Abstract
Background: Papillary thyroid carcinoma (PTC) is increasing in incidence while mortality is unchanged. Identifying patients with higher risk of recurrence and death is essential. Case series identify the hobnail variant of PTC (HVPTC), which is characterized by micropapillary architecture, apocrine features, and loss of cellular polarity. Herein, we describe the clinical course, pathologic features, and mutational profile of patients at our institution with HVPTC.
Methods: A query into the surgical pathologic database (2009–2012) was performed, and clinicopathologic data were collected on all patients carrying the diagnosis of HVPTC. BRAFV600E testing was performed on paraffin-embedded blocks using SNaPshot mutational analysis.
Results: Twelve patients with HVPTC were identified, with an average age of 54.1±18.8 years. Seven patients (63.6%) were AJCC Stage III or IV at presentation. Tumors were large (3.7±2.0 cm), some were multifocal (33.3%), and frequently with extrathyroidal extension (58.3%), lymphovascular invasion (41.7%), and lymph node metastasis (75%). Forty percent of the patients had concomitant tall cell features (TCF), and two had small foci of undifferentiated (anaplastic) thyroid carcinoma (ATC). Eighty percent of tumors undergoing mutational analysis had the BRAFV600E mutation, and the remaining 20% harbored a RET/PTC1 gene rearrangement. No other known thyroid cancer mutations were identified on SNaPshot analysis. At median follow-up of 26 months, four patients had recurrent or persistent disease, one of whom died from the disease one year after surgery.
Conclusions: The hobnail variant of PTC has an aggressive behavior, with a high incidence of infiltrative tumors and metastatic disease. Strikingly, all tumors in our series harbored a PTC-associated genetic abnormality, either a BRAFV600E mutation (80%) or a RET/PTC1 rearrangement (20%). This histologic variant warrants further study, and patients with this diagnosis should be observed closely for recurrence.
Introduction
Papillary thyroid carcinoma (PTC) is the most common endocrine malignancy, with thyroid cancer being the fifth most common malignancy in women and the fastest increasing cancer in both sexes over the past decade in the United States (1). The increasing incidence of thyroid cancer is reflected in the projected 60,220 new cases in the United States in 2013 (1). PTC is by far the most common, comprising up to 88% of thyroid malignancies (2,3). Including all variants, PTC patients have 96% survival at 10 years (1). While disease-specific mortality from well-differentiated thyroid carcinomas is low, approximately 15% of patients with thyroid cancer have local or regional recurrent disease following initial surgery (4,5). Patients with recurrent disease have worse outcomes, and recurrent tumors frequently behave more aggressively and often lose radioiodine avidity. Moreover, 35% of patients who develop a recurrence die of PTC (6,7).
Much current work is focused on determining which PTC patients require more aggressive surgery, adjuvant therapy, and closer surveillance. Although not a part of the current American Joint Committee on Cancer (AJCC) staging system, histologic variants portend varied prognoses. The importance of histologic variants was acknowledged in the most recent American Thyroid Association guidelines, which now cite “aggressive histology (e.g., tall cell, insular, columnar cell (papillary) carcinoma)” as a criterion for increased risk in the risk-stratification schema (8). There are numerous known variants of PTC with varying natural history, with follicular variant of papillary thyroid carcinoma (FVPTC) having a better prognosis and tall cell variant (TCV) a worse prognosis than classical PTC (9–11). Recently, small series of patients with an aggressive “hobnail” variant were reported with very poor disease-specific survival (43–66%) (12–14). Herein, we report the clinical presentation, tumor characteristics, and outcomes of patients undergoing thyroidectomy at our institution showing characteristics of this hobnail variant of PTC (HVPTC). Importantly, in addition to BRAFV600E and RET/PTC translocation testing in the majority of the cohort, we also performed extensive mutational analysis on a subset of patients using a clinical adaptation of the SNaPshot platform, a single nucleotide primer extension polymerase chain reaction (PCR)-based assay (15). This is the first study to examine such a broad molecular profile in HVPTC.
Materials and Methods
Patients
Query of the surgical pathology database at Massachusetts General Hospital from 2009 to 2012 for “hobnail” resulted in 12 patients with hobnail features described in the report with PTC. We included patients with diagnostic features of papillary carcinoma and hobnail morphology (Fig. 1) (16). A single case was reported as TCV with hobnail features, better classified as HVPTC on review, but this was the index case seen at our institution following its first report in the literature. All patients with hobnail morphology had their pathology (re)reviewed at our institution (P.M.S.). All data were obtained in accordance with the institutional internal review board at the Massachusetts General Hospital.
FIG. 1.
