Abstract
Since 2004, the country of Georgia has provided antiretroviral therapy (ART) to all patients in need. A nationwide retrospective cohort study was conducted to assess the effect of universal access to ART on patterns of mortality and causes of death among HIV-infected individuals in Georgia. All known HIV-infected adult individuals (age ≥18 years) diagnosed from 1989 through 2012 were included. Rates and causes of death were determined using routinely collected data from the national HIV/AIDS database. Causes of death were classified according to the Coding of Death in HIV (CoDe) protocol. Between 1989 and 2012, 3,554 HIV-infected adults were registered in Georgia contributing to 13,572 person-years (PY) of follow-up. A total of 779 deaths were registered during follow-up. The mortality rate peaked in 2004 with 10.74 deaths per 100 PY (95% CI: 7.92–14.24) and significantly decreased after the universal availability of ART to 4.02 per 100 PY (95% CI: 3.28–4.87) in 2012. In multivariate analysis the strongest predictor of mortality was having AIDS at the time of HIV diagnosis (hazard ratio: 5.69, 95% CI: 4.72–6.85). AIDS-related diseases accounted for the majority of deaths (n=426, 54.7%). Tuberculosis (TB) was the leading cause of death accounting for 21% of the total deaths reported. Universal access to ART significantly reduced mortality among HIV-infected patients in Georgia. However, overall mortality rates remain high primarily due to late diagnosis, and TB remains a significant cause of death. Improving rates of early HIV diagnosis and ART initiation may further decrease mortality as well as prevent new HIV and TB infections.
Introduction
The rapid expansion of antiretroviral therapy (ART) in resource-limited settings has led to significant survival gains previously seen only in developed countries.1–4 Despite this decline in the number of deaths, mortality rates remain significantly higher in low-income versus high-income countries.5 The timing of widespread ART introduction has also led to differences in the spectrum of cause-specific mortality. In settings where ART has been readily available since its introduction in the mid 1990s, HIV-infected persons are increasingly likely to die from non-HIV-related diseases, while in resource-limited countries AIDS-related events remain the primary cause of death.6
Georgia is a small Eastern European nation (population 4.5 million) located between Russia and Turkey with an HIV epidemic driven primarily by injection drug use (IDU). As of December 31, 2012, 3,642 cases of HIV infections have been registered in the national HIV/AIDS database of Georgia, including 3,554 cases among persons at least 18 years of age. The estimated adult HIV prevalence is 0.3%.7 While antiretroviral drugs have been available in the country since the 1990s, access was limited only to those able to afford them. In 2004, through support from the Global Fund to Fight AIDS, Tuberculosis and Malaria, Georgia ensured universal access to ART for all eligible patients in accordance with existing guidelines.8
With the widespread availability of ART, it is expected that HIV-positive persons in Georgia will live longer and, similar to industrialized countries, be at increased risk for non-AIDS-related morbidity and mortality. Therefore, monitoring causes of death can help to define priorities in the prevention and management of HIV and comorbidities. The objective of this analysis was to evaluate the effect of universal access to ART on temporal trends in mortality and causes of death.
Materials and Methods
Study design and setting
A nationwide retrospective cohort study design was utilized. All HIV-infected adults (age ≥18 years) diagnosed in Georgia from the start of the epidemic in 1989 through 2012 were included. The Infectious Diseases, AIDS and Clinical Immunology Research Center (National AIDS Center) located in Tbilisi, Georgia has coordinated the National HIV/AIDS Treatment and Care Program since its inception. The National AIDS Center was the sole provider of HIV clinical care services in Georgia until 2006 when three affiliated regional HIV clinics opened. All individuals with confirmed HIV diagnoses are first seen at the National AIDS Center, after which they have the option to continue clinical care at the National Center or transfer care to a regional facility.
For the majority of the study period ART was recommended for patients with a CD4 count <200 cells/mm3 or an AIDS-defining illness. In 2012, Georgia completed a phased implementation of the 2010 WHO guidelines changing ART initiation recommendations to all patients with a CD4 count <350 cells/mm3. Antiretroviral drugs are dispensed at the National AIDS Center and affiliated regional centers on a monthly basis. All HIV-related medical services are provided free of charge. The services provided to patients have been expanding over time and currently, in addition to ART, include out-patient and in-patient care for HIV-associated conditions, laboratory monitoring (including CD4 count, viral load, HIV drug resistance for those failing on ART), prevention, and treatment of opportunistic infections.
