Abstract
Dental pulp/dentin regeneration using dental stem cells combined with odontogenic factors may offer great promise to treat and/or prevent premature tooth loss. We previously demonstrated that bone morphogenetic protein 9 (BMP9) is one of the most potent factors in inducing bone formation. Here, we investigate whether BMP9 can effectively induce odontogenic differentiation of the stem cells from mouse apical papilla (SCAPs). Using a reversible immortalization system expressing SV40 T flanked with Cre/loxP sites, we demonstrate that the SCAPs can be immortalized, resulting in immortalized SCAPs (iSCAPs) that express mesenchymal stem cell markers. BMP9 upregulates Runx2, Sox9, and PPARγ2 and odontoblastic markers, and induces alkaline phosphatase activity and matrix mineralization in the iSCAPs. Cre-mediated removal of SV40 T antigen decreases iSCAP proliferation. The in vivo stem cell implantation studies indicate that iSCAPs can differentiate into bone, cartilage, and, to lesser extent, adipocytes upon BMP9 stimulation. Our results demonstrate that the conditionally iSCAPs not only maintain long-term cell proliferation but also retain the ability to differentiate into multiple lineages, including osteo/odontoblastic differentiation. Thus, the reversibly iSCAPs may serve as an important tool to study SCAP biology and SCAP translational use in tooth engineering. Further, BMP9 may be explored as a novel and efficacious factor for odontogenic regeneration.
Introduction
Premature tooth loss caused by caries, pulpitis, and apical periodontitis presents a formidable challenge in controlling health care costs and loss of economic productivity, in addition to its adverse effect on the quality of life. Teeth are highly mineralized organs resulting from sequential and reciprocal interactions between the oral epithelium and the underlying cranial neural-crest-derived mesenchyme [1–3]. While de novo tooth engineering may provide great promise for improving clinical outcomes of dental diseases, harnessing the natural regenerative potential of dental stem cells in dentin-pulp tissues may offer more practical solutions to enhance wound healing and maintain pulp vitality [4–6]. Any successful tissue engineering would require at least three components, including biocompatible scaffolding materials, effective biological factors, and progenitors that have differentiation potential of becoming intended tissue types.
Significant progresses have been made toward the identification and characterization of dental mesenchymal progenitors [1,7]. Conventional mesenchymal stem cells (MSCs) are nonhematopoietic multipotent cells, which have the capacity to differentiate into osteoblastic, chondrogenic, and adipogenic lineages although MSCs have been shown to differentiate into other lineages [8–10]. Besides bone marrow, MSCs have been isolated from other tissues, including periosteum, brain, liver, bone marrow, adipose, skeletal muscle, amniotic fluid, and hair follicle lineages [9,10]. While MSCs isolated from various tissues share many similar characteristics, they exhibit discernible differences in their expression profile and differentiation potential [9]. Most of dental structures are derived from dental ectomesenchyme, a zone of condensed cells derived from oral ectoderm during embryonic tooth development [1,4,7].
Dental stem cells are considered a population of MSC-like cells, and at least five types of dental stem/progenitor cells have been identified and characterized thus far [1,7], including dental pulp stem cells (DPSCs), stem cells from human exfoliated deciduous teeth, periodontal ligament stem cells, dental follicle progenitor cells, and stem cells from apical papilla (SCAPs). Although these postnatal populations have MSC-like characteristics, including the self-renewal capability and multilineage differentiation potential, the dental stem cells are isolated from specialized tissues with potent capacities to differentiate into odontogenic cells, and also have the ability to give rise to other cell lineages with different potency from that of bone-marrow-derived MSCs. Originally isolated from the apical part of the papilla [11], we previously demonstrated that bone morphogenetic protein 9 (BMP9; also known as growth and differentiation factor 2, or GDF2) is one of the most potent factors that can induce osteogenic, adipogenic, and to a lesser extent, chondrogenic differentiation [12–16]. Here, we investigate the effect of BMP9 on the osteo/odontogenic differentiation of mouse SCAPs.
To overcome the technical challenge of isolating sufficient stem cells for in vitro and in vivo studies, we sought to investigate whether reversibly immortalized SCAPs (iSCAPs) can maintain long-term cell proliferation without compromising the multipotent differentiation potential. Using the previously characterized reversible immortalization system, which expresses SV40 T antigen flanked with Cre/loxP sites [17–23], we demonstrated that mouse SCAPs can be effectively immortalized with an enhanced proliferative activity. The iSCAPs express most of the MSC markers, suggesting that the iSCAPs may be MSC like. BMP9 upregulates lineage-specific regulators Runx2 (osteogenic), Sox9 (chondrogenic), and PPARγ2 (adipogenic) and odontoblastic markers, and induces osteogenic marker alkaline phosphatase (ALP) activity and matrix mineralization in the iSCAPs in vitro. Cre recombinase-mediated removal of SV40 large T antigen results in a significant decrease in cell proliferation. The in vivo stem cell implantation studies indicate that the iSCAPs are able to form bone, cartilage, and, to a lesser extent, adipose tissues upon BMP9 stimulation. Taken together, our results demonstrate that the conditionally iSCAPs not only maintain long-term cell proliferation but also retain the ability to differentiate into multiple lineages, including osteo/odontoblastic differentiation. The reversibly iSCAPs may serve as an important tool to study SCAP biology and the SCAP translational use in tooth engineering. Further, BMP9 may be explored as a novel and efficacious factor for odontogenic regeneration.
Materials and Methods
Cell culture and chemicals
HEK-293 cell line was purchased from ATCC and maintained in complete Dulbecco's modified Eagle's medium (DMEM) containing 10% fetal bovine serum (Invitrogen), 100 U of penicillin, and 100 μg of streptomycin at 37°C in 5% CO2 [12,24–26]. Unless indicated otherwise, all chemicals were purchased from Sigma-Aldrich or Fisher Scientific.
Generation and amplification of recombinant adenoviruses expressing BMP9, Cre recombinase, and GFP
Recombinant adenoviruses were generated using the AdEasy technology as described [12,13,15,27]. The coding regions of human BMP9 and Cre recombinase were PCR amplified and cloned into an adenoviral shuttle vector, and subsequently used to generate recombinant adenoviruses in HEK-293 cells. The resulting adenoviruses were designated as AdBMP9 and AdCre, both of which also express GFP. Analogous adenovirus expressing only GFP (AdGFP) was used as controls [26–28].
Isolation and immortalization of SCAPs from mouse lower incisor teeth
All animal studies were conducted by following the guidelines approved by Institutional Animal Care and Use Committee (IACUC). Male CD1 mice, 4 weeks old, were euthanized immediately prior to tissue harvest. The lower incisor teeth were carefully extracted, rinsed in sterile phosphate-buffered saline (PBS), and kept in sterile 100-mm cell culture dishes. The apical papilla tissues were resected and minced into small tissue bits, followed by trypsin digestion (0.25% trypsin and 1 mM EDTA) at 37°C for 20–60 min. The dissociated cells were washed in complete DMEM, plated in six-well cell culture plates, and incubated for 24 h at 37°C. Adherent cells were used as SCAPs. Aliquots were kept in a liquid nitrogen tank. All SCAPs used in this study were within five passages.