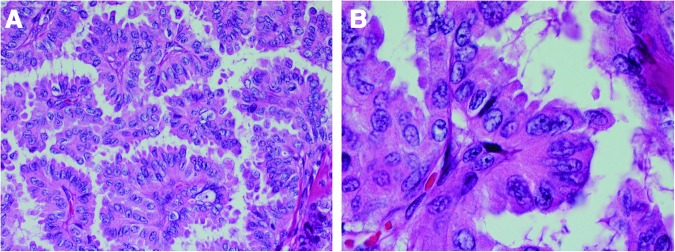
Case of hobnail variant papillary thyroid carcinoma (HVPTC) showing A the micropapillary architecture and B loss of cellular polarity with apocrine snouting of cells (hematoxylin and eosin, 100× and 200× respectively). Color images available online at www.liebertpub.com/thy
Clinical and pathologic characteristics
Demographic information and clinical data were obtained from the electronic medical record. Surgical pathology specimens were reviewed (P.M.S.) for tumor size and lymph node involvement, as well as the presence of multifocality, lymphovascular invasion, necrosis, and extrathyroidal extension. If multiple foci of tumor were present, the largest tumor dimension was noted. Tumor staging was based on the AJCC 7th edition TNM staging (17).
BRAF and SNaPshot mutational analysis
All available tumors were assessed for the BRAFV600E mutation, which leads to valine (V) being substituted for by glutamate (E) at codon 600. In 9 out of 10 patients in which BRAFV600E testing was performed, genotyping analysis was carried out using the SNaPshot platform as previously described (15). In two patients, BRAFV600E testing was ordered clinically at the discretion of the surgeon. One of those cases was tested at our institution using the SNaPshot platform, and the other tumor was tested at an outside facility for the BRAFV600E mutation. In brief, total nucleic acid (TNA) was isolated from formalin-fixed paraffin-embedded tissue (FFPE) and subject to multiplex PCR followed by a single nucleotide primer extension step that generates allele-specific fluorescently labeled probes (15). The SNaPshot tumor genotyping assay (Applied Biosystems, Foster City, CA) was developed at the MGH Translational Research Laboratory for clinical tumor genotyping. It can detect low-level somatic mutations in TNA extracted from archival material and was designed to query 68 hotspot loci in 14 genes commonly mutated in cancer (AKT1, APC, BRAF, CTNNB1, EGFR, IDH1, KIT, KRAS, MEK1, NOTCH1, NRAS, PIK3CA, PTEN, and TP53) (15).
RET/PTC rearrangements
All available tumor samples were also tested for RET/PTC1 and RET/PTC3 rearrangements, as these translocations are not assessed by SNaPshot analysis. To obtain pure RNA, 200 ng of TNA was first treated with DNase I (Invitrogen, Grand Island, NY). Then, 100–150 ng of total RNA was reverse transcribed using the SuperScript III First-Strand Synthesys Super Mix for qRT-PCR (Invitrogen). RT-PCR was performed using previously published conditions and gene-specific primers designed to flank the RET/PTC1 and RET/PTC3 fusion sites, as well as GAPDH control primers to ensure sample integrity (18). Samples were run in triplicate in conjunction with two positive control RNAs obtained from two tumors known to harbor the RET/PTC1 and RET/PTC3 rearrangements respectively.
Immunohistochemistry
The following immunostains were used, including clone information and stain preparation: Cytokeratin 7 (Dako IR619, Clone OV-TL 13/30, 1:3 citrate, pH 6, 20 min); epithelial membrane antigen (Leica RTU cat# PA0035 clone GP1.4); thyroid transcription factor 1 (TTF-1; Diagnostic Biosystems MOB 285 clone SPT24, 1:25 citrate, pH 6, 30 min); Ki-67 (Dako M7240 Clone MIB1, 1:200 EDTA pH 9, 20 min); E-cadherin (Leica RTU cat# PA0387 clone 36B5 ER2 pH 9, 30 min); Cytokeratin 19 (Leica RTU cat# PA0799 clone b170 ER1 pH 6, 30 min); β-catenin (Leica RTU cat# PA0083 clone 17C2 ER1 pH 6, 30 min); and p53 (Leica RTU cat# PA0057 clone DO7 ER2, EDTA pH 9, 20 min).
Statistical analysis
Continuous outcomes were expressed as mean±standard deviation (SD) or median, interquartile range (IQR) as needed. All data were analyzed using SPSS v20.0 (IBM Corp., Armonk, NY).
Results
Demographics and clinical characteristics
Twelve patients met our inclusion criteria, constituting 1% of PTC at our institution during the same time period (Table 1). Nine out of 12 patients (75%) were female, with a mean age of 54.1±18.8 years (range 21–80 years). Seven out of 12 patients (58.3%) were Stage III or IV at presentation. Eleven patients were treated with total thyroidectomy and one with lobectomy. Ten out of 12 patients (83.3%) had radioactive iodine (RAI; one patient did not return for treatment).
Table 1.
