnicity, % of Caucasians | 99.4 | 100 | 100 | 100 |
Values are median (range) unless otherwise specified.
BMI, body mass index; BSA, body surface area.
Table 2 shows the results of study site-specific and pooled analyses of PTC risk associated with continuous anthropometric measurements. Heterogeneity across the individual studies was evident (p<0.05 for all anthropometric measurements comparisons). All (individual and pooled) studies showed a significantly increased risk of PTC per 5% increase in body fat percentage and per 5 kg/m2 increase in BMI. The risk estimates for height, weight, and BSA varied by study site, but the pooled analyses showed a significantly reduced PTC risk per 5 cm increase in height and a significantly increased PTC risk per 10 kg increase in weight and per 0.5 m2 increase in BSA.
Table 2.
Study-Specific and Pooled Odds Ratios and Confidence Intervals for Papillary Thyroid Cancer Risk for Continuous Anthropometric Measurements
| U.S. study | Italy study | Germany study | ||||||
|---|---|---|---|---|---|---|---|---|
| Variable | OR [CI] | p | OR [CI] | p | OR [CI] | p | Pooled OR [CI] | p |
| Height (5 cm increment) | ||||||||
| Overall | 1.06 [0.96–1.17] | 0.287 | 0.83 [0.77–0.89] | <0.001 | 0.78 [0.69–0.88] | <0.001 | 0.85 [0.81–0.89] | <0.001 |
| Female | 1.05 [0.92–1.19] | 0.474 | 0.85 [0.77–0.93] | <0.001 | 0.86 [0.73–1.01] | 0.062 | 0.89 [0.83–0.94] | <0.001 |
| Male | 1.07 [0.91–1.26] | 0.423 | 0.80 [0.71–0.90] | <0.001 | 0.69 [0.58–0.83] | <0.001 | 0.80 [0.74–0.86] | <0.001 |
| Weight (10 kg increment) | ||||||||
| Overall | 1.33 [1.22–1.45] | <0.001 | 1.43 [1.32–1.55] | <0.001 | 1.19 [1.07–1.32] | <0.001 | 1.27 [1.22–1.34] | <0.001 |
| Female | 1.28 [1.15–1.43] | <0.001 | 1.42 [1.28–1.57] | <0.001 | 1.23 [1.08–1.39] | 0.002 | 1.27 [1.20–1.35] | <0.001 |
| Male | 1.39 [1.20–1.61] | <0.001 | 1.43 [1.25–1.63] | <0.001 | 1.12 [0.94–1.33] | 0.223 | 1.28 [1.19–1.39] | <0.001 |
| Body fat (5% increment) | ||||||||
| Overall | 1.44 [1.29–1.60] | <0.001 | 1.87 [1.68–2.08] | <0.001 | 1.42 [1.24–1.63] | <0.001 | 1.54 [1.45–1.64] | <0.001 |
| Female | 1.35 [1.18–1.53] | <0.001 | 1.73 [1.53–1.96] | <0.001 | 1.37 [1.16–1.60] | <0.001 | 1.46 [1.35–1.57] | <0.001 |
| Male | 1.67 [1.35–2.07] | <0.001 | 2.19 [1.79–2.69] | <0.001 | 1.58 [1.22–2.03] | <0.001 | 1.78 [1.58–2.00] | <0.001 |
| BSA (0.5 m2 increment) | ||||||||
| Overall | 3.03 [2.09–4.40] | <0.001 | 2.47 [1.83–3.32] | <0.001 | 1.33 [0.87–2.04] | 0.191 | 2.44 [2.00–2.97] | <0.001 |
| Female | 2.66 [1.67–4.25] | <0.001 | 2.56 [1.75–3.73] | <0.001 | 1.81 [1.06–3.12] | 0.031 | 2.41 [1.87–3.09] | <0.001 |
| Male | 3.63 [1.6–6.73] | <0.001 | 2.17 [1.33–3.54] | 0.002 | 0.78 [0.38–1.59] | 0.489 | 1.9 [1.44–2.75] | 0.001 |
| BMI (5 kg/m2 increment) | ||||||||
| Overall | 1.54 [1.35–1.76] | <0.001 | 2.12 [1.86–2.41] | <0.001 | 1.52 [1.29–1.80] | <0.001 | 1.77 [1.64–1.91] | <0.001 |
| Female | 1.43 [1.23–1.66] | <0.001 | 1.93 [1.66–2.25] | <0.001 | 1.45 [1.20–1.76] | <0.001 | 1.64 [1.50–1.80] | <0.001 |
| Male | 1.85 [1.43–2.40][ | <0.001 | 2.57 [2.01–3.29] | <0.001 | 1.72 [1.28–2.33] | <0.001 | 1.98 [1.71–2.30] | <0.001 |
Odds ratios were adjusted for age, sex, race/ethnicity, and study center.
