Abstract
Although adipose-derived stem cells (ASCs) show promise for cell therapy, there is a tremendous need for developing ASC activators. In the present study, we investigated whether or not vitamin C increases the survival, proliferation, and hair-regenerative potential of ASCs. In addition, we tried to find the molecular mechanisms underlying the vitamin C-mediated stimulation of ASCs. Sodium-dependent vitamin C transporter 2 (SVCT2) is expressed in ASCs, and mediates uptake of vitamin C into ASCs. Vitamin C increased the survival and proliferation of ASCs in a dose-dependent manner. Vitamin C increased ERK1/2 phosphorylation, and inhibition of the mitogen-activated protein kinase (MAPK) pathway attenuated the proliferation of ASCs. Microarray and quantitative polymerase chain reaction showed that vitamin C primarily upregulated expression of proliferation-related genes, including Fos, E2F2, Ier2, Mybl1, Cdc45, JunB, FosB, and Cdca5, whereas Fos knock-down using siRNA significantly decreased vitamin C-mediated ASC proliferation. In addition, vitamin C-treated ASCs accelerated the telogen-to-anagen transition in C3H/HeN mice, and conditioned medium from vitamin C-treated ASCs increased the hair length and the Ki67-positive matrix keratinocytes in hair organ culture. Vitamin C increased the mRNA expression of HGF, IGFBP6, VEGF, bFGF, and KGF, which may mediate hair growth promotion. In summary, vitamin C is transported via SVCT2, and increased ASC proliferation is mediated by the MAPK pathway. In addition, vitamin C preconditioning enhanced the hair growth promoting effect of ASCs. Because vitamin C is safe and effective, it could be used to increase the yield and regenerative potential of ASCs.
Introduction
Adipose-derived stem cells (ASCs) are located in the perivascular region and can be isolated from the stromal vascular fraction of adipose tissue [1–3]. ASCs show promise in tissue regeneration due to their ability to function as building blocks and their paracrine effects [4–6]. Although an unexpanded stromal vascular fraction of lipoaspirate can be used for clinical application [7,8], ASCs are usually expanded to acquire large numbers of cells required for therapeutic application. Cultured autologous ASCs have been used in clinical trials for the treatment of limb ischemia, diabetes mellitus, spinal cord injury, and Crohn's fistulan [8,9]. Transplanted cells encounter an inflammatory environment that mitigates their function and survival; therefore, treating the cells with exogenous stimuli to enhance their survival and paracrine function before transplantation is one strategy for overcoming this limitation [10,11]. For example, growth factors, such as basic fibroblast growth factor (bFGF) and platelet-derived growth factor-B (PDGF-B), have been used during ASC expansion to increase the proliferation and regenerative potential of these cells [1]. Hypoxia also increases ASC survival, and hypoxia preconditioning upregulated growth factor expression and induced a regenerative potential in animal experiments [12–14]. Alternatively, low-dose UVB treatment before transplantation increased the survival and hair growth promoting effect of ASCs in vivo [15]. We previously investigated the key mediators and signaling pathways involved in ASC stimulation under hypoxia, and found that reactive oxygen species (ROS) generation, the PI3K/Akt pathway, the mitogen-activated protein kinase (MAPK) pathway, and miR-210 play key roles in ASC stimulation [16–18]. However, there is a tremendous need for alternative ASC stimuli for use during cultivation to increase the survival, proliferation, paracrine effect, and therapeutic efficiency of ASCs. Here we propose vitamin C as a promising alternative ASC stimulus since it is much cheaper than growth factors, easy to handle, and physiologically safe. In addition, vitamin C exhibits comparable stimulating effect to other activators.
Vitamin C is an essential micronutrient and acts as a cofactor in numerous biosynthetic enzymes. Vitamin C plays both antioxidant and pro-oxidant roles depending on concentration, and enhances or inhibits cell proliferation. Vitamin C supplementation has diverse effects on stem cells, and it could be used to maintain stem cell properties. For example, vitamin C treatment enhances the generation of induced pluripotent stem cells, and improves the quality of somatic cell reprogramming via epigenetic modification [19,20]. Vitamin C also increased the cardiomyogenesis of embryonic stem cells through NADPH oxidase and nitric oxide synthase [21]. Vitamin C treatment increased ASC proliferation and upregulated Oct4 and Sox2 expression [22]. Likewise, growth medium supplemented with 2-phospho-l-ascorbic acid, a vitamin C derivative, induced ASC survival and Oct4 gene expression [23]. However, the signaling pathway and molecular mechanism(s) underlying the stimulatory effects of vitamin C on ASCs has not been fully determined. Therefore, in the present study, we investigated whether or not vitamin C increases the survival, proliferation, and hair-regenerative potential of ASCs. We also attempted to determine the molecular mechanisms underlying vitamin C-induced stimulation of ASCs.
Materials and Methods
Materials
Vitamin C, 2-phospho-l-ascorbic acid, dehydro-l-(+)-ascorbic acid dimer, and ascorbic acid 6-palmitate were obtained from Sigma (St. Louis, MO). Human recombinant hepatocyte growth factor (HGF) was purchased from PEPROTECH (Rocky Hill, NJ).
ASC culture
Human ASCs were isolated from liposuction of subcutaneous fat as described previously [24], and cultured in minimum essential medium-alpha (Invitrogen, Carlsbad, CA) with 10% fetal bovine serum (FBS; Invitrogen) and 1% penicillin and streptomycin (Invitrogen) at 37°C in a humidified atmosphere containing 5% CO2. ASCs were characterized by the presence of cell surface markers, such as CD34, CD73, CD90, and CD105, and multiple differentiation potential was tested in differentiation medium [4,24].
Cell proliferation assay
ASCs were seeded in a 48-well plate at a density of 5×103 cells/well. After 24 h, the medium was replaced with medium containing 0.2% FBS. The following day, cells were treated with vitamin C (10, 50, 100, and 200 μM) for 48 and 72 h. Then, the medium was removed and cell numbers were counted using the CCK-8 assay kit (Dojindo, Rockville, MD). Cells were treated with 10% CCK-8 solution in the medium for 2 h, and then the absorbance was measured at 450 nm with a microplate reader (TECAN, Männedorf, Switzerland). To inhibit ERK, cells were incubated with 1 and 5 μM U0126 (Calbiochem, San Diego, CA).
Cell survival assay
To measure the survival of ASCs in the presence of vitamin C, cells were cultured in a 48-well plate at a density of 8×103 cells/well for 24 h. The cultured cells were pretreated with vitamin C (200 μM) for 2 h. Then, the cells were treated with 500 μM hydrogen peroxide (H2O2; Sigma). After 4 h, the medium was changed and cells were incubated with vitamin C (200 μM) for 24 h. Then, cell survival was measured using the CCK-8 assay.
ROS measurement
Cellular ROS production was measured using 2′,7′-dichlorofluorescin diacetate (DCF-DA; Molecular probes, Eugene, OR). ASCs (2.5×105 cells) were seeded in a 60-mm culture dish. Cells were pretreated with DCF-DA (20 μM) for 20 min, and treated with vitamin C (200 μM) for 20 min at 37°C in the dark. Then, cells were harvested using trypsin-EDTA, and fluorescence intensity of DCF-DA was measured using flow cytometry (BD Biosciences, Franklin Lakes, NJ).
Polymerase chain reaction
Total RNA was extracted using the TRIzol reagent (Invitrogen) and mRNA was reverse-transcribed with the cDNA synthesis kit (Promega, Madison, WI). cDNA was synthesized from 500 ng of total RNA by 200 U RTase. The reaction protocol included a 5-min incubation at 95°C, and then 35 cycles of 95°C for 30 s, 56°C for 20 s, and 72°C for 40 s, followed by a final elongation at 72°C for 5 min. Primers used are listed in Table 1. Quantitative real-time polymerase chain reactions (QPCRs) were performed in a step-one plus real-time PCR system (Applied Biosystems, Carlsbad, CA) using SYBR green PCR master mix (TaKaRa, Seta, Otsu, Japan). GAPDH was used for sample standardization. Fold change was calculated using the ΔCt value.
