Abstract
Mechanisms leading to the observed immune dysregulation in chronic HIV infection are not well understood. The MHC-II class ligand, lymphocyte activation gene-3 (LAG-3, CD223), has been implicated in the complex regulation mechanism of immune functions. In this study, we describe a new population of HIV-specific CD8+ T cells expressing LAG-3. These LAG-3+CD8+ T cells do not display immunophenotypic patterns traditionally attributed to regulatory T cells. The LAG3+CD8+ T cells are CCR7+,CD127−, and display heterogeneous surface expressions of CD45RA and CD25. Interestingly, HIV-specific LAG-3+CD8+ T cells do not substantially express CTLA-4 and LAG-3 expression does not correlate with interleukin (IL)-10 or tumor growth factor (TGF)-β production. In addition, HIV-specific LAG3+CD8+ T cells do not produce interferon (IFN-γ) or express CD107a. The frequency of HIV-specific LAG3+CD8+ T cells negative correlated with plasma viral load. Our study introduces a new population of HIV-specific CD8+ T cells and proposes additional mechanisms of immune regulation in chronic HIV infection.
Introduction
Lymphocyte activation gene-3 (LAG-3, CD223) is an MHC class II ligand related to CD4 that forms part of the immunoglobulin superfamily.1–3 Many cells of the hematopoietic lineage such as B, NK, and γδ T cells and activated and regulatory CD4+ and CD8+ express LAG-3.1,2,4–8 LAG-3 is believed to play an important role in regulating T cell homeostasis and inhibiting antigen-driven T cell expansion.9–12 In addition, LAG-3 expression is required for maximal induction of regulatory T cell function.13
Chronic HIV-1 infection is characterized by the progressive loss of immune function and subsequent failure of the immune system to eradicate the infection. Impaired CD8+ T cell function, a common feature of chronic HIV disease, leads to suboptimal viral control.14 LAG-3 expression is associated with negative regulatory cell-surface patterns that are determinants of CD8+ T cell exhaustion in lymphocytic choriomeningitis virus and HIV infections.15,16 Elevated plasma LAG-3 levels and lymphatic tissue expression have been associated with advanced HIV disease.17,18 Simultaneous blockade of the T cell inhibitory receptors PD-1 and LAG-3 has shown improved T cell responses and viral control in vivo.15,19 Interestingly, LAG-3 is also a marker for recently activated effector T cells, although differential expression of LAG-3 on T cells has not been fully elucidated in HIV infection.9,15,20
In this study we evaluate the role of LAG-3 in CD8+ effector dysfunction and its association with other negative immune regulators, including PD-1, CTLA-4, and 2B4. We describe a new subpopulation of LAG-3+CD8+ T cells that is antigen specific. Expression of LAG-3 on HIV-specific T cells negatively correlates with viral load, suggesting a possible role in viral control.
Materials and Methods
Study subjects and samples
Peripheral blood mononuclear cell (PBMC) samples from HIV-positive volunteers (n=35) were obtained from the Research in Access to Care in the Homeless (REACH) cohort in San Francisco as previously described.21–23 Demographic information and CD4+ T cell count were obtained at the time of enrollment and blood draw. HIV-positive volunteers on antiretroviral treatment (ART) were usually on a protease inhibitor or a nonnucleoside reverse transcriptase inhibitor. CD4+ T cell count and viral loads were measured approximately every month. The CD4+ T cell counts for these samples were 422–1,283×106 cells/liter (median=494×106 cells/liter). Viral plasma loads were undetectable. Institutional Review Board approvals were obtained from the California Department of Public Health and University of California San Francisco Committee on Human Research. All participants in the study gave written and informed consent. None of the study participants had received antiretroviral therapy for at least 6 months. HIV RNA levels were determined from plasma using the Roche Amplicor 1.5 (generously donated by Roche Diagnostic Systems), per the manufacturer's instructions. PBMCs were separated and cryopreserved in liquid nitrogen until assay time.
Antigens
Peptides corresponding to the subtype B consensus sequences of HIV-1 for Gag and Nef were synthesized as 15 amino acids (a.a.) overlapping by 11 a.a. (AIDS Research and Reference Reagent Program, Division of AIDS, NIAID, www.aidsreagent.org). Synthetic peptides for Gag (total=123) and Nef (total=49) were pooled into single pools of peptides with a final concentration of 1 μg/ml per peptide. A single pool of overlapping peptides corresponding to the amino acid sequence of pp65 protein from JPT Peptide Technologies GmbH (Berlin, Germany) was used to detect human CMV-specific responses.24,25
Flow cytometry reagents
The following antibodies were used in the assays per the manufacturer's instructions (Becton Dickinson, San Jose, CA): AmCyan-conjugated anti-CD3 (clone SK7), PerCP-Cy5.5 or PE-Cy7-conjugated anti-CD4 (clone SK3), APC-H7-conjugated anti-CD8 (clone SK1), PE-Cy5-conjugated CTLA-4 (CD152; clone BNI3), PE-conjugated anti-CD25 (clone 2A3), PE-Cy7- or APC-conjugated anti-CD45RA clone (clone L48; clone HI100, respectively), PE-conjugated anti-CD107a (clone H4A3), Alexa Fluor 647-conjugated anti-CD127 (clone hIL-7R-M21), or APC-conjugated anti-interferon (IFN)-γ (clone 25723.11). FITC-conjugated anti-LAG-3 staining was used according to the manufacturer's instructions (CD223; clone 17B4, Burlington, NC). All samples were acquired using a multichannel (eight-color) BD LSRII flow cytometer (Becton Dickinson, San Jose, CA).