To establish the iSCAPs, early passage SCAPs (<3 passages) were seeded in 25 cm2 flasks and infected with packaged retrovirus SSR #69, which expresses SV40 T antigen flanked with Cre/loxP sites [17–23]. Stable iSCAP pools were established by selecting the infected cells with hygromycin B (at 4 μg/mL) for 1 week. Aliquots of iSCAPs were kept in liquid nitrogen tanks.
RNA isolation and semiquantitative RT-PCR
Total RNA was isolated by using TRIZOL Reagents (Invitrogen) and used to generate cDNA templates by reverse transcription reactions with hexamer and M-MuLV reverse transcriptase (New England Biolabs). The cDNA products were used as PCR templates. The semiquantitative RT-PCR was carried out as described [19,29–32]. PCR primers (Supplementary Table S1; Supplementary Data are available online at www.liebertpub.com/scd) were designed by using the Primer3 program and used to amplify the genes of interest (∼150–250 bp). A touchdown PCR program was performed as follows: 94°C for 2 min for 1 cycle; 92°C for 20 s, 68°C for 30 s, and 72°C for 12 cycles decreasing 1°C per cycle, and then at 92°C for 20 s, 57°C for 30 s, and 72°C for 20 s for 20–25 cycles, depending on the abundance of target genes. PCR products were resolved on 1.5% agarose gels. All samples were normalized by the expression level of GAPDH.
Crystal violet staining and quantitative analysis
Subconfluent cells were seeded in 12-well plates and subjected to crystal violet staining at the indicated time points. Images were taken from the plates. For quantitative measurement, the stained cells were dissolved in 10% acetic acid at room temperature for 20 min with agitation. Absorbance was measured at 570–590 nm [33–35].
MTT proliferation assay
A modified MTT assay was used as described [24,31,33,34,36,37]. Briefly, iSCAPs were infected with AdCre or AdGFP for 16 h, and replated in 96-well plates at a low density in triplicate. At the indicated time points, 15 μL of MTT dye solution was added to each well and incubated for an additional 4 h. Thereafter, 100 μL/well solubilization/Stop Solution was added to terminate the reactions and to dissolve formazan crystals in a humidified atmosphere overnight. Absorbance at 570 nm was measured using a 96-well microplate reader [33,34].
Immunofluorescence staining
Immunofluorescence staining was performed as described [15,18,19,24,26,38]. Briefly, cells were fixed with methanol, permeabilized with 1% NP-40, and blocked with 10% bovine serum albumin, followed by incubating with CD73, CD44, CD90, CD117/c-kit, CD29, CD133, CD105/endoglin, CD166/ALCAM, or BMPR-II antibody (Santa Cruz Biotechnology) for 1 h. After washing, cells were incubated with Texas Red-labeled secondary antibody (Santa Cruz Biotechnology) for 30 min. Cell nuclei were stained with DAPI. Stains were examined under a fluorescence microscope. Stains without primary antibodies, or with control IgG, were used as negative controls.
ALP activity assay
ALP activity was assessed quantitatively with a modified assay using the Great Escape SEAP Chemiluminescence assay kit (BD Clontech) and qualitatively with histochemical staining assay (using a mixture of 0.1 mg/mL napthol AS-MX phosphate and 0.6 mg/mL Fast Blue BB salt) as described [12,13,15,24,26,28,29,39]. Each assay condition was performed in triplicate and the results were repeated in at least three independent experiments. ALP activity was normalized by total cellular protein concentrations among the samples.
Matrix mineralization assay (Alizarin Red S staining)
The iSCAPs were seeded in 24-well cell culture plates and infected with AdBMP9 or AdGFP. Infected cells were cultured in the presence of ascorbic acid (50 mg/mL) and β-glycerophosphate (10 mM). At 10 days after infection, mineralized matrix nodules were stained for calcium precipitation by Alizarin Red S staining as described [12,13,15,24,26,28,29,39]. Cells were fixed with 0.05% (v/v) glutaraldehyde at room temperature for 10 min and washed with distilled water; fixed cells were incubated with 0.4% Alizarin Red S (Sigma-Aldrich) for 5 min, followed by extensive washing with distilled water. The staining of calcium mineral deposits was recorded under bright-field microscopy.
Oil Red O staining assay
The iSCAPs were seeded in 12-well cell culture plates and infected with AdBMP9 or AdGFP for 10 days. Oil Red O staining was performed as described [15]. Briefly, cells were fixed with 10% formalin at room temperature for 20 min and washed with PBS. The fixed cells were stained with freshly prepared Oil Red O solution (six parts saturated Oil Red O dye in isopropanol plus four parts water) at 37°C for 30–60 min, followed by washing with 70% ethanol and distilled water.
The iSCAP implantation and ectopic bone formation
All animal studies were conducted by following the guidelines approved by the Institutional Animal Care and Use Committee (IACUC). Stem cell-mediated ectopic bone formation was performed as described [13,15,20,32,39–41]. Briefly, iSCAPs were infected with AdBMP9 or AdGFP for 16 h and collected and resuspended in PBS for subcutaneous injection (5×106/injection) into the flanks, or intramuscular injection of quadriceps (1×106/injection) of athymic nude (nu/nu) mice (five animals per group, 4–6 weeks old, female, Harlan Sprague-Dawley). At 4 weeks after implantation, animals were sacrificed, and the implantation sites were retrieved for microcomputed tomography (μCT) imaging, histologic evaluation, and special stains.
μCT analysis
All retrieved specimens were fixed and imaged using the μCT component of a GE triumph (GE Healthcare) trimodality preclinical imaging system. All image data analyses were performed using Amira 5.3 (Visage Imaging, Inc.), and 3D volumetric data were obtained as described [20,29,32,40,41].
Histological evaluation, alcian blue, and trichrome staining
Retrieved tissues were fixed, decalcified in 10% buffered formalin, and embedded in paraffin. Serial sections of the embedded specimens were stained with hematoxylin and eosin (H & E). Trichrome and alcian blue stains were carried out as previously described [12,13,15].
Statistical analysis
The quantitative assays were performed in triplicate and/or repeated three times. Data were expressed as mean±SD. Statistical significances were determined by one-way analysis of variance and the Student's t-test. A value of P<0.05 was considered statistically significant.