CI, confidence interval; OR, odds ratio.
Table 3 shows the results of the pooled analyses using categorized anthropometric measurements. There was an inverse association between height and PTC risk for both men and women. The OR for women taller than 169 cm compared to those ≤160 cm tall was 0.76, and the OR for men taller than 183 cm compared to those ≤175 cm tall was 0.50. The tests for trend were significant for both sexes. Increased weight, body fat percentage, and BSA were all significantly associated with increased risk of PTC regardless of sex and in a dose-related manner. The most pronounced results were for body fat percentage, for which the OR was 3.83 for women whose body fat percentage was >37.8% compared to women whose body fat percentage was ≤29.0%, and the OR was 4.05 for men whose body fat percentage was >27.9% compared to men whose body fat percentage was ≤20.4%.
Table 3.
Pooled Odds Ratios and Confidence Intervals for Papillary Thyroid Cancer Risk According to Categories of Anthropometric Measurements
| Variable | Q1 | Q2 | Q3 | Q4 | |||||
|---|---|---|---|---|---|---|---|---|---|
| Case/Cntl | OR [CI] | Case/Cntl | OR [CI] | Case/Cntl | OR [CI] | Case/Cntl | OR [CI] | ptrend | |
| Height (cm), women | ≤160 | 161–165 | 166–169 | >169 | |||||
| 456/272 | 1.0 | 370/280 | 0.83 [0.67–1.04] | 226/300 | 0.50 [0.39–0.63] | 308/260 | 0.76 [0.60–0.96] | <0.001 | |
| Height (cm), men | ≤175 | 176–179 | 180–183 | >183 | |||||
| 255/287 | 1.0 | 100/276 | 0.56 [0.42–0.76] | 126/223 | 0.81 [0.60–1.08] | 76/229 | 0.50 [0.36–0.69] | <0.001 | |
| Weight (kg), women | ≤58 | 59–65 | 66–73 | >73 | |||||
| 265/289 | 1.0 | 293/290 | 1.15 [0.91–1.46] | 276/284 | 1.13 [0.88–1.44] | 526/248 | 2.53 [1.99–3.21] | <0.001 | |
| Weight (kg), men | ≤73 | 74–80 | 81–90 | >90 | |||||
| 92/257 | 1.0 | 107/261 | 1.24 [0.88–1.75] | 169/274 | 2.03 [1.47–2.80] | 187/223 | 2.53 [1.83–3.51] | <0.001 | |
| Body fat (%), women | ≤29.0 | 29.1–32.9 | 33.0–37.8 | >37.8 | |||||
| 218/280 | 1.0 | 218/273 | 1.12 [0.86–1.46] | 320/277 | 1.83 [1.40–2.40] | 601/281 | 3.83 [2.85–5.15] | <0.001 | |
| Body fat (%), men | ≤20.4 | 20.5–24.2 | 24.3–27.9 | >27.9 | |||||
| 69/255 | 1.0 | 86/252 | 1.12 [0.76–1.66] | 121/253 | 1.70 [1.14–2.52] | 279/255 | 4.05 [2.67–6.15] | <0.001 | |
| BSA (m2), women | ≤1.62 | 1.63–1.71 | 1.72–1.82 | >1.82 | |||||
| 282/263 | 1.0 | 272/282 | 0.93 [0.73–1.19] | 309/300 | 1.02 [0.80–1.29] | 494/266 | 1.92 [1.51–2.44] | <0.001 | |
| BSA (m2), men | ≤1.89 | 1.90–2.00 | 2.01–2.10 | >2.10 | |||||
| 115/248 | 1.0 | 137/259 | 1.32 [0.96–1.81] | 119/252 | 1.35 [0.97–1.88] | 184/256 | 1.85 [1.36–2.52] | <0.001 | |
| BMI (kg/m2), women | ≤21.4 | 21.5–23.8 | 23.9–26.4 | >26.4 | |||||
| 215/280 | 1.0 | 266/281 | 1.28 [1.00–1.65] | 273/269 | 1.38 [1.07–1.78] | 603/281 | 2.98 [2.34–3.80] | <0.001 | |
| BMI (kg/m2), men | ≤23.3 | 23.4–25.1 | 25.2–27.4 | >27.4 | |||||
| 61/254 | 1.0 | 101/249 | 1.60 [1.09–2.33] | 126/256 | 1.94 [1.34–2.80] | 267/256 | 4.01 [2.84–5.66] | <0.001 | |
| BMI (kg/m2) | <18.5 (underweight) | 18.5–24.9 (normal weight) | 25–29.9 (overweight) | ≥30 (obese) | |||||
| Women | 35/47 | 0.82 [0.52–1.30] | 581/671 | 1.0 | 422/292 | 1.67 [1.38–2.03] | 319/101 | 3.91 [3.02–5.05] | <0.001 |
| Men | 3/3 | — | 151/483 | 1.0 | 247/425 | 1.79 [1.39–2.30] | 154/104 | 4.28 [3.08–5.94] | <0.001 |
| <45 years old | 28/36 | 0.76 [0.45–1.29] | 425/697 | 1.0 | 246/343 | 1.58 [1.26–1.98] | 177/82 | 4.28 [3.11–5.89] | <0.001 |
| ≥45 years old | 10/14 | 0.92 [0.39–2.14] | 307/459 | 1.0 | 423/374 | 1.91 [1.54–2.36] | 296/123 | 4.49 [3.41–5.92] | <0.001 |
| Overall | 38/50 | 0.87 [0.56–1.36] | 732/1156 | 1.0 | 669/717 | 1.72 [1.48–2.00] | 473/205 | 4.17 [3.41–5.10] | <0.001 |
Odds ratios were adjusted for age, sex, race/ethnicity, and study center.