Flow-based CD107a degranulation assay
The degranulation assay was performed as previously described.23,26 Briefly, PBMCs (1×106) were stimulated with HIV (Gag or Nef) or human CMV peptide pools and incubated in the presence of anti-CD107a-PE (BD Biosciences), 1 μg/ml costimulatory anti-CD49d and anti-CD28, and Golgi-Stop (BD Biosciences) for 6 h at 37°C in 5% CO2. Staphylococcus enterotoxin B (SEB) (1 μg/ml; Sigma-Aldrich) and media alone were used as positive and negative controls, respectively. Cells were subsequently stained with anti-CD45RA PE, anti-CCR7 PE CY7, anti-CD3 PerCP Cy5.5, and anti-CD8 allophycocyanin Cy7, CD4 Pacific Blue (BD Pharmingen). A minimum of 30,000 CD8+ events/sample was acquired by flow cytometry. Percentages of CD3+CD8+ T cells expressing CD107a were determined after subtraction of background activity using preset gating. All volunteers demonstrated significant CD107a expression following SEB stimulation.
Intracellular cytokine flow cytometry
IFN-γ production was performed as previously described.23 PBMCs (1×106) were incubated with SEB or peptide pools for 6 h at 37°C in 5% CO2 in the presence of costimulatory anti-CD28 and anti-CD49d (1 μg/ml, Becton-Dickinson) and Golgi-Stop (BD Pharmigen). Alternatively, PBMCs were stimulated for 24 h in the absence of Golgi-Stop followed by restimulation with SEB or peptide pools in the presence of Golgi-Stop for an additional 6 h. A minimum of 30,000 CD3+ T cells per sample was acquired using eight-color flow cytometer (LSRII, BD Biosciences) and analysis was performed by FlowJo software (TreeStar, San Carlos, CA).
LAG-3 surface expression on antigen-specific CD8+ T cells
Detection of HIV-specific LAG-3 or CTLA-4 expressing CD8+ T cells was performed using PBMCs (1×106) incubated with either Gag, Nef, or CMV pp65 peptide pools incubated for 24 h at 37°C in 5% CO2 in the presence of costimulatory anti-CD49d and anti-CD28 (1 μg/ml, BD Biosciences). Cells were further stained with the following abs: LAG-3-FITC, CTLA-4-PE-Cy5, CD3-AmCyan, CD8-APC-H7, 2B4 PE (CD244), CD25-PE, CD127-Alexa Fluor 647, PD-1 APC (CD279), and CD45RA-PE-Cy7. PBMCs were fixed with BD FACS Lysing Solution and a minimum of 30,000 CD3+ T cells per sample was acquired on the flow cytometer (BD LSRII) and analyzed using FlowJo software. Stimulation with SEB (1 μg/ml, Sigma-Aldrich) was used as a positive control for the expression of LAG-3. Media alone was used as a negative control. To detect CD107a and IFN-γ on LAG-3-expressing CD8+ T cells, PBMCs were stimulated as described above, washed twice with phosphate-buffered saline (PBS) buffer, and restimulated with peptides in the presence of anti-CD28, anti-CD49, and Golgi-Stop (BD Biosciences) for an additional 6 h and analyzed for the expression of CD107a-PE and IFN-γ-APC. A minimum of 30,000 CD3+ T cells was acquired on the flow cytometer (BD LSRII) and analyzed using FlowJo software.
Statistical analysis
Groups were compared using the Mann–Whitney U test and analysis was performed with PRISM software version 5.0 (Graph-Pad). Correlations were determined using the Spearman rank correlation test (Graph-Pad). This statistical test like the Mann–Whitney U test is a nonparametric test and does not assume normality, homoscedasticity, or linearity and is calculated by rank. Statistical significance was defined as p<0.05.