Table 1.
List of Primers
| Gene | Forward | Reverse |
|---|---|---|
| Oct4 | 5′-CCCGCCGTATGAGTTCTGT-3′ | 5′-TCCAGCTTCTCCTTCTCCAG-3′ |
| Nanog | 5′-ACCTTCCAATGTGGAGCATC-3′ | 5′-GAATTTGGCTGGAACTGCA-3′ |
| Sox2 | 5′-GGGGAAAGTAGTTTGCTGCCTC-3′ | 5′-CCGCCGCCGATGATTGTTATT-3′ |
| SVCT1 | 5′-CCTTGCTTTCATACTTGACA-3′ | 5′-GAAAATCCTTTGAAGACTGG-3′ |
| SVCT2 | 5′-CAGCGAATTGTAATGGAAAG-3′ | 5′-TGGAAGTGAAGGCTTATTCA-3′ |
| HGF | 5′-CATGGACAAGATTGTTATCG-3′ | 5′-TCATTCAGCTTACTTGCATC-3′ |
| IGFBP6 | 5′-GAATCCAGGCACCTCTACCA-3′ | 5′-GGTAGAAGCCTCGATGGTCA-3′ |
| VEGF | 5′-TACCTCCACCATGCCAAGT-3′ | 5′-TGCATTCACATTTGTTGTGC-3′ |
| bFGF | 5′-AGGAGTGTGTGCTAACCGTT-3′ | 5′-CAGTTCGTTTCAGTGCCACA-3′ |
| KGF | 5′-TCTGTCGAACACAGTGGTACCT-3′ | 5′-GTGTGTCCATTTAGCTGATGCAT-3′ |
| GAPDH | 5′-CGAGATCCCTCCAAAATCAA-3′ | 5′-TGTGGTCATGAGTCCTTCCA-3′ |
siRNA transfection
A mixture of sodium-dependent vitamin C transporter 2 (SVCT2) siRNA (Santa Cruz Biotechnology, Dallas, TX), ERK1 siRNA (Santa Cruz Biotechnology), FOS siRNA (Invitrogen), or E2F2 siRNA (Dharmacon, Lafayette, LA) and Lipofectamine 2000 (Invitrogen) was prepared before cell trypsinization. Then, cells were transfected with the siRNA mixture for 48 h. Gene silencing was evaluated by QPCR and western blotting.
FITC-labeled vitamin C uptake assay
FITC-labeled vitamin C was synthesized according to a previous study [25], with some modifications. ASCs (1×104) were seeded in a 96-well plate and cultured. FITC-labeled vitamin C (100 μM) was mixed with the indicated concentrations of unlabeled vitamin C and incubated with ASCs for 15 min. Then, the cells were washed with cold phosphate-buffered saline, and the FITC signal was evaluated by measuring the absorbance at 488 nm.
Vitamin C concentration assay
To monitor intracellular vitamin C level, ASCs were seeded in a 96-well plate at a density of 3×103 cells/well, and treated with vitamin C for 24 h. Intracellular vitamin C concentration was measured with an Ascorbic Acid Assay kit (BioVision, Linda Vista, CA) according to the manufacturer's instructions.
Microarray
To measure the change in gene expression induced by vitamin C, cells were cultured in a 100-mm dish with vitamin C for 24 h, and then total RNA was extracted using the Total RNA isolation kit (Invitrogen). Total RNA (200 ng) was amplified with a Low Input Quick Amp Labeling kit (one-color; Agilent Technologies UK, Ltd., Wokingham, United Kingdom) and labeled with Cy3. Cy3-labeled cRNA (1.65 μg) was fragmented by incubation with fragmentation buffer at 60°C for 30 min. Labeled cRNA was hybridized to a Whole Human Genome Microarray (4×44K) at 60°C for 17 h. Then, the microarray chip was scanned using Agilent microarray scanner and analyzed by Agilent Feature Extraction software.
Identification of differentially expressed genes
To normalize the signals from the microarray analysis of control and vitamin C-treated ASCs, we processed the signals using 75% percentile normalization. Normalized signals of the two groups were compared by t-test, and adjusted by Bonferroni correction. All data processing and statistical analyses were performed using GeneSpring GX7 (Agilent Technologies UK, Ltd.). We selected the genes that showed a more than a 2-fold difference as differentially expressed genes (DEGs). We used GO miner (http://discover.nci.nih.gov/gominer) for GO term analysis of our DEGs.
Phospho-kinase chip array
Cells were seeded in a 100-mm dish with medium containing 0.2% FBS. Then, 80% confluent cells were treated with vitamin C (200 μM) for 10 min. Proteins were isolated from the cells using lysis buffer (R&D Systems, Minneapolis, MN). Phosphorylation of signaling molecules was detected by a phospho-kinase array kit (R&D Systems) according to the manufacturer's instructions.
Growth factor antibody array
To measure the effect of vitamin C on growth factor expression, cells were treated with vitamin C (200 μM) for 24 h. After treatment, signals indicating growth factor expression were detected using a growth factor antibody array kit according to the manufacturer's instructions (RayBiotech, Parkway Lane, NC).
Western blotting
Proteins were isolated using an sodium dodecyl sulfate (SDS) lysis buffer containing protease inhibitors. Lysates were incubated on ice for 30 min, and then soluble proteins were isolated by centrifugation at 4°C. Proteins were separated by 10% SDS–polyacrylamide gel electrophoresis (PAGE) and transferred to a PVDF membrane (Millipore, Billerica, MA). The membrane was then blocked using 5% non-fat milk for 1 h at room temperature. After blocking, the membrane was probed with the primary antibody (ERK1/2 and phosphor-ERK1/2; Cell Signaling Technology, Danvers, MA) overnight at 4°C, and then incubated with an HRP-conjugated secondary antibody (Cell Signaling) for 2 h at room temperature. The membrane was incubated in ECL solution (Millipore) and exposed.
Animal experiments
Mice were anesthetized according to a protocol approved by the United States Pharmacopoeia (USP) and the Institutional Animal Care and Use Committee (IACUC) of CHA University. An area on the back of 7-week-old C3H/HeN mice in the telogen stage of the hair cycle was shaved with clippers and electric shaver, and special care was taken to avoid damaging the bare skin [15,26]. Before injection into the mice, ASCs were treated with vitamin C (200 μM). These vitamin C-preconditioned ASCs (ASCvit.C) and untreated ASCs were maintained at 37°C in 5% CO2 for 4 h. ASCs (1×104) or ASCvit.C (1×104) were then injected into the dorsal skin of each mouse. Any darkening of the skin (indicative of hair growth) was carefully monitored by photography. After 12 days, the dorsal hair was shaved and weigh.
Preparation of conditioned medium
Conditioned medium derived from ASCs (ASC-CM) and vitamin C-treated ASCs (vit.C-CM) was prepared as previously described [27]. CM was collected, centrifuged at 1,800 rpm for 10 min, and filtered through a 0.22-μm syringe filter. The filtrate was concentrated in a 3-kDa molecular weight cut-off Vivaspin sample concentrator (Sartorius Stedim Biotech GmbH, Goettingen, Germany), and 10-fold concentrated CM was used for the subsequent experiments.
Hair organ culture
Anagen hair follicles were isolated from a volunteer and cultured ex vivo, as previously described [28,29]. Briefly, dissected hair follicles were cut into small pieces (∼2 mm in length from the bottom of the dermal papilla) and cultured in Williams E medium (Sigma) with 10 ng/mL hydrocortisone, 10 ng/mL insulin, 2 mM l-glutamine, and 100 U/mL penicillin at 37°C in 5% CO2. ASC-CM or vit.C-CM was added to the basal Williams E medium. After culturing for 6 days, hair follicles were harvested and hair length was measured. Hair follicles were also stained with an anti-Ki67 antibody (BD Biosciences) and DAPI.