Results
The iSCAPs from mouse lower incisor teeth can be maintained in long-term culture
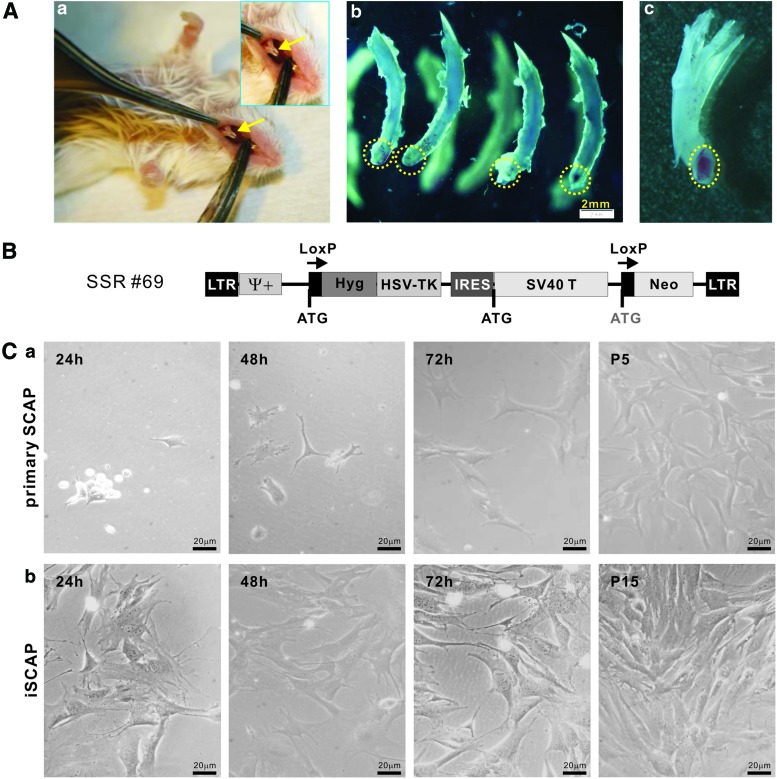
The dental pulp is a highly specialized mesenchymal tissue surrounded by a rigid mineralized tissue. The SCAPs represent one of the five types of progenitor cells that can be identified in dental tissues. Although it is well established that the induced pluripotency of differentiated fibroblasts can be achieved through reprogramming with a set of defined factors, such as Oct3/4, Sox9, Klf4, and c-Myc [42], we sought to investigate whether iSCAPs retain progenitor properties while gaining long-term proliferation capability. We first identified and isolated stem cells of dental apical papilla from mouse lower incisor teeth (Fig. 1A). The resected apical papilla tissues were collected, minced, and digested with Trypsin-EDTA. Adherent SCAPs were subjected to immortalization using the reversible immortalization vector SSR #69 that contains the hygromycin and SV40 T antigen expression cassette flanked with loxP sites (Fig. 1B) [17–23]. Primary SCAPs were shown to grow well, although at a significantly lower rate, up to at least five passages (Fig. 1C, panel a). The iSCAPs grew more rapidly and maintained a high proliferation rate after 15 passages (Fig. 1C, panel b). The iSCAPs have been passed more than 60 generations by now and proliferate well. Thus, these results indicate that we successfully immortalized SCAPs in vitro.
FIG. 1.
Isolation and immortalization of mouse stem cells from apical papilla cells (iSCAPs) from mouse lower incisor teeth. (A) Isolation of SCAPs from mouse lower incisor teeth. The incisor teeth of young adult CD1 mice were identified (a) and carefully extracted (b). The apical papilla areas were resected (b, dotted circles) and retrieved for cell isolation. A representative incisor tooth is shown after the removal of its dental apical papilla (c, the dotted circles). Arrows indicate mouse in cisor teeth. (B) Schematic representation of immortalization vector SSR #69. The retroviral vector expresses SV40 large T antigen flanked with Cre/loxP sites. The stable line should confer resistance to hygromycin B. (C) Morphology of primary SCAPs and the iSCAPs. Primary SCAPs (a) and the iSCAPs (b) were seeded at a low cell density and photographed at the indicated time. Primary SCAPs were maintained up to five passages (P5) while iSCAPs were readily maintained indefinitely (passage 15 is shown). Color images available online at www.liebertpub.com/scd
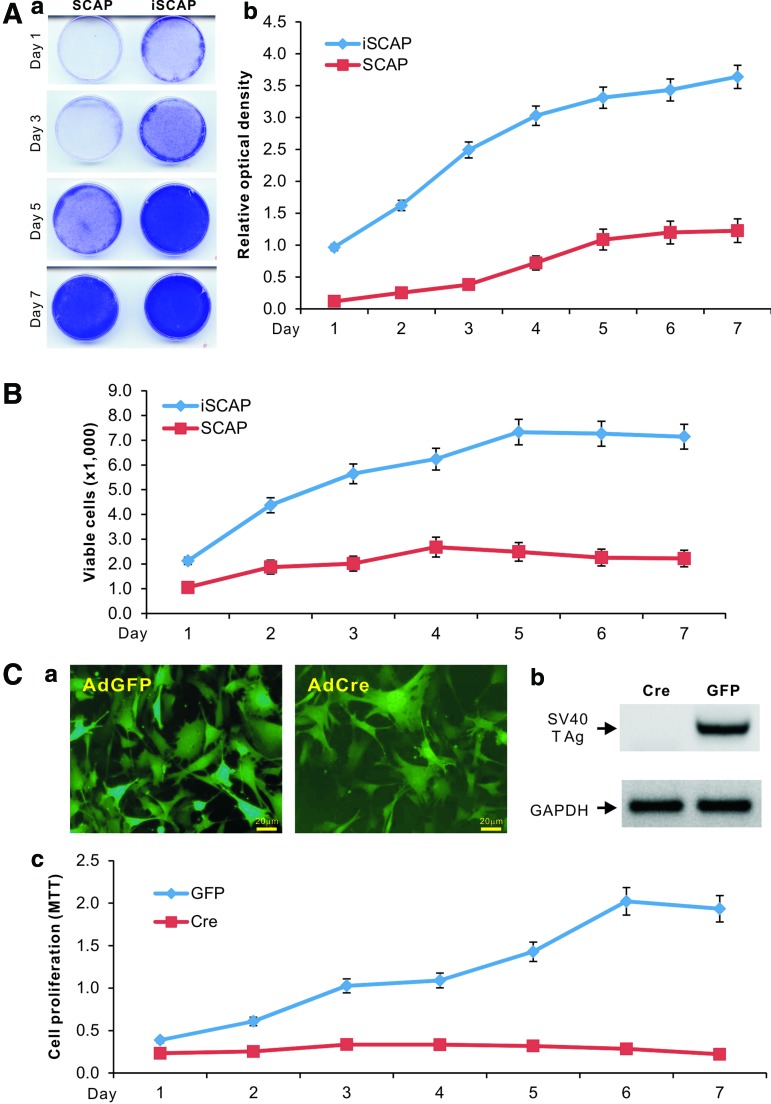
We next carried out quantitative analyses to compare the proliferative activity between primary SCAPs and iSCAPs. Using crystal violet staining assay, we found that iSCAPs reached complete confluence at day 5 while primary SCAPs reached complete confluence at day 7 when both started with a similar cell density (Fig. 2A, panel a). A quantitative assessment of the stained cells confirmed the staining results and revealed that iSCAPs had significantly higher cell staining at each given time point than that of the SCAPs (Fig. 2A, panel b). Direct cell counting experiment further confirmed that iSCAPs grow faster than primary SCAPs (Fig. 2B). Thus, these results demonstrate that iSCAPs can be maintained in long-term culture and exhibit much higher proliferation rate.
FIG. 2.