Cntl, control; Q, quartile.
BMI was also significantly associated with increased PTC risk (ptrend<0.001). The OR for women with a BMI>26.4 kg/m2 compared to those with a BMI≤21.4 kg/m2 was 2.98, and the OR for men with a BMI>27.4 kg/m2 compared to those with a BMI≤23.3 kg/m2 was 4.01. There was a significantly increased risk in both the overweight and obese categories compared to the normal-weight category for both men and women, and for subjects both younger than and older than 45 years of age. The ORs for overweight and obese subjects compared with normal-weight subjects were 1.72 [CI 1.48–2.00] and 4.17 [CI 3.41–5.10] respectively. The interactions of BMI with age and sex were not significant, whereas the interaction of BMI with race/ethnicity was significant (p=0.007). Therefore, the pooled analyses using categorized anthropometric measurements were repeated for the Caucasian subgroup.
Table 4 shows the results of the pooled analyses of categorized anthropometric measurements in association with PTC risk in Caucasians, who accounted for 93.4% of cases and 93.4% of controls. The category scales were the same as specified above. The significance and magnitude of risk estimates were similar to the results for the overall analysis. The associations between BMI, body fat percentage, and PTC risk remained significant among non-Caucasians (data not shown).
Table 4.
Pooled Odds Ratio and Confidence Intervals for Papillary Thyroid Cancer Risk in Caucasians According to Categories of Anthropometric Measurements
| Q1 | Q2 | Q3 | Q4 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Variable | Case/Cntl | OR [CI] | Case/Cntl | OR [CI] | Case/Cntl | OR [CI] | Case/Cntl | OR [CI] | ptrend |
| Height (cm), women | ≤160 | 161–165 | 166–169 | >169 | |||||
| 413/235 | 1.0 | 341/257 | 0.81 [0.64–1.02] | 210/273 | 0.49 [0.38–0.64] | 297/248 | 0.76 [0.60–0.98] | 0.002 | |
| Height (cm), men | ≤175 | 176–179 | 180–183 | >183 | |||||
| 242/269 | 1.0 | 94/267 | 0.54 [0.40–0.74] | 117/217 | 0.78 [0.58–1.05] | 73/221 | 0.49 [0.35–0.69] | <0.001 | |
| Weight (kg), women | ≤58 | 59–65 | 66–73 | >73 | |||||
| 245/268 | 1.0 | 274/270 | 1.17 [0.91–1.49] | 262/252 | 1.21 [0.94–1.56] | 479/222 | 2.57 [2.00–3.31] | <0.001 | |
| Weight (kg), men | ≤73 | 74–80 | 81–90 | >90 | |||||
| 86/242 | 1.0 | 104/248 | 1.29 [0.91–1.84] | 163/270 | 2.00 [1.43–2.78] | 172/214 | 2.45 [1.75–3.43] | <0.001 | |
| Body fat (%), women | ≤29.0 | 29.1–32.9 | 33.0–37.8 | >37.8 | |||||
| 205/263 | 1.0 | 202/253 | 1.09 [0.84–1.43] | 298/256 | 1.78 [1.35–2.36] | 553/240 | 3.89 [2.86–5.30] | <0.001 | |
| Body fat (%), men | ≤20.4 | 20.5–24.2 | 24.3–27.9 | >27.9 | |||||
| 60/248 | 1.0 | 81/242 | 1.10 [0.74–1.63] | 119/239 | 1.71 [1.14–2.56] | 259/245 | 3.70 [2.42–5.67] | <0.001 | |
| BSA (m2), women | ≤1.62 | 1.63–1.71 | 1.72–1.82 | >1.82 | |||||
| 255/238 | 1.0 | 258/262 | 0.96 [0.74–1.23] | 291/269 | 1.07 [0.84–1.38] | 454/243 | 1.96 [1.52–2.52] | <0.001 | |
| BSA (m2), men | ≤1.89 | 1.90–2.00 | 2.01–2.10 | >2.10 | |||||
| 108/229 | 1.0 | 131/252 | 1.26 [0.91–1.75] | 117/244 | 1.36 [0.97–1.91] | 169/249 | 1.71 [1.24–2.36] | 0.001 | |
| BMI (kg/m2), women | ≤21.4 | 21.5–23.8 | 23.9–26.4 | >26.4 | |||||
| 205/265 | 1.0 | 251/264 | 1.26 [0.97–1.63] | 256/244 | 1.38 [1.06–1.80] | 546/239 | 3.01 [2.34–3.88] | <0.001 | |
| BMI (kg/m2), men | ≤23.3 | 23.4–25.1 | 25.2–27.4 | >27.4 | |||||
| 56/245 | 1.0 | 97/238 | 1.70 [1.15–2.51] | 124/242 | 2.13 [1.46–3.11] | 248/249 | 3.98 [2.78–5.69] | <0.001 | |
| BMI (kg/m2) | <18.5 (underweight) | 18.5–24.9 (normal weight) | 25–29.9 (overweight) | ≥30 (obese) | |||||
| Women | 33/45 | 0.82 [0.51–1.31] | 547/624 | 1.0 | 397/255 | 1.73 [1.41–2.11] | 281/88 | 3.74 [2.84–4.92] | <0.001 |