Results
LAG-3 is expressed on HIV-specific CD8+ T cells and does not correspond with IFN-γ or CD107a expression
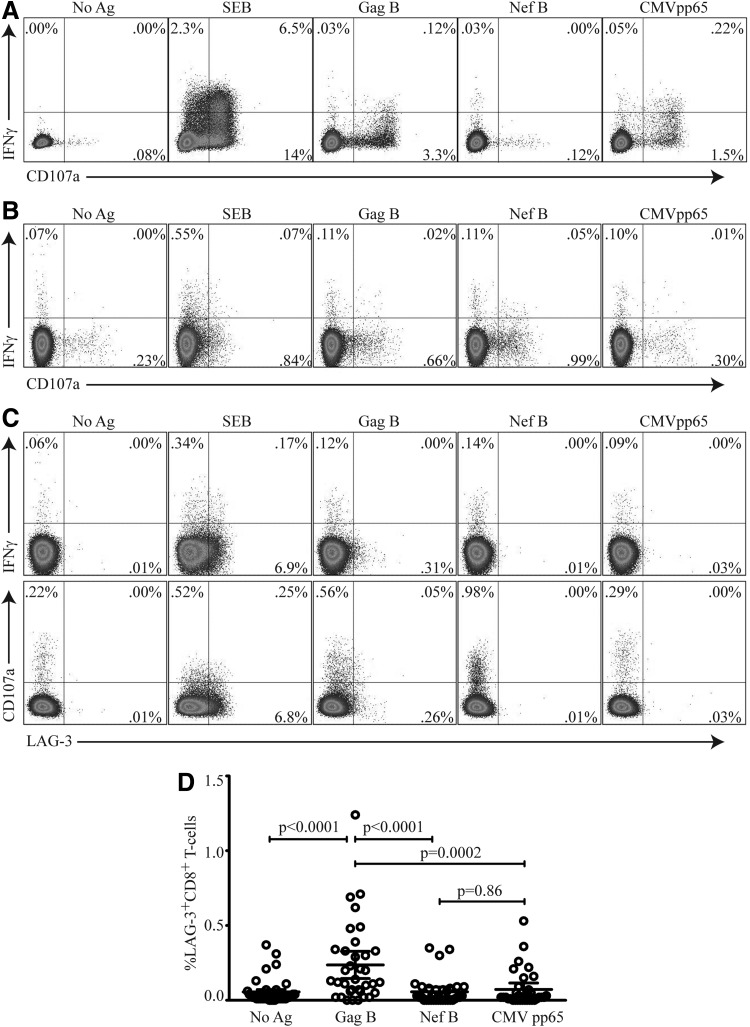
In this study we assessed the frequency of LAG-3, IFN-γ, and CD107a expression on Gag- and Nef-specific CD8+ T cells in 35 HIV-1-infected volunteers. Through the standard 6-h intracellular protocol we are able to detect robust IFN-γ and CD107a (Fig. 1A); however, we are unable to detect surface expression of LAG-3 (data not shown). We therefore developed an assay that would allowed the simultaneous measurement of surface expression of LAG-3 and intracellular IFN-γ and CD107a (Fig. 1B). Twenty-three and six volunteers demonstrated significant LAG-3 expression on Gag- and Nef-specific CD8+ T cell responses, respectively [median=0.29% (range, 0.07–1.24%) and 0.21% (range, 0.09–0.35) for Gag and Nef, respectively]. Eight volunteers demonstrated significant LAG-3 expression on CMV pp65-specific CD8+ T cell responses [median=0.22% (range, 0.09–0.53%)]. No overlap between HIV-specific CD8+ T cells expressing LAG-3 and IFN-γ or CD107a was observed (Fig. 1C). LAG-3 frequencies were significantly higher on Gag- but not on Nef- or CMV pp65-specific CD8+ T cells compared to no antigen-stimulated cells (p<0.0001, p=0.73, and p=0.91, respectively; data not shown). Differences in the frequencies of LAG-3 expression on Gag-specific were significantly higher compared to Nef-specific and CMV pp65-specific CD8+ T cell responses (p<0.0001 and p=0.0002, respectively) (Fig. 1D).
FIG. 1.
Lymphocyte activation gene-3 (LAG-3+) expression on HIV-specific CD8+ T cells does not express CD107a or produce interferon (IFN)-γ. (A) Peripheral blood mononuclear cells (PBMCs) were stimulated for 6 h with HIV antigens Gag B, Nef B, or CMV pp65 in the presence of anti-CD107a PE and Golgi-Stop or (B) restimulated following an initial 24-h stimulation, as described in Materials and Methods, then stained with Vivid viability dye for dead/live cell exclusion, anti-CD3 AmCyan, anti-CD4 PerCP Cy5.5, anti-CD8 APC-Cy7, anti-LAG-3 FITC, and IFN-γ APC, and analyzed by flow cytometry. Samples were gated on the CD3+/CD8+ lymphocyte population and then the percent of CD107a and IFN-γ-positive CD8+ T cells was determined. (C) Representative plots of the frequency LAG-3 on Gag- or Nef-specific CD8+ T cells and CMV pp65-specific CD8+ T cells expressing CD107a and IFN-γ are shown. Staphylococcus enterotoxin B (SEB) is used as a positive control. No significant overlap between Lag-3 expression and CD107a or IFN-γ was observed. (D) LAG-3+CD8+ T cell expression after antigen peptide stimulation compared to no antigen-stimulated control. Gag B-specific LAG-3+CD8+ T cells were statistically significant compared to Nef B or CMV pp65 (p<0.0001 and p=0.0002, respectively). Results were expressed as the percentage of CD107a or IFN-γ-positive CD8+ T cells (percent positive=percent Ag-specific – percent negative control). Responses ≥0.1% and two times the background were considered positive. p values <0.05 were considered significant (n=35).