Proliferative activity and Cre-mediated reversibility of the iSCAPs. (A) Crystal violet staining for cell proliferation and viability of iSCAPs. Subconfluent iSCAPs and primary SCAPs (<3 passages) were seeded in 12-well cell culture plates and stained with crystal violet at the indicated time points (a). The stained cells were dissolved in 10% acetic acid and optical absorbance was measured at 570 to 590 nm (b). Staining for each condition was carried out in triplicate. (B) Viable cell counting. Subconfluent iSCAPs and primary SCAPs (<3 passages) were seeded in 24-well cell culture plates. Cells were collected at the indicated time points and stained with Trypan blue; viable cells were counted in triplicate. (C) Cre-mediated removal of SV40 T antigen in iSCAPs. Subconfluent iSCAPs were effectively transduced with AdCre or control adenoviral vector AdGFP (a). The Cre recombinase-mediated removal of SV40 T antigen in iSCAPs was confirmed by RT-PCR analysis (b). The AdCre- or AdGFP-transduced iSCAPs were seeded at a low confluence and subjected to MTT assay at the indicated time points (c). Each assay condition was done in triplicate. Color images available online at www.liebertpub.com/scd
Cre recombinase can reverse the iSCAPs' proliferative activity
As shown in Fig. 1B, the immortalizing gene SV40 large T antigen can be excised via the action of Cre recombinase on the flanking loxP sites. To effectively express Cre in iSCAPs, we constructed a recombinant adenoviral vector AdCre, which was shown to transduce iSCAPs with high efficiency (Fig. 2C, panel a). The efficient removal of SV40 T antigen by Cre expression was confirmed by RT-PCR analysis in AdCre-infected iSCAPs, but not in the GFP control (Fig. 2C, panel b). When cell proliferation was analyzed using MTT assay, we found that cell proliferation rate of the AdCre-transduced iSCAPs was significantly decreased compared with the AdGFP-infected iSCAPs (Fig. 2C, panel c). Similar results were obtained from crystal violet staining assays and direct cell counting experiments (data not shown). Taken together, these results indicate that the proliferative activity of iSCAPs can be effectively reversed by Cre recombinase.
iSCAPs express MSC markers
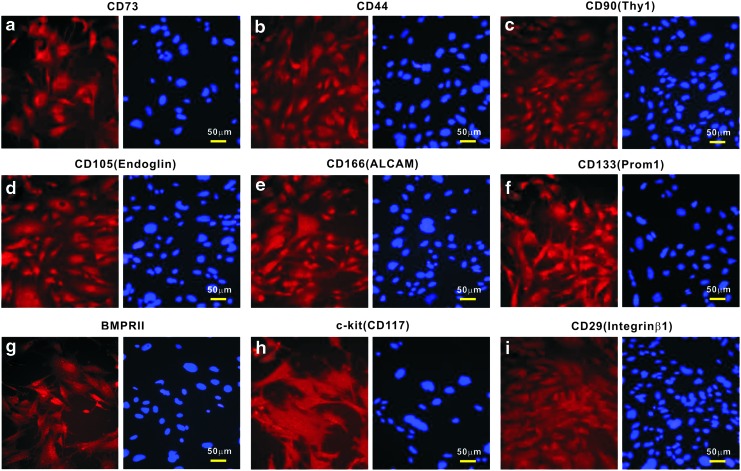
Considering that SCAPs should exhibit certain features of mesenchymal progenitors, we characterized the expression of MSC markers in iSCAPs using immunofluorescence staining. Consensus human MSC markers include CD73, CD44, CD90/Thy-1, CD105/Endoglin, and CD166/ALCAM [43]. We found that the just-mentioned markers were readily detectable in iSCAPs (Fig. 3, panel a–e). Further, we found the expression of other mesenchymal and/or progenitor markers, such as CD133/Prom1, BMPRII, CD117/c-kit, and CD29/integrin β1 (Fig. 3, panel f and i) [20]. We also conducted quantitative flow cytometry analysis of the iSCAPs stained with the antibodies against the five consensus MSC markers, and found that ∼88% to 94% of the iSCAPs were stained positive for these markers (Supplementary Fig. S1). In addition, the iSCAPs lacked the expression of CD45 and CD34 (data not shown). Taken together, these results demonstrate that the iSCAPs express most of the conventional MSC markers, suggesting that these cells may possess MSC-like phenotypes.
FIG. 3.
Endogenous expression of mesenchymal stem cell (MSC) markers in the iSCAPs. Subconfluent iSCAPs were seeded in 24-well cell culture plates for 24 h. Cells were fixed and subjected to immunofluorescence staining with the indicated primary antibodies (a–i), as described in the “Materials and Methods” section. Cell nuclei were counterstained with DAPI. Control IgG and minus primary antibody staining were performed as negative controls (not shown). Representative results are shown. Color images available online at www.liebertpub.com/scd
iSCAPs are capable of differentiating into multiple lineages upon BMP9 stimulation
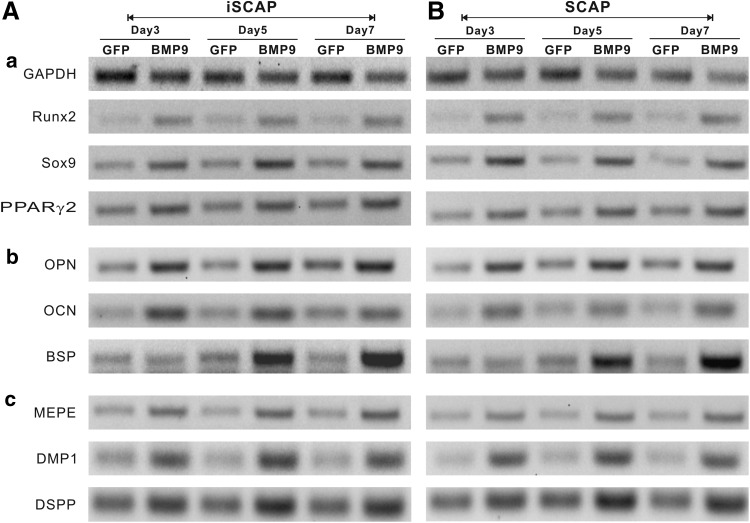
We previously demonstrated that BMP9 is one of the most potent factors that can induce osteogenic, adipogenic, and, to a lesser extent, chondrogenic differentiation [12–16]. Given the fact that iSCAPs express MSC markers, we tested whether the iSCAPs were able to differentiate into osteogenic, chondrogenic, and adipogenic lineages [8,9]. When the primary SCAPs and iSCAPs were transduced with BMP9 or GFP adenoviral vector, the three key lineage-specific regulators Runx2 (osteogenic), Sox9 (chondrogenic), and PPARγ2 (adipogenic) were significantly upregulated at as early as day 3 in both primary and immortalized SCAPs (Fig. 4, panel a). We further analyzed the expression of osteoblastic/odontoblastic differentiation markers, such as osteopontin (OPN), osteocalcin (OCN), and bone sialoprotein (BSP). OPN and OCN were upregulated by BMP9 at all examined time points in both primary and immortalized SCAPs, while BSP was significantly upregulated at later time points (Fig. 4, panel b). Lastly, we studied the BMP9-induced expression of odonotogenic differentiation markers, such as matrix extracellular phosphoglycoprotein (MEPE), dentin matrix acidic phosphoprotein 1 (DMP1), and dentin sialophosphoprotein (DSPP). We found that both MEPE and DMP1 were upregulated by BMP9, while DSPP expression was upregulated with higher endogenous expression (Fig. 4, panel c). These results indicate that the iSCAPs retain multipotency and are capable of differentiating into MSC lineages upon BMP9 stimulation.
FIG. 4.