| Men | 3/3 | — | 142/465 | 1.0 | 241/405 | 1.86 [1.44–2.41] | 139/101 | 4.00 [2.85–5.60] | <0.001 |
| <45 years old | 27/35 | 0.74 [0.43–1.27] | 393/661 | 1.0 | 231/324 | 1.67 [1.32–2.12] | 149/76 | 4.17 [2.97–5.85] | <0.001 |
| ≥45 years old | 9/13 | 0.84 [0.34–2.04] | 296/430 | 1.0 | 407/336 | 1.97 [1.58–2.46] | 271/113 | 4.26 [3.20–5.66] | <0.001 |
| Overall | 36/48 | 0.86 [0.55–1.36] | 689/1091 | 1.0 | 638/660 | 1.80 [1.54–2.10] | 420/189 | 4.00 [3.25–4.94] | <0.001 |
Odds ratios were adjusted for age, sex, and study center.
Discussion
The present study evaluated associations of anthropometric measurements with PTC risk on the basis of pooled analysis of three case–control populations. Of the anthropometric measurements examined, BMI and body fat percentage had the strongest association with PTC risk. Specifically, PTC risk in obese subjects was approximately four times that of normal-weight subjects, and men with a body fat percentage >27.9% and women with a body fat percentage >37.8% had approximately four times the PTC risk of men and women in the lowest quartile of body fat percentage. The associations remained significant across subgroups defined by study site, sex, and age. Body fat percentage is a better indicator of body fatness than BMI because body fat percentage takes age and sex into account, and lean mass is age- and sex-dependent (16), but BMI is more frequently used in epidemiological studies.
Our findings add to the existing evidence suggesting that a greater BMI is associated with increased PTC risk. This association between BMI and PTC risk is not consistent in the literature, however. Several prior studies have shown no association of BMI with thyroid cancer risk in men. Dal Maso et al., in a pooled analysis of 12 case–control studies from the United States, Europe, and Asia, found a significant moderate increase in thyroid cancer risk with higher BMI for women (relative risk for highest tertile, 1.2 [CI 1.0–1.4]) but not for men; the significant association for women was largely attributable to two U.S. studies, which had 325 female cases and only 51 male cases (10). Similarly, Clero et al. conducted a pooled analysis of two case–control studies including mainly Pacific Islander subjects (489 female cases and 65 male cases) and found a significantly increased risk of differentiated thyroid cancer with higher BMI for women (OR for highest tertile, 3.0 [CI 2.0–14.48]) but not for men (12). The nonsignificance of the association between BMI and thyroid cancer risk in men is likely due to the small numbers of male cases in previous studies. Our current pooled analysis, which included 557 male cases, is to our knowledge the largest case–control study to examine the association between BMI and thyroid cancer risk in men, and the results showed a statistically significant association between BMI and PTC risk in both men and women. Furthermore, our results, showing a slightly higher PTC risk in men in the highest BMI group than in women in the highest BMI group, are consistent with results of earlier pooled studies (13,18). Renehan et al., in a meta-analysis, found that a greater BMI was strongly associated with risk of thyroid cancer both among men (relative risk per 5 kg/m2 increase, 1.33 [CI 1.04–1.70]) and among women (1.14 [CI 1.06–1.23]) (18). Similarly, Kitahara et al., in a recent pooled analysis of five U.S. cohorts including 768 women and 388 men diagnosed with thyroid cancer, found that BMI was associated with thyroid cancer risk for women (hazard ratio per 5 kg/m2 increase, 1.2 [CI 1.1–1.3]) and men, for whom the association was slightly stronger (1.3 [CI 1.2–1.5]) after two years of follow-up (13).
