Abstract
Salmonella enterica serovar Typhimurium variant Copenhagen was isolated from 5 of 152 (3.3%) feral pigeons from the city of Ghent (Belgium) and from 26 pooled fecal samples from 114 pigeon lofts (22.8%). These isolates belonged to phage type (PT) 99. Seven of the pigeon isolates were further compared in vitro to five human variant Copenhagen isolates, 2 isolates of PT 208, 1 isolate each of PT 120 and U302, and a nontypeable isolate. No differences in invasiveness in human intestinal epithelial Caco-2 cells were found. The human strains, however, were able to multiply significantly more inside human THP-1 macrophages than the pigeon strains. After inoculation of mice with a pigeon PT 99 strain, high numbers of Salmonella bacteria were shed with the feces, the internal organs were heavily colonized, and the animals showed severe clinical symptoms resulting in death. In conclusion, the less-pronounced ability of the pigeon variant Copenhagen strains to multiply inside human macrophages than human strains as well as the lack of human PT 99 isolates during 2002, despite the relatively high frequency of this PT in the pigeon population, suggest these strains to be of low virulence to humans. However, the high virulence for mice of the tested strain implies that rodents may act as reservoirs.
Because of the high numbers of free-roaming and captive pigeons in Belgium and their well known association with Salmonella, pigeons might be a source of salmonellosis in humans. Pigeon isolates of Salmonella enterica subsp. enterica serovar Typhimurium variant Copenhagen are considered a host-adapted lineage of the serovar Typhimurium (5, 6, 8). As might be expected from host restricted Salmonella strains (3, 10), these pigeon isolates are highly virulent to pigeons (6, 7). Adaptation to one host often coincides with low virulence to other hosts, such as humans (3, 10). It was the aim of the present study to determine the virulence of pigeon-associated Salmonella strains for humans by using epidemiological data, in vitro enterocyte and macrophage models, and an in vivo murine model. The in vitro and in vivo models have been previously used to assess virulence of Salmonella strains for humans (1).
MATERIALS AND METHODS
Prevalence of pigeon-adapted variant Copenhagen strains in pigeons and humans.
For the pigeon strains, pooled fecal samples from 114 different pigeon lofts in Belgium and pooled samples from the intestines, livers, and spleens of 152 feral pigeons from the city of Ghent (Belgium) were examined for the presence of Salmonella. The samples were enriched overnight in tetrathionate brilliant green broth (Merck, Darmstadt, Germany) at 37°C and then streaked onto brilliant green agar (Lab-M, Bury, United Kingdom). Colonies with characteristics of Salmonella were further identified biochemically on the basis of glucose fermentation, sulfur reduction, the presence of lysine decarboxylation, the absence of lactose fermentation, and urease production. The isolates were serotyped by means of agglutination tests by using single-factor antisera, and the phage types (PTs) were determined.
For the human strains, clinical Salmonella isolates were obtained from 194 Belgian laboratories during the year 2002. The strains were serotyped, and the PTs were determined in 319 (13%) of 2,438 serovar Typhimurium isolates. The maximum number (B) of human salmonellosis cases due to an infection with pigeon-adapted strains with 95% probability (α) was determined with the following formula: B = [1 − (1 − α)1/n] × {N − [(n − 1)/2]}, where N is the total number of serovar Typhimurium strains and n is the number of serovar Typhimurium strains of which the PT was determined.
Invasion of pigeon versus human isolates of variant Copenhagen into Caco-2 cells.
Seven pigeon-derived variant Copenhagen PT 99 strains belonging to four different BlnI pulsed-field gel electrophoresis types (6) and five human variant Copenhagen isolates, 2 of PT 208, 1 of PT 120, 1 of PT U302, and 1 that was nontypeable, were used in a gentamicin protection assay. Briefly, overnight bacterial cultures grown in Luria-Bertani broth (37°C) were resuspended in cell culture medium (Dulbecco's modified Eagle's medium [Gibco, Paisley, Scotland], 10% fetal calf serum, 1% nonessential amino acids [Gibco]). Confluent monolayers of Caco-2 cells in a 96-well plate were exposed to 107 CFU of the different human and pigeon Salmonella isolates. The plate was centrifuged for 10 min at 37°C and incubated at 37°C and 5% CO2 for 1 h. The cells were rinsed five times with Hanks' balanced salt solution (Gibco) and incubated for another hour at 37°C and 5% CO2 in cell culture medium containing 50 μg of gentamicin (Gibco)/ml. Afterwards, the cells were rinsed five times with Hanks' balanced salt solution and lysed at room temperature for 10 min with 1% Triton X-100 (Acros, Geel, N.J.) in distilled water. Finally, 120 μl of 10-fold dilutions was plated on brilliant green agar, and Salmonella colonies were counted after incubation for 24 h at 37°C. The invasion assays were performed in triplicate.