ELISA for HGF concentration
The concentration of human HGF in ASC-CM and vit.C-CM was measured using the Quantikine ELISA kit (R&D Systems) according to the manufacturer's instructions.
Statistical analysis
All data are representative data from three independent experiments. The statistical significance of the differences among groups was tested using ANOVA or Student's t-test. P values<0.05 or 0.01 were considered statistically significant.
Results
Vitamin C increases the proliferation of ASCs
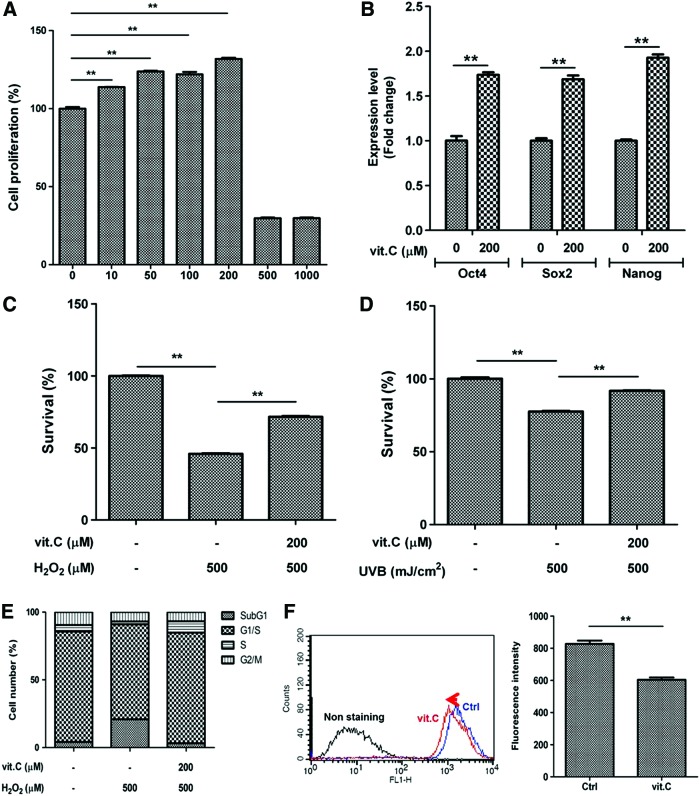
First, we compared the proliferative effect of vitamin C and its derivatives in ASCs. Although 2-phospho-l-ascorbic acid and dehydro-l(+)-ascorbic acid induced ASC proliferation, vitamin C showed the most potent stimulatory effect on ASC proliferation (Supplementary Fig. S1A; Supplementary Data are available online at www.liebertpub.com/scd). Vitamin C increased ASC proliferation in a dose-dependent manner (Fig. 1A, P<0.01); however, it did not induce proliferation at high concentrations (ie, >500 μM).
FIG. 1.
Vitamin C increases the proliferation and survival of adipose-derived stem cells (ASCs). We compared the stimulatory effects of vitamin C in vitro, and vitamin C increased the proliferation and survival of ASCs. (A) Cell proliferation was measured by the CCK-8 assay, and vitamin C increased the proliferation of ASCs in a dose-dependent manner. (B) The mRNA expression of pluripotency markers was measured by quantitative real-time polymerase chain reaction (QPCR), and vitamin C significantly induced the expression of Oct4, Sox2, and Nanog. (C, D) Cell damage was induced by hydrogen peroxide (H2O2) treatment (500 μM, C) and UVB irradiation (500 mJ/cm2, D) of ASCs, and vitamin C (200 μM) significantly increased the survival of ASCs, as determined by the CCK-8 assay. (E) Vitamin C attenuated the H2O2-induced sub-G1 of ASCs as measured by flow cytometry. (F) Reactive oxygen species generation was measured using 2′,7′-dichlorofluorescin diacetate, and vitamin C reduced the fluorescence intensity, as determined by flow cytometry. Data are mean±STD (**P<0.01, Student's t-test). Color images available online at www.liebertpub.com/scd
Vitamin C derivatives also upregulated the mRNA expression of pluripotency markers, as shown by QPCR (Supplementary Fig. S1B). Vitamin C (200 μM) significantly increased the mRNA expression of Oct4, Sox2, and Nanog (Fig. 1B, P<0.01), and upregulation of these pluripotency markers is related to vitamin C-induced ASC proliferation.
Vitamin C increases the survival of ASCs in vitro
We also investigated whether vitamin C increased the survival of ASCs. Cell damage was induced by H2O2 (500 μM for 3 h) and UVB (500 mJ/cm2) treatments in ASCs, and vitamin C (200 μM) significantly increased survival of ASCs in CCK-8 assay (Fig. 1C, D; P<0.01). In a flow cytometry analysis, vitamin C treatment reduced the H2O2-induced sub-G1 phase of ASCs (from 20.3% to 3.2%; Fig. 1E). We also measured intracellular ROS levels in ASCs. H2O2-induced ROS generation was significantly reduced by vitamin C treatment (Fig. 1F, P<0.01), and vitamin C acts as an ROS scavenger. These suggest that vitamin C increases the survival of ASCs from H2O2-induced oxidative stress.
In addition, we detected ASC survival in mouse skin tissue using immunofluorescence; however, there was no significant difference in the survival of PKH26-labeled ASCs between control and vitamin C-treated ASCs at days 3 and 5 after subcutaneous injection (data not shown).
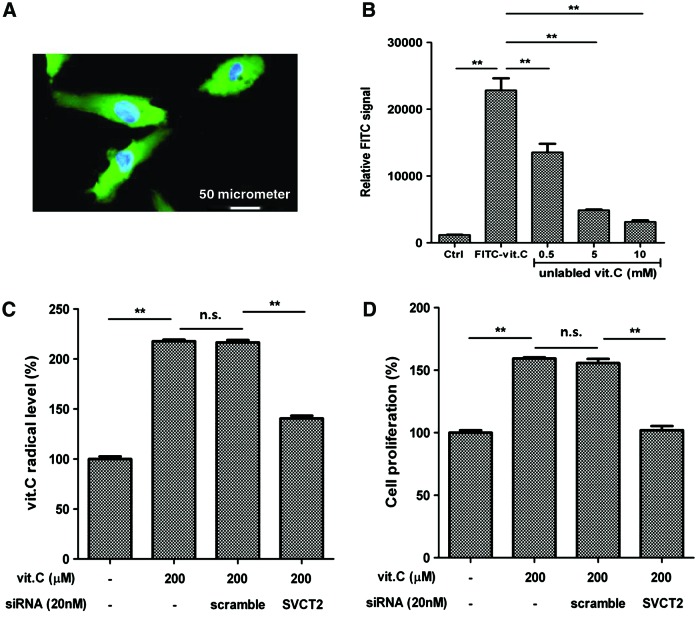
SVCT2 mediates vitamin C uptake
As vitamin C is hydrophilic and has been reported to be transported into cells via membrane transporters [30–32], we attempted to determine the cellular uptake mechanism of vitamin C in ASCs. Fluorescence microscopy clearly showed that FITC-labeled vitamin C was effectively incorporated into ASCs (green, Fig. 2A). Although the vitamin C signal is high in the cytosol, it is not in the nucleus (blue, Fig. 2A). This indicates that vitamin C is distributed in the cytosol of ASCs. In addition, fluorescence intensity decreased in the presence of unlabeled vitamin C (Fig. 2B), and this suggests that an active vitamin C transport system exists in ASCs. Therefore, the mRNA expression of the SVCT was measured by RT-PCR. SVCT1 is not expressed (not detected until cycle 40), but SVCT2 is expressed in ASCs (Ct value=30.68±0.20).
FIG. 2.