Immunophenotyping of HIV-specific LAG-3+CD8+ T cells
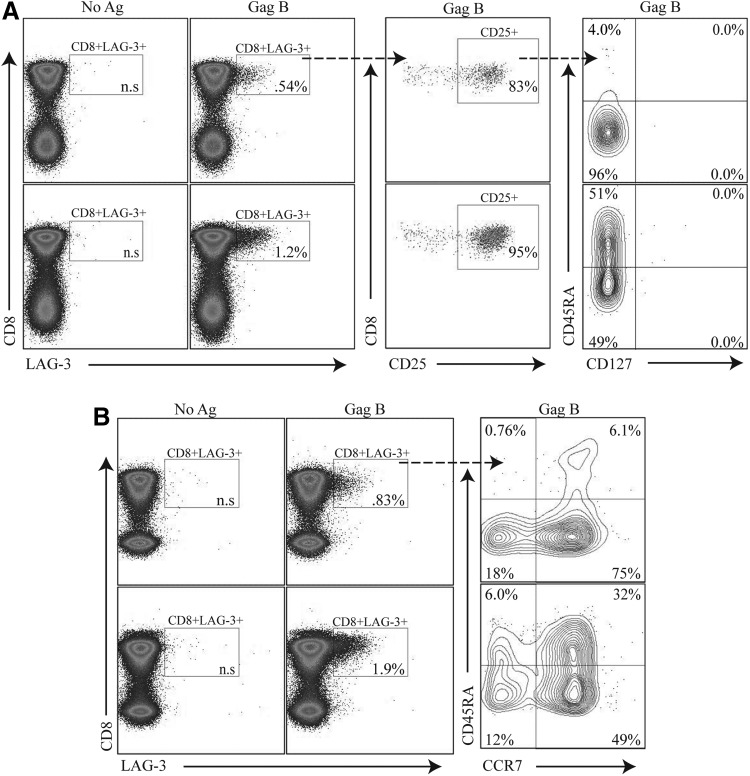
Specific CD8+ T cell functions have been ascribed to distinct populations defined by immunophenotyping, including expression of the homing receptor CCR7, the IL-7 receptor CD127, and different isoforms of CD45RA.27 We examined the level of CD25, CD127, and CD45RA expression on HIV-specific CD8+ T cells expressing LAG-3. Representative plots of the phenotype of the HIV-specific CD8+ T cell population expressing LAG-3 are shown in Fig. 2. LAG3+CD8+ T cells were CD25 positive, CD127 negative and demonstrated a heterogeneous phenotype expression of CD45RA (Fig. 2A) and CCR7 (Fig. 2B).
FIG. 2.
HIV-specific LAG-3+CD8+ T cells express a variable surface immunophenotype. PBMCs were stimulated with HIV peptides then stained with Vivid for live/dead cell exclusion, LAG-3 FITC, CTLA-4 PE-Cy5, CD3 AmCyan, CD8 APC H7, CD25 PE, CD127 Alexa Fluor 647, and CD45RA PE-Cy7 and analyzed by flow cytometry. Samples were first gated on SSC/FSC, followed by SSC/CD3+, and then on the CD3+/CD8+ lymphocyte population; the percent of LAG-3+CD8+ T cells was then determined. (A) Two representative plots of HIV-specific LAG-3+CD8+ T cells after Gag B peptide stimulations that express CD25+ and are CD127 low. (B) HIV-specific LAG-3+CD8+ T cells with variable expression of CD45RA and CCR7. Box inserts within each quadrant represent the indicated cell subpopulations. Dotted arrows indicate further subgating of indicated cell subpopulations.