Intracellular survival of pigeon versus human isolates of variant Copenhagen inside THP-1 cells.
The same strains as described for the Caco-2 invasion assay were used to compare the pigeon- and human-derived strains for the ability to survive inside the human macrophage cell line THP-1. Inoculation of the THP-1 cells was performed as described above, with the exception that at 1 h after the addition of 50 μg of gentamicin/ml, the medium was replaced by medium containing 20 μg of gentamicin/ml. At 0, 2, and 6 h after this medium replacement, the cells were lysed and the numbers of bacteria were counted. The assays were performed in triplicate.
Cytotoxicity of Salmonella strains for THP-1 cells.
The macrophages were exposed to the pigeon and the human strains as described above. At 6 h postinoculation (p.i.), 20 μl of the cell proliferation agent WST-1 (Roche, Mannheim, Germany) was added to each well and absorbencies were measured at 450 nm (Titertek, Helsinki, Finland). The number of cells per well was determined by using a standard curve prepared with a dilution series of the cell suspension.
In vivo virulence of a pigeon versus human variant Copenhagen strain for mice.
A pigeon-derived variant Copenhagen strain with known virulence for pigeons (DAB69) and a human variant Copenhagen strain (MB2504) were grown for 6 h in Luria-Bertani broth at 37°C. Ten 6-week-old BALB/c mice were inoculated orally with 104 CFU of one of the variant Copenhagen strains. The number of Salmonella bacteria was determined in feces, which were collected daily for 7 days. The clinical health of the animals was assessed daily. At 7 days p.i., the mice were humanely killed and the numbers of CFU of Salmonella per gram of cecum, spleen, and liver were determined. If only positive for Salmonella after enrichment, a bacterial count of 10 CFU/g was attributed.
RESULTS
Prevalence of pigeon-adapted variant Copenhagen strains in pigeons and humans.
Variant Copenhagen was isolated from 5 of 152 (3.3%) feral pigeons from the city of Ghent (Belgium) and from 26 pooled fecal samples from 114 pigeon lofts (22.8%). All of the isolates belonged to PT 99.
In 2002, 10,075 human Salmonella isolates were collected in Belgium, of which 2,438 (24.2%) belonged to serovar Typhimurium. Among the serovar Typhimurium isolates, 574 belonged to variant Copenhagen. None of 319 serovar Typhimurium strains (of which 42 were variant Copenhagen) belonged to PT 99. The maximum number of human salmonellosis cases in 2002 due to infection with PT 99 strains was estimated to be 21, this is a maximum of 0.87% of the infections with serovar Typhimurium and 0.21% of the Salmonella infections.
Invasion of pigeon versus human isolates of variant Copenhagen into Caco-2 cells.
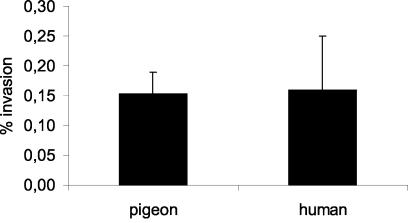
Results of the invasion assays are summarized in Fig. 1. All of the strains tested were able to invade the Caco-2 cells. No significant differences between human and pigeon strains were noticed.
FIG. 1.
Average magnitude of invasion ± standard error of the mean of pigeon- and human-derived strains of variant Copenhagen into Caco-2 cells. The results represent the percentages of bacteria recovered from the inoculum.
Intracellular survival of pigeon versus human isolates of variant Copenhagen inside THP-1 cells.