Sodium-dependent vitamin C transporter 2 (SVCT2) mediates vitamin C uptake. Vitamin C uptake is mediated by SVCT2, which increases the proliferation of ASCs. (A) Fluorescence microscopy showed that FITC-labeled vitamin C was effectively incorporated into ASCs. The vitamin C signal (green) was high in the cytosol, but not in the nucleus (blue). (B) Fluorescence intensity decreased in the presence of unlabeled vitamin C, which suggests the existence of a vitamin C transporter. (C) The intracellular vitamin C concentration was measured using a vitamin C radical assay kit, and a siRNA specific for SVCT2 (20 nM) was transfected into ASCs. SVCT2 knockdown significantly reduced the intracellular vitamin C concentration in ASCs. (D) In addition, transfection of SVCT2 siRNA attenuated the vitamin C-induced proliferation of ASCs. Data are mean±STD (n.s., not significant; **P<0.01, Student's t-test). Color images available online at www.liebertpub.com/scd
To determine the involvement of SVCT2 in vitamin C transport, we measured the intracellular vitamin C concentration in ASCs, and then siRNA was used to inhibit candidate vitamin C transporters. SVCT2 siRNA was transfected into ASCs, and SVCT2 knockdown significantly reduced the intracellular vitamin C radical concentration in ASCs (Fig. 2C, P<0.01). In addition, we investigated whether or not SVCT2 mediates the proliferation of ASCs. As expected, transfection of SVCT2 siRNA attenuated the vitamin C-induced proliferation (Fig. 2D) of ASCs (P<0.01). These results collectively indicate that cellular uptake of vitamin C is mediated by SVCT2, which is involved in the proliferation of ASCs.
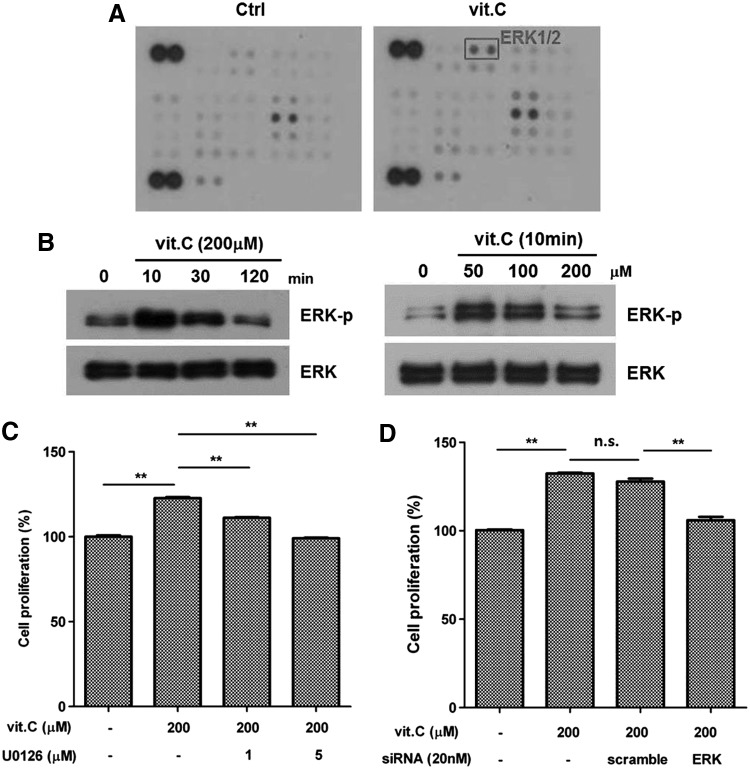
Vitamin C induces MAPK pathway
We attempted to identify the signaling pathway activated by vitamin C in ASCs using a phospho-kinase array. Although AMPK, stat3, and MSK1/2 were marginally induced, ERK1/2 was significantly induced by vitamin C (200 μM, 10 min) treatment (Fig. 3A). Therefore, we confirmed the phosphorylation of ERK1/2 by western blot analysis, and found that it was induced by vitamin C treatment in a time- and dose-dependent manner (Fig. 3B). Treatment of ASCs with a MAPK pathway inhibitor (U0126, 1–5 μM) reduced cell proliferation in a dose-dependent manner (Fig. 3C, P<0.01), indicating that this pathway plays a role in vitamin C-mediated stimulation of ASCs. Using ERK1 siRNA (20 nM), we also found that ERK1 knockdown attenuated the vitamin C-induced proliferation of ASCs (Fig. 3D, P<0.01). These results suggest that vitamin C primarily increases the proliferation of ASCs through the MAPK signaling pathway.
FIG. 3.
Vitamin C induces the mitogen-activated protein kinase (MAPK) pathway. Vitamin C increased the phosphorylation of ERK1/2, and inhibition of the MAPK pathway attenuates the proliferation of ASCs. (A) In a phospho-kinase array, the ERK1/2 signal was highly induced by vitamin C treatment (200 μM for 10 min). (B) Phosphorylation of ERK1/2 increased in a time- and dose-dependent manner. (C) Treatment with a MAPK pathway inhibitor (U0216, 1–5 μM) reduced the vitamin C-induced proliferation of ASCs. (D) In addition, inhibition of ERK1 using siRNA (20 nM) reduced the vitamin C-induced proliferation of ASCs. Data are mean±STD (n.s., not significant; **P<0.01, Student's t-test).
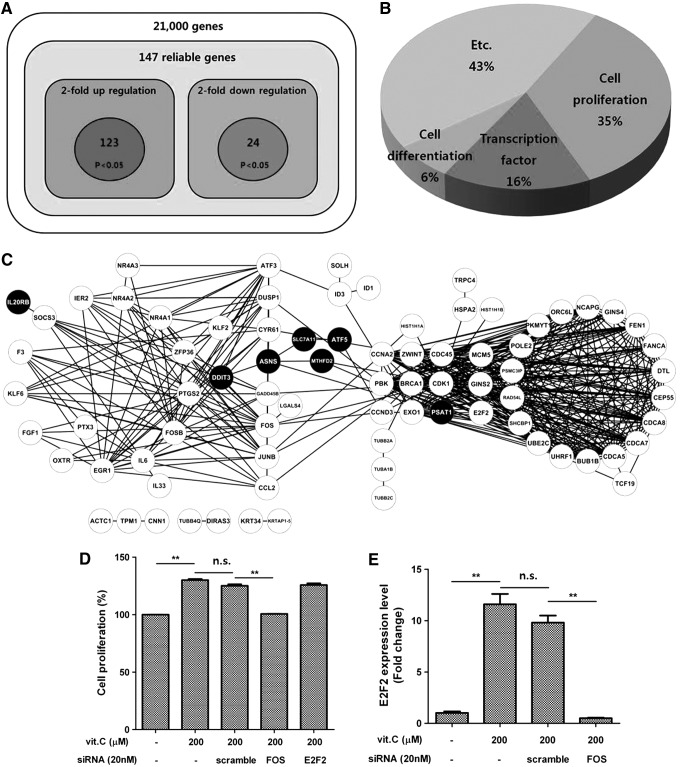
Vitamin C upregulates proliferation-related genes
Microarray analysis was used to clarify the vitamin C-regulated genes that mediate ASC proliferation (Fig. 4A). Vitamin C significantly upregulated 123 genes (>2-fold, P<0.05), and downregulated 24 genes (<2-fold, P<0.05; Fig. 4B) in ASCs. Accordingly, we selected 10 upregulated genes, and confirmed their upregulation using QPCR. As shown in Table 2, mRNA expression of Fos, E2F2, Mybl1, Cdc45, Junb, Cdca5, Fosb, and Ier2 was significantly increased by vitamin C treatment. Analysis of GO terms using the DEGs demonstrated that genes related to cell proliferation (35%), transcription (16%), and differentiation (6%) were primarily upregulated by vitamin C treatment (Fig. 4B). In addition, a possible interaction network among the DEGs related with cell proliferation (Fig. 4C) was analyzed by STRING 9.05 (http://string.embl.de) and plotted using Cytoscape 2.8.3. The predicted network diagram is well-organized, which indicates that the primary role of vitamin C in ASCs is the induction of cell cycle and proliferation.
FIG. 4.