A strong referral pathway between the HIV/AIDS and other health services, particularly with closely related fields such as drug abuse and tuberculosis (TB), ensures effective navigation of patients within the Georgian healthcare system. Collaborative TB/HIV activities include symptom screening of all HIV patients for TB at dedicated AIDS facilities, followed by culture confirmation of suspected cases in dedicated TB facilities and, if confirmed, TB drug sensitivity testing to select an appropriate treatment regimen.
To enhance linkage to and retention in clinical care, the National AIDS Center utilizes patient education, counseling, adherence monitoring and support, as well as active case follow-up through phone contact and/or outreach. Patient contact information is regularly updated and is used to contact patients or close contacts when needed in an effort to minimize noncompliance with clinic visits and loss to follow-up.
Data collection
The study used regularly collected data from the national HIV/AIDS database. The National AIDS Center has maintained and operated a national HIV/AIDS database since the first case of HIV was reported in Georgia in 1989. The database is a secure web-based system, allowing for real-time data entry for all HIV-infected patients at all clinical sites. Demographic, epidemiologic, clinical, and laboratory data are collected for each patient. Some of the specific information collected and used in the study included date of HIV diagnosis, age, sex, presumed mode of HIV transmission, CD4 cell counts, AIDS-defining illnesses, use of ART, and date of death. Quality control mechanisms including data consistency and missing value checks, as well as verification of entered data against source documents, are performed routinely by the database management team to ensure the accuracy of collected data.
Causes of death
Data from medical records were abstracted by researchers to classify causes of death according to the Coding of Death in HIV (CoDe) protocol.9,10 Results of the CoDe classification were compared to causes of death documented in medical records. The physician is responsible for assigning the underlying cause of death according to the International Classification of Diseases, 10th revision.11 If no information is available on the circumstances of death, verbal autopsies of family members or other reliable informants are performed using WHO recommended methodology12 to ascertain the cause of death. In the case of a discrepancy between the CoDe and medical record, a panel of three reviewers (primary clinician, research clinician, and independent clinician) was assembled to reach a consensus on the primary cause of death. If this could not be achieved the case was classified by majority vote or the case was classified unknown/unclassifiable if the decision of panelists diverged.
Deaths were attributed to AIDS-defining illnesses based on the 1993 CDC classification system.13 In addition, deaths due to visceral leishmaniasis, Hodgkin's lymphoma, and non-Hodgkin's lymphoma of all cell types were classified as AIDS related in accordance with the CoDe protocol. If the cause of death was unable to be classified, but the patient experienced an AIDS-defining illness or had a CD4 count <200 cells/mm3 within 6 months prior to death, the death was classified as AIDS related. All remaining deaths were classified either as non-AIDS-related causes per CoDe protocol or unknown if information on death was insufficient.
Statistical analysis
All statistical analyses were performed using SAS v9.2 (SAS Institute Inc., Cary, NC). Individuals were included starting from the date of HIV diagnosis and were followed until death or December 31, 2012 or 12 months after their last contact with the HIV care system (clinical visit or case follow-up contact), whichever occurred first. Patients were considered lost to follow-up if their status could not be ascertained by available medical and case follow-up records 12 months after their last documented contact with the system. ART was defined as a combination of at least three antiretroviral drugs prescribed solely for the treatment of HIV disease. Patients were defined as having AIDS at baseline if they had an AIDS-defining illness or CD4 count <200 cells/mm3 at entry into clinical care.
Mortality rates were calculated for the total follow-up period and for each calendar year as number of events divided by the number of total person-years (PY) of follow-up contributed to that calendar year. Factors associated with mortality were assessed in Cox proportional hazards regression analysis. The effect of widespread availability of ART on patterns of mortality was assessed by categorizing time periods into “preuniversal access era” (1989–2004) and “universal access era” (2005–2012). Comparisons were tested using Pearson's chi-square or Fisher's exact test as appropriate. All tests were two sided at a significance level of 0.05.
Ethical approval
The study was approved by the institutional review board (IRB) of the IDACIRC, which granted a waiver for patient consent/authorization. Only deidentified data were used in statistical analyses.
Results
Mortality rates and associated factors
A total of 3,554 HIV-positive adults were registered in Georgia from 1989 through 2012 contributing to 13,572 PY of follow-up. The median follow-up was 2.8 years [interquartile range (IQR): 0.9–5.8]. At the end of 2012, 2,378 (66.9%) patients were known to be alive based on clinical and case follow-up records, 779 (21.9%) patients died, and the status could not be ascertained for 397 (11.2%) patients.