The Clinical, Pathological, and Mutational Characteristics of 12 Hobnail Papillary Thyroid Carcinoma Cases
| Case/age/sex | FNA Dx | Surgery/EBRT | RAI | Size (cm) | % Hobnail | % Tall cell | % ATC | ETE | LVI | Multifocal | Lymph node metastasis | Distant metastasis | AJCC pTNM Stage* | BRAF status, % of BRAFV600E alleles | RET/PTC1/RET/PTC3 status | Local recurrence (mo) | Follow-up (mo) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1/22F | PTC | TT+CLND | Yes | 6.0 | 60 | 40 | 0 | Yes | No | No | Yes | No | T3 N1b M0 | V600E, 19% | –/– | No | NED (36) |
| 2/65F | PTC | TT+CLND | Yes | 4.5 | NA | NA | <5% | Yes | Yes | No | Yes | Lung, liver, bone | T4 N1a M1 | NA | NA | Yes (8) | DOD (13) |
| 3/71F | PTC/TCV | TT+CLND+MRND, tracheal, (redo*3)/ EBRT | Yes | 6.5 | 50 | 30 | <1% | Yes | Yes | No | Yes | Lung | T4b N1b M1 | V600E, 31% | –/– | No | NED (39) |
| 4/21F | SUSP PTC | TT | NA | 3.0 | 50 | 0 | 0 | No | No | No | No | No | T2 NX MX | V600E, 30% | –/– | NA | LTFU |
| 5/44M | PTC | TT+MRND W/IJ+SCM, Level VII | Yes | 0.5 | 90 | 0 | 0 | Yes | No | Yes | Yes | Axilla | T3 N1b MX | WT | +/− | Yes (19) | NED (57) |
| 6/62M | PTC | Lobectomy+CLND/EBRT | Yes | 3.2 | 40 | 20 | 0 | Yes | No | No | Yes | No | T3 N1a MX | V600E, 22% | –/– | Yes (2) | NED (35) |
| 7/68F | PTC | TT+CLND+MRND | Yes | 4.0 | 50 | 50 | 0 | Yes | Yes | No | Yes | No | T4a N1a MX | V600E | NA | No | SD (27) |
| 8/63F | PTC | TT+CLND | Yes | 4.5 | 60 | 0 | 0 | No | No | Yes | Yes | No | T3 N1 MX | V600E | NA | No | NED (14) |
| 9/42F | SUSP PTC | TT+CLND | No | 0.8 | NA | NA | 0 | No | No | No | Yes | No | T1 N0 MX | NA | NA | No | NED (20) |
| 10/80F | Redo, not primary | TT+CLND+MRND/(Redo *1)/EBRT | Yes | 5.7 | 100 | 0 | 0 | Yes | Yes | Yes | Yes | No | T3 N1b MX | V600E, 24% | –/– | Yes (2) | SD (26) |
| 11/48F | PTC | TT+CLND | Yes | 3.7 | 45 | 0 | 0 | No | Yes | No | Yes | No | T2 N1a MX | WT | +/− | No | SD (14) |
| 12/63M | PTC | TT | Yes | 1.5 | 70 | 0 | 0 | No | No | Yes | No | No | T1b NX MX | V600E, 26% | –/– | No | NED (12) |
FNA Dx, fine-needle aspiration diagnosis; EBRT, external beam radiotherapy; Postop RAI, postoperative radioactive iodine; ETE, extrathyroidal extension; LVI, lymphovascular invasion; FU, follow-up; mo, months; PTC, papillary thyroid carcinoma; TT, total thyroidectomy; CLND, central cervical lymph node dissection; MRND, modified radical neck dissection; TCF, tall cell features (<50%); ATC, anaplastic thyroid carcinoma; NED, no evidence of disease; DOD, dead of disease; LTFU, lost to follow-up; SD, stable disease; NA, not available. *American Joint Commission on Cancer (7th edition).
Pathology
Preoperative diagnosis with fine-needle aspiration (FNA) resulted in classical variant PTC in eight patients (66.6%), suspicious for PTC in two (16.6%), and TCV-PTC in one patient (8.3%). Tumors were large (3.7±2.0 cm), some multifocal (33.3%), and frequently with extrathyroidal extension (58.3%), lymphovascular invasion (41.7%), and lymph node metastasis (75%). Tumor necrosis was not identified in any of the 12 specimens. Forty percent of the patients had concomitant tall cell features (TCF), comprising <50% of tumor morphology, and two had small foci of undifferentiated (anaplastic) thyroid carcinoma (ATC; one with 5% ATC who died of disease at one year, and one with <1% who is currently without evidence of disease). This subset of patients with TCF had large tumors (4.1±2.2 cm), with 50% of them presenting with lymphovascular invasion (LVI) and 33.3% suffering a recurrence. The six patients without coexistent TCF had comparable clinicopathologic features: large tumors (3.2±1.8 cm), 33.3% of them having LVI, and 20% with recurrent locoregional disease. We performed a subgroup analysis excluding the two patients with ATC. The 10 patients without ATC had comparable clinicopathologic features to the whole cohort: large tumors (3.3±1.9 cm), 30% of them having LVI, and 22.2% with recurrent locoregional disease.