Bone morphogenetic protein 9 (BMP9) induces expression of lineage markers and osteo/odontogenic differentiation markers in iSCAPs. Subconfluent iSCAPs (A) and primary SCAPs (B) were transduced with AdBMP9 or AdGFP. Total RNA was isolated at the indicated time points and subjected to reverse transcription. The resulted RT products were used for semiquantitative PCR (sqPCR) analysis using primers specific for MSC-lineage-specific genes (a), late osteo/odontogenic markers (b), and odontoblast differentiation markers (c). Each sqPCRs were carried out at least in two independent batches of experiments. All samples were normalized with the endogenous GAPDH expression level. Representative results are shown.
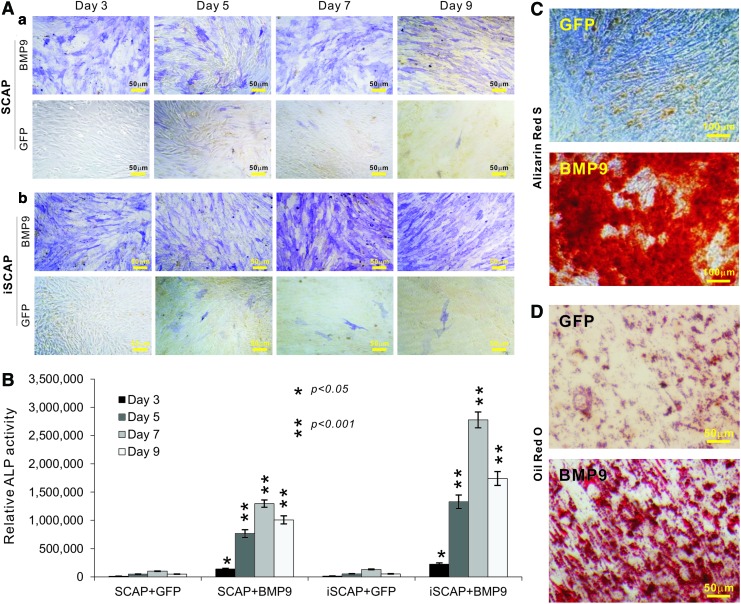
We further analyzed the osteogenic potential of the iSCAPs. When primary SCAPs and iSCAPs were stimulated with BMP9 or control GFP, we found that early osteogenic marker ALP was readily detectable in BMP9-, but not GFP, stimulated cells at as early as day 3, and that the BMP9-stimulated iSCAPs exhibited stronger ALP staining than that of primary SCAPs (Fig. 5A, panels a and b). BMP9-induced ALP activities in SCAPs and iSCAPs were also quantitatively determined and similar results were obtained (Fig. 5B). We further demonstrated that BMP9-transduced iSCAPs effectively underwent late stage of osteogenic differentiation as evidenced by matrix mineralization assessed with Alizarin Red S staining (Fig. 5C). When adipogenic potential was examined, we found that the iSCAPs were readily induced to differentiate into adipocytes upon BMP9 stimulation (Fig. 5D). Taking together, we demonstrate osteogenic and adipogenic potential of the iSCAPs upon BMP9 stimulation.
FIG. 5.
BMP9 induces alkaline phosphatase (ALP), matrix mineralization, and adipogenesis of iSCAPs. (A, B) BMP9-induced ALP activity in primary SCAPs (a) and iSCAPs (b). Subconfluent cells were seeded in 12-well cell culture plates and infected with AdBMP9 or AdGFP. At the indicated time points postinfection, cells were either stained for qualitative ALP activity (A) or lysed for quantitative measurement of ALP activity (B). Each assay condition was done in triplicate. Representative staining results are shown. (C) Alizarin Red S staining. Subconfluent iSCAPs were seeded in 24-well cell culture plates, infected with AdBMP9 or AdGFP, and maintained in matrix mineralization culture medium for 10 days. Matrix mineralization nodules were stained with Alizarin Red S as described in “Materials and Methods” section. Staining results were recorded under a microscope. (D) Oil Red O staining. Subconfluent iSCAPs were seeded in 12-well cell culture plates, infected with AdBMP9 or AdGFP, and cultured for 10 days. Oil Red O staining was performed as described in “Materials and Methods” section. All staining experiments were carried out in duplicate. Representative images are shown. Color images available online at www.liebertpub.com/scd
BMP9 induces effective ectopic bone formation, chondrogenesis, and, to a lesser extent, adipogenesis from iSCAPs
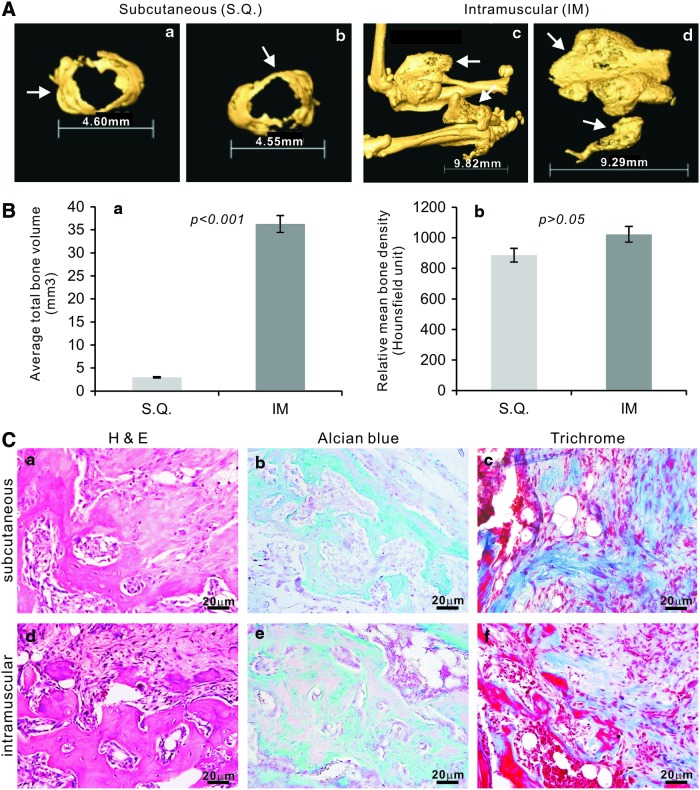
We further tested whether the iSCAPs are able to undergo multilineage differentiation in vivo. Using our previously established stem cell implantation assay [12,13,15,26,29,39], we transduced the iSCAPs with AdBMP9 or AdGFP in culture and collected and injected the cells subcutaneously (SQ) or intramuscularly (IM) into athymic nude mice. Bony masses were retrieved from mice injected with BMP9-transduced iSCAPs after 4 weeks (Fig. 6A), while no masses were formed in the cells transduced with AdGFP. We also tested the primary SCAPs for the in vivo studies and found that only very small masses were detected in BMP9 treatment group (data not shown). μCT imaging indicated that intramuscular injection of BMP9-transduced iSCAPs formed larger and more robust masses than that of the SQ injection (Fig. 6A, panels a, b vs. c, d). Analysis of the average total bone volume indicates that the bone masses were significantly larger than that of SQ injection (P<0.001) (Fig. 6B, panel a). However, there was no significant difference in mean bone density between the two injection routes (Fig. 6B, panel b). H & E staining of the retrieved masses from both SQ and IM injections indicates that BMP9-transduced iSCAPs formed mature and fully mineralized osteoid matrix (Fig. 6C, panels a and d). Interestingly, only sparsely distributed adipocytes were observed, which are significantly lower than that in BMP9-stimulated MSCs or immortalized mouse embryonic fibroblasts (iMEFs) we previously reported [13,15,20,32,39–41]. The presence of chondrocytes and chondroid matrix was evident in both groups with alcian blue staining (Fig. 6C, panels b and e). Trichrome staining further confirms that BMP9-transduced iSCAPs can form mature and highly mineralized osteoid matrix in vivo (Fig. 6C, panels c and f). Thus, the in vivo results strongly suggest that iSCAPs may give rise to osteo/odontogenic, chondrogenic, and, to a lesser extent, adipogenic lineages, which can be effectively initiated by BMP9.