LAG-3 expression on HIV-specific CD8+ T cells does not correlate with markers of T cell exhaustion
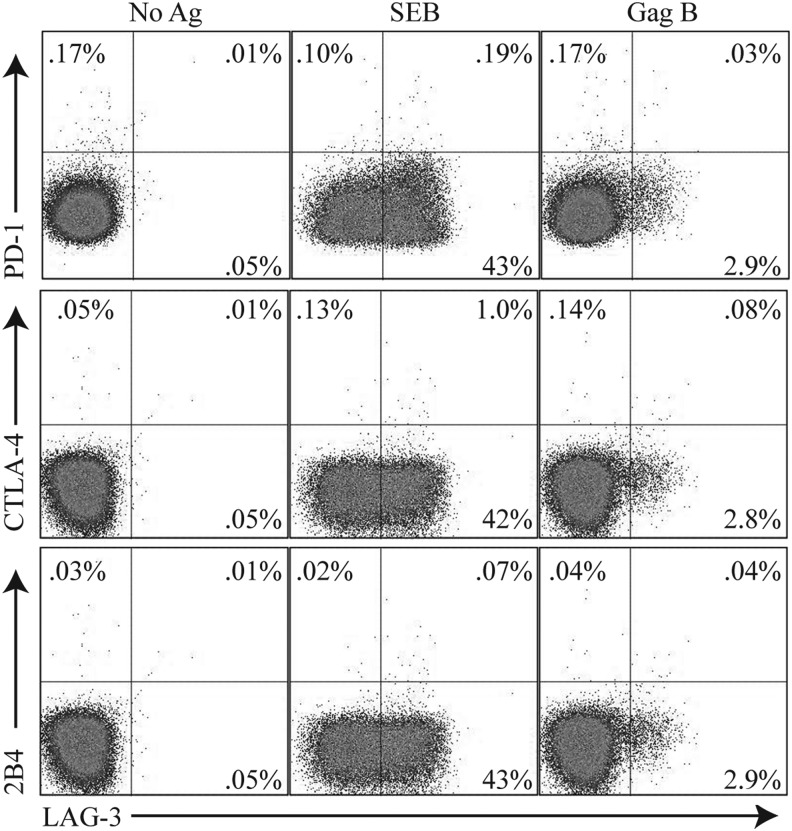
Exhausted HIV-specific CD8+ T cells express high levels of programmed death-1 (PD-1).28–30 The upregulation of CTLA-4 on HIV-specific T cells is also associated with immune dysfunction.31 Expression of 2B4 on CD8+ T cells has been associated with HIV disease progression and decreased killing activity.32,33 However, recent studies on HIV-specific CD8+ T cell exhaustion has been shown to be associated with the complex expression of PD-1, CTLA-4, and 2B4 but not LAG-3.16 We found no evidence of PD-1, CTLA-4, or 2B4 coexpression on CD8+ T cells that express LAG-3 (Fig. 3), consistent with previous studies.16 In addition, no tumor growth factor (TGF)-β or interleukin (IL)-10 production was detected upon peptide stimulation in these Lag3+CD8+ T cells (data not shown). These results suggest that HIV-specific LAG-3+CD8+ T cells represent a unique population distinct from previously described effector or suppressor CD8+ T cells.26,34,35
FIG. 3.
LAG-3+CD8+ T cell populations are distinct from PD1+, CTLA4+, or 2B4+ CD8+ T cells. PBMCs were stimulated with HIV-specific peptide Gag B peptides then stained with Vivid for live/dead cell exclusion, LAG-3 FITC, 2B4 PE, CD3 AmCyan, PD-1 APC, CTLA-4 PE-Cy5, CD4 PE-Cy7, and CD8 APC-H7. Samples were first gated on the CD3+/CD8+ lymphocyte population; the percent of LAG3+, PD-1+ CTLA4+, or 2B4+ cells was then determined. Representative plots of the frequencies of the LAG-3+ Gag-specific CD8+ T cells and expression of PD-1+ CTLA4+ or 2B4+ are shown. Responses ≥0.1% and two times the background were considered positive.
LAG-3 expression on HIV-specific CD8+ T cells negatively correlates with HIV RNA
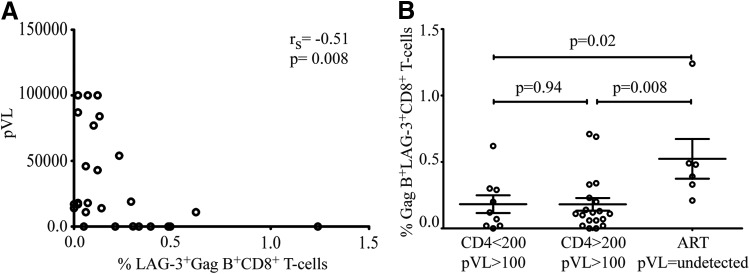
Plasma HIV-1 viral load is closely correlated with disease progression and the level of immune activation.36 Functional impairment of HIV-specific T cells during chronic HIV infection is closely linked to viral replication and is thought to be due to T cell exhaustion.28,37 Surprisingly, we found a significantly negative correlation between the frequency of the LAG-3 positive, HIV-specific CD8+ T cells, and plasma HIV RNA (rs=−0.51, p=0.0008) (Fig. 4A). No correlation between GAG-specific LAG-3-positive and CTLA-4-positive CD8+ T cells was observed (data not shown).
FIG. 4.
Negative correlation between the frequency of HIV-specific LAG-3+CD8+ T cells and HIV plasma RNA. The percentage of Gag-specific LAG-3 is plotted against the HIV plasma RNA. A significant negative correlation between HIV plasma RNA and Gag-specific LAG-3+CD8+ T cells in (A) was observed (rs=−0.51, p=0.0008). (B) Increased LAG-3 expression is also observed in antiretroviral treatment (ART)-mediated viral suppression compared to samples from individuals with CD4 <200 plasma viral load (pVL) >100 and CD4 >200 pVL >100 (p=0.02 and p=0.008, respectively); CD4 >200 (n=19), CD4 <200 (n=9), ART (n=5). Spearman rank correlations with p values<0.05 were considered significant.