Immunohistochemistry
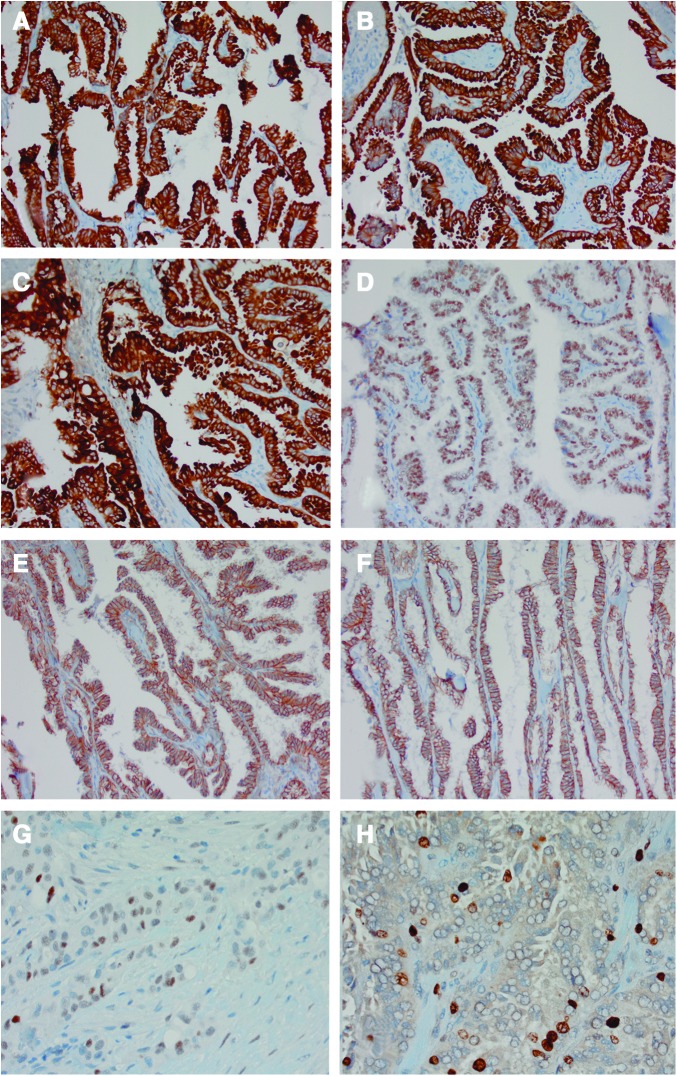
Immunohistochemistry was performed on select cases for comparison with the data seen in the initial paper by Asioli et al. (12). β-Catenin was membranous (intact), indicating no change in normal distribution often seen in mutated β-catenin, which manifests with nuclear retention/staining, including in undifferentiated areas of this tumor where β-catenin mutations are known to occur. There was increased expression of tumor suppressor p53 (nuclear), as seen in many high-grade thyroid cancers. E-cadherin expression was intact (membranous), and ki-67 proliferative index was moderate at 8% (increased is generally >5% in endocrine neoplasms). The cytokeratin profile was similar to other PTC variants, with strong cytoplasmic and membranous staining; TTF1 was positive (nuclear; Fig. 2).
FIG. 2.
Immunohistochemical staining of a patient with HVPTC. (A) cytokeratin 7, (B) cytokeratin 19, (C) epithelial membrane antigen, (D) thyroid transciption factor (TTF-1), (E) β-catenin, (F) E-cadherin, (G) p53, H. Ki-67. β-catenin is membranous (intact) with increased expression of tumor suppressor p53 (nuclear), normal E-cadherin, and Ki67 is moderate at 8%. The cytokeratin profile is similar to other PTC subtypes, with strong cytoplasmic and membranous stain; TTF-1 is positive (nuclear). Color images available online at www.liebertpub.com/thy
Mutational analysis and RET/PTC rearrangements
Tumor was available in 10 out of 12 patients for mutational testing. One case had conventional BRAF testing, and nine tumors were subject to SNaPshot analysis. Eight out of 10 (80%) tumors tested had the BRAFV600E mutation, including all three patients without coexistent TCF that were tested (P.M.S. identified areas of hobnail features in the mixed tumors for mutational analysis). The mean percentage of BRAFV600E alleles was 25.3%, ranging from 19% to 31%. Other than identifying BRAFV600E in the eight patients undergoing SNaPshot analysis, there were no other mutations identified in the additional 13 genes studied. Eight out of 12 patients were tested for RET/PTC1 and RET/PTC3 rearrangements, and the two tumors that were BRAF wild type were both found to be positive for RET/PTC1 (Table 1).
Follow-up
At median follow-up of 26.5 months (IQR 14–35.5), four patients had recurrent or gross persistent disease: one with coexistent ATC (5% of the tumor) and TCF, two with TCF alone, and one without ATC or TCF. The patient with coexistent 5% ATC who recurred died within one year of presentation of her disease. One patient was lost to follow-up.