FIG. 6.
BMP9 induces ectopic bone formation from iSCAPs. Subconfluent iSCAPs were infected with AdBMP9 or AdGFP for 16 h. Cells were harvested for subcutaneous injections [3×106 cells/site in 100 μL phosphate-buffered saline (PBS)] into the flanks, or intramuscular (quadriceps) injections (106 cells/site in 100 μL PBS) of athymic nude mice (n=5 each group). At 4 weeks after injection, the animals were sacrificed. Masses formed at the injection sites were retrieved, fixed in formalin, and subjected to μCT imaging (A). Representative 3-D isosurface reconstruction images for subcutaneous (a & b) and intramuscular (c & d) bone masses are shown. No masses were detected in the animals injected with GFP-transduced iSCAPs. The arrows indicate the formed heterotopic bone masses. The 3D reconstruction was performed for all scanned samples, and the average total bone volume (a) and relative mean bone density (b) were determined using the Amira 5.3 software (B). After μCT imaging was completed, the samples were decalcified and subjected to paraffin-embedded sectioning for histologic evaluation (C), including hematoxylin and eosin (H & E) staining (a & d), alcian blue staining (b & e), and trichrome staining (c & f). Representative results are shown. Color images available online at www.liebertpub.com/scd
Discussion
Using the SCAPs isolated from the apical papilla tissue of mouse lower incisor teeth, we demonstrate that these cells can be effectively immortalized with SV40 T antigen. The immortalization is reversible. Further, the iSCAPs express most of the examined MSC markers, and retain multipotent potential of differentiating into osteoblastic/odontoblastic, chondrogenic, and adipogenic lineages, upon BMP9 stimulation, both in vitro and in vivo. Thus, the iSCAPs may be used as a valuable tool to study SCAP biology and the possible utility of SCAPs in tooth engineering. Our results also suggest that BMP9 may be explored as a novel and efficacious factor for odontogenic regeneration.
Dental stem cells are considered as MSC-like progenitors. Thus, markers that are used for identifying MSCs are also used for dental stem cells. Unfortunately, MSCs usually lack specific markers. It is generally accepted that MSCs have to satisfy three criteria [43]. First, MSCs must be plastic adherent when maintained in standard culture conditions. Second, more than 95% of the MSC population should express CD105, CD73, and CD90, but lack expression (<2% positive) of CD45 and CD34. Third, the cells must be able to differentiate to osteoblasts, adipocytes, and chondroblasts under standard in vitro differentiating conditions [43]. We have shown that the iSCAPs express CD105 and CD73, and other stromal/progenitor cell markers, such as CD44, CD99, c-kit/CD117, CD29, CD133, and CD166. A recent study compared the osteo/odontogenic differentiation potential of human DPSCs and human SCAPs [44]. It was shown that both types of stem cells displayed an active potential for cellular migration, organization, and mineralization, producing 3D mineralized structures, progressively expressing differentiation markers, including DSPP, BSP, OCN, and ALP, with the characteristics of osteodentin [44]. Interestingly, SCAPs showed a significantly higher proliferation rate and mineralization potential [44], suggesting that SCAPs may serve a better source of progenitors for dental tissue engineering.
Two types of dental stem cells were previously immortalized. Saito et al. reported that a cementoblast progenitor cell line, BCPb8, was isolated from dental follicle cells and immortalized with Bmi-1 and hTERT [45]. The immortalized BCPb8 showed the potential to differentiate into cementoblasts on implantation in vivo [45]. It was further reported that mouse PDL progenitors of mouse dental follicle (MDF) cells from mouse incisor tooth germs could be immortalized by expressing a mutant HPV type 16 E6 gene without the PDZ-domain-binding motif [46]. The resultant MDF (E6-EGFP) cells had an extended life span, expressed tendon/ligament-phenotype-related genes, and had the capacity to generate PDL-like tissue that expressed periostin, Scx, and type XII collagen and the fibrillar assembly of type I collagen [46].
Because the life span of primary dental stem cells, such as SCAPs, is limited and the isolation of primary SCAPs is time consuming and labor intensive. Thus, there is an essential need to develop SCAPs with infinite growth. Cell immortalization and transformation strategies have been widely used [47]. In general, transformation approaches involve in overexpression of oncogenes and/or inactivation of tumor-suppressor genes. Commonly used oncogenes include KRas, c-myc, CDK4, and cyclin D1, to name a few, while the frequently inactivated tumor suppressor genes are p53, Rb, and p16INK. Another commonly used gene for immortalization is telomerase (TERT). Ectopic expression of the catalytic subunit of mouse telomerase (MTert) confers a growth advantage to primary MEFs and facilitates their spontaneous immortalization by targeting the TGFβ pathway [48].
In this study, we use SV40 large T antigen to immortalize SCAPs. Large T antigen plays essential roles in the infection of permissive cells, leading to production of progeny SV40 virions, and in the infection of nonpermissive cells, leading to malignant transformation [49]. The ability of SV40 large T antigen to immortalize cells is largely dependent on its ability to complex with p53. Unlike the oncogenes, SV40 T-antigen-transformed cells are in general less tumorigenic [50]. Thus, SV40 T antigen has become one of the most commonly used genes to immortalize primary mammalian cells. In fact, using the reported system [17], we have successfully immortalized MEFs, hepatic progenitor cells, melanoblastic progenitor cells, and fetal cardiomyogenic progenitors [18–22]. These immortalized cells can be reversed and maintained indefinitely in culture without losing their lineage-specific differentiation potential.
In summary, to overcome the challenges in maintaining sufficient SCAPs for in vitro and in vivo studies, we demonstrate that SCAPs can be efficiently immortalized by SV40 T antigen. The reversibly iSCAPs exhibit high proliferative activity and maintain long-term cell proliferation, which can be reversed by introducing Cre recombinase. The iSCAPs express most of the MSC markers and retain multipotent differentiation potential as they can differentiate into osteo/odontogenic, chondrogenic, and adipogenic lineages upon BMP9 stimulation. Thus, the iSCAPs should be a valuable tool for studying SCAP biology and the SCAP translational use in tooth engineering. Further, BMP9 may be explored as a novel and efficacious factor for odontogenic tissue engineering.
Supplementary Material
Acknowledgments
The authors thank Dr. Chad Hanley of the Department of Radiology at the University of Chicago for his assistance and advice on μCT scanning and imaging analysis. The reported work was supported in part by research grant from the National Institutes of Health (AT004418, AR50142, and AR054381 to T.C.H., R.C.H., and H.H.L.), and the 973 Program of Ministry of Science and Technology (MOST) of China (no. 2011CB707900 to T.C.H.), the Natural Science Foundation of China (no. 81271183 to F.D., and no. 81301551 to E.H.), and Chongqing Municipal Commissions on Education, and Science & Technology (no. KJ130303 and no. cstc2013jcyjA0093 to J.W.). This work was also supported in part by the University of Chicago Core Facility Subsidy Grant from the National Center for Advancing Translational Sciences (NCATS) of the National Institutes of Health through grant number UL1 TR000430.