Of the 3,554 registered patients 2,611 (73.5%) were men and the median age at HIV diagnosis was 36 years (IQR: 30–42). Among them 54.8% of cases had a history of IDU, 39.6% of patients acquired their infection via heterosexual contact, and 4.3% acquired their infection via male-to-male sex. Among the registered patients, baseline CD4 cell count was available for 2,947 (82.9%) patients and the median value was 238 cells/mm3 (IQR: 106–408). A total of 1,284 (36.1%) patients had an AIDS-defining illness, including 523 (14.7%) cases of TB. Overall, 1,715 (48.3%) patients had AIDS at entry into HIV care, defined as a CD4 count <200 cells/mm3 or the presence of an AIDS-defining illness. Almost half (n=1,680, 47.3%) of the patients had antibodies against hepatitis C virus (HCV) and 221 (6.2%) patients had hepatitis B surface antigen (HBsAg). Information on the stage of liver disease was not available. A small majority of (n=1,877, 52.8%) patients ever received ART.
Temporal comparison of baseline characteristics showed that patients diagnosed in the preuniversal access era (1989–2004) were younger (median 33 years vs. 37 years, p<0.0001), more likely to be male (82.3% vs. 71.6%, p<0.0001), and IDU (68.5% vs. 51.9%, p<0.0001) compared to those diagnosed in the universal access era (2005–2012). The proportion of patients with baseline AIDS increased from 36.8% in the preuniversal access era to 50.7% in the universal access era (p<0.0001). There was also a significant increase in the proportion of patients receiving ART (42.5% vs. 55.1%, p<0.0001). Notably, of 268 patients who were diagnosed in the preuniversal access era and ever received ART, only 33 (12.3%) initiated therapy before 2005.
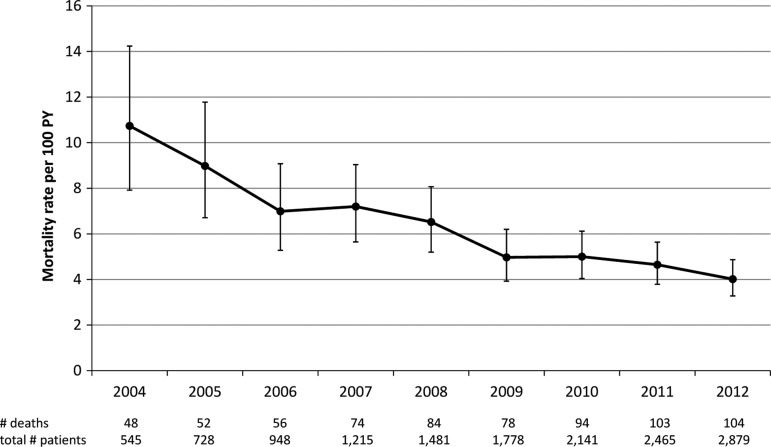
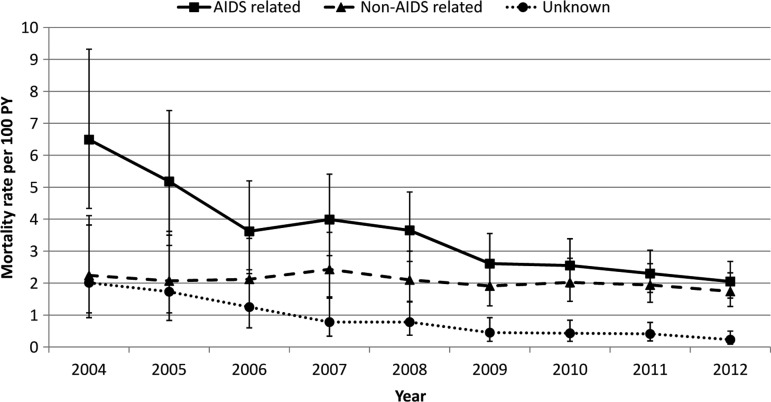
Among the 779 deaths, the median time to death was 6 (IQR: 1–24) months. Twenty-three percent of patients died within 1 month and 51% within 6 months after HIV diagnosis. The cumulative mortality rate was 5.74 per 100 PY [95% confidence interval (CI): 5.34–6.16]. The all-cause mortality rate peaked in 2004 with 10.74 deaths per 100 PY (95% CI: 7.92–14.24) and significantly decreased after the widespread availability of ART to 4.02 per 100 PY (95% CI: 3.28–4.87) reported in 2012 (p<0.0001) (Fig. 1). There was more than a 3-fold decrease in AIDS-related mortality from 6.49 deaths per 100 PY (95% CI: 4.34–9.32) in 2004 to 2.05 deaths per 100 PY (95% CI: 1.53–2.68) in 2012 (p<0.0001). Death due to unknown causes also significantly decreased over this period (p=0.001), while deaths due to non-AIDS causes remained stable (Fig. 2).