Next we stratified our data based on CD4 count and viral suppression into three groups and analyzed Gag B LAG-3+CD8+ frequency. We find that ART-mediated viral suppression correlated with a higher expression of LAG-3 on Gag B-specific CD8+ T cells in individuals with CD4 counts >200 compared to samples from individual not receiving ART with CD4 counts <200 or CD4 >200 counts (p=0.02 and p=0.008, respectively).
Discussion
LAG-3, a transmembrane protein that binds MHC class II, has been previously associated with negative regulation of cellular proliferation, activation, and homeostasis.38 Although LAG-3 has been reported on regulatory T cells, it remains unclear whether LAG-3 expression on CD8+ T cells confers similar regulatory functions in HIV infection. In vitro studies have shown that CD8+LAG-3+CD25+ T cells have a suppressor function imparted through CCL4. These cells also expressed CTLA-4, FoxP3, and CD107a but no IFN-γ. Additionally these cells were described to be mostly CD45RA− and CD127−.38 In these studies we show that Gag-B-specific CD8+LAG-3+CD25+ cells were induced and are CTLA-4, CD107a, and IFN-γ negative. Unlike the above mentioned study we found CD45RA to be heterogeneously expressed and CD127−. In addition, these cells did not produce IL-10 or TGF-β.
Although it is tempting to label these Gag-B specific CD8+LAG-3+CD25+ T cells as suppressor T cells, we cannot rule out the possibility that these cells are activated effector. LAG-3 expression has also been shown to be a marker of recently activated effector cells.9 The expression of CD25 has also been shown to be upregulated on activated T cells.27 Moreover, phenotypic analysis of these cells shows them to be CD127− and a heterogeneous expression of CD45RA and CCR7. The mix phenotypic profile of these Gag-B-specific CD8+LAG-3+ T cells points to an effector-like [effector (CD45RA+CCR7−) or effector memory (CD45RA−CCR7−)] phenotype.39,40
Here, we show that LAG-3 expression is antigen specific and can be delineated from other CD8+ T cells, both inhibitory and effector. Surface LAG-3 expression on activated human T cells is upregulated by interleukin IL-2, IL-7, and IL-12 and thus may play an essential role during activation.41 LAG-3 has been associated as an inhibitory marker and in immune suppression.3,8,9,11,15,16,42,43
Given the similarities in downstream targets, it is tempting to speculate that inhibitory molecules may act antagonistically against other positive costimulation. The presence of LAG-3+ HIV-specific-positive CD8+ T cells was associated with a significantly lower HIV RNA level in our study, arguing that these cells do not have a similar regulatory effect previously ascribed to suppressor T cells. Analysis of the surface marker expression of the LAG-3+ Gag-specific CD8+ T cells displayed a heterogeneous pattern of surface immunophenotypes that does not allow distinction between inhibitory and effector phenotypes. It is not yet clear whether accumulation of T cells expressing various inhibitory or effector molecules is a consequence of chronic HIV-specific immune activation. The direct and indirect role of LAG-3+ HIV-specific CD8-positive cells in cytokine production and CD8+ lytic ability remains unknown. It is likely that many pathways in addition to those activated by inhibitory receptors are involved in the events leading to T cell exhaustion.
We propose that LAG-3 expression by HIV-specific CD8+ T cells identifies a new antigen-specific CD8+ T cell population. Larger longitudinal studies will provide more conclusive answers on the functional role of these CD8+ T cells in T cell homeostasis and immune recovery.
In conclusion, our data show that LAG-3 is upregulated on CD8+ T cells that are HIV specific. Levels of LAG-3 are highest in individuals with lower viral load. A mechanistic understanding of how immune modulation receptors restrain or promote T cell responses would not only aid in our understanding of HIV immunopathogenesis, but could also provide novel targets for therapeutics.