Vitamin C upregulates proliferation-related genes. Microarray was used to clarify the vitamin C target genes that mediate ASC stimulation, and Fos was identified as a key transcription factor. (A) Vitamin C treatment significantly upregulated 123 genes (>2-fold, P<0.05), but downregulated 24 genes. (B) Analysis of gene ontology using the differentially expressed genes (DEGs) demonstrated that genes related to cell proliferation (35%), transcription (16%), and differentiation were primarily upregulated by vitamin C treatment. (C) Using these altered proliferation-related genes, a possible interaction network among the DEGs was analyzed by STRING 9.05 (http://string.embl.de) and plotted using Cytoscape 2.8.3. White circles indicate the upregulated genes, while the black circles indicate the downregulated genes. (D) Transfection of Fos siRNA significantly reduced the proliferation of ASCs, whereas transfection of E2F2 did not. (E) Further, Fos silencing reduced the vitamin C-induced E2F2 mRNA expression. Data are mean±STD (n.s., not significant; **P<0.01, Student's t-test).
Table 2.
List of Differentially Expressed Genes
| Real-time PCR (fold change) | |||
|---|---|---|---|
| Genes | 4 h | Microarray (fold change) 24 h | 24 h |
| FOS | 4.60 (±0.54) | 2.27 (±0.13) | 147.69 |
| E2F2 | 6.85 (±1.61) | 22.6 (±3.21) | 8.45 |
| MYBL1 | 1.58 (±0.33) | 1.71 (±0.56) | 2.33 |
| CDC45 | 2.32 (±0.46) | 1.61 (±0.09) | 5.07 |
| JUNB | 2.17 (±0.49) | 1.34 (±0.46) | 2.52 |
| CDCA5 | 1.93 (±0.31) | 2.56 (±0.67) | 2.09 |
| FOSB | 2.83 (±0.34) | 1.86 (±0.58) | 27.05 |
| IER2 | 1.70 (±0.08) | 1.73 (±0.17) | 3.11 |
PCR, polymerase chain reaction.
Vitamin C upregulates Fos
Because the mRNA expression of two transcription factors (E2F2 and Fos) was strongly increased as indicated by QPCR (Table 2), we investigated the involvement of these genes in ASC proliferation. Although transfection of E2F2 siRNA did not attenuate ASC proliferation, Fos siRNA significantly reduced ASC proliferation (Fig. 4D, P<0.01). Pharmacological inhibition of MAPK pathway by U0126 significantly reduced the mRNA expression of Fos (Supplementary Fig. S2), which suggests that Fos expression is regulated by the MAPK pathway. Further, Fos silencing reduced the vitamin C-induced E2F2 mRNA expression (Fig. 4E, P<0.01).
Vitamin C induces the hair-regenerative potential of ASCs
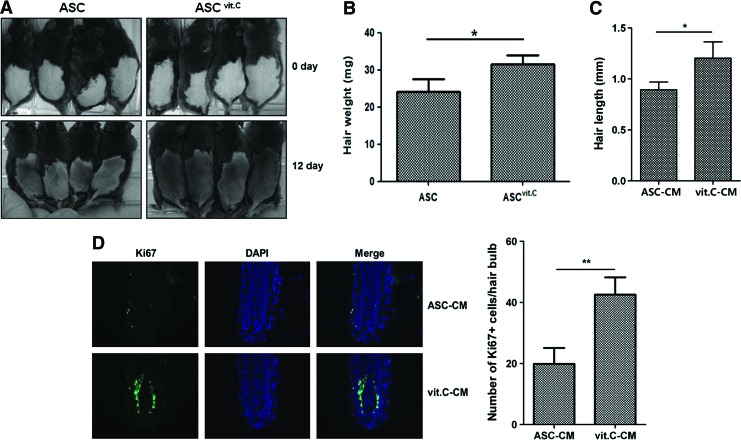
Next, we examined the ability of vitamin C-treated ASCs and the CM from these cells to enhance hair generation in vivo and in vitro. Compared with the injection of ASCs (1×104), subcutaneous injection of vitamin C-treated ASCs (ASCvit.C) accelerated the telogen-to-anagen transition in C3H mice (Fig. 5A, n=6 for each group). We measured the regenerated hair weight on day 12, and found that hair weight was significantly increased in the ASCvit.C-injected group (Fig. 5B, P<0.05).
FIG. 5.
Vitamin C induces the hair-regenerative potential of ASCs. Vitamin C-treated ASCs (ASCvit.C) induced the telogen-to-anagen transition in C3H/HeN mice, and ASC-CM harvested after vitamin C treatment (vit.C-CM) increased hair length and the number of Ki67-positive cells in hair organ culture. (A) Compared with ASC injection (1×104 cells), subcutaneous injection of ASCvit.C accelerated the telogen-to-anagen transition in C3H/HeN mice. (B) Hair weight in C3H mice was significantly increased in the ASCvit.C-injected group on day 12. (C) Ex vivo hair organ culture was performed after the addition of conditioned medium derived from ASCs (ASC-CM) or vit.C-CM to William's E medium. Compared with hair length in ASC-CM culture, hair length was significantly increased in vit.C-CM-enriched medium after 6 days of organ culture. (D) Anti-Ki67 antibody staining of hair follicles showed that the number of Ki67-positive matrix keratinocytes around the dermal papilla was significantly increased by vit.C-CM treatment. Data are mean±SEM (*P<0.05, **P<0.01, Student's t-test). Color images available online at www.liebertpub.com/scd
As the increased secretion of growth factors could lead to enhancement of hair regeneration, we compared the hair-regenerative potential between ASC-CM and that from vitamin C-treated ASCs (vit.C-CM). After the addition of ASC-CM and vit.C-CM to William's E medium, an ex vivo hair organ culture was performed. After 6 days of culture, hair length was significantly increased in vit.C-CM-enriched medium, compared with ASC-CM (Fig. 5C, P<0.05). In addition, anti-Ki67 antibody staining (a marker of proliferating cells) of hair follicles showed that the number of Ki67-positive matrix keratinocytes around the dermal papilla was significantly increased by vit.C-CM treatment (Fig. 5D, n=7 for each group; P<0.01).
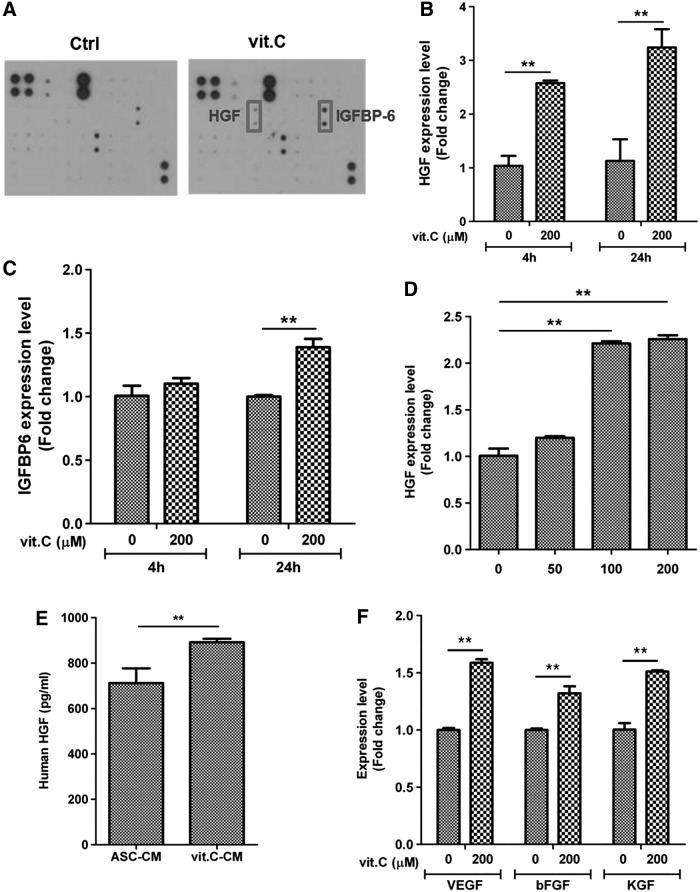
Vitamin C upregulates growth factor expression and enhances hair growth
As hair regeneration is mainly mediated by paracrine factors, we attempted to identify the key secretory factors that mediate hair growth. Using a growth factor antibody array, we investigated which growth factors were induced by vitamin C treatment in ASCs. Of the 41 growth factors tested, expression of HGF and insulin-like growth factor binding protein 6 (IGFBP6) was upregulated by vitamin C treatment (Fig. 6A). We then confirmed the upregulation of these genes by QPCR. Although the expression of HGF mRNA was significantly increased by vitamin C treatment at 4 and 24 h (200 μM, P<0.01; Fig. 6B), IGFBP6 mRNA expression was only slightly upregulated at 24 h (Fig. 6C, P<0.01). Vitamin C treatment induced HGF mRNA expression in a dose-dependent manner at 24 h (Fig. 6D, P<0.01). In addition, we measured the concentration of HGF protein in ASC-CM and vit.C-CM using an ELISA. The protein concentration of HGF was ∼30% higher in vit.C-CM than ASC-CM (Fig. 6E). Further, we measured the mRNA expression of VEGF, bFGF, and keratinocyte growth factor (KGF) in ASCs, and found that the expression increased 4 h after vitamin C treatment (Fig. 6F, P<0.01). These growth factors might function together to increase the hair-regenerative potential of ASCs.