FIG. 1.
The rates of all-cause mortality among HIV-infected adults in the country of Georgia, 2004–2012.
FIG. 2.
AIDS-related and non-AIDS-related mortality among HIV-infected adults in the country of Georgia, 2004–2012.
In multivariate Cox regression analysis baseline diagnosis of AIDS [hazard ratio (HR): 5.69, 95% CI: 4.72–6.85] was the strongest predictor of mortality. Increasing age, a history of IDU, and receiving an HIV diagnosis before universal access to ART were also significantly associated with death (Table 1).
Table 1.
Factors Associated with Mortality Among HIV-Infected Adults in the Country of Georgia (1989–2012)
| Univariate | Multivariate | |||
|---|---|---|---|---|
| HR | 95% CI | HR | 95% CI | |
| Age (per year increase) | 1.05 | 1.04–1.06 | 1.03 | 1.02–1.04 |
| Gender | ||||
| Female | 1 | 1 | ||
| Male | 2.16 | 1.77–2.63 | 1.18 | 0.91–1.53 |
| Mode of transmission | ||||
| Non-IDU | 1 | 1 | ||
| IDU | 2.05 | 1.75–2.39 | 1.47 | 1.20–1.80 |
| Period of HIV diagnosis | ||||
| After universal access to ART | 1 | 1 | ||
| Before universal access to ART | 1.10 | 0.93–1.31 | 1.56 | 1.30–1.86 |
| Disease stage at diagnosis | ||||
| HIV | 1 | 1 | ||
| AIDS | 6.39 | 5.33–7.66 | 5.69 | 4.72–6.85 |
HR, hazard ratio; IDU, injection drug use; ART, antiretroviral therapy.
Causes of death
AIDS-related diseases accounted for the majority of deaths (n=426, 54.7%). End-stage liver disease due to chronic viral hepatitis (n=118, 15.1%, including 111 cases due to HCV infection) and non-AIDS-related malignancies (n=40, 5.1%) were the most frequent non-AIDS-related causes of death. Overall, TB was the leading cause of death accounting for 21% of total cases of death. In 92 (11.8%) cases the cause of death was unclassifiable or unknown (Table 2).
Table 2.
Causes of Death Among HIV-Infected Adults in the Country of Georgia, 1989–2012 (n=779)
| Cause (CoDe classification) | n | % |
|---|---|---|
| AIDS | 426 | 54.7 |
| Infection (non-AIDS) | 10 | 1.3 |
| Chronic viral hepatitis | 118 | 15.1 |
| Malignancy (non-AIDS) | 40 | 5.1 |
| Ischemic heart disease | 19 | 2.4 |
| Stroke | 17 | 2.2 |
| Gastrointestinal hemorrhage | 6 | 0.8 |
| Pulmonary embolism | 3 | 0.4 |
| Renal failure | 6 | 0.8 |
| Accident or other violent death | 7 | 0.9 |
| Suicide | 6 | 0.8 |
| Substance abuse | 16 | 2.1 |
| CNS disease | 3 | 0.4 |
| Heart or vascular | 3 | 0.4 |
| Respiratory disease | 4 | 0.5 |
| Digestive system disease | 3 | 0.3 |
| Unclassifiable/unknown | 92 | 11.8 |
CNS, central nervous system.
Of 426 AIDS-related deaths, the majority were from AIDS-related opportunistic infections including 166 (39.0%) due to TB, 46 (10.8%) from Pneumocystis jiroveci pneumonia (PCP), 35 (8.2%) from cryptococcal meningitis, and 32 (7.5%) from cerebral toxoplasmosis. There was also a significant number of deaths secondary to AIDS-related malignancies (n=40, 9.4%) and wasting syndrome (n=27, 6.3%). In 41 cases (9.6%) death was classified as AIDS related based on a history of AIDS-defining illness or CD4 count <200 cells/mm3 within 6 months prior to death.