Acknowledgments
This work was supported by NIH grants AI43885, MH54907, AI71772, and K24 MH87227.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Triebel F, Jitsukawa S, Baixeras E, et al. : LAG-3, a novel lymphocyte activation gene closely related to CD4. J Exp Med 1990;171:1393–1405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Workman CJ, Rice DS, Dugger KJ, Kurschner C, and Vignali DAA: Phenotypic analysis of the murine CD4-related glycoprotein, CD223 (LAG-3). Eur J Immunol 2002;32:2255–2263 [DOI] [PubMed] [Google Scholar]
- 3.Workman CJ, Dugger KJ, and Vignali DA: Cutting edge: Molecular analysis of the negative regulatory function of lymphocyte activation gene-3. J Immunol 2002;169:5392–5395 [DOI] [PubMed] [Google Scholar]
- 4.Baixeras E, Huard B, Miossec C, et al. : Characterization of the lymphocyte activation gene 3-encoded protein. A new ligand for human leukocyte antigen class II antigens. J Exp Med 1992;176:327–337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kisielow M, Kisielow J, Capoferri-Sollami G, and Karjalainen K: Expression of lymphocyte activation gene 3 (LAG-3) on B cells is induced by T cells. Eur J Immunol 2005;35:2081–2088 [DOI] [PubMed] [Google Scholar]
- 6.Huard B, Gaulard P, Faure F, Hercend T, and Triebel F: Cellular expression and tissue distribution of the human LAG-3-encoded protein, an MHC class II ligand. Immunogenetics 1994;39:213–217 [DOI] [PubMed] [Google Scholar]
- 7.Triebel F: LAG-3: A regulator of T-cell and DC responses and its use in therapeutic vaccination. Trends Immunol 2003;24:619–622 [DOI] [PubMed] [Google Scholar]
- 8.Workman CJ. and Vignali DA: Negative regulation of T cell homeostasis by lymphocyte activation gene-3 (CD223). J Immunol 2005;174:688–695 [DOI] [PubMed] [Google Scholar]
- 9.Workman CJ, Cauley LS, Kim IJ, Blackman MA, Woodland DL, and Vignali DA: Lymphocyte activation gene-3 (CD223) regulates the size of the expanding T cell population following antigen activation in vivo. J Immunol 2004;172:5450–5455 [DOI] [PubMed] [Google Scholar]
- 10.Macon-Lemaitre L. and Triebel F: The negative regulatory function of the lymphocyte-activation gene-3 co-receptor (CD223) on human T cells. Immunology 2005;115:170–178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hannier S, Tournier M, Bismuth G, and Triebel F: CD3/TCR complex-associated lymphocyte activation gene-3 molecules inhibit CD3/TCR signaling. J Immunol 1998;161:4058–4065 [PubMed] [Google Scholar]
- 12.Hannier S. and Triebel F: The MHC class II ligand lymphocyte activation gene-3 is co-distributed with CD8 and CD3-TCR molecules after their engagement by mAb or peptide-MHC class I complexes. Int Immunol 1999;11:1745–1752 [DOI] [PubMed] [Google Scholar]
- 13.Huang CT, Workman CJ, Flies D, et al. : Role of LAG-3 in regulatory T cells. Immunity 2004;21:503–513 [DOI] [PubMed] [Google Scholar]
- 14.Shin H. and Wherry EJ: CD8 T cell dysfunction during chronic viral infection. Curr Opin Immunol 2007;19:408–415 [DOI] [PubMed] [Google Scholar]
- 15.Blackburn SD, Shin H, Haining WN, et al. : Coregulation of CD8+ T cell exhaustion by multiple inhibitory receptors during chronic viral infection. Nat Immunol 2009;10:29–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yamamoto T, Price DA, Casazza JP, et al. : Surface expression patterns of negative regulatory molecules identify determinants of virus-specific CD8+ T-cell exhaustion in HIV infection. Blood 2011;117:4805–4815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Price P, Keane N, Gray L, Lee S, Gorry PR, and French MA: Brief Report: CXCR4 or CCR5 tropism of human immunodeficiency virus type 1 isolates does not determine the immunological milieu in patients responding to antiretroviral therapy. Viral Immunol 2006;19:734–740 [DOI] [PubMed] [Google Scholar]
- 18.Li Q, Smith AJ, Schacker TW, et al. : Microarray analysis of lymphatic tissue reveals stage-specific, gene expression signatures in HIV-1 infection. J Immunol 2009;183:1975–1982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grosso JF, Goldberg MV, Getnet D, et al. : Functionally distinct LAG-3 and PD-1 subsets on activated and chronically stimulated CD8 T cells. J Immunol 2009;182:6659–6669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lim AYF, Price P, Beilharz MW, and French MA: Cell surface markers of regulatory T cells are not associated with increased forkhead box p3 expression in blood CD4+ T cells from HIV-infected patients responding to antiretroviral therapy. Immunol Cell Biol 2006;84:530–536 [DOI] [PubMed] [Google Scholar]
- 21.Moss AR, Hahn JA, Perry S, et al. : Adherence to highly active antiretroviral therapy in the homeless population in San Francisco: A prospective study. Clin Infect Dis 2004;39:1190–1198 [DOI] [PubMed] [Google Scholar]
- 22.Elrefaei M, Ventura FL, Baker CA, Clark R, Bangsberg DR, and Cao H: HIV-specific IL-10-positive CD8+ T cells suppress cytolysis and IL-2 production by CD8+ T cells. J Immunol 2007;178:3265–3271 [DOI] [PubMed] [Google Scholar]