FIG. 6.
Vitamin C upregulates growth factor expression. The expression of diverse growth factors was induced by vitamin C treatment, and they collectively enhanced the hair growth promoting effect of ASCs. (A) In a growth factor antibody array, the expression of hepatocyte growth factor (HGF) and insulin-like growth factor binding protein 6 (IGFBP6) protein was increased by vitamin C treatment. (B) QPCR showed that the expression of HGF mRNA was significantly induced at 4 and 24 h after vitamin C (200 μM) treatment. (C) IGFBP6 mRNA was slightly upregulated at 24 h. (D) Vitamin C treatment induced HGF mRNA expression in a dose-dependent manner at 24 h. (E) In addition, an ELISA measure of HGF protein levels in CM showed that HGF secretion was ∼30% higher in vit.C-CM than in ASC-CM. (F) mRNA expression of VEGF, bFGF, and KGF increased significantly after 4 h of vitamin C treatment. Data are mean±STD (**P<0.01, Student's t-test).
Since HGF has been shown to be involved in hair regeneration [33–35], and HGF mRNA expression is significantly upregulated by vitamin C, we investigated whether HGF treatment induces hair growth in hair organ culture. As expected, HGF (1 and 10 ng/mL) treatment increased hair length (Supplementary Fig. S3A), and increased the number of Ki67-positive matrix keratinocytes around the dermal papilla in hair organ culture (Supplementary Fig. S3B). We also investigated whether or not HGF expression was regulated by the MAPK pathway, but pharmacological inhibition by U0126 only slightly reduced HGF expression (Supplementary Fig. S2B).
Discussion
Previous studies have reported the proliferative and reprogramming effects of vitamin C on stem cells [36–38]; however, the molecular mechanism underlying vitamin C-induced stem cell stimulation has not been fully elucidated. Here, we demonstrated that vitamin C induces the proliferation, and vitamin C preconditioning enhanced the hair-regenerative potential of ASCs. We investigated the cellular and molecular mechanisms underlying vitamin C-induced ASC stimulation, and found that cellular uptake of vitamin C is mediated by SVCT2, and that vitamin C activates MAPK signaling. Vitamin C upregulated Fos, E2F2, Ier2, Mybl1, Cdc45, JunB, FosB, and Cdca5 mRNA levels, and the transcription factor Fos plays a key role in ASC proliferation. Moreover, vitamin C increased the expression of HGF, IGFBP6, VEGF, bFGF, and KGF, which collectively enhanced the hair-regenerative potential of ASCs. These signaling and molecular changes induced by vitamin C treatment enhance ASC proliferation and regenerative potential.
At high concentrations, vitamin C is cytotoxic to many types of cancer cells due to cellular ROS generation and significantly impedes tumor progression in vivo without being toxic to normal tissues [39,40]. In addition, vitamin C cytotoxicity in cancer cells was ameliorated by the addition of catalase and ROS scavengers [40]. In a previous study, we found that the generation of low-level or mild ROS in ASCs increased their proliferation and migration via the MAPK and PI3K/Akt signal pathways [16–18]. Therefore, we hypothesized that physiological levels of vitamin C could stimulate ASCs through ROS generation. However, in our experiment, low concentration of vitamin C did not act as an ROS generator, but instead reduced ROS generation in ASCs. Therefore, vitamin C protected ASCs from oxidative stresses such as H2O2 and UVB irradiation (Fig. 1C, D). Because vitamin C could act as either a pro-oxidant or antioxidant depending on its concentration, we should be careful to interpret the redox regulation of vitamin C in ASCs.
We previously demonstrated that miR-210 upregulation plays a key role in ASC stimulation under hypoxia and that miR-210 upregulation downregulates Ptpn2 [16], which is involved in the proliferation of ASCs. Therefore, we investigated whether vitamin C could increase miR-210 levels in a preliminary study, and found that vitamin C treatment slightly increased miR-210 levels. However, compared with hypoxia, which increased miR-210 more than 4-fold, miR-210 upregulation following vitamin C treatment was very low (Supplementary Fig. S4). In addition, we used a microRNA array to investigate which microRNA(s) is/are involved in ASC stimulation by vitamin C; however, we did not find any differentially expressed microRNAs in our experiment. Therefore, further studies are needed on the microRNA regulation.
The Fos gene family consists of four members: Fos, FosB, FosL1, and FosL2. Fos genes encode leucine zipper proteins that can dimerize with proteins of the Jun family, and form the transcription factor complex AP-1 [41,42]. Consequently, Fos proteins have been implicated as regulators of cell proliferation, differentiation, apoptosis, and transformation. In this study, we found that vitamin C treatment upregulated Fos mRNA in addition to both FosB and JunB mRNA (Table 2). Therefore, it is reasonable to conclude that vitamin C induces the AP-1 transcription factor complex in ASCs, and thus increases their proliferation by upregulating cell-cycle-regulating genes. For example, vitamin C-induced Fos upregulation is involved in E2F2 expression (Fig. 4E), which may contribute to the cell cycle progression of ASCs. In addition, we found that inhibition of the MAPK pathway using U0126 significantly reduced the mRNA expression of Fos. Therefore, the SVCT2/ERK/Fos axis plays a pivotal role in vitamin C-induced proliferation of ASCs.
In this study, we compared the stimulatory effect of vitamin C and its derivatives on ASCs. In addition to the proliferation and survival of ASCs, we measured the expression of SVCT2, Fos, and HGF mRNA after treatment with vitamin C derivatives (Supplementary Fig. S1). Although 2-phospho-l-ascorbic acid and dehydro-l(+)-ascorbic acid slightly upregulated the expression of SVCT2 mRNA, vitamin C treatment strongly upregulated SVCT2, Fos, and HGF expression in ASCs. Taken together with the proliferation and survival results, it is reasonable to conclude that vitamin C has the strongest stimulatory effects on ASCs.
Growth factors have diverse effects on the self-renewal capability and proliferation of ASCs [16,43,44]. In a previous study, we tested and compared the proliferation-promoting effects of growth factors, and we showed that PDGF-B and PDGF-D have strongest effects on ASC proliferation during short-term culture [16,43]. bFGF acts as a self-renewal factor, increases the proliferation of ASCs, and facilitates long-term culture (more than 20 passages without senescence) [44]. However, recombinant HGF treatment (<10 ng/mL) did not induce the proliferation of ASCs (data not shown). In the present study, we also compared the proliferating effect of vitamin C to that of other growth factors (Supplementary Fig. S5). Although more investigation is required to identify the growth factor that is directly related to ASC proliferation, vitamin C (200 μM) increased ASC proliferation comparable to bFGF (5 ng/mL). However, the effect of PDGF-B and PDGF-D on ASC proliferation was superior to that of vitamin C. Considering that vitamin C is safe and very inexpensive, it is a promising option for a culture medium supplement to increase ASC proliferation.