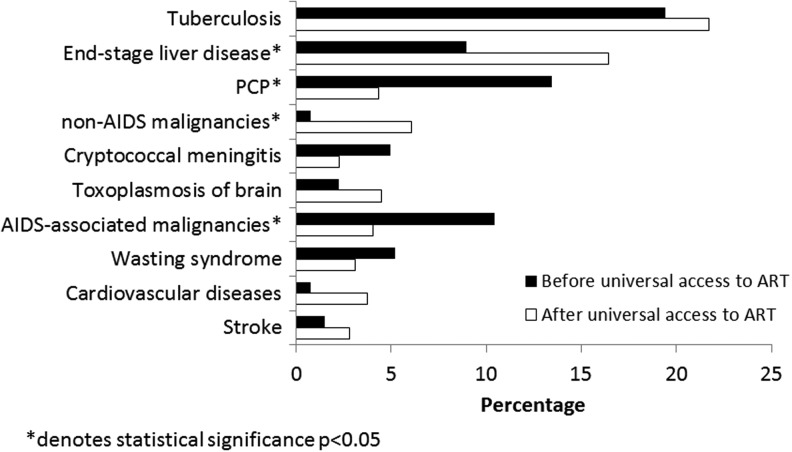
There were temporal changes in causes of death. The proportion of patients who died from an AIDS-related illness decreased significantly in the universal access era (52.7% vs. 64.2%, p<0.02). There was a corresponding increase in the proportion of deaths attributable to non-AIDS conditions in the universal versus preuniversal access era (36.7% vs. 17.9%, p<0.0001) as well as a decrease in the proportion of unknown causes of death (10.5% vs. 17.9%, p<0.02). TB was the leading cause of death in both periods. There was a statistically significant decrease in the proportion of deaths attributable to PCP and AIDS-related malignancies in the universal access era, while deaths due to end-stage liver disease and non-AIDS-related malignancies increased significantly (Fig. 3).
FIG. 3.
Comparison of causes of death before and after universal access to antiretroviral therapy (ART).
Discussion
In this study we report on the causes of and risk factors for death among all reported HIV-infected adults in the country of Georgia since the beginning of the HIV epidemic. We found a significant and progressive decrease in mortality after antiretroviral drugs became universally available in 2004. The mortality rate in 2004 was 10.74 deaths per 100 PY compared to 4.02 per 100 PY reported in 2012 (p<0.0001). Additionally, while we found an increasing rate of non-AIDS-related death, TB continues to be the number one cause of death (21%). Georgia remains the only country in the region that has achieved and sustained universal access to ART,8 and our results are a testament to these benefits. To achieve further decreases in mortality earlier HIV diagnosis and improved TB prevention are needed.
Despite the reduction in death we found among HIV-infected patients in Georgia, mortality rates remain significantly higher than those reported from industrialized countries.14–17 A recent EuroSIDA report found that mortality among HIV-infected patients in Eastern Europe (composed of countries from the former Soviet Union) was significantly higher than in Western Europe and Argentina.16 The regional difference in the rate of mortality most likely stems from differences in health systems and infrastructure as well as patient characteristics (higher rates of coinfections with viral hepatitis and TB and higher rates of late diagnosis in Georgia).16,18 It is also worth noting that within the same EuroSIDA report, the mortality rate in Eastern Europe over the period of 2002–2011 was less than 2.5 deaths per 100 PY of follow-up, which is substantially lower than total mortality of 5.74/100 PY reported in our study and the lowest reported mortality of 4.02/100 PY in 2012. This may be due to the fact that EuroSIDA collects data from selected clinical sites, most of which are affiliated with academic institutions, and these sites and the care they provide may not be representative of countrywide data. Thus the EuroSIDA study results may be an underestimation of mortality in the region.19 Our analysis provides a more accurate description of the situation at a national level in the region.
Our results highlight the problem of late HIV diagnosis and its association with a high mortality rate. Overall, 48% of patients already had AIDS at the time of HIV diagnosis and 51% of deaths occurred within the first 6 months after entering care. Similar to previous reports,20,21 late diagnosis in this study was the strongest predictor of death (HR 5.69, 95% CI: 4.72–6.85). This finding emphasizes the urgent need for expanding HIV testing efforts in the country in order to diagnose patients before they develop AIDS.
More than half of the patients in our cohort were IDUs, and similar to other reports we found IDU to be a risk factor for death (HR 1.47, 95% CI 1.20–1.80).22,23 There are data, however, suggesting that equal access to care may reduce this disparity. For example, researchers from British Columbia found no difference in rates of survival among HIV-infected IDUs and non-IDUs who were started on ART.24 It has also been stated that physicians often defer ART from IDUs because of adherence concerns.25 In Georgia all person have equal access to ART. According to a 2012 country report for the UN Global AIDS Response Progress Reporting, 98% of IDUs with a CD4 count <200 were receiving ART in Georgia.26 In this analysis 52% of IDUs and 53% of non-IDUs were initiating ART (p=0.18). Thus the higher mortality among IDUs in our cohort did not result from issues of ART access, but rather from presenting with more advanced disease than non-IDUs. Taking into account the very low HIV testing coverage of this population,27 it is not surprising that more IDUs were diagnosed late than non-IDUs (55% vs. 40%, p<0.0001). In addition, TB was much more common among persons who had a history of drug abuse (27.9% vs. 13.5%, p<0.0001) and IDUs were more likely to be coinfected with HCV (68.6% vs. 21.4%, p<0.0001). Other unmeasured confounders, such as sociobehavioral risk factors, could also account for the differences in mortality among IDUs and non-IDUs seen in our study.