Discussion
In this paper, we describe the clinical course, pathologic features, and mutational profile of 12 patients with HVPTC, defined as >30% of examined tumor morphology. While this represents a limited, heterogeneous group, there are a number of commonalities: namely, in half of the patients, the hobnail features were also associated with tall cell and/or undifferentiated components. Extrathyroidal extension and locoregional lymph node metastases were common, while multifocality of the hobnail component was not. Interestingly, all 10 tumors available for molecular testing were positive for genetic abnormalities known to be associated with thyroid malignancy. Eight out of 10 (80%) patients tested for the BRAFV600E mutation were positive, a prevalence much higher than the one cited in the literature for other PTC variants (19). The mean percentage of BRAFV600E alleles in our study was 25.3%, ranging from 19% to 31%. This is comparable to the group of patients in the Guerra et al. study, which recently showed that recurrence of PTC was 5.3-fold higher in the tumors with percentages of the BRAFV600E allele of 30% compared with tumors with percentages of the BRAFV600E allele of <30% (20). The two BRAFV600E-negative tumors carried the RET/PTC1 gene rearrangement, which is present in 20–70% of PTC but has not been previously described in HVPTC (21). No other “known” thyroid carcinoma mutations were identified by the SNaPshot profile, examining 68 commonly mutated loci in 14 cancer genes. Of note, the SNAPshot clinical assay was developed to cover recurrent mutations across multiple tumor types and was particularly focused on genetic abnormalities targeted by FDA-approved therapies or by drugs in clinical trials. The panel is well suited to test oncogenes, which are affected by mutations at a very limited number of loci, but has limited coverage for tumor suppressors such as TP53.
HVPTC has been described in other case reports and case series (12–14,22–30). The architectural growth pattern for HVPTC is micropapillary (i.e., small papillae). Other common characteristics include loss or inversion of cellular polarity, increased eosinophilic cytoplasm, increased nuclear to cytoplasmic ratios, and pleomorphic nuclei mostly located in the apex of the cytoplasm that gives the characteristic “hobnail” appearance (Fig. 1). Kakudo et al. were the first to describe a thyroid tumor with micropapillary structures and hobnail features (22). Motosugi et al. reported a case of an aggressive PTC with “micropapillary structure and hobnail appearance” (13). In this case, there were no associated TCF, and the tumor stained positively for TTF-1, Tg, and focally for p53. The largest series to date reported 24 patients from the Mayo Clinic and University of Turin (29). Their inclusion criteria included patients with loss of polarity/cohesiveness with hobnail/micropapillary features in 10% or more of the tumor, and availability of clinical and follow-up data and of pathologic material (Table 2) (29). A subgroup of eight patients from this cohort had been included in a previous study (12). Another retrospective study reported on seven PTC cases from Mexico with micropapillary features, and concluded that emphasizing the micropapillary component of PTC in the diagnosis is important, as it is associated with lymphovascular invasion, lymph node and distant metastases, and reduced survival rates (14).
Table 2.
Comparison of Clinical and Pathological Characteristics Between Hobnail PTC Cases of Published Case Series and the Cases in the Present Study
| Study (# cases) | F:M | Age (years)* | Inclusion criteria | Tumor size (cm)* | % pTNM Stage III/IV | LVI (%) | Lymph node metastasis (%) | Distant metastasis (%) | Follow-up (mo)# | Disease-specific survival (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| Asioli et al. 2012 (29) (n=24) | 18:6 | 57.3±12.0 | Nonsolid type PTC; ≤10% tall/columnar or diffuse sclerosing features; loss of polarity/cohesiveness with hobnail/micropapillary features in ≥10% of tumor cells; available clinical and follow-up data and pathologic material | 3.0±1.7 | 58.3 | 70.8 | 60.9 | 43.5 | 95 (21–165) | 66.6 |
| Lino-Silva et al. 2012 (14) (n=7) | 3:4 | 45.9±13.4 | Neoplastic cells with papillary morphology, abundant, clear or oncocytic cytoplasm, without true fibrovascular cores, surrounded by a clear halo of fibers surrounded by fibrous stroma; pleomorphic and hyperchromatic nuclei; nuclear grooves; reverse polarity | 4.4±0.3 | 71.4 | 71.4 | 57.1 | 42.9 | 69 (64–74) | 42.9 |
| Present study (n=12) | 9:3 | 54.1±18.8 | Hobnail features in >30% of the tumor; TCF in <30% of the tumors | 3.7±2.0 | 58.3 | 41.7 | 75.0 | 25.0 | 26 (14–35.5) | 90.9 |
Mean age±standard deviation; #median (interquartile range).
The clinical and pathologic characteristics of HVPTC cases in our institution showed similarities and differences with those published in the largest case series (Table 2). Tumors were larger (3.7 cm vs. 3.3 cm) on average, had less lymphovascular invasion (41.7% vs. 71%), increased incidence of lymph node metastasis (75% vs. 60%), and decreased incidence of distant metastasis (25% vs. 43.3%) compared to prior published data. Disease-specific survival was also higher (90.9% vs. 61.3%), but our median follow-up was shorter (2.2 years vs. >6 years).