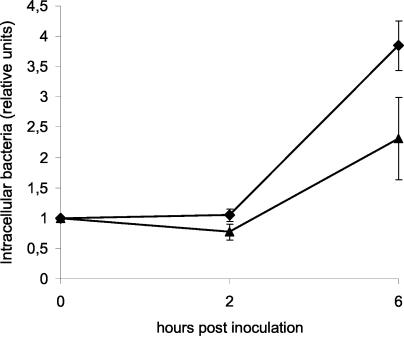
All of the strains tested were able to survive and multiply inside the THP-1 cells. Results are shown in Fig. 2. At 6 h p.i., significantly less Salmonella bacteria were isolated from cells inoculated with the pigeon strains than from cells inoculated with the human strains (P < 0.05, Mann-Whitney U test).
FIG. 2.
Average intracellular survival ± standard error of the mean of pigeon-derived (triangles) and human-derived (diamonds) strains of variant Copenhagen inside THP-1 cells. Intracellular survival is expressed relative to the number of bacteria recovered at 0 h p.i..
Cytotoxicity of Salmonella strains for THP-1 cells.
None of the strains induced cytotoxic effects in the THP-1 cells at 6 h p.i., as measured by the WST-1 assay.
In vivo virulence of a pigeon versus human variant Copenhagen strain for mice.
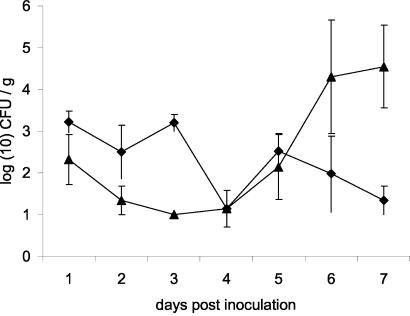
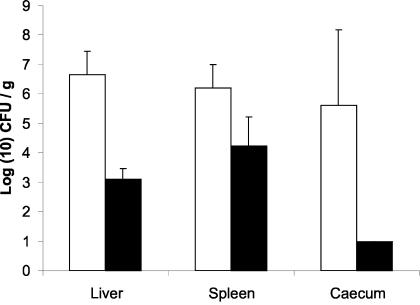
Fecal shedding of the two strains was comparable during the first 5 days p.i. (Fig. 3). From day 5 p.i. on, shedding of the human strain decreased, whereas shedding of the pigeon strain increased and reached a plateau phase. Mice inoculated with the human strain did not show any clinical symptoms. Mice inoculated with the pigeon strain showed severe clinical symptoms from day 5 p.i.: rugged appearance, apathia, and anorexia. Four of these five mice inoculated with the pigeon strain were moribund at day 7 p.i. and were euthanized. Significantly higher numbers of Salmonella bacteria were found in the ceca, livers, and spleens of mice inoculated with the pigeon strain than in those of mice inoculated with the human strain (Fig. 4) (P < 0.05, Mann-Whitney U test).
FIG. 3.
Average number of bacteria ± standard error of the mean recovered from fecal pellets of BALB/c mice inoculated with either a human-derived (diamonds) or pigeon-derived (triangles) strain of variant Copenhagen.
FIG. 4.
Average number of bacteria ± standard error of the mean recovered from internal organs of BALB/c mice at 7 days p.i. inoculated with either a human-derived (black bars) or pigeon-derived (white bars) strain of variant Copenhagen.
DISCUSSION
The prevalence of variant Copenhagen in the city pigeon population appears to be fairly low. However, taking into consideration the huge numbers of these birds in the Belgian cities and their rather close contact with humans, the risk of transfer of zoonotic agents to humans is not unthinkable. It would, therefore, be useful to be able to estimate the public health risk of pigeons as carriers of Salmonella. The results of the in vitro and in vivo assessment of the virulence of pigeon-derived Salmonella strains, however, were rather ambiguous. The in vitro assays suggested that, whereas invasiveness was equal for both human and pigeon strains, the human-derived strains were more capable of multiplying inside human macrophages. The association of the ability of intracellular multiplication with host adaptation has been proven previously (2, 9, 12). In contrast, a clear association of invasiveness into intestinal epithelial cells and host adaptation has not been found (4, 11). Hence, our in vitro results suggest that the pigeon-derived variant Copenhagen strains are less adapted to and, therefore, probably less virulent to humans than the human strains. This hypothesis is supported by the lack of human isolates of PT 99 in the 2002 period in Belgium and its estimated low prevalence in the human population, despite the relatively high prevalence in the pigeon population.