The findings of our study are in agreement with reports from high- and middle-income countries that demonstrated a decrease in AIDS-related mortality and an increase in non-AIDS-related causes of death.28–32 Similar to these reports we found a significant increase in deaths due to liver diseases, largely due to HCV infection. Recently Georgia initiated a free HCV treatment program with pegylated interferon and ribavirin for HIV/HCV-coinfected patients. It is essential to maintain this free program and also to make new direct-acting antivirals available for HIV patients to achieve the dramatic reduction in liver-related mortality. Analysis also showed a significant increase in deaths due to non-AIDS-related malignancies in the universal access era. Although not statistically significant, there was nearly a 5-fold increase in the proportion of deaths due to cardiovascular diseases. These findings emphasize the importance of fostering a multidisciplinary approach to HIV disease management. Deaths due to unknown causes have been decreasing progressively, which is likely due to the strengthened capacity of the national HIV/AIDS treatment and care program, including better diagnostic capabilities, improved data management systems, and case follow-up resources.
Temporal comparisons showed a significant reduction in AIDS-related mortality due to PCP and AIDS-related malignancies, but overall more than half of the cases of death in the universal access era were attributable to AIDS. With regard to this, the persistently high mortality from TB is of particular concern. TB accounted for 21% of total deaths reported among HIV patients in Georgia and continued to be the leading cause of death despite the universal availability of free treatment for both HIV and TB.
Our study provides further evidence of the dramatic effect of TB on HIV disease outcomes in the Eastern European region. A recent systematic assessment showed that the Eastern European region has the second highest TB death rate among HIV-positive persons.33 Multinational comparison of outcomes of TB/HIV coinfection showed a 5-fold increased risk of death among patients from Eastern Europe compared to those living in Western Europe.34 This high TB/HIV mortality rate in Eastern Europe is likely related to delayed TB and HIV diagnosis and high rates of multidrug resistant (MDR) TB in the region. For instance, 14 of 15 former Soviet Union republics, including Georgia, are among the world's 27 high burden MDR TB countries.35 In Georgia 11% of new and 32% of treated TB cases had MDR TB, which along with delays in TB and HIV diagnosis substantially contributed to increased mortality.35,36 Our analysis emphasizes that the poor HIV/TB outcomes in Georgia require an urgent health system-wide response.
The major limitation of our study is the possible underascertainment of mortality among patients lost to follow-up. Despite the effective mechanisms to retain patients in care and find those lost to follow-up, the status could not be ascertained in 11% of patients. A recent systematic analysis of primarily African studies showed that the proportion of patients who died among those lost to follow-up may range between 9% and 50%.37 It was also shown that reported deaths only for patients remaining in care underestimates mortality on a population level.38 Thus it is valid to assume that some patients lost to follow-up in our study could have died, which has not been accounted for in the analysis, thus resulting in an underestimation of mortality. Overall, the proportion of lost to follow-up in the Georgian HIV program is at least similar to or in many cases is lower than that reported elsewhere.19,39 Another limitation of the study is the fact that of total deaths 92 (11.6%) could not be classified because of lack of information and a further 41 (5.3%) cases were categorized as AIDS related based on a history of an AIDS-defining illness or CD4 count <200 cells/mm3 within 6 months prior to death. Despite this important limitation our analysis provides a comprehensive population-level picture of patterns of mortality in Georgia.