Given that HVPTC was only recently characterized, it is likely that reported series of other aggressive variants include not previously identified hobnail variants or other PTC variants with hobnail features, which may account for <30% of overall tumor morphology but comprise an appreciable tumor component. With these limitations in mind, it appears as though, in HVPTC, tumors on average are larger (3.7 cm) compared with FVPTC (2.8 cm), classical PTC (2.6 cm), and TCV-PTC (2.6 cm) (31,32). Moreover, HVPTC shows extrathyroidal extension (58.3% vs. 15% in FVPTC vs. 25% in PTC vs. 54% TCV-PTC) and metastasize to the lymph nodes more frequently (75% vs. 16% in FVPTC vs. 34% in PTC vs. 40% in TCV-PTC) (31,32).
Our study is retrospective, with a relatively short follow-up (median, 26 months) due to the novelty of the described entity. However, it is the second largest case series published to date from a single institution and extends our knowledge of this rare variant of PTC, especially in respect of the mutational profile of these tumors.
HVPTC maintains characteristic nuclear features of PTC with well-formed fibrovascular cores. Micropapillary architecture is found in a subset of adenocarcinomas where it is often associated with poor prognosis, such as in the breast, ovary, lung, and urinary bladder (33–36). HVPTC is biologically aggressive, with prominent primary tumor infiltration, metastatic disease, and BRAFV600E mutation. We along with others have reported that BRAF positive mutations correlate with aggressive features, recurrence, and, on univariate analysis, disease-specific mortality (4,16,19,37). The impact of RET/PTC rearrangements for PTC aggressiveness and prognosis is controversial. While our knowledge of this rare PTC variant is in its infancy, our results suggest that some patients with hobnail morphology may have better outcomes if treated early in the natural history of the disease, since, in our cohort, only one out of three patients recurred, and there has been only one disease-related death to date. However, this also may be due to the relatively short follow-up in comparison to other large case series (Table 2). Asioli et al. (29) included cases with hobnail features in ≥10% of tumor cells and investigated the association of clinicopathologic variables with the presence of a hobnail pattern in at least 30% of the tumors, concluding that even a small percentage of hobnail features can be associated with aggressive tumor behavior. Lino-Silva et al. (14) used cases with neoplastic cells with papillary morphology; abundant, clear, or oncocytic cytoplasm; pleomorphic and hyperchromatic nuclei; nuclear grooves; and reverse polarity.
HVPTC clearly warrants further study, and patients with this diagnosis should be observed closely for recurrent disease. Identification of the hobnail pattern, as either HVPTC in cases of >30% of the tumor morphology or hobnail features in cases with a smaller component, and labeling as such in surgical pathology reports and future prognostic analyses will be essential in elucidating the true natural history of this “new” aggressive PTC variant.
Acknowledgments
Funded, in part, by the Program in Cancer Outcomes Research Training Grant (NCI R25CA092203), Massachusetts General Hospital Department of Surgery, and Massachusetts General Hospital American Cancer Society Institutional Research Grant.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.National Cancer Institute 2013 Fast stats: an interactive tool for access to SEER cancer statistics. Available at http://seer.cancer.gov/faststats (accessed on September18, 2013)
- 2.Aschebrook-Kilfoy B, Ward MH, Sabra MM, Devesa SS.2011Thyroid cancer incidence patterns in the United States by histologic type, 1992–2006. Thyroid 21:125–134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Howlader N, Noone AM, Krapcho M, Garshell J, Nwebeyman N, Altekruse SF, Kosary CL, Yu M, Ruhl J, Tatalovich Z, Cho H, Mariotto A, Lewis DR, Chen HS, Feuer EJ, Cronin KA. (eds) SEER cancer statistics review, 1975–2010. Available at http://seer.cancer.gov/csr/1975_2010/ (accessed August20, 2013)