The regenerative potential of ASCs and the paracrine effect of ASCs differ according to the stimulators used [12,15,45–47]. For example, hypoxia upregulated VEGF and bFGF, which enhanced wound healing and hair-regenerative potential of ASCs [12]. Low-dose UVB irradiation also upregulated bFGF, KGF, VEGF, and HGF, which enhanced the hair-regenerative potential of ASCs [15]. In addition, pharmacological priming of ASCs using deferoxamine induces HIF-1α and VEGF expression, and enhanced the migration and in vitro wound-healing activities of endothelial cells [47]. In the present study, we showed that vitamin C primarily increased HGF, IGFBP6, VEGF, bFGF, and KGF, which collectively enhanced the hair-regenerative potential of ASCs.
In summary, vitamin C increases ASC proliferation, and vitamin C preconditioning enhances the hair growth promoting effect of ASCs in vivo. Vitamin C is transported via SVCT2, activates MAPK signaling, and upregulates Fos to increase the proliferation of ASCs. In addition, vitamin C upregulates the expression of HGF, IGFBP6, VEGF, bFGF, and KGF to enhance the paracrine effect of ASCs. Vitamin C is very inexpensive, safe, and easy to handle. Therefore, vitamin C, either alone or in combination with growth factors, could be used as a supplement for ASC cultivation.
Supplementary Material
Acknowledgments
This research was supported by the Bio & Medical Technology Development Program of the National Research Foundation (NRF) funded by the Korean government (MEST) (2011-0019634 and 2011-0019636). Sun U. Song was supported by the Korea Healthcare Technology R&D Project, Ministry for Health, Welfare & Family Affairs (A110076).
Author Disclosure Statement
The authors declare no competing financial interests.
References
- 1.Kaewsuwan S, Song SY, Kim JH. and Sung JH. (2012). Mimicking the functional niche of adipose-derived stem cells for regenerative medicine. Expert Opin Biol Ther 12:1575–1588 [DOI] [PubMed] [Google Scholar]
- 2.Mendel TA, Clabough EB, Kao DS, Demidova-Rice TN, Durham JT, Zotter BC, Seaman SA, Cronk SM, Rakoczy EP, et al. (2013). Pericytes derived from adipose-derived stem cells protect against retinal vasculopathy. PLoS One 8:e65691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zuk PA, Zhu M, Ashjian P, De Ugarte DA, Huang JI, Mizuno H, Alfonso ZC, Fraser JK, Benhaim P. and Hedrick MH. (2002). Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell 13:4279–4295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim WS, Park BS, Sung JH, Yang JM, Park SB, Kwak SJ. and Park JS. (2007). Wound healing effect of adipose-derived stem cells: a critical role of secretory factors on human dermal fibroblasts. J Dermatol Sci 48:15–24 [DOI] [PubMed] [Google Scholar]
- 5.Song SY, Chung HM. and Sung JH. (2010). The pivotal role of VEGF in adipose-derived-stem-cell-mediated regeneration. Expert Opin Biol Ther 10:1529–1537 [DOI] [PubMed] [Google Scholar]
- 6.Yang JA, Chung HM, Won CH. and Sung JH. (2010). Potential application of adipose-derived stem cells and their secretory factors to skin: discussion from both clinical and industrial viewpoints. Expert Opin Biol Ther 10:495–503 [DOI] [PubMed] [Google Scholar]
- 7.Mazo M, Cemborain A, Gavira JJ, Abizanda G, Arana M, Casado M, Soriano M, Hernandez S, Moreno C, et al. (2012). Adipose stromal vascular fraction improves cardiac function in chronic myocardial infarction through differentiation and paracrine activity. Cell Transplant 21:1023–1037 [DOI] [PubMed] [Google Scholar]
- 8.Gimble JM, Bunnell BA, Chiu ES. and Guilak F. (2011). Concise review: adipose-derived stromal vascular fraction cells and stem cells: let's not get lost in translation. Stem Cells 29:749–754 [DOI] [PubMed] [Google Scholar]
- 9.Mizuno H, Tobita M. and Uysal AC. (2012). Concise review: adipose-derived stem cells as a novel tool for future regenerative medicine. Stem Cells 30:804–810 [DOI] [PubMed] [Google Scholar]
- 10.Rota C, Imberti B, Pozzobon M, Piccoli M, De Coppi P, Atala A, Gagliardini E, Xinaris C, Benedetti V, et al. (2012). Human amniotic fluid stem cell preconditioning improves their regenerative potential. Stem Cells Dev 21:1911–1923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Francis KR. and Wei L. (2010). Human embryonic stem cell neural differentiation and enhanced cell survival promoted by hypoxic preconditioning. Cell Death Dis 1:e22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee EY, Xia Y, Kim WS, Kim MH, Kim TH, Kim KJ, Park BS. and Sung JH. (2009). Hypoxia-enhanced wound-healing function of adipose-derived stem cells: increase in stem cell proliferation and up-regulation of VEGF and bFGF. Wound Repair Regen 17:540–547 [DOI] [PubMed] [Google Scholar]
- 13.Rehman J, Traktuev D, Li J, Merfeld-Clauss S, Temm-Grove CJ, Bovenkerk JE, Pell CL, Johnstone BH, Considine RV. and March KL. (2004). Secretion of angiogenic and antiapoptotic factors by human adipose stromal cells. Circulation 109:1292–1298 [DOI] [PubMed] [Google Scholar]
- 14.Thangarajah H, Vial IN, Chang E, El-Ftesi S, Januszyk M, Chang EI, Paterno J, Neofytou E, Longaker MT. and Gurtner GC. (2009). IFATS collection: Adipose stromal cells adopt a proangiogenic phenotype under the influence of hypoxia. Stem Cells 27:266–274 [DOI] [PubMed] [Google Scholar]
- 15.Jeong YM, Sung YK, Kim WK, Kim JH, Kwack MH, Yoon I, Kim DD. and Sung JH. (2013). Ultraviolet B preconditioning enhances the hair growth-promoting effects of adipose-derived stem cells via generation of reactive oxygen species. Stem Cells Dev 22:158–168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim JH, Park SG, Song SY, Kim JK. and Sung JH. (2013). Reactive oxygen species-responsive miR-210 regulates proliferation and migration of adipose-derived stem cells via PTPN2. Cell Death Dis 4:e588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim JH, Park SH, Park SG, Choi JS, Xia Y. and Sung JH. (2011). The pivotal role of reactive oxygen species generation in the hypoxia-induced stimulation of adipose-derived stem cells. Stem Cells Dev 20:1753–1761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim JH, Song SY, Park SG, Song SU, Xia Y. and Sung JH. (2012). Primary involvement of NADPH oxidase 4 in hypoxia-induced generation of reactive oxygen species in adipose-derived stem cells. Stem Cells Dev 21:2212–2221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Esteban MA. and Pei D. (2012). Vitamin C improves the quality of somatic cell reprogramming. Nat Genet 44:366–367 [DOI] [PubMed] [Google Scholar]
- 20.Yin R, Mao SQ, Zhao B, Chong Z, Yang Y, Zhao C, Zhang D, Huang H, Gao J, et al. (2013). Ascorbic acid enhances Tet-mediated 5-methylcytosine oxidation and promotes DNA demethylation in mammals. J Am Chem Soc 135:10396–10403 [DOI] [PubMed] [Google Scholar]