Author Disclosure Statement
The authors declare no conflicts of interest.
References
- 1.Huang GT, Gronthos S. and Shi S. (2009). Mesenchymal stem cells derived from dental tissues vs. those from other sources: their biology and role in regenerative medicine. J Dent Res 88:792–806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bluteau G, Luder HU, De Bari C. and Mitsiadis TA. (2008). Stem cells for tooth engineering. Eur Cell Mater 16:1–9 [DOI] [PubMed] [Google Scholar]
- 3.Rai S, Kaur M. and Kaur S. (2013). Applications of stem cells in interdisciplinary dentistry and beyond: an overview. Ann Med Health Sci Res 3:245–254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang GT. (2011). Dental pulp and dentin tissue engineering and regeneration: advancement and challenge. Front Biosci (Elite Ed) 3:788–800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goldberg M. (2011). Pulp healing and regeneration: more questions than answers. Adv Dent Res 23:270–274 [DOI] [PubMed] [Google Scholar]
- 6.Smith AJ, Smith JG, Shelton RM. and Cooper PR. (2012). Harnessing the natural regenerative potential of the dental pulp. Dent Clin North Am 56:589–601 [DOI] [PubMed] [Google Scholar]
- 7.Tziafas D. and Kodonas K. (2010). Differentiation potential of dental papilla, dental pulp, and apical papilla progenitor cells. J Endod 36:781–789 [DOI] [PubMed] [Google Scholar]
- 8.Prockop DJ. (1997). Marrow stromal cells as stem cells for nonhematopoietic tissues. Science 276:71–74 [DOI] [PubMed] [Google Scholar]
- 9.Rastegar F, Shenaq D, Huang J, Zhang W, Zhang BQ, He BC, Chen L, Zuo GW, Luo Q, et al. (2010). Mesenchymal stem cells: molecular characteristics and clinical applications. World J Stem Cells 2:67–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shenaq DS, Rastegar F, Petkovic D, Zhang BQ, He BC, Chen L, Zuo GW, Luo Q, Shi Q, et al. (2010). Mesenchymal progenitor cells and their orthopedic applications: forging a path towards clinical trials. Stem Cells Int 2010:519028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sonoyama W, Liu Y, Yamaza T, Tuan RS, Wang S, Shi S. and Huang GT. (2008). Characterization of the apical papilla and its residing stem cells from human immature permanent teeth: a pilot study. J Endod 34:166–171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cheng H, Jiang W, Phillips FM, Haydon RC, Peng Y, Zhou L, Luu HH, An N, Breyer B, et al. (2003). Osteogenic activity of the fourteen types of human bone morphogenetic proteins (BMPs). J Bone Joint Surg Am 85-A:1544–1552 [DOI] [PubMed] [Google Scholar]
- 13.Kang Q, Sun MH, Cheng H, Peng Y, Montag AG, Deyrup AT, Jiang W, Luu HH, Luo J, et al. (2004). Characterization of the distinct orthotopic bone-forming activity of 14 BMPs using recombinant adenovirus-mediated gene delivery. Gene Ther 11:1312–1320 [DOI] [PubMed] [Google Scholar]
- 14.Luu HH, Song WX, Luo X, Manning D, Luo J, Deng ZL, Sharff KA, Montag AG, Haydon RC. and He TC. (2007). Distinct roles of bone morphogenetic proteins in osteogenic differentiation of mesenchymal stem cells. J Orthop Res 25:665–677 [DOI] [PubMed] [Google Scholar]
- 15.Kang Q, Song WX, Luo Q, Tang N, Luo J, Luo X, Chen J, Bi Y, He BC, et al. (2009). A comprehensive analysis of the dual roles of BMPs in regulating adipogenic and osteogenic differentiation of mesenchymal progenitor cells. Stem Cells Dev 18:545–559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Luther G, Wagner ER, Zhu G, Kang Q, Luo Q, Lamplot J, Bi Y, Luo X, Luo J, et al. (2011). BMP-9 induced osteogenic differentiation of mesenchymal stem cells: molecular mechanism and therapeutic potentia. Curr Gene Ther 11:229–240 [DOI] [PubMed] [Google Scholar]
- 17.Westerman KA. and Leboulch P. (1996). Reversible immortalization of mammalian cells mediated by retroviral transfer and site-specific recombination. Proc Natl Acad Sci U S A 93:8971–8976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bi Y, Huang J, He Y, Zhu GH, Su Y, He BC, Luo J, Wang Y, Kang Q, et al. (2009). Wnt antagonist SFRP3 inhibits the differentiation of mouse hepatic progenitor cells. J Cell Biochem 108:295–303 [DOI] [PubMed] [Google Scholar]
- 19.Huang J, Bi Y, Zhu GH, He Y, Su Y, He BC, Wang Y, Kang Q, Chen L, et al. (2009). Retinoic acid signalling induces the differentiation of mouse fetal liver-derived hepatic progenitor cells. Liver Int 29:1569–1581 [DOI] [PubMed] [Google Scholar]
- 20.Huang E, Bi Y, Jiang W, Luo X, Yang K, Gao JL, Gao Y, Luo Q, Shi Q, et al. (2012). Conditionally immortalized mouse embryonic fibroblasts retain proliferative activity without compromising multipotent differentiation potential. PLoS One 7:e32428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang K, Chen J, Jiang W, Huang E, Cui J, Kim SH, Hu N, Liu H, Zhang W, et al. (2012). Conditional immortalization establishes a repertoire of mouse melanocyte progenitors with distinct melanogenic differentiation potential. J Invest Dermatol 132:2479–2483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li M, Chen Y, Bi Y, Jiang W, Luo Q, He Y, Su Y, Liu X, Cui J, et al. (2013). Establishment and characterization of the reversibly immortalized mouse fetal heart progenitors. Int J Med Sci 10:1035–1046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang X, Cui J, Zhang BQ, Zhang H, Bi Y, Kang Q, Wang N, Bie P, Yang Z, et al. (2013). Decellularized liver scaffolds effectively support the proliferation and differentiation of mouse fetal hepatic progenitors. J Biomed Mater Res A [Epub ahead of print]; DOI: 10.1002/jbm.a.34764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Luo X, Chen J, Song WX, Tang N, Luo J, Deng ZL, Sharff KA, He G, Bi Y, et al. (2008). Osteogenic BMPs promote tumor growth of human osteosarcomas that harbor differentiation defects. Lab Invest 88:1264–1277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Haydon RC, Zhou L, Feng T, Breyer B, Cheng H, Jiang W, Ishikawa A, Peabody T, Montag A, Simon MA. and He TC. (2002). Nuclear receptor agonists as potential differentiation therapy agents for human osteosarcoma. Clin Cancer Res 8:1288–1294 [PMC free article] [PubMed] [Google Scholar]