In summary, universal access to ART significantly decreased mortality among HIV patients in Georgia. However, AIDS remains the leading cause of death, largely resulting from a late HIV diagnosis. Sustaining universal access to ART plus earlier HIV diagnosis and ART initiation can bring even greater benefits in terms of preventing premature mortality and preventing new HIV and TB infections.40
Acknowledgments
This work was supported in part by the WHO Regional Office for Europe, NIH/FIC (Emory AIDS International Training and Research Program, grant D43 TW01042, Emory NIH Fogarty International Center, grant D43 TW007124, the New York State International Training and Research Program, grant D43 TW000233), and NIH NIAID K23AI103044.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Palella FJ, Jr, Delaney KM, Moorman AC, et al. : Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. HIV Outpatient Study Investigators. N Engl J Med 1998;338(13):853–860 [DOI] [PubMed] [Google Scholar]
- 2.Sterne JA, Hernan MA, Ledergerber B, et al. : Long-term effectiveness of potent antiretroviral therapy in preventing AIDS and death: A prospective cohort study. Lancet 2005;366(9483):378–384 [DOI] [PubMed] [Google Scholar]
- 3.Mills EJ, Bakanda C, Birungi J, et al.: Life expectancy of persons receiving combination antiretroviral therapy in low-income countries: A cohort analysis from Uganda. Ann Intern Med 2011;155(4):209–216 [DOI] [PubMed] [Google Scholar]
- 4.Reniers G, Araya T, Davey G, et al. : Steep declines in population-level AIDS mortality following the introduction of antiretroviral therapy in Addis Ababa, Ethiopia. AIDS 2009;23(4):511–518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Braitstein P, Brinkhof MW, Dabis F, et al. : Mortality of HIV-1-infected patients in the first year of antiretroviral therapy: Comparison between low-income and high-income countries. Lancet 2006;367(9513):817–824 [DOI] [PubMed] [Google Scholar]
- 6.Lucas S: Causes of death in the HAART era. Curr Opin Infect Dis 2012;25(1):36–41 [DOI] [PubMed] [Google Scholar]
- 7.UNAIDS: Global Report: UNAIDS Report on the Global AIDS epidemic 2013. UNAIDS, Geneva, 2013 [Google Scholar]
- 8.Tsertsvadze T, Chkhartishvili N, Sharvadze L, et al. : Outcomes of universal access to antiretroviral therapy (ART) in Georgia. AIDS Res Treat 2011;2011:621078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kowalska JD, Friis-Moller N, Kirk O, et al.: The Coding Causes of Death in HIV (CoDe) Project: Initial results and evaluation of methodology. Epidemiology 2011;22(4):516–523 [DOI] [PubMed] [Google Scholar]
- 10.The CoDe Project: Coding Causes of Death in HIV, Protocol version 1.0. The CoDe Project, www.cphiv.dk/CoDe/tabid/55/Default.aspx Accessed November12, 2012
- 11.World Health Organization: ICD-10: International Statistical Classification of Diseases and Related Health Problems, 10th rev., 2nd ed. World Health Organization, Geneva, 2005 [Google Scholar]
- 12.World Health Organization. Verbal autopsy standards: ascertaining and attributing cause of death. World Health Organization, Geneva, 2007 [Google Scholar]
- 13.Centers for Diseases Control and Prevention: 1993 revised classification system for HIV infection and expanded surveillance case definition for AIDS among adolescents and adults. MMWR Recomm Rep 1992;41(RR-17):1–19 [PubMed] [Google Scholar]
- 14.Weber R, Ruppik M, Rickenbach M, et al. : Decreasing mortality and changing patterns of causes of death in the Swiss HIV Cohort Study. HIV Med 2013;14(4):195–207 [DOI] [PubMed] [Google Scholar]
- 15.Palella FJ, Jr, Baker RK, Moorman AC, et al. : Mortality in the highly active antiretroviral therapy era: Changing causes of death and disease in the HIV outpatient study. J Acquir Immune Defic Syndr 2006;43(1):27–34 [DOI] [PubMed] [Google Scholar]
- 16.Reekie J, Kowalska JD, Karpov I, et al. : Regional differences in AIDS and non-AIDS related mortality in HIV-positive individuals across Europe and Argentina: The EuroSIDA Study. PLoS One 2012;7(7):e41673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Krentz HB, Kliewer G, and Gill MJ: Changing mortality rates and causes of death for HIV-infected individuals living in Southern Alberta, Canada from 1984 to 2003. HIV Med 2005;6(2):99–106 [DOI] [PubMed] [Google Scholar]
- 18.European Centre for Disease Prevention and Control, WHO Regional Office for Europe: HIV/AIDS Surveillance in Europe 2012. European Centre for Disease Prevention and Control, Stockholm, 2013 [Google Scholar]
- 19.Mocroft A, Kirk O, Aldins P, et al. : Loss to follow-up in an international, multicentre observational study. HIV Med 2008;9(5):261–269 [DOI] [PubMed] [Google Scholar]