Mechanisms by which BMI affects thyroid cancer risk are still unknown, though several plausible explanations exist, as recently summarized by Pappa and Alevizaki (19). A greater BMI (and obesity) is a marker for insulin resistance and chronic low-grade inflammation, which are the major driving forces behind carcinogenesis and tumor progression (18). A greater BMI has also been associated with a higher serum thyrotropin (TSH) level (20), which is an independent predictor of differentiated thyroid cancer, regardless of age and sex (21,22). Specifically linked to thyroid cancer, TSH cooperates with insulin and insulin-like growth factor-1 signaling to activate downstream MAPK and PI3K pathways that are central in thyroid carcinogenesis (23). Estrogens are known to promote thyroid tumor growth (24), and this relationship has been taken as a possible biological explanation for increased thyroid cancer risk in postmenopausal women observed in earlier studies (25,26). However, estrogen promotion of thyroid tumor growth is unlikely to explain the results of the current study, in which increased PTC risk was found in women both younger than and older than 45 years of age. A greater BMI has also been linked with certain dietary behaviors, such as excess protein and carbohydrate intake, that have been found to increase thyroid cancer risk (27). Low levels of physical activity have been linked with a higher BMI and increased risk of some cancers, but most studies showed little or no association of physical activity with thyroid cancer risk (28,29). Further studies assessing various parameters in conjunction with BMI are warranted to clarify the intermediate factors and mechanisms underlying the relationship between BMI and PTC risk.
In the pooled analysis, a greater BSA was also significantly associated with PTC risk, but the magnitudes of association were different across study sites, especially among men, and this finding is not easily explained. BSA is considered a better indicator than BMI of metabolic mass, and BSA is used in determining drug dose. In another case–control study of differentiated thyroid cancer risk, BSA was found to be the dominant anthropometric factor associated with thyroid cancer risk, but the conclusion was based on women only (12).
In contrast to weight, height is a stable measurement that is thought to be determined during childhood and adolescence by genetic predisposition, nutrition and physical behaviors, and socioeconomic status and other factors (30). In the pooled analysis, height was inversely associated with PTC risk, but the inverse association was not consistent across study sites. The inverse association is in contrast with some previous studies that reported a positive association between height and thyroid cancer risk (31–33), but other studies have provided inconsistent findings (14,34), suggesting that population-specific factors may influence associations between thyroid cancer risk and height. Additionally, it is possible that obesity is confounding these observations and is less prevalent among those of taller stature than those of short or normal stature. In fact, we found a significant inverse association between BMI and height among these subjects in this study (ptrend<0.01).
Our study has several strengths, including the reasonably large sample size, narrow case definition, and on-site measurement of height and weight, which enabled us to determine significant associations between anthropometric measurements and PTC risk in men and women separately. However, several potential limitations should be considered. First, although a significant increased PTC risk associated with body fat and BMI was found in all three case–control populations, we could not exclude the possibility that these associations may be biased due to confounding by unmeasured risk factors for PTC. A history of benign thyroid diseases (including hypothyroidism and hyperthyroidism) has been associated with both subsequent thyroid cancer risk and weight change. However, our study did not evaluate the possible extent of such bias. Although age and sex, the major risk factors for PTC, were adjusted for in the model, other possible confounders, such as smoking, education status, dietary and physical behaviors, and genetic predisposition factors, were not assessed in our analysis. We also lack information on other anthropometric measurements, such as waist-to-hip ratio, which may be a more accurate measurement of central adiposity than BMI and body fat percentage. However, previous studies did not show waist-to-hip ratio to be associated more strongly than BMI with thyroid cancer risk (14,33,35). A further limitation is that height and weight information was collected shortly before or after cancer diagnosis, making it difficult to establish temporality. Furthermore, interpretation of our findings is based on the assumption that these measurements reflect the long-term status of adiposity, which may not be the case; misclassification bias could be introduced. Although there is evidence supporting that change in adiposity over time is unlikely to impact the association between obesity and PTC risk substantially, as previous studies did not find weight gain to be a significant risk factor for PTC (35,36) and weight loss is uncommon for patients with newly diagnosed PTC, there are also findings suggesting that weight gain over life, especially 10–20 years before cancer occurrence, was associated with increased thyroid cancer risk (37,38). Reverse causation is also possible given the fact that mild weight gain is a common symptom of hypothyroidism, but the possibility is unlikely because hypothyroidism is not frequently observed in patients with PTC. Our study is also subject to the other limitations inherent to case–control study design. Since all three studies used case–control study design and there were mismatches in age and sex between cases and controls in the U.S. and German studies, the possibility of selection bias, especially among controls, should be considered. Since most subjects were Caucasian, our results are not generalizable to a racially diverse population. Finally, the possibility of screening bias cannot be excluded, but the possibility is less likely given the observation that a higher BMI was associated with a more advanced stage of thyroid cancer at diagnosis (39).