- 23.Elrefaei M, Baker CA, Jones NG, Bangsberg DR, and Cao H: Presence of suppressor HIV-specific CD8+ T cells is associated with increased PD-1 expression on effector CD8+ T cells. J Immunol 2008;180:7757–7763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maecker HT, Dunn HS, Suni MA, et al. : Use of overlapping peptide mixtures as antigens for cytokine flow cytometry. J Immunol Methods 2001;255:27–40 [DOI] [PubMed] [Google Scholar]
- 25.Elrefaei M, El-sheikh N, Kamal K, and Cao H: HCV-specific CD27− CD28− memory T cells are depleted in hepatitis C virus and Schistosoma mansoni co-infection. Immunology 2003;110:513–518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Elrefaei M, Barugahare B, Ssali F, Mugyenyi P, and Cao H: HIV-specific IL-10-positive CD8+ T cells are increased in advanced disease and are associated with decreased HIV-specific cytolysis. J Immunol 2006;176:1274–1280 [DOI] [PubMed] [Google Scholar]
- 27.Chattopadhyay PK. and Roederer M: Good cell, bad cell: Flow cytometry reveals T-cell subsets important in HIV disease. Cytometry A 2010;77:614–622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Day CL, Kaufmann DE, Kiepiela P, et al. : PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature 2006;443:350–354 [DOI] [PubMed] [Google Scholar]
- 29.Petrovas C, Casazza JP, Brenchley JM, et al. : PD-1 is a regulator of virus-specific CD8+ T cell survival in HIV infection. J Exp Med 2006;203:2281–2292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Trautmann L, Janbazian L, Chomont N, et al. : Upregulation of PD-1 expression on HIV-specific CD8+ T cells leads to reversible immune dysfunction. Nat Med 2006;12:1198–1202 [DOI] [PubMed] [Google Scholar]
- 31.Kaufmann DE, Kavanagh DG, Pereyra F, et al. : Upregulation of CTLA-4 by HIV-specific CD4+ T cells correlates with disease progression and defines a reversible immune dysfunction. Nat Immunol 2007;8:1246–1254 [DOI] [PubMed] [Google Scholar]
- 32.Aldy KN, Horton NC, Mathew PA, and Mathew SO: 2B4+ CD8+ T cells play an inhibitory role against constrained HIV epitopes. Biochem Biophys Res Commun 2011;405:503–507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Peritt D, Sesok-Pizzini DA, Schretzenmair R, et al. : C1.7 antigen expression on CD8+ T cells is activation dependent: Increased proportion of C1.7+CD8+ T cells in HIV-1-infected patients with progressing disease. J Immunol 1999;162:7563–7568 [PubMed] [Google Scholar]
- 34.Zanussi S, Simonelli C, D'Andrea M, et al. : CD8+ lymphocyte phenotype and cytokine production in long-term non-progressor and in progressor patients with HIV-1 infection. Clin Exp Immunol 1996;105:220–224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Garba ML, Pilcher CD, Bingham AL, Eron J, and Frelinger JA: HIV antigens can induce TGF-beta(1)-producing immunoregulatory CD8+ T cells. J Immunol 2002;168:2247–2254 [DOI] [PubMed] [Google Scholar]
- 36.D'Souza M, Fontenot AP, Mack DG, et al. : Programmed death 1 expression on HIV-specific CD4+ T cells is driven by viral replication and associated with T cell dysfunction. J Immunol 2007;179:1979–1987 [DOI] [PubMed] [Google Scholar]
- 37.Zhang JY, Zhang Z, Wang X, et al. : PD-1 up-regulation is correlated with HIV-specific memory CD8+ T-cell exhaustion in typical progressors but not in long-term nonprogressors. Blood 2007;109:4671–4678 [DOI] [PubMed] [Google Scholar]
- 38.Joosten SA, van Meijgaarden KE, Savage NDL, et al. : Identification of a human CD8+ regulatory T cell subset that mediates suppression through the chemokine CC chemokine ligand 4. Proc Natl Acad Sci USA 2007;104:8029–8034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Addo MM, Draenert R, Rathod A, et al. : Fully differentiated HIV-1 specific CD8+ T effector cells are more frequently detectable in controlled than in progressive HIV-1 infection. PLoS One 2007;2:e321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Paiardini M, Cervasi B, Albrecht H, et al. : Loss of CD127 expression defines an expansion of effector CD8+ T cells in HIV-infected individuals. J Immunol 2005;174:2900–2909 [DOI] [PubMed] [Google Scholar]
- 41.Bruniquel D, Borie N, Hannier S, and Triebel F: Regulation of expression of the human lymphocyte activation gene-3 (LAG-3) molecule, a ligand for MHC class II. Immunogenetics 1998;48:116–124 [DOI] [PubMed] [Google Scholar]
- 42.Iouzalen N, Andreae S, Hannier S, and Triebel F: LAP, a lymphocyte activation gene-3 (LAG-3)-associated protein that binds to a repeated EP motif in the intracellular region of LAG-3, may participate in the down-regulation of the CD3/TCR activation pathway. Eur J Immunol 2001;31:2885–2891 [DOI] [PubMed] [Google Scholar]
- 43.Workman CJ. and Vignali DA: The CD4-related molecule, LAG-3 (CD223), regulates the expansion of activated T cells. Eur J Immunol 2003;33:970–979 [DOI] [PubMed] [Google Scholar]