- 26.Tang N, Song WX, Luo J, Luo X, Chen J, Sharff KA, Bi Y, He BC, Huang JY, et al. (2009). BMP9-induced osteogenic differentiation of mesenchymal progenitors requires functional canonical Wnt/beta-catenin signaling. J Cell Mol Med 13:2448–2464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Luo J, Deng ZL, Luo X, Tang N, Song WX, Chen J, Sharff KA, Luu HH, Haydon RC, et al. (2007). A protocol for rapid generation of recombinant adenoviruses using the AdEasy system. Nat Protoc 2:1236–1247 [DOI] [PubMed] [Google Scholar]
- 28.Luo Q, Kang Q, Si W, Jiang W, Park JK, Peng Y, Li X, Luu HH, Luo J, et al. (2004). Connective tissue growth factor (CTGF) is regulated by Wnt and bone morphogenetic proteins signaling in osteoblast differentiation of mesenchymal stem cells. J Biol Chem 279:55958–55968 [DOI] [PubMed] [Google Scholar]
- 29.Zhang W, Deng ZL, Chen L, Zuo GW, Luo Q, Shi Q, Zhang BQ, Wagner ER, Rastegar F, et al. (2010). Retinoic acids potentiate BMP9-induced osteogenic differentiation of mesenchymal progenitor cells. PLoS One 5:e11917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rastegar F, Gao JL, Shenaq D, Luo Q, Shi Q, Kim SH, Jiang W, Wagner ER, Huang E, et al. (2010). Lysophosphatidic acid acyltransferase beta (LPAATbeta) promotes the tumor growth of human osteosarcoma. PLoS One 5:e14182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Su Y, Wagner ER, Luo Q, Huang J, Chen L, He BC, Zuo GW, Shi Q, Zhang BQ, et al. (2011). Insulin-like growth factor binding protein 5 suppresses tumor growth and metastasis of human osteosarcoma. Oncogene 30:3907–3917 [DOI] [PubMed] [Google Scholar]
- 32.Huang E, Zhu G, Jiang W, Yang K, Gao Y, Luo Q, Gao JL, Kim SH, Liu X, et al. (2012). Growth hormone synergizes with BMP9 in osteogenic differentiation by activating the JAK/STAT/IGF1 pathway in murine multilineage cells. J Bone Miner Res 27:1566–1575 [DOI] [PubMed] [Google Scholar]
- 33.He BC, Chen L, Zuo GW, Zhang W, Bi Y, Huang J, Wang Y, Jiang W, Luo Q, et al. (2010). Synergistic antitumor effect of the activated PPARgamma and retinoid receptors on human osteosarcoma. Clin Cancer Res 16:2235–2245 [DOI] [PubMed] [Google Scholar]
- 34.He BC, Gao JL, Zhang BQ, Luo Q, Shi Q, Kim SH, Huang E, Gao Y, Yang K, et al. (2011). Tetrandrine inhibits Wnt/beta-catenin signaling and suppresses tumor growth of human colorectal cancer. Mol Pharmacol 79:211–219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hu N, Jiang D, Huang E, Liu X, Li R, Liang X, Kim SH, Chen X, Gao JL, et al. (2013). BMP9-regulated angiogenic signaling plays an important role in the osteogenic differentiation of mesenchymal progenitor cells. J Cell Sci 126:532–541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Luu HH, Kang Q, Park JK, Si W, Luo Q, Jiang W, Yin H, Montag AG, Simon MA, et al. (2005). An orthotopic model of human osteosarcoma growth and spontaneous pulmonary metastasis. Clin Exp Metastasis 22:319–329 [DOI] [PubMed] [Google Scholar]
- 37.Su Y, Luo X, He BC, Wang Y, Chen L, Zuo GW, Liu B, Bi Y, Huang J, et al. (2009). Establishment and characterization of a new highly metastatic human osteosarcoma cell line. Clin Exp Metastasis 26:599–610 [DOI] [PubMed] [Google Scholar]
- 38.Zhu GH, Huang J, Bi Y, Su Y, Tang Y, He BC, He Y, Luo J, Wang Y, et al. (2009). Activation of RXR and RAR signaling promotes myogenic differentiation of myoblastic C2C12 cells. Differentiation 78 195–204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sharff KA, Song WX, Luo X, Tang N, Luo J, Chen J, Bi Y, He BC, Huang J, et al. (2009). Hey1 basic helix-loop-helix protein plays an important role in mediating BMP9-induced osteogenic differentiation of mesenchymal progenitor cells. J Biol Chem 284:649–659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Luo J, Tang M, Huang J, He BC, Gao JL, Chen L, Zuo GW, Zhang W, Luo Q, et al. (2010). TGFbeta/BMP type I receptors ALK1 and ALK2 are essential for BMP9-induced osteogenic signaling in mesenchymal stem cells. J Biol Chem 285:29588–29598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen L, Jiang W, Huang J, He BC, Zuo GW, Zhang W, Luo Q, Shi Q, Zhang BQ, et al. (2010). Insulin-like growth factor 2 (IGF-2) potentiates BMP-9-induced osteogenic differentiation and bone formation. J Bone Miner Res 25:2447–2459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K. and Yamanaka S. (2007). Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 131:861–872 [DOI] [PubMed] [Google Scholar]
- 43.Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop D. and Horwitz E. (2006). Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 8:315–317 [DOI] [PubMed] [Google Scholar]
- 44.Bakopoulou A, Leyhausen G, Volk J, Tsiftsoglou A, Garefis P, Koidis P. and Geurtsen W. (2011). Comparative analysis of in vitro osteo/odontogenic differentiation potential of human dental pulp stem cells (DPSCs) and stem cells from the apical papilla (SCAP). Arch Oral Biol 56:709–721 [DOI] [PubMed] [Google Scholar]
- 45.Saito M, Handa K, Kiyono T, Hattori S, Yokoi T, Tsubakimoto T, Harada H, Noguchi T, Toyoda M, Sato S. and Teranaka T. (2005). Immortalization of cementoblast progenitor cells with Bmi-1 and TERT. J Bone Miner Res 20:50–57 [DOI] [PubMed] [Google Scholar]
- 46.Yokoi T, Saito M, Kiyono T, Iseki S, Kosaka K, Nishida E, Tsubakimoto T, Harada H, Eto K, Noguchi T. and Teranaka T. (2007). Establishment of immortalized dental follicle cells for generating periodontal ligament in vivo. Cell Tissue Res 327:301–311 [DOI] [PubMed] [Google Scholar]
- 47.vom Brocke J, Schmeiser HH, Reinbold M. and Hollstein M. (2006). MEF immortalization to investigate the ins and outs of mutagenesis. Carcinogenesis 27:2141–2147 [DOI] [PubMed] [Google Scholar]
- 48.Geserick C, Tejera A, Gonzalez-Suarez E, Klatt P. and Blasco MA. (2006). Expression of mTert in primary murine cells links the growth-promoting effects of telomerase to transforming growth factor-beta signaling. Oncogene 25:4310–4319 [DOI] [PubMed] [Google Scholar]
- 49.Prives C. (1990). The replication functions of SV40 T antigen are regulated by phosphorylation. Cell 61:735–738 [DOI] [PubMed] [Google Scholar]
- 50.Gee CJ. and Harris H. (1979). Tumorigenicity of cells transformed by Simian virus 40 and of hybrids between such cells and normal diploid cells. J Cell Sci 36:223–240 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.