In summary, anthropometric factors, especially BMI and body fat percentage, were significantly associated with increased risk of PTC. Future studies employing more precise measurement of body fat using novel techniques (including dual-energy X-ray absorptiometry) could more accurately assess the magnitude of body fat in association with PTC risk. Future studies of anthropometric factors and PTC that incorporate intermediate factors, including adiposity and hormone biomarkers, are essential to help clarify potential mechanisms of the relationship.
Acknowledgments
This work was supported by an American Thyroid Association Thyroid Cancer grant (principal investigator, E.M.S.), MD Anderson Start-up Funds (principal investigator, E.M.S.), Italian Ministry of Research, Programmi di Ricerca di Interesse Nazionale (PRIN) 2009–2011, and Tuscany Cancer Institute (Istituto Toscano Tumori) 2010–2013. The authors thank Margaret Lung, Kathryn Patterson, Liliana Mugartegui, and Jenny Vo for their help with subject recruitment at MD Anderson, and Stephanie P. Deming for editing the manuscript.
Author Disclosure Statement
The authors declare no conflicts of interest.
References
- 1.Kilfoy BA, Zheng T, Holford TR, Han X, Ward MH, Sjodin A, Zhang Y, Bai Y, Zhu C, Guo GL, Rothman N.2009International patterns and trends in thyroid cancer incidence, 1973–2002. Cancer Causes Control 20:525–531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.National Cancer Institute 2013 SEER cancer statistics review, 1975–2010. Available at http://seer.cancer.gov/csr/1975_2010 (accessed August2013)
- 3.Aschebrook-Kilfoy B, Kaplan EL, Chiu BC, Angelos P, Grogan RH.2013The acceleration in papillary thyroid cancer incidence rates is similar among racial and ethnic groups in the United States. Ann Surg Oncol 20:2746–2753 [DOI] [PubMed] [Google Scholar]
- 4.Davies L, Welch HG.2006Increasing incidence of thyroid cancer in the United States, 1973–2002. JAMA 295:2164–2167 [DOI] [PubMed] [Google Scholar]
- 5.Chen AY, Jemal A, Ward EM.2009Increasing incidence of differentiated thyroid cancer in the United States, 1988–2005. Cancer 115:3801–3807 [DOI] [PubMed] [Google Scholar]
- 6.Li N, Du XL, Reitzel LR, Xu L, Sturgis EM.2013Impact of enhanced detection on the increase in thyroid cancer incidence in the United States: review of incidence trends by socioeconomic status within the Surveillance, Epidemiology, and End Results registry, 1980–2008. Cancer 23:103–110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ron E, Lubin JH, Shore RE, Mabuchi K, Modan B, Pottern LM, Schneider AB, Tucker MA, Boice JD., Jr1995Thyroid cancer after exposure to external radiation: a pooled analysis of seven studies. Radiat Res 141:259–277 [PubMed] [Google Scholar]
- 8.Ogden CL CM, Kit BK, Flegal KM.2012Prevalence of obesity in the United States, 2009–2010. NCHS data brief, no 82 Hyattsville, MD: National Center for Health Statistics; [PubMed] [Google Scholar]
- 9.Wang Y, Beydoun MA.2007The obesity epidemic in the United States—gender, age, socioeconomic, racial/ethnic, and geographic characteristics: a systematic review and meta-regression analysis. Epidemiol Rev 29:6–28 [DOI] [PubMed] [Google Scholar]
- 10.Dal Maso L, La Vecchia C, Franceschi S, Preston-Martin S, Ron E, Levi F, Mack W, Mark SD, McTiernan A, Kolonel L, Mabuchi K, Jin F, Wingren G, Galanti MR, Hallquist A, Glattre E, Lund E, Linos D, Negri E.2000A pooled analysis of thyroid cancer studies. V. Anthropometric factors. Cancer Causes Control 11:137–144 [DOI] [PubMed] [Google Scholar]