- 4.Prescott JD, Sadow PM, Hodin RA, Le LP, Gaz RD, Randolph GW, Stephen AE, Parangi S, Daniels GH, Lubitz CC.2012BRAF V600E status adds incremental value to current risk classification systems in predicting papillary thyroid carcinoma recurrence. Surgery 152:984–990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hay ID, Thompson GB, Grant CS, Bergstralh EJ, Dvorak CE, Gorman CA, Maurer MS, McIver B, Mullan BP, Oberg AL, Powell CC, van Heerden JA, Goellner JR.2002Papillary thyroid carcinoma managed at the Mayo Clinic during six decades (1940–1999): temporal trends in initial therapy and long-term outcome in 2444 consecutively treated patients. World J Surg 26:879–885 [DOI] [PubMed] [Google Scholar]
- 6.Grogan RH, Kaplan SP, Cao H, Weiss RE, Degroot LJ, Simon CA, Embia OM, Angelos P, Kaplan EL, Schechter RB.2013A study of recurrence and death from papillary thyroid cancer with 27 years of median follow-up. Surgery 154:1436–1446 [DOI] [PubMed] [Google Scholar]
- 7.Ortiz S, Rodriguez JM, Parrilla P, Perez D, Moreno-Gallego A, Rios A, Soria T.2001Recurrent papillary thyroid cancer: analysis of prognostic factors including the histological variant. Eur J Surg 167:406–412 [DOI] [PubMed] [Google Scholar]
- 8.Cooper DS, Doherty GM, Haugen BR, Kloos RT, Lee SL, Mandel SJ, Mazzaferri EL, McIver B, Pacini F, Schlumberger M, Sherman SI, Steward DL, Tuttle RM.2009Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid 19:1167–1214 [DOI] [PubMed] [Google Scholar]
- 9.LiVolsi VA.2010Papillary carcinoma tall cell variant (TCV): a review. Endocr Pathol 21:12–15 [DOI] [PubMed] [Google Scholar]
- 10.Morris LG, Shaha AR, Tuttle RM, Sikora AG, anly I Tall-cell variant of papillary thyroid carcinoma: a matched-pair analysis of survival. Thyroid 20:153–158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lin HW, Bhattacharyya N.2010Clinical behavior of follicular variant of papillary thyroid carcinoma: presentation and survival. Laryngoscope 120:712–716 [DOI] [PubMed] [Google Scholar]
- 12.Asioli S, Erickson LA, Sebo TJ, Zhang J, Jin L, Thompson GB, Lloyd RV.2010Papillary thyroid carcinoma with prominent hobnail features: a new aggressive variant of moderately differentiated papillary carcinoma. A clinicopathologic, immunohistochemical, and molecular study of eight cases. Am J Surg Pathol 34:44–52 [DOI] [PubMed] [Google Scholar]
- 13.Motosugi U, Murata S, Nagata K, Yasuda M, Shimizu M.2009Thyroid papillary carcinoma with micropapillary and hobnail growth pattern: a histological variant with intermediate malignancy? Thyroid 19:535–537 [DOI] [PubMed] [Google Scholar]
- 14.Lino-Silva LS, Dominguez-Malagon HR, Caro-Sanchez CH, Salcedo-Hernandez RA.2012Thyroid gland papillary carcinomas with “micropapillary pattern,” a recently recognized poor prognostic finding: clinicopathologic and survival analysis of 7 cases. Hum Pathol 43:1596–1600 [DOI] [PubMed] [Google Scholar]
- 15.Dias-Santagata D, Akhavanfard S, David SS, Vernovsky K, Kuhlmann G, Boisvert SL, Stubbs H, McDermott U, Settleman J, Kwak EL, Clark JW, Isakoff SJ, Sequist LV, Engelman JA, Lynch TJ, Haber DA, Louis DN, Ellisen LW, Borger DR, Iafrate AJ.2010Rapid targeted mutational analysis of human tumours: a clinical platform to guide personalized cancer medicine. EMBO Mol Med 2:146–158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li C, Lee KC, Schneider EB, Zeiger MA.2012BRAF V600E mutation and its association with clinicopathological features of papillary thyroid cancer: a meta-analysis. J Clin Endocrinol Metab 97:4559–4570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Edge S, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A. (eds) 2009AJCC Cancer Staging Manual. Springer, New York [Google Scholar]
- 18.Nikiforov YE, Steward DL, Robinson-Smith TM, Haugen BR, Klopper JP, Zhu Z, Fagin JA, Falciglia M, Weber K, Nikiforova MN.2009Molecular testing for mutations in improving the fine-needle aspiration diagnosis of thyroid nodules. J Clin Endocrinol Metab 94:2092–2098 [DOI] [PubMed] [Google Scholar]
- 19.Xing M, Alzahrani AS, Carson KA, Viola D, Elisei R, Bendlova B, Yip L, Mian C, Vianello F, Tuttle RM, Robenshtok E, Fagin JA, Puxeddu E, Fugazzola L, Czarniecka A, Jarzab B, O'Neill CJ, Sywak MS, Lam AK, Riesco-Eizaguirre G, Santisteban P, Nakayama H, Tufano RP, Pai SI, Zeiger MA, Westra WH, Clark DP, Clifton-Bligh R, Sidransky D, Ladenson PW, Sykorova V.2013Association between BRAF V600E mutation and mortality in patients with papillary thyroid cancer. JAMA 309:1493–1501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guerra A, Sapio MR, Marotta V, Campanile E, Rossi S, Forno I, Fugazzola L, Budillon A, Moccia T, Fenzi G, Vitale M.2012The primary occurrence of BRAF(V600E) is a rare clonal event in papillary thyroid carcinoma. J Clin Endocrinol Metab 97:517–524 [DOI] [PubMed] [Google Scholar]