The in vivo virulence assay in BALB/c mice demonstrated that the pigeon strain used was more virulent to mice than the human strain. Mice have been used previously as a model to assess virulence of serovar Typhimurium for humans (1). Highly virulent murine strains, however, do not necessarily have to be virulent in humans and vice versa (e.g., S. enterica serovar Typhi). Possibly, in the case of pigeon-derived Salmonella strains, rodents act as reservoirs for the bacterium to reside in, helping to maintain and spread the infection in the pigeon population, for example, by contaminating food sources. Although supposedly host adapted, at least some of the pigeon-derived strains of variant Copenhagen are able to cause severe clinical salmonellosis in the murine host. This finding resembles the situation encountered with S. enterica serovar Dublin, which occasionally causes severe infections in hosts other than cattle (10).
REFERENCES
- 1.Allen, C. A., P. J. Fedorka-Cray, A. Vazquez-Torres, M. Suyemoto, C. Altier, L. R. Ryder, F. C. Fang, and S. J. Libby. 2001. In vitro and in vivo assessment of Salmonella enterica serovar Typhimurium DT104 virulence. Infect. Immun. 69:4673-4677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barrow, P. A., M. B. Huggins, and M. A. Lovell. 1994. Host specificity of Salmonella infection in chickens and mice is expressed in vivo primarily at the level of the reticuloendothelial system. Infect. Immun. 62:4602-4610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bäumler, A. J., R. M. Tsolis, T. A. Ficht, and L. G. Adams. 1998. Evolution of host adaptation in Salmonella enterica. Infect. Immun. 66:4579-4587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bolton, A. J., M. P. Osborne, T. S. Wallis, and J. Stephen. 1999. Interaction of Salmonella choleraesuis, Salmonella dublin and Salmonella typhimurium with porcine and bovine terminal ileum in vivo. Microbiology 145:2431-2441. [DOI] [PubMed] [Google Scholar]
- 5.Nastasi, A., C. Mammina, and M. R. Villafrate. 1993. Epidemiology of Salmonella typhimurium: ribosomal DNA analysis of strains from human and animal sources. Epidemiol. Infect. 110:553-565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pasmans, F., F. van Immerseel, M. Heyndrickx, A. Martel, C. Godard, C. Wildemauwe, R. Ducatelle, and F. Haesebrouck. 2003. Host adaptation of pigeon isolates of Salmonella enterica subsp. enterica serovar Typhimurium variant Copenhagen PT 99 is associated with enhanced macrophage cytotoxicity. Infect. Immun. 71:6068-6074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Proux, K., F. Humbert, M. Guittet, P. Colin, and G. Bennejean. 1998. Vaccination of pigeon by Salmonella typhimurium. Avian Pathol. 27:161-167. [DOI] [PubMed] [Google Scholar]
- 8.Rabsch, W., H. L. Andrews, R. A. Kingsley, R. Prager, H. Tschäpe, L. G. Adams, and A. J. Bäumler. 2002. Salmonella enterica serotype Typhimurium and its host-adapted variants. Infect. Immun. 70:2249-2255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schwan, W. R., X. Z. Huang, L. Hu, and D. J. Kopecko. 2000. Differential bacterial survival, replication, and apoptosis-inducing ability of Salmonella serovars within human and murine macrophages. Infect. Immun. 68:1005-1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Uzzau, S., D. J. Brown, T. Wallis, S. Rubino, G. Leori, S. Bernard, J. Casadesús, D. J. Platt, and J. E. Olsen. 2000. Host adapted serotypes of Salmonella enterica. Epidemiol. Infect. 125:229-255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Uzzau, S., G. S. Leori, V. Petruzzi, P. R. Watson, G. Schianchi, D. Bacciu, V. Mazzarello, T. S. Wallis, and S. Rubino. 2001. Salmonella enterica serovar-host specificity does not correlate with the magnitude of intestinal invasion in sheep. Infect. Immun. 69:3092-3099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vladoianu, I. R., H. R. Chang, and J. C. Pechere. 1990. Expression of host resistance to Salmonella typhi and Salmonella typhimurium: bacterial survival within macrophages of murine and human origin. Microb. Pathog. 8:83-90. [DOI] [PubMed] [Google Scholar]