- 11.Meinhold CL, Ron E, Schonfeld SJ, Alexander BH, Freedman DM, Linet MS, Berrington de Gonzalez A.2010Nonradiation risk factors for thyroid cancer in the US Radiologic Technologists Study. Am J Epidemiol 171:242–252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Clero E, Leux C, Brindel P, Truong T, Anger A, Teinturier C, Diallo I, Doyon F, Guenel P, de Vathaire F.2010Pooled analysis of two case-control studies in New Caledonia and French Polynesia of body mass index and differentiated thyroid cancer: the importance of body surface area. Thyroid 20:1285–1293 [DOI] [PubMed] [Google Scholar]
- 13.Kitahara CM, Platz EA, Freeman LE, Hsing AW, Linet MS, Park Y, Schairer C, Schatzkin A, Shikany JM, Berrington de Gonzalez A.2011Obesity and thyroid cancer risk among U.S. men and women: a pooled analysis of five prospective studies. Cancer Epidemiol Biomarkers Prev 20:464–472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rinaldi S, Lise M, Clavel-Chapelon F, Boutron-Ruault MC, Guillas G, Overvad K, Tjonneland A, Halkjaer J, Lukanova A, Kaaks R, Bergmann MM, Boeing H, Trichopoulou A, Zylis D, Valanou E, Palli D, Agnoli C, Tumino R, Polidoro S, Mattiello A, Bueno-de-Mesquita HB, Peeters PH, Weiderpass E, Lund E, Skeie G, Rodriguez L, Travier N, Sanchez MJ, Amiano P, Huerta JM, Ardanaz E, Rasmuson T, Hallmans G, Almquist M, Manjer J, Tsilidis KK, Allen NE, Khaw KT, Wareham N, Byrnes G, Romieu I, Riboli E, Franceschi S.2012Body size and risk of differentiated thyroid carcinomas: findings from the EPIC study. Int J Cancer 131:E1004–E1014 [DOI] [PubMed] [Google Scholar]
- 15.Iribarren C, Haselkorn T, Tekawa IS, Friedman GD.2001Cohort study of thyroid cancer in a San Francisco Bay area population. Int J Cancer 93:745–750 [DOI] [PubMed] [Google Scholar]
- 16.Deurenberg P, Weststrate JA, Seidell JC.1991Body-mass index as a measure of body fatness—age-specific and sex-specific prediction formulas. Br J Nutr 65:105–114 [DOI] [PubMed] [Google Scholar]
- 17.Du Bois D, Du Bois EF.1916A formula to estimate the approximate surface area if height and weight be known. Arch Intern Med 17:863–871 [PubMed] [Google Scholar]
- 18.Renehan AG, Tyson M, Egger M, Heller RF, Zwahlen M.2008Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet 371:569–578 [DOI] [PubMed] [Google Scholar]
- 19.Pappa T, Alevizaki M.2014Obesity and thyroid cancer: a clinical update. Thyroid 24:190–199 [DOI] [PubMed] [Google Scholar]
- 20.Nyrnes A, Jorde R, Sundsfjord J.2006Serum TSH is positively associated with BMI. Int J Obes (Lond) 30:100–105 [DOI] [PubMed] [Google Scholar]
- 21.Haymart MR, Repplinger DJ, Leverson GE, Elson DF, Sippel RS, Jaume JC, Chen H.2008Higher serum thyroid stimulating hormone level in thyroid nodule patients is associated with greater risks of differentiated thyroid cancer and advanced tumor stage. J Clin Endocrinol Metab 93:809–814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim HK, Yoon JH, Kim SJ, Cho JS, Kweon SS, Kang HC.2013Higher TSH level is a risk factor for differentiated thyroid cancer. Clin Endocrinol (Oxf) 78:472–477 [DOI] [PubMed] [Google Scholar]
- 23.Tramontano D, Cushing GW, Moses AC, Ingbar SH.1986Insulin-like growth factor-I stimulates the growth of rat thyroid cells in culture and synergizes the stimulation of DNA synthesis induced by TSH and Graves'-IgG. Endocrinology 119:940–942 [DOI] [PubMed] [Google Scholar]
- 24.Manole D, Schildknecht B, Gosnell B, Adams E, Derwahl M.2001Estrogen promotes growth of human thyroid tumor cells by different molecular mechanisms. J Clin Endocrinol Metab 86:1072–1077 [DOI] [PubMed] [Google Scholar]