- 21.Romei C, Elisei R.2012RET/PTC Translocations and clinico-pathological features in human papillary thyroid carcinoma. Front Endocrinol (Lausanne) 3:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kakudo K, Tang W, Ito Y, Mori I, Nakamura Y, Miyauchi A.2004Papillary carcinoma of the thyroid in Japan: subclassification of common type and identification of low risk group. J Clin Pathol 57:1041–1046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Albores-Saavedra J, Wu J.2006The many faces and mimics of papillary thyroid carcinoma. Endocr Pathol 17:1–18 [DOI] [PubMed] [Google Scholar]
- 24.Liu Z, Bai Y, Li Y, Ohba K, Nakamura H, Ozaki T, Taniguchi E, Mori I, Kakudo K.2010Non-solid type thyroid carcinoma: a case report of moderately differentiated adenocarcinoma of the thyroid. Pathol Int 60:524–527 [DOI] [PubMed] [Google Scholar]
- 25.Liu Z, Kakudo K, Bai Y, Li Y, Ozaki T, Miyauchi A, Taniguchi E, Mori I.2011Loss of cellular polarity/cohesiveness in the invasive front of papillary thyroid carcinoma, a novel predictor for lymph node metastasis; possible morphological indicator of epithelial mesenchymal transition. J Clin Pathol 64:325–329 [DOI] [PubMed] [Google Scholar]
- 26.Bai Y, Kakudo K, Nakamura M, Ozaki T, Li Y, Liu Z, Mori I, Miyauchi A, Zhou G.2009Loss of cellular polarity/cohesiveness in the invasive front of papillary thyroid carcinoma and periostin expression. Cancer Lett 281:188–195 [DOI] [PubMed] [Google Scholar]
- 27.Bai Y, Kakudo K, Li Y, Liu Z, Ozaki T, Ito Y, Kihara M, Miyauchi A.2008Subclassification of non-solid-type papillary thyroid carcinoma identification of high-risk group in common type. Cancer Sci 99:1908–1915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tang W, Nakamura Y, Zuo H, Yasuoka H, Yang Q, Wang X, Nakamura M, Mori I, Miyauchi A, Kakudo K.2003Differentiation, proliferation and retinoid receptor status of papillary carcinoma of the thyroid. Pathol Int 53:204–213 [DOI] [PubMed] [Google Scholar]
- 29.Asioli S, Erickson LA, Righi A, Lloyd RV.2012Papillary thyroid carcinoma with hobnail features: histopathologic criteria to predict aggressive behavior. Hum Pathol 44:320–328 [DOI] [PubMed] [Google Scholar]
- 30.Yang GC, Fried K, Scognamiglio T.2013Cytological features of clear cell thyroid tumors, including a papillary thyroid carcinoma with prominent hobnail features. Diagn Cytopathol 41:757–761 [DOI] [PubMed] [Google Scholar]
- 31.Yu XM, Schneider DF, Leverson G, Chen H, Sippel RS.2013Follicular variant of papillary thyroid carcinoma is a unique clinical entity: a population-based study of 10,740 cases. Thyroid 23:1263–1268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Morris LG, Shaha AR, Tuttle RM, Sikora AG, Ganly I.2010Tall-cell variant of papillary thyroid carcinoma: a matched-pair analysis of survival. Thyroid 20:153–158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen L, Fan Y, Lang RG, Guo XJ, Sun YL, Cui LF, Liu FF, Wei J, Zhang XM, Fu L.2008Breast carcinoma with micropapillary features: clinicopathologic study and long-term follow-up of 100 cases. Int J Surg Pathol 16:155–163 [DOI] [PubMed] [Google Scholar]
- 34.Chang SJ, Ryu HS, Chang KH, Yoo SC, Yoon JH.2008Prognostic significance of the micropapillary pattern in patients with serous borderline ovarian tumors. Acta Obstet Gynecol Scand 87:476–481 [DOI] [PubMed] [Google Scholar]
- 35.Sanchez-Mora N, Presmanes MC, Monroy V, Moreno N, Lara-Martinez JM, Aladro MH, Alvarez-Fernandez E.2008Micropapillary lung adenocarcinoma: a distinctive histologic subtype with prognostic significance. Case series. Hum Pathol 39:324–330 [DOI] [PubMed] [Google Scholar]
- 36.Amin MB, Ro JY, el-Sharkawy T, Lee KM, Troncoso P, Silva EG, Ordonez NG, Ayala AG.1994Micropapillary variant of transitional cell carcinoma of the urinary bladder. Histologic pattern resembling ovarian papillary serous carcinoma. Am J Surg Pathol 18:1224–1232 [DOI] [PubMed] [Google Scholar]
- 37.Elisei R, Viola D, Torregrossa L, Giannini R, Romei C, Ugolini C, Molinaro E, Agate L, Biagini A, Lupi C, Valerio L, Materazzi G, Miccoli P, Piaggi P, Pinchera A, Vitti P, Basolo F.2012The BRAF(V600E) mutation is an independent, poor prognostic factor for the outcome of patients with low-risk intrathyroid papillary thyroid carcinoma: single-institution results from a large cohort study. J Clin Endocrinol Metab 97:4390–4398 [DOI] [PubMed] [Google Scholar]