- 20.Sabin CA, Smith CJ, Youle M, et al. : Deaths in the era of HAART: Contribution of late presentation, treatment exposure, resistance and abnormal laboratory markers. AIDS 2006;20(1):67–71 [DOI] [PubMed] [Google Scholar]
- 21.Simmons R, Ciancio B, Kall M, Rice B, and Delpech V: Ten-year mortality trends among persons diagnosed with HIV infection in England and Wales in the era of antiretroviral therapy: AIDS remains a silent killer. HIV Med 2013;14(10):596–604 [DOI] [PubMed] [Google Scholar]
- 22.Larsen MV, Omland LH, Gerstoft J, et al. : Impact of injecting drug use on mortality in Danish HIV-infected patients: A nation-wide population-based cohort study. Addiction 2010;105(3):529–535 [DOI] [PubMed] [Google Scholar]
- 23.Murray M, Hogg RS, Lima VD, et al. : The effect of injecting drug use history on disease progression and death among HIV-positive individuals initiating combination antiretroviral therapy: Collaborative cohort analysis. HIV Med 2012;13(2):89–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wood E, Hogg RS, Lima VD, et al. : Highly active antiretroviral therapy and survival in HIV-infected injection drug users. JAMA 2008;300(5):550–554 [DOI] [PubMed] [Google Scholar]
- 25.Westergaard RP, Ambrose BK, Mehta SH, and Kirk GD: Provider and clinic-level correlates of deferring antiretroviral therapy for people who inject drugs: A survey of North American HIV providers. J Int AIDS Soc 2012;15(1):10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.UNAIDS: 2012 Progress reports submitted by countries. www.unaids.org/en/dataanalysis/knowyourresponse/countryprogressreports/2012countries/ Accessed December31, 2013
- 27.Chikovani I, Goguadze K, Ranade S, Wertlieb M, Rukhadze N, and Gotsadze G: Prevalence of HIV among injection drug users in Georgia. J Int AIDS Soc 2011;14:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Crum NF, Riffenburgh RH, Wegner S, et al. : Comparisons of causes of death and mortality rates among HIV-infected persons: Analysis of the pre-, early, and late HAART (highly active antiretroviral therapy) eras. J Acquir Immune Defic Syndr 2006;41(2):194–200 [DOI] [PubMed] [Google Scholar]
- 29.Sackoff JE, Hanna DB, Pfeiffer MR, and Torian LV: Causes of death among persons with AIDS in the era of highly active antiretroviral therapy: New York City. Ann Intern Med 2006;145(6):397–406 [DOI] [PubMed] [Google Scholar]
- 30.Simard Edgar P. and Engels Eric A: Cancer as a cause of death among people with AIDS in the United States. Clin Infect Dis 2010;51(8):957–962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lewden C, May T, Rosenthal E, et al. : Changes in causes of death among adults infected by HIV between 2000 and 2005: The “Mortalite 2000 and 2005” surveys (ANRS EN19 and Mortavic). J Acquir Immune Defic Syndr 2008;48(5):590–598 [DOI] [PubMed] [Google Scholar]
- 32.Pacheco AG, Tuboi SH, May SB, et al. : Temporal changes in causes of death among HIV-infected patients in the HAART era in Rio de Janeiro, Brazil. J Acquir Immune Defic Syndr 2009;51(5):624–630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Au-Yeung C, Kanters S, Ding E, et al. : Tuberculosis mortality in HIV-infected individuals: A cross-national systematic assessment. Clin Epidemiol 2011;3:21–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Podlekareva DN, Mocroft A, Post FA, et al. : Mortality from HIV and TB coinfections is higher in Eastern Europe than in Western Europe and Argentina. AIDS 2009;23(18):2485–2495 [DOI] [PubMed] [Google Scholar]
- 35.World Health Organization: Global Tuberculosis Report 2012. WHO, Geneva, 2012 [Google Scholar]
- 36.Rabin AS, Kuchukhidze G, Sanikidze E, Kempker RR, and Blumberg HM: Prescribed and self-medication use increase delays in diagnosis of tuberculosis in the country of Georgia. Int J Tuberc Lung Dis 2013;17(2):214–220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brinkhof MWG, Pujades-Rodriguez M, and Egger M: Mortality of patients lost to follow-up in antiretroviral treatment programmes in resource-limited settings: Systematic review and meta-analysis. PLoS One 2009;4(6):e5790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brinkhof MWG, Spycher BD, Yiannoutsos C, et al. : Adjusting mortality for loss to follow-up: Analysis of five ART programmes in sub-Saharan Africa. PLoS One 2010;5(11):e14149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brinkhof MW, Dabis F, Myer L, et al.: Early loss of HIV-infected patients on potent antiretroviral therapy programmes in lower-income countries. Bull World Health Organ 2008;86(7):559–567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Granich R, Gupta S, Suthar AB, et al. : Antiretroviral therapy in prevention of HIV and TB: Update on current research efforts. Curr HIV Res 2011;9(6):446–469 [DOI] [PMC free article] [PubMed] [Google Scholar]