- 25.Guignard R, Truong T, Rougier Y, Baron-Dubourdieu D, Guenel P.2007Alcohol drinking, tobacco smoking, and anthropometric characteristics as risk factors for thyroid cancer: a countrywide case-control study in New Caledonia. Am J Epidemiol 166:1140–1149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Goodman MT, Kolonel LN, Wilkens LR.1992The association of body size, reproductive factors and thyroid cancer. Br J Cancer 66:1180–1184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marcello MA, Sampaio AC, Geloneze B, Vasques AC, Assumpcao LV, Ward LS.2013Obesity and excess protein and carbohydrate consumption are risk factors for thyroid cancer. Nutr Cancer 64:1190–1195 [DOI] [PubMed] [Google Scholar]
- 28.Cash SW, Ma H, Horn-Ross PL, Reynolds P, Canchola AJ, Sullivan-Halley J, Beresford SA, Neuhouser ML, Vaughan TL, Heagerty PJ, Bernstein L.2013Recreational physical activity and risk of papillary thyroid cancer among women in the California Teachers Study. Cancer Epidemiol 37:46–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kitahara CM, Platz EA, Beane Freeman LE, Black A, Hsing AW, Linet MS, Park Y, Schairer C, Berrington de Gonzalez A.2012Physical activity, diabetes, and thyroid cancer risk: a pooled analysis of five prospective studies. Cancer Causes Control 23:463–471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Silventoinen K, Zdravkovic S, Skytthe A, McCarron P, Herskind AM, Koskenvuo M, de Faire U, Pedersen N, Christensen K, Kaprio J.2006Association between height and coronary heart disease mortality: a prospective study of 35,000 twin pairs. Am J Epidemiol 163:615–621 [DOI] [PubMed] [Google Scholar]
- 31.Kabat GC, Heo M, Kamensky V, Miller AB, Rohan TE.2013Adult height in relation to risk of cancer in a cohort of Canadian women. Int J Cancer 132:1125–1132 [DOI] [PubMed] [Google Scholar]
- 32.Sung J, Song YM, Lawlor DA, Smith GD, Ebrahim S.2009Height and site-specific cancer risk: a cohort study of a Korean adult population. Am J Epidemiol 170:53–64 [DOI] [PubMed] [Google Scholar]
- 33.Kabat GC, Kim MY, Thomson CA, Luo J, Wactawski-Wende J, Rohan TE.2012Anthropometric factors and physical activity and risk of thyroid cancer in postmenopausal women. Cancer Causes Control 23:421–430 [DOI] [PubMed] [Google Scholar]
- 34.Han JM, Kim TY, Jeon MJ, Yim JH, Kim WG, Song DE, Hong SJ, Bae SJ, Kim HK, Shin MH, Shong YK, Kim WB.2013Obesity is a risk factor for thyroid cancer in a large, ultrasonographically screened population. Eur J Endocrinol 168:879–886 [DOI] [PubMed] [Google Scholar]
- 35.Kitahara CM, Platz EA, Park Y, Hollenbeck AR, Schatzkin A, Berrington de Gonzalez A.2012Body fat distribution, weight change during adulthood, and thyroid cancer risk in the NIH-AARP Diet and Health Study. Int J Cancer 130:1411–1419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Suzuki T, Matsuo K, Hasegawa Y, Hiraki A, Kawase T, Tanaka H, Tajima K.2008Anthropometric factors at age 20 years and risk of thyroid cancer. Cancer Causes Control 19:1233–1242 [DOI] [PubMed] [Google Scholar]
- 37.Clavel-Chapelon F, Guillas G, Tondeur L, Kernaleguen C, Boutron-Ruault MC.2010Risk of differentiated thyroid cancer in relation to adult weight, height and body shape over life: the French E3N cohort. Int J Cancer 126:2984–2990 [DOI] [PubMed] [Google Scholar]
- 38.Brindel P, Doyon F, Rachedi F, Boissin JL, Sebbag J, Shan L, Chungue V, Bost-Bezeaud F, Petitdidier P, Paoaafaite J, Teuri J, de Vathaire F.2009Anthropometric factors in differentiated thyroid cancer in French Polynesia: a case-control study. Cancer Causes Control 20:581–590 [DOI] [PubMed] [Google Scholar]
- 39.Kim HJ, Kim NK, Choi JH, Sohn SY, Kim SW, Jin SM, Jang HW, Suh S, Min YK, Chung JH, Kim SW.2013Associations between body mass index and clinico-pathological characteristics of papillary thyroid cancer. Clin Endocrinol (Oxf) 78:134–140 [DOI] [PubMed] [Google Scholar]