- 21.Bartsch C, Bekhite MM, Wolheim A, Richter M, Ruhe C, Wissuwa B, Marciniak A, Muller J, Heller R, et al. (2011). NADPH oxidase and eNOS control cardiomyogenesis in mouse embryonic stem cells on ascorbic acid treatment. Free Radic Biol Med 51:432–443 [DOI] [PubMed] [Google Scholar]
- 22.Potdar PD. and D'Souza SB. (2010). Ascorbic acid induces in vitro proliferation of human subcutaneous adipose tissue derived mesenchymal stem cells with upregulation of embryonic stem cell pluripotency markers Oct4 and SOX 2. Hum Cell 23:152–155 [DOI] [PubMed] [Google Scholar]
- 23.Lin TM, Tsai JL, Lin SD, Lai CS. and Chang CC. (2005). Accelerated growth and prolonged lifespan of adipose tissue-derived human mesenchymal stem cells in a medium using reduced calcium and antioxidants. Stem Cells Dev 14:92–102 [DOI] [PubMed] [Google Scholar]
- 24.Kim WS, Park BS, Park SH, Kim HK. and Sung JH. (2009). Antiwrinkle effect of adipose-derived stem cell: activation of dermal fibroblast by secretory factors. J Dermatol Sci 53:96–102 [DOI] [PubMed] [Google Scholar]
- 25.Kim SY, Kwon YW, Jung IL, Sung JH. and Park SG. (2011). Tauroursodeoxycholate (TUDCA) inhibits neointimal hyperplasia by suppression of ERK via PKCalpha-mediated MKP-1 induction. Cardiovasc Res 92:307–316 [DOI] [PubMed] [Google Scholar]
- 26.Muller-Rover S, Handjiski B, van der Veen C, Eichmuller S, Foitzik K, McKay IA, Stenn KS. and Paus R. (2001). A comprehensive guide for the accurate classification of murine hair follicles in distinct hair cycle stages. J Invest Dermatol 117:3–15 [DOI] [PubMed] [Google Scholar]
- 27.Kim WS, Park BS, Kim HK, Park JS, Kim KJ, Choi JS, Chung SJ, Kim DD. and Sung JH. (2008). Evidence supporting antioxidant action of adipose-derived stem cells: protection of human dermal fibroblasts from oxidative stress. J Dermatol Sci 49:133–142 [DOI] [PubMed] [Google Scholar]
- 28.Kwack MH, Shin SH, Kim SR, Im SU, Han IS, Kim MK, Kim JC. and Sung YK. (2009). l-Ascorbic acid 2-phosphate promotes elongation of hair shafts via the secretion of insulin-like growth factor-1 from dermal papilla cells through phosphatidylinositol 3-kinase. Br J Dermatol 160:1157–1162 [DOI] [PubMed] [Google Scholar]
- 29.Kwack MH, Sung YK, Chung EJ, Im SU, Ahn JS, Kim MK. and Kim JC. (2008). Dihydrotestosterone-inducible dickkopf 1 from balding dermal papilla cells causes apoptosis in follicular keratinocytes. J Invest Dermatol 128:262–269 [DOI] [PubMed] [Google Scholar]
- 30.Harrison FE, Dawes SM, Meredith ME, Babaev VR, Li L. and May JM. (2010). Low vitamin C and increased oxidative stress and cell death in mice that lack the sodium-dependent vitamin C transporter SVCT2. Free Radic Biol Med 49:821–829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rajan DP, Huang W, Dutta B, Devoe LD, Leibach FH, Ganapathy V. and Prasad PD. (1999). Human placental sodium-dependent vitamin C transporter (SVCT2): molecular cloning and transport function. Biochem Biophys Res Commun 262:762–768 [DOI] [PubMed] [Google Scholar]
- 32.Wang H, Dutta B, Huang W, Devoe LD, Leibach FH, Ganapathy V. and Prasad PD. (1999). Human Na(+)-dependent vitamin C transporter 1 (hSVCT1): primary structure, functional characteristics and evidence for a non-functional splice variant. Biochim Biophys Acta 1461:1–9 [DOI] [PubMed] [Google Scholar]
- 33.Jindo T, Tsuboi R, Imai R, Takamori K, Rubin JS. and Ogawa H. (1994). Hepatocyte growth factor/scatter factor stimulates hair growth of mouse vibrissae in organ culture. J Invest Dermatol 103:306–309 [DOI] [PubMed] [Google Scholar]
- 34.Jindo T, Tsuboi R, Takamori K. and Ogawa H. (1998). Local injection of hepatocyte growth factor/scatter factor (HGF/SF) alters cyclic growth of murine hair follicles. J Invest Dermatol 110:338–342 [DOI] [PubMed] [Google Scholar]
- 35.Shimaoka S, Tsuboi R, Jindo T, Imai R, Takamori K, Rubin JS. and Ogawa H. (1995). Hepatocyte growth factor/scatter factor expressed in follicular papilla cells stimulates human hair growth in vitro. J Cell Physiol 165:333–338 [DOI] [PubMed] [Google Scholar]
- 36.Chen J, Guo L, Zhang L, Wu H, Yang J, Liu H, Wang X, Hu X, Gu T, et al. (2013). Vitamin C modulates TET1 function during somatic cell reprogramming. Nat Genet 45:1504–1509 [DOI] [PubMed] [Google Scholar]
- 37.Shi Y, Zhao Y. and Deng H. (2010). Powering reprogramming with vitamin C. Cell Stem Cell 6:1–2 [DOI] [PubMed] [Google Scholar]
- 38.Wang T, Chen K, Zeng X, Yang J, Wu Y, Shi X, Qin B, Zeng L, Esteban MA, Pan G. and Pei D. (2011). The histone demethylases Jhdm1a/1b enhance somatic cell reprogramming in a vitamin-C-dependent manner. Cell Stem Cell 9:575–587 [DOI] [PubMed] [Google Scholar]
- 39.Chen Q, Espey MG, Sun AY, Lee JH, Krishna MC, Shacter E, Choyke PL, Pooput C, Kirk KL, Buettner GR. and Levine M. (2007). Ascorbate in pharmacologic concentrations selectively generates ascorbate radical and hydrogen peroxide in extracellular fluid in vivo. Proc Natl Acad Sci U S A 104:8749–8754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen Q, Espey MG, Sun AY, Pooput C, Kirk KL, Krishna MC, Khosh DB, Drisko J. and Levine M. (2008). Pharmacologic doses of ascorbate act as a prooxidant and decrease growth of aggressive tumor xenografts in mice. Proc Natl Acad Sci U S A 105:11105–11109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chiu R, Boyle WJ, Meek J, Smeal T, Hunter T. and Karin M. (1988). The c-Fos protein interacts with c-Jun/AP-1 to stimulate transcription of AP-1 responsive genes. Cell 54:541–552 [DOI] [PubMed] [Google Scholar]
- 42.Curran T. and Franza BR., Jr. (1988). Fos and Jun: the AP-1 connection. Cell 55:395–397 [DOI] [PubMed] [Google Scholar]
- 43.Kang YJ, Jeon ES, Song HY, Woo JS, Jung JS, Kim YK. and Kim JH. (2005). Role of c-Jun N-terminal kinase in the PDGF-induced proliferation and migration of human adipose tissue-derived mesenchymal stem cells. J Cell Biochem 95:1135–1145 [DOI] [PubMed] [Google Scholar]
- 44.Zaragosi LE, Ailhaud G. and Dani C. (2006). Autocrine fibroblast growth factor 2 signaling is critical for self-renewal of human multipotent adipose-derived stem cells. Stem Cells 24:2412–2419 [DOI] [PubMed] [Google Scholar]
- 45.Chung HM, Won CH. and Sung JH. (2009). Responses of adipose-derived stem cells during hypoxia: enhanced skin-regenerative potential. Expert Opin Biol Ther 9:1499–1508 [DOI] [PubMed] [Google Scholar]
- 46.Zhou J, Lu P, Ren H, Zheng Z, Ji J, Liu H, Jiang F, Ling S, Heng BC, Hu X. and Ouyang H. (2013). 17β-estradiol protects human eyelid-derived adipose stem cells against cytotoxicity and increases transplanted cell survival in spinal cord injury. J Cell Mol Med 18:326–343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liu GS, Peshavariya HM, Higuchi M, Chan EC, Dusting GJ. and Jiang F. (2013). Pharmacological priming of adipose-derived stem cells for paracrine VEGF production with deferoxamine. J Tissue Eng Regen Med. DOI: 10.1002/term.1796 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.